Abstract
Endotoxemia is a causal factor in the development of alcoholic liver injury. The present study aimed at determining the interactions of ethanol with different fat sources at the gut-liver axis. Male Sprague-Dawley rats were pair fed control or ethanol liquid diet for 8 wk. The liquid diets were based on a modified Lieber-DeCarli formula, with 30% total calories derived from corn oil (rich in polyunsaturated fatty acids). To test the effects of saturated fats, corn oil in the ethanol diet was replaced by either cocoa butter (CB, rich in long-chain saturated fatty acids) or medium-chain triglycerides (MCT, exclusively medium-chain saturated fatty acids). Ethanol feeding increased hepatic lipid accumulation and inflammatory cell infiltration and perturbed hepatic and serum metabolite profiles. Ethanol feeding with CB or MCT alleviated ethanol-induced liver injury and attenuated ethanol-induced metabolic perturbation. Both CB and MCT also normalized ethanol-induced hepatic macrophage activation, cytokine expression, and neutrophil infiltration. Ethanol feeding elevated serum endotoxin level, which was normalized by MCT but not CB. In accordance, ethanol-induced downregulations of intestinal occludin and zonula occludens-1 were normalized by MCT but not CB. However, CB normalized ethanol-increased hepatic endotoxin level in association with upregulation of an endotoxin detoxifying enzyme, argininosuccinate synthase 1 (ASS1). Knockdown ASS1 in H4IIEC3 cells resulted in impaired endotoxin clearance and upregulated cytokine expression. These data demonstrate that the protection of saturated fats against alcohol-induced liver injury occur via different actions at the gut-liver axis and are chain length dependent.
Keywords: alcohol, saturated fat, endotoxemia, inflammation, liver injury
alcoholic liver disease (ALD) is a major health problem worldwide (19, 41). Endotoxemia is closely associated with the pathogenesis of ALD through the stimulation of proinflammatory cytokine production (6, 37). Significantly increased levels of endotoxin were found in both alcoholic patients and experimental animal models of ALD, and the levels correlated well with the development of liver injury (7). Our previous study showed that neutralization of endotoxin by endotoxin neutralizing protein attenuates acute alcohol-induced cytokine production and liver injury, suggesting the importance of endotoxins in mediating alcoholic hepatotoxicity (51). Mechanistic studies have suggested that intestinal bacterial overgrowth and intestinal permeability increase contribute to the development of endotoxemia in alcoholics (35, 37). Our previous work showed that orally administrated lipopolysaccharide was detectable in the plasma of alcohol-intoxicated mice but not in control mice (52), providing direct evidence that alcohol increases gut permeability to endotoxins. Animal studies also showed that prevention of gut leakiness resulted in suppression of alcohol exposure-induced endotoxemia and liver damage (18, 44), suggesting that gut leakiness is a causal factor in the development of alcoholic endotoxemia and liver injury.
Accumulating evidence has indicated that dietary fats are important determinants in the pathogenesis of ALD. The experimental models of ALD are commonly generated by feeding animals the Lieber-DeCarli liquid diet, in which fats in the diet are rich in unsaturated fatty acids (3, 25). Studies of alcohol exposure with dietary fats enriched with saturated fatty acids have been shown to produce much less liver damage compared with the originally used unsaturated fatty acids. These studies applied saturated fatty acids with different carbon chain lengths, including medium-chain triglycerides (MCT) (17, 22, 24, 30–32), long-chain saturated fats (cocoa butter, CB) (47, 48), or a mixture of long-chain saturated fats and monounsaturated fats (beef tallow) (16, 38). However, whether saturated fat sources with different chain lengths make a difference in protection against alcohol-induced liver injury has not been elucidated. Mechanistic studies on the protective effects of saturated fats against alcoholic steatosis have shown that fatty acid oxidation was accelerated through activation of adiponectin and the SIRT1-AMPK pathway and the restoration of hepatocyte nuclear receptor-4α (HNF-4α) (22, 38, 48). Dietary saturated fat reduced alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition (38). Recent studies showed that dietary fats differentially modulate the intestinal tight junctions and hepatic inflammatory response, suggesting that dietary fats have an impact on the gut-liver axis (16). Although saturated fats have been shown to attenuate alcohol-induced endotoxemia and hepatic inflammation, the mechanisms have not been fully defined.
The fatty acid compositions of MCT and CB are clearly distinguished by chain length. All the fatty acids in MCT have less than 12 carbons, with the most abundant being C8:0 fatty acid (67%). All the fatty acids in CB have more than 16 carbons, and C16:0 (25.3%) and C18:0 (33.5%) are the major saturated fatty acids. The characterizations of MCT and CB enable us to address the question of whether saturated fats of different chain lengths may differentially modulate ethanol-induced pathogenesis. The present study used a liquid ethanol diet with corn oil (CO) as dietary fat to produce alcoholic liver injury in rats, and the effects of dietary CB and MCT on ethanol-induced generation of endotoxin-cytokine signaling at the gut-liver axis were investigated.
MATERIALS AND METHODS
Animals and treatments.
Animal experiments were carried out according to experimental procedures approved by the Institutional Animal Care and Use Committee. Three-month-old male Sprague-Dawley rats (Charles River, Wilmington, MA) were fed the modified Lieber-DeCarli liquid diets (3, 25) containing ethanol (alcohol-fed, AF; n = 8) or isocaloric maltose dextrin as control (pair-fed, PF; n = 6) for 8 wk. Liquid diets were prepared as 1 kcal/ml; protein content was constant at 16% of total calories, fat 34%; and each diet had identical mineral and vitamin content. For ethanol consumption groups, the ethanol content (%, wt/vol) was initially 5% for the first 2 wk and was increased by about 0.14% every 2 wk up to a concentration of 5.43% during the final 2 wk (equals 35% to 38% of total calories in the diet), whereas ethanol was replaced isocalorically with maltose dextrin in control group (Table 1). To determine whether dietary fat sources modulate alcohol-induced liver injury, 30% of total calories were provided by either CO (rich in polyunsaturated fatty acids), CB (rich in long-chain saturated fatty acids), or MCT (rich in medium-chain saturated fatty acids), respectively. Detailed fat compositions of each dietary fat are listed in Table 2. Because the ethanol feeding group with MCT showed the lowest food intake, the other three groups were pair fed the same amount that AF/MCT rats had in the previous day. All ingredients for the liquid diets were obtained from Dyets (Bethlehem, PA) with the exception of ethanol, which was purchased from Sigma-Aldrich (St. Louis, MO). At the end of the experiment, rats were anesthetized with isoflurane after 4-h fasting, and blood, liver, and intestinal samples were harvested for assays.
Table 1.
Nutrient compositions in the liquid diets
| PF/CO | AF/CO | AF/CB | AF/MCT | |
|---|---|---|---|---|
| Protein | 16 | 16 | 16 | 16 |
| Carbohydrate | 50 | 12 | 12 | 12 |
| Ethanol | 38 | 38 | 38 | |
| Safflower oil | 4 | 4 | 4 | 4 |
| Corn oil | 30 | 30 | ||
| Cocoa butter | 30 | |||
| MCT | 30 |
Values are % of total calories. PF/CO, pair-fed with corn oil; AF/CO, alcohol-fed with corn oil; AF/CB, alcohol-fed with cocoa butter; AF/MCT, alcohol-fed with medium chain triglycerides (MCT).
Table 2.
Fat compositions of dietary fats contained in liquid diets
| Fats | Corn Oil | Cocoa Butter | MCT |
|---|---|---|---|
| < C8:0 | 4.0% | ||
| C8:0 | 67.0% | ||
| C10:0 | 27.0% | ||
| C12:0 | 2.0% | ||
| C16:0 | 11.0% | 25.7% | |
| C18:0 | 2.0% | 33.9% | |
| C18:1 | 28.0% | 36.9% | |
| C18:2 | 58.0% | 3.2% | |
| C18:3 | 0.2% | ||
| C20:0 | 1.0% | 0.1% | |
| Saturated fats | 14.0% | 59.7% | 100% |
| Unsaturated fats | 86.0% | 40.3% |
MCT, alcohol-fed with medium chain triglycerides.
Cell culture and treatments.
H4IIEC3 rat hepatoma cells (American Type Culture Collection, Rockville, MD) were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% (vol/vol) fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen), at 37°C in a 5% CO2 environment. Culture medium was changed every 2 or 3 days. For silencing argininosuccinate synthase 1 (ASS1), ASS1 small interfering RNA (siRNA) transfection was conducted with rat ASS1 siRNA or negative control siRNA (Invitrogen) by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. The silencing effect was validated after 24 h of transfection. Fluorescein isothiocyanate (FITC)-labeled LPS (Sigma-Aldrich) was added to medium at 5 μg/ml 9 h post-siRNA transfection and treated for 15 h. Intracellular LPS was evaluated by both endotoxin assay and microscopy of FITC and 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen), which counterstains the nucleus. Cell viability was determined by the Cell Counting Kit-8 (CCK-8) (Enzo Life Sciences, Farmingdale, NY) relative to the negative control group (negative control siRNA without LPS treatment).
Histopathological analysis of liver.
Liver tissues were fixed in 10% formalin and processed for paraffin embedding. Paraffin sections were cut at 5 μm and processed for hematoxylin and eosin (H&E) staining to assess the histological features of steatosis and inflammation.
Detection of hepatic macrophages and neutrophils.
For detection of hepatic macrophages, liver tissues were frozen in Tissue-Tek CRYO-OCT compound (Fisher Scientific, Waltham, MA). Cryostat tissue sections were cut at 7 μm and fixed with methanol for 10 min at −20°C. Tissue sections were incubated with mouse anti-CD68 or anti-CD163 antibody at 4°C overnight, followed by incubation with Alexa Fluor 594-conjugated donkey anti-mouse IgG (Invitrogen) for 30 min at room temperature. The nuclei were counterstained by DAPI (Invitrogen). The number of positively stained macrophages and area of fluorescence intensity were quantified by Image Pro Premier (Media Cybernetics, Rockville, MD). For detection of hepatic neutrophils, liver tissues were fixed with 10% formalin and cut at 5 μm. The sections were incubated with rabbit anti-myeloperoxidase (MPO) antibody (LifeSpan Biosciences, Seattle, WA) at 4°C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG for 30 min at room temperature. Diaminobenzidine was used as HRP substrate for visualization, and the nuclei were counterstained by methyl green (Sigma-Aldrich). The negative controls were conducted by omitting the primary antibody.
Determination of hepatic lipid accumulation.
