Abstract
The cation cotransporters Na+-K+-2Cl− cotransporter 1 and 2 (NKCC1 and NKCC2) and Na+-Cl cotransporter (NCC) are phosphorylated and activated by the kinases Ste20-related proline alanine-rich kinase (SPAK) and oxidative stress-responsive kinase (OSR1), and their targeted disruption in mice causes phenotypes resembling the human disorders Bartter syndrome and Gitelman syndrome, reflecting reduced NKCC2 and NCC activity, respectively. We previously cloned a kinase-inactive kidney-specific SPAK isoform, kidney-specific (KS)-SPAK, which lacks the majority of the kinase domain present in full-length SPAK. Another putative inactive SPAK isoform, SPAK2, which only lacks the initial portion of the kinase domain, is also highly expressed in kidney. The functional relevance of inactive SPAK isoforms is unclear. Here, we tested whether KS-SPAK and SPAK2 differentially affect cation cotransporter activity. While KS-SPAK and SPAK2 both strongly inhibited NKCC1 activity, SPAK2 was a much weaker inhibitor of NKCC2 activity. Removal of the catalytic loop from SPAK2 resulted in an inhibitory effect on NKCC2 similar to that of KS-SPAK. Full-length SPAK is phosphorylated and activated by members of the with-no-lysine[K] (WNK) kinase family. Mutation of a WNK phosphorylation in KS-SPAK did not alter its ability to inhibit NKCC2 activity. In contrast, we found that residues involved in KS-SPAK interactions with cation cotransporters are required for it to inhibit cotransporter activity. Finally, both KS-SPAK and SPAK2 associated with NKCC2, as demonstrated by coimmunoprecipitation. Together, these data identify the structural basis for the differential effects of KS-SPAK and SPAK2 on cation cotransporter activity that may be physiologically important.
Keywords: cation cotransporter, kinase, hypertension
ste20- and sps1-related proline alanine-rich kinase (SPAK) and oxidative stress response kinase 1 (OSR1) are closely related members of the STE20 kinase subfamily that regulate renal ion transport. Hypertonic or hypotonic low-chloride conditions activate members of the with-no-lysine[K] (WNK) kinase family (1, 10, 14, 20, 29), leading to phosphorylation of SPAK and OSR1 at two sites, SPAK T243 and OSR1 T185, within the T loop, and at SPAK S383 and OSR1 S325, within the S-motif. These phosphorylation events activate SPAK and OSR1, which are then primed to phosphorylate downstream targets, the best-characterized being members of the cation cotransporter family including the Na+-K+-2Cl− cotransporter 1 (NKCC1, SLC12a2; Refs. 2, 16), the Na+-K+22Cl− cotransporter 2 (NKCC2, SLC12a1), and the Na+-Cl cotransporter (NCC, SLC12a3; Refs. 3, 6, 19). The ubiquitously expressed NKCC1 plays a role in osmoregulation and cellular ion homeostasis, whereas NKCC2 and NCC are expressed exclusively at the apical membrane of the thick ascending limb (TAL) and distal convoluted tubule (DCT), respectively. While these renal segments also express NKCC1, it is only localized to the basolateral membrane. In humans, loss-of-function mutations in NKCC2 cause Bartter syndrome, whereas NCC mutations cause Gitelman syndrome (reviewed in Ref. 23). These two diseases present clinically with hypochloremic metabolic alkalosis, hypokalemia, hypomagnesemia, and normal to low blood pressure, but Bartter syndrome presents with hypercalciuria and Gitelman syndrome with hypocalciuria.
Several mouse models have recently shed light on the physiological roles of SPAK and OSR1 in regulating renal function. Firstly, knockin mice bearing a SPAK mutation in the T loop (T243A), which prevents its activation by WNK kinases (18), display salt-sensitive hypotension accompanied by markedly reduced phosphorylation of both NCC and NKCC2 at SPAK/OSR1 phosphorylation sites (18). Targeted disruption of SPAK leads to a Gitelman-like phenotype (8, 13, 28), with a significant reduction in levels of total and phospho-NCC but an increase in phospho-NKCC2 expression. In contrast, renal epithelia-specific disruption of OSR1 results in a Bartter-like phenotype, with reduced phospho-NKCC2 levels but increased phospho-NCC levels (11). Together, these data suggest that in vivo OSR1 plays a more important role in the regulation of NKCC2 along the TAL, whereas SPAK is the key regulator of NCC.
Following our observation that SPAK disruption increases NKCC2 phosphorylation and activity led, we cloned a novel SPAK isoform highly expressed at the mRNA level in the kidney [kidney-specific (KS)-SPAK]. Immunofluorescence, Western blotting, and coimmunoprecipitation studies revealed that KS-SPAK is more highly expressed along the TAL (13, 22). KS-SPAK lacks the T loop as well as the catalytic site present in full-length (FL)-SPAK and inhibits OSR1-dependent phosphorylation of NKCC2 in vitro (13). We proposed that KS-SPAK and another truncated SPAK isoform, SPAK2, act as inhibitors of FL-SPAK/OSR1 and hence NKKC2 activity along the TAL. Thus, in SPAK knockout mice which lack all SPAK isoforms (13), removal of KS-SPAK and SPAK2 along the TAL disinhibits OSR1 resulting in increased phosphorylation of NKCC2 at SPAK/OSR1 phosphorylation sites. Along the DCT where inhibitory SPAK isoforms are not expressed and FL-SPAK is the key activator of NCC, phospho-NCC levels decrease. Similarly, in the SPAK T243A knockin mouse, all SPAK isoforms expressed are inactive and act as dominant-negative inhibitors of OSR1 along the TAL and DCT, leading to reductions in both phospho-NKCC2 and phospho-NCC (18). In further support of the idea that SPAK isoforms and OSR1 exert segment-specific effects, we recently reported that FL-SPAK rather than OSR1 is the key mediator of vasopressin-mediated NCC activation (22).
Although KS-SPAK inhibits the ability of FL-SPAK or OSR1 to phosphorylate cation cotransporters in vitro, there is some evidence that phosphorylation status might not be an accurate index of NKCC1 and NKCC2 activity (9). Having reported that KS-SPAK inhibits NKCC2 phosphorylation in vitro (13), we wished to explore the functional consequences of this effect on cotransporter activity. It was recently reported that SPAK2 potently inhibits activity of NKCC1 (8), but the effect of this isoform on the activity of NKCC2 has also not been tested. Although the SPAK/OSR1 phosphorylation sites that lead to increased transport activity are conserved between NKCC1 and NKCC2, it is possible that they are differentially regulated by SPAK isoforms. It is also unclear why two inhibitory SPAK isoforms exist. One possibility is that KS-SPAK primarily inhibits NKCC2 at the apical membrane, whereas SPAK2 mainly targets NKCC1 at the basolateral membrane, permitting finer tuning of transepithelial sodium transport. To address these issues, we therefore tested the ability of inhibitory SPAK isoforms to regulate the activities of both NKCC1 and NKCC2, using the well-validated Xenopus laevis oocyte coexpression system.
MATERIALS AND METHODS
DNA constructs.
