Abstract
The present study investigates the relative ability of α-, γ-, and δ-tocopherol (Toc) to modulate cell signaling events that are associated with inflammatory responses in fetal-derived intestinal (FHs 74 Int) cells. Secretion of the proinflammatory cytokine IL-8 in FHs 74 Int cells was stimulated in the following order: α-Toc < γ-Toc < δ-Toc. A similar proinflammatory response was observed when inflammation was induced in FHs 74 Int cells. Modulation of IL-8 expression by Toc corresponded to an isoform-specific modulation of NF-κB and nuclear factor-erythroid 2-related factor 2 (Nrf2) cell signaling pathways involved in expression of proinflammatory cytokines and antioxidant enzymes, respectively. δ-Toc and, to a lesser extent, γ-Toc activated NF-κB and Nrf2 signaling, as indicated by the greater nuclear translocation of transcription factors. Activation of NF-κB signaling by γ- and δ-Toc was accompanied by upregulation of NF-κB target genes, such as IL-8 and prostaglandin-endoperoxide synthase 2, with and without a prior IFNγ-PMA challenge. Nevertheless, γ- and δ-Toc, particularly δ-Toc, concurrently downregulated glutamate-cysteine ligase, a Nrf2 target gene that encodes for glutathione biosynthesis. This observation was substantiated by confirmation that γ- and δ-Toc were effective at decreasing glutamate-cysteine ligase protein expression and cellular glutathione content. Downregulation of glutathione content in fetal intestinal cells corresponded to induction of apoptosis-mediated cytotoxicity. In conclusion, γ- and δ-Toc are biologically active isoforms of vitamin E and show superior bioactivity to α-Toc in modulating cell signaling events that contribute to a proinflammatory response in fetal-derived intestinal cells.
Keywords: IL-8, Nrf2, NF-κB, tocopherol, inflammation
since the discovery of vitamin E in 1922, eight structurally related compounds, including four tocopherol (α-, β-, γ-, and δ-Toc) and four tocotrienol isoforms, have been identified (8, 21). Of these, α-Toc has garnered considerable public interest, largely because this form of vitamin E has the greatest relative biological activity and, also, because it is the dominant form of vitamin E in the systemic circulation (6, 7). Similarly, human milk contains mostly α-Toc, minor levels of γ-Toc, and a barely quantifiable level of δ-Toc (18). In contrast, infant formula consists of substantial amounts of γ- and δ-Toc, which can be attributed to the use of specific sources of vegetable oils to constitute the lipid component of the infant food, along with mixed Toc isoforms (1, 11, 19, 47). It should also be noted that the major form of vitamin E consumed in the United States is γ-Toc, although the major circulating Toc is α-Toc (53). Given the high concentrations of γ- and δ-Toc in infant formula, it can be implied that more vitamin E is consumed by formula- than breast-fed infants and also that the mixture of vitamin E forms consumed by formula-fed infants is markedly different from that consumed by breast-fed infants. The potential health effects of differences in Toc isoform proportions and related concentrations in the infant formula are not known, since little information exists on the biological activity of non-α-Toc isoforms in infant cells. Since dietary Toc isoforms are only metabolized in the liver after intestinal absorption, thus leading to excretion of non-α-Toc, the intestine represents a unique organ where all intact forms of vitamin E exist in concentrations that can manifest a physiological effect (31, 49).
The current study aims to determine the effect of γ- and δ-Toc, relative to α-Toc, in modulating baseline and IFNγ-PMA-induced inflammatory responses (10) in infant intestinal cells. A primary fetal-derived intestinal cell line (FHs 74 Int) was employed, with IL-8 secretion used as the end-point measure for inflammation, and the effect was evaluated in recognition of the relative cellular uptake of each Toc isoform (19). Two stress-activated cell signaling pathways, known to contribute to expression of the proinflammatory cytokine IL-8, namely, NF-κB and nuclear factor-erythroid 2-related factor 2 (Nrf2) pathways (2, 30, 46), were also examined. Activation of the NF-κB pathway leads to expression of proinflammatory cytokines, including IL-8 (30). On the other hand, the Nrf2 signaling pathway regulates expression of the antioxidant enzymes that boost cellular defense systems and mitigate an inflammatory response (2, 39). These two signaling pathways have also been reported to work together to modulate the overall inflammatory response (4, 35). The affinity of Toc isoforms to modulate Nrf2 and NF-κB signaling pathways was therefore investigated as a possible mechanism regulating IL-8 expression. Lastly, the extent to which different Toc isoforms are taken up by the cells and the effect of Toc isoforms on cell viability were evaluated in response to the relative potential to modulate inflammation.
MATERIALS AND METHODS
Materials.
RRR-α-Toc was purchased from Fisher (Fairlawn, NJ) and (+)-γ-Toc, (+)-δ-Toc, protease inhibitor cocktail (P8340), and EGF from Sigma-Aldrich (St. Louis, MO). FHs 74 Int cells (CCL-241) and Hybri-Care medium were purchased from Cedarlane (Hornby, ON, Canada). NF-κB and Nrf2 TransAM ELISA kits (catalog nos. 40096 and 50296, respectively) were obtained from Active Motif (Carlsbad, CA), NE-PER nuclear protein extraction kit (product no. 78835) from Pierce (Rockford, IL), and DC protein assay from Bio-Rad Laboratories (Hercules, CA). Detection limits for NF-κB and Nrf2 TransAM ELISA are 0.15 and 0.5 μg of nuclear extract per well, respectively. Custom PCR array (CAPH10994) and IL-8 single-analyte ELISA kits were purchased from Qiagen (Valencia, CA) and APO-BrdU terminal deoxynucleotidyl transferase dUTP-mediated nick end labeling (TUNEL; catalog no. AU1001) and glutathione enzyme-based (catalog no. 703002) assay kits from Phoenix Flow Systems (San Diego, CA) and Cayman Chemical (Ann Arbor, MI), respectively. Rabbit polyclonal anti-glutamate-cysteine ligase (GCLC) primary antibody (ab41463) and mouse monoclonal anti-GAPDH primary antibody (ab8245) were obtained from Abcam (Cambridge, MA).
Cell culture.
FHs 74 Int cells represent a primary cell line derived from the small intestine of a 3- to 4-mo-gestation human fetus. FHs 74 Int cells were maintained in Hybri-Care medium containing 10% FBS, 100 μg/ml streptomycin, 100 U of penicillin, and 30 ng/ml EGF. Initial seeding density for FHs 74 Int cells was 1.2 × 106 cells/well in 2 ml of complete medium in six-well plates that were incubated for 2 days at 37°C. Cells were subsequently placed in serum-free medium for 18 h and then treated with Toc isoforms in reduced-serum (1%) medium. After 24 h, Toc-containing medium was removed, and the cells were challenged with a combination of IFNγ (4,000 U/ml) and PMA (0.05 μg/ml) in 1.5 ml of medium cocultured with Toc isoforms. Toc isoforms were delivered in ethanol at a maximum concentration of 0.25% in culture medium.
Cellular uptake of Toc.
The relative cellular uptake of different Toc isoforms in FHs 74 Int cells was determined using HPLC coupled with fluorescence detection based on previously established methods (19, 38, 42). At the end of each incubation period with different Toc isoforms, the culture medium was aspirated, and cells were washed once with 2% BSA in PBS on ice and then twice with PBS. The cells were lysed using a radioimmunoprecipitation assay (RIPA) buffer, and the protein content was measured by DC protein assay. The same cell extract was also analyzed for vitamin E content using HPLC (1100 series, Agilent Technology, Palo Alto, CA) (18). Briefly, separation of Toc isoforms was achieved using methanol as the mobile phase (1 ml/min) and a 3-μm SphereClone column (150 × 4.6 mm). Individual Toc isoforms were identified at an excitation wavelength of 292 nm and an emission wavelength of 330 nm.
IL-8 measurement.
Cells were pretreated with IFNγ-PMA cocktail for 24 h, the culture medium was recovered and centrifuged at 1,000 g for 10 min at 4°C, and the supernatant was measured for IL-8 using an ELISA kit according to the manufacturer's instructions (19). We repeated this experiment in the absence of IFNγ-PMA to determine the effect of individual Toc isoforms on baseline IL-8 expression.