Hepatic lipid accumulation was assessed by histochemical staining of neutral lipids and biochemical assay of lipid concentrations. For histochemical staining of neutral lipids, cryostat tissue sections were cut at 7 μm and fixed with 10% formalin for 10 min. Tissue sections were stained following the Oil Red O procedure. To quantitatively analyze hepatic lipids, liver tissues were extracted by use of chloroform/methanol (2:1 vol/vol). Protein in the homogenate was assayed by using protein assay reagent (Bio-Rad, Hercules, CA) to normalize the amount of lipid extracted. Triglyceride, cholesterol, and free fatty acid (FFA) levels were measured with assay kits from BioVision (Milpitas, CA).
Blood parameters.
Blood samples were drawn from the dorsal vena cava, and serum was obtained by centrifuging the blood at 8,000 g for 15 min at 4°C. Glucose and β-hydroxybutyrate were determined by using commercial kits purchased from Cayman Chemical (Ann Arbor, MI) following the manufacturer's instructions. Serum alanine aminotransferase (ALT) activity and aspartate aminotransferase (AST) activity were colorimetrically measured with Infinity ALT Reagent and Infinity AST Reagent (Fisher Scientific), respectively. Serum triglycerides were determined by Infinity Triglyceride Reagent (Fisher Scientific).
Metabolomic profiles and data analysis.
Serum and liver samples were prepared and analyzed with high-performance liquid chromatography-time of flight mass spectrometry (HPLC-TOFMS) as described previously (45). An Agilent HPLC 1200 system equipped with a binary solvent delivery manager and a sample manager (Agilent, Santa Clara, CA) was used with chromatographic separations performed on a 4.6 × 150 mm 5-μm Agilent ZoRbax Eclipse XDB-C18 chromatography column. The liquid chromatography elution conditions were optimized as follows: isocratic at 1% B (0–0.5 min), linear gradient from 1% to 20% B (0.5–9.0 min), 20–75% B (9.0–15.0 min), 75–100% B (15.0–18.0 min), isocratic at 100% B (18–19.5 min); linear gradient from 100% to 1% B (19.5–20.0 min) and isocratic at 1% B (20.0–25.0 min). For positive ion mode (ESI+) A = water with 0.1% formic acid and B = acetonitrile with 0.1% formic acid, whereas A = water and B = acetonitrile for negative ion mode (ESI−). Mass spectrometry is performed using an Agilent model 6220 MSD TOF mass spectrometer equipped with a dual-sprayer electrospray ionization source (Agilent). The TOF mass spectrometer was operated with the following optimized conditions: 1) ES+ mode, capillary voltage 3,500 V, nebulizer 45 psig, drying gas temperature 325°C, drying gas flow 11 l/min; and 2) ES− mode, similar conditions as ES+ mode except the capillary voltage is adjusted to 3,000 V. During metabolite profiling experiments, both plot and centroid data were acquired for each sample from 50 to 1,000 Da over a 25-min analysis time.
Principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were carried out between groups. The differential metabolites were selected when they meet the requirements of variable importance in the projection > 1 in OPLS model and P < 0.05 from Student's t-test. The corresponding fold change shows how these selected differential metabolites varied between groups. Compounds identification was performed by comparing the accurate mass and retention time with reference standard available in our laboratory, or comparing the accurate mass with online database such as the Human Metabolome Database (http://www.hmdb.ca/).
Entodoxin assay.
The endotoxin levels in the blood, liver, and intestinal contents as well as in H4IIEC3 cells were assessed by use of a chromogenic kinetic limulus amoebocyte lysate (LAL) assay kit (QCL-1000, Lonza, Walkersville, MD). Blood samples were drawn from the dorsal vena cava. Serum was obtained by centrifuging the blood at 8,000 g for 15 min at 4°C. Liver samples and intestinal contents were prepared as described elsewhere (1). Briefly, liver tissues and intestinal contents from ileum, cecum, and colon lumen were weighed, collected in sterile, endotoxin-free tubes, diluted with limulus LAL reagent water (1:10 for liver tissues, 1:1,000 for intestinal contents samples), and homogenized. The samples were centrifuged at 14,000 g, for 10 min, and the supernatants were collected for the measurements of endotoxin. H4IIEC3 cells were lysed by Nonidet P-40 (NP-40) lysis buffer (20 mM Tris·HCl pH 7.4, 150 mM NaCl, 0.1 mM EDTA, 1% NP-40). After sonication and centrifugation (14,000 g, for 10 min), the supernatants were collected for assay. Inactivation of inhibitors was obtained by heating all samples for 15 min at 75°C. Standards and samples were incubated with LAL for 10 min at 37°C followed by 6-min incubation with colorimetric substrate. The reaction was stopped with 25% acetic acid, and the absorbance at 405 nm was read. The concentration of endotoxin was expressed in endotoxin units (EU) per milliliter for serum, EU per milligram hepatic tissues, and EU per 106 cells.
Quantitative PCR.
Total RNA from liver or intestinal (ileum, cecum, colon) mucosa was isolated by using TRIzol reagent (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. The isolated RNA was then reverse transcribed with the TaqMan Reverse Transcription Reagents (Life Technologies) after assessment of RNA quantity. Semiquantitative analysis of relative gene expressions were performed on the Applied Biosystems 7500 Real Time PCR Systems (Applied Biosystems, Carlsbad, CA) using SYBR green PCR Master Mix (Qiagen, Valencia, CA). Primers were designed and synthesized by Integrated DNA Technologies (Coralville, CA); sequences are listed in Table 3. All primer pairs were validated by demonstrating high amplification efficiency, consistent single-peak melt curve, and the presence of single product of the expected amplicon size on agarose gels. The relative gene expression was normalized to 18s rRNA expression, and calculated by the 2−ΔΔCt method (26) setting the values of PF/CO as 1.
Table 3.
Primer sequences used for qPCR analysis
| Gene | Genebank Accession No. | Forward Primer (5́-3́)/Reverse Primer (5́-3́) | Amplicon Size |
|---|---|---|---|
| CINC-1/Cxcl1 | NM_030845 | ACCCAAACCGAAGTCATAGCCAC/ACTAGTGTTGTCAGAAGCCAGCGT | 181 bp |
| MCP-1/Ccl2 | NM_031530 | TGCTGTCTCAGCCAGATGCAGTTA/TACAGCTTCTTTGGGACACCTGCT | 131 bp |
| Occludin/Ocln | NM_031329 | ATGCGGAAAGAGTCGACAGTCCAA/AAGTCATCCACGGACAAGGTCAGA | 148 bp |
| ZO-1/Tjp1 | NM_001106266 | AAGATGGGATTCTTAGGCCCAGCA/TCTTTGGCTGCAGGGCTATCTTCT | 136 bp |
| LBP/Lbp | NM_017208 | TGACATGTTACCGCCTGACTCCAA/AGACCACTGTTCCAAGAAGCTCCA | 119 bp |
| CD14/Cd14 | NM_021744 | TGAGTTGGGCGAGAAAGGACTGAT/TCCCGCAGTGAATTGTGACTGAGA | 174 bp |
| ASS1/Ass1 | NM_013157 | TCGTATACACCGGTTTCTGGCACA/ATGTACACCTGGCCCTTGAAGACA | 121 bp |
| 18 s rRNA/Rn18 s | NR_046237 | ACGGACCAGAGCGAAAGCAT/TGTCAATCCTGTCCGTGTCC | 152 bp |
qPCR, quantitative PCR.
Western blot.
Protein lysates of the liver or H4IIEC3 cells were extracted by using NP-40 lysis buffer plus protease and phosphatase inhibitor. Protein concentrations were estimated by the Bradford method (Bio-Rad). Aliquots containing 60 μg total proteins were loaded onto a 8–12% sodium dodecyl sulfate-polyacrylamide gel, transblotted onto polyvinylidene difluoride membrane (Bio-Rad), blocked with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20, and then incubated with rabbit anti-ASS1 (Novus Biologicals, Littleton, CO), rabbit anti-CD14 (Santa Cruz Biotechnologies, Santa Cruz, CA), or mouse anti-β-actin (Sigma-Aldrich). The membrane was then incubated with HRP-conjugated donkey anti-rabbit or goat anti-mouse immunoglobulin G (Thermo Scientific, Rockford, IL). The bound complexes were detected with enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) and quantified by densitometry analysis.
Statistical analysis.
All data are expressed as means ± SD (n = 8–10). The results were analyzed by Student's t-test and one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test. Differences between groups were considered significant at P < 0.05.
RESULTS
Effects of dietary fat sources on routine parameters and histopathological changes in the liver of alcohol-fed rats.
Routine parameters are listed in Table 4. There were no significant differences in average daily food intake and final body weight among all treatments. Alcohol feeding increased liver weight as well as liver-to-body weight ratio regardless of dietary fat sources. Chronic alcohol exposure with CO as the fat source significantly decreased blood glucose level, which was prevented by replacing CO with either CB or MCT. Regardless of dietary fat sources, chronic alcohol exposure dramatically elevated serum β-hydroxybutyrate level compared with controls, although the value in the AF/CB group was relatively lower than AF/CO and AF/MCT groups. Serum ALT and AST levels were increased in all the three groups fed ethanol. Serum triglyceride level was significantly decreased in AF/CO group compared with PF/CO group, which was normalized after replacement of CO with either CB or MCT.
Table 4.
Routine parameters and blood metabolites of rats fed liquid diets for 8 wk
| Measurements | PF/CO | AF/CO | AF/CB | AF/MCT |
|---|---|---|---|---|
| Food intake, g/day per rat | 68.72 ± 3.57 | 66.44 ± 4.52 | 67.40 ± 5.27 | 65.59 ± 4.71 |
| Body weight, g | 394.20 ± 30.35 | 393.14 ± 17.12 | 385.02 ± 29.37 | 375.57 ± 18.98 |
| Liver weight, g | 10.64 ± 0.69a | 12.90 ± 0.96b | 11.73 ± 0.73b | 12.52 ± 0.80b |
| LW/BW, % | 2.82 ± 0.15a | 3.21 ± 0.15b | 3.12 ± 0.12b | 3.39 ± 0.11c |
| Blood glucose, mg/dl | 151.22 ± 12.07a | 132.50 ± 7.79b | 151.74 ± 20.76ab | 149.05 ± 16.57ab |
| β-Hydroxybutyrate, mM | 0.18 ± 0.04a | 1.10 ± 0.33b | 0.72 ± 0.28c | 1.31 ± 0.32b |
| Alanine aminotransferase, U/l | 25.23 ± 3.41a | 44.09 ± 4.94b | 42.46 ± 2.82b | 42.70 ± 2.76b |
| Aspartate aminotransferase, U/l | 38.12 ± 6.38a | 46.26 ± 5.59b | 41.79 ± 6.90b | 45.54 ± 4.76b |
| Serum triglycerides, mg/dl | 150.96 ± 20.64a | 98.53 ± 13.04b | 139.86 ± 28.74c | 154.36 ± 24.28c |
Results are means ± SD (n = 6–8). LW, liver weight; BW, body weight. Significant differences (P < 0.05) between groups were determined by ANOVA. Means without a common letter differ at P < 0.05.