3xFLAG-GFP-Human NKCC2A in polvector was a kind gift from Biff Forbush, and mouse NKCC1-pSPORT1 and NKCC2F-pSPORT1 were kind gifts from Gerardo Gamba. Wild-type KS-SPAK, full-length SPAK2, and SPAK2 truncation oocyte expression constructs were made by using a mouse FL-SPAK construct (FL-SPAK pMO-myc) we previously generated (13) as a PCR template. PCR products were amplified using Phusion HSII Polymerase (Finnzymes), digested with EcoR, and then cloned into the vector pMO-myc, which had been linearized with EcoRI. Cloning into pMO-myc resulted in the addition of an NH2-terminal myc tag. Following isolation of miniprep DNA, clones with the insert in the correct orientation as determined by restriction digestion underwent DNA sequencing. The following primers were used, with the same reverse primer being used for all constructs. mKS-SPAKF: 5′-CTGAGAATTCGCCCCTGAAGTCATGGAACAGGTGA; mSPAK2F: 5′-TCGAGAATTCGATGAACTCCTGAAAGAAATTCAAGCC; mSPAK2T1F: 5′-GTCAGAATTCGTCAAAGATGAGCTGTGGCTGGTCATG; mSPAK2T2F: 5′-GTCAGAATTCGGAGAACATAAAAATGGTGTCCTAGAAGAGGCG; mSPAK2T3F: 5′-GTCAGAATTCCACAGAAACGGTCAGATCCATAGGGATTTG; mSPAK2T4F: 5′-GTCAGAATTCGGAGTAAGCGCATTCTTAGCCACAGGG; and mSPAKSTOPRRI: 5′-CTGAGAATTCTCAGCTCACACTCAACTGGGCGGAAC.
To generate KS-SPAK mutants, the Quikchange Lightning kit (Stratagene) was used according to the manufacturer's protocol, with the following forward primers (and a reverse complement primer for each): mKS-SPAKS133A: 5′-GCGAGTTCCTGGGTCGGCCGGTCACCTTCATAAG; mKS-SPAKSD238A: 5′-GTTTACTCCAGGAAGAGCTACAGCAGACGGTGTGT; and mKS-SPAKSL252A: 5′-GCTCTTCTCTGCTGGCGCGGTTGACGGCCATGAT.
Capped RNA synthesis.
Templates for in vitro transcription were prepared by restriction digest with PmeI (SPAK isoforms), NheI (NKCC2A), or XbaI (NKCC1 and NKCC2F) followed by phenol:chloroform extraction. Capped RNA (cRNA) for injection into X. laevis oocytes was prepared using the mMessage mMachine T7 polymerase kit (Ambion), according to the manufacturer's protocol, and purified using lithium chloride precipitation. The concentration of cRNA was determined by spectrophotometery, and integrity was confirmed by agarose gel electrophoresis.
86Rubidium uptake assays.
The X. laevis coexpression system was used to determine the activity of endogenous NKCC1, human NKCC2A, or mouse NKCC2F in the absence and presence of different SPAK isoforms. All animal studies were submitted to, and approved by, the Oregon Health and Science University Institutional Review Committee for Animal Welfare. Note that for all uptakes, the data presented are not representative experiments, but rather the combined uptake data from several different frogs, as indicated in figure legends. In each experiment, all injected oocytes for each group were pooled for the uptake experiment. The average uptake per oocyte was calculated, and this value was normalized to the value from water- or cotransporter-only-injected oocytes (set to 100%).
Oocytes were harvested surgically from adult female X. laevis under 0.2% tricaine anesthesia and incubated in ND96 (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES-Tris, pH 7.4) in the presence of collagenase B (2 mg/ml) for 1 h. After four washes in ND96, the oocytes were manually defolliculated and incubated at 18°C in ND96 supplemented with 2.5 mM sodium pyruvate and 5 mg/dl gentamicin overnight. The next day, stage V-VI oocytes were sorted and injected with cRNA as described in the figure legends. While the total amount (in ng) of cRNA varied, in all cases the total volume injected was 50 nl. ND96 was replaced daily for two days before changing to uptake preincubation solutions.
For initial experiments using hypertonic uptake solution, the night before the uptake, injected oocytes were transferred to ND96 Cl− free solution (in mM: 96 Na isethionate, 2 mM K gluconate, 1.8 mM Ca gluconate, 1 mM Mg gluconate, 5 mM HEPES, 2.5 mM Na pyruvate, and 5 mg/dl gentamicin, pH 7.4) 24 h before the uptake assay. Thirty minutes before the addition of uptake medium, the oocytes incubated in Cl− and K+ free-ND96 with inhibitors (1 mM ouabain and 0.1 mM amiloride), according to the protocol of Gamba (7); 1 mM bumetanide was added as indicated to confirm uptake was specifically through NKCC1 or NKCC2. Oocytes were then transferred to hypertonic uptake medium (in mM: 58 NaCl, 38 N-methyl-d-glucamine, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, with inhibitors, pH 7.4, osmolarity increased to 300 mosM by the addition of sucrose) and 5 mM 86RbCl (Rb+ is a congener for K+) and incubated at 30°C for 1 h. The oocytes were washed four times with 1 ml ice-cold uptake medium, lysed in 1% SDS, added to 5 ml of liquid scintillant (Scintiverse, Fisher Scientific), and counted for 1 min in a liquid scintillation counter. Ten to twenty oocytes were analyzed for each uptake condition during each experiment, and the number of each experiment reflects the number of different female X. laevis used to harvest oocytes.
For experiments comparing the effects of tonicity and all other experiments using hypotonic low Cl− uptake medium, a modified version of the protocol of Gamba and colleagues (17) was used. Control groups of oocytes injected with SPAK isoforms and mutants but without NKCC2. The degree of inhibition of uptake in these groups was then added to the uptake in groups injected with SPAK isoforms and mutants coinjected with NKCC2 to adjust for their effects on endogenous NKCC1. For tonicity experiments, at least 30 oocytes were injected per cRNA group, and the night before uptake were divided into groups of 10 for preincubation in the appropriate solution. Preincubation solutions were as follows: hypotonic low Cl− preincubation buffer (170 mosM, in mM: 79 Na isethionate, 2 mM K gluconate, 1.8 mM Ca gluconate, 1 mM Mg gluconate, and 5 mM HEPES, pH 7.4), isotonic ND96, or hypertonic ND96 (340 mosM), made by adding 1 g/50 ml N-methyl d-glucamine (NMDG) to isotonic ND96. The next day uptakes were performed using a basic uptake medium (in mM: 40 NaCl, 10 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, 0.1 amiloride, 1 ouabain, and 2.5 bumetanide as indicated) with 32 mM NMDG (hypotonic, 170 mosM), 51.1 mM NMDG (isotonic, 210 mosM), and 119 mM NMDG (hypertonic, 340 mosM). The pH of each solution was adjusted to pH 7.4, and osmolarity was confirmed using an osmometer. The uptake was then performed with 5 mM 86RbCl as above, except there was no 30 min preincubation in Cl− and K+ free-ND96 with inhibitors before the uptake period, which was for 30 min rather than 1 h.
Oocyte protein extraction for immunoblotting.
For immunoblotting to confirm protein expression, four to five oocytes, selected from those injected with cRNA for uptake experiments, were transferred to Eppendorf tubes and homogenized in 30 μl/oocyte of homogenization buffer [20 mM Tris·HCl pH 7.6, 100 mM NaCl, and 2% NP-40, 10 ul/ml Calbiochem protease inhibitor cocktail 2 (Novagen), 10 μg/ml leupeptin, 0.2 mM sodium orthovanadate, and 43 μg/ml leupeptin] by repeated pipetting. The yolk and cellular debris were pelleted at 3,600 g for 10 min, and the supernatant was centrifuged at 3,600 g four more times to remove additional yolk. All centrifugation steps were performed at 4°C. Oocyte homogenates were stored at −70°C before use.
Coimmunoprecipitation.
Oocytes were injected (25 per group) and incubated as for uptake studies but were maintained in ND96 for the duration of the incubation period (3 days). Protein extraction was performed as for immunoblotting except 50 μl/oocyte of homogenization buffer was used, and extracts were used immediately (with aliquots also stored at −70°C for input immunoblotting). Five-hundred microliters of lysate (equivalent to 10 oocytes) were precleared by incubating with 30 μl of mouse IgG-Agarose (Sigma no. A0919) for 1 h at 4°C in an end-over-end rotator. The precleared lysate was then transferred to a fresh Eppendorf tube and incubated with 30 μl of a 1:1 slurry of anti-FLAG M2 Affinity Gel (Sigma no. A2220)/homogenization buffer overnight at 4°C in an end-over-end rotator. The next day, beads were washed with 1 ml of homogenization buffer for 10 min at 4°C in an end-over-end rotator five times. Precipitated proteins were eluted from the beads by incubating in 25 μl of 2× SDS loading buffer at 95°C for 10 min. The eluate was then analyzed by immunoblotting (see below).
