Abstract
Regulation of urea transporter UT-A1 in the kidney is important for the urinary concentrating mechanism. We previously reported that activation of the cAMP/PKA pathway by forskolin (FSK) leads to UT-A1 ubiquitination, endocytosis, and degradation. In this study, we discovered that FSK-induced UT-A1 ubiquitination is monoubiquitination as judged by immunoblotting with specific ubiquitin antibodies to the different linkages of the ubiquitin chain. UT-A1 monoubiquitination induced by FSK was processed mainly on the cell plasma membrane. Monoubiquitination facilitates UT-A1 endocytosis, and internalized UT-A1 is accumulated in the early endosome. Inhibition of ubiquitination by E1 ubiquitin-activating enzyme inhibitor PYR-41 significantly reduced FSK-induced UT-A1 endocytosis and degradation. Interestingly, FSK-stimulated UT-A1 degradation occurs through a lysosomal protein degradation system. We further found that the PKA phosphorylation sites of UT-A1 at Ser486 and Ser499 are required for FSK-induced UT-A1 monoubiquitination. The physiological significance was confirmed using rat kidney inner medullary collecting duct suspensions, which showed that vasopressin treatment promotes UT-A1 ubiquitination. We conclude that unlike under basal conditions in which UT-A1 is subject to polyubiquitination and proteasome-mediated protein degradation, activation of UT-A1 by FSK induces UT-A1 monoubiquitination and protein lysosomal degradation.
Keywords: membrane protein, vasopressin, phosphorylation, trafficking, endocytosis
vasopressin-regulated urea transporter UT-A1, exclusively expressed in the inner medullary collecting duct (IMCD), plays an important role in urea reabsorption and generation of the corticomedullary osmolarity gradient (25, 27). UT-A1 is physiologically significant in the kidney as has been shown by the UT-A1/UT-A3 knockout mouse, which has seriously impaired urea reabsorption and urine concentration ability(12).
Arginine vasopressin (AVP, also known as antidiuretic hormone, ADH) is the major hormone that regulates UT-A1 transporter activity in vivo. Vasopressin rapidly increases urea permeability in isolated rat terminal IMCDs within 5–10 min (34). Vasopressin binds to IMCD epithelial cell V2-receptors; stimulates adenylyl cyclase III and VI, which convert ATP into cAMP; activates protein kinase A (PKA); and subsequently increases UT-A1 phosphorylation and urea transport activity (36). UT-A1 phosphorylation by PKA occurs mainly at two sites, Ser486 and Ser499, within the large intracellular loop (3). These two sites are important for AVP-regulated accumulation of UT-A1 in the cell membrane and urea transporter activity. Treatment of UT-A1-MDCK cells with forskolin (FSK), an adenylyl cyclase stimulator, elevates cAMP level and increases cell urea flux. This effect occurs through increasing UT-A1 phosphorylation and membrane trafficking (5, 13).
As a membrane protein, the function of UT-A1 transport activity relies on its presence in the plasma membrane. Although UT-A1 trafficking to the apical membrane of polarized epithelial cells is crucial for regulation of urea transport, the net UT-A1 abundance on the cell membrane is determined by both the biosynthetic delivery of proteins to the plasma membrane via the secretory pathway and the removal of proteins from the plasma membrane via the endocytic pathway. Numerous mechanisms for endocytosis exist in mammalian cells. Caveolae and clathrin-coated pits (CCP) are the two major routes for endocytosis in eukaryotic cells. One protein can be internalized via one or multiple endocytic pathways depending on the different cellular situations (9). Previous work from our group revealed that UT-A1 internalization is dynamin-dependent and occurs through both caveolae- and clathrin-mediated pathways. Blocking either pathway increases UT-A1 abundance in the cell membrane (20).
Endocytosis, postendocytic trafficking, and degradation of membrane proteins are frequently associated with its ubiquitination state. The resulting ubiquitination of membrane proteins leads to the attenuation of signaling processes (19, 21). Ubiquitination is a posttranslational modification carried out by a cascade of three enzymes: E1, ubiquitin-activating enzyme, which binds to ubiquitin to generate a high energy E1-ubiquitin intermediate; E2, ubiquitin-conjugating enzyme, a ubiquitin carrier protein; and E3, ubiquitin ligase, which transfers ubiquitin to a target protein (14a). The targeted membrane proteins on the cell surface can be modified by the addition of either monoubiquitin or polyubiquitin chains (2). Protein modification by ubiquitin often occurs with different consequences. Monoubiquitination, which chiefly occurs on the plasma membrane, often involves the trafficking and lysosomal degradation of membrane proteins, whereas polyubiquitinated proteins in cytosol are usually targeted to the proteasome for degradation (20a, 26).
Vasopressin regulates urea permeability in the IMCD through increases in UT-A1 phosphorylation and apical plasma-membrane accumulation (3). We recently found that FSK, the adenylyl cyclase stimulator often being used for in vitro experiments to stimulate UT-A1 urea transport activity, can promote UT-A1 ubiquitination and protein degradation, revealing the coin's other side of UT-A1 phosphorylation by vasopressin or FSK (29). This suggests that activation of the cAMP/PKA pathway causing UT-A1 phosphorylation also triggers the protein ubiquitination and degradation machinery for UT-A1. This negative feedback of UT-A1 activation may have important physiological roles in vivo in attenuating hormonal response by promoting UT-A1 ubiquitination and endocytosis, and facilitating protein degradation, thereby allowing the cell to return to a basal condition after vasopressin stimulation.