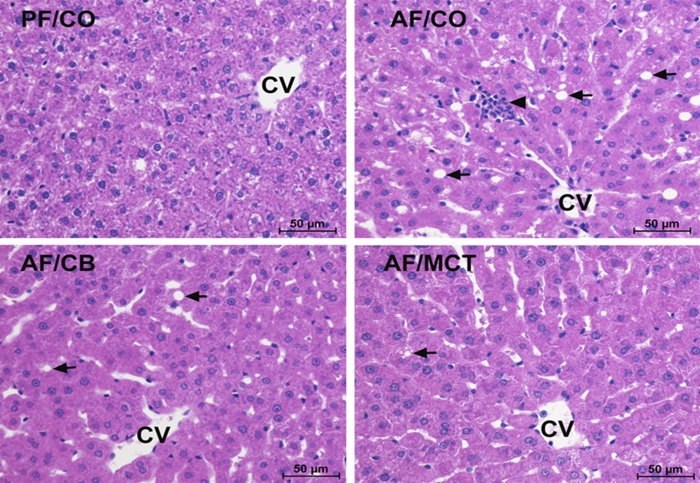
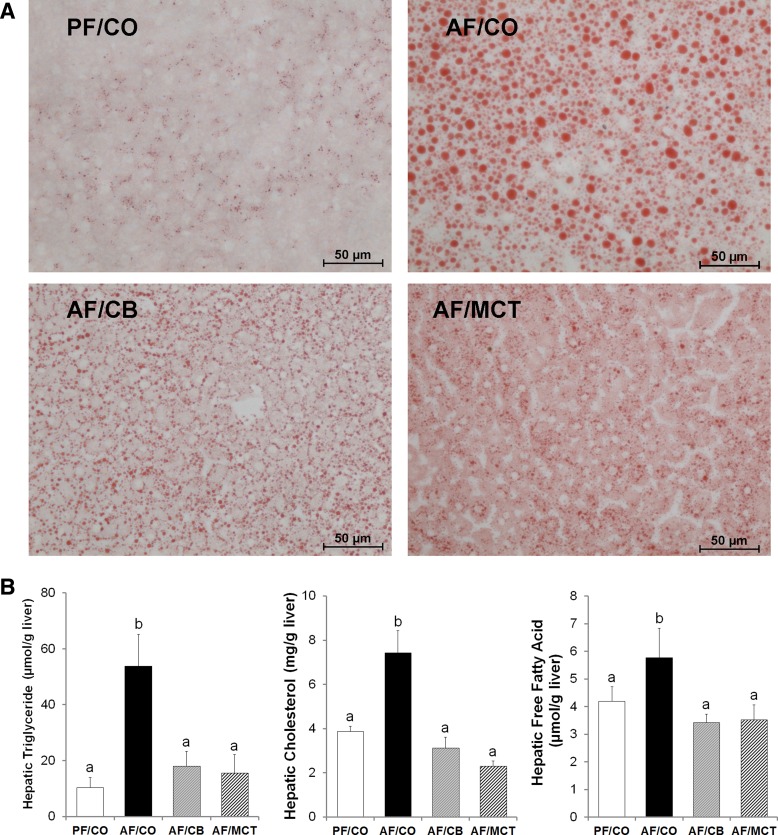
Histopathological changes of the liver were assessed by light microscopy of tissue sections with H&E staining. As shown in Fig. 1, alcohol exposure with CO caused liver injury, as indicated by lipid droplet accumulation (arrows) and inflammatory cell infiltration (arrowheads). These histopathological changes were alleviated by replacing CO with either CB or MCT. Hepatic lipid accumulation was further assessed by Oil Red O staining of neutral lipids and quantitative analysis of hepatic triglyceride, cholesterol, and FFAs. Ethanol feeding with CO induced remarkable accumulation of lipid droplets, whereas dietary CB or MCT substitution prevented this effect (Fig. 2A). The hepatic concentrations of triglyceride, cholesterol, and FFAs were all significantly higher in AF/CO group than PF/CO group, which were normalized in both AF/CB and AF/MCT groups (Fig. 2B).
Fig. 1.
Liver histopathology in rats fed ethanol with different dietary fats for 8 wk. Liver sections were stained by hematoxylin and eosin. Light microscopy showed lipid accumulation (arrows) and inflammatory cell infiltration (arrowheads) in the liver. Scale bar: 50 μm. CV, central vein; PF/CO, pair fed with corn oil; AF/CO, alcohol fed with corn oil; AF/CB, alcohol fed with cocoa butter; AF/MCT, alcohol fed with medium-chain triglycerides.
Fig. 2.
Lipid accumulation in the liver of rats chronically fed ethanol with different fat sources for 8 wk. A: neutral lipid accumulation in the liver. Neutral lipids were stained with Oil Red O. Scale bar = 50 μm. B: hepatic lipid concentrations. Triglyceride, cholesterol, and free fatty acid were measured with assay kits. Results are means ± SD (n = 6–8). Significant differences (P < 0.05, ANOVA) are indicated by different letters.
Impact of dietary fat sources on metabolite profiles in liver and serum in alcohol-fed rats.
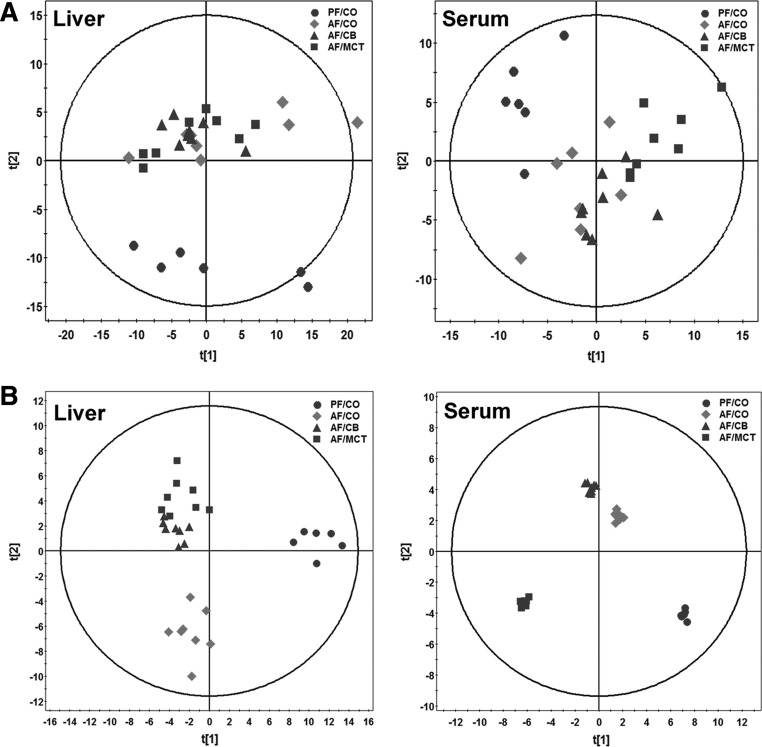
A total of 220 metabolites in liver samples and 167 metabolites in serum samples were identified by HPLC-TOFMS, of which 69 liver tissue metabolites and 71 serum metabolites were further validated by reference standards. The metabolite profiles among groups were evaluated with unsupervised statistics, PCA. Trends of separation between ethanol feeding groups and pair feeding groups were found in both liver and serum metabolites (Fig. 3A). We then constructed the OPLS-DA models. Regardless of dietary fat sources, the three ethanol feeding groups were all clearly separated from the PF/CO group. Moreover, the clusters of the AF/CB and AF/MCT groups distributed closely, whereas they were separated clearly from the AF/CO group in liver samples. In serum samples, the clusters of the AF/MCT group distributed further to AF/CO than the AF/CB group (Fig. 3B).
Fig. 3.
Principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) plots of spectral data of liver and serum metabolites in rats. Metabolite profiles of liver tissue homogenates and serum were analyzed by high-performance liquid chromatography-time of flight mass spectrometry (HPLC-TOFMS) (n = 6–8). A: PCA plots of liver and serum metabolites. B: OPLS-DA plots of liver and serum metabolites.
Table 5 lists liver metabolites with significant changes greater than or equal to twofold in alcohol-fed groups. Alcohol feeding with CO as the fat source increased hepatic concentrations of 27 metabolites and decreased concentrations of 13 metabolites. The most profound increase was estrone glucuronide, with a 449-fold increase. Metabolites showing more than 10-fold increase were bile acids, including cholic acid (15.40-fold), glycocholic acid (10-fold), glycodeoxycholic acid (41-fold), 2-methylglutaric acid (25-fold), and 3-methylglutary carnitine (14-fold). Although dietary CB and MCT attenuated alcohol-increased hepatic estrone glucuronide, cholic acid level was further increased by both CB and MCT, and 3-methylglutary carnitine was elevated by MCT. Metabolites showing reduced concentration in the AF/CO group include four glycerophospholipids (lysoPC 16:1, lysoPC 18:2, lysoPC 20:4, and lysoPC 20:5), three fatty acids (palmitoleic acid, linoelaidic acid, and 5,8,11-eicosatrienoic acid), two monosaccharides (glucose 6-phosphate and 2,3-dihydroxyvaleric acid), an organic oxoanionic compound (phosphoenolpyruvic acid), a purine nucleoside (xanthosine), a keto acid (pyruvic acid), and a sulfonic acid (taurine). Dietary CB and MCT attenuated the reduced levels of all the glycerophospholipids and fatty acids induced by alcohol consumption.
Table 5.
