Abstract
The renal proximal tubule (PT) is a major site for maintaining whole body pH homeostasis and is responsible for reabsorbing ∼80% of filtered HCO3−, the major plasma buffer, into the blood. The PT adapts its rate of HCO3− reabsorption (JHCO3−) in response to acute acid-base disturbances. Our laboratory previously showed that single isolated perfused PTs adapt JHCO3− in response to isolated changes in basolateral (i.e., blood side) CO2 and HCO3− concentrations but, surprisingly, not to pH. The response to CO2 concentration can be blocked by the ErbB family tyrosine kinase inhibitor PD-168393. In the present study, we exposed enriched rabbit PT suspensions to five acute acid-base disturbances for 5 and 20 min using a panel of phosphotyrosine (pY)-specific antibodies to determine the influence of each disturbance on pan-pY, ErbB1-specific pY (four sites), and ErbB2-specific pY (two sites). We found that each acid-base treatment generated a distinct temporal pY pattern. For example, the summated responses of the individual ErbB1/2-pY sites to each disturbance showed that metabolic acidosis (normal CO2 concentration and reduced HCO3− concentration) produced a transient summated pY decrease (5 vs. 20 min), whereas metabolic alkalosis produced a transient increase. Respiratory acidosis (normal HCO3− concentration and elevated CO2 concentration) had little effect on summated pY at 5 min but produced an elevation at 20 min, whereas respiratory alkalosis produced a reduction at 20 min. Our data show that ErbB1 and ErbB2 in the PT respond to acute acid-base disturbances, consistent with the hypothesis that they are part of the signaling cascade.
Keywords: proximal tubule, ErbB1, ErbB2, acid-base, phosphotyrosine
the maintenance of ph inside cells [intracellular pH (pHi)] and in the extracellular fluid [extracellular pH (pHo)] is vital for almost every biological process. Thus, uncompensated pH disturbances affect a wide range of processes (21, 30, 58, 74, 91) and such parameters as neuronal excitability (37, 40, 61), blood pressure regulation (6, 7), apoptosis (10, 20, 23), and ventilation (53, 66). Moreover, solid tumors create local acid-base disturbances to provide a selective advantage over their normal competitors (85, 86).
In mammals, CO2/HCO3− is by far the most important buffer system. The two major components interact via the reactions CO2 + H2O ↔ H2CO3 ↔ HCO3− + H+, and thus blood pH depends on the ratio of the CO2 concentration ([CO2]) to the HCO3− concentration ([HCO3−]). Controlling blood [CO2] is the respiratory system, which responds to elevations in [CO2] or decreases in pH by increasing ventilation and thereby promoting the excretion of metabolically generated CO2 (8). Controlling blood [HCO3−] is the urinary system, which responds to acidosis by secreting H+ into the lumen of renal tubules (28). The majority of this secreted H+ titrates the HCO3− filtered by the glomeruli and converts it to CO2; the equivalent amount of HCO3− moves into the blood, representing “reabsorbed HCO3−.” The kidney also secretes an amount of H+ equivalent to the H+ absorbed in the diet, the OH− excreted in the feces, and the nonvolatile acids produced by metabolism; the equivalent amount of HCO3− transferred into the blood represents “new HCO3−.”
The renal proximal tubule (PT) is responsible for ∼80% of total H+ secretion. The extrusion of H+ into the PT lumen occurs via Na+/H+ exchanger 3 (NHE3) and the vacuolar-type H+ pump, both in the brush-border membrane (9, 29, 97). Carbonic anhydrase (CA) IV converts luminal H+ and HCO3− to H2O and CO2. H2O enters the PT cell via the water channel [aquaporin 1 (AQP1)] in the apical membrane (54, 64, 76). CO2 also enters the PT cell, probably largely via AQP1 (9, 19, 48, 52). Inside the PT cell, CA II converts CO2 and H2O to HCO3− and H+. The aforementioned acid extruders transfer H+ to the lumen, and electrogenic Na+-HCO3− cotransporter (NBC)e1-A completes the process by transferring Na+ and what appears to be 3 HCO3− across the basolateral (BL) membrane.
Three landmark studies (11, 24, 69) have described the effects of respiratory acidosis (RAc; a fall in arterial pH caused by a rise in [CO2]) on dogs, showing that the kidney responds rapidly by increasing acid secretion. Metabolic acidosis (MAc; a fall in arterial [HCO3−] at constant [CO2]) elicits short-term and long-term adaptations by the kidney. For example, when rabbits are challenged with 2-h MAc, the subsequently isolated apical/brush-border PT membrane vesicles exhibit an increase in NHE3, whereas BL vesicles exhibit an increase in NBC activity (80). Because RAc and MAc in a living animal involve changes in [CO2], [HCO3−], and H+ concentration, a remaining puzzle was how to distinguish the extent to which tubule cells sense changes in each of these three parameters.
In 1995, our laboratory developed a rapid mixing technique for generating temporarily out-of-equilibrium (OOE) solutions, enabling one to make isolated changes in [CO2], [HCO3−], or pH (104). The method can be easily adapted for BL or bath exposure of single, isolated perfused PTs (105). A surprising observation was that varying BL pH (pHBL) at fixed BL [CO2] ([CO2]BL) and BL [HCO3−] ([HCO3−]BL) had no effect on the rate of HCO3− reabsorption (JHCO3−). However, either lowering [HCO3−]BL at constant [CO2]BL or raising [CO2]BL at constant [HCO3−]BL caused a substantial increase in JHCO3− (107). The opposite changes in [HCO3−]BL or [CO2]BL caused JHCO3− to decrease (107). This study demonstrated that the key parameters sensed by the PT, at least over a period of ∼20 min, are [CO2]BL and [HCO3−]BL but not pHBL. Further work showed that the irreversible ErbB family inhibitor PD-168393 (35 nM) or the reversible ErbB inhibitor BPIQ-1 (10 nM) each prevent the PT from responding to isolated changes in [CO2]BL (106).
The ErbB family comprises ErbB1/HER1/EGF receptor (EGFR), ErbB2/HER2/Neu, ErbB3/HER3, and ErbB4/HER4, which form homo- or heterodimers. Ligand-induced conformational changes within the extracellular ligand-binding domain activate cytosolic tyrosine kinase domains (26, 57, 100, 103). ErbB2 is an orphan receptor with no known ligand; its extracellular domain adopts an extended conformation, similar to that of invertebrate EGFR and ligand-bound human ErbB1 (2, 17). However, ErbB2 has an autoinhibition mechanism (25). ErbB3 lacks key residues required for appreciable catalytic activity but can elicit downstream signaling through heterodimerization with other ErbB family members (38, 78). Kinase activation initiates transphosphorylation at tyrosine residues along the COOH-terminal portions of each receptor, forming docking platforms for Src homology 2 (SH2) and phosphotyrosine (pY)-binding domains of target signaling molecules (45, 84, 101). The ErbB family regulates diverse cellular processes, including proliferation, differentiation, adhesion, and migration (1, 56). Deregulated expression and mutation of ErbB receptors occurs frequently in cancerous tissues (71, 99). The PTs of normal adult kidneys express ErbB1 and ErbB2, with a strong BL localization of ErbB1 and a weaker, more diffuse pattern for ErbB2 (49, 63, 102). Low levels of ErbB3 expression have been reported in the adult PT (65, 102). ErbB4, which regulates cell polarity and tubulogenesis during development, maintains moderate expression in the adult PT (81, 93, 102).
The purpose of the present work was to test the hypothesis that changes in [CO2]/[HCO3−] signal in part via ErbB1 and ErbB2. Our approach was to prepare suspensions of the rabbit renal cortex enriched in PTs and to expose them to a series of in-equilibrium CO2/HCO3− solutions mimicking RAc, MAc, respiratory alkalosis (RAlk), metabolic alkalosis (MAlk), and fully compensated respiratory acidosis (cRAc), which is the same as fully compensated MAlk. We found that each acid-base disturbance produced a characteristic set of time courses (5 and 20 min) for four ErbB1 pY sites (pY845, pY992, pY1068, and pY1173) and two ErbB2 pY sites (pY1221 and pY1222).
MATERIALS AND METHODS
Solutions
For the isolation of tubules, we used a variant of DMEM-Ham's F-12 medium (DMEM-F-12, 50:50), obtained as a powder without l-glutamine or NaHCO3 (catalog no. 90-091-PB, Corning, Cellgro Mediatech, Manassas, VA). After diluting the powder with Milli-Q water, we lowered the temperature to 0°C in an ice bath and supplemented the medium with 15 mM HEPES, 2 mM l-glutamine, 2 mM l-(+)-lactic acid, and 2 mM heptanoic acid (catalog no. 75190, Sigma-Aldrich, St. Louis, MO). After titrating the pH to 7.40 at 0°C, we added 15 mM NaHCO3 and gassed the solution with 3% CO2-balance air. Finally, we adjusted the osmolality to 310 ± 5 mosM with H2O or NaCl. For the Percoll gradient, 10× PBS was made from tablets without calcium or magnesium (catalog no. 2810305, MP Biomedicals, Solon, OH).
Table 1 shows the compositions of our test solutions. CO2-containing solutions contained the indicated percentages of CO2, 21% O2, and balance N2. We created the gas mixtures in a computer-controlled gas-mixing system (Series 4000, Environics, Tolland, CT) and bubbled the solutions for 30–40 min. Nominally CO2-free solutions (for which CO2 is not specified in Table 1) were equilibrated with room air.
Table 1.
Compositions of test solutions
| Solution | HEPES | HEPES + 20 mM Butyrate | HEPES + 20 mM Butyrate | HEPES + 1 ng/ml EGF | 2.5% CO2/22 mM HCO3− | 5% CO2/11 mM HCO3− | 5% CO2/22 mM HCO3− | 5% CO2/44 mM HCO3− | 10% CO2/22 mM HCO3− | 10% CO2/44 mM HCO3− |
|---|---|---|---|---|---|---|---|---|---|---|
| Solutes | HEPES | Butyrate (pH 7.1) | Butyrate (pH 7.4) | EGF | Respiratory alkalosis | Metabolic acidosis | Control | Metabolic alkalosis | Respiratory acidosis | Fully compensated respiratory acidosis |
| Nominal CO2 | ∼0 | ∼0 | ∼0 | ∼0 | 0.6 | 1.2 | 1.2 | 1.2 | 2.4 | 2.4 |
| NaHCO3 | ∼0 | ∼0 | ∼0 | ∼0 | 22 | 11 | 22 | 44 | 22 | 44 |
| pH* | 7.4 | 7.1 | 7.4 | 7.4 | 7.7 | 7.4 | 7.4 | 7.7 | 7.1 | 7.4 |
| NaCl | 133 | 115 | 115 | 133 | 111 | 123 | 111 | 89 | 111 | 89 |
| KCl | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| H3PO4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| CaCl2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| MgSO4·7H2O | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Glucose | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 |
| l-Lactic acid | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| l-Glutamine | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| HEPES | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| EGF | 0 | 0 | 0 | 1 ng/ml | 0 | 0 | 0 | 0 | 0 | 0 |
| N-butyric acid (i.e., butyrate) | 0 | 20 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Values are in mM (except for pH and where indicated). In some experiments, we added 50 nM PD-168393 to the solutions.
We titrated all solutions to the indicated pH at 37°C and adjusted the osmolality to 310 ± 5 mosM.
In butyrate-containing solutions, we titrated 20 mM N-butyric acid with NaOH to produce butyrate. Our laboratory has previously used 10 mM (73) or 16 mM (18, 31) butyrate/butyric acid as a surrogate for the pHi-lowering effects of 1.5% CO2 in Xenopus oocytes and 30 mM butyrate/butyric acid for 5% CO2 (94). However, in PTs, BL CO2/HCO3− produces only a transient fall in pHi (due to CO2 entry) followed by a sustained alkalinization (51). Also in PTs (based on results with 10 mM acetate), Na+-monocarboxylate cotransport at the apical membrane (which is of unknown accessibility in PT suspensions) is expected to alkalinize the cell (50). We chose 20 mM as the concentration of butyrate/butyric acid that (acting at the exposed BL membrane) is likely to reproduce at least a transient fall in pHi created by CO2/HCO3− in PT suspensions, perhaps (acting at the apical membrane) followed by a sustained pHi increase.
We purchased l-(+)-lactic acid solution (30% in water by weight; L1875) and butyric acid (+99%) from Sigma-Aldrich (B103500), lyophilized purified mouse EGF1 from AbD Serotec (MorphoSys, Martinsried/Planegg, Germany), and PD-168393 (catalog no. 513033) from Calbiochem (La Jolla, CA).
Harvesting of tissue.
Female New Zealand White rabbits were used as the source of kidneys for renal PT suspensions using a method adapted from Doctor et al. (22) and others (33, 44, 72). Protocols for the housing and handling of New Zealand White rabbits were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Rabbits under anesthesia [∼10 ml iv (5%) pentobarbital sodium in H2O] were euthanized by exsanguination. The kidneys were removed, decapsulated, sliced into 5- to 6-mm coronal sections, and placed into ice-cold DMEM-F-12. The renal cortex was trimmed and finely diced. We digested the tissue using 0.5 mg/ml collagenase type IV (catalog no. C5138, Sigma-Aldrich) in DMEM-F-12, shaking at 225 rpm for 10 min at 37°C, harvested by centrifugation at 150 g for 1 min, and washed three times with DMEM-F-12 to remove collagenase.