NF-κB and Nrf2 activation.
To establish the effect of different Toc isoforms on the activation of NF-κB and Nrf2 signaling, we first established the effect of IFNγ-PMA on NF-κB and Nrf2 activation. Cells pretreated with IFNγ-PMA were harvested to obtain the nuclear fraction. The effect of Toc on modulation of NF-κB and Nrf2 signaling was determined after 90 min and 24 h of IFNγ-PMA stimulation, respectively. We repeated this experiment in the absence of IFNγ-PMA challenge to determine the role of different Toc isoforms on NF-κB and Nrf2 signaling in FHs 74 Int cells. Cells were then scraped into ice-cold PBS, and the nuclear pellet was obtained using the NE-PER kit. A nuclear extract was reconstituted in the lysis buffer supplied with the Nrf2 or NF-κB (p65) ELISA kit. Specifically, nuclei were lysed by vortexing once every 10 min for up to 40 min, and a final extract was recovered by centrifugation at 18,500 g for 10 min at 4°C. The relative presence of NF-κB and Nrf2 in the nuclear fraction was established using the TransAM ELISA kits according to the manufacturer's instructions.
Effects of Toc isoforms on gene transcription.
The ability of different Toc isoforms to modify expression of selected genes involved in Nrf2 and NF-κB signaling was determined using real-time PCR array. We prepared cDNA from 725 ng of mRNA obtained from FHs 74 Int cells as previously described (20). The custom PCR array consisted of 19 genes that transcribed proteins that regulate oxidative stress, inflammation, or cell proliferation; two housekeeping genes, one positive and one reverse transcription control, and a genomic contamination control were also present (Table 1). Each experiment consisted of three independent replicates of FHs 74 Int cells incubated with different Toc isoforms and challenged with IFNγ-PMA for 4 or 24 h. Untreated cells served as the negative control, and the positive control consisted of cells that were incubated with IFNγ-PMA. Reverse transcription, genomic DNA, and positive PCR controls were included for each sample. The experiment was also performed in the absence of IFNγ-PMA challenge to determine the role of individual Toc isoforms in transcription of the select genes. PCR amplification proceeded as described using the ABI7500 fast real-time PCR system (Applied Biosystems, Foster City, CA), and the data were analyzed using a software tool downloaded from the Qiagen website (44).
Table 1.
Genes tested in custom PCR array
| Gene | Full Name | Function of Encoded Protein | Ref. No. |
|---|---|---|---|
| Nrf2 target genes | |||
| GPX1 | Glutathione peroxidase 1 | Classic GPX; detoxification of H2O2 | 27 |
| GPX2 | Glutathione peroxidase 2 (gastrointestinal) | Gastrointestinal GPX; detoxification of H2O2 | 3 |
| GPX4 | Glutathione peroxidase 4 (phospholipid hydroperoxidase) | Reduces H2O2, lipid hydroperoxides | 27 |
| GSTA1 | Glutathione S-transferase-α1 | Detoxification mechanism of reactive xenobiotic | 29 |
| GCLC | Glutamate-cysteine ligase, catalytic subunit | Glutathione biosynthesis; rate-limiting enzyme | 56 |
| GSS | Glutathione synthetase | Glutathione biosynthesis | 23 |
| GSR | Glutathione reductase | Reduction of oxidized glutathione | 54 |
| Genes involved in NF-κB signaling | |||
| NFKB1 | Nuclear factor of κ-light polypeptide gene enhancer in B cells 1 | Precursor to p50 subunit of NF-κB complex Inactive p105 subunit acts as NF-κB inhibitor |
45, 51 |
| RELA | V-rel reticuloendotheliosis viral oncogene homolog A (avian) | p65 subunit of NF-κB transcription factor | 41 |
| NF-κB target genes | |||
| IL-8 | Interleukin 8 | Proinflammatory chemokine; recruitment of neutrophil | 34 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | Synthesis of prostaglandin (PGE2) | 52 |
| IRAK2 | Interleukin-1 receptor-associated kinase 2 | Component of Toll-like/IL-1 receptor signaling; positive regulator of NF-κB | 14 |
| BID | BH3-interacting domain death agonist | Proapoptotic protein | 33 |
| BAX | BCL2-associated X protein | Proapoptotic protein | 33 |
| TP53 | Tumor protein p53 | Transcription factor mediating cell death | 43 |
| Genes in other signaling pathways | |||
| ALOX15B | Arachidonate 15-lipoxygenase, type B | Lipoxygenase that converts arachidonic acid to 15S-hydroperoxyeicosatetraenoic acid | 9 |
| PKCA | Protein kinase Cα | Serine/threonine kinase; proliferation, apoptosis, differentiation | 40 |
| MAPK1 | Mitogen-activated protein kinase 1 | ERK; p38; proliferation, differentiation, transcription regulation | 25 |
| MAPK8 | Mitogen-activated protein kinase 8 | JNK; proliferation, differentiation, transcription regulation | 25 |
| Housekeeping Genes | |||
| RPL13A | Ribosomal protein L13a | Housekeeping gene | 22 |
| ACTB | β-Actin | Housekeeping gene | 22 |
GCLC protein and glutathione content.
To determine the relative effect of different Toc isoforms on GCLC protein expression, FHs 74 Int cells were rinsed twice with PBS and lysed with RIPA buffer containing protease inhibitor cocktail. The total protein content was normalized after measurement by DC protein assay. Proteins from cell lysates were separated in a 12% resolving SDS-polyacrylamide gel and subjected to Western blotting with anti-GCLC antibody (1:1,000 dilution); GAPDH was used as a loading control. The effect of different Toc isoforms on the glutathione content of FHs 74 Int cells was determined using a glutathione assay kit according to the manufacturer's instructions.
Effect of Toc on cell viability and apoptosis.
The effects of different Toc isoforms on FHs 74 Int cell viability were evaluated using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay, as previously described (17). To determine whether the Toc-induced cell death was attributed to apoptosis, DNA fragmentation was examined using APO-BrdU TUNEL assay kits according to the manufacturer's instruction. Flow cytometry analysis was performed using a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA), and flow cytometry data were analyzed using FlowJo (Tree Star, Ashland, OR) (19).
Statistics.
All experiments were performed in triplicate, and data are expressed as means ± SD. Significant differences between Toc isoforms at specific concentrations were identified using a one-way ANOVA (P < 0.05) followed by Tukey's post hoc analysis to determine differences between individual groups. In addition, the effect of different Toc isoforms on modulation of IL-8 expression, Nrf2 and NF-κB signaling, cell viability, and glutathione content relative to control cells was determined using a t-test (P < 0.05).
RESULTS
Cellular uptake of Toc.
