Abstract
Parenteral nutrition (PN)-associated liver disease (PNALD) is a life-threatening complication of the administration of PN. The development of PNALD may be partly due to the composition of the lipid emulsion administered with PN: soybean oil-based lipid emulsions (SOLE) are associated with liver disease, while fish oil-based lipid emulsions (FOLE) are associated with prevention and improvement of liver disease. The objective of this study was to determine how the choice of lipid emulsion modified the production of bioactive lipid mediators (LMs). We utilized a mouse model of steatosis to study the differential effect of FOLE and SOLE. We subsequently validated these results in serum samples from a small cohort of human infants transitioning from SOLE to FOLE. In mice, FOLE was associated with production of anti-inflammatory, proresolving LMs; SOLE was associated with increased production of inflammatory LMs. In human infants, the transition from SOLE to FOLE was associated with a shift toward a proresolving lipidome. Together, these results demonstrate that the composition of the lipid emulsion directly modifies inflammatory homeostasis.
Keywords: parenteral nutrition, liver disease, lipid mediators, omega-3, omega-6
intravenous lipid emulsions are coadministered with parenteral nutrition (PN) to provide nonprotein calories and essential fatty acids. The administration of intravenous lipids is associated with a severe liver disease, PN-associated liver disease (PNALD), which ranges in pathology from steatosis to cholestasis to fibrosis and cirrhosis. Neonates and infants receiving PN are at the highest risk for liver disease, with PNALD occurring in up to two-thirds of infants (35). One-year mortality in children with PNALD approaches 100% if they are not weaned from PN or do not receive a liver and/or intestinal transplant (39).
The composition of the lipid emulsion has been implicated in the development of liver disease. The only US Food and Drug Administration-approved lipid emulsions are plant-derived (manufactured from safflower oil, soybean oil, or a combination of safflower and soybean oils). Soybean oil-based lipid emulsion (SOLE) is associated with steatosis and liver dysfunction in mice, while fish oil-based lipid emulsion (FOLE) prevents steatosis and normalizes liver function (1, 18). Subsequently, FOLE was shown to prevent and reverse PNALD in children, leading to a reduction in rates of PNALD-related liver transplants and mortality (23).
The beneficial effect of FOLE is thought to arise from the fact that ω-3 polyunsaturated fatty acids (PUFAs), including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are present in high concentration in FOLE, are the parent molecules for anti-inflammatory eicosanoids and proresolving lipid mediators (LMs). Conversely, ω-6 PUFAs, including arachidonic acid (AA), which is present in high concentration in SOLE, are the parent molecules for predominantly proinflammatory eicosanoids (27). The direct relationship between lipid emulsion content and inflammatory homeostasis is unknown.
More recently, the paradigm for understanding the anti-inflammatory effects of ω-3 PUFAs has shifted with the discovery of novel families of LMs termed “specialized proresolving mediators” (SPMs) (31, 32). These molecules are autacoid endogenous mediators of cellular programs to restore tissue homeostasis and actively resolve inflammation. This novel class includes the ω-6 fatty acid-derived lipoxins (LXs), as well as LM autacoids derived from ω-3 fatty acids: resolvins (Rvs), protectins, and maresins (MaRs) (30, 33, 34). The properties and actions of each of these molecules have been recently reviewed and are briefly summarized in Table 1 (32).
Table 1.
Summary of key lipid mediators
| Lipid Mediator | Description |
|---|---|
| PUFAs | |
| DHA | ω-3 fatty acid in cold water fish; widely studied for anti-inflammatory properties and proposed health benefits, particularly in cardiovascular disease, neurological disease, and cancer; essential for nervous system development; precursor to D-series resolvins, protectins, and maresins |
| EPA | ω-3 fatty acid in cold water fish; widely studied for anti-inflammatory properties and proposed health benefits, particularly in cardiovascular disease, neurological disease, and cancer; precursor to E-series resolvins |
| AA | ω-6 fatty acid that serves as a precursor to inflammatory eicosanoids, including PGs and leukotrienes, in addition to serving as a precursor to the counterregulatory LXs |
| Specialized proresolving mediators | LM autacoids derived from ω-3 PUFAs that resolve inflammation and restore tissue homeostasis |
| Resolvins (e.g., RvD1, RvD2, RvD5, RvE1) | Inhibits inflammatory cell infiltration and reduces proinflammatory cytokine expression; protective in numerous disease states, including peritonitis, atherosclerosis, colitis, insulin resistance, and ischemia-reperfusion |
| PD1 | Most well-known for neuroprotective properties; also reduces neutrophil recruitment, blocks T cell migration, and inhibits TNF-α and IFN-γ production; in murine asthma, PD1 decreases eosinophilic and lymphocytic infiltration of the airways, airway hyperreactivity, and inflammatory cytokine production |
| MaR1 | Limits neutrophil infiltration in murine peritonitis and enhances macrophage uptake of apoptotic neutrophils; also accelerates surgical regeneration and controls inflammatory- and chemotherapy-induced pain |
| LX | Counterregulates leukocyte trafficking, reduces neutrophil chemotaxis, and increases macrophage phagocytosis of apoptotic leukocytes |
| HDHAs | Oxygenated products of DHA; 4-HDHA is antiangiogenic and mediates protective effects of dietary DHA in a mouse model of retinopathy of prematurity; 14- and 17-HDHA are precursors to MaR1 and PD1, respectively; 17-HDHA suppresses murine macrophage production of TNF-α in response to LPS and is protective in mouse models of arthritis, colitis, and renal reperfusion injury |
| Other lipid mediators | |
| HETEs | Possesses proinflammatory properties that mediate the pathophysiology of hypertension, thrombosis, cancer, atherosclerosis, and inflammation |
| HEPEs | Lipoxygenase metabolites of EPA; 18-HEPE is a biomarker of the activation of proresolving E-series resolvins |
| PGs | Increase in concentration at the site of acute inflammation; promote cellular growth, decrease vascular smooth muscle tone, and increase pain sensitization and thrombus formation |
| TXs | AA is metabolized to TXA2, which is rapidly degraded to the more inert, but stable, TXB2; TXs promote hemostasis through platelet aggregation, vasoconstriction, leukocyte adhesion, and TNF-α production |
| LTB4 | Proinflammatory; promotes recruitment and activation of neutrophils, macrophages, and eosinophils; elevated LTB4 concentrations have been found in a variety of inflammatory states, including asthma, chronic obstructive pulmonary disease, and rheumatoid arthritis |
PUFAs, polyunsaturated fatty acids; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; AA, arachidonic acid; LM, lipid mediator; PD1, protectin D1; MaR1, maresin 1; LX, lipoxin; HDHAs, hydroxydocosahexaenoic acids; HETEs, hdroxyeicosatetrienoic acids; HEPEs, hydroxyeicosapentaenoic acids; TXs, thromboxanes; LTB4, leukotriene B4. See Refs. 3, 5, 30–34, and 40.
Local tissue biosynthesis of LMs is an important regulator of inflammatory homeostasis. The differential regulation of LM pathways directly mediates tissue damage and repair in a variety of pathological states. AA-derived mediators, such as leukotrienes (LTs), PGs, and thromboxane (TXs), largely promote the recruitment and activation of inflammatory cells. SPMs serve as counterregulatory signals to limit the extent of an inflammatory response by restricting inflammatory cell chemotaxis and limiting the production of inflammatory cytokines. SPMs have a protective role in a variety of disease states, including peritonitis, colitis, pain, arthritis, asthma, ischemia-reperfusion, atherosclerosis, acute lung injury, retinopathy, and insulin resistance (3, 31, 32).
