Abstract
The purpose of this study was to determine whether duloxetine [a serotonin (5-HT)-norepinephrine reuptake inhibitor] combined with transcutaneous foot stimulation or WAY-100635 (a 5-HT1A antagonist) can enhance inhibition of bladder overactivity in cats. Cystometrograms were performed on eight cats under α-chloralose anesthesia by infusing saline and then 0.25% acetic acid (AA) to induce bladder overactivity. To inhibit bladder overactivity, foot stimulation (5 Hz) was applied via transcutaneous pad electrodes to the right hindfoot at two and four times the threshold intensity for inducing a toe twitch. Duloxetine (0.003–3 mg/kg) was administered intravenously to determine the effect of combination treatment. After the 3 mg/kg dose of duloxetine, WAY-100635 (0.5 mg/kg) was given intravenously. AA irritation significantly (P < 0.0001) reduced bladder capacity to 42.7 ± 7.4% of the saline control capacity. Foot stimulation alone at both two and four times the threshold intensity significantly (P < 0.0001) inhibited bladder overactivity and increased bladder capacity to 66.7 ± 6.3% and 85.7 ± 6.5% of the saline control, respectively. Duloxetine alone dose dependently inhibited bladder overactivity and completely restored bladder capacity to the saline control (109 ± 15.5%) at 3 mg/kg. Although duloxetine combined with foot stimulation did not further enhance inhibition, WAY-100635 (0.5 mg/kg) given after 3 mg/kg duloxetine further increased (P = 0.008) bladder capacity to 162.2 ± 22.5% of the saline control. Although duloxetine and foot stimulation independently inhibited bladder overactivity, combined treatment did not enhance inhibition. Duloxetine combined with WAY-100635, however, synergistically enhanced bladder inhibition, indicating a potential novel treatment for overactive bladder if duloxetine is combined with a 5-HT1A receptor antagonist drug.
Keywords: duloxetine, WAY-100683, bladder, neuromodulation, cat
overactive bladder (OAB) is a syndrome characterized by urinary urgency, frequency, and incontinence, which occurs in 16–17% of the population and is difficult to manage medically (22). Antimuscarinic drugs remain the first line of therapy, but side effects and relatively low efficacy have resulted in a continued search for alternative treatments for OAB (1, 5). Duloxetine [a serotonin (5-HT)-norepinephrine reuptake inhibitor] that is approved in Europe for the treatment of stress urinary incontinence (24) has also shown efficacy for the treatment of OAB symptoms in multiple animal (35) and clinical studies, including a randomized controlled clinical trial of 306 women (8, 29). However, side effects such as nausea, dry mouth, and dizziness resulted in high patient dropout rates, which appeared to be dose dependent (24).
Neuromodulation therapy (sacral or tibial), which is an alternative to drug treatment, is an effective second line option for refractory OAB (25, 37). However, neuromodulation therapies can be invasive, inconvenient, and costly (25, 37). Our previous studies in cats have revealed a novel, noninvasive method to suppress bladder overactivity by stimulating somatic afferent nerves in the foot with skin surface electrodes. Although foot stimulation alone does not completely suppress bladder overactivity (6, 33), we have recently identified another treatment strategy that combines foot stimulation with low doses of drugs such as tolterodine or tramadol to enhance OAB treatment efficacy while lowering the potential for unwanted side effects (18, 27). The additive effect of neuromodulation and drug therapy has also been demonstrated clinically in patients who had incomplete responses to neuromodulation therapy alone (13). Therefore, in this study, we determined if inhibition of bladder overactivity induced by noninvasive foot stimulation might be augmented by the addition of a low dose of duloxetine that is not effective in inhibiting the bladder. Reducing the dose of duloxetine could minimize the side effects and thus reduce the patient dropout rate. We also investigated whether the effect of duloxetine on bladder overactivity can be augmented when combined with the 5-HT1A antagonist WAY-100635 to inhibit 5-HT1A autoreceptors in the raphe nucleus. Previous studies (2, 19) investigating antidepressant effects of 5-HT-norepinephrine reuptake inhibitors have shown that their action on central serotonergic pathways can be amplified when combined with WAY-100635. Because the primary mechanism of action of duloxetine on the bladder is also believed to be through central serotonergic regulation of bladder function (34), we determined if WAY-100635 can enhance the effect of duloxetine on bladder overactivity.