Immunoblotting.
Oocyte lysates were resolved on either 10% Bio-Rad mini-protean gels with a running buffer of 1 g/l SDS, 25 mM Tris, and 192 mM glycine, pH 8.3, or Invitrogen Nupage 10% or 4–12% Bis-Tris gels with Nupage MOPS SDS running buffer. Resolved proteins were transferred to PDVF, 0.2 um (Immobilon-PSQ transfer membrane) with Bio-Rad (25 mM Tris and 192 mM glycine, pH 8.3) or Invitrogen Nupage transfer buffers. For coimmunoprecipitations, the Invitrogen system was used exclusively. For both immunoblotting to confirm expression of injected cRNAs for uptake experiments and coimmunoprecipitations, the amount of lysate used was equivalent to 1/10th of an oocyte (1% of coimmunoprecipitation input). After transfer, membranes were blocked for 1 h at room temperature in 5% fat-free milk powder/phosphate buffered saline/0.1% Tween-20. The following primary antibodies were then used, diluted in blocking buffer: 1:3,500 mouse monoclonal anti-Flag M2 (Sigma F3165) overnight at 4°C, 1:3,000 mouse monoclonal anti-C-myc (Sigma M5546) 1 h at room temperature, and 1:500 rabbit anti-C-SPAK 1 h at room temperature. The affinity-purified rabbit anti-C-SPAK antibody was generated in our laboratory using the same epitope as E. Delpire (16), who kindly gifted the DNA construct to generate the fusion protein. After three 15-min washes in blocking buffer, membranes were incubated with 1:5,000 (in blocking buffer) horseradish peroxidase-conjuated anti-rabbit IgG or anti-mouse IgG secondary antibodies (both Invitrogen) for 1 h at room temperature. After three 15-min washes in blocking buffer, membranes were incubated with Western Lightning ECL (Perkin Elmer) and subjected to autoradiography.
Statistical analysis.
The significance of the differences between groups was tested by one-way ANOVA with multiple comparisons using Bonferroni's correction.
RESULTS
KS-SPAK potently inhibits NKCC1 and NKCC2 activity.
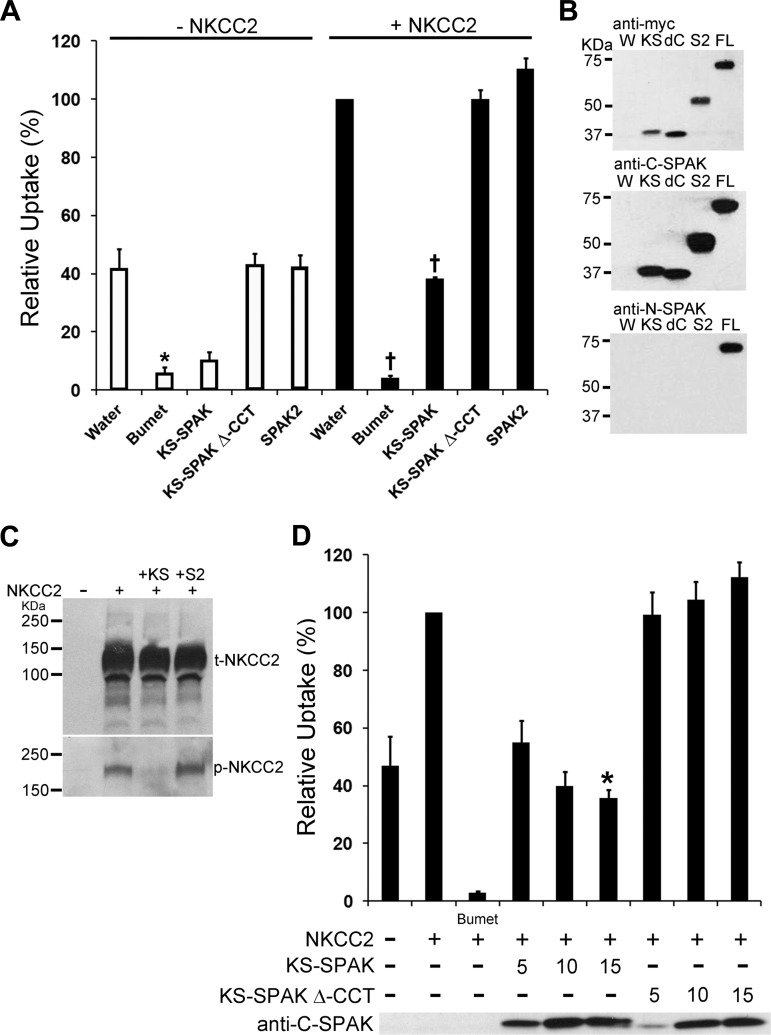
To determine whether the putative inhibitory SPAK isoform KS-SPAK inhibits the activity of NKCC2, 86Rb uptake assays were performed in X. laevis oocytes. In these initial experiments, uptakes were performed under hypertonic conditions (300 mosM), which have been previously reported to activate FL-SPAK and OSR1. In Xenopus oocytes NKCC1 is endogenously expressed and active (24), and OSR1 but not FL-SPAK is also expressed (G. Gamba, personal communication). In the absence of coinjected NKCC2, KS-SPAK inhibited 86Rb uptake via endogenous NKCC1 by 74%, a level similar to that observed following treatment with bumetanide (82%), which specifically inhibits NKCC1 and NKCC2 activity (Fig. 1A). SPAK and OSR1 interact with cation cotransporters through a conserved carboxy-terminal region (CCT; Ref. 26). Deletion of this region (KS-SPAK Δ-CCT) blocked the inhibitory action of KS-SPAK; in addition, SPAK2 exerted no inhibitory effect on endogenous NKCC1 (Fig. 1A). We next examined the effects of inhibitory SPAK isoforms on the activity of coinjected mouse NKCC2. In these experiments, the total 86Rb uptake is the sum of uptake through coinjected NKCC2 and endogenous NKCC1. KS-SPAK inhibited 86Rb uptake when coinjected with NKCC2 by 60% (Fig. 1A). Accounting for the fact that KS-SPAK inhibits endogenous NKCC1, the inhibitory effect of KS-SPAK on 86Rb uptake through NKCC2 is ∼50%, but it did not inhibit uptake to the same extent as bumetanide. KS-SPAK Δ-CCT and SPAK2 had no effect on 86Rb uptakes in oocytes coinjected with NKCC2 under these conditions and Western blotting confirmed that this was not due to differences in expression (Fig. 1B). The inhibitory effect of KS-SPAK on NKCC2 activity correlated with a reduction in phospho-NKCC2, but not in total NKCC2, as shown by Western blotting (Fig. 1C). Consistent with its relatively weak effect on NKCC2 activity, SPAK2 had no obvious effect on phospho-NKCC2 levels. Titration of KS-SPAK revealed that its inhibitory effect appears to be dose-dependent (Fig. 1D).
Fig. 1.