Recently, Mas and others (17, 22) established that healthy human subjects receiving 3 wk of oral ω-3 fatty acid supplementation have concentrations of SPMs that are within the biological range known to have proresolving activity in human leukocytes and mice. We hypothesized that FOLE would promote tissue formation of anti-inflammatory and proresolving mediators in vivo; therefore, we sought to describe lipidomic profiles in a murine model of hepatic steatosis and human infants receiving PN.
MATERIALS AND METHODS
Animal Studies
Animal protocols complied with the National Institutes of Health Animal Research Advisory Committee guidelines and were approved by the Boston Children's Hospital Animal Care and Use Committee.
A murine model of hepatic steatosis was induced by oral administration of a PN solution to mice, as previously described (1, 18). Male 6-wk-old C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) were randomized to three groups. All groups were placed on an exclusive ad libitum liquid, fat-free, high-carbohydrate diet (HCD). This solution was identical to typical PN solutions used at Boston Children's Hospital. It contained 20% dextrose, a mixture of 2% essential and nonessential amino acids (TrophAmine, B. Braun Medical, Irvine, CA), 0.2% pediatric trace elements (American Reagent, Shirley, NY), pediatric multivitamins (Hospira, Lake Forest, IL), 30 meq of sodium, 20 meq of potassium, 15 meq of calcium, 10 meq of magnesium, 10 mmol of phosphate, 36.67 meq of chloride, and 19.4 meq of acetate per liter. This liquid diet was placed in a single bottle per cage and replaced daily to avoid contamination. Mice received no other source of nutrition or hydration.
Group 1 received the HCD plus intravenous normal saline (hereafter designated “HCD”). Groups 2 and 3 received commercial lipid emulsions via tail vein injections every other day (2.4 g fat·kg body wt−1·day−1): group 2 received the HCD plus FOLE (Omegaven, Fresenius Kabi, Bad Homburg vor der Höhe, Germany), and group 3 received the HCD plus SOLE (Intralipid, Fresenius Kabi, Uppsala, Sweden, for Baxter Healthcare, Deerfield, IL). The composition of the lipid emulsions is shown in Table 2.
Table 2.
Composition of FOLE and SOLE
| Intralipid | Omegaven | |
|---|---|---|
| Oil source, g | ||
| Soybean oil | 10 | 0 |
| Fish oil | 0 | 10 |
| α-Tocopherol, mg/l | 38 | 150–296 |
| Phytosterols, mg/l | 348 ± 33 | 0 |
| Fat composition, g | ||
| Linoleic | 5.0 | 0.1–0.7 |
| α-Linolenic | 0.9 | <0.2 |
| Arachidonic | 0 | 0.1–0.4 |
| DHA | 0 | 1.44–3.09 |
| EPA | 0 | 1.28–2.82 |
| Oleic | 2.6 | 0.6–1.3 |
| Stearic | 0.35 | 0.05–0.2 |
| Palmitic | 1.0 | 0.25–1 |
Fish oil-derived lipid emulsion (FOLE, Omegaven) and soybean oil-derived lipid emulsion (SOLE, Intralipid) contain 10 g fat/100 ml.
Mice were housed five per wire-bottom cage in a barrier room with regulated temperature (21 ± 1.6°C) and humidity (45 ± 10%) and an alternating 12:12-h light-dark cycle. After 19 days, the animals were euthanized by carbon dioxide inhalation. At the time of animal euthanasia, a designated lobe of the liver was collected as frozen sections, placed in embedding medium (Tissue-Tek O.C.T. Compound, Sakura Finetek, Torrance, CA), and promptly immersed in liquid nitrogen. Sections were stained at the Department of Pathology, Boston Children's Hospital with Oil Red O for hepatic fat.
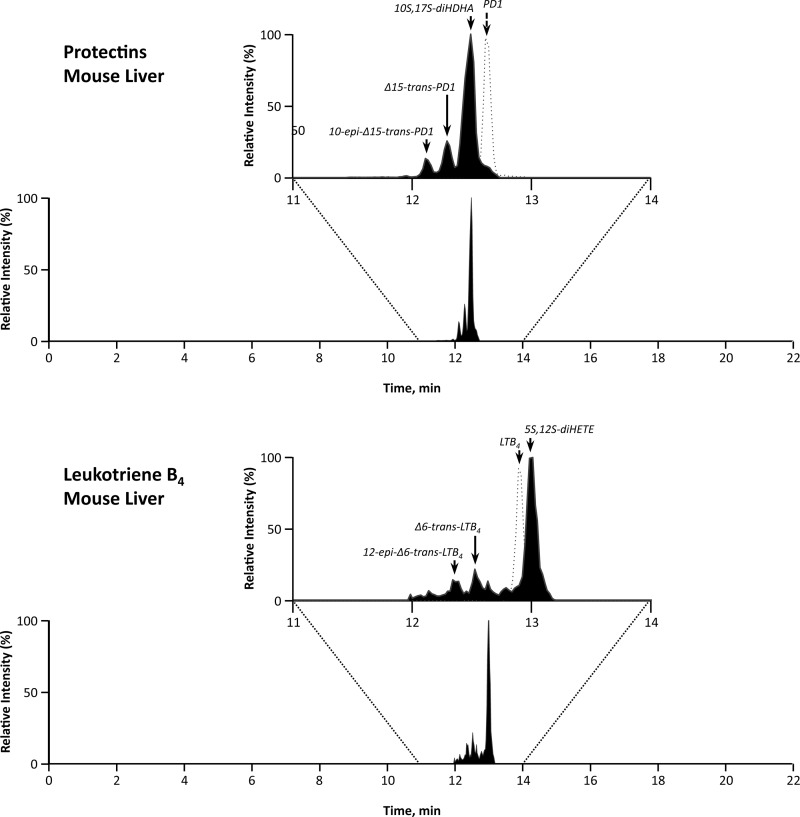
The remaining liver sections were immediately snap-frozen and stored at −80°C. LMs were extracted from murine liver tissues via solid-phase extraction. Liquid chromatography (LC)-tandem mass spectrometry (MS/MS) was performed with a Shimadzu LC-20AD HPLC (Shimadzu Scientific Instruments, Columbia, MD) equipped with an Agilent Eclipse Plus C18 column (4.6 mm × 50 mm × 1.8 μm) paired with a Sciex Instruments 5500 QTRAP linear ion-trap triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). Analyst 1.5 software (Applied Biosystems) was used for instrument control and data acquisition. The mobile phase consisted of 60:40:0.01 (vol/vol/vol) methanol-water-acetic acid and was ramped to 100:0:0.01 (vol/vol/vol) methanol-water-acetic acid after 16.5 min at a rate of 500 μl/min to wash and equilibrate the column. Ion pairs from multiple-reaction monitoring methods carried out previously were used to profile and quantify individual LMs. The criteria for identification of each LM are described elsewhere (40). Representative spectra are displayed in Fig. 1. Briefly, each LM from murine tissues was matched using retention time and at least six diagnostic ions compared with synthetic standards and, where available, authentic standards. Quantification was performed using calibration curves for each product and LM, and recoveries were determined using deuterated internal standards for each chromatographic region of interest. Data were analyzed using one-way ANOVA with Bonferroni's multiple comparison test. Statistical significance was defined as P < 0.05.
Fig. 1.
Representative multiple reaction monitoring chromatograms for lipid mediators (LMs) in leukotriene B4 (LTB4) and protectin (PD1) pathways. HDHA, hydroxydocosahexaenoic acid; HETE, hydroxyeicosatetraenoic acid.