Isolation of PTs
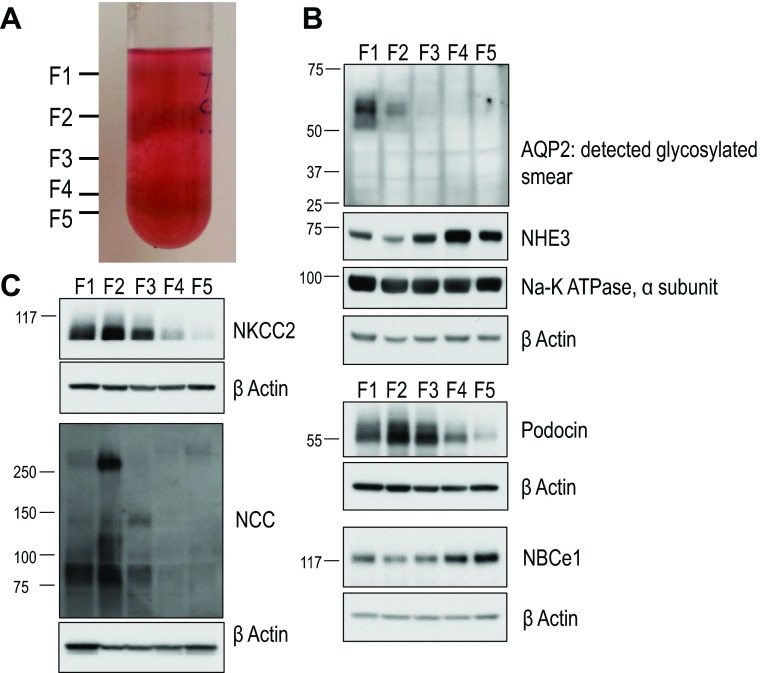
Digested cortical tubules were placed on ice and separated from one another by gentle pressure with a flat glass pestle followed by trituration through a 10-ml pipette. Once dispersed, cortical tubules were washed three times in DMEM-F-12, with harvesting each time at 150 g for 2 min. Cortical tubules were shaken gently in freshly gassed DMEM-F-12 at 4°C for 1 h, resuspended in 4 × 25-ml aliquots of ice-cold 45% Percoll solution (45 ml Percoll, 50 ml freshly gassed DMEM-F-12, and 4.5 ml of 10× PBS), and separated on a self-forming gradient by centrifugation at 25,000 g for 35 min at 4°C. The material migrated into strata (see Fig. 1A). As shown in Fig. 1, B and C, immunoblots of tubule fractions F1 to F5 isolated from individual strata showed gradual enrichment of NHE3 (present in PTs) and NBCe1 (PT marker) and depletion of AQP2 (collecting duct), podocin (podocytes in glomerulus), Na+-K+-Cl− cotransporter 2 (NKCC2; thick ascending limb), and Na+-Cl− cotransporter (NCC; distal convoluted tubule) from tubule fraction F1 (highest stratum) to tubule fraction F5 (lowest stratum). It was the two most dense, PT-enriched fractions (tubule fractions F4 and F5) that we used for experimentation. Enriched PTs were washed several times in freshly gassed, ice-cold DMEM-F-12.
Fig. 1.
Characteristics of our renal cortical tubule preparation. A: a mixture of renal tubules from the rabbit kidney cortex was applied to a 45% self-forming Percoll gradient and subjected to centrifugation at 25,000 × g for 35 min at 4°C. Tubules typically migrated into tubule fractions F1–F5, with proximal tubules (PTs) migrating in lower tubule fractions F4 and F5. B and C: characterization of tubule fractions F1–F5 by Western blot. Lysates were prepared from material isolated from tubule fractions F1–F5, resolved by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and probed with the indicated antibodies. From top to bottom in B: aquaporin 2 (AQP2; collecting duct marker), Na+/H+ exchanger 3 (NHE3; brush-border membrane), α-subunit of Na+-K+-ATPase (basolateral transporter, renal tubule epithelium), β-actin (loading control), podocin (podocyte marker), β-actin (loading control), Na+-HCO3− cotransporter (NBC)e1-A (PT marker), and β-actin (loading control). From top to bottom in C: Na+-K+-Cl− cotransporter 2 (NKCC2; thick ascending limb marker), β-actin (loading control), Na+-Cl− cotransporter (NCC; distal convoluted tubule marker), and β-actin (loading control).
Challenging PTs With Acid-Base Disturbances
The enriched PTs-fractions, tubule fraction F4 and F5, from four Percoll gradients (from two kidneys) were pooled and mixed end over end to create a uniform suspension (typically of 70–100 ml), divided into aliquots of ∼5 ml each containing ∼3–8 mg protein (see Preparation of Lysate below), placed in a 50-ml, conical-bottom polypropylene tube with a screw cap (catalog no. 352070, BD Biosciences, Durham, NC), diluted to a total volume of ∼10 ml in freshly gassed DMEM-F-12, and allowed to rest at room temperature for 30 min before experimentation. Each PT aliquot was briefly pelleted at 150 g for 1 min, resuspended (after decanting) in 50 ml (virtually to the plastic screw cap) of one of our prewarmed, pregassed test solutions (Table 1), and then incubated for 5 or 20 min at 37°C in a water bath.1 After the incubation, PTs were harvested by centrifugation at 1,000 g for 30 s, test solutions were decanted, and PT pellets were flash frozen in liquid nitrogen and stored at −80°C, pending analysis by Western blot.
Antibodies
ErbB1.
We used rabbit polyclonal anti-EGFR (no. 2232, Cell Signaling Technology, Danvers, MA).
ErbB2.
We used rabbit polyclonal anti-ErbB2 (no. 18299-1-AP, ProteinTech Group, Chicago, IL) and goat polyclonal anti-Neu C-14 (sc-31154, Santa Cruz Biotechnology, Santa Cruz, CA).
Pan-pY.
We used two antibodies that target generic pY motifs: mouse monoclonal anti-pY phospho-Tyr100 (no. 9411, Cell Signaling Technology) and mouse monoclonal cocktail anti-pY 4G10 Platinum (no. 05–1050, EMD Millipore, Billerica, MA). These produced similar staining patterns and, because of our normalization procedure (see Data Analysis below), were used interchangeably.
Phosphospecific ErbB1-pY.
We used two antibodies that target pY845 of ErbB1. In early experiments, we used mouse monoclonal anti-ErbB1 pY-845 clone 12A3 (no. 04-283, EMD Millipore). Later, we found greater sensitivity, although a similar staining pattern, with rabbit polyclonal anti-ErbB1 pY-845 (no. sc-101669, Santa Cruz Biotechnology). To target pY992, we used a single rabbit polyclonal antibody (no. 40-8250, Invitrogen, Grand Island, NY). To target pY1068, we used a single rabbit polyclonal antibody (no. 324867, Calbiochem). To target pY1173, we used a single rabbit polyclonal antibody (XBP-4088, ProSci, Poway, CA).
Phosphospecific ErbB2-pY.
To target the tandem site pY1221/pY1222, we used a single rabbit polyclonal antibody (sc-101694, Santa Cruz Biotechnology).
Actin.
To normalize sample loading on protein gels, we used mouse monoclonal anti-β-actin antibody clone AC-15 (no. A1978, Sigma-Aldrich).
Tubule fraction markers.
We used AQP2 rabbit polyclonal antibody (sc-28629, Santa Cruz Biotechnology), NHE3 mouse monoclonal antibody (3H3) to opossum NHE3 (kindly provided by Dr. Daniel Biemesderfer, Yale University), Na+-K+-ATPase mouse monoclonal antibody (464.6) to the α1-subunit (ab7671, Abcam, Cambridge, MA), podocin rabbit polyclonal antibody (sc-21009, Santa Cruz Biotechnology), NBCe1 rabbit polyclonal antibody [NBC-3 (75)], NKCC2 rabbit polyclonal antibody, NKCC2 rabbit polyclonal antibody (sc-133823, Santa Cruz Biotechnology), and NCC rabbit polyclonal antibody (AB3553, Millipore).
Secondary antibodies.
We used a goat affinity-purified antibody to rabbit IgG, horseradish peroxidase (HRP) conjugated (AP132P, Millipore), and goat affinity-purified antibody to mouse IgG, HRP conjugated (no. 55563, MP Biomedicals).
Preparation of Lysate
PT pellets were thawed and rapidly placed on ice before the addition of ice-cold lysis buffer of the following composition (in mM): 25 HEPES (pH 7.50), 100 NaCl, 50 NaF, 10 Na-pyrophosphate (Na4P2O7·10 H2O), 1 EDTA, and 1% Nonidet P-40 (AB01425, American Bioanalytical, Natick, MA), to which we added protease inhibitor cocktail (Sigma-Aldrich) at 1:25 (vol/vol). Using a 21-gauge needle and syringe, we sheared and rapidly lysed the PT pellet. After thorough homogenization, we added 2 mM Na3VO4 to each sample to stabilize pY sites, and the cell debris was removed by centrifugation. Finally, a Pierce BCA protein assay (catalog no. 23227, Thermo Scientific) was performed to determine total protein.
Protein Gels and Western Blot Analysis
Equal amounts of protein were loaded onto NuPAGE Novex 4–12% Bis-Tris gels (NP0322 and NP0366, Invitrogen), resolved by electrophoresis, electrophoretically transferred to a polyvinylidene difluoride membrane, and blocked in Tris-buffered saline plus Tween 20 [containing 10 mM Tris·HCl (pH 7.50), 150 mM NaCl, and 0.01% Tween 20] supplemented with 5% milk and 2 mM Na3VO4 at room temperature for 1 h. We probed overnight with an anti-pY primary antibody (see above). After washing well to remove unbound primary antibodies, we incubated blots with secondary antibodies linked to HRP (see above), washed, and detected immunoreactive bands with Pierce ECL2 reagent (catalog no. 80196, Thermo Scientific) on X-ray film or using a FluorChem E gel-documentation device (Protein Simple, San Jose, CA). Before reprobing (e.g., with antibodies to actin, ErbB1, or ErbB2), we stripped blots by an incubation in Pierce Restore Plus Western blot stripping buffer (catalog no. 46430, Thermo Scientific) at 37°C for 30 min.
Image Acquisition
For densitometry, we scanned films as 16-bit grayscale images using an Epson Perfection V500 photo scanner at a positive film setting at high resolution (600–800 dpi). The densitometry of β-actin on each blot was used as a loading control with which to normalize pY immunoreactivity. We performed all densitometry analyses, both after scanning film or imaging with the FluorChem E, using ImageJ software (National Institutes of Health).
Data Analysis
After densitometry analysis, we normalized the intensity of pY immunoreactivity to the intensity of actin immunoreactivity in the same lane. In the analyses shown in Figs. 4A, 5A, 7A, 8A, 10A, 11A, and 12, A and C, we divided the normalized pY-to-actin ratio for any “solution treatment” by the normalized pY-to-actin ration after nominally CO2/HCO3−-free HEPES treatment. In the analyses shown in Figs. 4B, 5B, 7B, 8B, 10B, 11B, and 12, B and D, we divided the normalized pY-to-actin ratio for each solution treatment by the normalized pY-to-actin ratio after our control (Ctrl) 5% CO2/22 mM HCO3− treatment. In the analyses shown in Figs. 4C, 5C, 7C, 8C, 10C, and 11C, we divided the normalized pY-to-actin ratio for each solution treatment in the presence of PD-168393 by the normalized pY-to-actin ratio after treatment in the same solution without PD-168393. For statistical analysis of each data set, we log2 transformed each point (so that that a twofold increase is treated the same as a twofold decrease) and discarded the ∼5% of data points that were >2 SD away from the mean of each set, as previously described by others (67). We performed one-tailed, paired t-tests using Excel 2010.
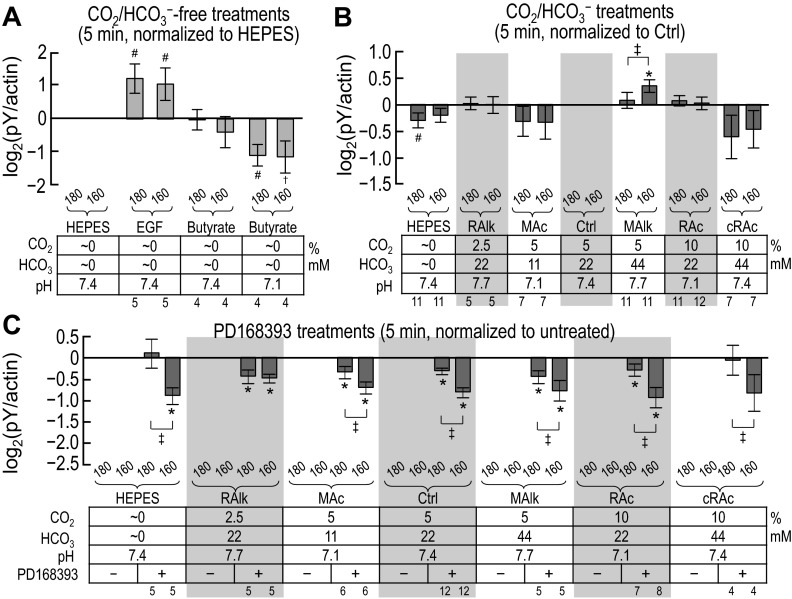
Fig. 4.
Quantification of pY immunoreactivity after solution treatments of 5 min. Shown are the results of densitometry of blots similar to those shown in Fig. 3. For each treatment, pan-pY was first normalized to actin. A: ratios of pan-pY to actin (pan-pY/actin) for nominally CO2/HCO3−-free treatments, normalized to pan-pY/actin for the nominally CO2/HCO3−-free HEPES treatment. B: pan-pY/actin for nominally CO2/HCO3−-free HEPES and CO2/HCO3− solutions, normalized to pan-pY/actin for our Ctrl treatment (5% CO2/22 mM HCO3−). C: pan-pY/actin for treatments like those in B but with and without the ErbB inhibitor PD-168393, normalized to pan-pY/actin for the equivalent treatment without PD-168393. Sample sizes (n) are shown beneath each graph. †Bar for which all constituent data points are <0 (or >0) but not statistically different from 0. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.” ‡Pair of bars for which P < 0.05 (compared with each other by a one-tailed, paired t-test).
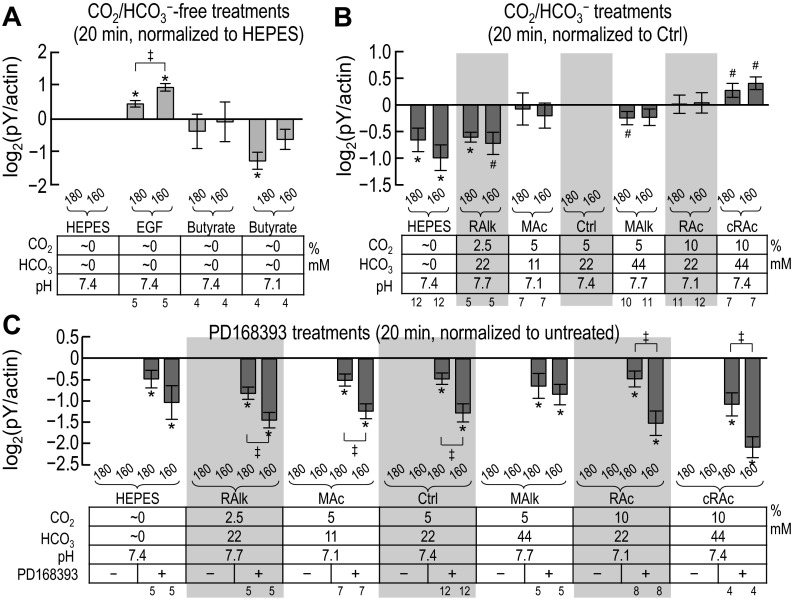
Fig. 5.