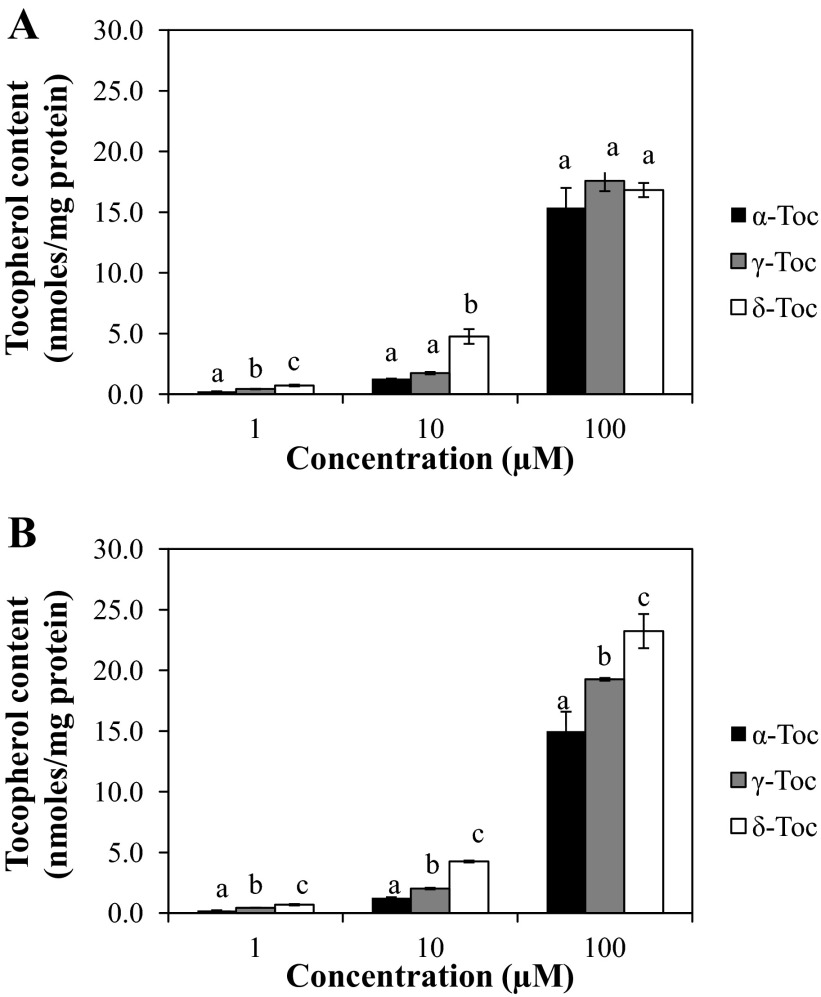
Uptake of different Toc isoforms by FHs 74 Int cells was concentration- and isoform-dependent (Fig. 1). Using this cell model system, we found that δ-Toc was retained by cells to the greatest (P < 0.05) extent, followed by γ- and α-Toc, respectively. The Toc isoform content measured in FHs 74 Int cells was used to establish whether a relative bioactive function could be attributed to the extent of uptake in intestinal cells. Distinct isoform- and concentration-dependent cellular Toc uptake patterns were also observed when cells were exposed to different isoforms in the absence of IFNγ-PMA (Fig. 1).
Fig. 1.
Cellular uptake of α-, γ-, and δ-tocopherol (Toc) in FHs 74 Int cells cultured under conditions used to evaluate Toc isoform modulation of the inflammatory response without (A) or with (B) IFNγ-PMA stimulation. Cells were treated with Toc isoforms for 24 h and then exposed for 24 h to replenished Toc isoforms in the presence or absence of IFNγ-PMA challenge. Different letters (a, b, c) indicate significant (P < 0.05) differences between Toc isoforms at the same concentration.
Modulation of IL-8.
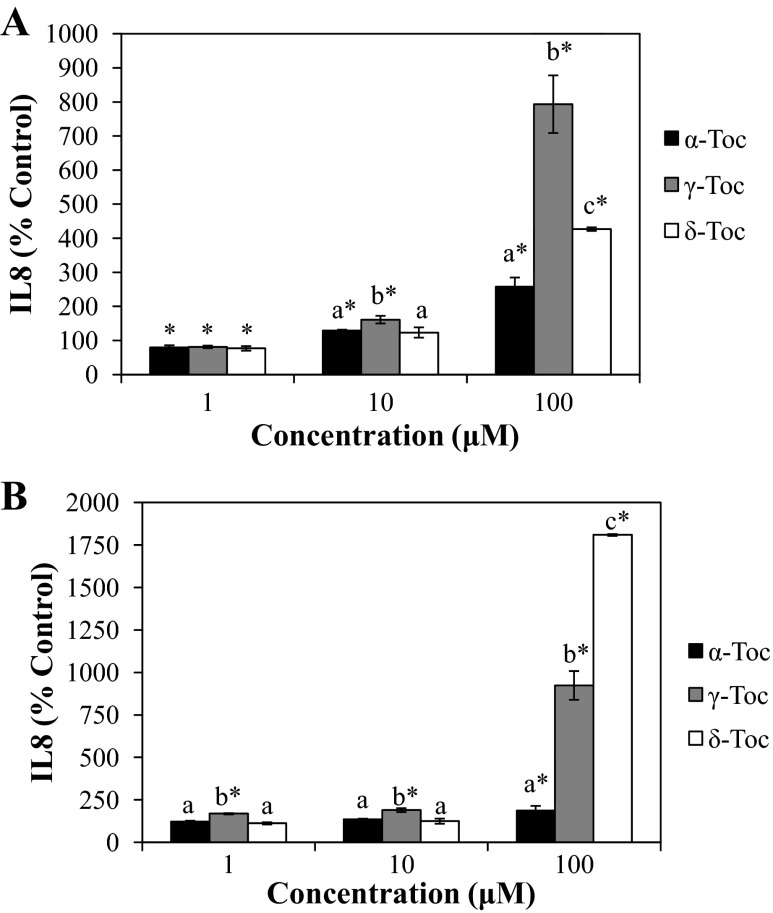
To determine the relative effect of the Toc isoforms on the inflammatory response in IFNγ-PMA-challenged FHs 74 Int cells, we measured the inhibition of IL-8 secretion (Fig. 2). Toc isoforms were found to differentially promote IL-8 production in IFNγ-PMA-treated cells in a concentration-dependent manner (Fig. 2A). At 100 μM, γ-Toc significantly (P < 0.05) promoted IL-8 production by about eightfold, whereas δ-Toc and α-Toc stimulated IL-8 secretion by three- and twofold, respectively, relative to control cells incubated with IFNγ-PMA alone. To further compare the proinflammatory effects of different Toc isoforms, we evaluated each Toc isoform on non-IFNγ-PMA-challenged FHs 74 Int cells. Significantly (P < 0.05) greater IL-8 secretion was observed in Toc-treated cells than in control non-Toc-treated cells (Fig. 2B). Exposure of cells to 100 μM Toc isoforms, in particular, resulted in an isoform-dependent stimulation of IL-8 secretion from cells: δ-Toc > γ- Toc > α-Toc (P < 0.05).
Fig. 2.
Effect of different tocopherol isoforms on IL-8 secretion in FHs 74 Int cells in the presence (A) or absence (B) of IFNγ-PMA challenge. IL-8 was quantified from supernatants of cells that were treated with Toc isoforms prior to IFNγ-PMA challenge or no challenge. Different letters indicate significant (P < 0.05) differences between Toc isoforms at the same concentration. *P < 0.05 relative to control (19). In A, IL-8 in Toc-treated cells was compared with IL-8 in IFNγ-PMA-stimulated control cells (IL-8 = 1,067 pg/ml). In B, IL-8 in Toc-treated cells was compared with IL-8 in non-IFNγ-PMA-stimulated control cells (IL-8 = 173 pg/ml).
Modulation of NF-κB and Nrf2 signaling.
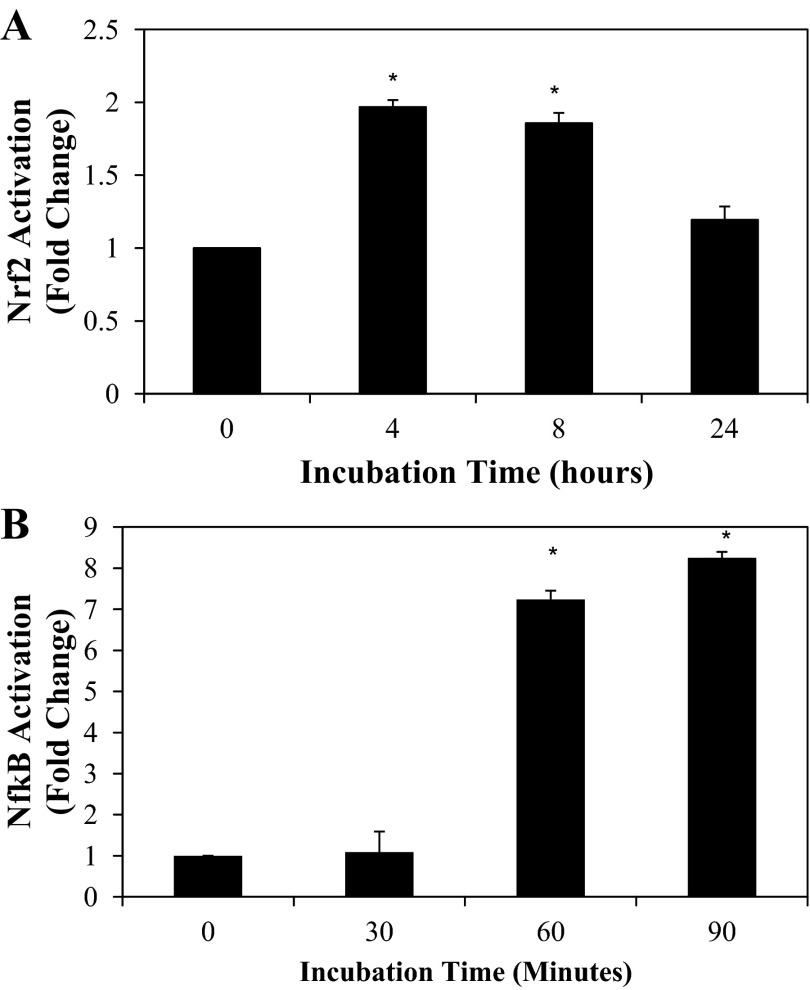
Incubation of FHs 74 Int cells with IFNγ-PMA stimulated a time-dependent translocation of Nrf2 and NF-κB (p65) to the nucleus. After 90 min of incubation with IFNγ-PMA, NF-κB was maximally translocated to the nucleus, while the Nrf2 transcription factor was translocated to the nucleus 4–8 h after IFNγ-PMA exposure (Fig. 3).