Fold changes of hepatic metabolites in rats subjected to alcohol with different dietary fat sources for 8 wk
| Compound | Library | M/Z | RT (min) | Class | FC AF/CO vs. PF/CO | P AF/CO vs. PF/CO | FC AF/CB vs. AF/CO | P AF/CB vs. AF/CO | FC AF/MCT vs. AF/CO | P AF/MCT vs. AF/CO |
|---|---|---|---|---|---|---|---|---|---|---|
| Phosphoenolpyruvic acid | Std | 166.99 | 4.01 | Aliphatic Acyclic Compounds | 0.46 | 7.8E-03* | 0.92 | 6.4E-01 | 1.01 | 9.4E-01 |
| Ophthalmic acid | HMDB05765 | 290.13 | 4.54 | Amino Acids, Peptides, and Analogues | 5.52 | 3.4E-04* | 1.19 | 3.8E-01 | 2.33 | 6.4E-03* |
| Hexanoylglycine | HMDB00701 | 174.11 | 19.04 | Amino Acids, Peptides, and Analogues | 4.48 | 1.9E-02* | 0.57 | 1.0E-01 | 0.62 | 1.6E-01 |
| N-Acetyl-l-phenylalanine | HMDB00512 | 208.10 | 19.28 | Amino Acids, Peptides, and Analogues | 2.89 | 4.8E-02* | 0.51 | 7.1E-02 | 0.53 | 1.1E-01 |
| Succinyladenosine | HMDB00912 | 382.10 | 17.68 | Amino Acids, Peptides, and Analogues | 2.32 | 3.2E-03* | 1.20 | 2.3E-01 | 1.29 | 2.1E-01 |
| γ-Aminobutyric acid | Std | 102.07 | 3.85 | Amino Acids, Peptides, and Analogues | 2.15 | 6.4E-04* | 0.81 | 7.8E-02 | 0.89 | 4.1E-01 |
| Glutamic acid | Std | 146.06 | 3.85 | Amino Acids, Peptides, and Analogues | 2.11 | 3.4E-04* | 0.81 | 5.2E-02 | 0.89 | 3.9E-01 |
| Phenylacetylglycine | HMDB00821 | 192.07 | 19.12 | Amino Acids, Peptides, and Analogues | 2.06 | 1.8E-02* | 0.70 | 9.7E-02 | 1.20 | 3.5E-01 |
| S-Nitrosoglutathione | HMDB04645 | 335.06 | 4.54 | Amino Acids, Peptides, and Analogues | 2.03 | 4.3E-03* | 1.55 | 1.4E-02* | 1.56 | 2.2E-02* |
| Ureidosuccinic acid | HMDB00828 | 175.03 | 13.29 | Amino Acids, Peptides, and Analogues | 1.53 | 5.5E-01 | 3.41 | 1.5E-02* | 1.62 | 2.4E-01 |
| Nicotinic acid | HMDB01488 | 124.04 | 6.32 | Aromatic Heteromonocyclic Compounds | 1.20 | 5.8E-01 | 0.63 | 1.6E-01 | 0.38 | 1.9E-02* |
| Indoxyl sulfate | HMDB00682 | 212.00 | 18.80 | Aromatic Heteropolycyclic Compounds | 2.55 | 8.4E-03* | 1.33 | 1.2E-01 | 1.76 | 5.1E-03* |
| 5-Hydroxyindoleacetic acid | HMDB00763 | 190.05 | 18.53 | Aromatic Heteropolycyclic Compounds | 2.39 | 9.1E-03* | 0.64 | 2.8E-02* | 0.64 | 3.3E-02* |
| N-Acetyl-7-O-acetylneuraminic acid | HMDB00785 | 352.12 | 8.82 | Carbohydrates and Carbohydrate Conjugates | 2.72 | 1.0E-03* | 0.86 | 3.4E-01 | 0.53 | 6.4E-03* |
| Glucose 6-phosphate | Std | 261.03 | 3.88 | Carbohydrates and Carbohydrate Conjugates | 0.50 | 5.0E-02* | 0.98 | 9.4E-01 | 0.95 | 8.5E-01 |
| 2,3-Dihydroxyvaleric acid | HMDB00421 | 135.06 | 3.74 | Carbohydrates and Carbohydrate Conjugates | 0.37 | 8.9E-04* | 1.10 | 7.2E-01 | 0.85 | 4.7E-01 |
| Estrone glucuronide | HMDB04483 | 445.18 | 12.13 | Lipids | 449.12 | 2.4E-06* | 0.63 | 1.7E-02* | 0.41 | 1.5E-04* |
| Glycodeoxycholic acid | Std | 448.30 | 21.93 | Lipids | 40.85 | 3.5E-04* | 1.10 | 6.3E-01 | 1.10 | 7.5E-01 |
| 2-Methylglutaric acid | HMDB00422 | 145.05 | 15.92 | Lipids | 25.14 | 1.4E-05* | 0.77 | 1.5E-01 | 1.39 | 3.9E-01 |
| α-Linolenic acid | HMDB01388 | 277.21 | 24.24 | Lipids | 23.30 | 1.4E-02* | 1.44 | 2.4E-01 | 0.65 | 2.8E-01 |
| Cholic acid | Std | 407.27 | 21.63 | Lipids | 15.40 | 2.4E-03* | 2.55 | 3.6E-02* | 2.64 | 3.6E-02* |
| 3-Methylglutarylcarnitine | HMDB00552 | 288.14 | 9.22 | Lipids | 13.94 | 4.7E-04* | 1.06 | 7.9E-01 | 2.63 | 1.1E-02* |
| Glycocholic acid | Std | 464.30 | 20.39 | Lipids | 9.50 | 8.4E-04* | 1.15 | 5.2E-01 | 0.68 | 1.7E-01 |
| LysoPC(18:3(6Z;9Z;12Z)) | HMDB10387 | 518.32 | 22.12 | Lipids | 8.28 | 4.8E-03* | 1.02 | 9.7E-01 | 0.69 | 2.1E-01 |
| Cholesterol sulfate | HMDB00653 | 467.32 | 20.39 | Lipids | 7.50 | 4.5E-04* | 1.14 | 5.3E-01 | 0.62 | 7.2E-02 |
| LPA(0:0/18:2(9Z;12Z)) | HMDB07852 | 435.25 | 22.95 | Lipids | 5.35 | 1.4E-06* | 0.90 | 3.4E-01 | 0.89 | 4.8E-01 |
| Leukotriene A4 | HMDB01337 | 317.21 | 24.01 | Lipids | 2.60 | 5.2E-02* | 1.03 | 9.2E-01 | 0.93 | 8.3E-01 |
| Stearoylcarnitine | HMDB00848 | 428.37 | 23.77 | Lipids | 2.17 | 3.1E-02* | 1.42 | 9.2E-02 | 0.69 | 1.9E-01 |
| Propionylcarnitine | HMDB00824 | 218.14 | 15.29 | Lipids | 2.04 | 4.0E-02* | 0.54 | 2.5E-02* | 0.43 | 9.1E-03* |
| LysoPC(22:4(7Z;10Z;13Z;16Z)) | HMDB10401 | 572.37 | 20.85 | Lipids | 1.86 | 3.9E-02* | 0.69 | 1.2E-01 | 0.18 | 5.9E-04* |
| α-Tocotrienol | HMDB06327 | 425.35 | 22.10 | Lipids | 1.56 | 1.2E-01 | 0.27 | 2.2E-06* | 0.27 | 3.3E-06* |
| Linoleyl carnitine | HMDB06469 | 424.34 | 22.10 | Lipids | 1.42 | 2.4E-01 | 0.28 | 8.9E-05* | 0.26 | 1.6E-04* |
| LysoPC(15:0) | HMDB10381 | 482.32 | 22.62 | Lipids | 1.28 | 5.6E-01 | 1.22 | 5.9E-01 | 0.10 | 2.6E-03* |
| Oleoylcarnitine | HMDB05065 | 426.36 | 22.52 | Lipids | 1.19 | 7.2E-01 | 2.54 | 4.2E-03* | 1.38 | 2.9E-01 |
| Adrenic acid | HMDB02226 | 333.28 | 22.13 | Lipids | 1.12 | 7.5E-01 | 0.16 | 4.9E-03* | 0.63 | 1.3E-01 |
| LysoPC(22:5(7Z;10Z;13Z;16Z;19Z)) | HMDB10403 | 570.35 | 21.02 | Lipids | 0.94 | 8.9E-01 | 0.07 | 1.0E-02* | 0.38 | 1.2E-01 |
| l-Octanoylcarnitine | HMDB00791 | 288.22 | 18.91 | Lipids | 0.89 | 4.2E-01 | 1.11 | 5.8E-01 | 4.93 | 8.8E-05* |
| Palmitic acid | HMDB00220 | 255.23 | 24.51 | Lipids | 0.85 | 4.7E-01 | 0.18 | 3.4E-04* | 1.26 | 2.9E-01 |
| Oleamide | HMDB02117 | 282.28 | 22.08 | Lipids | 0.75 | 3.8E-01 | 0.38 | 2.1E-03* | 1.43 | 1.4E-01 |
| Palmitoleic acid | HMDB03229 | 255.23 | 22.51 | Lipids | 0.40 | 4.9E-02* | 3.58 | 8.5E-04* | 4.04 | 2.0E-02* |
| LysoPC(18:2(9Z;12Z)) | HMDB10386 | 520.34 | 20.83 | Lipids | 0.31 | 1.5E-02* | 2.96 | 1.8E-02* | 2.40 | 4.1E-02* |
| LysoPC(20:4(5Z;8Z;11Z;14Z)) | HMDB10395 | 544.34 | 20.98 | Lipids | 0.30 | 2.6E-02* | 3.10 | 7.8E-03* | 2.21 | 1.0E-01 |
| LysoPC(20:5(5Z;8Z;11Z;14Z;17Z)) | HMDB10397 | 542.32 | 21.68 | Lipids | 0.27 | 6.6E-04* | 2.09 | 1.3E-02* | 2.44 | 2.2E-03* |
| LysoPC(16:1(9Z)) | HMDB10383 | 494.32 | 19.84 | Lipids | 0.25 | 2.8E-02* | 3.84 | 1.6E-02* | 3.74 | 1.6E-02* |
| 5,8,11-Eicosatrienoic acid | HMDB10378 | 305.25 | 24.53 | Lipids | 0.21 | 1.1E-02* | 1.92 | 1.8E-01 | 4.58 | 3.6E-03* |
| Linoelaidic acid | HMDB06270 | 279.23 | 23.50 | Lipids | 0.20 | 1.0E-02* | 5.14 | 1.7E-03* | 5.75 | 9.5E-04* |
| Adenosine monophosphate | HMDB00045 | 346.05 | 4.60 | Nucleosides, Nucleotides, and Analogues | 2.13 | 1.1E-04* | 0.99 | 8.7E-01 | 1.16 | 2.8E-01 |
| Deoxyguanosine | Std | 266.10 | 4.13 | Nucleosides, Nucleotides, and Analogues | 2.02 | 4.3E-03* | 0.76 | 8.8E-02 | 0.78 | 1.3E-01 |
| Nicotinamide ribotide | HMDB00229 | 335.06 | 4.20 | Nucleosides, Nucleotides, and Analogues | 1.29 | 5.0E-01 | 2.37 | 2.3E-02* | 2.19 | 6.3E-02* |
| Xanthosine | HMDB00299 | 285.08 | 17.01 | Nucleosides, Nucleotides, and Analogues | 0.50 | 2.9E-02* | 0.98 | 8.7E-01 | 0.74 | 8.8E-02* |
| 1,11-Undecanedicarboxylic acid | HMDB02327 | 245.18 | 3.64 | Organic Acids and Derivatives | 2.16 | 1.6E-02* | 0.60 | 1.8E-02* | 0.94 | 7.4E-01 |
| Pyruvic acid | Std | 87.01 | 4.65 | Organic Acids and Derivatives | 0.43 | 4.2E-03* | 0.80 | 4.8E-01 | 1.27 | 4.2E-01 |
| Taurine | Std | 126.02 | 3.73 | Organic Acids and Derivatives | 0.14 | 8.5E-05* | 0.86 | 4.2E-01 | 1.15 | 4.6E-01 |
Results are means ± SD (n = 6–8).