Quantification of pY immunoreactivity after solution treatments of 20 min. Except for the duration of the treatments, this figure is identical to Fig. 4. Sample sizes (n) are shown beneath each graph. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.” ‡Pair of bars for which P < 0.05 (compared with each other by a one-tailed, paired t-test).
Fig. 7.
Quantification of ErbB1 immunoreactivity at individual pY sites after solution treatments of 5 min. These are the results of densitometry of blots similar to those shown in Fig. 6. For each treatment, pY-specific immunoreactivity at each ErbB1 site was first normalized to actin. A: ratios of ErbB1-pY to actin (ErbB1-pY/actin) for nominally CO2/HCO3−-free treatments, normalized to ErbB1-pY/actin for the nominally CO2/HCO3−-free HEPES treatment. B: ErbB1-pY/actin for nominally CO2/HCO3−-free HEPES and CO2/HCO3− solutions, normalized to ErbB1-pY/actin for our Ctrl treatment (5% CO2/22 mM HCO3−). C: ErbB1-pY/actin for treatments like those in B but with and without the ErbB inhibitor PD-168393, normalized to ErbB1-pY/actin for the equivalent treatment without PD-168393. Sample sizes (n) are shown beneath each graph. †Bar for which all constituent data points are <0 (or >0) but not statistically different from 0. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.”
Fig. 8.
Quantification of ErbB1 immunoreactivity at individual pY sites after solution treatments of 20 min. Except for the duration of the treatments, this figure is identical to Fig. 7. Sample sizes (n) are shown beneath each graph. †Bar for which all constituent data points are <0 (or >0) but not statistically different from 0. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.”
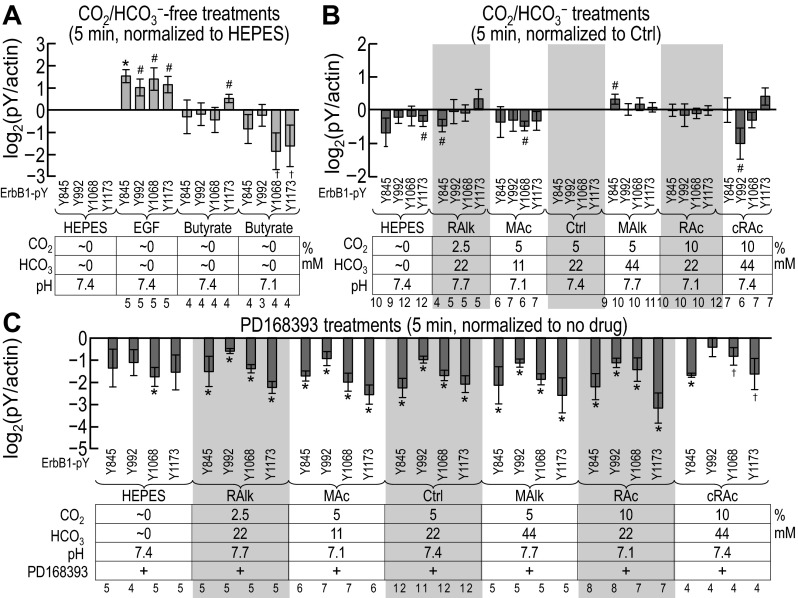
Fig. 10.
Quantification of ErbB2 immunoreactivity at pY1221/pY1222 after solution treatments of 5 min. These are the results of densitometry of blots similar to those shown in Fig. 9. For each treatment, pY-specific immunoreactivity at ErbB2 pY1221/pY1222 was first normalized to actin. A: ratios of ErbB2-pY1221/pY1222 to actin [(ErbB2-pY1221/2)/actin] for nominally CO2/HCO3−-free treatments normalized to (ErbB2-pY1221/2)/actin for the nominally CO2/HCO3−-free HEPES treatment. B: ratios of pY1221/pY1222 to actin [(pY1221/2)/actin] for nominally CO2/HCO3−-free HEPES and CO2/HCO3− solutions, normalized to (pY1221/2)/actin for our Ctrl treatment (5% CO2/22 mM HCO3−). C: (ErbB2-pY1221/2)/actin for treatments like those in B but with and without the ErbB inhibitor PD-168393, normalized to (pY1221/2)/actin for the equivalent treatment without PD-168393. Sample sizes (n) are shown beneath each graph. †Bar for which all constituent data points are <0 (or >0) but not statistically different from 0. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.”
Fig. 11.
Quantification of ErbB2 immunoreactivity at pY1221/pY1222 after solution treatments of 20 min. Except for the duration of the treatments, this figure is identical to Fig. 10. Sample sizes (n) are shown beneath each graph. †Bar for which all constituent data points are <0 (or >0) but not statistically different from 0. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.”
Fig. 12.
Quantification of pseudo-pan-ErbB1/2-pY (ErbB-pY′) after solution treatments of 5 and 20 min. The responses of ErbB1-pY845, ErbB1-pY992, ErbB1-pY1068, ErbB1-pY1173 (Figs. 7 and 8), and ErbB2-pY1221/pY1222 (Figs. 10 and 11) were summed to provide an approximation of the pan-ErbB1/2-pY response to the various acid-base disturbances. A: ErbB-pY′ at 5 min for nominally CO2/HCO3−-free treatments, normalized to ErbB-pY′ for the nominally CO2/HCO3−-free HEPES treatment at 5 min. B: ErbB-pY′ at 5 min for nominally CO2/HCO3−-free HEPES and CO2/HCO3− solutions, normalized to ErbB-pY′ for our Ctrl treatment (5% CO2/22 mM HCO3−). C: ErbB-pY′ at 20 min for nominally CO2/HCO3−-free treatments, normalized to ErbB-pY′ for the HEPES treatment at 20 min. D: ErbB-pY′ at 20 min for HEPES and CO2/HCO3− solutions, normalized to ErbB-pY′ for our control treatment (5% CO2/22 mM HCO3−). Sample sizes (n) are shown beneath each graph. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.”
RESULTS
Detection of Major pY Bands
Figure 2A, left, shows an anti-pan-pY Western blot of protein prepared from an aliquot of a PT suspension that had been transferred from DMEM-F-12 and then incubated in our Ctrl 5% CO2/22 mM HCO3−/pH 7.4 solution for 5 min. In this sample, we detected prominent pan-pY bands with molecular weights (MWs) of ∼180, ∼160, ∼130, and ∼120 kDa. We also saw a dim band at ∼65 kDa and an inconsistent band (present in this blot but not all others) at ∼55 kDa. The bands below 150 kDa do not appear to be related to ErbB1 or ErbB2 because, unlike the ∼180 and ∼160-kDa bands, as shown below, the pan-pY signals for bands < 150 kDa seldom responded to acid-base disturbances or the inhibitor PD-168393. For this reason, we did not further pursue these lower MW bands.
Fig. 2.
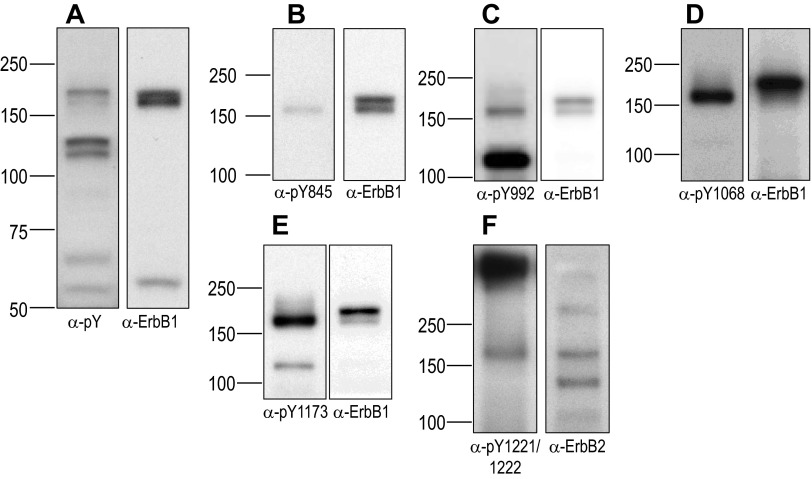
Western blots of rabbit renal PT preparations. Tubules were exposed to our control (Ctrl) 5% CO2/22 mM HCO3− solution for 5 min, and aliquots of extracted protein were resolved by SDS-PAGE, transferred to PVDF membranes, probed with the indicated phosphotyrosine (pY) antibodies, stripped, and then reprobed with an antibody to ErbB1 or ErbB2. A: blots were probed first with anti-pan-pY (left) and reprobed with anti-ErbB1 (right). B: blots were probed first with anti-ErbB1/pY845. C: blots were probed first with anti-ErbB1/pY992. D: blots were probed first with anti-ErbB1/pY1068. E: blots were probed first with anti-ErbB1/pY1173. F: blots were probed first with anti-ErbB2/pY1221/pY1222.
The same blot, reprobed with an anti-ErbB1 antibody, Fig. 2A, right, shows that ErbB1 immunoreactivity was located in the vicinity of both the 180- and 160-kDa pan-pY bands shown in Fig. 2A, left; thus, ErbB1-pY likely contributes to these two bands. Indeed, similar blots probed with antibodies directed against specific pY sites in the COOH-terminal tail of ErbB1 (pY845, pY992, pY1068, and pY1173 in Fig. 2, B–E) showed ErbB1-pY immunoreactivity in this MW range. The ErbB2-pY1221/2 immunoreactivity was also in the 160- to 180-kDa range (Fig. 2F).
In addition to the bands at ∼180 and ∼160 kDa, our phosphospecific antibodies sometimes showed reactivity between 100 and 120 kDa, often with ErbB1-pY992 (Fig. 2C), rarely with ErbB1-pY1068 (not present in Fig. 2D), often with ErbB1-pY1173 (Fig. 2E), and often with ErbB2-pY1221/2 (not present in Fig. 2F). The ErbB1 antibody, raised against an epitope surrounding Y1068, never reacted with any of these bands at 100–120 kDa. Because the appearance of these bands was inconsistent, their identity is unknown, and because these fragments cannot be part of an intact ErbB receptor, we excluded them from further analysis. We also excluded from further analysis high MW (>250 kDa) material detected with ErbB2-pY1221/2 (Fig. 2F, left) but that was less detectable when probing total ErbB2 (Fig. 2F, right). Thus, we focused our attention on pY immunoreactivity at 180 kDa (pY180kDa) and 160 kDa (pY160kDa).
The ratio of pY180kDa-to-pY160kDa intensities varied among rabbits and PT treatments. However, in the majority of cases, as evidenced by the example blots shown in Figs. 2A and 3, pY180kDa was greater in intensity than pY160kDa. For example, the ratio pY180kDa to pY160kDa was 1.7 ± 0.2 after a 5-min exposure to our Ctrl solution (5% CO2/22 mM HCO3−/pH 7.4, n = 12 rabbits).
Fig. 3.
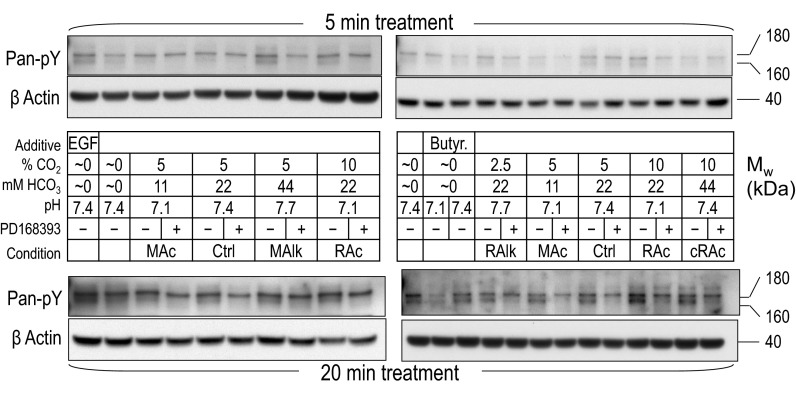
Pan-pY immunoreactivity at 180 and 160 kDa in rabbit PT preparations subjected to acid-base disturbances (5 and 20 min). Tubules were exposed to one of several treatment solutions, and protein was subjected to Western blot analysis. For each lane, we show (from top to bottom) the pan-pY immunoreactivity from tubules that were exposed to treatments for 5 min and a reprobe of the same blot with an anti-actin antibody (top), a summary of the treatment conditions (table in middle), and the pan-pY immunoreactivity from tubules that were exposed to treatments for 20 min and a reprobe of the same blot with an anti-actin antibody (bottom). MAc, metabolic acidosis; MAlk, metabolic alkalosis; RAc, respiratory acidosis; RAlk, respiratory alkalosis; cRAc, fully compensated respiratory acidosis.
The Pan-pY Profile of PT Suspensions Varies with Acid-Base Disturbances and PD-168393 Treatment
General approach.
We divided a PT suspension into multiple equivalent aliquots and exposed each to one of a series of solutions designed to mimic diverse acid-base disturbances (see Table 1) for either 5 or 20 min in the presence or absence of the ErbB inhibitor PD-168393. Because of physical limitations on the amount of PT material that we could isolate from a single rabbit and on the number of samples that we could process simultaneously, we performed our study in a series of overlapping assays. Figure 3 shows Western blots of material isolated from two such assays that, between them, covered the full array of solutions shown in Table 1.
Note that each assay set included one sample treated with 1) our nominally CO2/HCO3−-free/pH 7.4 solution (HEPES) and another sample treated with 2) our Ctrl 5% CO2/22 mM HCO3−/pH 7.4 solution.
Five-minute treatment.
Figure 4A shows the pan-pY signal intensity at 180 and 160 kDa (measured from blots such as those shown in Fig. 3) for nominally CO2/HCO3−-free treatments, normalized first to the intensity of actin immunoreactivity in the same sample and normalized second to the pan-pY signal of the HEPES-treated sample (i.e., HEPES = 1.0). Thus, these data represent the CO2/HCO3−-independent effects of nominally CO2/HCO3−-free EGF or butyrate challenges on pY180kDa and pY160kDa signals.