METHODS
All protocols involving the use of animals in the present study were approved by the Animal Care and Use Committee of the University of Pittsburgh.
Experimental setup.
Experiments were performed in a total of eight adult anesthetized cats (five female cats and three male cats between 3.1 and 4.4 kg). Each cat was anesthetized with isoflurane (2–3% in O2) during surgery and then changed to α-chloralose (65 mg/kg, supplemented as necessary) anesthesia during data collection. A pulse oximeter (9847V, Nonin Medical, Plymouth, MN) with the sensor attached to the tongue was used to monitor heart rate and blood O2 saturation. Catheters were inserted in the right cephalic vein and right carotid artery for the intravenous infusion of drugs and monitoring systemic blood pressure, respectively. Airway access was secured with a tracheostomy tube. Ureters were accessed through a midline abdominal incision and drained externally. The bladder was then cannulated with a double lumen catheter through a small cut at the proximal urethra to infuse saline or 0.25% acetic acid (AA) and simultaneously measure bladder pressure. The proximal urethra was tied to prevent leakage. Fur was removed from the right hindfoot, and two self-adhesive pad electrodes (diameter: 1 cm, Grass FE10ND, Astro-Medical, Mentor, OH) were attached to the skin at the bottom of the foot. One electrode was at the front of the foot, and the other was at the heel (6, 33).
Stimulation protocol and drug administration.
Uniphasic rectangular pulses (5-Hz frequency, 0.2-ms pulse width) were delivered to the surface electrodes on the foot. The stimulation intensity threshold was defined as the minimal intensity to induce a toe twitch. Foot stimulation of two and four times the stimulation intensity threshold (2T and 4T, respectively) was used in this study since our previous studies (6, 18, 33) demonstrated that this intensity range was effective in inhibiting reflex bladder contractions.
Initially, the bladder capacity was determined during cystometrograms (CMGs) by slowly infusing the bladder with saline. Multiple CMGs were performed to ensure reproducibility of the saline control capacity. Bladder capacity was defined as the bladder volume threshold required to induce a micturition contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Repeated CMGs were then performed with an infusion of 0.25% AA to irritate the bladder, activate nociceptive bladder afferent C-fibers, and induce bladder overactivity. When the bladder capacity stabilized, the following four CMGs were performed with AA infusion before the administration of duloxetine: 1) control without stimulation, 2) during 2T stimulation, 3) during 4T stimulation, and 4) control without stimulation to determine any poststimulation effect. The bladder was emptied at the end of each CMG, and a 3- to 5-min rest period was inserted between CMGs. After the predrug CMGs were performed, increasing cumulative doses (0.003, 0.01, 0.03, 0.1, 0.3, 1, and 3 mg/kg iv) of duloxetine (Selleck Chemicals, Houston, TX) were given to determine the drug effect on bladder capacity. Ten minutes after the administration of each dose of duloxetine, the four CMGs were again performed with AA infusion under different conditions (control, 2T stimulation, 4T stimulation, and poststimulation control). The four repeated CMGs were completed within 40–60 min.
In four cats, 50–70 min after the maximal dose of duloxetine (3 mg/kg) was given, WAY-100635 (Sigma-Aldrich, St. Louis, MO), a 5-HT1A antagonist, was administered (0.5 mg/kg iv) to block 5-HT1A inhibitory autoreceptors on serotonergic neurons in the raphe nucleus in an attempt to enhance the serotonergic inhibitory influence of duloxetine on bladder reflexes. Five minutes after the administration of WAY-100635, we performed control CMGs without stimulation to examine the combined effect of WAY-100635 and duloxetine on bladder capacity.
Data analysis.
For each CMG, bladder capacity was normalized to the initial saline control capacity in the same animal, which allowed for comparisons between animals. Bladder capacities were averaged for each condition and reported with SEs. Student's t-test, one-way ANOVA followed by Dunnett posttests, or two-way ANOVA followed by Bonferroni posttests was used to determine the statistical significance (P < 0.05).
RESULTS
Suppression of bladder overactivity by foot stimulation.