Fig. 3.
Time-course activation of Nrf2 (A) and NF-κB (B) signaling by a mixture of IFNγ (4,000 U/ml) and PMA (0.05 μg/ml) in FHs 74 Int cells. Values are means ± SD (n = 3). *P < 0.05 vs. time 0.
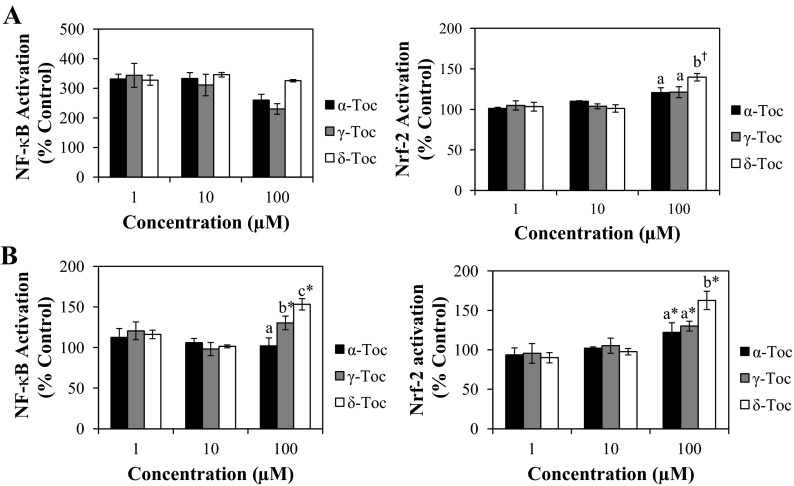
Tocs influenced NF-κB signaling in FHs 74 Int cells in an isoform-specific manner. In the absence of IFNγ-PMA stimulation, δ-Toc at 100 μM was the most effective activator of the NF-κB signaling pathway, followed by γ-Toc, while α-Toc had no effect (Fig. 4B). In the presence of IFNγ-PMA challenge, however, we did not observe further stimulation of NF-κB nuclear translocation beyond that elicited by IFNγ-PMA alone (Fig. 4A). It is possible that the effect of IFNγ-PMA had maximized NF-κB activity and, therefore, that an additional effect of Toc treatment on NF-κB signaling only extended the duration of NF-κB activation, rather than further upregulating nuclear NF-κB synthesis.
Fig. 4.
Modulation of NF-κB and Nrf2 activation by Toc isoforms in FHs 74 Int cells in the presence (A) or absence (B) of IFNγ-PMA challenge. Different letters indicate significant (P < 0.05) differences between Toc isoforms at the same concentration. NF-κB and Nrf2 were recovered from the nuclear fraction of cells that were treated with Toc isoforms and then challenged with IFNγ-PMA or left unchallenged for 90 min and 24 h, respectively. †P < 0.05, Toc-treated vs. IFNγ-PMA-treated cells. *P < 0.05, Toc-treated vs. untreated cells.
In regulating the Nrf2 signaling pathway, δ-Toc at 100 μM was shown to stimulate Nrf2 activation, as evidenced by the enhanced nuclear translocation that occurred regardless of IFNγ-PMA challenge (Fig. 4). In the absence of IFNγ-PMA challenge, this Nrf2 signaling was promoted to the greatest extent by δ-Toc, followed by γ- and α -Toc, which increased Nrf2 signaling to a similar extent.
Modulation of NF-κB and Nrf2 target genes.
Toc isoforms significantly (P < 0.05) increased transcription of genes involved in NF-κB signaling, including IL-1 receptor-associated kinase 2 (IRAK2), NFKB1, prostaglandin-endoperoxide synthase 2 (PTGS2), and IL-8 (Table 2), in FHs 74 Int cells challenged with IFNγ-PMA for 4 h. Extending IFNγ-PMA exposure to 24 h produced a more pronounced effect of Toc exposure, particularly on IL-8 and PTGS2 gene transcription (Table 2). The effect was isoform-dependent: γ- and δ-Toc increased the levels of IL-8 and PTGS2 transcripts, whereas α-Toc exerted no significant effects compared with IFNγ-PMA-treated FHs 74 Int cells. The increase in NF-κB target genes was accompanied by a downregulated transcription of numerous Nrf2-related genes that are involved in glutathione biosynthesis (GCLC and glutathione synthetase) and detoxification [glutathione S-transferase (GSTA1)] and the antioxidant enzyme glutathione peroxidase 1. A downregulation of genes expressing signaling involved in cell proliferation (tumor protein 53 and BAX) and other cell signaling mediators such as MAPK1 (Table 2) was also observed.
Table 2.
Effects of tocopherol isoforms on modulation of gene transcription in IFNγ-PMA-challenged FHs 74 Int cells
| Fold Regulation |
||||
|---|---|---|---|---|
| Gene | IFNγ-PMA | + α-Toc | + γ-Toc | + δ-Toc |
| 4 h | ||||
| IRAK2 | 3.2a | 3.6a,b | 4.0b | 4.9c |
| NFKB1 | 4.6a | 5.4a,b | 5.8a,b | 6.2b |
| IL8 | 1.0a | 2.4b | 2.9b | 9.2c |
| PTGS2 | 5.5a | 10.3b | 11.1b | 27.3c |
| 24 h | ||||
| GPX1 | 1.3a | 1.2a | 1.3a | −3.0b |
| GSTA1 | −5.4 | ND | ND | ND |
| GCLC | −1.2b | 1.2a | −1.8c | −8.2d |
| GSS | 1.2a | 1.3a | 1.2a | −3.0b |
| BAX | −1.1a | −1.2a | −1.4a | −4.2b |
| TP53 | 1.1a | 1.1a | −1.1b | −4.3c |
| IRAK2 | −1.7a | 1.2a | 5.3b | 5.6b |
| NFKB1 | 1.3a | 1.6a | 3.1b | 1.9a |
| IL8 | 5.4a | 6.2a | 169.5b | 377.4c |
| PTGS2 | 1.8a | 28.9a | 820.2b | 2,118.3c |
| MAPK1 | 1.2a | 1.2a | 1.2a | −3.6b |
Fold regulation is expressed relative to untreated control cells. Only genes that were significantly regulated >3-fold are listed. ND, not detectable. Different letters (a, b, c) indicate significant (P < 0.05) differences between treatments.
In the absence of IFNγ-PMA challenge, fewer genes were significantly regulated than when IFNγ-PMA was used to induce an inflammatory response in FHs 74 Int cells (Table 3). Nevertheless, we observed that γ- and δ-Toc upregulated NF-κB target genes, such as IL-8 and PTGS2, and downregulated Nrf2 target genes, such as GCLC and GSTA1, despite the absence of the IFNγ-PMA challenge. In addition, the effect of δ-Toc was most pronounced, followed by γ-Toc, while α-Toc had no effect, in modulating the gene expression of IL-8 and GCLC, a finding consistent with that observed in FHs 74 Int cells challenged with IFNγ-PMA.
Table 3.