In the present study, we present new findings that FSK stimulation-induced UT-A1 ubiquitination is monoubiquitination and that internalized UT-A1 subsequently is targeted to the lysosome system for degradation. Furthermore, we demonstrate that the PKA phosphorylation sites of UT-A1, at S486 and S499, are required for FSK-induced UT-A1 monoubiquitination and protein degradation. Therefore, the monoubiquitination-dependent degradation of UT-A1 could represent an important mechanism that underlies the downregulation of cell response to AVP/FSK stimulation.
MATERIALS AND METHODS
Construct.
The pcDNA3 FLAG-Tac-UT-A1, an external FLAG-tagged UT-A1, has been described before (29). Amino acids of serine at 486 and 499 of UT-A1 PKA sites were mutated to alanine by site-directed mutagenesis.
Cell culture and treatment.
MDCK cells stably expressing UT-A1 (13) and HEK 293 cells were maintained in DMEM supplemented with 10% FCS, 25 mM HEPES, and antibiotics in a 5% CO2 incubator. After confluency, the cells were treated with the different compounds alone or in combination as described in detail for the relevant experiments. Cells were then processed for Western blot, immunoprecipation, cell surface biotinylation, internalization, or subcellular organelle isolation. FSK, chlorpromazine, and chloroquine were purchased from Sigma. MG132, lactacystin, and PYR-41 were from Calbiochem.
Plasma membrane and early endosome preparation.
Cell plasma membrane from UT-A1-MDCK cells was prepared by a sucrose gradient (2.0, 1.6, 1.4, 1.2, and 0.8 M) ultracentrifugation as described elsewhere(5). Fractions from the interfaces of 1.6/1.4 density were collected as plasma membrane.
Early endosome was prepared according to the protocol reported by Butterworth et al. (4). Briefly, after cell lysis, the postnuclear supernatant was diluted 1:1 with 62% sucrose in HEPES buffer and placed at the bottom of a 5-ml ultracentrifuge tube. Sucrose (35%; 1.5 ml) was layered on top followed by 1.5 ml of 25% sucrose and 0.5 ml of HEPES buffer. The gradients were centrifuged in a SW50.1 rotor at 37,000 rpm (∼167,000 g) for 75 min at 4°C. The interfaces between 25% and 35% sucrose were collected as the early endosomal fractions.
Cell surface biotinylation and endocytosis assay.
Cell surface biotinylation assays were carried out as described (29). UT-A1 internalization was measured by biotinylation and sodium 2-mercaptoethane sulfonate (MesNa) treatment (29). Briefly, the cells were first biotin-labeled with a freshly prepared solution of 0.5 mg/ml EZ-Link sulfo-NHS-SS-biotin (Pierce 21331) in a borate buffer for 30 min at 4°C. The biotin reaction was quenched for 10 min with 0.1 mM lysine (Sigma). After washing with PBS, cells were added with prewarmed culture medium (without or with different treatments) and incubated at 37°C to allow protein endocytosis for the indicated period. The control cells with no endocytosis were kept on ice. The noninternalized biotin was cleaved three times with the cell-impermeable reducing agent MesNa (50 mM MesNa, 1 mM EDTA, 0.2% BSA in 50 mM Tris, pH 8.6) for 20 min at 4°C on a rocking platform. The biotin bound to the endocytosed proteins is protected from MesNa cleavage. After quenching with iodoacetamide and PBS washing, cells were solubilized in a radioimmunoprecipitation assay (RIPA) buffer. The biotinylated proteins were retrieved by streptavidin-agarose affinity precipitation, and the UT-A1 was detected by Western blotting with UT-A1 antibody.
Confocal microscopy.
The transient transfection of pcDNA3 FLAG-Tac-UT-A1 into MDCK cells was carried out by using GenJet In Vitro DNA Transfection Reagent (SignaGen Laboratories) according to the manufacturer's instructions. After 48 h the transfected cells were washed with ice-cold PBS and then incubated with FLAG antibody (1:200) in DMEM without serum for 60 min at 4°C. Unbound antibody was removed with three washes of ice-cold PBS. Prewarmed complete culture medium with or without different treatments was added. The cells were then placed at 37°C for 60 min to allow internalization. The control cells with no endocytosis were kept on ice. The internalization was stopped by washing with ice-cold PBS followed by fixation with 3% paraformaldehyde. The cells were permeabilized with 0.2% Tween for 5 min and incubated with AlexaFluor 568-conjugated secondary antibody (Invitrogen) for 60 min. LysoTracker Green DND-26 (Invitrogen) was used to label the lysosome according to the manufacturer's instructions. Cells were mounted with Vectashield (Vector Laboratories) and the images were captured by confocal microscopy at identical microscopic settings.
IMCD suspension preparation and vasopressin treatment.