Human Studies
We selected infants who were admitted to the Boston Children's Hospital Neonatal Intensive Care Unit for liver failure and PN. All the infants had received prolonged intravenous lipid therapy with SOLE. According to standard clinical care at our institution, these infants were transitioned to FOLE (1 g·kg−1·day−1). This study was approved by the Institutional Review Board at Boston Children's Hospital.
Three blood samples were collected: 1) immediately prior to initiation of FOLE (after several weeks of SOLE), 2) 1 wk after initiation of FOLE, and 3) 4 wk after initiation of FOLE. Blood was collected in Vacutainer serum separator tubes (BD Biosciences, Franklin Lakes, NJ). Blood was allowed to clot at room temperature for 10 min and then centrifuged at 1,000 relative centrifugal force for 10 min. Serum was extracted and frozen at −80°C.
LM autacoids were extracted using a solid-phase extraction technique with a SampliQ ODS-C18 cartridges (Agilent Technologies). Eicosanoids and docosanoids were identified and quantified by LC-MS/MS-based lipidomics, as described elsewhere (11, 38a, 28, 40). Deuterated internal standards [PGE2, LXA4, LTB4, 15-hydroxyeicosatetraenoic acid (HETE), AA, and DHA] were added prior to extraction to correct for class-specific recovery and extraction efficiency. Briefly, using a triple-quadrupole linear ion-trap LC-MS/MS system (MDS Sciex 3200 QTRAP) equipped with a Kinetex C18 minibore column, we analyzed solid-phase extracted samples. The mobile phase consisted of gradients A [72:28:0.01 water-acetonitrile-acetic acid (by volume)] and B [60:40 propan-2-ol-acetonitrile (vol/vol)] with a flow rate of 450 μl/min. MS/MS analyses were performed in negative-ion mode, and prominent fatty acid-derived bioactive products and biosynthesis pathway markers were quantified in multiple-reaction monitoring mode. Calibration curves (1–1,000 pg) and specific LC retention times for each lipid mediator and pathway markers were established with synthetic standards (Cayman Chemical).
RESULTS
Animal Studies
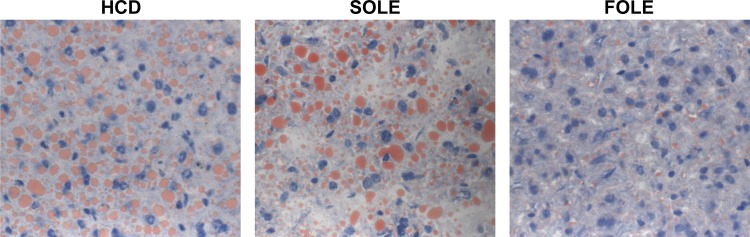
The administration of an oral HCD without lipid induced hepatic steatosis. This fatty infiltration is consistent with a high-glucose diet and an essential fatty acid deficiency (Fig. 2). Administration of SOLE was associated with macro- and microvesicular hepatic steatosis, whereas administration of FOLE reduced the degree of steatosis (Fig. 2). Liver MRI analysis demonstrated hepatic fat content (mean ± SD) to be 28.7 ± 6.1% in HCD, 15.2 ± 4.8% in SOLE, and 8.5 ± 2.1% in FOLE (P < 0.05).
Fig. 2.
Representative liver sections from mice fed high-carbohydrate diet (HCD), fish oil-based lipid emulsion (FOLE), and soybean oil-based lipid emulsion (SOLE). Liver sections were stained with Oil Red O for intracellular lipid and photographed at ×400 magnification. HCD and SOLE resulted in macro- and microvesicular steatosis; FOLE resulted in reduction of visible lipid droplets.
Eicosanoid metabolome.
Levels of 12- and 15-HETE increased by 51.9% and 103.1%, respectively, in SOLE compared with HCD (Table 3). HETEs were lower in FOLE than SOLE, with a 34.6% reduction in 12-HETE and a 42.7% reduction in 15-HETE.
Table 3.
Levels of specialized proresolving and proinflammation mediators in liver tissue of mice fed PN with SOLE or FOLE
| Pathway Marker Transitions |
LM, pg/500 mg liver |
||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 3 | HCD | SOLE | FOLE | |
| DHA bioactive metabolome | |||||
| RvD1 | 375 | 215 | * | * | * |
| RvD2 | 375 | 141 | * | * | * |
| RvD3 | 375 | 147 | * | * | * |
| RvD5 | 359 | 199 | * | * | * |
| RvD6 | 359 | 159 | * | * | * |
| PD1 | 359 | 153 | * | * | * |
| 10S,17S-diHDHA | 359 | 153 | 222 ± 93 | 173 ± 20 | 493 ± 217 |
| 10-epi-Δ15-trans-PD1 | 359 | 153 | 38.4 ± 17.5 | 20.5 ± 0.03 | 72.7 ± 46.8 |
| Δ15-trans-PD1 | 359 | 153 | 25.2 ± 11.0 | 24.4 ± 2.7 | 68.6 ± 27.3 |
| 22-OH-PD1 | 375 | 153 | * | * | * |
| 22-COOH-PD1 | 389 | 153 | * | * | * |
| MaR1 | 359 | 250 | * | * | * |
| 7S,14S-diHDHA | 359 | 250 | 50.3 ± 29.7 | 49.9 ± 7.1 | 233 ± 120 |
| 7-epi-Δ12-trans-MaR1 | 359 | 250 | 74.3 ± 35.5 | 77.8 ± 6.9 | 136 ± 40 |
| Δ12-trans-MaR1 | 359 | 250 | 28.7 ± 15.1 | 46.6 ± 0.3 | 99.5 ± 24.8 |
| 4S,14S-diHDHA | 359 | 101 | 80.8 ± 39.1 | 41.6 ± 16.2 | 250 ± 101 |
| DHA pathway markers | |||||
| 17-HDHA | 343 | 245 | 383 ± 64 | 243 ± 58 | 1,006 ± 141†§§ |
| 14-HDHA | 343 | 205 | 2,891 ± 311 | 2,375 ± 527 | 6,363 ± 1,300§ |
| 7-HDHA | 343 | 141 | 47.9 ± 15.2 | 35.0 ± 3.0 | 145 ± 32†§ |
| 4-HDHA | 343 | 101 | 124 ± 32 | 68.0 ± 12.0 | 309 ± 89 |
| EPA bioactive metabolome | |||||
| RvE1 | 349 | 195 | * | * | * |
| RvE2 | 333 | 253 | * | * | * |
| RvE3 | 333 | 201 | * | * | * |
| LXA5 | 349 | 215 | * | * | * |
| LXB5 | 349 | 221 | * | * | * |
| 5S,15S-diHEPE | 333 | 253 | 61.0 ± 50.7 | 30.4 ± 15.2 | 471 ± 399 |
| EPA pathway markers | |||||
| 18-HEPE | 317 | 259 | 35.4 ± 21.6 | 323 ± 113 | 4,678 ± 388†††§§§ |
| 15-HEPE | 317 | 219 | 93.8 ± 54.9 | 480 ± 157 | 2,543 ± 152†††§§§ |
| 12-HEPE | 317 | 179 | 311 ± 151 | 3,042 ± 691 | 2,2809 ± 5,514††§ |
| 5-HEPE | 317 | 115 | 14.8 ± 0.8 | 59.9 ± 15.5 | 337 ± 43†††§§§ |
| AA bioactive metabolome | |||||
| LXA4 | 335 | 115 | * | * | * |
| LXB4 | 351 | 221 | * | * | * |
| 5S,15S-diHETE | 335 | 235 | 96.8 ± 37.3 | 203 ± 28 | 162 ± 65 |
| LTB4 | 335 | 195 | * | * | * |
| 5S,12S-diHETE | 335 | 195 | 16.3 ± 5.1 | 19.1 ± 4.5 | 69.9 ± 34.6 |
| 12epi-Δ6-trans-LTB4 | 335 | 195 | 11.1 ± 5.0 | 8.0 ± 1.0 | 2.6 ± 1.3 |
| Δ6-trans-LTB4 | 335 | 195 | 13.3 ± 5.2 | 10.9 ± 0.4 | 3.3 ± 1.6 |
| 20-OH-LTB4 | 351 | 195 | * | * | * |
| 20-COOH-LTB4 | 365 | 195 | * | * | * |
| PGD2 | 351 | 189 | 770 ± 489 | 792 ± 168 | 198 ± 72 |
| PGE2 | 351 | 189 | 525 ± 393 | 197 ± 16 | 123 ± 56 |
| PGF2α | 353 | 193 | 925 ± 413 | 1,731 ± 668 | 283 ± 105 |
| TXB2 | 369 | 169 | 60.8 ± 45.8 | 24.6 ± 5.6 | 17.2 ± 6.3 |
| AA pathway markers | |||||
| 15-HETE | 319 | 219 | 681 ± 135 | 1,383 ± 374 | 792 ± 85 |
| 12-HETE | 319 | 179 | 3,325 ± 374 | 5,051 ± 769 | 3,302 ± 246 |
| 5-HETE | 319 | 115 | 138 ± 29 | 113 ± 10 | 52.4 ± 3.7† |
| Sum of proresolving mediators | 677 ± 250 | 667 ± 19 | 1,984 ± 1,040 | ||
| Sum of proinflammatory mediators | 2,261 ± 1,130 | 2,758 ± 831 | 680 ± 253 | ||
| Proresolution index | 0.3 | 0.2 | 2.9 | ||
| (sum of proresolving mediators/sum of inflammatory mediators) | |||||
LM values are means ± SE (n = 3). Quartile 1, M-H (parent ion); quartile 3, diagnostic ions in the MS-MS (daughter ion).