Effects of tocopherol isoforms on modulation of gene transcription in non-IFNγ-PMA-challenged FHs 74 Int cells
| Fold Regulation |
||||
|---|---|---|---|---|
| Gene | Control | + α-Toc | + γ-Toc | + δ-Toc |
| 4 h | ||||
| IL8 | 1.0a | 3.0b | 1.6a | 1.8a |
| 24 h | ||||
| GSTA1 | 1.0a | 1.1a | −1.1a | −10.6b |
| GCLC | 1.0a | 1.1a | −1.5b | −6.4c |
| IL8 | 1.0a | 1.3a | 21.5b | 28.6c |
| PTGS2 | 1.0a | 2.2a | 9.4a | 147.8b |
Fold regulation is expressed relative to untreated control cells. Control group consists of untreated cells. Only genes that were significantly regulated >3-fold are listed. Different letters indicate significant (P < 0.05) differences between treatment groups.
Modulation of cellular glutathione content.
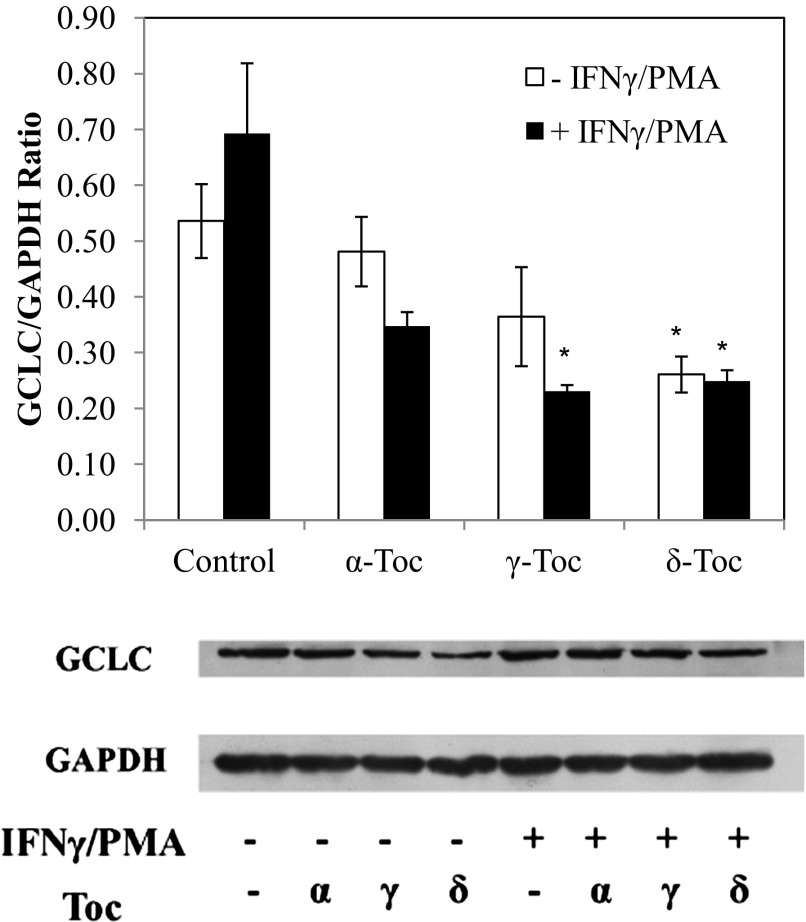
On the basis of the finding that γ- and δ-Toc modulated GCLC (the gene that encodes for γ-glutamylcysteine synthetase, a rate-limiting enzyme in glutathione biosynthesis) to the greatest extent, we examined the effect of different Toc isoforms on regulation of γ-glutamylcysteine synthetase protein expression and glutathione content in Toc-treated FHs 74 Int cells. Treatment of FHs 74 Int cells with IFNγ-PMA resulted in a significant (P < 0.05) decrease in the expression of GCLC protein (Fig. 5). γ- and δ-Toc significantly reduced GCLC expression in FHs 74 Int cells stimulated with IFNγ-PMA (P < 0.05; Fig. 6). In the absence of IFNγ-PMA challenge, however, only δ-Toc significantly reduced glutathione expression in FHs 74 Int cells (Fig. 5).
Fig. 5.
Effect of 100 μM α-, γ-, and δ-Toc on glutamate-cysteine ligase (GCLC) protein expression of FHs 74 Int cells incubated for 24 h in the presence or absence of IFNγ-PMA challenge. *P < 0.05, α-, γ-, and δ-Toc-treated vs. control cells.
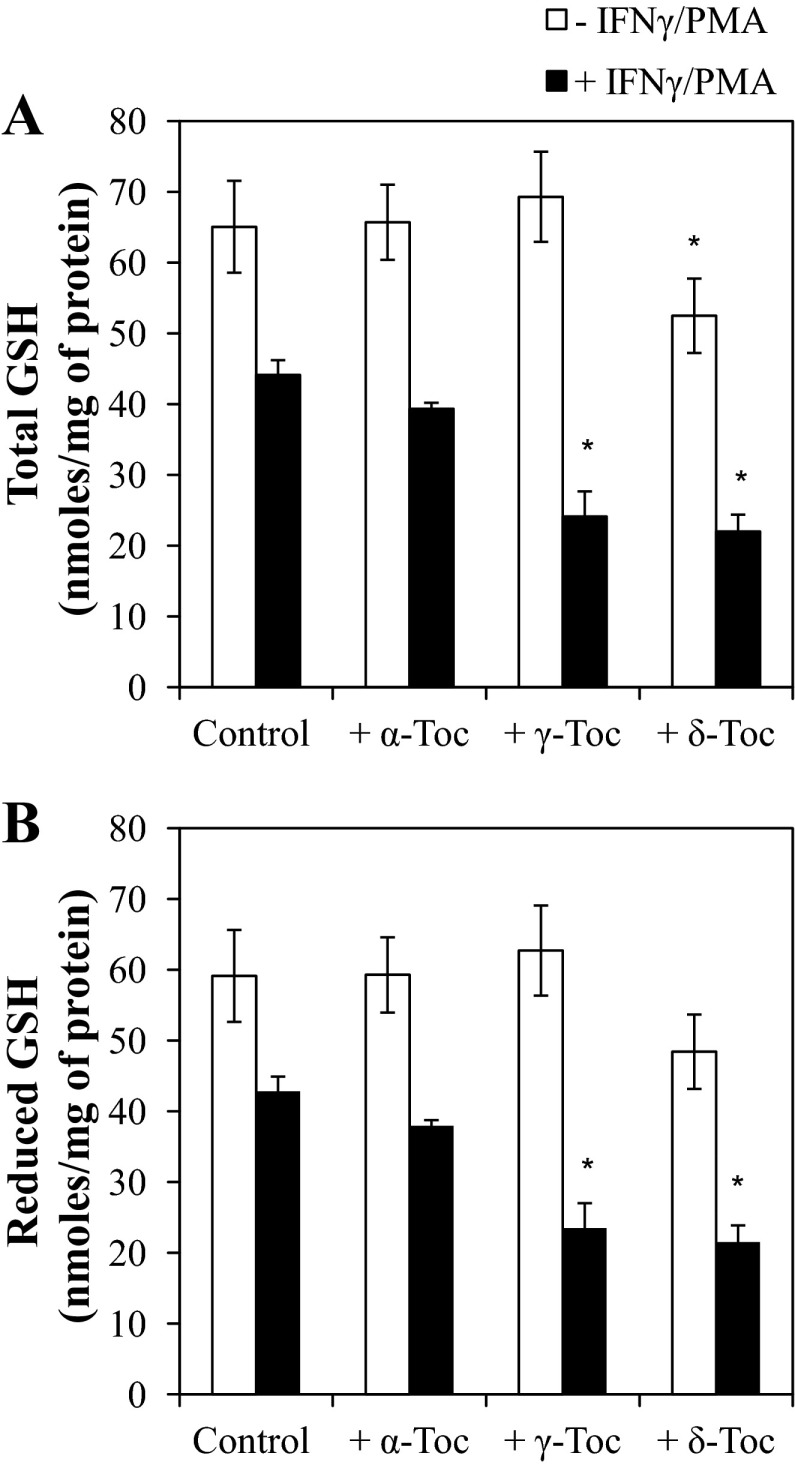
Fig. 6.