All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River Laboratories) weighing 125–200 g were used in this study. Rat kidney IMCD suspensions were prepared by digestion with hyaluronidase and collagenase B (Sigma) as described (6).The IMCD suspensions were then treated with 0.1 μM vasopressin (Sigma) at 37°C in a 5% CO2 incubator for 0, 1, 2, or 4 h. Total proteins were extracted and used for immunoprecipitation with UT-A1 antibody.
Cell lysate preparation, immunoprecipitation, and Western blot.
After treatment, cells were lysed in a modified RIPA buffer (150 mM NaCl, 10 mM Tris·HCl pH 7.5; 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and protease inhibitors). The protein concentration of the cleared lysate was determined using the BCA Protein Assay (Pierce). For immunoprecipitation, equal amounts (0.5–1 mg) of total proteins were incubated with UT-A1 antibody at 4°C overnight with gentle mixing, followed by the addition of 10 μl of protein A beads (Pierce) and continued incubation for another 2 h. The beads were pelleted by centrifugation at 3,000 rpm for 1 min and washed three times with RIPA buffer. The precipitated proteins were eluted in 40 μl of Laemmli sample buffer. For Western blot analysis, the proteins were separated by 4–15% SDS-PAGE and electrotransferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were routinely processed by blocking with 5% milk/PBS, incubation overnight with primary antibody, and incubation for 1 h with horseradish peroxidase-conjugated secondary antibody. Immunoreacting proteins were detected using an enhanced chemiluminescence kit (Amersham). The antibodies used in this study included polyclonal rabbit UT-A1 antibody (6), monoclonal mouse ubiquitin (P4D1) antibody (SC-8017; Santa Cruz), FK1 and FK2 antibodies (BML-PW8805 and BML-PW8805; Enzo Life Sciences), monoclonal mouse FLAG antibody (F1804; Sigma); Rab5 (SC-598; Santa Cruz), and actin (A2066; Sigma). Western blot band densities were quantitated with the ImageJ program (National Institutes of Health, Bethesda, MD), and statistical significance was conducted by one-way ANOVA for multiple group analysis.
RESULTS
Forskolin stimulation induces UT-A1 monoubiquitination.
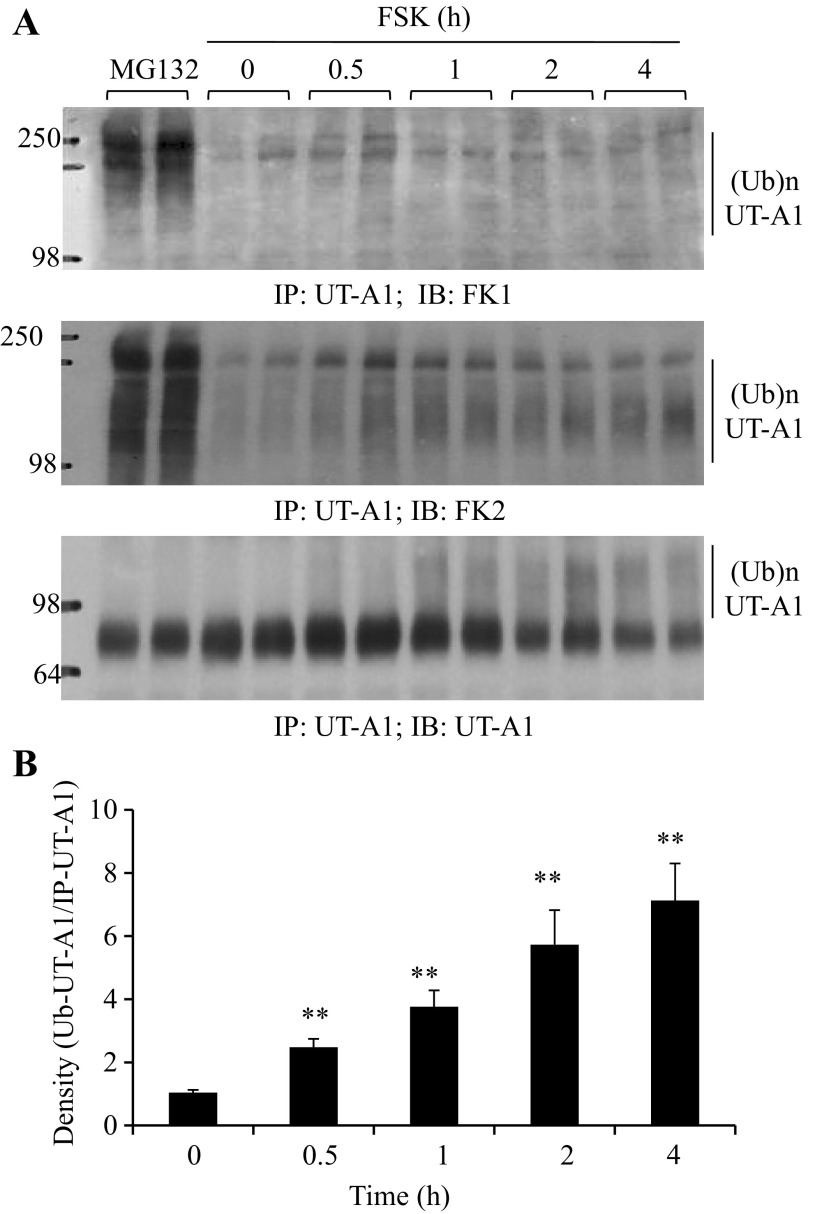
FSK stimulation promotes UT-A1 ubiquitination (29). Interestingly, when compared with ubiquitinated UT-A1 induced by proteasome inhibitor treatment, we observed a much smaller size of ubiquitinated UT-A1 induced by FSK treatment (data not shown). This prompted us to investigate whether FSK-induced UT-A1 ubiquitination is different. We took advantage of the two specific ubiquitin antibodies for this study. FK1 recognizes only polyubiquitinated proteins, whereas FK2 detects both monoubiquitinated and polyubiquitinated proteins (14). UT-A1-MDCK cells were treated with FSK for the indicated times. The proteasome inhibitor MG132 that induced ubiquitinated UT-A1 (7, 28) was used as a control. As shown in Fig. 1A, strong signals were detected in cells treated with MG132, but a very weak signal was observed in FSK-treated cells. However, when blotted with FK2 antibody, sustained ubiquitin signals were demonstrated in FSK-treated cells. This indicated that FSK-induced UT-A1 ubiquitination is monoubiquitination. Figure 1B shows the densitometry quantification of UT-A1 monoubiquitination after FSK stimulation.