Below limits of detection (detection limit was ∼2 pg in tissue matrix). Ion suppression effect was 85–92%.
P < 0.05,
P < 0.01,
P < 0.001 vs. HCD.
P < 0.05,
P < 0.01,
P < 0.001 vs. SOLE.
Relative to HCD, levels of 12-epi-Δ6-trans-LTB4 and Δ6-trans-LTB4 were 27.9% and 18.0% lower, respectively, in SOLE, and 76.6% and 75.2% lower, respectively, in FOLE.
The proinflammatory and thrombotic mediators PGE2 and TXB2 decreased in SOLE relative to HCD by 62.5% and 59.5%. FOLE decreased levels of PGD2, PGE2, PGF2α, and TXB2 by 75.0%, 37.6%, 83.7%, and 30.1% relative to SOLE.
EPA metabolome.
Relative to HCD, SOLE increased levels of 5-, 12-, 15-, and 18-hydroperoxyeicosapentaenoic acid (HEPE) by 304.7%, 878.1%, 411.7%, and 812.4%, respectively (Table 3). Compared with SOLE, FOLE further increased levels of 5-, 12-, 15-, and 18-HEPE by 462.6%, 649.8%, 429.8%, and 1,348.3%, respectively.
Compared with HCD, the level of 5S,15S-diHEPE was 50.2% lower in SOLE and 672.1% higher in FOLE.
DHA metabolome.
Levels of 4-, 7-, 14-, and 17-hydroxydocosahexaenoic acid (HDHA) were lower in SOLE (45.2%, 26.9%, 17.8%, and 36.6%, respectively) than HCD (Table 3). FOLE increased levels of 4-, 7-, 14-, and 17-HDHA compared with SOLE by 354.4%, 314.3% (P < 0.05), 167.9% (P < 0.05), and 314.0% (P < 0.01), respectively.
Levels of 10S,17S-diHDHA, 10-epi-Δ15-trans-protectin D1 (PD1), and Δ15-trans-PD1 increased in FOLE by 185.0%, 254.6%, and 181.1%, respectively, compared with SOLE. Similarly, levels of 7S,14S-diHDHA, 7-epi-Δ12-trans-MaR1, Δ12-trans-MaR1, and 4S,14S-diHDHA increased in FOLE by 363.2%, 74.8%, 113.5%, and 501.0%, respectively.
Summary of LM analysis.
The proresolution index (ratio of summation of proresolving mediators to summation of inflammatory mediators) was 0.3 in HCD, 0.2 in SOLE, and 2.9 in FOLE (Table 3).
Human Studies
Patient 1.
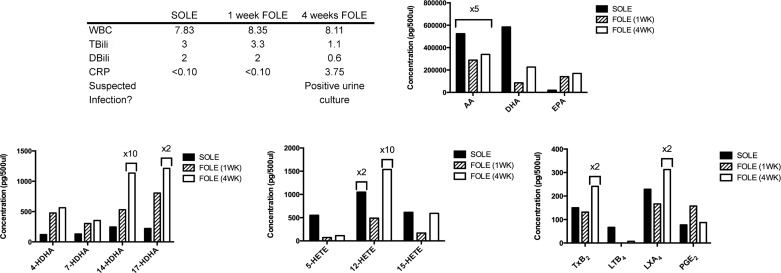
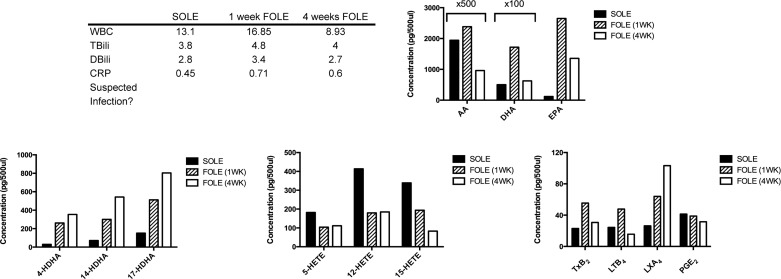
Patient 1 was a male born at 36 wk gestation with jejunal atresia. Multiple abdominal operations resulted in short bowel syndrome. Patient 1 was dependent on SOLE for 6.1 wk prior to initiation of FOLE. Clinical parameters at the three time points of blood collection are summarized in Fig. 3. Patient 1 was diagnosed with a urinary tract infection on the day prior to collection of the third blood sample. Patient 1 was weaned from PN 5.1 wk after initiation of FOLE.
Fig. 3.
Bioactive lipid mediator (LM) profile for patient 1. Table outlines selected laboratory values collected immediately prior to transition from SOLE to FOLE, 1 wk after FOLE, and 4 wk after FOLE and notes whether infection was suspected at the time of sample collection. WBC, white blood cells (103 cells/μl); TBili, total bilirubin (mg/dl); DBili, direct bilirubin (mg/dl); CRP, C-reactive protein (mg/dl). Bar graphs display results from analysis of polyunsaturated fatty acids (PUFAs), resolvin (Rv) precursors (HDHA), HETEs, and eicosanoids. Columns are labeled with multiplicative values (e.g., ×2, ×5, ×10) to indicate changes in scale appropriate for the column and denote a concentration equal to the displayed value multiplied by the factor. Increase in inflammatory eicosanoids [such as 12-HETE and thromboxane (TX) TXB2] in the 4-wk sample corresponded with a positive urine culture shortly prior to blood sample collection. AA, arachadonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LTB4, leukotriene B4; LXA2, lipoxin A2.