Kidney-specific (KS)-Ste20-related proline alanine-rich kinase (SPAK) inhibits cation cotransporter activity in Xenopus oocytes. A: 13–14 oocytes per group were injected with water or 10 ng of each capped RNA (cRNA) as shown. After 3 days 86Rb uptake was measured for pooled groups of oocytes under hypertonic (300 mosM) conditions. Data are expressed relative to water-injected oocytes (100%) ± SE. Water-injected oocytes treated with 1 mM bumetanide (Bumet) confirmed that the uptake in the water-injected group was through endogenous Na+-K+-2Cl− cotransporter 1 (NKCC1) or coinjected NKCC1 or NKCC2. Deletion of the conserved carboxy terminus (CCT), required for interactions with both cation cotransporters and with-no-lysine[K] (WNK) kinases, prevented inhibition of uptake by KS-SPAK (KS-SPAK Δ-CCT). *P < 0.001, compared with water injected; †P < 0.001, compared with NKCC2 only-injected; n = 4 (different frogs). B: Western blotting confirmed expression of the appropriate SPAK isoforms. W, water injected; KS, KS-SPAK; dC, KS-SPAK Δ-CCT; S2, SPAK2; FL, FL-SPAK. All isoforms have an NH2-terminal myc tag (top); each SPAK possesses the SPAK COOH terminus (middle). Anti-N-SPAK confirmed that only FL-SPAK has the SPAK NH2 terminus. C: immunoblotting showed that KS-SPAK, but not SPAK2, reduced phosphorylation of NKCC2 at the SPAK/OSR1 phosphorylation sites T95/T100 (human numbering), without an effect on total NKCC2 expression levels. D: titration of KS-SPAK revealed a dose-dependent inhibitory effect of KS-SPAK and confirmed the requirement for the Δ-CCT. *P = 0.05, compared with 5 ng KS-SPAK; n = 4 (different frogs).
SPAK2 is a relatively weak inhibitor of NKCC1 and NKCC2 activity.
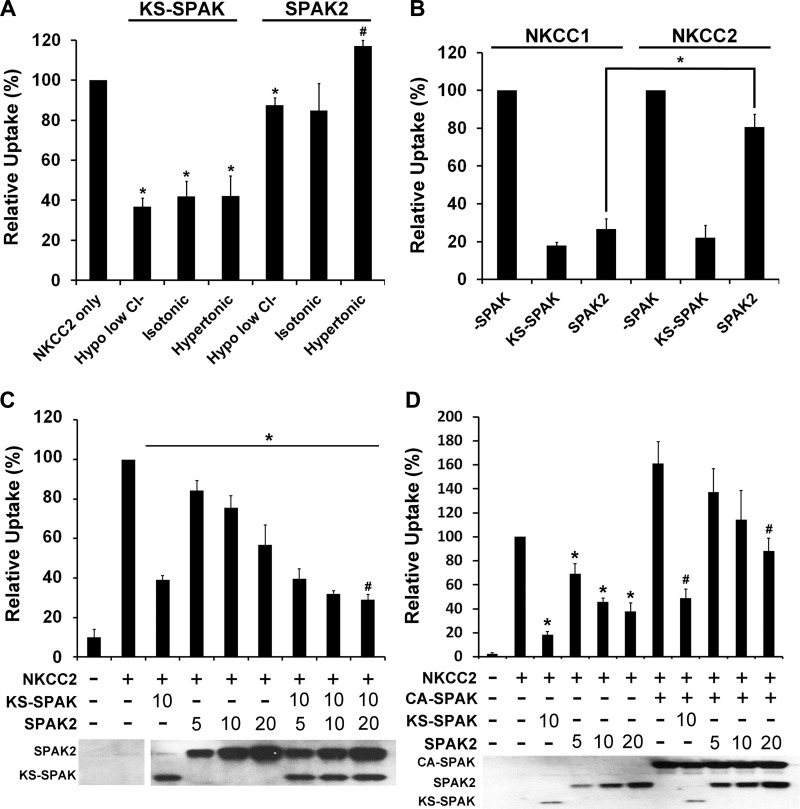
As described above, KS-SPAK clearly inhibited NKCC2 activity, but SPAK2 had no effect on the activities of endogenous NKCC1 or coinjected mouse NKCC2 (Fig. 1A) under hypertonic conditions. These data are in contrast to those recently reported by Grimm et al. (8), who reported that SPAK2 inhibited activity of coinjected NKCC1 and that this observation could be extrapolated to NKCC2 due to its similarity to NKCC1. However, there is evidence suggesting that NKCC1 and NKCC2 are differentially regulated by the ionic milieu (9) as well as by phosphatase inhibitors (9). Since our data indicate that the lack of effect of SPAK2 was not due to a problem with expression (Fig. 1B), we considered that the differences in findings may be due to the uptake conditions used. To examine this, we next performed 86Rb uptakes in oocytes at different tonicities: hypotonic low Cl− (170 mosM), isotonic (210 mosM), and hypertonic (340 mosM) conditions (all relative to normal oocyte tonicity). For each condition, oocytes were injected with the relevant cRNAs as a large batch then separated into groups the night before the uptake for preincubation in the appropriate solution; this avoids variability due to differences in cRNA injection. The preincubation and uptake conditions used precisely followed those described in (17), with the exception that a hypertonic preincubation solution (340 mosM) was prepared by adding NMDG to isotonic ND96. The uptake solutions used were identical in all respects except tonicity, which was also varied by adding NMDG. Under these very carefully controlled conditions, KS-SPAK consistently inhibited NKCC2 activity by 58–63% under all tonicities (Fig. 2A). In contrast, SPAK2 mildly but significantly inhibited NKCC2 activity under hypotonic low Cl− conditions, which maximally activate FL-SPAK and OSR1. The level of inhibition of 86Rb uptake was only 13% for SPAK2 vs. 63% inhibition for KS-SPAK under these conditions (P < 0.005). Under isotonic conditions there was a nonsignificant trend to lower NKCC2 activity in SPAK2 coinjected oocytes. Under the hypertonic conditions used in this study, SPAK2 exerted a mild stimulatory effect on 86Rb uptake (17% higher than NKCC2 only, P < 0.005), although this effect was not observed in a subsequent set of experiments performed under the same condition (Fig. 2C).
Fig. 2.
SPAK2 is a strong inhibitor of NKCC1 but a relatively weak inhibitor of NKCC2. A–D: data are expressed relative to uptake by NKCC2-injected oocytes, set to 100% for each condition ± SE; n = 4–5 (different frogs). Uptake by water-injected oocytes was only 3–17% of that of oocytes injected with NKCC2 alone; bumetanide inhibited uptake by oocytes injected with NKCC2 alone by 90–96% (data not shown), confirming that the majority of uptake is through injected NKCC2 (A) At least 30 oocytes were injected as indicated with 20 ng of NKCC2 and 10 ng of KS-SPAK or SPAK2 cRNAs used. In this experiment, the preincubation and uptake conditions differ from those used in Fig. 1 (see materials and methods). The night before the uptake experiment, each injection group was divided into groups of 10 and preincubated in the appropriate solution. 86Rb uptake was then measured for the pooled groups of oocytes in the appropriate uptake medium. *P ≤ 0.01, lower activity, and #P < 0.01, higher activity, compared with the NKCC2-injected group at the same tonicity. B: 10–20 oocytes were injected as indicated with 20 ng of NKCC1 or NKCC2 cRNA and 10 ng of KS-SPAK or SPAK2 cRNA. 86Rb uptake was performed after 3 days under hypotonic conditions, following an overnight preincubation in hypotonic low Cl− solution. *P ≤ 0.001, lower degree of inhibition of uptake by SPAK2 in NKCC2- than in NKCC1-coinjected oocytes. C: 12–20 oocytes were injected with 20 ng of NKCC2 cRNA and KS-SPAK and/or SPAK2 cRNA (in ng) as indicated. 86Rb uptake was performed after 3 days under hypertonic conditions, following an overnight preincubation in hypertonic solution. *P < 0.01, lower uptake compared with oocytes injected with NKCC2 only; #P < 0.05, higher uptake by oocytes injected with NKCC2, KS-SPAK and SPAK2 (20 ng), compared with water-injected oocytes. D: 10–22 oocytes were injected with 20 ng of NKCC2 cRNA and constitutively active FL-SPAK (CA-SPAK), KS-SPAK and/or SPAK2 cRNA (in ng) as indicated. 86Rb uptake was performed after 3 days under hypotonic conditions, following an overnight preincubation in hypotonic low Cl− solution. *P < 0.001, lower uptake compared with oocytes injected with NKCC2 only; #P < 0.05, lower uptake by oocytes injected with NKCC2 and CA-SPAK with either KS-SPAK (10 ng) or SPAK2 (20 ng), compared with oocytes injected with NKCC2 and CA-SPAK only. C and D, bottom: representative Western blots confirming expression of SPAK isoforms.