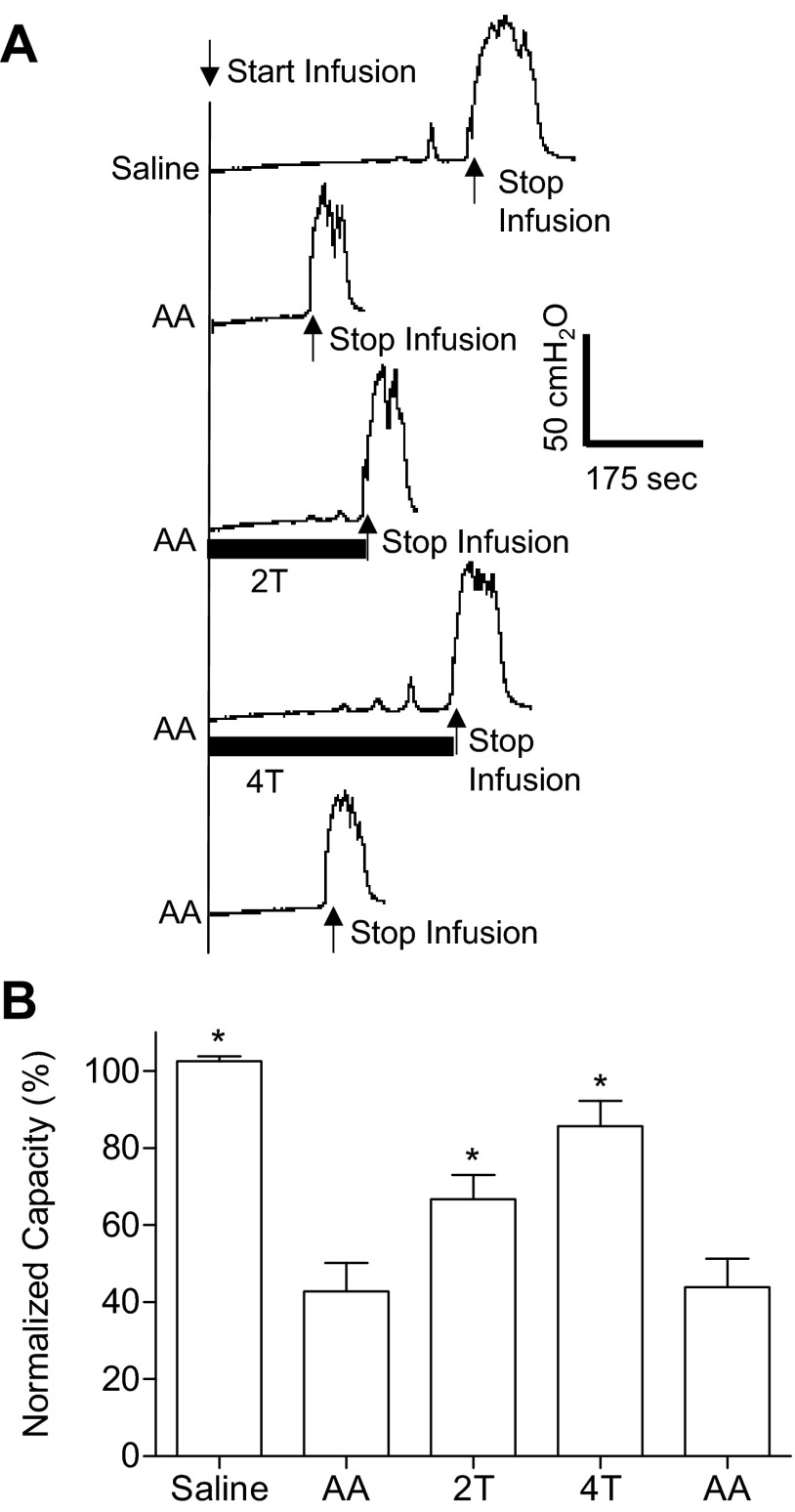
After saline control CMGs were performed, AA-induced bladder irritation significantly (P < 0.0001) reduced bladder capacity to a mean of 42.7 ± 7.4% (5.7 ± 1.5 ml) of the saline control capacity (11.8 ± 1.8 ml; Fig. 1). Foot stimulation significantly (P < 0.0001) inhibited bladder overactivity and increased the capacity to 66.7 ± 6.3% at 2T and 85.7 ± 6.5% at 4T of the saline control. The poststimulation AA control capacity was not different from the prestimulation AA control, demonstrating that there was no poststimulation effect on bladder capacity (Fig. 1).
Fig. 1.