All DHA metabolome markers increased dramatically when FOLE was substituted for SOLE. After 4 wk of FOLE, levels of 4-, 7-, 14-, and 17-HDHA increased 367.5%, 171.1%, 4,529.3%, and 993.4%, respectively, compared with the SOLE baseline.
Institution of FOLE resulted in an initial decrease in HETEs, with 5-, 12-, and 15-HETE decreasing by 79.7%, 76.7%, and 72.4%, respectively, at 1 wk of FOLE compared with the SOLE baseline. However, the HETEs increased at 4 wk of FOLE: 5-, 12-, and 15-HETE increased by 55.9%, 3,042.9%, and 248.8%, respectively, compared with the 1-wk FOLE sample.
Levels of AA and DHA decreased by 35% and 61.2%, respectively, at 4 wk of FOLE compared with the SOLE baseline. The level of EPA increased by 755.1% at 4 wk of FOLE compared with the SOLE baseline.
LTB4 decreased by 89.3% at 4 wk of FOLE relative to the SOLE baseline. TXB2 and LXA4 increased by 221.0% and 173.3% at 4 wk of FOLE relative to the SOLE baseline. PGE2 initially increased by 104.5% at 1 wk of FOLE and then returned to near the SOLE baseline by 4 wk of FOLE (87.3 pg/500 μl).
Patient 2.
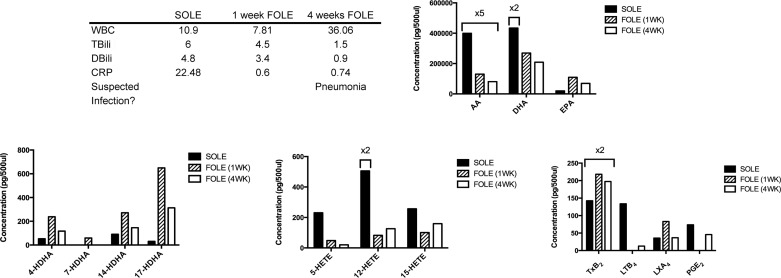
Patient 2 was a female born at 26 wk gestation. She had a history of intrauterine growth restriction, respiratory distress syndrome, and presumed necrotizing enterocolitis (managed medically). She was dependent on SOLE for 5.7 wk prior to initiation of FOLE. Clinical parameters at the three time points of blood collection are summarized in Fig. 4. Patient 2 was weaned from PN 16.4 wk after initiation of FOLE.
Fig. 4.
Bioactive LM profile for patient 2. Table outlines selected laboratory values collected immediately prior to transition from SOLE to FOLE, 1 wk after FOLE, and 4 wk after FOLE and notes whether infection was suspected at the time of sample collection. See Fig. 3 legend for abbreviations and units of measure. Bar graphs display results from analysis of PUFAs, Rv precursors (HDHA), HETEs, and eicosanoids. Columns are labeled with multiplicative values to indicate changes in scale appropriate for the column and denote a concentration equal to the displayed value multiplied by the factor. AA-derived proinflammatory HETEs were highest during administration of SOLE. LTB4 levels declined dramatically with transition from SOLE to FOLE.
The most dramatic increase in HDHA concentrations was observed between the SOLE baseline and 1 wk of FOLE, with 4-, 14-, and 17-HDHA increasing by 353.5%, 200.3%, and 2,058.2%, respectively. 7-HDHA was undetectable in the SOLE sample and the 4-wk FOLE sample but was 58.8 pg/500 μl at 1 wk of FOLE. HDHA compounds decreased at 4 wk of FOLE, but levels of 4-, 14-, and 17-HDHA were still 123.2%, 60.8%, and 941.3% higher, respectively, than the SOLE baseline.
The ω-6 AA-derived HETEs showed a trend similar to HDHA. At 1 wk of FOLE, the concentrations of 5-, 12-, and 15-HETE were 87.0%, 76.7%, and 72.4% lower, respectively, than the SOLE baseline. At 4 wk of FOLE, the level of 5-HETE was 79.7% of the SOLE baseline, while 12-HETE increased by 632.2% compared with the SOLE baseline. 15-HETE returned to near the SOLE baseline (592.6 pg/500 μl).
AA and DHA decreased at 4 wk of FOLE compared with the SOLE baseline (79.8% and 76.0%, respectively). At 4 wk of FOLE, the concentration of EPA was 253.4% higher than the SOLE baseline.
After 1 wk of FOLE, LTB4 and PGE2 had substantially decreased compared with the SOLE baseline (both were undetectable at 1 wk). TXB2 and LXA4 increased at 1 wk of FOLE by 53.1% and 131.9%, respectively, relative to the SOLE baseline. By 4 wk of FOLE, LXA4 had returned near the SOLE baseline, while TXB2 remained 38.6% higher than the SOLE baseline.
Patient 3.
Patient 3 was a male born at 37 wk with gastroschisis, requiring multiple small bowel resections resulting in short bowel syndrome. Patient 3 was dependent on SOLE for 19.1 wk prior to transfer from an outside hospital with sepsis and multiorgan failure. FOLE was initiated immediately. At 1 day after initiation of FOLE, patient 3 had a positive urine culture for Candida. Clinical parameters at the three time points of blood collection are summarized in Fig. 5. After 3.4 wk of FOLE, patient 3 developed bradycardia in the setting of gastrointestinal bleeding, required CPR, and died. Postmortem blood cultures grew gram-positive cocci.
Fig. 5.
Bioactive LM profile for patient 3. Table outlines selected laboratory values collected immediately prior to transition from SOLE to FOLE, 1 wk after FOLE, and 4 wk after FOLE and notes whether infection was suspected at the time of sample collection. See Fig. 3 legend for abbreviations and units of measure. Bar graphs display results from analysis of PUFAs, Rv precursors (HDHA), HETEs, and eicosanoids. Columns are labeled with multiplicative values to indicate changes in scale appropriate for the column and denote a concentration equal to the displayed value multiplied by the factor. Increase in proinflammatory LMs (including HETEs, PGE2, and LTB4) at the 4-wk time point corresponded with a state of sepsis and systemic inflammation, resulting in the patient's death shortly after collection of the 4-wk blood sample.
After 1 wk of FOLE, levels of 4-, 14-, and 17-HDHA were 1,442.9%, 13,111.2%, and 1,880.6% higher, respectively, than the SOLE baseline. After 4 wk of FOLE, levels of these same compounds remained elevated compared with the SOLE baseline (1,496.6%, 8,537.8%, and 663.5%, respectively). Only 7-HDHA decreased at 1 and 4 wk of FOLE compared with the SOLE baseline.
After 1 wk of FOLE, 12- and 15-HETE increased compared with the SOLE baseline (86.7% and 3.9%, respectively). After 4 wk of FOLE, levels of 12- and 15-HETE were lower than the SOLE baseline (24.2% and 54.8%, respectively). 5-HETE was 205.3% higher than the SOLE baseline.
After 4 wk of FOLE, AA and EPA increased by 109% and 2,733.6%, respectively, compared with the SOLE baseline. The level of DHA after 4 wk of FOLE was similar to the SOLE baseline.
After 4 wk of FOLE, LTB4 and PGE2 levels were 736.7% and 54.8% higher, respectively, than the SOLE baseline. TXB2 was near the SOLE baseline (257.9 pg/500 μl), while LXA4 was 52.3% lower than the SOLE baseline.