Fig. 1.
Forskolin stimulation induces UT-A1 monoubiquitination. A: UT-A1-MDCK cells were treated with 10 μM forskolin (FSK) for the indicated times, then solubilized in RIPA buffer. The lysates were immunoprecipitated with UT-A1 antibody and followed by immunoblotting with FK1, FK2, or UT-A1 antibodies. Proteasome inhibitor MG132 (10 μM) was used as a control. B: ubiquitinated UT-A1 detected by FK2 antibody was quantified (n = 4) by densitometry and normalized to the immunoprecipitated UT-A1. The relative intensity of time 0 was set as 1 (compare with time 0; **P < 0.01).
FSK-induced UT-A1 monoubiquitination largely occurs on the cell membrane.
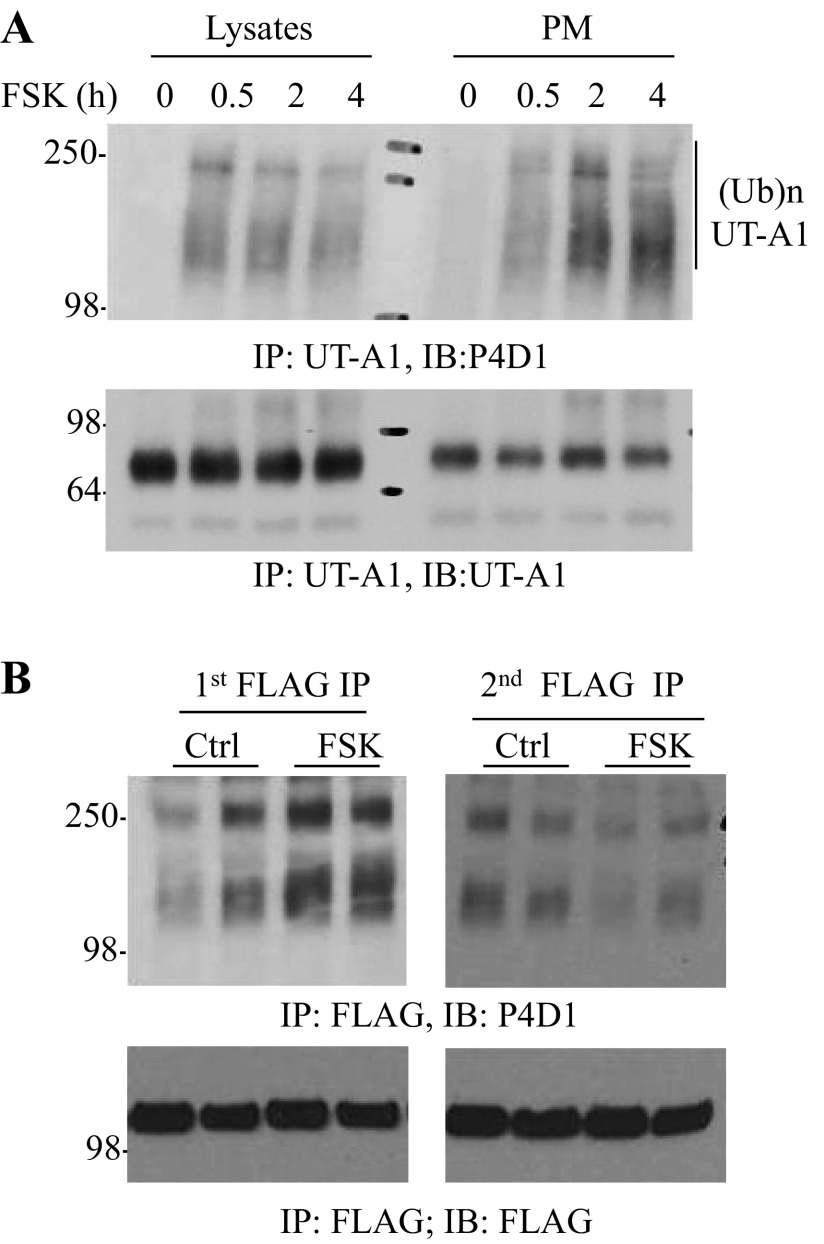
Next we explored the subcellular compartment of UT-A1 monoubiquitination under FSK stimulation. The plasma membranes of UT-A1-MDCK cells were isolated by sucrose gradient ultracentrifugation. The whole cell lysate and plasma membrane fractions were immunoprecipitated with UT-A1 antibody, then immunoblotted with ubiquitin antibody (P4D1). Figure 2A shows that FSK-induced ubiquitinated UT-A1 is predominantly located on the plasma membrane.