Foot inhibition of bladder overactivity induced by 0.25% acetic acid (AA) irritation before duloxetine treatment. A: cystometrogram (CMG) pressure traces during saline or AA infusion with and without foot stimulation. The infusion rate was 2 ml/min. The solid bars under the bladder pressure traces represent the duration of stimulation. The foot stimulation threshold was defined as the minimal intensity to induce observable toe twitch. 2T and 4T, two and four times the threshold intensity (T). Stimulation: 5 Hz, 0.2 ms; T: 11 V. B: summarized bladder capacity under different CMG conditions (n = 8 cats). Foot stimulation: 5 Hz, 0.2 ms; T: 4–15V. *Significant difference compared with the AA control before stimulation (by one-way ANOVA).
Dose-dependent effect of duloxetine on bladder overactivity with and without stimulation.
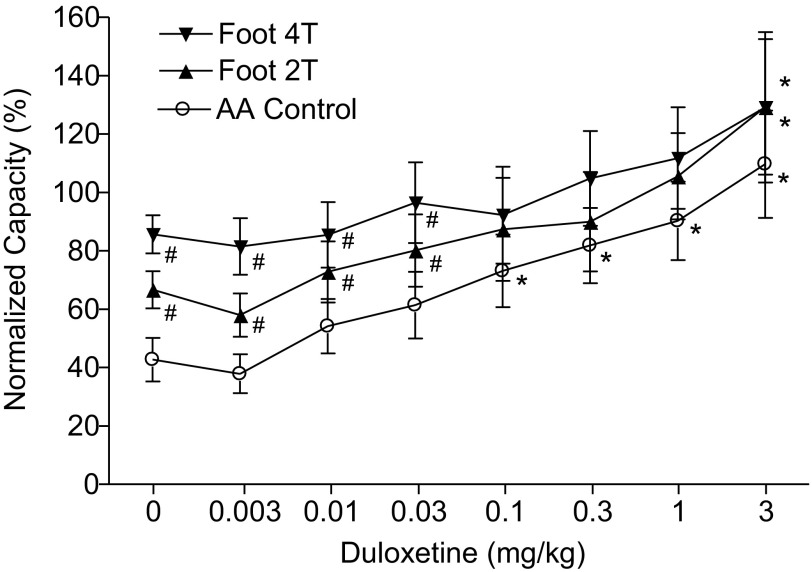
In the absence of stimulation, duloxetine dose dependently and significantly (P < 0.05) increased bladder capacity during AA infusion at doses of 0.1–3 mg/kg and completely removed AA-induced overactivity at 3 mg/kg, increasing bladder capacity to 109 ± 15.5% of the saline control (Figs. 2A and 3). When foot stimulation was combined with duloxetine, the stimulation significantly (P < 0.05) increased the capacity after doses ranging from 0.003 to 0.03 mg/kg but did not significantly increase the capacity after doses between 0.1 and 3 mg/kg (Figs. 2 and 3). After the highest dose (3 mg/kg), 2T and 4T stimulation significantly (P < 0.05) increased bladder capacity to 129.3 ± 23.2% and 129.2 ± 25.8% of the saline control, respectively. After 2T and 4T stimulation in duloxetine-treated animals, bladder capacity returned to prestimulation levels, indicating the absence of a poststimulation effect.
Fig. 2.
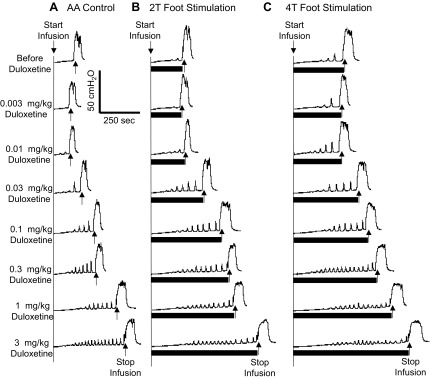
CMG traces showing the dose-dependent effect of a range of intravenous doses of duloxetine and foot inhibition on bladder overactivity caused by AA irritation. CMGs were performed in sequence from left to right in A–C and from top to bottom to examine a possible interaction between duloxetine and foot stimulation. The solid bars under the bladder pressure traces represent the duration of stimulation. A: control CMGs without foot stimulation. B: CMGs during 2T stimulation. C: CMGs during 4T stimulation. Stimulation: 5 Hz, 0.2 ms; T: 11 V. The infusion rate was 2 ml/min.