Since it was reported that SPAK2 strongly inhibits NKCC1 activity (8), we sought to determine whether the effects of KS-SPAK and SPAK2 on oocytes injected with NKCC1 or NKCC2 differed under hypotonic low Cl− conditions. KS-SPAK inhibited uptake by both NKCC1 and NKCC2 to a similar degree (82 and 78%, respectively; Fig. 2B). In contrast, SPAK2 exerted a significantly greater effect on NKCC1 (73% inhibition of uptake) than on NKCC2 (19% inhibition); the degree of inhibition of NKCC1 by KS-SPAK or SPAK2 did not differ significantly. The effect of SPAK2 on NKCC1 activity was thus broadly in agreement with the findings of Grimm et al. (8), but our data suggest that effects on NKCC1 cannot be extrapolated to NKCC2. SPAK2 appeared to stimulate NKCC2 activity under hypertonic conditions (Fig. 2A), so we next determined the effect of coinjecting both KS-SPAK and SPAK2 on NKCC2 activity under the same conditions (Fig. 2C). The amount of KS-SPAK was fixed at 10 ng, while SPAK2 was titrated from 5 to 20 ng. In this set of experiments, SPAK2 (in the absence of KS-SPAK) did not stimulate NKCC2 activity but had an inhibitory effect at all doses that was greatest at 20 ng (43% inhibition). The reasons for this discrepancy are unclear, but most likely reflect differences between batches of oocytes. Overall, however, these data were consistent with the idea that SPAK2 has a relatively mild effect on NKCC2 activity. Coinjection of SPAK2 with KS-SPAK did not reduce NKCC2 activity further (KS-SPAK in the absence of SPAK2, 61% inhibition, KS-SPAK with 20 ng SPAK2, 71% inhibition, P = 0.32), and activity was still significantly higher than in oocytes injected with water only (P < 0.05). One possibility is that this residual NKCC2 activity that cannot be inhibited by KS-SPAK and SPAK2 represents NKCC2 activity regulated by SPAK/OSR1-independent pathways (21). To begin to determine the mechanism by which KS-SPAK and SPAK2 inhibit NKCC2 activity, we next examined their effect on FL-SPAK-stimulated uptake under hypotonic low Cl− conditions (Fig. 2D). In pilot studies, wild type FL-SPAK did not have a significant effect on uptake (data not shown), in agreement with previous reports, so a constitutively active mutant (CA-SPAK, T243E/S383D) was used. This mutant stimulated uptake by 61% (P = 0.005). Titration of SPAK2 in the absence of CA-SPAK resulted in a dose-dependent inhibition of uptake (increasing from 31–62% inhibition), which was lower than the amount of inhibition achieved by 10 ng KS-SPAK (82%). When CA-SPAK was coinjected, the degree of inhibition was reduced for both KS-SPAK and SPAK2, with 5 or 10 ng SPAK2 no longer significantly inhibiting 86Rb uptake. In the presence of CA-SPAK, inhibition by 10 ng SPAK2 was 25% lower than in its absence (P < 0.05); for 10 ng KS-SPAK, this difference was 12% (P < 0.05). With 5 or 20 ng SPAK2, there was a similar trend, which did quite reach significance (P < 0.2 for each). Coinjection of KS-SPAK or SPAK2 with CA-SPAK increases the amount of active SPAK/OSR1 in the oocytes, which most likely out competes the inhibitory isoforms for NKCC2 binding, resulting in less inhibition of uptake. For the weaker inhibitor, SPAK2, a significant inhibitory effect was only observed when 20 ng were coinjected; 10 ng of KS-SPAK still significantly inhibited uptake, albeit more weakly.
Deletion of the SPAK2 catalytic loop enhances its inhibitory effect on NKCC2.
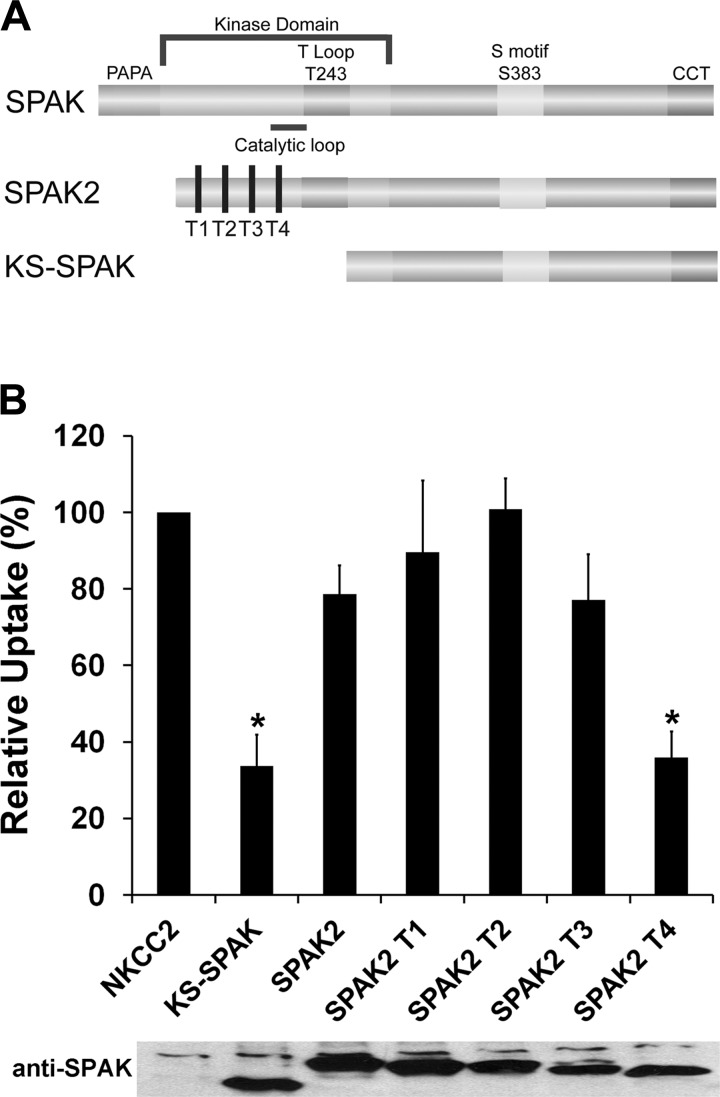
Since SPAK2 exerts a relatively weak inhibitory effect on the activity of NKCC2 compared with KS-SPAK, we attempted to identify possible differences between the two isoforms that might explain this. A truncation series of SPAK2 was generated, with each truncation removing 27–28 amino acids (Fig. 3A). 86Rb uptake of oocytes coinjected with NKCC2 and SPAK2 truncations T1-T3 did not differ significantly from that of oocytes injected with NKCC2 and full-length SPAK2 (Fig. 3B). However, uptakes by oocytes injected with NKCC2 and SPAK2 T4 or KS-SPAK were significantly lower than those by oocytes coinjected with NKCC2 and SPAK2 (KS-SPAK, 66 ± 8% lower than SPAK2, P = 0.001; SPAK2 T4 64 ± 7% lower than SPAK2, P < 0.001). Thus T4 appears to behave identically to KS-SPAK with regards to inhibition of NKCC2. Generation of T4 removed the catalytic loop that is present in SPAK2 and also in FL-SPAK but is absent from KS-SPAK. These data suggest that the catalytic loop might play a role in limiting the inhibitory effect of SPAK2 on NKCC2.
Fig. 3.