Fig. 2.
FSK stimulation promotes cell surface UT-A1 ubiquitination. A: UT-A1 MDCK cells were treated with 10 μM FSK for different times. The plasma membrane (PM) was isolated by a sucrose gradient ultracentrifugation. Equal amounts of lysates or the PM were immunoprecipitated with UT-A1 antibody followed by immunoblotting with antiubiquitin (Ub) antibody P4D1. B: FLAG-Tac-UT-A1 transfected HEK 293 cells were treated without or with 10 μM FSK for 4 h at 37°C. The cells were incubated with FLAG antibody on ice for 1 h then lysed in RIPA buffer. The lysates were incubated with protein G beads overnight (first immunoprecipitation of the cell surface UT-A1). The supernatant was collected and processed for a second immunoprecipitation with FLAG antibody (cytoplasmic UT-A1). The immunoprecipitated samples were analyzed by Western blot with ubiquitin antibody. This experiment was repeated two times with similar results.
Both amino and carboxyl termini of UT-A1 are present in the intracellular space. We recently engineered an extracellular N-terminal FLAG-tagged UT-A1 (29). By using this unique FLAG-tagged UT-A1, we assessed the cell membrane UT-A1 ubiquitination induced by FSK. HEK 293 cells transfected with pcDNA3 FLAG-Tac-UT-A1 were treated with or without FSK for 2 h at 37°C. Cell surface UT-A1 was labeled with FLAG antibody on ice for 30 min. The cells were then lysed, and the cell surface UT-A1 conjugated with FLAG antibody was precipitated by protein G beads. The postimmunoprecipitation supernatants were collected and used for secondary immunoprecipitation by FLAG antibody to pull down the cytosolic UT-A1. The immunoprecipitated samples were probed with ubiquitin antibody. Figure 2B shows that FSK-induced UT-A1 ubiquitination is largely observed in the first FLAG antibody immunoprecipitated samples; namely from the cell surface UT-A1.
FSK-induced UT-A1 endocytosis and degradation is regulated by ubiquitination.
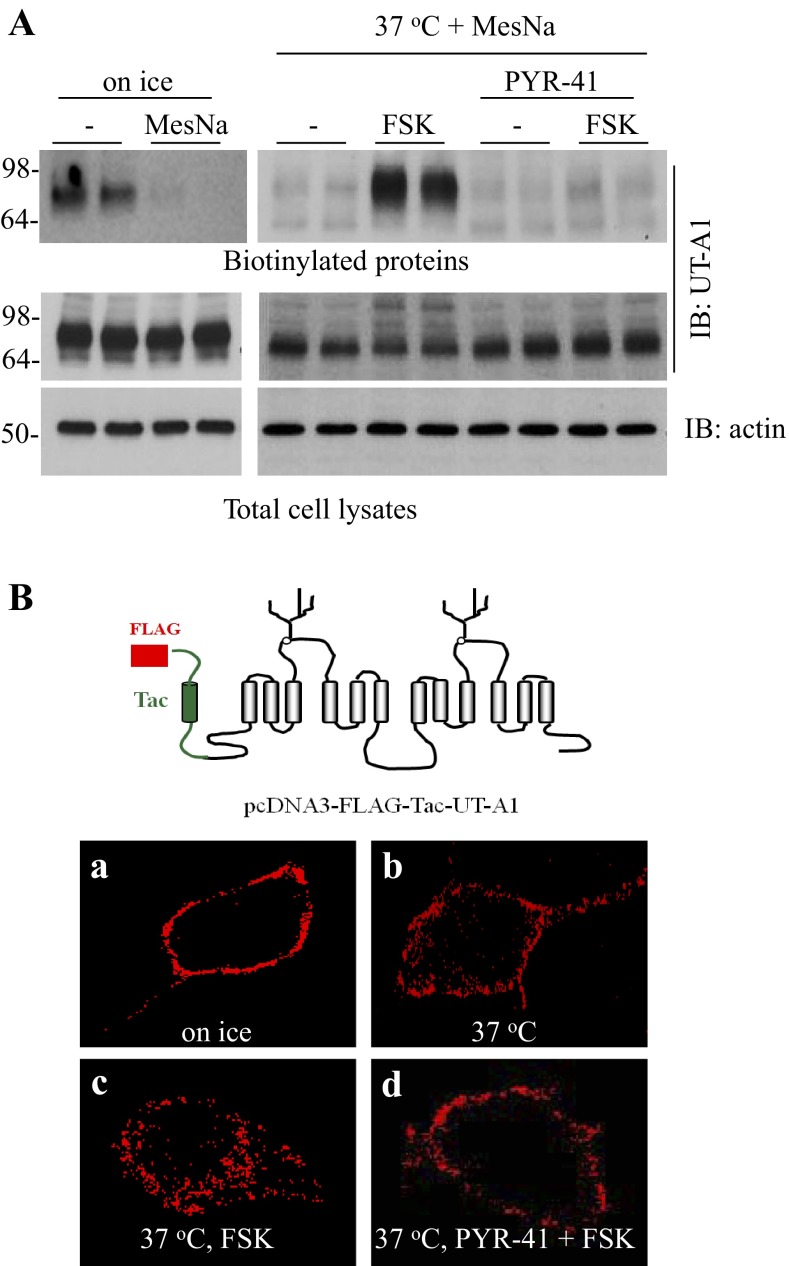
Ubiquitination has been shown to be necessary for endocytosis of many transporters (10, 16, 17). FSK stimulation promotes UT-A1 ubiquitination, endocytosis, and degradation (29). We then asked whether ubiquitin modification mediates UT-A1 endocytosis upon FSK stimuli. The cell surface UT-A1 was labeled with biotin on ice, and then shifted to 37°C in the presence of different treatments. The noninternalized biotin was cleaved by MesNa. As measured by cell surface biotinylation, after FSK stimulation for 2 h, both UT-A1 internalization and degradation were increased. However, this effect was inhibited by PYR-41, a specific inhibitor of the E1 