Patient 4.
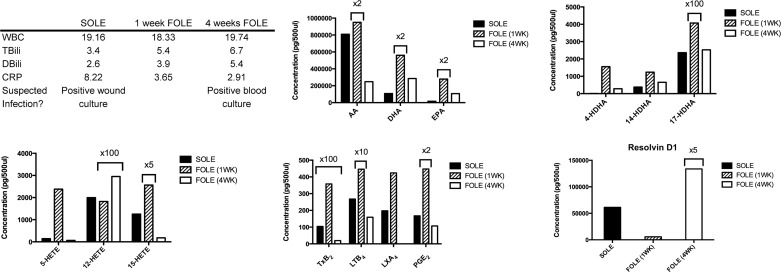
Patient 4 was a female born at 33 wk gestation with gastroschisis and multiple intestinal atresias. Patient 4 developed short bowel syndrome after multiple abdominal operations. She was dependent on SOLE for 25.3 wk prior to transfer to Boston Children's Hospital in liver failure. FOLE was initiated, and clinical parameters at the three time points of blood collection are summarized in Fig. 6. Patient 4 had a positive blood culture 3 wk after initiation of FOLE. Patient 4 remains on FOLE.
Fig. 6.
Bioactive LM profile for patient 4. Table outlines selected laboratory values collected immediately prior to transition from SOLE to FOLE, 1 wk after FOLE, and 4 wk after FOLE and notes whether infection was suspected at the time of sample collection. See Fig. 3 legend for abbreviations and units of measure. Bar graphs display results from analysis of PUFAs, Rv precursors (HDHA), HETEs, and eicosanoids. Columns are labeled with multiplicative values to indicate changes in scale appropriate for the column and denote a concentration equal to the displayed value multiplied by the factor. Rv precursor compounds (4-, 14-, and 17-HDHA) rose progressively with greater duration during FOLE administration. Proresolving mediator LXA4 increased with FOLE administration.
After 4 wk of FOLE, the concentrations of all HDHA compounds steadily increased. Levels of 4-, 14-, and 17-HDHA were 1,102.4%, 659.9%, and 432.3% higher, respectively, than the SOLE baseline. 7-HDHA was undetectable in all three samples.
Levels of 5-, 12-, and 15-HETE were lower after 4 wk of FOLE than the SOLE baseline, with reductions of 38.8%, 55.3%, and 75.5%, respectively.
The amount of AA decreased by 50.5% after 4 wk of FOLE compared with the SOLE baseline. DHA and EPA increased at 4 wk by 24.7% and 1,037.4%, respectively.
The amount of LTB4 and PGE2 decreased by 35.7% and 23.7%, respectively, at 4 wk of FOLE compared with the SOLE baseline. TXB2 and LXA4 increased after 4 wk of FOLE by 33.3% and 291.5%, respectively, compared with the SOLE baseline.
Patient 5.
Patient 5 was a male born at 24.7 wk gestation with respiratory distress syndrome. Prior to transfer to Boston Children's Hospital, patient 5 had a history of necrotizing enterocolitis with bowel perforation; multiple abdominal procedures left him with short bowel syndrome. He was dependent on SOLE for 15.7 wk prior to transfer to Boston Children's Hospital in liver failure. Patient 5 had a positive wound culture on the day prior to initiation of FOLE and a positive blood culture 3.1 wk after initiation of FOLE. Clinical parameters at the three time points of blood collection are summarized in Fig. 7. Patient 5 was weaned from PN after 24.4 wk on FOLE.
Fig. 7.
Bioactive LM profile for patient 5. Table outlines selected laboratory values collected immediately prior to transition from SOLE to FOLE, 1 wk after FOLE, and 4 wk after FOLE and notes whether infection was suspected at the time of sample collection. See Fig. 3 legend for abbreviations and units of measure. Bar graphs display results from analysis of PUFAs, Rv precursors (HDHA), HETEs, and eicosanoids. Levels of the Rv precursor 17-HDHA were most notably elevated with administration of FOLE. The specialized proresolving mediator RvD1 was increased ∼1,000-fold after 4 wk of FOLE.
Levels of 4-, 7-, 14-, and 17-HDHA increased dramatically by 15,343.5%, 226.2%, 17,131.3%, and 143,107.1%, respectively, at 1 wk of FOLE compared with the SOLE baseline. After 4 wk of FOLE, 4-, 7-, 14-, and 17-HDHA remained elevated by 2,735.1, 73.2%, 6.9%, and 1,869.8%, respectively, above the SOLE baseline.
After 4 wk of FOLE, levels of 5-, 12-, and 15-HETE decreased relative to the SOLE baseline by 54.2%, 85.2%, and 85.4%, respectively.
The amount of AA decreased by 69.4% at 4 wk of FOLE compared with the SOLE baseline. The level of DHA and EPA increased by 164.3% and 551.1%, respectively, relative to the SOLE baseline.
Levels of TXB2, LTB4, and PGE2 decreased by 81.5%, 40.6%, and 36.1%, respectively, at 4 wk of FOLE relative to the SOLE baseline. The concentration of LXA4 initially increased by 115.1% at 1 wk of FOLE compared with SOLE baseline, but LXA4 was undetectable at 4 wk of FOLE.
The level of RvD1 increased at 4 wk of FOLE relative to the SOLE baseline by 992.3%.
DISCUSSION
In the present study we demonstrate that FOLE and SOLE induce functionally distinct LM profiles in our murine model of hepatic steatosis. FOLE promoted local tissue production of proresolution precursors that serve as pathway markers of the active resolution of inflammation (32, 34). Additionally, we demonstrate for the first time in humans that change from SOLE to FOLE results in an increase in proresolving LMs and a decrease in proinflammatory LMs (i.e., PGE2 and LTB4). In patient 5, the transition to FOLE resulted in the direct increase in RvD1 biosynthesis.
Several elements of PN, including the change in enterohepatic circulation due to the absence of enteral intake, hypercaloric PN solutions, and high carbohydrate and glucose loads, contribute to the development of liver disease. Numerous risk factors for PNALD, including prematurity, sepsis, and duration of PN, have been proposed, but associations in the literature are inconsistent (25).
Clinically, FOLE have shown great promise in the treatment of PNALD in children. Infants treated with FOLE demonstrate reversal of cholestasis six times faster than infants who received only SOLE (23). The mechanism by which FOLE are hepatoprotective is likely multifactorial. One notable difference between SOLE and FOLE is the concentration of α-tocopherol, a potent antioxidant. Intralipid contains ∼87 μmol/l of α-tocopherol, while Omegaven contains ∼505 μmol/l. SOLE are also rich in phytosterols, compounds that are similar to cholesterol and are found in plants. Phytosterols are toxic to the liver; in neonatal piglets, intravenous phytosterols induce cholestasis (8). Furthermore, the provision of predominantly ω-3 or ω-6 lipids alters the composition of the cell membrane, which then alters the downstream production of LMs. About 30% of the cell membrane is PUFAs (5). An increased proportion of ω-3 PUFAs limits the ω-6 substrate for the production of AA and subsequent LM autacoids that amplify acute inflammation, as demonstrated in the present study.
In the current study the most dramatic finding in the murine model was the increase in the DHA metabolome. 14-HDHA is the precursor to MaR formation, while 17-HDHA is the precursor to RvD1 biosynthesis (31). Therefore, these products serve as biomarkers of endogenous biosynthesis from DHA and in vivo conversion to proresolving mediators. 17-HDHA can be converted locally and is protective in mouse models of arthritis, colitis, and renal reperfusion injury (4, 9, 16). RvD1 and 17-HDHA enhance the humoral immune response via an increase in activated B cell IgM and IgG production (24). Fat-1 mice with high levels of DHA and EPA have elevated concentrations of 18-HEPE and 17-HDHA (14).