Effect of 100 μM α-, γ-, and δ-Toc on total (A) and reduced (B) glutathione (GSH) content of FHs 74 Int cells incubated for 24 h in the presence or absence of IFNγ-PMA challenge. *P < 0.05, α-, γ-, and δ-Toc-treated vs. control cells.
The reduction of GCLC expression in FHs 74 Int cells was found to correspond to significant changes in cellular glutathione content (Fig. 6). Specifically, γ- and δ-Toc were effective in reducing the glutathione content of IFNγ-PMA-challenged FHs 74 Int cells. Similar to the trend observed with the changes in GCLC protein expression, only δ-Toc suppressed (P < 0.05) the glutathione content of FHs 74 Int cells in the absence of IFNγ-PMA challenge (Fig. 6).
Modulation of apoptosis-mediated cytotoxicity.
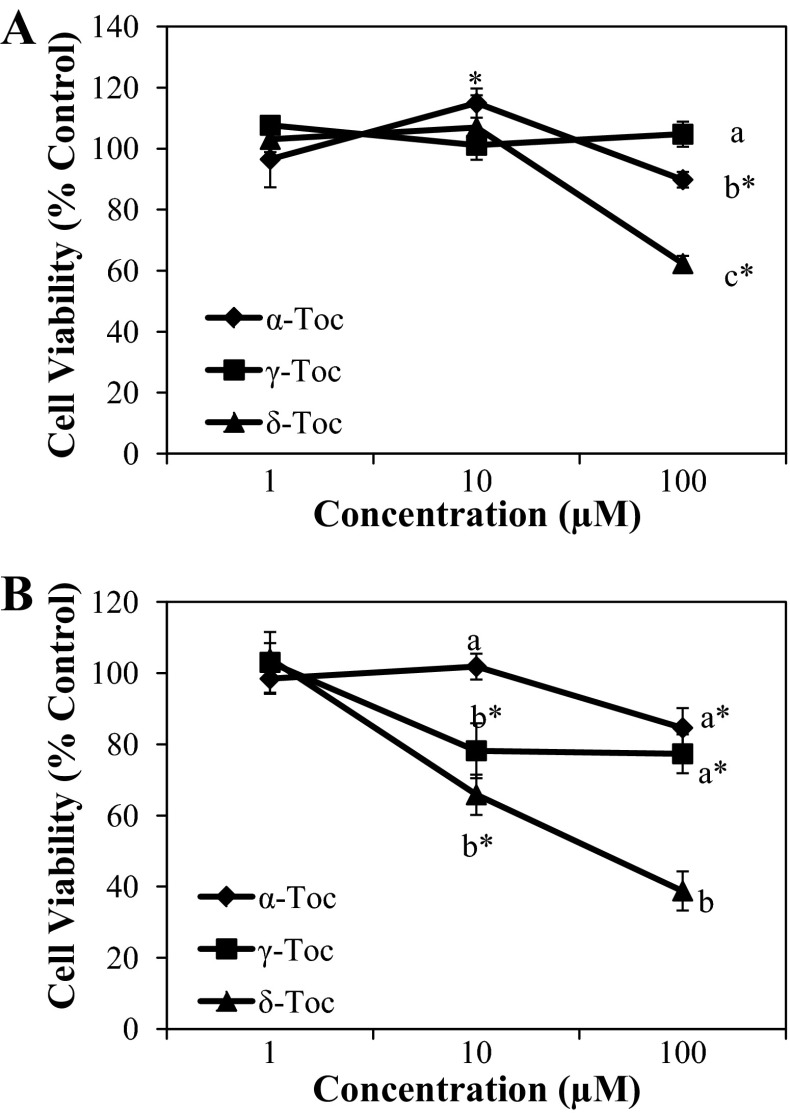
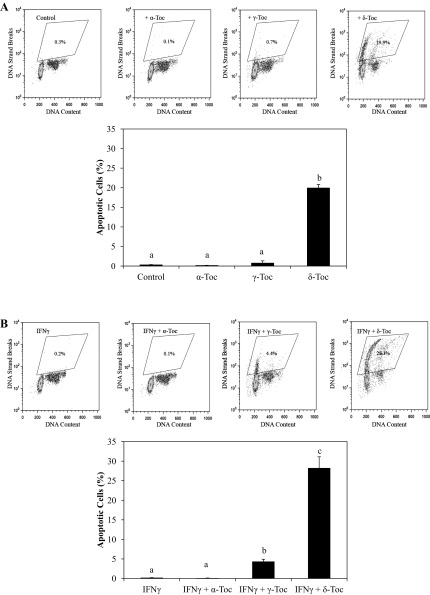
Non-α-Toc isoforms exerted a cytotoxic effect on FHs 74 Int cells (Fig. 7) that was exacerbated by the addition of IFNγ-PMA. For example, whereas γ- and δ-Toc effectively reduced cell viability only at 100 μM, a minimum concentration of 10 μM was found to be sufficient to exert cytotoxic effects in FHs 74 Int cells that had been pretreated with IFNγ-PMA. In particular, δ-Toc reduced FHs 74 Int cell viability to the greatest extent, regardless of IFNγ-PMA stimulation. To determine whether programmed cell death or apoptosis was involved in the mechanism of cell death caused by different Toc isoforms, DNA fragmentation was assessed in FHs 74 Int cells using TUNEL assays (Fig. 8). Apoptosis was induced in IFNγ-PMA-challenged FHs 74 Int cells to the greatest extent by δ-Toc (Fig. 8), followed by γ-Toc, whereas α-Toc was ineffective. On the other hand, only δ-Toc elicited apoptosis-mediated cytotoxicity in FHs 74 Int cells in the absence of IFNγ-PMA stimulation.
Fig. 7.
Effect of α-, γ-, and δ-Toc on FHs 74 Int cell viability in the absence (A) or presence (B) of IFNγ-PMA challenge as determined using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. Cells were treated with α-, γ-, and δ-Toc prior to challenge in the presence or absence of IFNγ-PMA. Different letters denote significant (P < 0.05) difference between α-, γ-, and δ-Toc at a given concentration. *P < 0.05, α-, γ-, and δ-Toc-treated vs. control cells.
Fig. 8.
A: effect of 100 μM α-, γ-, and δ-Toc on apoptosis of FHs 74 Int cells as shown by flow cytometry dot plot (top) and summarized as percentage of total apoptotic cells (bottom). FHs 74 Int cells were treated with α-, γ-, and δ-Toc in the absence of IFNγ-PMA. Different letters denote significant difference (P < 0.05) between Toc isoforms. B: effect of 100 μM α-, γ-, and δ-Toc on apoptosis of FHs 74 Int cells as shown by flow cytometry dot plot (top) and summarized as percentage of total apoptotic cells. FHs 74 Int cells were treated with α-, γ-, and δ-Toc and then challenged with IFNγ-PMA. Different letters denote significant (P < 0.05) difference between Toc isoforms.
DISCUSSION
Very recently, γ- and δ-Toc were shown to promote an inflammatory response in primary fetal-derived intestinal FHs 74 Int cells (19). In this study we relate the inflammatory response of FHs 74 Int cells exposed to equal concentration of Toc isoforms to the cellular concentration of Toc. Our approach enabled us to compare Toc isoform effects on inflammatory responses that occurred within a range of concentration-dependent uptakes for each isoform. The fact that Toc uptake by FHs 74 Int cells was isoform-dependent points to a specific structure-function activity for different Toc isoforms for cellular uptake. For example, cellular uptake was greatest for δ-Toc, the most polar Toc tested, followed by γ- and α-Toc, respectively. This finding corresponds to the observation that a similar order of different Toc isoforms (e.g., δ-Toc > γ-Toc > α-Toc) promoted an inflammatory response in the primary fetal-derived intestinal FHs 74 Int cells. Thus it can be inferred that the relative uptake of different Toc isoforms contributed at least in part to the corresponding greater bioactivity of γ- and δ-Toc in FHs 74 Int cells.