ubiquitin-activating enzyme (Fig. 3A), supporting the role of ubiquitination in FSK-induced UT-A1 endocytosis and degradation. To directly visualize UT-A1 endocytosis, MDCK cells were transfected with pcDNA3-FLAG-Tac-UT-A1. Two days later, cells were incubated with FLAG antibody on ice to specifically label cell surface UT-A1 then placed at 37°C to allow for endocytosis. As demonstrated by confocal microscopy, FSK increases the internalization of UT-A1, and the treatment of PYR-41 almost completely abrogated FSK-induced UT-A1 internalization (Fig. 3B).
Fig. 3.
E1 ubiquitin enzyme inhibitor PYR-41 prevents FSK-induced UT-A1 endocytosis. A: UT-A1-MDCK cells were biotinylated on ice then placed at 37°C for 1 h to induce endocytosis in the presence or absence of 10 μM FSK and/or 10 μM PYR-41. The uninternalized cell surface biotin was cleaved by MesNa at 4°C. The biotinylated proteins eluted from streptavidin beads and the whole cell lysates were blotted with UT-A1 antibody (n = 3). B: MDCK cells were transiently transfected with pcDNA3-FLAG-Tac-UT-A1. After 48 h, cell surface UT-A1 was labeled with anti-FLAG antibody at 4°C for 1 h. Cells were left on ice (a) or switched to 37°C for 30 min to allow endocytosis in the absence (b) or presence (c) of 10 μM FSK or (d) pretreated with 10 μM PYR-41 for 30 min prior to addition of FSK. The cells were then fixed and permeabilized. UT-A1 was detected by the Alexa Fluor 568-conjugated secondary antibody (in red) and examined via confocal microscopy.
Internalized UT-A1 stimulated by FSK highly accumulates in the early endosome.
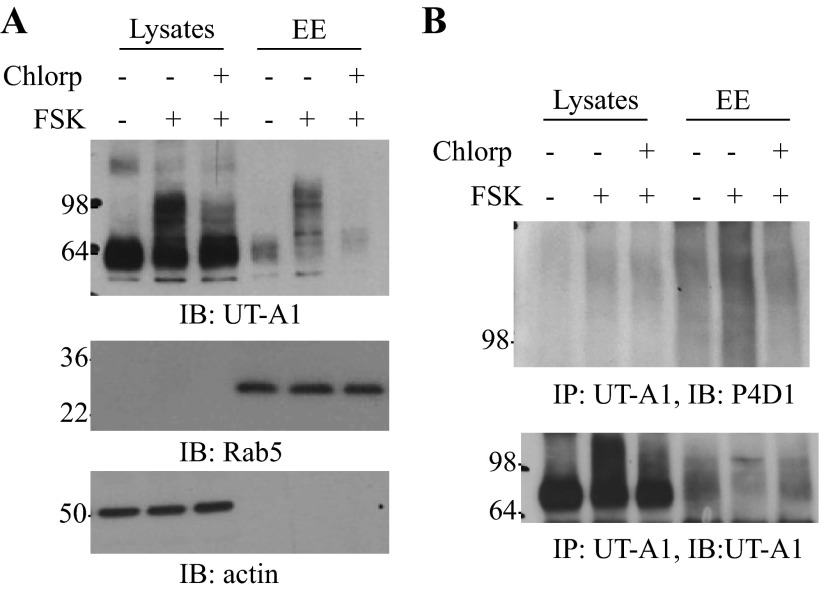
We then tracked the postendocytic trafficking of UT-A1 after internalization. UT-A1-MDCK cells were treated without or with FSK plus chlorpromazine. Early endosome was isolated (4), and expression of UT-A1 in the total cell lysate and early endosome fractions were examined by immunoblotting. As shown in Fig. 4A, the abundance of UT-A1 in early endosome was increased upon FSK stimulation. Chlorpromazine, which blocks clathrin-mediated endocytosis, reduced FSK-induced UT-A1 accumulation in early endosome. This indicates that UT-A1 in early endososome is largely retrieved from the cell membrane. Rab5 was used as an early endosome marker. The state of UT-A1 ubiquitination in the early endosome was further examined by immunoprecipitation with UT-A1 antibody followed by immunoblotting with ubiquitin antibody (Fig. 4B). FSK stimulation caused a high amount of ubiquitinated UT-A1 accumulation in early endosome, and this effect was ameliorated when clathrin-mediated endocytosis was inhibited by chlorpromazine.