Significant differences (P < 0.05) between groups were determined by Student's t-test. M/Z, mass-to-charge ratio; RT, retention time; FC, fold change; P, P value.
Table 6 lists serum metabolites with changes greater or equal to twofold in alcohol-fed groups. Alcohol feeding with CO as the fat source increased serum concentrations of 22 metabolites. The most profound increase was pantetheine, an intermediate in the production of coenzyme A. Eight metabolites were increased more than fivefold, including five bile acids, one indole metabolite (indoxyl), one amino acid/derivative metabolite (N-acetylornithine), and one fatty acid/derivative metabolite (tetracosahexaenoic acid). Alcohol feeding with CO as the fat source decreased serum concentrations of three metabolites, including butyrylcarnitine, linoelaidic acid, and pyruvic acid. Dietary CB and MCT showed limited effects on alcohol-induced alterations in serum metabolites. Compared with the AF/CO group, alcohol feeding with CB showed positive effects on seven metabolites and negative effects on two metabolites, whereas alcohol feeding with MCT showed positive effects on nine metabolites and negative effects on six metabolites.
Table 6.
Fold changes of serum metabolites in rats subjected to alcohol with different dietary fat sources for 8 wk
| AF/CO vs. PF/CO |
AF/CB vs. AF/CO |
AF/MCT vs. AF/CO |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Library | M/Z | RT (min) | Class | FC | P | FC | P | FC | P |
| Trimethylamine N-oxide | Std | 76.07 | 3.81 | Aliphatic Acyclic Compounds | 2.54 | 4.1E-03* | 2.96 | 1.7E-04* | 0.84 | 3.8E-01 |
| N-Acetylornithine | HMDB03357 | 175.11 | 4.27 | Amino Acids, Peptides, and Analogues | 6.69 | 7.9E-03* | 1.27 | 3.3E-01 | 2.68 | 1.1E-04* |
| Hexanoylglycine | HMDB00701 | 174.11 | 18.98 | Amino Acids, Peptides, and Analogues | 2.37 | 2.0E-02* | 0.96 | 8.4E-01 | 0.89 | 6.1E-01 |
| Isoputreanine | HMDB06009 | 161.13 | 4.23 | Amino Acids, Peptides, and Analogues | 2.26 | 2.6E-02* | 0.96 | 8.6E-01 | 0.66 | 4.8E-02* |
| Indoxyl | HMDB04094 | 134.06 | 19.49 | Aromatic Heteropolycyclic Compounds | 7.40 | 2.1E-02* | 0.92 | 8.0E-01 | 1.89 | 1.7E-02* |
| Phenol | HMDB00228 | 93.03 | 18.66 | Aromatic Homomonocyclic Compounds | 3.08 | 3.6E-02* | 2.36 | 1.2E-02* | 1.51 | 2.8E-01 |
| 4-Methoxyphenylacetic acid | HMDB02072 | 165.05 | 19.53 | Aromatic Homomonocyclic Compounds | 2.22 | 2.6E-02* | 1.95 | 3.1E-03* | 0.53 | 4.2E-02* |
| Indoxyl sulfate | HMDB00682 | 212.00 | 18.84 | Aromatic Heteropolycyclic Compounds | 2.09 | 6.1E-03* | 1.58 | 1.7E-03* | 2.16 | 1.4E-04* |
| Inodxyl glucuronide | HMDB10319 | 308.08 | 18.66 | Carbohydrates and Carbohydrate Conjugates | 2.73 | 2.4E-04* | 1.41 | 3.3E-02* | 1.97 | 3.2E-04* |
| Deoxycholic acid | Std | 391.28 | 23.86 | Lipids | 9.60 | 3.7E-03* | 1.12 | 5.9E-01 | 2.45 | 7.5E-02 |
| Tetracosahexaenoic acid | HMDB02007 | 357.28 | 21.80 | Lipids | 8.74 | 3.1E-02* | 0.98 | 9.5E-01 | 2.16 | 3.1E-02* |
| Hyodeoxycholic acid | Std | 391.28 | 21.84 | Lipids | 7.97 | 1.2E-03* | 1.12 | 6.1E-01 | 2.20 | 2.8E-03* |
| Glycocholic acid | Std | 464.29 | 20.39 | Lipids | 7.33 | 4.4E-02* | 0.99 | 9.7E-01 | 0.81 | 6.3E-01 |
| Cholic acid | Std | 407.27 | 21.66 | Lipids | 6.34 | 6.5E-03* | 1.17 | 5.2E-01 | 1.05 | 8.7E-01 |
| 3a;7b;12a-Trihydroxyoxocholanyl-Glycine | HMDB00331 | 466.32 | 20.35 | Lipids | 5.90 | 2.5E-02* | 0.80 | 5.3E-01 | 0.80 | 5.4E-01 |
| LysoPC(18:3(9Z;12Z;15Z)) | HMDB10388 | 518.32 | 22.24 | Lipids | 3.54 | 7.5E-03* | 1.01 | 9.8E-01 | 1.14 | 5.4E-01 |
| LysoPC(14:0) | HMDB10379 | 468.31 | 22.30 | Lipids | 3.36 | 4.2E-02* | 1.08 | 7.2E-01 | 1.30 | 2.3E-01 |
| 18-Oxocortisol | HMDB00332 | 375.18 | 21.53 | Lipids | 2.30 | 3.3E-03* | 1.13 | 3.8E-01 | 0.37 | 5.1E-04* |
| Elaidic carnitine | HMDB06464 | 426.36 | 22.08 | Lipids | 1.21 | 3.6E-01 | 0.93 | 7.0E-01 | 0.49 | 4.0E-02* |
| Linoleyl carnitine | HMDB06469 | 424.34 | 21.55 | Lipids | 1.20 | 5.3E-01 | 0.22 | 2.6E-03* | 0.23 | 3.2E-03* |
| Stearoylcarnitine | HMDB00848 | 428.37 | 22.84 | Lipids | 1.04 | 7.8E-01 | 2.35 | 2.1E-06* | 0.64 | 6.4E-03* |
| Butyrylcarnitine | HMDB02013 | 232.15 | 17.86 | Lipids | 0.60 | 5.2E-03* | 1.47 | 2.1E-02* | 2.76 | 7.7E-06* |
| Linoelaidic acid | HMDB06270 | 279.23 | 23.52 | Lipids | 0.23 | 4.2E-02* | 1.41 | 5.1E-01 | 4.63 | 1.1E-02 |
| Pantetheine | HMDB03426 | 277.13 | 19.30 | Organic Acids and Derivatives | 101.62 | 3.4E-06* | 0.47 | 6.5E-03* | 1.06 | 8.4E-01 |
| 2-Hydroxybutanoic acid | Std | 103.04 | 10.80 | Organic Acids and Derivatives | 3.49 | 9.3E-05* | 0.84 | 2.7E-01 | 2.53 | 2.6E-03* |
| 4-Hydroxybutyric acid | HMDB00710 | 103.04 | 12.47 | Organic Acids and Derivatives | 3.28 | 1.0E-04* | 0.86 | 3.0E-01 | 2.51 | 3.0E-03* |
| 3-Succinoylpyridine | HMDB00992 | 180.07 | 18.86 | Organic Acids and Derivatives | 2.20 | 5.0E-03* | 0.98 | 9.1E-01 | 1.05 | 7.5E-01 |
| Pyruvic acid | Std | 87.01 | 4.56 | Organic Acids and Derivatives | 0.44 | 2.0E-03* | 1.29 | 3.3E-01 | 0.93 | 7.2E-01 |
Results are means ± SD (n = 6–8).
Significant differences (P < 0.05) between groups were determined by Student's t-test.
Dietary saturated fats attenuated hepatic macrophage activation, chemokine expression, and neutrophil infiltration in alcohol-fed rats.
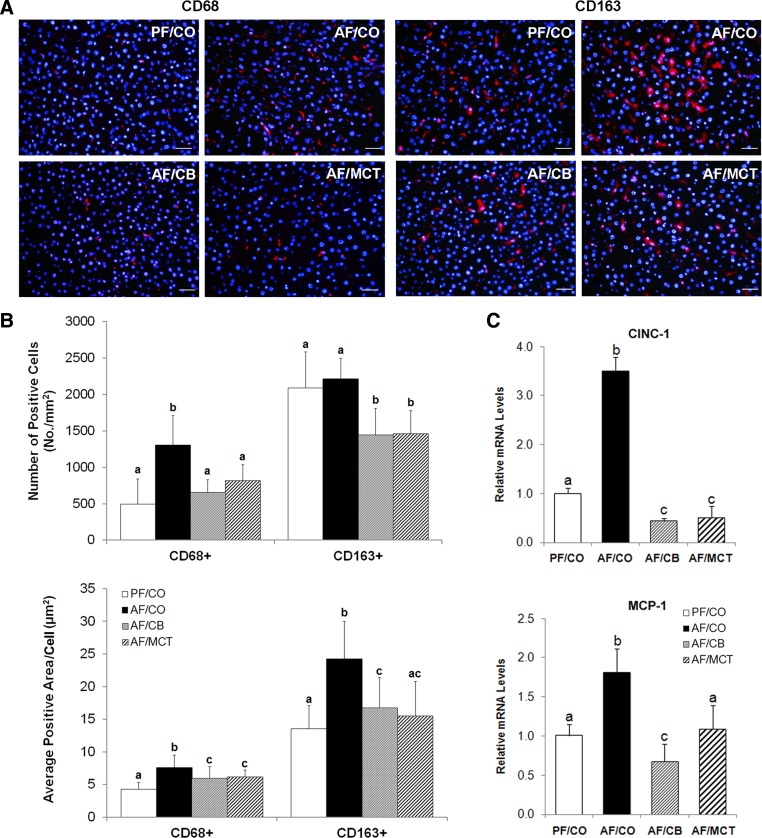
Immunofluorescence staining showed that CD68+ macrophages were significantly increased and enlarged in the livers of rats fed ethanol with CO as the fat source for 8 wk (Fig. 4, A and B). Although ethanol feeding with CO did not affect CD163+ macrophage cell numbers, the cells were significantly expanded. Dietary CB or MCT reduced the cells numbers and cell sizes of both CD68+ and CD163+ macrophages. In accordance, the gene expressions of cxcl1/CINC-1 (the rat analog to human IL-8) and ccl2/MCP-1 were significantly upregulated in the AF/CO group compared with the PF/CO group and normalized in the AF/CB and AF/MCT groups (Fig. 4C). As shown in Fig. 5, ethanol feeding with CO induced neutrophil infiltration in the liver. With dietary substitution of saturated fats, the numbers of neutrophils were decreased.