Fig. 3.
Summarized results of the dose-dependent effect of duloxetine administered intravenously and foot inhibition on bladder overactivity induced by AA irritation (n = 8 cats). Stimulation: 5 Hz, 0.2 ms; T: 4–15 V. #Significantly different from the AA control (by two-way ANOVA); *significantly different from the bladder capacity measured before drug treatment (i.e., at 0 mg/kg of duloxetine) (by one-way ANOVA).
Combined effect of duloxetine and WAY-100635.
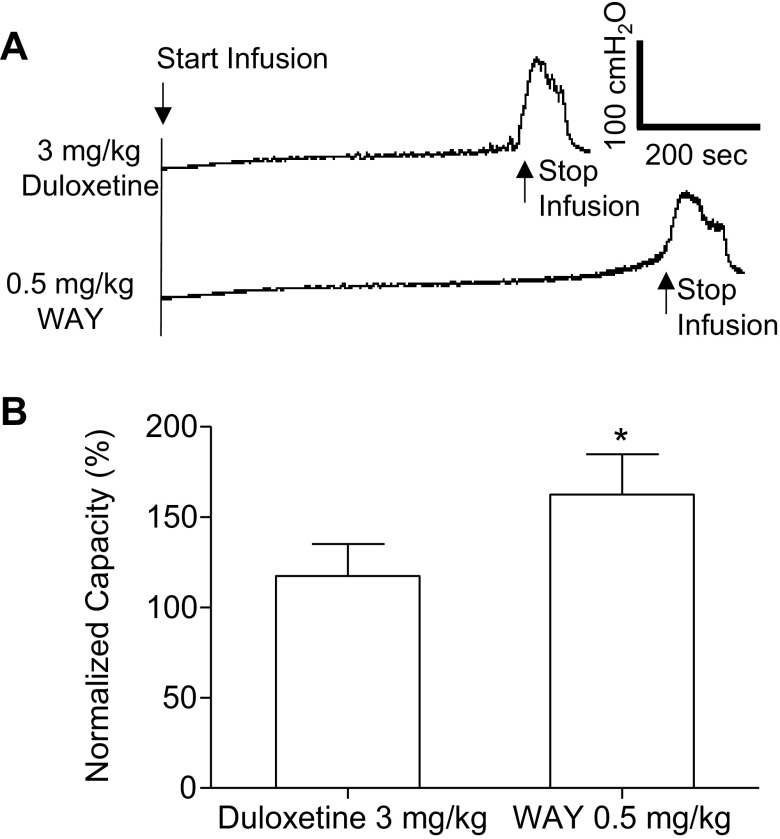
After the final 3 mg/kg dose of duloxetine, intravenous injection of WAY-100635 (0.5 mg/kg), which is a dose that alone does not affect bladder capacity (20, 31, 36), significantly (P = 0.008) increased bladder capacity from a mean of 117.3 ± 17.6% to 162.2 ± 22.5% of the saline control (Fig. 4).
Fig. 4.
Inhibition of bladder overactivity induced by the combination of duloxetine and WAY-100635 (WAY) treatment. A: CMG pressure traces during AA infusion after 3 mg/kg duloxetine and then after 0.5 mg/kg WAY intravenously. B: summarized bladder capacities measured before and after WAY treatment (n = 4 cats). *Significant increase in capacity after the administration of WAY (by Student's t-test).
DISCUSSION
This study demonstrates that foot stimulation is effective in inhibiting bladder overactivity induced by AA irritation (Fig. 1) and that duloxetine alone can also dose dependently increase bladder capacity, completely reversing the overactivity at the highest dose (3 mg/kg; Figs. 2A and 3). However, the combination of duloxetine and foot stimulation did not exhibit a statistically significant additive benefit at increasing doses of duloxetine. Interestingly, the administration of WAY-100635 (a 5-HT1A antagonist) after the highest dose of duloxetine increased bladder capacity an additional 45% (Fig. 4), whereas the same dose of WAY-100636 alone in previous studies (20, 31, 36) did not increase capacity.