The ω-3 PUFAs and the downstream bioactive LMs are protective in other animal models of liver disease. In vitro, DHA supplementation reduces hepatocyte oxidative stress and hydrogen peroxide-induced DNA damage (12). DHA limits carbon tetrachloride-induced hepatic injury, with a significant increase in the formation of 17-HDHA and PD1 (12). 17-HDHA specifically reduces oxidative damage and macrophage TNF-α release. Additionally, ω-3 PUFAs are protective in murine obesity-induced hepatic steatosis (11). In ob/ob mice, provision of ω-3 PUFAs is associated with an increase in 17-HDHA, PD1, and RvD1 and a decrease in ω-6 fatty acid-derived PGE2 and TXB2, as well as 5-, 12-, and 15-HETE (11). The findings of the current study support the role of ω-3 PUFAs in the prevention of hepatic steatosis.
In addition to their proresolution properties, 4-, 7-, and 17-HDHA are peroxisome proliferator-activated receptor-γ (PPARγ) ligands (12). The role of PPARγ in steatosis is controversial. PPARγ expression in mice is prosteatotic, and PPARγ knockout is protective against steatosis (20). Paradoxically, synthetic PPARγ, such as thiazolidendiones, are capable of reducing hepatic fat content by 30–50% (2, 36–38). The contribution of PPARγ to any benefit of 4-, 7-, and 17-HDHA in the resolution of steatosis in this murine model may be of interest for future studies.
FOLE not only increased the biosynthesis of proresolving mediators, but it also markedly reduced the proinflammatory metabolome. FOLE reduced the AA-derived PGE2, PGD2, and PGF2α, as well as TXB2. The PGs, in particular, are known to dramatically increase at the site of acute inflammation, and levels are increased by the administration of SOLE in our model. Besides the well-characterized role in inflammatory exudates and immune cell chemotaxis, PGE2 from Kuppfer cells is thought to regulate lipid synthesis in the liver. In ethanol-induced liver injury, Kuppfer cell-derived PGE2 increases cAMP levels in hepatocytes and induces triglyceride accumulation (10).
TXB2 is the stable, intermediate metabolite of TXA2. TXB2 measures TXA2 levels, since it is not possible to directly measure TXA2 by any method because of its short half-life. TXA2 is produced by Kuppfer cells in response to stress and promotes hepatic inflammation, portal vasoconstriction, and leukocyte adhesion in the sinusoids (6, 15, 21). Inhibition of TX is hepatoprotective in models of endotoxemia, ischemia-reperfusion, cirrhosis, and alcoholic liver disease (41). Dietary fish oil has previously been shown to lower plasma TXB2 in septic rats. The reduction in TX by FOLE in our study may be therapeutically relevant and warrants additional study.
The shift in inflammatory homeostasis was further supported by the HETE profile. SOLE increased the concentration of proinflammatory HETEs, while FOLE reduced the concentration of these bioactive lipids. Plasma HETE levels are elevated in patients with alcoholic liver disease compared with healthy controls (26).
In the present study, FOLE was associated with decreased levels of Δ6-trans-LTB4 and 12-epi-Δ6-trans-LTB4, the isomers of LTB4. LTB4 is a key regulator of the initiation and amplification of the immune response. Deficiency of the LTB4 receptor is protective against the sequelae of diet-induced obesity, including steatosis and hepatic triglyceride accumulation. The ω-3 PUFAs are known to increase the degradation of LTB4 (12, 29). In the present study the decreased production of LTB4-related biosynthesis isomers may reflect decreased biosynthesis of LTB4 in FOLE.
Importantly, the analysis of the murine model of hepatic steatosis mirrored many of the trends we observed in our cohort of infants transitioning from SOLE to FOLE. In humans, the most consistent effect of FOLE was the rise in proresolving precursors (HDHA compounds), which reflects the activation of a cascade of immune-modulating lipids. The counterregulatory eicosanoid mediator LXA4 also consistently rose with the administration of FOLE, which is consistent with prior studies that demonstrate a rise in LXA4 with RvE1 treatment (13). Together with the present study, this is the clearest evidence for the switch to inflammation resolution with the transition from SOLE to FOLE.
SPMs are key mediators of the immune response to infection; therefore, ongoing infection or physiological stress may alter bioactive LM profiles independently of the lipid emulsion. Several patients in this study developed infectious complications during the course of sample collection. Specific SPMs are differentially regulated during infection, enhance bacterial containment, and reduce antibiotic requirements (7). RvD1 and RvD5 each reduce bacterial titers in blood and exudates, and RvD1, RvD5, and PD1 enhance phagocytosis of Escherichia coli in mice and isolated human macrophages (7). The relationship between infection and serum lipidome may be of diagnostic significance and warrants further study.
Patient 1 developed a urinary tract infection on the day prior to collection of the 4-wk sample. There was a spike in TXB2, as well as 12- and 15-HETE, in this sample. 15-HETE is a precursor to LXA4, and the increase in 15-HETE corresponded with a rise in LXA4. Patient 2 was diagnosed with pneumonia shortly before collection of the 4-wk sample; the 4-wk sample demonstrated mild-to-moderate increases in LTB4 and PGE2, which were undetectable in the 1-wk sample. Patient 4 had no infectious or inflammatory-related complications during the course of the study. Patient 5 developed a culture-positive wound infection just before collection of the SOLE sample and a positive blood culture ∼1 wk before the 4-wk sample collection. Patient 5 had no episodes of sepsis. The most profound rise in proinflammatory markers, including LTB4, PGE2, and TXB2, was observed in the 1-wk sample. It is unclear whether this rise was anticipating the brewing septic episode or signaled an otherwise-undiagnosed inflammatory stressor.
Patient 3, who suffered from multiorgan failure and sepsis, succumbed to his illness during the study period. Lipidomic profiling revealed dramatic increases in LTB4 and PGE2, key mediators of acute inflammation, and a decrease in the SPM LXA4 in the sample collected hours prior to death. von Moltke et al. (38a) described the phenomenon “eicosanoid storm,” which is characterized by the pathological release of LMs after triggering of a critical innate immune response. Eicosanoids are recognized as key signaling mediators in the activation of the inflammasome, a protein platform in the innate immune system that initiates inflammatory cascades in response to stress or pathogens. The rise in proinflammatory LMs in patient 3 may reflect such an eicosanoid storm in the hours prior to his unfortunate death.
There are several limitations to the present study. We employed a murine model of steatosis, and the degree to which this model mimics human PNALD is uncertain. This model likely only reflects the “first hit,” steatosis, that occurs in the development of PNALD. Future work with the addition of a “second hit,” sepsis, may provide insight into the role of resolution in PNALD. In the current study, PN was administered orally, rather than intravenously, due to the high morbidity associated with intravenous administration in mice and the confounding of infectious complications. All animals consumed identical volumes of oral PN solution and, therefore, had identical energy delivery. The only difference between the groups was the intravenous lipid emulsion.
This study does not mechanistically define the role of LMs in steatosis or PNALD, nor does it define the relative contributions of different body compartments to the unique LM signatures in this model. The altered production of LMs does not imply causation in terms of therapy. Rather, FOLE and SOLE are associated with different LM signatures. The mechanism by which the differential production of LMs affects liver disease and patient survival is under active investigation. The downstream signaling and cellular changes that occur as a result of changes in LM signature are beyond the scope of this study.