Fig. 4.
Hepatic inflammation in rats after ethanol feeding with different dietary fats for 8 wk. A: macrophages in the liver detected by immunofluorescence staining. Scale bar: 20 μm. Red: CD68+ or CD163+ macrophages; blue: 4′,6-diamidino-2-phenylindole (DAPI) counterstaining of nuclei. B: quantitative analysis of CD68+ and CD163+ macrophages in the liver by Image Pro Premier (n = 25–35). C: hepatic cytokine/chemokine expressions of cxcl1/CINC-1 and ccl2/MCP-1 measured by quantitative PCR (qPCR). The relative gene expression was normalized to 18s rRNA expression and calculated by the 2−ΔΔCt method setting the value of PF/CO as 1. Results are means ± SD (n = 6). Significant differences (P < 0.05, ANOVA) are indicated by different letters.
Fig. 5.
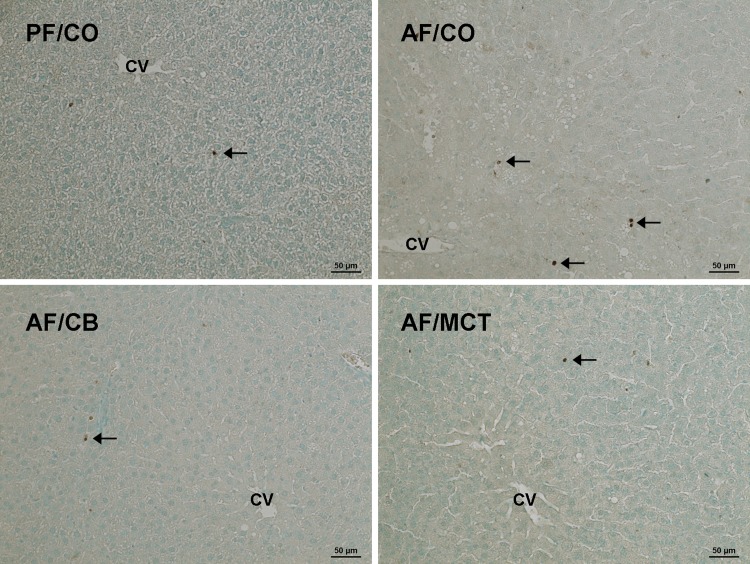
Hepatic neutrophil infiltration in rats fed ethanol with different dietary fats for 8 wk. Neutrophils (arrows) were detected by immunohistochemistry using anti-myeloperoxidase antibody. Nuclei were counterstained by methyl green. Scale bar = 50 μm.
Dietary medium-chain triglyceride but not CB normalized serum endotoxin level and tight junction protein expression in alcohol-fed rats.
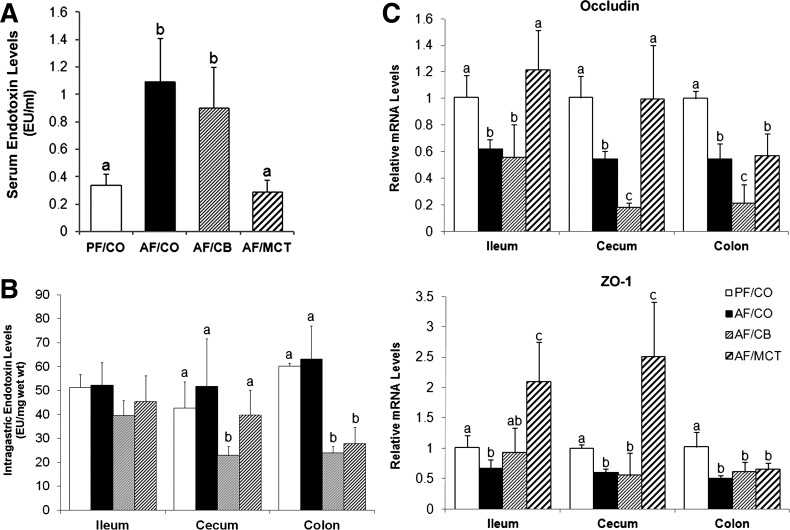
To determine whether CB or MCT prevention from alcohol-induced inflammatory response was through inhibition of endotoxemia, serum endotoxin levels were measured. As shown in Fig. 6A, alcohol feeding with CO as the fat source increased serum endotoxin levels by more than twofold. Although the alcohol-induced elevation of serum endotoxin levels was normalized by MCT, it was not affected by dietary CB.
Fig. 6.
Endotoxemia and intestinal tight junction expression in rats fed ethanol with different dietary fats for 8 wk. A: serum endotoxin levels. Endotoxin levels were measured by the limulus amoebocyte lysate method, and results were expressed in endotoxin units (EU) per milliliter serum. B: intestinal luminal endotoxin levels. Endotoxin levels within the intestinal lumen were expressed in EU per milligram wet weight. C: gene expressions of occludin and zonula occludens (ZO-1) measured by qPCR. The relative gene expression was normalized to 18s rRNA expression and calculated using the 2−ΔΔCt method, setting the value of PF/CO as 1. Results are means ± SD (n = 6–8). Significant differences (P < 0.05, ANOVA) are indicated by different letters.
To discriminate whether the elevated serum endotoxin level in the AF/CO group was due to bacterial overgrowth or impaired intestinal barrier function, the intestinal luminal endotoxin levels were measured. Chronic ethanol feeding with CO did not affect intragastric endotoxin level within the ileum, cecum, or colon compared with PF/CO rats. Dietary CB lowered cecal and colonic endotoxin levels, whereas MCT decreased endotoxin level within the colonic lumen (Fig. 6B). To further define the mechanisms of how CB and MCT impact intestinal barrier function, the mRNA expression of tight junction proteins were measured. As shown in Fig. 6C, alcohol feeding with CO as the fat source led to dramatic reduction of occludin and zonula occludens (ZO)-1 in all the intestinal segments tested (ileum, cecum, and colon). Dietary MCT upregulated gene expressions of occludin and ZO-1 to normal levels, or even higher, in the ileum and cecum. However, dietary CB did not show protective effects on alcohol-perturbed gene expression of tight junction proteins.
Dietary CB normalized hepatic endotoxin level and endotoxin signaling in association with upregulating ASS1 in alcohol-fed mice.
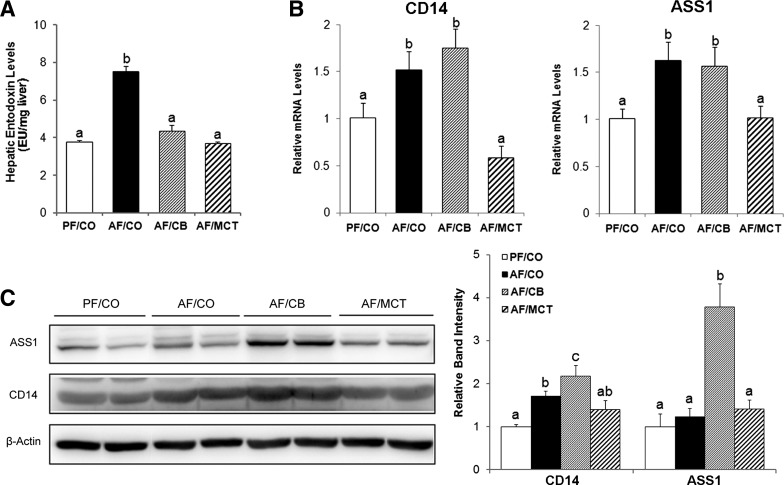
To determine how CB abrogated the alcohol-induced inflammatory response without preventing the elevation of serum endotoxin, hepatic endotoxin level was measured. As shown in Fig. 7A, alcohol feeding with CO as the fat source increased hepatic endotoxin level, which were normalized by both dietary CB and MCT. To further determine the mechanism of how CB abrogated hepatic endotoxin elevation in alcohol-fed mice, hepatic expression of endotoxin binding (CD14) and detoxification (ASS1) genes/proteins were examined. The gene expressions of CD14 and ASS1 were upregulated in rats fed alcohol with either CO or CB as the fat source (Fig. 7B) and were normalized by MCT. The protein levels of CD14 and ASS1 were measured by immunoblot analysis (Fig. 7C). Although the protein level of CD14 was elevated by alcohol feeding with either CO or CB as the fat source, the protein level of ASS1 was only increased by alcohol feeding with CB as the fat source.
Fig. 7.
Hepatic LPS signaling pathway in rats fed ethanol with different dietary fats for 8 wk. A: hepatic endotoxin levels. Endotoxin levels in the liver were measured by the limulus amoebocyte lysate method, and results were expressed in EU per milligram liver tissue. B: expression of genes related to hepatic LPS signaling and detoxification. The mRNA levels of CD14 and argininosuccinate synthase 1 (ASS1) were measured by qPCR. The relative gene expression was normalized to 18s rRNA expression, and calculated using the 2−ΔΔCt method setting the value of PF/CO as 1. C: Western blot analysis of hepatic ASS1 and CD14. Protein bands were quantified by densitometry analysis and the ratio to β-actin was calculated by setting the value of PF/CO as 1. Results are means ± SD (A: n = 6–8; B: n = 6; C: n = 4). Significant differences (P < 0.05, ANOVA) are indicated by different letters.