The left bar of each pair in Fig. 4A represents the normalized pY180kDa signal and the right bar of each pair represents the normalized pY160kDa signal. Analysis of the data shown in Fig. 4A revealed the following:
1. Each treatment affects the intensities of pY180kDa and pY160kDa to similar extents (results of a one-tailed, paired t-test showed no differences between pY180kDa and pY160kDa in each pair, P > 0.05).
2. After 5 min of nominally CO2/HCO3−-free EGF treatment (which ought to activate ErbB1 and ErbB2), pY180kDa and pY160kDa intensities tend to increase, consistent with the hypothesis that ErbB activation contributes to pY180kDa and pY160kDa intensities in nominally CO2/HCO3−-free solutions (HEPES vs. EGF in Fig. 4A).2
3. After 5 min of nominally CO2/HCO3−-free butyrate treatment (which ought to cause pHi to fall) accompanied by a decrease in pHo to 7.1, pY180kDa tends to fall, mirrored by pY160kDa, which is consistently reduced but with a high variance (HEPES vs. butyrate, pH 7.1, in Fig. 4A).
Figure 4B shows pan-pY signal intensities (measured from blots such as those shown in Fig. 3) for HEPES and Ctrl CO2/HCO3− conditions as well as five acid-base disturbances imposed in the presence of CO2/HCO3−. In each case, we normalized to the intensity of actin immunoreactivity from each sample and then normalized to the pan-pY signal of the Ctrl-treated sample (i.e., Ctrl = 1.0). Analysis of the data shown in Fig. 4B revealed that:
1. Nominally CO2/HCO3−-free HEPES treatment, which tends to reduce pY180kDa, is the only condition that influences the intensity of pY180kDa at 5 min (i.e., HEPES is less than Ctrl in Fig. 4B).
2. MAlk is the only treatment that significantly influences pY160kDa at 5 min (i.e., MAlk/160 is significantly greater than Ctrl/160 in Fig. 4B).2
3. MAlk enhances pY160kDa more than pY180kDa (i.e., MAlk/180 vs. MAlk/160 in Fig. 4B).
Figure 4C shows pan-pY signal intensities (measured from blots such as those shown in Fig. 3) for a subset of experiments that we performed with and without the ErbB inhibitor PD-168393. For each sample, we normalized to the intensity of actin immunoreactivity and then normalized to the PD-168393-free signal (i.e., PD-168393 untreated = 1.0 for each solution type). Thus, these data represent the PD-168393 sensitivity of each pY signal. Analysis of the data shown in Fig. 4C revealed that:
1. PD-168393 significantly reduces the intensity of pY160kDa but not pY180kDa in HEPES.
2. PD-168393 reduces both pY180kDa and pY160kDa in RAlk, MAc, Ctrl, MAlk, and RAc. This observation is consistent with the hypothesis that ErbB1/2 activation can contribute to pY180kDa and pY160kDa intensity in the presence of CO2/HCO3−.2
3. PD-168393 does not significantly influence either pY180kDa or pY160kDa in cRAc.
4. With the exception of RAlk, the inhibitory effect of PD-168393 is greater for pY160kDa than for pY180kDa. For RAlk, pY180kDa and pY160kDa are equally sensitive to inhibition.
In summary, a 5-min treatment with nominally CO2/HCO3−-free EGF tends to raise both pY180kDa and pY160kDa (Fig. 4A), whereas after a 5-min treatment with nominally CO2/HCO3−-free butyrate (pH 7.1), the simultaneous decrease in pHo and pHi, pY180kDa tends to fall. MAlk raises pY160kDa (Fig. 4B). Finally, PD-168393 generally lowers both pY180kDa and pY160kDa (Fig. 4C).
Twenty-minute treatment.
Figure 5, A–C, shows data equivalent to those shown in Fig. 4, A–C but for a 20-min exposure rather than a 5-min exposure to each treatment solution. We provide a comparison of the 5- and 20-min data in the discussion (see Fig. 14).
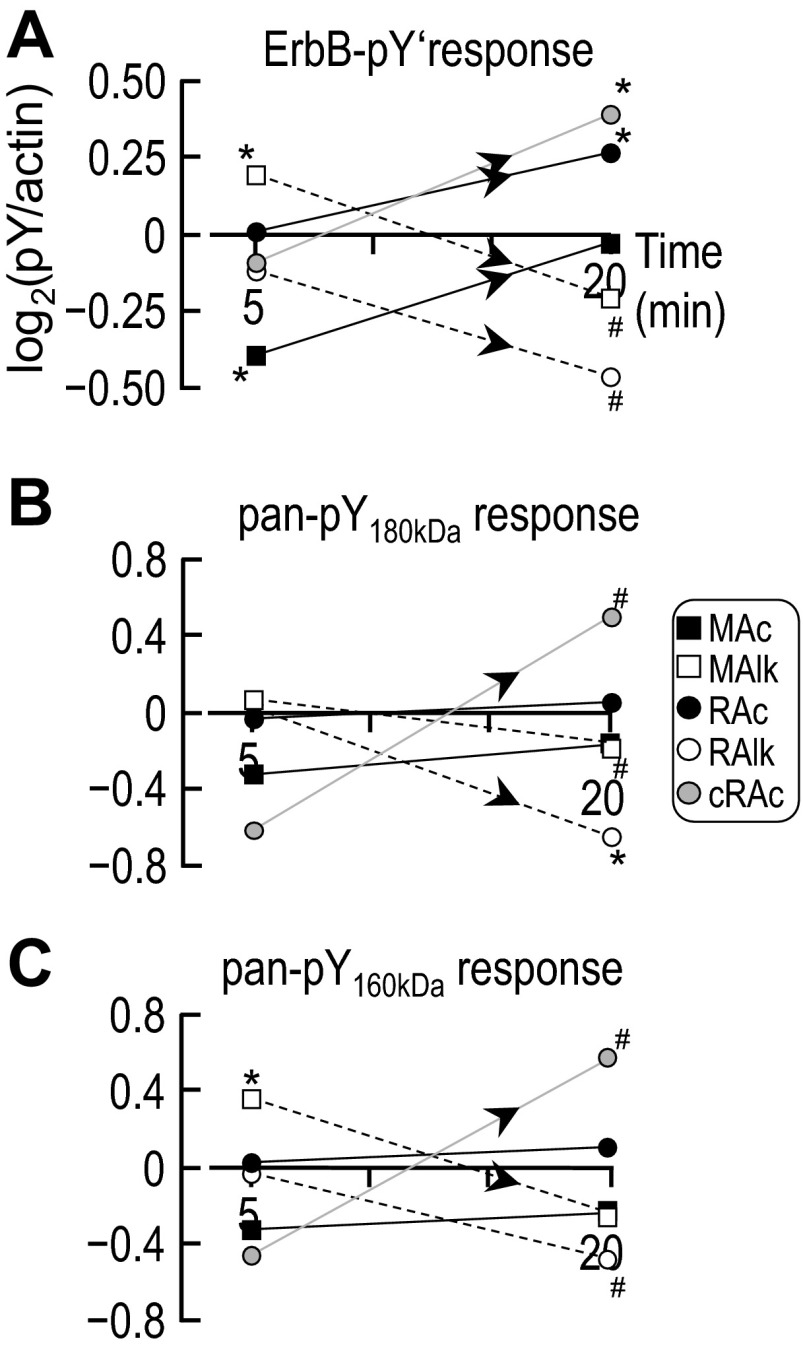
Fig. 14.
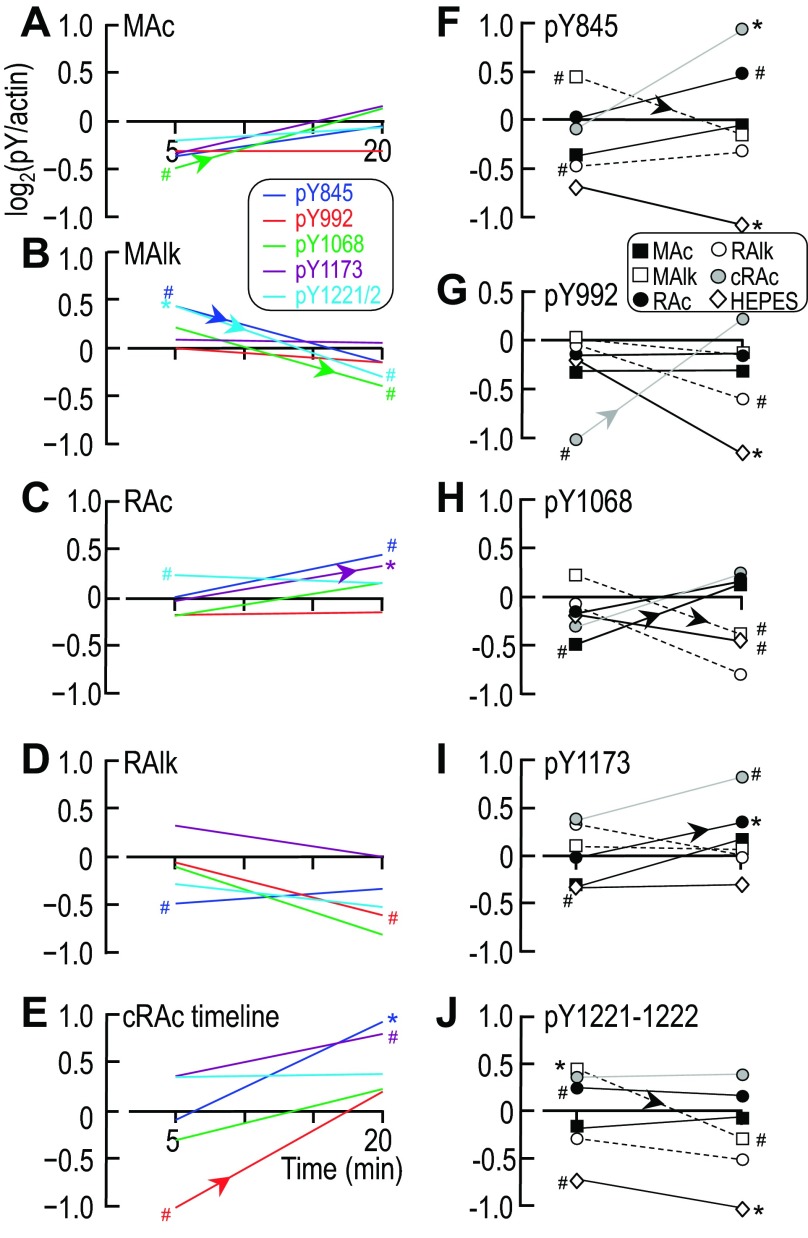
Time-dependent changes of ErbB-pY′ and pan-pY signals at ∼180 and ∼160 kDa for each acid-base disturbance. Each line represents the time course of the log2-transformed parameter for one of the five acid-base disturbances. A: ErbB-pY′. B: pan-pY180kDa. C: pan-pY160kDa. In these analyses, we assume that the parameter value at 0 min (i.e., before imposition of one of the acid-base disturbances) is equivalent to the parameter value for our Ctrl treatment (5% CO2/22 mM HCO3−) at 5 or 20 min, where, by definition, log2(pY/actin) = 0. Sample sizes (n) are shown in Figs. 4B, 5B, 12B and 12D. The upward-sloping or downward-sloping arrows indicate a statistically significant difference between the corresponding values at 5 and 20 min. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.”
Figure 5A shows pY180kDa and pY160kDa signal intensities (measured from blots such as those shown in Fig. 3) after PT exposure to nominally CO2/HCO3−-free solutions normalized to the HEPES (pH 7.4) solution. Analysis of these data revealed that:
1. As with the 5-min treatment (Fig. 4A), nominally CO2/HCO3−-free EGF enhances the intensity of both pY180kDa and pY160kDa (i.e., EGF is significantly greater than HEPES in Fig. 5A); however, different from the effect of a 5-min exposure, at 20 min, pY160kDa is enhanced to a greater extent than pY180kDa (i.e., EGF/180 vs. EGF/160 in Fig. 5A).2
2. In contrast to the 5-min treatment (Fig. 4A), nominally CO2/HCO3−-free butyrate at pHo 7.1 reduces pY180kDa but not pY160kDa (i.e., at pY180kDa, butyrate at pHo 7.1 is significantly less than HEPES in Fig. 5A).
Figure 5B shows pY180kDa and pY160kDa signal intensities (measured from blots such as those shown in Fig. 3) after a 20-min exposure of PT preparations to nominally CO2/HCO3−-free HEPES or various CO2/HCO3− solutions. In each case, we normalized to the Ctrl solution. Analysis of these data revealed that:
1. MAlk after 20 min, in contrast to the situation at 5 min (Fig. 4B), where MAlk is the only CO2/HCO3− treatment that elicits an effect (an increase in pY160kDa), tends to decrease pY180kDa and has no effect on pY160kDa.2
2. RAlk reduces pY180kDa and also tends to reduce pY160kDa (i.e., RAlk/180 vs. Ctrl/180 and RAlk/160 vs. Ctrl/160 in Fig. 5B).2
3. In contrast, neither MAc nor RAc (the two acidosis treatments) influences either pY180kDa or pY160kDa (i.e., MAc/180 or RAc/180 vs. Ctrl/180 and MAc/160 or RAc/160 vs. Ctrl/160 in Fig. 5B).
4. cRAc tends to enhance both pY180kDa and pY160kDa (i.e., cRAc/180 vs. Ctrl/180 and cRAc/160 vs. Ctrl/160 in Fig. 5B).
5. Nominally CO2/HCO3−-free HEPES after 20 min, in contrast to the 5-min exposure (Fig. 4B), reduces not only pY180kDa but also pY160kDa (i.e., HEPES/180 vs. Ctrl/180 and HEPES/160 vs. Ctrl/160 in Fig. 5B).
Figure 5C shows, for a subset of the data shown in Fig. 5B, the PD-168393 sensitivities of pY180kDa and pY160kDa after 20 min of treatment. Analysis of these data revealed that:
1. PD-168393 reduces both pY180kDa and pY160kDa for all treatments.2
2. As was the trend at 5 min (Fig. 4C), the inhibition by PD-168393 at 20 min tends to be greater for pY160kDa than for pY180kDa.