Deletion of the SPAK2 catalytic loop enhances its inhibitory effect on cation cotransporter activity. A: schematic of SPAK isoforms and SPAK2 truncations. The NH2 terminus of SPAK2 truncations used in the experiments presented in B are shown by vertical black bars (T1-T4); note that the truncation from T1 to T4 removes the catalytic loop, and that SPAK2 T4 is still 28 amino acids longer than KS-SPAK. B: 15–20 oocytes were injected with 20 ng of NKCC2 cRNA and with 10 ng of KS-SPAK, SPAK2, or SPAK2 truncations (T1-T4) cRNA, as indicated. 86Rb uptake was performed after 3 days under hypotonic low Cl− conditions. A representative immunoblot using an antibody against the COOH terminus of SPAK is shown, confirming similar expression levels. Data are expressed relative to NKCC2-injected oocytes (100%) ± SE; n = 4–5 (different frogs); *P < 0.01, lower activity compared with the SPAK2-injected group. Uptake by water-injected oocytes was only 15% of that by NKCC2-injected oocytes, and bumetanide inhibited uptake by NKCC2-injected oocytes by 85% (data not shown).
Inhibition of NKCC2 by KS-SPAK is independent of its phosphorylation status but requires residues involved in its interaction with cation cotransporters.
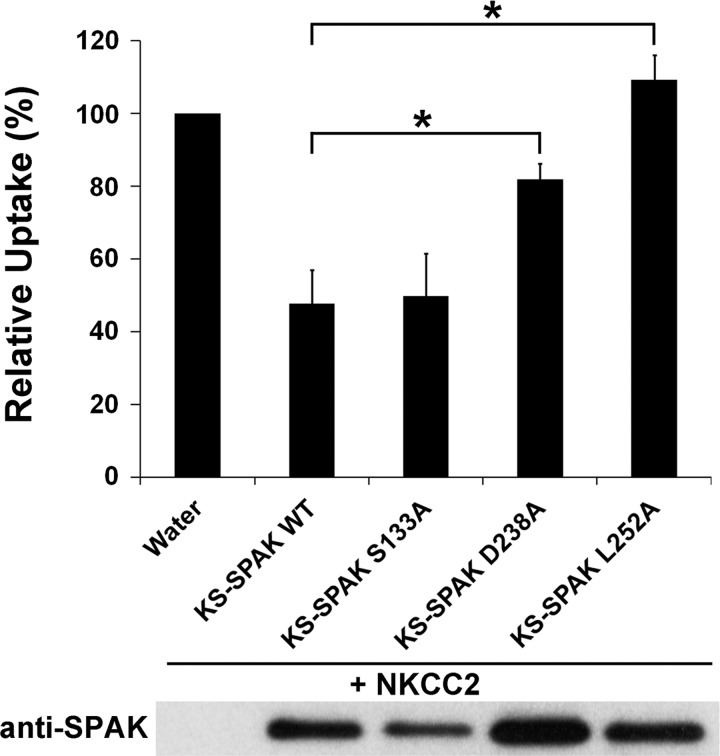
Next, we tested the effects of mutating several KS-SPAK residues that have been previously reported to play important roles in the function of FL-SPAK. FL-SPAK can be phosphorylated by WNK kinases at two residues, T243 and S383, which results in its activation; phosphomimetic mutations (T243E/S383D) result in constitutive activity of the kinase (5). KS-SPAK lacks the residue equivalent to T243 since the KS-SPAK START codon is M251 in FL-SPAK. The homologous site to S383 in KS-SPAK, S133, was mutated this residue to an alanine (S133A) by site-directed mutagenesis. This mutant allowed us to determine whether the inhibitory effect of KS-SPAK requires prior phosphorylation by FL-SPAK or OSR1. Under hypotonic low Cl− conditions, KS-SPAK S133A displayed an inhibitory effect on NKCC2 indistinguishable to that of wild-type KS-SPAK (KS-SPAK WT, 52% ± 9% inhibition, P < 0.001; KS-SPAK S133A 50% ± 11% inhibition, P = 0.002 vs. NKCC2 only; KS-SPAK WT vs. KS-SPAK S133A, P = 0.89; Fig. 4), suggesting the effect of KS-SPAK is independent of its phosphorylation status at this site.
Fig. 4.
KS-SPAK inhibition of cation cotransporter activity is independent of its phosphorylation, but requires specific residues required for protein-protein interactions. 10–20 oocytes were injected as indicated with 20 ng of NKCC2 cRNA and 10 ng of wild-type (WT) or mutant KS-SPAK cRNA. 86Rb uptake was performed after 3 days under hypotonic conditions, following an overnight preincubation in hypotonic low Cl− solution. KS-SPAK S133A is mutated at a residue equivalent to S383 in full-length SPAK, which following phosphorylation by WNK kinase, leads to its activation. The residues in full-length SPAK equivalent to KS-SPAK residues D238 and L252 have been reported to play major roles its interactions with both NKCC1 and WNK kinase. Data are expressed relative to NKCC2-injected oocytes (100%) ± SE; n = 5 (different frogs); *P < 0.05, lower activity compared with the NKCC2-injected group. Uptake by water-injected oocytes was only 20% of that by NKCC2-injected oocytes, and bumetanide inhibited uptake by NKCC2-injected oocytes by 85% (data not shown). A representative immunoblot using an antibody against the COOH terminus of SPAK is shown to confirm expression.
We next sought to examine the roles of individual residues important for mediating interactions between FL-SPAK and NKCC1 and WNK kinases (26) in the inhibitory effect of KS-SPAK. We selected two residues (D479 and L493, human FL-SPAK numbering, D488 and L502, mouse FL-SPAK numbering) that showed the greatest reduction in binding to both WNK1 or NKCC1 for mutagenesis studies to allow a more refined approach compared with complete deletion of the CCT (see KS-SPAK Δ-CCT; Fig. 1). Note that these residues lie upstream of the CCT. Under hypotonic low Cl− conditions, mutation of KS-SPAK D238 (equivalent to D488 in mouse FL-SPAK) greatly reduced inhibition of NKCC2 activity (KS-SPAK WT 52% ± 9% inhibition, P < 0.001; KS-SPAK D238A 20% ± 5% inhibition, P < 0.05; KS-SPAK WT vs. KS-SPAK D238A, P < 0.01). Mutation of KS-SPAK L252 (equivalent to L502 in mouse FL-SPAK) completely abolished the ability of KS-SPAK to inhibit 86Rb uptake. These data provide further evidence that the inhibitory effect of KS-SPAK on cation cotransporter activity involves protein-protein interactions between them.
KS-SPAK and SPAK2 interact with NKCC2.
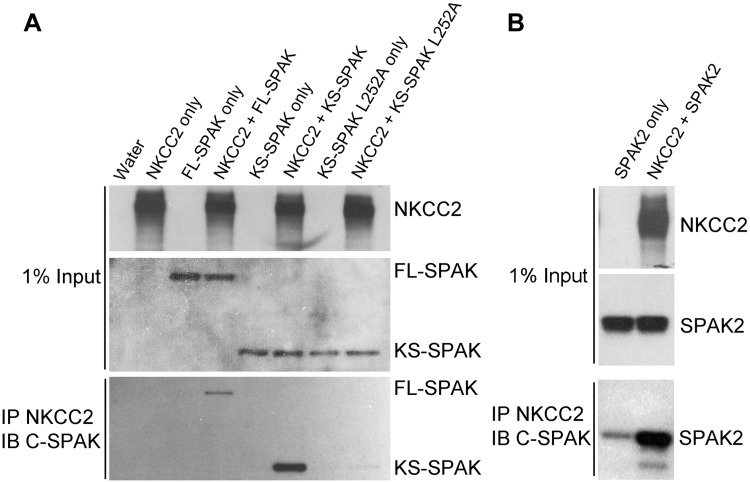
Since mutation of KS-SPAK L252, a site previously reported to play an important role in mediating interactions between NKCC1 and WNK1, prevented KS-SPAK from inhibiting 86Rb uptake in NKCC2-injected oocytes (Fig. 5), these data suggest that the mechanism by which KS-SPAK inhibits cation cotransporter activity could be competition with endogenous FL-SPAK or OSR1 for cotransporter binding. Further evidence for this mechanism is illustrated by our previous finding that KS-SPAK significantly impairs the ability of FL-SPAK or OSR1 to phosphorylate NH2-terminal fragments of NCC and NKCC2 in vitro (13). To determine whether KS-SPAK interacts with NKCC2, we performed coimmunoprecipitation studies using Xenopus oocytes. An initial experiment showed that both FL-SPAK and KS-SPAK interact with NKCC2 (Fig. 5A). Neither SPAK isoform was precipitated unless NKCC2 was coinjected, confirming the specificity of the interaction. Mutation of L252 (L252A) dramatically reduced the amount of KS-SPAK coimmunoprecipitated, despite a similar expression level (Fig. 5). In a similar experiment we found that SPAK2 also strongly interacts with NKCC2 (Fig. 5B), suggesting its relatively weaker inhibitory effect on NKCC2 activity is not due a lack of interaction.