Fig. 4.
Internalized ubiquitinated UT-A1 accumulated mainly in the early endosome (EE). UT-A1-MDCK cell were treated without or with 10 μM FSK for 3 h alone or together with chlorpromazine (Chlorp, 10 μg/ml, pretreated for 1 h), then the cells were subjected to EE isolation. The lysates from the whole cells and EE were blotted with UT-A1 antibody (A) or immunoprecipitated by UT-A1 antibody, then detected by ubiquitin antibody (B). These results were reproduced two additional times.
FSK-stimulated UT-A1 is degraded through a lysosomal degradation system.
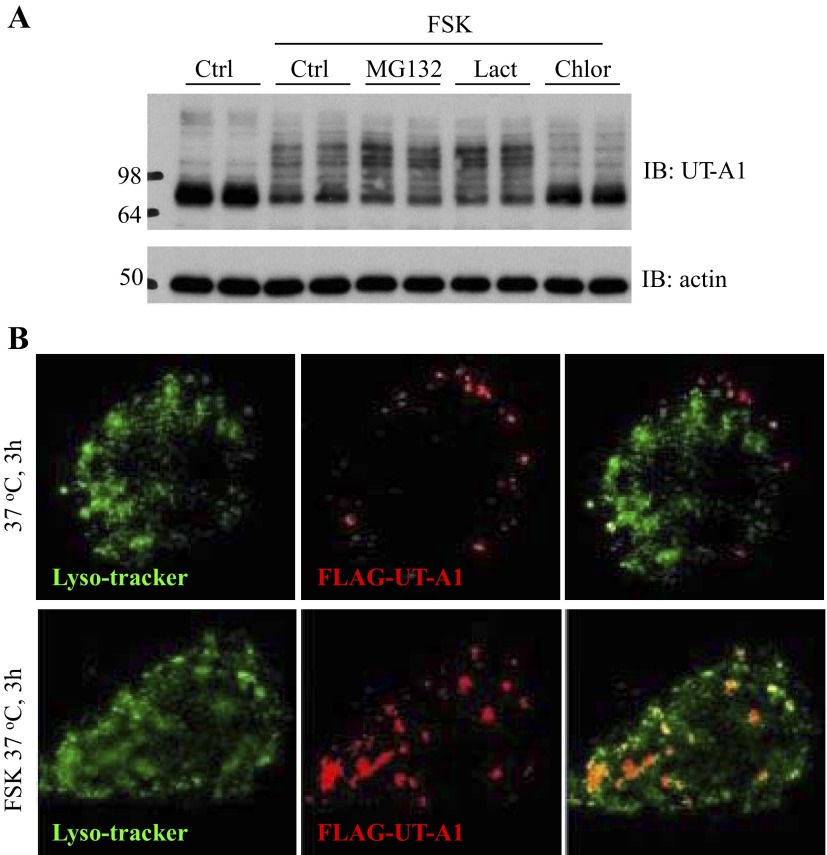
Early studies (7, 28) revealed that UT-A1 urea transporter is ubiquitinated and degraded in proteasome but not lysosome systems. We evaluated whether FSK induces UT-A1 ubiquitination and, subsequently, degradation through a proteasome-mediated protein degradation system. UT-A1-MDCK cells treated with FSK or together with proteosomal or lysosomal inhibitor for the indicated time were examined for abundance of UT-A1 protein by Western blot. FSK stimulation promotes UT-A1 degradation. Surprisingly, the proteasome inhibitor MG132 and lactacystin did not prevent FSK-induced UT-A1 degradation; in contrast, the lysosome inhibitor chloroquine blocked FSK-induced UT-A1 degradation (Fig. 5A). To clarify the FSK-stimulated UT-A1 targeting lysosome, we employed immunostaining and confocal microscopy. MDCK cells were transfected with pcDNA3-FLAG-Tac-UT-A1. The cells were incubated with FLAG antibody on ice to specifically label the cell membrane UT-A1. Antibody labeled UT-A1 was allowed to internalize for 1 h at 37°C. Internalized UT-A1 was identified by using Alexa 568-conjugated secondary antibody (Fig. 5B, in red). Lysosome was demonstrated by LysoTracker Green DND-26 (Fig. 5B, in green). As observed in these antibody labeling experiments (Fig. 5B), FSK stimulation increased internalized UT-A1 colocalization with lysosome tracker.