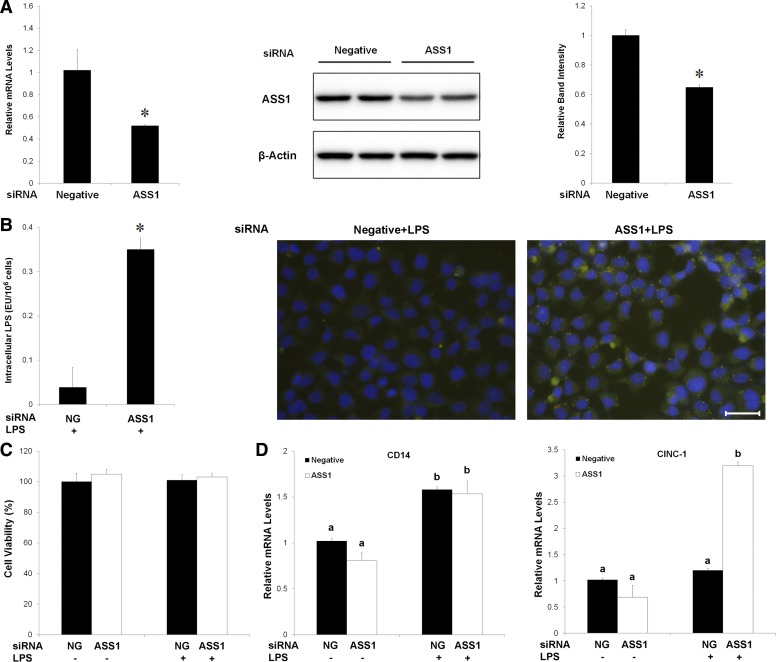
Knockdown of ASS1 caused endotoxin accumulation and inflammatory cytokine expression in hepatoma H4IIEC3 cells.
To elucidate the mechanistic link between ASS1 induction and endotoxin clearance, the effects of ASS1 knockdown on endotoxin clearance as well as cytokine expression were measured in rat hepatoma H4IIEC3 cells. As shown in Fig. 8A, siRNA transfection reduced the mRNA and protein levels of ASS1. Treatment with FITC-LPS for 15 h caused more endotoxin accumulation in ASS1 siRNA-transfected cells compared with negative siRNA-transfected cells, as indicated by quantification of cellular endotoxin level and fluorescent microscopy of intracellular FITC-endotoxin (Fig. 8B). Neither siRNA transfection nor endotoxin treatment affected cell viability (Fig. 8C). ASS1 siRNA transfection did not affect endotoxin-induced CD14 mRNA level but significantly upregulated CINC-1 expression (Fig. 8D).
Fig. 8.
Effect of ASS1 in detoxifying LPS in H4IIEC3 cells. A: ASS1 siRNA transfection reduced cellular ASS1 levels. H4IIEC3 were transfected with ASS1 siRNA or negative control for 24 h. Messenger RNA and protein levels of ASS1 were measured by qPCR and Western blot with 18s rRNA and β-actin as the internal control, respectively. B: role of ASS1 in LPS detoxification. H4IIEC3 cells were transfected with ASS1 siRNA or negative control (NG) for 24 h with FITC-LPS treatment during the last 15 h. Intracellular LPS was evaluated by both endotoxin assay and microscopy of FITC (green) and DAPI (blue) nuclear staining. C: Cell viability assay. Cell viability was determined by the Cell Counting Kit-8 (CCK-8) relative to the negative control group (negative control siRNA without LPS treatment). D: gene expression of CD14 and CINC-1. The mRNA levels of CD14 and CINC-1 were measured by qPCR. The relative gene expression was normalized to 18s rRNA expression and calculated by the 2−ΔΔCt method, setting the values of negative control as 1. Results are means ± SD (A: n = 4; B: n = 3; C: n = 6; D: n = 6). Significant differences (P < 0.05) between groups were determined by Student's t-test (A and B; indicated by *) or ANOVA (C and D; indicated by different letters). NG, negative control.
DISCUSSION
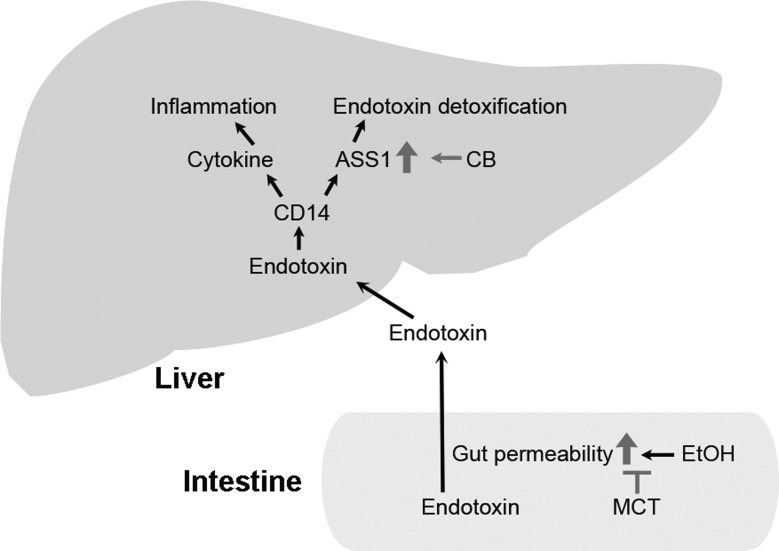
The present study demonstrates that the dietary saturated fats CB and MCT alleviated alcohol consumption-induced liver damage through differential actions at the gut-liver axis as illustrated in Fig. 9. Dietary MCT attenuated alcohol-induced downregulation of tight junction genes of the intestinal epithelium and consequently prevented alcohol-induced endotoxemia and hepatic inflammation. Dietary CB also attenuated the alcohol-induced hepatic inflammatory response. However, it did not improve the detrimental effects of alcohol on tight junction or the development of alcoholic endotoxemia. Unexpectedly, dietary CB normalized hepatic endotoxin level in association with upregulation of ASS1. Cell culture study demonstrated that ASS1 is a critical determinant of hepatocyte endotoxin clearance and endotoxin-induced chemokine expression. These results suggest that MCT and CB prevent the alcohol-induced endotoxin-chemokine cascade through differential mechanisms.
Fig. 9.
Schematic diagram of the potential mechanisms that underlie the protective actions of dietary saturated fats against alcoholic liver injury in rats. EtOH, ethanol; CB, cocoa butter; MCT, medium-chain triglyceride.
Endotoxin is a trigger of Kupffer cell activation that leads to production of proinflammatory cytokines in the liver. Several hypotheses have been suggested to be involved in the pathogenesis of endotoxemia, including bacterial overgrowth in the intestine and increased gut permeability (6, 37). Although overgrowth of bacteria in the intestine has been reported in alcoholic patients (28), only a minority of ALD patients had intestinal bacterial overgrowth. On the other hand, all the patients with different stages of ALD, including alcoholic steatosis, hepatitis, and cirrhosis, showed an increase in gut permeability to macromolecules (34). Similarly, increased permeability of isolated gut sac to macromolecules has been shown in alcohol-fed animals (49). The present study demonstrated that alcohol feeding increased serum endotoxin level without affecting the intestinal endotoxin level. Thus intestinal endotoxin overproduction is not an indispensable cause of serum endotoxin elevation in our rat model. Dietary saturated fats have been shown to attenuate alcoholic endotoxemia by sealing the leaky gut (16). A liquid alcohol diet with MCT as the fat source reversed alcohol/fish oil feeding elevated serum endotoxin levels (30, 31). The present study also showed that dietary MCT normalized the serum endotoxin elevation. However, dietary CB had no effect on alcohol-induced serum endotoxin elevation. These data suggest that the beneficial effects of saturated fats on the gut are chain length dependent.
Prevention of endotoxin penetration from the gut lumen to the blood is controlled by the intestinal barrier. The intestinal barrier function is provided by the epithelial cells and the paracellular apical junction complex, including tight junctions and adherence junctions (20). Tight junctions are the most apical part of the apical junction complex and are primarily involved in the regulation of epithelial permeability (43). Tight junctions are assembled by the organization of proteins such as occludin, claudins, and ZO (20, 43). Disassembling of proteins at tight junctions disrupts the intestinal barrier to allow the diffusion of macromolecules such as endotoxin and other pathogens from the intestinal lumen into the blood. Decreased distribution of tight junction proteins, including ZO-1, occludin, and claudin-1, in the intestinal epithelium has been reported in ALD patients as well as animal models (2, 49). A recent study demonstrated that replacing CO with saturated fats (MCT-beef tallow 82:18) attenuates alcohol-induced downregulation of intestinal tight junction genes (16). The present study found that dietary MCT, but not CB, normalized gene expression of tight junction genes in ileum and cecum. Our previous report showed that reduction of intestinal HNF-4α accounts for alcohol-induced downregulation of tight junction proteins (50). We also found that polyunsaturated fatty acid is an inhibitor and MCT is an activator of HNF-4α (22). Thus activation of HNF-4α may account for the beneficial effects of MCT on intestinal tight junction gene expression.
Previous studies have shown that MCT alleviates hepatic cytokine production and inflammation through abrogating alcohol-induced serum endotoxin elevation. The present study demonstrated that dietary CB attenuated the alcohol-induced inflammatory response without affecting serum endotoxin level, suggesting a different mechanism from MCT. The liver plays a paramount role in systemic clearance and detoxification of endotoxin (13, 27, 29). Either Kupffer cells or hepatocytes recognize bacterial components such as LPS through Toll-like receptors after lipopolysaccharide binding protein and membrane-bound CD14 binding (29). The expression of CD14 is linked with LPS responsiveness both in experimental models and patients with ALD. The mRNA level of CD14 in the liver was increased in ethanol-fed rats (42). In patients with alcoholic cirrhosis, CD14 is present in elevated amounts in the circulation (33). Moreover, the degree of injury correlates with the level of endotoxemia and with the level of CD14 expression (42). In the present study, we also found increased hepatic CD14 in rats fed ethanol with CO or CB, which correlated with the serum endotoxin levels. However, the ethanol-elevated hepatic endotoxin level was normalized by dietary CB. These findings indicated that dietary CB abrogated the alcohol-induced inflammatory response via detoxifying hepatic endotoxin instead of preventing the elevation of serum endotoxin.
ASS1 is a soluble enzyme responsible for a critical biochemical reaction in the citrulline-urea and nitric oxide cycles, which catalyzes the reversible ATP-dependent ligation of citrulline and aspartate to generate argininosuccinate, and is highly conserved among species (11, 36). ASS1 is expressed in many tissues, with the highest levels in the liver and kidney (11). Hepatic ASS1 acts as a sensitive biomarker of liver responses to bacterial endotoxin. Lipid A is the endotoxic portion of LPS. ASS1 physically binds to the lipid A portion of LPS, inactivates the biological activity and clears circulating LPS (39, 40). ASS1 was reported to be upregulated in rat hepatocytes by chronic ethanol feeding (21). We detected increased mRNA levels of ASS1 in AF/CO and AF/CB rats. However, the protein level of ASS1 was only upregulated in the AF/CB group. Cell culture studies further demonstrated that knockdown of ASS1 in rat H4IIEC3 hepatoma cells impaired intracellular clearance of LPS and upregulated cytokine expression. In addition to ASS1, acyloxyacyl hydrolase (AOAH) also degrades LPS (29). However, we did not observe differences in AOAH mRNA or protein levels among all experimental groups (data not shown). These results indicate that CB may speed up endotoxin detoxification via activating ASS1, thereby abrogating endotoxin signaling in the liver. A recent study demonstrated that partial ASS1 ablation protected acute but not chronic ethanol-induced liver injury in mice (21). This discrepancy might be due to the use of different animal models and the stages of disease.