Our results with duloxetine alone on bladder overactivity agree very well with those of a previous study (35) showing a significant increase in bladder capacity in a similar AA-induced bladder overactivity model in cats. That study also demonstrated a return to saline control capacity at 3 mg/kg duloxetine (iv). Our observations also correlate with clinical trials testing duloxetine in OAB patients, which showed a significant improvement in overactive symptoms, including a reduction of daily voids, incontinence episodes, and improvement in quality of life scores (8, 29). Duloxetine, a 5-HT-norepinephrine reuptake inhibitor, is thought to elicit bladder inhibition by facilitating central serotonergic inhibitory pathways. This mechanism is supported by the observation that methiothepin, a nonselective 5-HT receptor antagonist, reversed the effect of duloxetine on bladder capacity (35). Therefore, duloxetine, by increasing extracellular 5-HT levels, may augment the function of bulbospinal serotonergic pathways, which have an overall inhibitory effect on bladder activity (7, 21, 30). In addition, because duloxetine only affects bladder capacity during AA irritation and not during saline infusion (34), the inhibition seems to preferentially modulate the nociceptive C-fiber afferent-evoked micturition reflex at the spinal level as opposed to the Aδ-fiber supraspinal micturition pathway, which controls reflex bladder activity under physiological conditions during saline CMGs (10). The inhibition by duloxetine of the nociceptive C-fiber micturition reflex also appears to correlate with its antinociceptive activity in animal models of pain (17, 23) and its clinical efficacy in the treatment of multiple pain syndromes, such as neuropathic pain, fibromyalgia, and osteoarthritis (4, 26, 38). Animal studies (16, 17) have shown that duloxetine can produce analgesia by modulating descending serotonergic and noradrenergic pathways involved in endogenous pain suppression.
Our previous study (32) has shown that opioid receptors play a major role in the inhibition of bladder overactivity by foot stimulation. Tramadol, which has opioid agonist activity, can synergistically enhance foot inhibition and produce a long-lasting poststimulation inhibitory effect (18). However, tramadol also acts as a 5-HT-norepinephrine reuptake inhibitor (15). This raises the possibility that tramadol might also influence foot stimulation by enhancing 5-HT and norepinephrine mechanisms. However, this possibility seems less likely based on our present results showing that duloxetine, a 5-HT-norepinephrine reuptake inhibitor, failed to significantly enhance foot inhibition and did not unmask a poststimulation effect (Fig. 3). This dramatic contrast between the effects of tramadol and duloxetine when combined with foot stimulation suggests that other mechanisms, such as opioid receptor activation, play a major role in foot inhibition of bladder overactivity.
However, even though the effect of foot stimulation on bladder capacity was reduced rather than increased after higher doses (0.1–3 mg/kg) of duloxetine (Fig. 3), this combination experiment where both treatments have an inhibitory effect is complicated and might be open to different interpretations. For example, after doses of duloxetine that significantly increase bladder capacity by enhancing 5-HT levels, it is possible that this action partially occludes a serotonergic mechanism that underlies the effect of foot stimulation. Thus, it is still possible that 5-HT mechanisms may contribute to the inhibition of bladder overactivity by foot stimulation. In addition, duloxetine blocks the reuptake of norepinephrine and enhances noradrenergic synaptic mechanisms, which could also alter the response to foot stimulation. Further studies using selective 5-HT or norepinephrine receptor antagonists are needed to resolve this question.
While duloxetine combined with foot stimulation does not have potential for a clinical application, duloxetine combined with WAY-100635 further increased bladder capacity (Fig. 4). This result suggests that suppressing 5-HT1A receptors can enhance the inhibitory effect of duloxetine on bladder overactivity and might be used to reduce the effective treatment doses of duloxetine to limit both side effects and patient dropout rates. In a large randomized controlled clinical trial investigating duloxetine as a treatment for OAB, 79.1% of treated patients experienced side effects, including nausea (30.7%), dry mouth (16.3%), dizziness (14.4%), and constipation (13.7%), which contributed to a dropout rate of 41% (29). Previous clinical trials of duloxetine demonstrated progressively higher discontinuation rates as the dose increased due to side effects (24). Consequently, lower dropout rates may be achieved by combining a lower dose of duloxetine with a 5-HT1A antagonist drug. While these results are promising, further studies are required to determine the efficacy of low doses of duloxetine in combination with a 5-HT1A antagonist drug for the treatment of bladder overactivity.