3. The inhibition by PD-168393 is generally more robust at 20 min (Fig. 5C) than at 5 min (Fig. 4C).
In summary, a 20-min treatment with nominally CO2/HCO3−-free butyrate (pH 7.1) reduces pY180kDa (Fig. 5A, butyrate at pHo 7.1). Nominally CO2/HCO3−-free EGF enhances both pY180kDa and pY160kDa (Fig. 5A). Moreover, PD-168393 generally reduces the two pY signals across the treatments (Fig. 5C). These trends at 20 min are similar to (but tend to be more robust than) those at 5 min. The 20-min data differ most strikingly from the 5-min data for acid-base disturbances in the presence of CO2/HCO3−. At 5 min (Fig. 4B), only MAlk/160 shows a response. At 20 min (Fig. 5B), pY180kDa and pY160kDa exhibit a significant decrease or tend to decrease at low percent CO2 (HEPES and RAlk) and tend to increase at elevated percent CO2 and [HCO3−] (HEPES vs. Ctrl vs. cRAc) independent of pHo.
For ErbB1, the Time Course of pY at Specific Sites Depends on the Acid-Base Status and Inhibition With PD-168393
General approach.
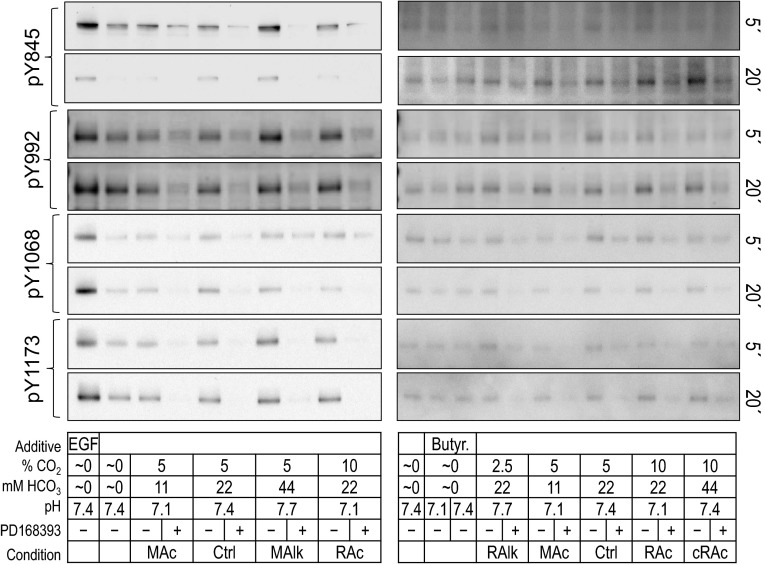
We hypothesized that the pY180kDa and pY160kDa signals shown in Figs. 4 and 5 reflect, in large measure, changes in the pY status of sites on ErbB1 and ErbB2. In parallel with the pan-pY analyses shown in Figs. 4 and 5, we also analyzed tyrosine phosphorylation on four specific tyrosine residues in ErbB1-Y845 in the activation loop of the kinase domain as well as Y992, Y1068, and Y1173 in the COOH-terminal tail of ErbB1. Figure 6 shows representative examples of Western blots probed with phosphospecific antibodies at 5 and 20 min. (Below, we present a similar analysis for the Y1221/Y1222 doublet in the COOH-terminal tail of ErbB2.)
Fig. 6.
ErbB1 immunoreactivity at individual pY sites in rabbit PT preparations subjected to acid-base disturbances (5 and 20 min). Tubules were exposed to one of several treatment solutions, and protein was subjected to Western blot analysis. For each lane, we show (from top to bottom) pY845 at 5 and 20 min, pY992 at 5 and 20 min, pY1068 at 5 and 20 min, pY1173 at 5 and 20 min, and a summary of the treatment conditions. Note that the blots on the right repeat some of the conditions on the left. We do not show the reprobes of the blots for actin.
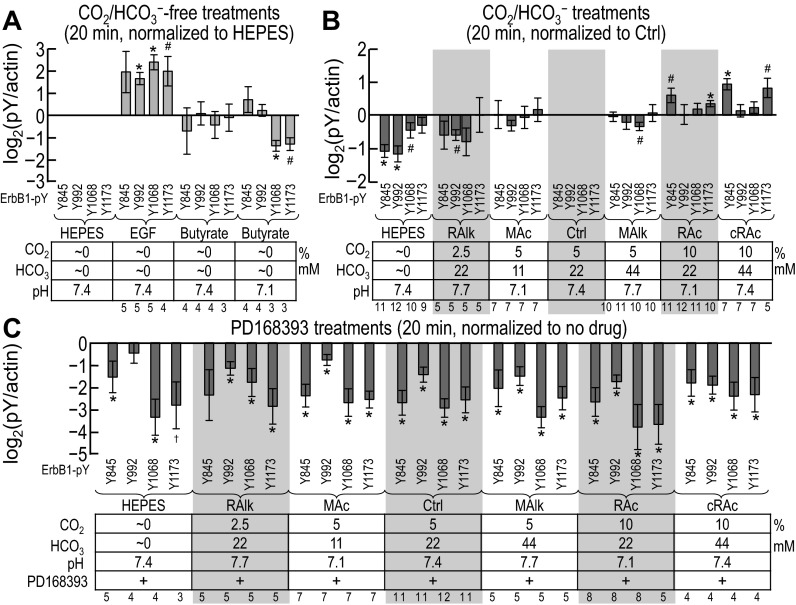
Five-minute treatment.
Figure 7A shows site-specific pY signal intensities (measured from blots such as those shown in Fig. 6) for nominally CO2/HCO3−-free treatments. We normalized these first to the intensity of actin immunoreactivity in the same lane and then normalized again to the corresponding normalized signal from the HEPES-treated sample (i.e., HEPES = 1.0). Thus, these data represent the effect of nominally CO2/HCO3−-independent acid-base disturbances on the pY status of each of the four ErbB1 tyrosine residues. Analysis of the data shown in Fig. 7A revealed that:
1. After 5 min of nominally CO2/HCO3−-free EGF treatment, pY immunoreactivity at all four sites tends to increase, as expected (HEPES vs. EGF in Fig. 7A); pY845 exhibits the most robust increase.2
2. Nominally CO2/HCO3−-free butyrate treatment at pHo 7.4 tends to increase pY1173 [HEPES/Y1173 vs. butyrate (7.4/1173) in Fig. 7A] but does not affect pY845, pY992, or pY1068.
3. Nominally CO2/HCO3−-free butyrate at pHo 7.1 does not alter pY845 or pY992 [HEPES vs. butyrate (pH 7.1) in Fig. 7A]. However, we note that this treatment consistently reduced pY1068 and pY1173, although with too great a variance for statistical significance.
Figure 7B shows site-specific pY signal intensities (measured from blots such as those shown in Fig. 6) for nominally CO2/HCO3−-free HEPES plus acid-base disturbances in the presence of CO2/HCO3−. We normalized values to actin and the Ctrl sample, as shown in Fig. 5B. Analysis of the data shown in Fig. 7B revealed that the four ErbB1 sites responded differently to each acid-base disturbances at 5 min:
1. The nominally CO2/HCO3−-free HEPES treatment tends to reduce only pY1173 (HEPES/Y1173 vs. Ctrl/Y1173 in Fig. 7B).
2. RAlk tends to reduce only pY845 (RAlk/Y845 vs. Ctrl/Y845 in Fig. 7B).
3. MAc tends to reduce only pY1068 (MAc/Y1068 vs. Ctrl/Y1068 in Fig. 7B).
4. MAlk tends to increase only pY845 (HEPES/Y845 vs. Ctrl/Y845 in Fig. 7B).
5. RAc did not cause an alteration in the pY status of any of the four sites (RAc vs. Ctrl in Fig. 7B).2
6. cRAc tends to reduce only pY992 (HEPES/Y992 vs. Ctrl/Y992 in Fig. 7B).
In summary, acid-base disturbances at 5 min tend to produce modest pY effects at specific sites.
Figure 7C shows, for a subset of the data shown in Fig. 7B, the PD-168393 sensitivity of pY at the four ErbB1 residues after 5-min treatments. The inhibition by the drug is reflected by the extent to which the values are <1.0. Analysis of these data revealed that:
1. For the nominally CO2/HCO3−-free HEPES treatment, the only significant effect of PD-168393 is a reduction in pY1068.
2. For RAlk, MAc, Ctrl, MAlk, and RAc, PD-168393 reduces each of the four pY signals.2
3. For cRAc, the only significant effect of the drug is a reduction in pY845.
In summary, and not surprisingly, for nearly all of the acid-base conditions, PD-168393 reduces the phosphorylation at all four pY sites. In other words, over a range of acid-base disturbances, PD-168393-sensitive kinases are active and required to maintain pY levels. However, under conditions of cRAc, a PD-168393-sensitive kinase is less critical to maintain the phosphorylation of Y992; cRAc at 5 min has eliminated that kinase activity.
Twenty-minute treatment.
Figure 8, A–C, shows equivalent data to those shown in Fig. 7, A–C, but for a 20-min exposure rather than a 5-min exposure to each treatment solution. We provide a comparison of the 5- and 20-min data in the discussion (see ErbB1 data in Fig. 13).
Fig. 13.
Time-dependent changes of ErbB1/2 immunoreactivity at individual pY sites. A–E: log2-transformed data for the four individual ErbB1 sites and one compound ErbB2 site, normalized to our Ctrl treatment (5% CO2/22 mM HCO3−), were plotted as a function of time and grouped according to acid-base disturbance. A: MAc. B: MAlk. C: RAc. D: RAlk. E: cRAc. F–J: the same data grouped according to pY site. F: ErbB1-pY845. G: ErbB1-pY992. H: ErbB1-pY1068. I: ErbB1-pY1173. J: ErbB2-pY1221/pY1222. In these analyses, we assume that the pY status at 0 min (i.e., before incubation with each treatment solution) is equivalent to the pY status of that site at 5 or 20 min under Ctrl conditions, where, by definition, log2(pY/actin) = 0. Sample sizes (n) are shown in Figs. 7B, 8B, 10B, and 11B. The upward-sloping or downward-sloping arrows indicate a statistically significant difference between the corresponding values at 5 and 20 min. #Bar for which P < 0.05 (compared with 0 by a one-tailed, paired t-test); in the text, we state that the parameter “tends or tended” to rise/fall. *Bar that is significantly different from 0, even after the very conservative Bonferroni correction (P < 0.05 divided by the number of treatment solutions considered); in the text, we refer to the change as “significant.”
Figure 8A shows the effect of nominally CO2/HCO3−-free treatments on each of the four ErbB1 tyrosine residues (measured from blots such as those shown in Fig. 6). Analysis of these data revealed trends similar to those found in the 5-min data shown in Fig. 7A:
1. Nominally CO2/HCO3−-free EGF tends to increase pY immunoreactivity at pY1173 and significantly at pY992 and pY1068 (HEPES vs. EGF in Fig. 8A).2
2. Nominally CO2/HCO3−-free butyrate treatment at pHo 7.4 does not significantly affect the pY status of any of the four sites.
3. Nominally CO2/HCO3−-free butyrate treatment at pHo 7.1 significantly reduces pY1068 and tends to reduce pY1173 (HEPES vs. butyrate at pHo 7.1 in Fig. 8A).
Figure 8B shows site-specific pY signal intensities (measured from blots such as those shown in Fig. 6) after 20-min treatments in nominally CO2/HCO3−-free HEPES or CO2/HCO3−. Analysis of the data shown in Fig. 8B revealed that the pY status evolved since the 5-min mark (see Fig. 7B):
1. The nominally CO2/HCO3−-free HEPES treatment significantly reduces pY845, and pY992 and tends to reduce pY1068 but not pY1173 (HEPES vs. Ctrl in Fig. 8B), in contrast to 5 min, where pY1173 tends to be reduced (Fig. 7B).
2. RAlk tends to reduce only pY992 (RAlk/Y992 vs. Ctrl/Y992 in Fig. 8B).
3. MAc does not alter the pY status of any of the four sites (MAc vs. Ctrl in Fig. 8B), although it had tended to reduce pY1068 at 5 min.
4. MAlk treatment tends to reduce only pY1068 (MAlk/1068 vs. Ctrl/1068 in Fig. 8B), a pattern different from 5 min.
5. RAc and cRAc now increase or tend to increase pY845 and pY1173, with cRAc-pY845 being the most robust change (cRAc/Y845 vs. Ctrl/Y845 in Fig. 8B).2
In summary, acid-base disturbances at 20 min produce specific effects on pY levels that are different from those at 5 min and are generally more robust.
Fig. 8C shows, for a subset of the data shown in Fig. 8B, the PD-168393 sensitivity of pY at the four ErbB1 residues after 20-min treatments. Analysis of these data revealed that:
1. For almost all conditions, PD-168393 robustly reduces pY levels at all four sites; HEPES-pY1173 and RAlk-pY845 may be special cases, either because of a small number of successful assays or a large variance.2
2. HEPES-pY992 is not significantly altered by PD-168393. Thus, the PD-168393-sensitive kinases responsible for phosphorylating Y992 have become relatively inactive after 20 min in HEPES.
In summary, as at 5 min, the four pY sites exhibit largely independent responses to acid-base disturbances, although the patterns are different from those at 5 min. The ability of PD-168393 to reduce pY levels becomes more robust by 20 min, especially for cRAc (where the effect of PD-168393 was less robust at 5 min).
For ErbB2, the Time Course of pY1221/2 Depends on the Acid-Base Status and Inhibition With PD-168393
General approach.