In addition to the relative disparities in the extent to which different Toc isoforms were taken up by FHs 74 Int cells, we also observed specific Toc isoform-induced modification of the NF-κB signaling pathway in FHs 74 Int cells. NF-κB activation was promoted to the greatest extent by δ-Toc, followed by γ-Toc, while α-Toc was ineffective. This pattern of NF-κB activation corresponds to the stimulatory effect of Toc on IL-8 secretion in FHs 74 Int cells and confirms the indispensable role of the NF-κB signaling pathway in expression of the proinflammatory cytokine IL-8. When we further challenged the cells using IFNγ-PMA stimulation, NF-κB nuclear translocation was not further enhanced by Toc. Nevertheless, the finding that Toc modulates baseline IL-8 secretion regardless of IFNγ-PMA challenge suggests that activation of NF-κB signaling did indeed occur. We suggest therefore that it is possible that NF-κB activation in IFNγ-PMA-treated cells reached a maximum nuclear translocation at the time of analysis and, furthermore, that the duration of NF-κB activation was specific to the source of Toc isoform treatment.
Despite the clear relationship between activation of NF-κB signaling and IL-8 secretion, our finding that α-Toc promoted IL-8 secretion without promoting activation of NF-κB signaling is difficult to explain. A possible role of Nrf2 signaling in IL-8 expression in FHs 74 Int cells was thus determined. All Toc isoforms tested, including α-Toc, were found to enhance Nrf2 signaling in FHs 74 Int cells, with the most pronounced effect elicited by δ-Toc, followed by α- and γ-Toc, both of which were less effective. In the presence of an inflammatory challenge induced by IFNγ-PMA treatment of FHs 74 Int cells, only δ-Toc enhanced Nrf2 activation. The current finding that γ- and δ-Toc promoted Nrf2 activation in FHs 74 Int cells while continuing to promote IL-8 expression was unexpected, since Nrf2 activation is largely believed to be associated with promotion of antioxidant enzyme expression. Our findings can be explained by results from an earlier study that reported an increase in Nrf2 expression associated with a concurrent increase in IL-8 expression, attributable to the stabilization of IL-8 mRNA (57). It is entirely possible, therefore, that the Nrf2 activation by different Toc isoforms in our study was due to the different abilities to stabilize IL-8 mRNA, which translated to a Toc isoform-dependent expression of IL-8 in FHs 74 Int cells.
The characteristic involvement of different Toc isoforms in modifying IFNγ-PMA-induced activation of Nrf2 and NF-κB was confirmed by comparing the relative ability of Toc isoforms to modulate expression of several Nrf2 and NF-κB target genes. Consistent with the finding that γ- and δ-Toc promoted NF-κB translocation to the nucleus in FHs 74 Int cells was the observation that γ- and δ-Toc also enhanced the expression of NF-κB-related genes. We show evidence of a Toc isoform-dependent increase in the transcription of NF-κB target genes, such as IL-8 and PTGS2, that occurred regardless of IFNγ-PMA stimulation.
While the Nrf2 signaling pathway was activated in FHs 74 Int cells treated with different Toc isoforms, as shown by the increased translocation of Nrf2 to the nucleus, treatment of cells with γ- and δ-Toc resulted in downregulation of numerous Nrf2 target genes. The gene that was most prominently altered by Toc isoforms in our array was GCLC, a Nrf2 target gene (29, 30). GCLC is the first rate-limiting enzyme in the two-step reaction of glutathione biosynthesis, which catalyzes the ligation of glutamate and cysteine to form γ-glutamylcysteine (56). The addition of glycine to the dipeptide produces the tripeptide glutathione, which is regulated to maintain normal cellular antioxidant status (56).
Consistent with the effect of Toc isoforms on GCLC transcription, γ- and δ-Toc suppressed the expression of GCLC protein, which corresponded to a decreased glutathione content of IFNγ-PMA-challenged FHs 74 Int cells. In the absence of IFNγ-PMA, δ-Toc continued to reduce the GCLC protein expression, as well as lower the total glutathione content, of FHs 74 Int cells. These findings confirm that non-α-Toc isoforms tested in this study have the capacity to downregulate glutathione biosynthesis in FHs 74 Int cells.
Our finding that Toc induced glutathione depletion in FHs 74 Int cells explains the observation that γ- and δ-Toc were cytotoxic to FHs 74 Int cells. The cytotoxicity of these Toc isoforms was mediated through apoptosis, as determined by its DNA fragmentation in FHs 74 Int cells when incubated with γ- and δ-Toc, respectively (15). Glutathione, a low-molecular-weight thiol present at high concentrations in all mammalian cells, functions in the cellular defense system against the generation of reactive species and xenobiotic-derived electrophiles (26). Intracellular levels of glutathione represent a balance between synthesis and consumption, as well as extracellular transport (26). Glutathione depletion in cells is an early hallmark of apoptosis in response to various stimuli (24). Since glutathione reacts directly or catalytically with reactive species, a reduction of the intracellular glutathione content is associated with the level of oxidative stress, which induces apoptosis (24, 48).
A notable finding related to the downregulation of GCLC was the increase in Nrf2 translocation to the nucleus. One explanation for this finding concerns the fact that activation of NF-κB signaling could suppress the transcription of Nrf2 target genes, despite the increased translocation of Nrf2 to the nucleus. Activation of the NF-κB pathway was shown to antagonize Nrf2 signaling at the level of transcription through the p65 subunit of the NF-κB complex, which could deprive Nrf2 of cAMP response element-binding protein-binding protein (CBP; a common coactivator of NF-κB and Nrf2) (36) or promote recruitment of the corepressor histone deacetylase 3 to the CBP and Nrf2 dimerization partner MafK (36). Alternatively, if p65 interacts with Keap1, a repressor protein that facilitates ubiquitination of Nrf2 in the cytoplasm, then this interaction would lead to proteosomal degradation (55). During NF-κB activation, increased amounts of p65 transcription factor are translocated to the nucleus. Physical interaction between Keap1 and p65 could explain our finding of an increase in nuclear Keap1, which in turn facilitates the shuttling of Keap1 from the nucleus to the cytoplasm to bind with Nrf2. The net effect would be repressed activity of antioxidant enzyme pathways.
Taken together, our findings show that γ- and δ-Toc, the dominant vitamin E isoforms found in infant formula, evoked a greater proinflammatory response in the fetal-derived intestinal cell line, than α-Toc, the dominant form of vitamin E in human milk. This may be of particular importance to formula-fed infants, who are at a relatively greater risk of developing necrotizing enterocolitis (37, 50), an inflammatory intestinal disorder.
The current finding that γ-Toc may promote an inflammatory response in the fetal-derived intestinal cell contradicts many studies that report the anti-inflammatory property of γ-Toc, including our finding in the adult-derived Caco-2 intestinal cell line (16, 19, 32). The anti-inflammatory activity of γ-Toc is touted to be superior to that of α-Toc, which is often attributed to the lack of a methyl group in the C-5 position in the chromanol ring (13, 28). Nevertheless, the ability of γ-Toc to oppose the anti-inflammatory effect of α-Toc has previously been reported (5, 12). In vitro, α-Toc was found to block, while γ-Toc was found to promote, VCAM-1-dependent lymphocyte transmigration. In combination, γ-Toc suppressed α-Toc inhibition of lymphocyte transmigration (5). The opposing effect of α- and γ-Toc was further confirmed in vivo, where α-Toc inhibits, while γ-Toc increases, leukocyte recruitment in the bronchoalveolar lavage and lung tissue during ovalbumin-induced lung inflammation in mice (5). An explanation for the apparent contradictory action of Toc to induce inflammation may involve Toc isoform-specific effects, as well as cell-specific responses. For example, Toc-induced lymphocyte migration has only been observed in endothelial cells, but not lymphocytes, when exposed to α- and γ-Toc (5). Thus there is a clear need for more research on the specific roles of γ- and δ-Toc in regulating inflammatory events.
Conclusion.
We have shown that γ- and δ-Toc evoke a greater inflammatory response in fetal-derived intestinal cell lines than α-Toc. Modification of the inflammatory response was attributed, at least in part, to the isoform-dependent uptake of Toc in FHs 74 Int cells and also to their characteristic affinities to alter Nrf2 and NF-κB cell signaling pathways. γ- and δ-Toc specifically triggered NF-κB activation, which antagonized Nrf2-mediated transcription and resulted in a downregulation of glutathione biosynthesis. The depletion of glutathione content corresponded to apoptosis-mediated cytotoxicity, which was induced to a greater extent by γ- and δ-Toc. γ- and δ-Toc, therefore, represent biologically active vitamin E forms in fetal-derived intestinal cells that can induce a proinflammatory effect. This finding should prompt further investigation into the safety of these vitamin E forms, which are present at high levels in infant formula compared with mother's milk.