It is reasonable to speculate that the additional inhibitory effect on bladder overactivity elicited by WAY-100635 is due to blockade of 5-HT1A autoreceptors expressed by 5-HT neurons in the raphe nucleus, thus enhancing the central serotonergic influence of duloxetine on bladder overactivity. Blockade of these receptors with WAY-100635 removes a negative feedback mechanism and increases the firing of bulbospinal serotonergic neurons (9, 14). In the dorsal raphe nucleus, these 5-HT1A autoreceptors are activated after the administration of 5-HT-norepinephrine reuptake inhibitors, such as duloxetine and venlafaxine, as well as selective 5-HT reuptake inhibitors, resulting in an initial decline in firing due to increased extracellular concentrations of 5-HT caused by reuptake inhibition (2, 3, 11, 12). During continuous administration of 5-HT-norepinephrine reuptake inhibitors, raphe neuron firing remains depressed for months before recovering due to 5-HT1A receptor desensitization (2, 11). Multiple studies (2, 11, 19) have demonstrated that an immediate recovery of raphe neuron firing is elicited in animals treated with 5-HT-norepinephrine reuptake inhibitors. This enhances the central serotonergic drive of duloxetine and likely mediates the enhanced inhibition of bladder overactivity after the administration of WAY-100635 in our experiments. However, other sites of action for the effect of WAY-100635 are also possible because the drug was administered systematically in this study rather than locally in the raphe nucleus.
On the other hand, previous investigations of the functions of 5-HT1A receptors have shown that subcutaneous or intravenous administration of 8-hydroxy-2-di-N-propylaminotetralin (8-OH-DPAT; a 5-HT1A receptor agonist) inhibits bladder activity; this inhibition was reversed by the administration of WAY-100635 (31, 36). However, when WAY-100635 was administered alone, it did not affect bladder overactivity, indicating that 5-HT1A receptors are not tonically active in the micturition reflex pathway (20, 31, 36). 8-OH-DPAT suppresses the micturition reflex in chronic spinal cats (31), indicating that the inhibitory effect is mediated by 5-HT1A receptors located in the spinal cord.
Our recent study (28) has shown that the repeated CMGs as performed in this study are very stable and reproducible. The bladder capacities measured during these repeated CMGs were not changed over seven dosages of vehicle (saline) intravenous injections during a several hour-long experiment (28). Therefore, the effects observed in this study are unlikely to be influenced by variations in bladder capacity during the repeated CMG protocol.
This study demonstrates that 5-HT-norepinephrine mechanisms might play a very limited role in foot inhibition of bladder overactivity, which contrasts with the prominent involvement of opioid mechanisms (6, 18, 33). When duloxetine was combined with the 5-HT1A antagonist WAY-100635, inhibition of bladder overactivity was further enhanced, which reveals a promising synergistic mechanism that could be used for a novel treatment for OAB. Understanding the neurotransmitter mechanisms underlying bladder overactivity and neuromodulation is important for developing new therapies for OAB.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-094905, DK-090006, DK-068566, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. conception and design of research; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; Z.S., Y.M., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urology 63, Suppl 1: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bjorvatn B, Fornal CA, Martin FJ, Metzler CW, Jacobs BL. Venlafaxine and its interaction with WAY 100635: effects on serotonergic unit activity and behavior in cats. Eur J Pharmacol 404: 121–132, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bonnefont J, Chapuy E, Clottes E, Alloui A, Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain 114: 482–890, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chappell AS, Desaiah D, Liu-Seifert H, Zhang S, Skljarevski V, Belenkov Y, Brown JP. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract 11: 33–41, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54: 543–562, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Larson JA, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Post-stimulation inhibitory effect on reflex bladder activity induced by activation of somatic afferent nerves in the foot. J Urol 187: 338–343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SY, Wang SD, Cheng CL, Kuo JS, De Groat WC, Chai CY. Glutamate activation of neurons in CV-reactive areas of cat brain stem affects urinary bladder motility. Am J Physiol Renal Fluid Electrolyte Physiol 265: F520–F529, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Di Rezze S, Frasca V, Inghilleri M, Durastanti V, Cortese A, Giacomelli E, et al. Duloxetine for the treatment of overactive bladder syndrome in multiple sclerosis: a pilot study. Clin Neuropharmacol 35: 231–234, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Fornal CA, Metzler CW, Gallegos RA, Veasey SC, McCreary AC, Jacobs BL. WAY-100635, a potent and selective 5-hydroxytryptamine1A antagonist, increases serotonergic neuronal activity in behaving cats: comparison with (S)-WAY-100135. J Pharmacol Exp Ther 278: 752–762, 1996 [PubMed] [Google Scholar]