Fig. 5.
KS-SPAK and SPAK2 interact with NKCC2. A: coimmunoprecipitations were performed in Xenopus laevis oocytes injected with 10 ng each of cRNA for NKCC2 (3× FLAG-NKCC2), FL-SPAK, KS-SPAK, or KS-SPAK L252A as indicated. 3 days after injection, lysate from 10 oocytes per group was precleared with mouse IgG-Agarose then incubated with anti-FLAG M2 Affinity Gel at 4°C overnight. After 5 washes with homogenization buffer, precipitated proteins were eluted and analyzed by immunoblotting to detect interactions, as were aliquots of the original lysate (1% of input), to confirm expression. NKCC2 was detected with an anti-Flag antibody, and SPAK isoforms were detected with an anti C-SPAK antibody. FL-SPAK and KS-SPAK only immunoprecipitated in the presence of NKCC2, and KS-SPAK L252A, which is mutated at a residue important for FL-SPAK interactions with NKCC1 (26), had greatly reduced binding compared with wild type KS-SPAK. B: same experiment was performed with SPAK2 instead of KS-SPAK L252A. Data obtained for KS-SPAK and FL-SPAK are not shown, but the results were similar to those shown in A.
DISCUSSION
There is now a significant body of data indicating that the WNK-SPAK/OSR1 kinase network plays a key role in regulating the activities of the renal cation cotransporters NKCC2 and NCC and hence sodium and potassium balance and blood pressure (4, 12). The prevailing paradigm has been that activation of SPAK/OSR1 by WNK kinases results in a stimulatory effect on cotransporter activity (19). However, our observation that three forms of SPAK protein exist in kidney led us to clone a novel inhibitory SPAK isoform, KS-SPAK, which is expressed at highest levels in this tissue (13). KS-SPAK lacks the majority of the SPAK kinase domain including the catalytic loop, and we demonstrated that it inhibits phosphorylation of NCC and NKCC2 by SPAK/OSR1 in vitro (13). We therefore proposed that a significant portion of SPAK expressed along the nephron (KS-SPAK) is inhibitory rather than stimulatory with regards to cation cotransporters (13). In addition to KS-SPAK, Delpire and colleagues (16) proposed the existence of another catalytically inactive SPAK isoform, which is generated by usage of an alternative translation initiation site on the full-length SPAK mRNA transcript SPAK2.
Several lines of evidence suggest that KS-SPAK and SPAK2 are predominantly expressed along the TAL, whereas FL-SPAK is the major form along the DCT. First, Western blotting of dissected medulla and cortex revealed that KS-SPAK and SPAK2 are enriched in the medulla, while FL-SPAK is more highly expressed in the cortex (13). Next, immunofluorescence using antibodies against the amino terminus of SPAK suggested that the majority of SPAK expressed along the TAL lacks the amino terminus of FL-SPAK (and is therefore KS-SPAK or SPAK2) (13, 22). In contrast, along the DCT, the majority of SPAK detected possesses the amino terminus (and is therefore FL-SPAK). Finally, we recently showed by coimmunoprecipitation on whole kidney lysates that KS-SPAK and SPAK2 display a greater interaction with NKCC2 than with NCC, while the opposite was observed for FL-SPAK (22). This pattern of SPAK isoform expression is consistent with the observation that SPAK disruption leads to a dramatic reduction in NCC phosphorylation and activity but an increase in NKCC2 phosphorylation (8, 13, 28). In contrast, kidney-specific disruption of OSR1 results in the opposite effects on NCC and NKCC2 phosphorylation to SPAK disruption, suggesting that OSR1 is the major activator of NKCC2 in vivo. This led us to propose that KS-SPAK acts as an inhibitor of OSR1 along the TAL. However, the recent report that SPAK2 inhibits NKCC1 activity in the X. laevis oocyte coexpression system (thus confirming that it is indeed an inhibitory SPAK isoform) indicated that SPAK2 is also likely to play a role in suppressing the effects of OSR1 along the TAL (8).
Here, we present novel insights into the roles of KS-SPAK and SPAK2 in the regulation of cation cotransporter activity. Studies with phosphatase and kinase inhibitors have shown that reduced NKCC1 or NKCC2 phosphorylation correlates with changes in transport, and phosphorylation above baseline is required to stimulate transport (9). However, increased phosphorylation does not always lead to cotransporter stimulation, and hyperphosphorylation may even inhibit activity. Therefore, to extend our previous studies in which we showed that KS-SPAK inhibits phosphorylation of NCC and NKCC2 by SPAK/OSR1, we first tested the ability of KS-SPAK and SPAK2 to inhibit cotransporter activity. Consistent with its inhibitory effect on cotransporter phosphorylation, KS-SPAK potently inhibited activity of endogenous NKCC1 and injected NKCC2 in X. laevis oocytes. This inhibitory effect required the extreme COOH terminus of KS-SPAK, which mediates interactions between FL-SPAK and WNK kinases or cation cotransporters (26). Mutagenesis of individual residues (D238 and L252) previously reported to be important for interactions between FL-SPAK and NKCC2 or WNK1 (26) also abrogated the inhibitory effect of KS-SPAK. Coimmunoprecipitation showed that KS-SPAK interacts with NKCC2, and mutation of L252 almost completely abolished this interaction. These observations suggest that KS-SPAK and SPAK2 may inhibit NKCC2 by competing with endogenous OSR1 (oocytes lack SPAK) for binding to NKCC2, thereby preventing its phosphorylation and subsequent activation. Further support for this possibility comes from our previous finding that KS-SPAK inhibits phosphorylation of NKCC2 by OSR1 in the absence of WNK1 (13). However, since these studies used constitutively activated OSR1, the possibility that KS-SPAK prevents activation of OSR1 by competing for binding to endogenous WNK1, which limits the availability of OSR1 to phosphorylate and activate NKCC2, cannot be excluded.
Phosphorylation of FL-SPAK or OSR1 at a threonine in the T loop (SPAK T243 or OSR1 T185, mouse numbering) dramatically stimulates their activity (25). However, the major WNK1 phosphorylation site in FL-SPAK and OSR1 is a conserved serine located near the COOH terminus (S383/S325). The OSR1 mutants S325A or S325E mutants were initially reported to have no effect on basal activity or the ability of WNK1 to activate OSR1 (25). However, a more detailed analysis of the equivalent residue in FL-SPAK (S383) suggested that this residue may form part of an autoinhibitory domain since mutation of S383 and surrounding residues in combination with the FL-SPAK T243E to alanines led to activation of the kinase (5). In the present study, mutation of the equivalent residue in KS-SPAK to an alanine (S133A) had no effect on the ability of KS-SPAK to inhibit NKCC2 activity. This is perhaps not surprising since in addition to lacking T243, the effects of FL-SPAK S383A on NKCC1 activity do not differ from those of wild type FL-SPAK (5). Another residue that may play a role in FL-SPAK activation, S321 has also been identified, but we did not examine the effect of mutating this site in the present studies (5).