Metabolome analysis in the present study demonstrated that the most significantly perturbed metabolites in AF/CO rats were fat related, i.e., bile acids, lipids (especially lysoPC), and fatty acid metabolites. A lipidomic study reported significant changes in phospholipid composition in the plasma of ethanol-fed male Fischer 334 rats compared with controls (5). Another study showed that plasma glycerophosphocholines and glycerophosphoserines were decreased after ethanol administration in rodents (8). A metabolomics study of high-fat diet-induced obese mice demonstrated that, among the 16 lysoPCs detected, only 1 was increased and the other 15 were dramatically reduced in the liver (15). Low phosphatidylcholine-to-phosphatidylethanolamine (PC/PE) ratio is believed to lead to increased hepatocyte membrane permeability, which contributes to steatosis and liver failure (23). Restoring PC generation in the liver was reported to attenuate alcoholic steatosis (14). Apart from the role in regulating lipid homeostatis, lysoPCs are known to exert anti-inflammatory actions (12). Research in sepsis has shown that lysoPC inversely correlates with the severity of infection (4) and that lysoPC administration in mouse models of sepsis protects them against lethality (46). The present study showed that substituting CO with CB or MCT prevented ethanol plus CO-induced lysoPC reduction in the liver. This might contribute to the alleviated steatosis in AF/CB and AF/MCT rats through normalizing PC and balancing PC/PE ratio and also attenuated hepatic inflammation due to the anti-inflammation action of lysoPC. Moreover, the dramatically decreased palmitic acid and increased palmitoleic acid in the liver in AF/CB rats indicated the activation of stearoyl-CoA desaturase-1 (SCD1). SCD1 is a rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids (MUFAs). MUFAs are believed to be protective against cardiovascular disease risk factors for decades (9). Consumption of dietary MUFA promotes healthy blood lipid profiles, mediates blood pressure, improves insulin sensitivity and regulates glucose levels (9). Guo et al. (10) reported that palmitoleate supplementation suppresses high-fat diet-induced liver inflammatory response in male C57BL/6J mice. Thus the protective roles of MUFA may also account for the beneficial effects of dietary CB substitution. However, the exact mechanisms of how the altered metabolites by dietary CB or MCT affect the progression of ALD require further investigation.
In conclusion, the present study demonstrates the important role of different types of dietary fat in the gut-liver pathology associated with chronic alcohol consumption. The protective effects of dietary saturated fats in preventing alcohol-induced cytokine production and inflammation in the liver occur via different mechanisms at the gut-liver axis. In contrast to MCT, which abrogates the inflammatory response at the intestine level, the protective actions of dietary CB against endotoxin signaling mainly occurred in the liver, at least partially through activating ASS1.
GRANTS
This research was supported by the National Institutes of Health (R01AA020212 and R01AA018844).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.Z., W.J., and Z.Z. conception and design of research; W.Z., Q.L., G.X., Xiuhua Sun, X.T., and Xinguo Sun performed experiments; W.Z. and G.X. analyzed data; W.Z. interpreted results of experiments; W.Z. and G.X. prepared figures; W.Z. drafted manuscript; W.Z., Q.L., Xiuhua Sun, and Z.Z. edited and revised manuscript; W.Z., Q.L., G.X., Xiuhua Sun, X.T., Xinguo Sun, W.J., and Z.Z. approved final version of manuscript.
REFERENCES
- 1.Aker S, Belosjorow S, Konietzka I, Duschin A, Martin C, Heusch G, Schulz R. Serum but not myocardial TNF-α concentration is increased in pacing-induced heart failure in rabbits. Am J Physiol Regul Integr Comp Physiol 285: R463–R469, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and l-glutamine. Am J Physiol Gastrointest Liver Physiol 289: G367–G375, 2005 [DOI] [PubMed] [Google Scholar]
- 3.DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr 91: 331–336, 1967 [DOI] [PubMed] [Google Scholar]
- 4.Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res 44: 754–761, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Fernando H, Kondraganti S, Bhopale KK, Volk DE, Neerathilingam M, Kaphalia BS, Luxon BA, Boor PJ, Shakeel Ansari GA. 1H and 31P NMR lipidome of ethanol-induced fatty liver. Alcohol Clin Exp Res 34: 1937–1947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita T. Endotoxemia in alcoholic liver disease. Hepatology 50: 1319; author reply 1319, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol 12: 162–169, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Gika HG, Ji C, Theodoridis GA, Michopoulos F, Kaplowitz N, Wilson ID. Investigation of chronic alcohol consumption in rodents via ultra-high-performance liquid chromatography-mass spectrometry based metabolite profiling. J Chromatogr A 1259: 128–137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 46: 209–228, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG, Dong H, Lu F, Wei L, Huo Y, Wu C. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PloS One 7: e39286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol 2: 8–23, 2011 [PMC free article] [PubMed] [Google Scholar]
- 12.Hung ND, Kim MR, Sok DE. Mechanisms for anti-inflammatory effects of 1-[15(S)-hydroxyeicosapentaenoyl] lysophosphatidylcholine, administered intraperitoneally, in zymosan A-induced peritonitis. Br J Pharmacol 162: 1119–1135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jirillo E, Caccavo D, Magrone T, Piccigallo E, Amati L, Lembo A, Kalis C, Gumenscheimer M. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res 8: 319–327, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol 46: 314–321, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang JT, Park JH, Yang HJ, Kim MS, Kwon DY, Yoon SH. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res 10: 722–731, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res 36: 835–846, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kono H, Enomoto N, Connor HD, Wheeler MD, Bradford BU, Rivera CA, Kadiiska MB, Mason RP, Thurman RG. Medium-chain triglycerides inhibit free radical formation and TNF-α production in rats given enteral ethanol. Am J Physiol Gastrointest Liver Physiol 278: G467–G476, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther 305: 880–886, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Laramee P, Kusel J, Leonard S, Aubin HJ, Francois C, Daeppen JB. The economic burden of alcohol dependence in Europe. Alcohol Alcohol 48: 259–269, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol 22: 85–89, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Leung TM, Lu Y, Yan W, Moron-Concepcion JA, Ward SC, Ge X, Conde de la Rosa L, Nieto N. Argininosuccinate synthase conditions the response to acute and chronic ethanol-induced liver injury in mice. Hepatology 55: 1596–1609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Zhong W, Qiu Y, Kang X, Sun X, Tan X, Zhao Y, Jia W, Zhou Z. Preservation of hepatocyte nuclear factor-4alpha contributes to the beneficial effect of dietary medium chain triglyceride on alcohol-induced hepatic lipid dyshomeostasis in rats. Alcohol Clin Exp Res 37: 587–598, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 3: 321–331, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lieber CS, Cao Q, DeCarli LM, Leo MA, Mak KM, Ponomarenko A, Ren C, Wang X. Role of medium-chain triglycerides in the alcohol-mediated cytochrome P450 2E1 induction of mitochondria. Alcohol Clin Exp Res 31: 1660–1668, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology 10: 501–510, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Mimura Y, Sakisaka S, Harada M, Sata M, Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology 109: 1969–1976, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Morencos FC, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci 40: 1252–1256, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Munford RS. Detoxifying endotoxin: time, place and person. J Endotoxin Res 11: 69–84, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J Pharmacol Exp Ther 299: 638–644, 2001 [PubMed] [Google Scholar]
- 31.Nanji AA, Zakim D, Rahemtulla A, Daly T, Miao L, Zhao S, Khwaja S, Tahan SR, Dannenberg AJ. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology 26: 1538–1545, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Nanji AA, Zhao S, Lamb RG, Dannenberg AJ, Sadrzadeh SM, Waxman DJ. Changes in cytochromes P-450, 2E1, 2B1, and 4A, and phospholipases A and C in the intragastric feeding rat model for alcoholic liver disease: relationship to dietary fats and pathologic liver injury. Alcohol Clin Exp Res 18: 902–908, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Oesterreicher C, Pfeffel F, Petermann D, Muller C. Increased in vitro production and serum levels of the soluble lipopolysaccharide receptor sCD14 in liver disease. J Hepatol 23: 396–402, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32: 742–747, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 42: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qualls JE, Subramanian C, Rafi W, Smith AM, Balouzian L, DeFreitas AA, Shirey KA, Reutterer B, Kernbauer E, Stockinger S, Decker T, Miyairi I, Vogel SN, Salgame P, Rock CO, Murray PJ. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe 12: 313–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50: 638–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr 134: 904–912, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Satoh M, Ando S, Shinoda T, Yamazaki M. Clearance of bacterial lipopolysaccharides and lipid A by the liver and the role of argininosuccinate synthase. Innate Immun 14: 51–60, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Satoh M, Iwahori T, Sugawara N, Yamazaki M. Liver argininosuccinate synthase binds to bacterial lipopolysaccharides and lipid A and inactivates their biological activities. J Endotoxin Res 12: 21–38, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Shield KD, Rylett M, Gmel G, Kehoe-Chan TA, Rehm J. Global alcohol exposure estimates by country, territory and region for 2005—a contribution to the Comparative Risk Assessment for the 2010 Global Burden of Disease Study. Addiction 108: 912–922, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Su GL, Rahemtulla A, Thomas P, Klein RD, Wang SC, Nanji AA. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol 152: 841–849, 1998 [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 70: 631–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y, Forsyth CB, Banan A, Fields JZ, Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. J Pharmacol Exp Ther 329: 952–958, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie G, Zhong W, Zheng X, Li Q, Qiu Y, Li H, Chen H, Zhou Z, Jia W. Chronic ethanol consumption alters Mammalian gastrointestinal content metabolites. J Proteome Res 12: 3297–3306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med 10: 161–167, 2004 [DOI] [PubMed] [Google Scholar]
- 47.You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr 138: 497–501, 2008 [DOI] [PubMed] [Google Scholar]
- 48.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol 298: G625–G633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor-4α mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol 299: G643–G651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol 163: 1137–1146, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Abrogation of nuclear factor-kappaB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-alpha production and liver injury. Am J Pathol 164: 1547–1556, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]