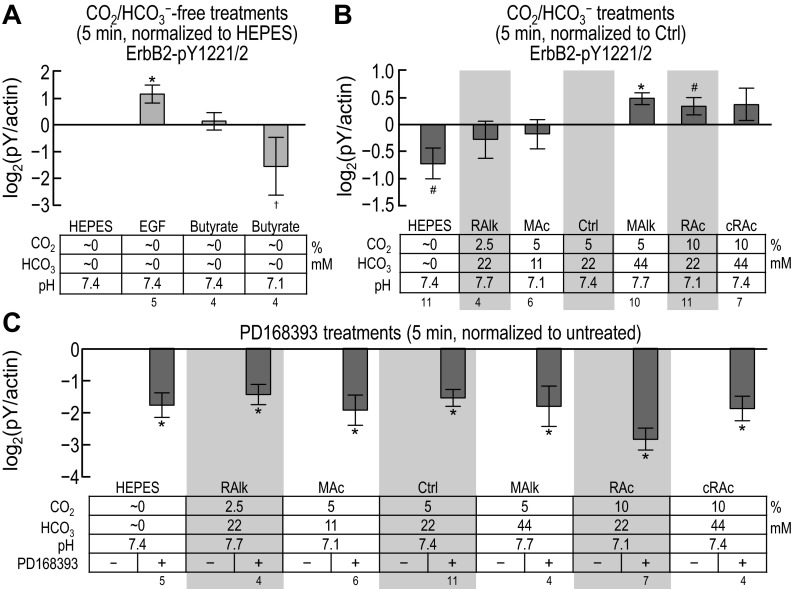
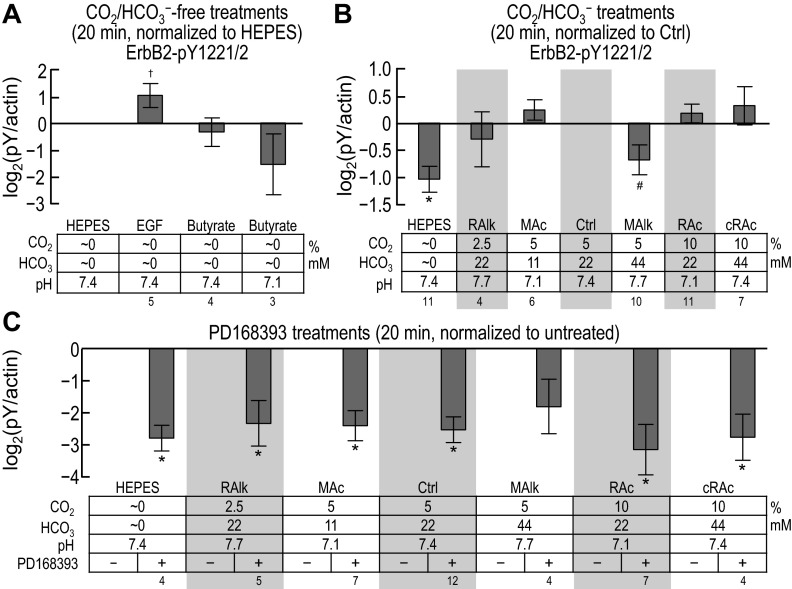
In parallel with the pan-pY and ErbB1-pY analyses shown in Figs. 4–8, we assessed tyrosine phosphorylation for the Y1221/2 compound site of ErbB2. Figure 9 shows representative Western blots probed at 5 and 20 min with a pY1221/2 phosphospecific antibody.
Fig. 9.
ErbB2 immunoreactivity at pY1221/pY1222 in rabbit PT preparations subjected to acid-base disturbances (5 and 20 min). Tubules were exposed to one of several treatment solutions, and protein was subjected to Western blot analysis. For each lane, we show (from top to bottom) the compound pY1221/pY1222 site at 5 and 20 min and a summary of the treatment conditions. Note that the blots on the right repeat some of the conditions on the left. We do not show the reprobes of the blots for actin.
Five-minute treatment.
Figure 10, A–C, shows, for ErbB2 pY1221/pY1222, data (measured from blots such as those shown in Fig. 9) similar to those shown for ErbB1-specific pY sites in Fig. 7, A–C. Note that pY1221/pY1222 significantly rose with nominally CO2/HCO3−-free EGF treatment (Fig. 10A, HEPES vs. EGF), tended to fall in nominally CO2/HCO3−-free HEPES (Fig. 10B, HEPES vs. Ctrl), rose in MAlk, tended to rise in RAc (Fig. 10B, MAlk and RAc vs. Ctrl), and fell in all conditions with PD-168393 (Fig. 10C).
Twenty-minute treatment.
Figure 11, A–C, shows, for ErbB2 pY1221/2, data (measured from blots such as those shown in Fig. 9) similar to those presented for ErbB1-specific pY sites in Fig. 8, A–C. Nominally CO2/HCO3−-free EGF treatment appeared to increase ErbB2 pY1221/pY1222, although with too high a variance to reach significance. Note that, as at 5 min (Fig. 10, B and C), pY1221/pY1222 fell in nominally CO2/HCO3−-free HEPES (Fig. 11B, HEPES vs. Ctrl) and generally fell with exposure to PD-168393 (Fig. 11C). In contrast to the 5-min data, where MAlk increased pY1221/pY1222, after 20 min, MAlk tended to decrease pY1221/2. We provide a comparison of the 5- and 20-min data in the discussion (see ErbB2 data in Fig. 13).
In summary, the pY status of ErbB2 pY1221/pY1222 is responsive to acid-base disturbances, and, as is the case for the ErbB1-specific sites, the pattern evolves between 5 min and 20 min. However, the response pattern of ErbB2 pY1221/pY1222 differs from that of any of the ErbB1-specific sites.
Comparison of CTRL Data at 5 and 20 min
To test whether the PT suspensions were stable between the 5- and 20-min incubations, we performed Western blots on PT aliquots incubated under Ctrl conditions for 5 and 20 min. In tissue from three rabbits, we found no noticeable differences (not shown) between the 5- and 20-min samples, when run side by side, for any of the five pY sites (i.e., 845, 992, 1068, and 1173 on ErbB1 and 1221/1222 on ErbB2). Thus, even 5 min at 37°C was sufficient for PTs to establish a steady state with respect to these pY sites. Indeed, work on isolated perfused rabbit PTs has indicated that 2 min is sufficient for pHi to fully recover from a sizeable acid load (15).
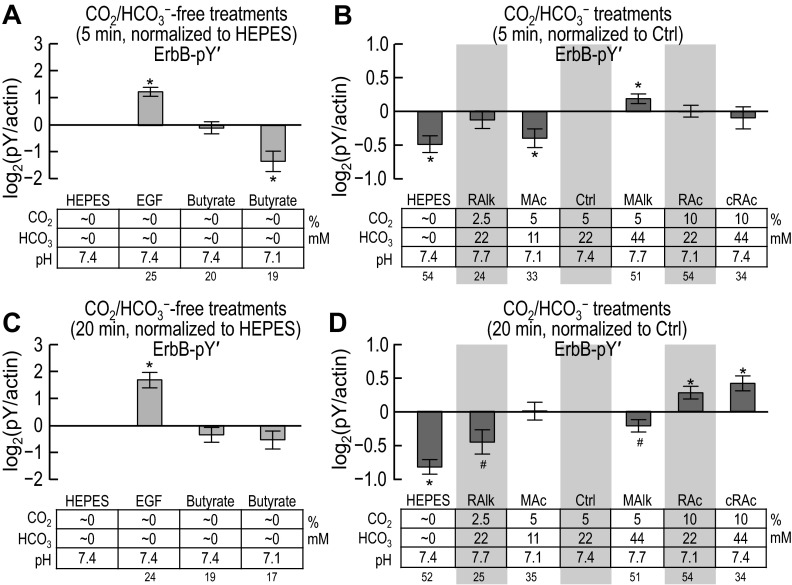
The “Total” ErbB1/2 pY (Pseudo-Pan-ErbB1/2-pY) Profile of PT Suspensions Varies With Acid-Base Disturbance and PD-168393 Treatment
Motivation.
Recall that in the experiments with the pan-pY antibody (shown in Figs. 4 and 5), we found that acid-base disturbances produced characteristic changes in pY levels of proteins near the MWs of ErbB1 and ErbB2. One way of evaluating whether these pan-pY signals do indeed reflect ErbB1/2 is to compare them with the sum of the five site-specific pY signals for ErbB1 and ErbB2 (shown in Figs. 7, 8, 10, and 11). Moreover, if the pan-pY data match up well with the summation data, it would suggest that our analysis of the five pY sites covers a representative portion of the pY space modulated by acid-base disturbances.
General approach.
Adding the responses of ErbB1-pY845, ErbB1-pY992, ErbB1-pY1068, ErbB1-pY1173 (Figs. 7 and 8), and ErbB2-pY1221/2 (Figs. 10 and 11) provides a pseudo-pan-ErbB1/2-pY (ErbB-pY′) response to acid-base disturbances. Figure 12, A–D, shows the results of this analysis. Note that, because of the normalization procedures that we used, the following analysis weights all site-specific antibodies equally.
Five-minute treatment.
The response of ErbB-pY′ to nominally CO2/HCO3−-free EGF and butyrate treatments (Fig. 12A) recapitulated the pan-pY response (Fig. 4A), inasmuch as EGF caused pY to rise (Fig. 4A, HEPES vs. EGF), whereas nominally CO2/HCO3−-free butyrate caused pY to fall at pHo 7.1 but not at pHo 7.4 (Fig. 4A, HEPES vs. butyrate). Similarly, the response of the summated ErbB-pY′ signal to CO2/HCO3−-dependent acid-base disturbances (Fig. 12B) largely recapitulated the pan-pY response, but with greater statistical strength, to those same treatments (Fig. 4B). Specifically:
1. Nominally CO2/HCO3−-free HEPES treatment significantly reduces pY/ErbB (Fig. 12B, HEPES vs. Ctrl).
2. MAc causes ErbB-pY′ to fall (Fig. 12B, MAc vs. Ctrl).2
3. MAlk causes ErbB-pY′ to rise (Fig. 12B, MAlk vs. Ctrl).2
4. RAlk, RAc, and cRAc do not significantly affect ErbB-pY′ (Fig. 12B, RAlk, RAc, or cRAc vs. Ctrl).2
In summary, at 5 min, ErbB-pY′ falls significantly in nominally CO2/HCO3−-free HEPES and MAc but rises in MAlk, consistent with the pan-pY signal at 5 min (Fig. 4).
Twenty-minute treatment.
As at 5 min, the response of ErbB-pY′ at 20 min to nominally CO2/HCO3−-free EGF (Fig. 12C) generally recapitulated that of pan-pY (Fig. 5A). However, different from the pan-pY response, ErbB-pY′ was not significantly affected by nominally CO2/HCO3−-free butyrate treatment at pHo 7.1 (Figs. 12C vs. 5A). The response of ErbB-pY′ signal to CO2/HCO3−-dependent acid-base disturbances (Fig. 12D) generally matched well with the pan-pY response to those same treatments (Fig. 5B). Specifically:
1. Nominally CO2/HCO3−-free HEPES treatment significantly reduces ErbB-pY′ (Fig. 12D, HEPES vs. Ctrl).
2. RAlk tends to decrease ErbB-pY′ (Fig. 12D, RAlk vs. Ctrl).
3. MAc causes no significant alteration of ErbB-pY′ (Fig. 12D, MAc vs. Ctrl).2
4. MAlk tends to decrease ErbB-pY′ (Fig. 12D, MAlk vs. Ctrl).
5. RAc and cRAc treatments significantly increase ErbB-pY′ (Fig. 12D, RAc or cRAc vs. Ctrl).2
One inconsistency with the pan-pY data is the significant increase in ErbB-pY′ with RAc (Fig. 12D, RAc vs. Ctrl), which contrasts with the lack of response to the same treatment in the pan-pY study (Fig. 5B, RAc vs. Ctrl). If our pan-pY and site-specific-pY data and analyses can be taken at face value, we could conclude that RAc must cause a substantial fall in pY at some site other than the five sites that we examined on ErbB1, ErbB2, or in pY on another protein that runs at the same MW. We provide a comparison of the 5- and 20-min data in the discussion (see Fig. 14A).
In summary, at 20 min, ErbB-pY′ falls or tends to fall with nominally CO2/HCO3−-free HEPES, RAlk, and MAlk but rises with RAc and cRAc. We also note that ErbB-pY′ exhibits a different pattern of responses at 5 and 20 min, as we detailed in the discussion.
DISCUSSION
Zhou et al. (107), who flowed OOE CO2/HCO3− solutions over the BL surface of isolated perfused PTs, found that the PT responds to independent changes in [CO2]BL and [HCO3−]BL with compensatory alterations in JHCO3− that would restore normal arterial pH. However, JHCO3− is not responsive to alterations in pHBL per se (107). Later, Zhou et al. (106) found that the ErbB inhibitors BPIQ-I and PD-168393 eliminate JHCO3− responses to alterations in [CO2]BL. These authors proposed that unidentified BL sensors for [CO2]BL and [HCO3−]BL trigger signaling cascades (dependent on receptor tyrosine kinases) that rapidly modulate both brush-border NHE3 and BL NBCe1-A and thereby regulate JHCO3−. Indeed, Quigley and Baum (68) had previously demonstrated that two ErbB1 receptor ligands, EGF and transforming growth factor-α, can each increase JHCO3− (68).
Based on the above discussion, it is reasonable to hypothesize that changes in [CO2]BL or [HCO3−]BL result in time-dependent changes in the pY status of one or more sites (the Y/pY fingerprint) on ErbB family members in the PT. In the present study, we chose to examine ErbB1 and ErbB2 because these are the two most abundant ErbB family members in the adult PT.
Scope of the Study
We chose to work on suspensions of native PTs because these most closely approximate the isolated perfused PTs that we have used in physiological studies. A disadvantage of native tissue is that changes in pY signals will not be as robust as in artificial systems in which ErbB1/2 might be heavily overexpressed. On the other hand, one might question the biological relevance of observations made in such artificial systems.
We worked on total ErbB1/2. It is possible that acid-base disturbances affect the Y/pY status of only a subset of ErbB1/2. If so, our results would underestimate the magnitude of the changes in the Y/pY status in the relevant subpopulation of ErbB1/2.
Of the well-characterized ErbB1 autophosphorylation sites, we focused on three sites involved with major signal transduction pathways and for which reliable antibodies were available: pY992 [which recruits phospolipase C-γ (58, 59)], pY1068 [which recruits growth factor receptor-bound protein 2 (4)], and pY1173 [which recruits Shc and Shp1 (35, 39, 98)]. We unsuccessfully attempted to study pY1045 [which recruits the E3 ubiquitin ligase Cbl (32)] with two different antibodies. We also studied pY845 [which is phosphorylated by Src (5, 88)].
Of the well-characterized sites on ErbB2, we focused on the compound 1221/1222 pY site [which recruits Shc (70)].
We chose to examine our samples at 5 and 20 min because 5 min was the shortest practical time for resuspending PTs and 20 min approximates the duration of experiments on isolated, perfused PTs (105–107).