The effects of SPAK2 on NKCC2 activity were unexpected, since it has been reported that SPAK2 strongly inhibited activity of coinjected NKCC1 in Xenopus oocytes (8). In our initial studies under hypertonic conditions SPAK2 inhibited neither endogenous NKCC1 nor NKCC2 activity. We ruled out the possibility that this was due to the level of SPAK2 expression by using antibodies against the NH2 and COOH termini of FL-SPAK for Western blotting. We next tested the effects of SPAK2 under different tonicities and found that it inhibited NKCC2 following hypotonic low chloride preincubation with hypotonic uptake medium, and the degree of inhibition was low compared with that of KS-SPAK (16% inhibition by SPAK2 vs. 70% inhibition by KS-SPAK). While we initially observed a mild stimulatory effect under hypertonic conditions, a more extensive experiment (Fig. 2C) showed that SPAK2 does in fact inhibit NKCC2 under hypertonic conditions, although the degree of inhibition was still significantly less than that by KS-SPAK. Similar to the observation of Grimm et al. (8), we found that SPAK2 strongly inhibited activity of coinjected NKCC1. While KS-SPAK inhibited both NKCC1 and NKCC2 to a similar degree, SPAK2 exerted a significantly greater effect on NKCC1 activity than on NKCC2. Together, these data suggest that in Xenopus oocytes, inhibition of NKCC2 is dependent on the mechanism by which endogenous OSR1 is activated (i.e., the preincubation conditions), and SPAK2 differentially regulates NKCC1 and NKCC2. These data further emphasize that in vitro findings regarding NKCC1 regulation should not be extrapolated to regulation of NKCC2. NKCC2 has higher baseline activity (7, 9), a lower responsiveness to changes in tonicity than NKCC1 (7), and reduced responsiveness to several stimuli including intracellular chloride (9). In particular, since it has been shown that NKCC1 is much more sensitive to changes in its phosphorylation status than NKCC2, as reflected by differences in their activity profiles at different tonicities (7), a relatively weak inhibitor like SPAK2 may have a more dramatic effect on NKCC1 activity than on NKCC2 activity. Another possibility is that the difference in regulation arises directly from differences between the cotransporters themselves. Indeed, while the SPAK/OSR1 phosphorylation sites are conserved the remainder of the amino termini of NKCC1 and NKCC2 diverge considerably.
To examine interactions between SPAK isoforms with regard to regulation of NKCC2 activity, we performed two titration studies. In the first, we examined the effects of increasing SPAK2 expression in the presence of fixed KS-SPAK expression. There was no additive effect of coinjecting SPAK2 and KS-SPAK on NKCC2 activity, suggesting that inhibition of SPAK/OSR1-dependent pathways was maximal. Furthermore, uptake was always significantly higher by oocytes injected with NKCC2 inhibitory SPAK isoforms than by water-injected oocytes, suggesting that residual NKCC2 activity is the result of SPAK/OSR1-independent activation (21). Next, we stimulated NKCC2 activity by coinjecting a constitutively active FL-SPAK mutant (CA-SPAK, T243E/S383D) and then examined the effects of KS-SPAK, and increasing amounts of SPAK2, on uptake. When coinjected with CA-SPAK, the ability of both KS-SPAK and SPAK2 to inhibit NKCC2 activity was reduced, with the weaker inhibitor SPAK2 losing its ability to inhibit when injected at low amounts. These data suggest that neither SPAK2 nor KS-SPAK displace CA-SPAK or endogenous OSR1 from NKCC2 but rather compete with them for binding to NKCC2.
While KS-SPAK displayed similar effects on both NKCC1 and NKCC2 activity, we explored possible mechanisms for the differences in the effects of SPAK2 on NKCC1 and NKCC2. A series of truncations revealed that SPAK2 deletion of up to 80 amino acids from its NH2 terminus did not enhance its ability to inhibit NKCC2. However, removal of 108 residues that included the catalytic loop resulted in an inhibitory effect equivalent to that of KS-SPAK. One possibility is that the presence of the catalytic loop, lacking in KS-SPAK but present in SPAK2, permits SPAK2 to interact with targets other than NKCC1 and NKCC2, resulting in a weaker inhibitory effect (27). While SPAK2 is a stronger inhibitor of NKCC1 in oocytes, these findings cannot be extrapolated to the whole organism. In mouse kidney, the majority of SPAK (all isoforms) along the TAL appears to localize to the apical membrane (8, 13, 22). However, a role for SPAK isoforms in regulating NKCC1 at the basolateral membrane cannot be ruled out. We previously reported that maneuvers that reduce extracellular fluid volume, dietary NaCl restriction, and targeted disruption of NCC lead to increased FL-SPAK expression but reduced KS-SPAK expression (13); the effect of dietary NaCl restriction on KS-SPAK expression has been independently confirmed (15). While NaCl restriction appears to reduce SPAK2 expression, its expression is relatively unchanged in NCC knockout mice. The precise roles of SPAK2 at baseline and following manipulations that would be predicted to activate or inhibit NKCC2 are thus unclear. However, we propose that in vivo, SPAK2 provides a tonic level of inhibition of NKCC2, while the more potent inhibitor KS-SPAK responds to stimuli that promote or inhibit NaCl retention In summary, the data reported here further support our hypothesis that inhibitory SPAK isoforms suppress NKCC2 activity and will further aid understanding the physiological significance of this novel SPAK/OSR1 isoform network.
GRANTS
This work was funded by a Beginning Grant-in-Aid from the American Heart Association (12BGIA9280029), a Mentored Research Scientist Development Award from National Institute of Diabetes and Digestive and Kidney Diseases (1K01-DK-07661701A1), and a New Investigator Award from the Medical Research Foundation of Oregon (all to J. A. McCormick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.J.P., J.N.C., and J.A.M. performed experiments; H.J.P., J.N.C., and J.A.M. approved final version of manuscript; J.A.M. conception and design of research; J.A.M. analyzed data; J.A.M. interpreted results of experiments; J.A.M. prepared figures; J.A.M. drafted manuscript; J.A.M. edited and revised manuscript.
REFERENCES
- 1.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+,K+,2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA 103: 10883–10888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem 278: 27347–27353, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Flatman PW. Cotransporters, WNKs and hypertension: an update. Curr Opin Nephrol Hypertens 17: 186–192, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Gagnon KB, Delpire E. Molecular physiology of SPAK and OSR1: two Ste20-related protein kinases regulating ion transport. Physiol Rev 92: 1577–1617, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagnon KB, Delpire E. On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl− cotransport activity. Am J Physiol Cell Physiol 299: C614–C620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Gimenez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannemann A, Flatman PW. Phosphorylation and transport in the Na-K-2Cl cotransporters, NKCC1 and NKCC2A, compared in HEK-293 cells. PLoS One 6: e17992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem 280: 26653–26658, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS. Impaired phosphorylation of Na(+)-K(+)-2Cl(−) cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci USA 108: 17538–17543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91: 177–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen MT, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA. Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters abundance along the nephron. Am J Physiol Renal Physiol 303: F92–F104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO J 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121: 3293–3304, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saritas T, Borschewski A, McCormick JA, Paliege A, Dathe C, Uchida S, Terker A, Himmerkus N, Bleich M, Demaretz S, Laghmani K, Delpire E, Ellison DH, Bachmann S, Mutig K. SPAK differentially mediates vasopressin effects on sodium cotransporters. J Am Soc Nephrol 24: 407–418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol 26: 1789–1802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shetlar RE, Scholermann B, Morrison AI, Kinne RK. Characterization of a Na(+)-K(+)-2Cl- cotransport system in oocytes from Xenopus laevis. Biochim Biophys Acta 1023: 184–190, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397: 223–231, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Won AP, Garbarino JE, Lim WA. Recruitment interactions can override catalytic interactions in determining the functional identity of a protein kinase. Proc Natl Acad Sci USA 108: 9809–9814, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zagorska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Thastrup J, Deak M, Campbell DG, Morrice NA, Prescott AR, Alessi DR. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol 176: 89–100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]