There were differences between the human serum and mouse liver lipidome, which is not surprising, since they reflect temporal snap shots in distinct tissues. Eicosanoids and docosanoids are not circulating hormones and are inactivated within minutes; hence, it is not surprising that many SPMs are not detected, but we did detect changes in their stable pathway markers. The vasculature has a prominent platelet-leukocyte LXA4 biosynthetic pathway, which may be the reason why we detected it readily in blood but not in the liver.
The human data represent five patients cared for at a single institution, which limits the generalizability of these findings. The clinical status of the patients varied simultaneously with changes to the lipid regimen during the study period. Many of the known SPMs were largely undetectable in the patients, which may be the result of low sample volume. The volume of blood obtained from these critically ill neonates was only ∼400 μl. Hence, larger studies are necessary to validate these initial results.
These results add novel information on ω-3 PUFAs in liver disease. Prior studies have demonstrated that provision of ω-3 PUFAs resulted in formation of SPMs. This study has the unique advantage of comparing the differential downstream lipid production of SOLE vs. FOLE, which to our knowledge has not been previously done. On the basis of results of this study, the choice of lipid for PN may significantly impact inflammatory status and resolution capability in patients. The use of SOLE is largely historical and does not reflect evidence for physiological superiority. The direct modulation of the inflammatory state by FOLE provides a physiological rationale for the therapeutic benefit of these emulsions.
GRANTS
B. T. Kalish was supported by a Howard Hughes Medical Institute Research Fellowship. S. Wang, K. Seamon, and K. Gronert were supported by National Eye Institute Grant EY-022208. J. M. Fitzgerald was supported by National Institute of General Medical Sciences Grant PO1 GM-095467 (to C. N. Serhan and Brigham and Women's Hospital). M. Puder was supported by the Children's Hospital Surgical Foundation and The Vascular Biology Program.
DISCLOSURES
A license agreement for the use of Omegaven has been signed by Boston Children's Hospital and Fresenius Kabi, and a patent has been submitted by Boston Children's Hospital on behalf of M. Puder and K. Gura.
AUTHOR CONTRIBUTIONS
B.T.K. and M.P. are responsible for conception and design of the research; B.T.K., H.D.L., J.M.F., S.W., K.S., K.M.G., and K.G. performed the experiments; B.T.K., J.M.F., S.W., and K.G. analyzed the data; B.T.K., K.G., and M.P. interpreted the results of the experiments; B.T.K. and J.M.F. prepared the figures; B.T.K. drafted the manuscript; B.T.K., H.D.L., J.M.F., K.M.G., K.G., and M.P. edited and revised the manuscript; B.T.K., K.G., and M.P. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Charles N. Serhan (Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women's Hospital, Harvard Medical School) for helpful discussions and Romain A. Colas (Center for Experimental Therapeutics and Reperfusion Injury) for assistance in sample analysis. The authors thank Marykate O'Malley for technical assistance with figures and graphics.
REFERENCES
- 1.Alwayn IP, Gura K, Nosé V, Zausche B, Javid P, Garza J, Verbesey J, Voss S, Ollero M, Andersson C, Bistrian B, Folkman J, Puder M. ω-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res 57: 445–452, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes 52: 1364–1370, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 5: 174ra26, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. ω-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol 187: 1957–1969, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bulger EM, Maier RV. Lipid mediators in the pathophysiology of critical illness. Crit Care Med 28: N27–N36, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Buxton DB, Fisher RA, Robertson SM, Olson MS. Stimulation of glycogenolysis and vasoconstriction by adenosine and adenosine analogues in the perfused rat liver. Biochem J 248: 35–41, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484: 524–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition 14: 158–164, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol 177: 5902–5911, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU, Rivera CA, Zhong Z, Thurman RG. Kupffer cell-derived prostaglandin E2 is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol 279: G100–G106, 2000 [DOI] [PubMed] [Google Scholar]
- 11.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: a role for resolvins and protectins. FASEB J 23: 1946–1957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V, Rodés J, Clària J. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J 20: 2537–2539, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-γ and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol 9: 873–879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous ω-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA 103: 11276–11281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katagiri H, Ito Y, Ishii KI, Hayashi I, Suematsu M, Yamashina S, Murata T, Narumiya S, Kakita A, Majima M. Role of thromboxane derived from COX-1 and -2 in hepatic microcirculatory dysfunction during endotoxemia in mice. Hepatology 39: 139–150, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol 164: 278–293, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem 58: 1476–1484, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Meisel JA, Le HD, de Meijer VE, Nosé V, Gura KM, Mulkern RV, Akhavan Sharif MR, Puder M. Comparison of 5 intravenous lipid emulsions and their effects on hepatic steatosis in a murine model. J Pediatr Surg 46: 666–673, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Morán-Salvador E, López-Parra M, García-Alonso V, Titos E, Martínez-Clemente M, González-Périz A, López-Vicario C, Barak Y, Arroyo V, Clària J. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J 25: 2538–2550, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol 142: 367–373, 1993 [PMC free article] [PubMed] [Google Scholar]
- 22.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLos One 6: e16957, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, Zhou J, Duggan C, Gura KM. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg 250: 395–402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol 189: 1036–1042, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangel SJ, Calkins CM, Cowles RA, Barnhart DC, Huang EY, Abdullah F, Arca MJ, Teitelbaum DH, 2011 American Pediatric Surgical Association Outcomes and Clinical Trials Committee Parenteral nutrition-associated cholestasis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg 47: 225–240, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Raszeja-Wyszomirska J, Safranow K, Milkiewicz M, Milkiewicz P, Szynkowska A, Stachowska E. Lipidic last breath of life in patients with alcoholic liver disease. Prostaglandins Other Lipid Mediat 99: 51–56, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Samuelsson B, Dahlén SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237: 1171–1176, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci Transl Med 3: 69ra12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schacky von C, Kiefl R, Marcus AJ, Broekman MJ, Kaminski WE. Dietary n-3 fatty acids accelerate catabolism of leukotriene B4 in human granulocytes. Biochim Biophys Acta 1166: 20–24, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 26: 1755–1765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of ω-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196: 1025–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev 111: 5922–5943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent anti-inflammatory and proresolving actions. J Exp Med 206: 15–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serhan CN. Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolving mediators. J Thromb Haemost 7 Suppl 1: 44–48, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr 27: 131–137, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Sutinen J, Kannisto K, Korsheninnikova E, Fisher RM, Ehrenborg E, Nyman T, Virkamäki A, Funahashi T, Matsuzawa Y, Vidal H, Hamsten A, Yki-Järvinen H. Effects of rosiglitazone on gene expression in subcutaneous adipose tissue in highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab 286: E941–E949, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Teranishi T, Ohara T, Maeda K, Zenibayashi M, Kouyama K, Hirota Y, Kawamitsu H, Fujii M, Sugimura K, Kasuga M. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metab Clin Exp 56: 1418–1424, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169–2176, 2004 [DOI] [PubMed] [Google Scholar]
- 38a.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490: 107–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wales PW, de Silva N, Kim JH, Lecce L, Sandhu A, Moore AM. Neonatal short bowel syndrome: a cohort study. J Pediatr Surg 40: 755–762, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol Chapt 14: Unit 14.26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoyama Y, Nimura Y, Nagino M, Bland KI, Chaudry IH. Role of thromboxane in producing hepatic injury during hepatic stress. Arch Surg 140: 801–807, 2005 [DOI] [PubMed] [Google Scholar]