GRANTS
This work was funded by a research grant from the Food, Nutrition, and Health Vitamin Research Fund (D. D. Kitts), the Natural Science and Engineering Council of Canada (D. D. Kitts), and the Four Year Fellowship (I. Elisia).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.E. and D.D.K. are responsible for conception and design of the research; I.E. performed the experiments; I.E. analyzed the data; I.E. interpreted the results of the experiments; I.E. prepared the figures; I.E. drafted the manuscript; I.E. and D.D.K. edited and revised the manuscript; I.E. and D.D.K. approved the final version of the manuscript.
REFERENCES
- 1.Anderson SA, Chinn HI, Fisher KD. History and current status of infant formulas. Am J Clin Nutr 35: 381–397, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Baird L, Dinkova-Kostova A. The cytoprotective role of the Keap1/Nrf2 pathway. Arch Toxicol 85: 241–272, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohé R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol 25: 4914–4923, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellezza I, Mierla AL, Minelli A. Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers 2: 483–497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills JM. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol 182: 4395–4405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieri JG, McKenna MC. Expressing dietary values for fat-soluble vitamins: changes in concepts and terminology. Am J Clin Nutr 34: 289–295, 1981 [DOI] [PubMed] [Google Scholar]
- 7.Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J 13: 1145–1155, 1999 [PubMed] [Google Scholar]
- 8.Brigelius-Flohé R. Bioactivity of vitamin E. Nutr Res Rev 19: 174–186, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Campbell SE, Musich PR, Whaley SG, Stimmel JB, Leesnitzer LM, Dessus-Babus S, Duffourc M, Stone W, Newman RA, Yang P, Krishnan K. γ-Tocopherol upregulates the expression of 15-S-HETE and induces growth arrest through a PPAR-γ-dependent mechanism in PC-3 human prostate cancer cells. Nutr Cancer 61: 649–662, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Chen XM, Kitts DD. Determining conditions for nitric oxide synthesis in Caco-2 cells using Taguchi and factorial experimental designs. Anal Biochem 381: 185–192, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Committee on the Evaluation of the Addition of Ingredients New to Infant Formula Infant Formula: Evaluating the Safety of New Ingredients. Washington, DC: National Academies Press, 2004, p. 7, 41–44 [PubMed] [Google Scholar]
- 12.Cook-Mills M, McCary A. Isoforms of vitamin E differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets 10: 348–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. γ-Tocopherol detoxification of nitrogen dioxide: superiority to α-tocopherol. Proc Natl Acad Sci USA 90: 1771–1775, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ. Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-κB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem 285: 38951–38960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darzynkiewicz Z, Galkowski D, Zhao H. Analysis of apoptosis by cytometry using TUNEL assay. Methods 44: 250–254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaraj S, Leonard S, Traber MG, Jialal I. γ-Tocopherol supplementation alone and in combination with α-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med 44: 1203–1208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elisia I, Popovich DG, Hu C, Kitts DD. Evaluation of viability assays for anthocyanins in cultured cells. Phytochem Anal 19: 479–486, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Elisia I, Kitts DD. Quantitation of hexanal as an index of lipid oxidation in human milk and association with antioxidant components. J Clin Biochem Nutr 49: 147–152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elisia I, Kitts DD. Different tocopherol isoforms vary in capacity to scavenge free radicals, prevent inflammatory response and induce apoptosis in both adult- and fetal-derived intestinal epithelial cells. Biofactors.In press [DOI] [PubMed] [Google Scholar]
- 20.Elisia I, Tsopmo A, Friel JK, Diehl-Jones W, Kitts DD. Tryptophan from human milk induces oxidative stress and upregulates the Nrf-2-mediated stress response in human intestinal cell lines. J Nutr 141: 1417–1423, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 56: 650–651, 1922 [DOI] [PubMed] [Google Scholar]
- 22.Foldager C, Munir S, Ulrik-Vinther M, Søballe K, Bünger C, Lind M. Validation of suitable housekeeping genes for hypoxia-cultured human chondrocytes. BMC Mol Biol 10: 1–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forman HJ, Dickinson DA. Oxidative signaling and glutathione synthesis. Biofactors 17: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 16: 1303–1314, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 11: 211–218, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27: 922–935, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31: 273–300, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA. New perspectives on vitamin E: γ-tocopherol and carboxyethylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med 36: 1–15, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev 43: 92–137, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol 72: 847–855, 2002 [PubMed] [Google Scholar]
- 31.Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett 409: 105–108, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. γ-Tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA 97: 11494–11499, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kluck RM, Esposti MD, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR, Newmeyer DD. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J Cell Biol 147: 809–822, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunsch C, Rosen CA. NF-κB subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 13: 6137–6146, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76: 1485–1489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu GH, Qu J, Shen X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta 1783: 713–727, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 336: 1519–1523, 1990 [DOI] [PubMed] [Google Scholar]
- 38.McCormick CC, Parker RS. The cytotoxicity of vitamin E is both vitamer- and cell-specific and involves a selectable trait. J Nutr 134: 3335–3342, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Motohashi H, Yamamoto M. Nrf2/Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Nakashima S. Protein kinase Cα (PKCα): regulation and biological function. J Biochem 132: 669–675, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Nishikori M. Classical and alternative NF-κB activation pathways and their roles in lymphoid malignancies. J Clin Exp Hematop 45: 15–24, 2005 [Google Scholar]
- 42.Reboul E, Thap S, Perrot E, Amiot MJ, Lairon D, Borel P. Effect of the main dietary antioxidants (carotenoids, γ-tocopherol, polyphenols, and vitamin C) on α-tocopherol absorption. Eur J Clin Nutr 61: 1167–1173, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-κB in p53-mediated programmed cell death. Nature 404: 892–897, 2000 [DOI] [PubMed] [Google Scholar]
- 44.SABiosciences: A Qiagen Company RT2 Profiler™ PCR Array (Online). http://www.sabiosciences.com/PCRArrayPlate.php [27 June 2013].
- 45.Savinova OV, Hoffmann A, Ghosh G. The Nfkb1 and Nfkb2 proteins p105 and p100 function as the core of high-molecular-weight heterogeneous complexes. Mol Cell 34: 591–602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreck R, Albermann K, Baeuerle PA. Nuclear factor κB: an oxidative stress-responsive transcription factor of eukaryotic cells. Free Radic Res 17: 221–237, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Senanayake SP, Fichtali J. Single-cell oils as sources of nutraceutical and specialty lipids: processing technologies and applications. In: Nutraceutical and Specialty Lipids and Their Co-Products, edited by Shahidi F. Boca Raton, FL: CRC, 2006 [Google Scholar]
- 48.Slater AF, Stefan C, Nobel I, Van Den Dobbelsteen DJ, Orrenius S. Signalling mechanisms and oxidative stress in apoptosis. Toxicol Lett 82/83: 149–153, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Sontag TJ, Parker RS. Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism. J Biol Chem 277: 25290–25296, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM, Laroia N, Ehrenkranz RA, Dudell G, Cristofalo EA, Meier P, Lee ML, Rechtman DJ, Lucas A. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 156: 562–567, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Ten RM, Paya CM, Israel N, Bail OL, Mattei MG, Virelizier JL, Kourilsky P, Israel A. The characterization of the promoter of the gene encoding the p50 subunit of NF-κB indicates that it participates in its own regulation. EMBO J 11: 195–203, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol 38: 1654–1661, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Wagner KH, Kamal-Eldin A, Elmadfa I. γ-Tocopherol—an underestimated vitamin? Ann Nutr Metab 48: 169–188, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 134: 489–492, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Yu M, Li H, Liu Q, Liu F, Tang L, Li C, Yuan Y, Zhan Y, Xu W, Li W, Chen H, Ge C, Wang J, Yang X. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal 23: 883–892, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Forman HJ. Glutathione synthesis and its role in redox signaling. Semin Cell Dev Biol 23: 722–728, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Chen X, Song H, Chen HZ, Rovin B. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur J Immunol 35: 3258–3267, 2005 [DOI] [PubMed] [Google Scholar]