- 10.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol 115: 1064–1070, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gartside SE, Umbers V, Sharp T. Inhibition of 5-HT cell firing in the DRN by non-selective 5-HT reuptake inhibitors: studies on the role of 5-HT1A autoreceptors and noradrenergic mechanisms. Psychopharmacology 130: 261–268, 1997 [DOI] [PubMed] [Google Scholar]
- 13.George E, Lane F, Noblett K. Use of combined anticholinergic medication and sacral neuromodulation in the treatment of refractory overactive bladder. Female Pelvic Med Reconst Surg 17: 97–99, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Gobert A, Lejeune F, Rivet JM, Audinot V, Newman-Tancredi A, Millan MJ. Modulation of the activity of central serotoninergic neurons by novel serotonin1A receptor agonists and antagonists: a comparison to adrenergic and dopaminergic neurons in rats. J Pharmacol Exp Ther 273: 1032–1046, 1995 [PubMed] [Google Scholar]
- 15.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 43: 879–923, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther 311: 576–584, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Jones CK, Peters SC, Shannon HE. Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents. J Pharmacol Exp Ther 312: 726–732, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Mally AD, Zhang F, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Combination of foot stimulation and tramadol treatment reverses irritation induced bladder overactivity in cats. J Urol 188: 2426–2432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchand F, Pelissier T, Eschalier A, Ardid D, Alloui A, Soto-Moyano R, Mondaca M, Laurido C, Constandil L, Hernández A. Blockade of supraspinal 5-HT1A receptors potentiates the inhibitory effect of venlafaxine on wind-up activity in mononeuropathic rats. Brain Res 1008: 288–292, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Matsuta Y, Schwen Z, Mally AD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Effect of methysergide on pudendal inhibition of micturition reflex in cats. Exp Neurol 247: 250–258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon SB, Spillane K. Brain stem influences on the parasympathetic supply to the urinary bladder of the cat. Brain Res 234: 237–249, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87: 760–766, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Mixcoatl-Zecuatl T, Jolivalt CG. A spinal mechanism of action for duloxetine in a rat model of painful diabetic neuropathy. Br J Pharmacol 164: 159–169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norton PA, Zinner NR, Yalcin I, Bump RC. Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol 187: 40–48, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1061, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Russell IJ, Mease PJ, Smith TR, Kajdasz DK, Wohlreich MM, Detke MJ, Walker DJ, Chappell AS, Arnold LM. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 136: 432–444, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Schwen Z, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Combination of foot stimulation and tolterodine treatment eliminates bladder overactivity in cats. Neurourol Urodyn; 10.1002/nau.22479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwen Z, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of 5-HT3 receptors in pudendal inhibition of bladder overactivity in cats. Am J Physiol Renal Physiol 305: F663–F671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steers WD, Herschorn S, Kreder KJ, Moore K, Strohbehn K, Yalcin I, Bump RC. Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int 100: 337–345, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Sugaya K, Ogawa Y, Hatano T, Koyama Y, Miyazato T, Oda M. Evidence for involvement of the subcoeruleus nucleus and nucleus raphe magnus in urine storage and penile erection in decerebrate rats. J Urol 159: 2172–2176, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp Neurol 199: 427–437, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Tai C, Ogagan PD, Chen G, Larson JA, Shen B, Wang J, Roppolo JR, de Groat WC. Involvement of opioid receptors in inhibition of bladder overactivity induced by foot stimulation in cats. J Urol 188: 1012–1016, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai C, Shen B, Chen M, Wang J, Liu H, Roppolo JR, de Groat WC. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int 107: 303–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thor KB. Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: implications for treating stress urinary incontinence. Urology 62, Suppl 1: 3–9, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther 274: 1014–1024, 1995 [PubMed] [Google Scholar]
- 36.Thor KB, Katofiasc MA, Danuser H, Springer J, Schaus JM. The role of 5-HT1A receptors in control of lower urinary tract function in cats. Brain Res 946: 290–297, 2002 [DOI] [PubMed] [Google Scholar]
- 37.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama A, Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, Raskin J. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 67: 1411–1420, 2006 [DOI] [PubMed] [Google Scholar]