Although it would have been ideal to use OOE solutions, which allow one to alter [CO2]BL, [HCO3−]BL, or pHBL one at a time, this approach is impractical with PT suspensions. Instead, we examined a series of classical acid-base disturbances, RAlk, MAc, MAlk, RAc, and cRAc, to examine how changes in acid-base parameters affect the five chosen pY sites on ErbB1 and ErbB2.
Effect of Acid-Base Disturbances on the pY Time Course for Individual ErbB1/2
Here, we consider the differences in pY at specific sites as the system moves from a state of having its strongest acid-base correlation with HCO3− (at 5 min) to a state of having its strongest correlation with CO2 (at 20 min).
To examine the dynamic response of a portion of the ErbB1/2 Y/pY fingerprint to changes in the acid-base status, we plotted the log2-transformed data shown in Figs. 7B, 8B, 10B, and 11B versus time in Fig. 13, A–E and F–J. Under Ctrl conditions, log2(pY/actin) = 0. In Fig. 13, A–E, we grouped all pY sites together for each of the acid-base disturbances.
MAc.
The results shown in Fig. 13A (the time course for MAc) revealed that pY1068 tended to be less than Ctrl at 5 min and to rise significantly between 5 and 20 min (indicated by the upward arrowhead).
MAlk.
The results shown in Fig. 13B (the time course for MAlk) revealed a pattern that generally tended to be opposite to that of MAc. After 5 min of MAlk, pY845 tended to be greater than Ctrl, and pY1221/pY1222 was significantly greater. Between 5 and 20 min, pY845, pY1068, and pY1221/2 all fell significantly (downward arrowheads). Finally, at 20 min, pY1068 and pY1221/2 both tended to be less than Ctrl.
RAc.
The results shown in Fig. 13C (the time course of RAc) were distinct from those of either MAc or MAlk. After 5 min of RAc, pY1221/pY1222 tended to be greater than Ctrl. Between 5 and 20 min, pY1173 rose significantly. Finally, at 20 min, pY845 tended to be greater than Ctrl, and pY1173 was significantly greater.
RAlk.
The results shown in Fig. 13D (the time course of RAlk) were distinct from RAc as well as MAc and MAlk. After 5 min of RAlk, pY845 tended to be less than Ctrl, and, after 20 min, pY992 tended to be less than Ctrl.
cRAc.
Figure 13E shows the time course of cRAc, which, in terms of chemistry, has the elevated [CO2] of RAc and the elevated [HCO3−] of MAlk but without the pH changes. The pY response of cRAc was unique but most resembled that of RAc. After 5 min of cRAc, pY992 tended to be less than Ctrl. Between 5 and 20 min, pY992 significantly increased. Finally, after 20 min, pY845 was significantly greater than Ctrl, and pY1173 tended to be greater.
Thus, Fig. 13, A–E, shows that each acid-base disturbance causes a unique pattern of pY changes between 5 and 20 min. In Fig. 13, F–J, we plotted the inverse analysis, grouping all acid-base disturbances (including HEPES) together for each of the pY sites. We found that each pY site has a unique response to each of the acid-base disturbances.
Effect of Acid-Base Disturbances on Summated and Pan-pY Time Courses
As noted in the results, the responses of the summated pY status of the individual ErbB1/2 sites to various acid-base disturbances (ErbB-pY′ in Fig. 12) largely reproduce, in those same PT preparations, the responses of pY180kDa and pY160kDa (Figs. 4 and 5). This result implies that the responses of the ErbB1-pY and ErbB-2 pY sites that we did not examine in the present study generally were either similar to the sites that we did examine or were sufficiently weak so as to not interfere with the overall pattern. The lone exception was the response to RAc, where ErbB-pY′ rose at 20 min (Fig. 12D), whereas pan-pY was unchanged (Fig. 5B). Presumably, RAc led to the dephosphorylation of one or more of the unexplored ErbB1/2 pY sites or of a pY site on another protein of similar MW.
ErbB-pY′.
To assess the overall trends in the cumulative pY status of ErbB1/2 over time, we replotted the log2-transformed ErbB-pY′ data for each acid-base treatment, as shown in Fig. 14A. After 5 min, MAc and MAlk (metabolic-type disturbances) exerted significant but opposite effects on ErbB-pY′. Between 5 and 20 min, we found that ErbB-pY′ significantly rose with MAc, RAc, and cRAc (acidosis-related disturbances) but significantly fell with MAlk and RAlk (alkalosis-related disturbances).
Pan-pY.
Figure 14, B and C, show analyses similar to that shown in A except that we examined pan-pY signals at ∼180 and ∼160 kDa, respectively. The major difference was the lack of a MAlk response at 5 min in the case of pY180kDa.
In summary, the time courses of the summated ErbB-pY′ signals (Fig. 14A) and of the pY180kDa signals (Fig. 14B) and pY160kDa signals (Fig. 14C) are unique for each disturbance. As shown in Fig. 13, A–E, we found that the Y/pY fingerprints for ErbB1/2 (i.e., the collection of pY properties), as far as we have been able to determine them, evolved over time in a unique fashion for each of the five acid-base disturbances. Viewed from the opposite perspective (Fig. 13, F–J), only pY845 exhibited a unique response to each of the acid-base disturbances. For example, the pY992 response was flat for MAc, RAc, and MAlk. In other words, aside from pY845, it takes a combination of pY sites to encode the acid-base disturbances.
PD-168393
PD168393 is a highly specific, potent, and irreversible inhibitor of ErbB1 and ErbB2 (27) that blocks the increase (or decrease) in JHCO3− that normally occurs when isolated perfused PTs are subjected to an increase (or decrease) in [CO2]BL (106). Several interesting insights arose from our results with PD-168393.
First, from our experiments with pan-pY antibodies, the largest PD-168393-induced changes in pY levels occur in the MW range of the ErbB family (i.e., pY180kDa and pY160kDa), and here the drug generally reduces pY160kDa more than pY180kDa (Figs. 4C and 5C). Second, in our experiments with site-specific pY-ErbB antibodies, PD-168393 nearly always produces a significant reduction in pY (Figs. 7C and 8C). From these two sets of observations, we can conclude that a PD-168393-dependent process must maintain the ErbB1/2 pY status across a wide range of acid-base states.
Third, at 5 min, cRAc tends to reduce pY992 (Fig. 7B), but PD-168393 has no effect on cRAc/pY992 (Fig. 7C). At 20 min, nominally CO2/HCO3−-free HEPES reduces pY992 (Fig. 8B), but PD-168393 has no effect on HEPES/pY992 (Fig. 8C). These results are consistent with the hypothesis that the PD-168393-sensitive kinases responsible for the phosphorylation of Y992 have a relatively low activity under conditions of cRAc at 5 min and nominally CO2/HCO3−-free HEPES at 20 min.
Acute Acid-Base Feedback in the PT
The present study may be the first to examine cell signaling in a broad matrix of acid-base conditions (six conditions total: HEPES, RAlk, MAc, Ctrl, MAlk, RAc, and cRAc). Moreover, as far as we are aware, the present data are the first evidence showing that any acute acid-base disturbance can produce a change in any phosphorylation level (specifically pY and specifically on ErbB1/2) in a native tissue. Our work with ErbB1/2 could be an important step in understanding how PT cells transduce acute acid-base disturbances to compensatory changes in acid-base transport.
Any feedback control system requires a sensor, an integrator, and an effector. In the case of the PT, the acid-base effectors include NHE3 at the apical membrane and NBCe1-A at the BL membrane; together, the two transporters produce HCO3− reabsorption. But what are the sensors and integrators?
Acid-base sensors.
Vertebrates have a wide range of mechanisms for sensing changes in acid-base status, and most of these are present in the kidney (for reviews, see Refs. 12, 79, and 89). First, G protein-coupled receptors (GPCRs) [including GPR4 (43, 83), OGR1 (43, 47), and TDAG8 (36)] can sense a decrease in pHo. GPR4 is expressed predominantly in collecting ducts and thick ascending limbs and mediates the pH-sensitive accumulation of cAMP (83). OGR1, which is widely distributed in the mouse nephron, including the PT, acts through phospholipase C (47). Second, several ion channels [including TWIK1 (14, 34), TASK2 (34, 41), ASIC (34, 96), and TRPV1 (34, 95)] sense decreases in pHo. Many are present in the kidney (12, 79, 89), including the PT (12, 79). Third, soluble adenylyl cyclase, which is present in intercalated cells of the collecting duct of the kidney (60), binds cytosolic HCO3− and responds by generating cAMP (16). Fourth, soluble guanylyl cyclases in the olfactory system (GC-D and GC-G) respond to HCO3− by generating cGMP (13, 77, 82). Fifth, the tyrosine kinase Pyk2, which is present in the PT (46), responds to in vitro decreases in pHi with increases in kinase activity (42, 62). Finally, receptor protein tyrosine phosphatase (RPTP)-γ has a ligand-binding domain that is homologous to canonical CAs (3) and is a candidate CO2/HCO3− sensor.
Of the six general mechanisms outlined above, we can rule out, as sensors of acute acid-base challenges in the PT, all those that sense pHo or pHi per se (i.e., GPCRs, ion channels, and Pyk2) because work with OOE solutions has revealed that PTs are sensitive not to pHBL or pHi but to changes in [CO2]BL and [HCO3−]BL per se (107). We can rule out soluble adenylyl cyclase because increases in the intracellular concentration would inhibit, not stimulate, JHCO3− (59). There is no evidence showing that HCO3−-dependent guanylyl cyclases are present in the PT.3 RPTP-γ is an attractive candidate because its mRNA is present in the PT3, and RPTP-γ could potentially influence the ErbB1/2 Y/pY fingerprint and thereby produce rapid changes in JHCO3−.
Integrators.
The present study shows that acid-base disturbances modulate the pY fingerprint of ErbB1/2. Thus, ErbB1/2 and their downstream signaling cascades are in a position to integrate acid-base signals in the PT. Work on OKP cells has revealed that an acute exposure to acidic media (i.e., MAc) rapidly activates Pyk2, which is required for the activation, after a short delay, of Src (42). Independently, acidic media activate the MEK-ERK-c-fos pathway in OKP cells (90). If the work on OKP cells translates to native PTs, which appear not to be intrinsically pH sensitive in the short term, then it is possible that CO2 and HCO3− sensors could lead to the activation of Pyk2 in the PT.
The major observation of the present study is that the four classical acid-base disturbances (RAlk, MAc, MAlk, and RAc) as well as nominally CO2/HCO3−-free HEPES (an extreme cRAlk) and cRAc each produce a distinctive ErbB1/2 Y/pY fingerprint, a pattern of Y/pY at specific sites that evolves over time. This conclusion is corroborated by the time courses of summated ErbB-pY′ as well as pan-pY180kDa and pan-pY160kDa for each of the conditions. At present, we do not know which of these changes in Y/pY status, and at which times, are important for transducing increases in extracellular [CO2] to increases in JHCO3− versus transducing increases in extracellular [HCO3−] to decreases in JHCO3− and so on. However, the PD-168393 sensitivity of changes in both JHCO3− and Y/pY are consistent with the hypothesis that at least some of the Y/pY changes that we report here are likely to be physiologically important. We also note that ErbB1 localizes strongly to the BL membrane, which is in direct contact with the OOE solutions in a physiological study (107) of JHCO3− in the PT.
We caution that, in addition to the five sites already examined, other ErbB1/2 Y/pY sites may be important in responses to acid-base disturbances. A major challenge will be to examine the importance of each site for each disturbance. Given the complexity of Y/pY responses to each acid-base disturbance, we think it unlikely that future research will point to a simple relationship among acid-base disturbances, Y/pY sites, and JHCO3− responses (i.e., that any one Y/pY site would be solely responsible for mediating the response to any one disturbance). Instead, with each disturbance, the time-dependent Y/pY pattern on ErbB1/2 would determine the time-dependent recruitment of diverse signaling proteins containing SH2 and pY-binding domains. We hypothesize that each disturbance, with its unique Y/pY fingerprint, initiates a unique signaling cascade, altering Y/pY, S/pS, and T/pT patterns on other PT proteins, and ultimately changes the activity of NBCe1-A, NHE3, and other transport-related proteins, which leads to the appropriate and observed modification in JHCO3− while stabilizing volume reabsorption (107).
The present work provides the first insights into how ErbB1/2 tyrosine residues are affected by specific acid-base disturbances at 5 or 20 min. We are in a position to begin to dissect the downstream effectors that lead to the HCO3−-reabsorbing machinery itself. In addition, it will be important to identify acid-base sensors upstream to ErbB1/2. Finally, although a major technical challenge, it would be especially powerful to develop approaches for using OOE solutions to address questions of evolving pY status.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant DK81567 and a National Kidney Foundation Postdoctoral Fellowship (to L. A. Skelton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.A.S. and W.F.B. conception and design of research; L.A.S. performed experiments; L.A.S. analyzed data; L.A.S. and W.F.B. interpreted results of experiments; L.A.S. prepared figures; L.A.S. drafted manuscript; L.A.S. and W.F.B. edited and revised manuscript; L.A.S. and W.F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Mark Parker for extraordinarily helpful discussions. The authors also thank Gerald Babcock and Brian Zeise for performing the Pco2 measurements.
Footnotes
Using an arterial blood gas machine (ABL80 Flex Analyzer, Radiometer, Westlake, OH), we assayed the Pco2 in samples taken from four sealed 50-ml, conical-bottom polypropylene tubes that contained 5% CO2/33 mM HCO3−/pH 7.5 solution. The initial Pco2 was 39 ± 1 mmHg (n = 4). We measured Pco2 after incubation for 20 min (39 ± 1 mmHg, n = 4, P = 0.40 by one-tailed paired t-test) or 120 min (39 ± 1 mmHg, n = 4, P = 0.16) at room temperature. These values were indistinguishable, from which we concluded that the polypropylene tubes had a negligible CO2 permeability over the course of 2 h.
A noteworthy item.
National Heart Lung and Blood Institute Proximal Tubule Database (http://helixweb.nih.gov/ESBL/Database/Transcriptomic/PTdatabase.html).
REFERENCES
- 1.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett 410: 83–86, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Alvarado D, Klein DE, Lemmon MA. ErbB2 resembles an autoinhibited invertebrate epidermal growth factor receptor. Nature 461: 287–291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnea G, Silvennoinen O, Shaanan B, Honegger AM, Canoll PD, D'Eustachio P, Morse B, Levy JB, Laforgia S, Huebner K. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTPγ defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol 13: 1497–1506, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batzer AG, Rotin D, Ureña JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol 14: 5192–5201, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274: 8335–8343, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Boedtkjer E, Aalkjær C. Intracellular pH in the resistance vasculature: regulation and functional implications. J Vasc Res 49: 479–496, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Boedtkjer E, Damkier HH, Aalkjær C. NHE1 knockout reduces blood pressure and arterial media/lumen ratio with no effect on resting pHi in the vascular wall. J Physiol 590: 1895–1906, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boron W. Organization of the respiratory system. In: Medical Physiology, edited by Boron W, Boulpaep E. Philadelphia, PA: Saunders Elsevier, 2012, p. 613–629 [Google Scholar]
- 9.Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol 17: 2368–2382, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Bouyer P, Sakai H, Itokawa T, Kawano T, Fulton CM, Boron WF, Insogna KL. Colony-stimulating factor-1 increases osteoclast intracellular pH and promotes survival via the electroneutral Na/HCO3 cotransporter NBCn1. Endocrinology 148: 831–840, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Brazeau P, Gilman A. Effect of plasma CO2 tension on renal tubular reabsorption of bicarbonate. Am J Physiol 175: 33–38, 1953 [DOI] [PubMed] [Google Scholar]
- 12.Brown D, Wagner CA. Molecular mechanisms of acid-base sensing by the kidney. J Am Soc Nephrol 23: 774–780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao YC, Cheng CJ, Hsieh HT, Lin CC, Chen CC, Yang RB. Guanylate cyclase-G, expressed in the Grueneberg ganglion olfactory subsystem, is activated by bicarbonate. Biochem J 432: 267–273, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Chatelain FC, Bichet D, Douguet D, Feliciangeli S, Bendahhou S, Reichold M, Warth R, Barhanin J, Lesage F. TWIK1, a unique background channel with variable ion selectivity. Proc Natl Acad Sci USA 109: 5499–5504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LK, Boron WF. Acid extrusion in S3 segment of rabbit proximal tubule. II. Effect of basolateral CO2/HCO3−. Am J Physiol Renal Fluid Electrolyte Physiol 268: F193–F203, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the herceptin Fab. Nature 421: 756–760, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Cooper GJ, Boron WF. Effect of pCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol Cell Physiol 275: C1481–C1486, 1998 [DOI] [PubMed] [Google Scholar]
- 20.D'Arcangelo D, Gaetano C, Capogrossi MC. Acidification prevents endothelial cell apoptosis by Axl activation. Circ Res 91: e4–12, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Doctor RB, Chen J, Peters LL, Lux SE, Mandel LJ. Distribution of epithelial ankyrin (Ank3) spliceoforms in renal proximal and distal tubules. Am J Physiol Renal Physiol 274: F129–F138, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Dong Z, Wang J, Zhong Q. Postmitochondrial regulation of apoptosis by bicarbonate. Exp Cell Res 288: 301–312, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Dorman PJ, Sullivan WJ, Pitts RF. The renal response to acute respiratory acidosis. J Clin Invest 33: 82–90, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan YX, Wong L, Ding J, Spiridonov NA, Johnson RC, Johnson GR. Mutational activation of ErbB2 reveals a new protein kinase autoinhibition mechanism. J Biol Chem 283: 1588–1596, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell 11: 507–517, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, McNamara DJ, Nelson JM, Sherwood V, Smaill JB, Trumpp-Kallmeyer S, Dobrusin EM. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci USA 95: 12022–12027, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giebisch G, Windhager EE. Urine Concentration and Dilution. In: Medical Physiology: a Cellular and Molecular Approach, edited by Boron WF, Boulpaep EL. Philadelphia, PA: Elsevier Saunders, 2009, p. 835–850 [Google Scholar]
- 29.Gluck SL, Underhill DM, Iyori M, Holliday LS, Kostrominova TY, Lee BS. Physiology and biochemistry of the kidney vacuolar H+-ATPase. Annu Rev Physiol 58: 427–445, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Goldberg R, Reshef-Bankai E, Coleman R, Green J, Maor G. Chronic acidosis-induced growth retardation is mediated by proton-induced expression of Gs protein. J Bone Miner Res 21: 703–713, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem 276: 8358–8363, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Grøvdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res 300: 388–395, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Groves CE, Suhre WB, Cherrington NJ, Wright SH. Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J Pharmacol Exp Ther 316: 743–752, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol: 283–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu JM, Chen CT, Chou CK, Kuo HP, Li LY, Lin CY, Lee HJ, Wang YN, Liu M, Liao HW, Shi B, Lai CC, Bedford MT, Tsai CH, Hung MC. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat Cell Biol 13: 174–181, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem 280: 9083–9087, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Jacobs S, Ruusuvuori E, Sipilä ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynelä J, Praetorius J, Voipio J, Hübner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci USA 105: 311–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci USA 106: 21608–21613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keilhack H, Tenev T, Nyakatura E, Godovac-Zimmermann J, Nielsen L, Seedorf K, Böhmer FD. Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signaling. J Biol Chem 273: 24839–24846, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Kelly T, Church J. Relationships between calcium and pH in the regulation of the slow afterhyperpolarization in cultured rat hippocampal neurons. J Neurophysiol 96: 2342–2353, 2006 [DOI] [PubMed] [Google Scholar]
- 41.L'Hoste S, Barriere H, Belfodil R, Rubera I, Duranton C, Tauc M, Poujeol C, Barhanin J, Poujeol P. Extracellular pH alkalinization by Cl−/HCO3− exchanger is crucial for TASK2 activation by hypotonic shock in proximal cell lines from mouse kidney. Am J Physiol Renal Physiol 292: F628–F638, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–1789, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature 425: 93–98, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Lungkaphin A, Chatsudthipong V, Evans KK, Groves CE, Wright SH, Dantzler WH. Interaction of the metal chelator DMPS with OAT1 and OAT3 in intact isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol 286: F68–F76, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys 58: 903–913, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Mitaka T, Shindoh M, Mochizuki Y, Sasaki H, Ishino M, Matsuya M, Ninomiya T, Sasaki T. Restricted expression of cell adhesion kinase-beta in rat tissues. Am J Pathol 150: 267–281, 1997 [PMC free article] [PubMed] [Google Scholar]
- 47.Mohebbi N, Benabbas C, Vidal S, Daryadel A, Bourgeois S, Velic A, Ludwig MG, Seuwen K, Wagner CA. The proton-activated G protein coupled receptor OGR1 acutely regulates the activity of epithelial proton transport proteins. Cell Physiol Biochem 29: 313–324, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakanishi K, Sweeney W, Jr, Avner ED. Segment-specific c-ErbB2 expression in human autosomal recessive polycystic kidney disease. J Am Soc Nephrol 12: 379–384, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Nakhoul NL, Boron WF. Acetate transport in the S3 segment of the rabbit proximal tubule and its effect on intracellular pH. J Gen Physiol 92: 395–412, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakhoul NL, Chen LK, Boron WF. Effect of basolateral CO2/HCO3− on intracellular pH regulation in the rabbit S3 proximal tubule. J Gen Physiol 102: 1171–1205, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol Cell Physiol 274: C543–C548, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59: 299–331, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 120: 371–383, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nogami M, Yamazaki M, Watanabe H, Okabayashi Y, Kido Y, Kasuga M, Sasaki T, Maehama T, Kanaho Y. Requirement of autophosphorylated tyrosine 992 of EGF receptor and its docking protein phospholipase C gamma 1 for membrane ruffle formation. FEBS Lett 536: 71–76, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366: 2–16, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 110: 775–787, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pastoriza-Munoz E, Harrington RM, Graber ML. Parathyroid hormone decreases HCO3− reabsorption in the rat proximal tubule by stimulating phosphatidylinositol metabolism and inhibiting base exit. J Clin Invest 89: 1485–1495, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HAJ, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Pavlov I, Kaila K, Kullmann DM, Miles R. Cortical inhibition, pH and cell excitability in epilepsy: what are optimal targets for antiepileptic interventions? J Physiol 591: 765–774, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preisig PA. The acid-activated signaling pathway: starting with Pyk2 and ending with increased NHE3 activity. Kidney Int 72: 1324–1329, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 5: 953–962, 1990 [PubMed] [Google Scholar]
- 64.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88: 11110–11114, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prigent SA, Lemoine NR, Hughes CM, Plowman GD, Selden C, Gullick WJ. Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene 7: 1273–1278, 1992 [PubMed] [Google Scholar]
- 66.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Quackenbush J. Microarray data normalization and transformation. Nat Genet 32, Suppl: 496–501, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Quigley R, Baum M. Effects of epidermal growth factor and transforming growth factor-α on rabbit proximal tubule solute transport. Am J Physiol Renal Fluid Electrolyte Physiol 266: F459–F465, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Relman AS, Etsten B, Schwartz WB. The regulation of renal bicarbonate reabsorption by plasma carbon dioxide tension. J Clin Invest 32: 972–978, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ricci A, Lanfrancone L, Chiari R, Belardo G, Pertica C, Natali PG, Pelicci PG, Segatto O. Analysis of protein-protein interactions involved in the activation of the Shc/Grb-2 pathway by the ErbB-2 kinase. Oncogene 11: 1519–1529, 1995 [PubMed] [Google Scholar]
- 71.Riely GJ. The use of first-generation tyrosine kinase inhibitors in patients with NSCLC and somatic EGFR mutations. Lung Cancer 60, Suppl 2: S19–S22, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Rodeheaver DP, Aleo MD, Schnellmann RG. Differences in enzymatic and mechanical isolated rabbit renal proximal tubules: comparison in long-term incubation. In Vitro Cell Dev Biol 26: 898–904, 1990 [DOI] [PubMed] [Google Scholar]
- 73.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature 387: 409–413, 1997 [DOI] [PubMed] [Google Scholar]
- 74.Roos A, Boron WF. Intracellular pH. Physiol Rev 61: 296–434, 1981 [DOI] [PubMed] [Google Scholar]
- 75.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol Renal Physiol 276: F27–F38, 1999 [DOI] [PubMed] [Google Scholar]
- 76.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA 95: 9660–9664, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schulz S, Wedel BJ, Matthews A, Garbers DL. The cloning and expression of a new guanylyl cyclase orphan receptor. J Biol Chem 273: 1032–1037, 1998 [DOI] [PubMed] [Google Scholar]
- 78.Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA 107: 7692–7697, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skelton LA, Boron WF, Zhou Y. Acid-base transport by the renal proximal tubule. J Nephrol 23, Suppl 16: S4–S18, 2010 [PMC free article] [PubMed] [Google Scholar]
- 80.Soleimani M, Grassi SM, Aronson PS. Stoichiometry of HCO3− cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest 79: 1276–1280, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol 185: 236–245, 1998 [DOI] [PubMed] [Google Scholar]
- 82.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA 106: 2041–2046, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, Petrovic S. Deletion of the pH sensor GPR4 decreases renal acid excretion. J Am Soc Nephrol 21: 1745–1755, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sweeney C, Carraway KL., 3rd Ligand discrimination by ErbB receptors: differential signaling through differential phosphorylation site usage. Oncogene 19: 5568–5573, 2000 [DOI] [PubMed] [Google Scholar]
- 85.Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem 284: 20299–20310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, Vaughan-Jones RD. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem 283: 20473–20483, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Taylor R. Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr 6: 35–39, 1990 [Google Scholar]
- 88.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA 96: 1415–1420, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tresguerres M, Buck J, Levin LR. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflügers Arch 460: 953–964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsuganezawa H, Sato S, Yamaji Y, Preisig PA, Moe OW, Alpern RJ. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int 62: 41–50, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol 46: 318–331, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Vega QC, Cochet C, Filhol O, Chang CP, Rhee SG, Gill GN. A site of tyrosine phosphorylation in the C terminus of the epidermal growth factor receptor is required to activate phospholipase C. Mol Cell Biol 12: 128–135, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veikkolainen V, Naillat F, Railo A, Chi L, Manninen A, Hohenstein P, Hastie N, Vainio S, Elenius K. ErbB4 modulates tubular cell polarity and lumen diameter during kidney development. J Am Soc Nephrol 23: 112–122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2). Am J Physiol Cell Physiol 282: C1278–C1289, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Vulcu SD, Liewald JF, Gillen C, Rupp J, Nawrath H. Proton conductance of human transient receptor potential-vanilloid type-1 expressed in oocytes of Xenopus laevis and in Chinese hamster ovary cells. Neuroscience 125: 861–866, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 97.Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol 281: F1117–F1122, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Ward CW, Gough KH, Rashke M, Wan SS, Tribbick G, Wang J. Systematic mapping of potential binding sites for Shc and Grb2 SH2 domains on insulin receptor substrate-1 and the receptors for insulin, epidermal growth factor, platelet-derived growth factor, and fibroblast growth factor. J Biol Chem 271: 5603–5609, 1996 [DOI] [PubMed] [Google Scholar]
- 99.Yamaguchi H, Chang SS, Hsu JL, Hung MC. Signaling cross-talk in the resistance to HER family receptor targeted therapy. Oncogene; 10.1038/onc.2013.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yarden Y, Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry 26: 1434–1442, 1987 [DOI] [PubMed] [Google Scholar]
- 101.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 102.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125: 1137–1149, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Zhao J, Hogan EM, Bevensee MO, Boron WF. Out-of-equilibrium CO2/HCO3− solutions and their use in characterizing a new K/HCO3 cotransporter. Nature 374: 636–639, 1995 [DOI] [PubMed] [Google Scholar]
- 105.Zhao J, Zhou Y, Boron WF. Effect of isolated removal of either basolateral HCO3− or basolateral CO2 on HCO3− reabsorption by rabbit S2 proximal tubule. Am J Physiol Renal Physiol 285: F359–F369, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Zhou Y, Bouyer P, Boron WF. Role of a tyrosine kinase in the CO2-induced stimulation of HCO3− reabsorption by rabbit S2 proximal tubules. Am J Physiol Renal Physiol 291: F358–F367, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Zhou Y, Zhao J, Bouyer P, Boron WF. Evidence from renal proximal tubules that and solute reabsorption are acutely regulated not by pH but by basolateral and CO2. Proc Natl Acad Sci USA 102: 3875–3880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]