Fig. 5.
FSK-stimulated UT-A1 degradation is blocked by lysosome inhibitors. A: UT-A1-MDCK cells were treated with 10 μM FSK or together with 10 μM MG132, or 10 μM lactacystin (Lact), or 50 μg/ml chloroquine (Chlor) for 8 h. The total cell lysates were immunoblotted with UT-A1 and actin antibodies. Representative Western blots from three experiments (n = 3) are shown. B: MDCK cells were transfected with pcDNA3 FLAG-Tac-UT-A1. Cell surface UT-A1 was labeled with anti-FLAG antibody at 4°C, then switched to 37°C in the presence or absence of 10 μM FSK for 3 h. UT-A1 was detected by the Alexa Fluor 568-conjugated secondary antibody (in red). LysoTracker Green DND-26 was used to label lysosome (in green).
Serine 486 and 499 are required for FSK-induced UT-A1 ubiquitination.
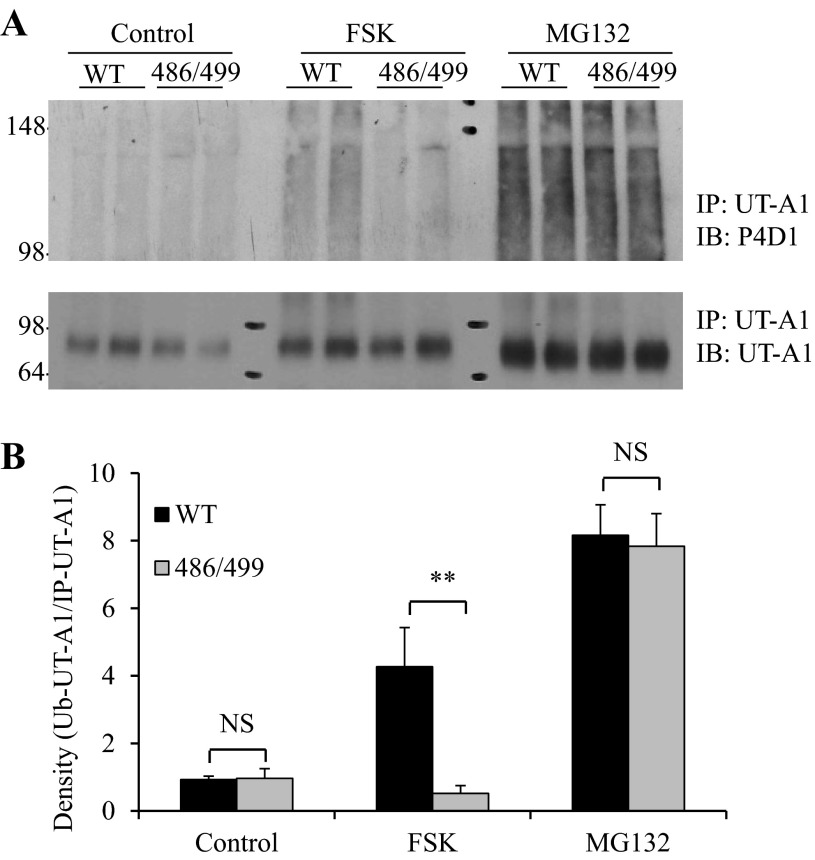
To assess whether the serines at 486 and 499, the two major PKA-mediated phosphorylation sites of UT-A1, are involved in FSK-stimulated UT-A1 ubiquitination, wild-type UT-A1 and S486A/S499A-UT-A1-MDCK cells were treated with FSK or MG132. UT-A1 ubiquitination was analyzed by immunoprecipitation with UT-A1 antibody followed by Western blots probed with ubiquitin antibody. As shown in Fig. 6, mutation of 486 and 499 PKA phosphorylation sites caused a sustained decrease in FSK-induced UT-A1 ubiquitination. However, mutation of serines 486 and 499 did not influence UT-A1 ubiquitination caused by the proteasome inhibitor MG132, indicating that the two PKA phosphorylation sites mediate FSK- but not MG132-induced UT-A1 ubiquitination.
Fig. 6.
Mutation of PKA sites at serine 486 and 499 reduces FSK-induced UT-A1 ubiquitination. A: wild-type UT-A1 or S486A/S499A-UT-A1 MDCK cells were treated with 10 μM FSK for 3 h or 10 μM MG132 for 6 h. The cells were lysed in RIPA buffer and equal amounts of cell lysates were immunoprecipitated with UT-A1 antibody, then blotted with ubiquitin or UT-A1 antibodies. B: densitometry analysis. Ubiquitinated UT-A1 was quantified and normalized to the total immunoprecipitated UT-A1 (n = 4). The relative intensity of the wild-type cells without treatment was set as 1 (NS, not significant; **P < 0.01).
Vasopressin treatment induces native UT-A1 ubiquitination.
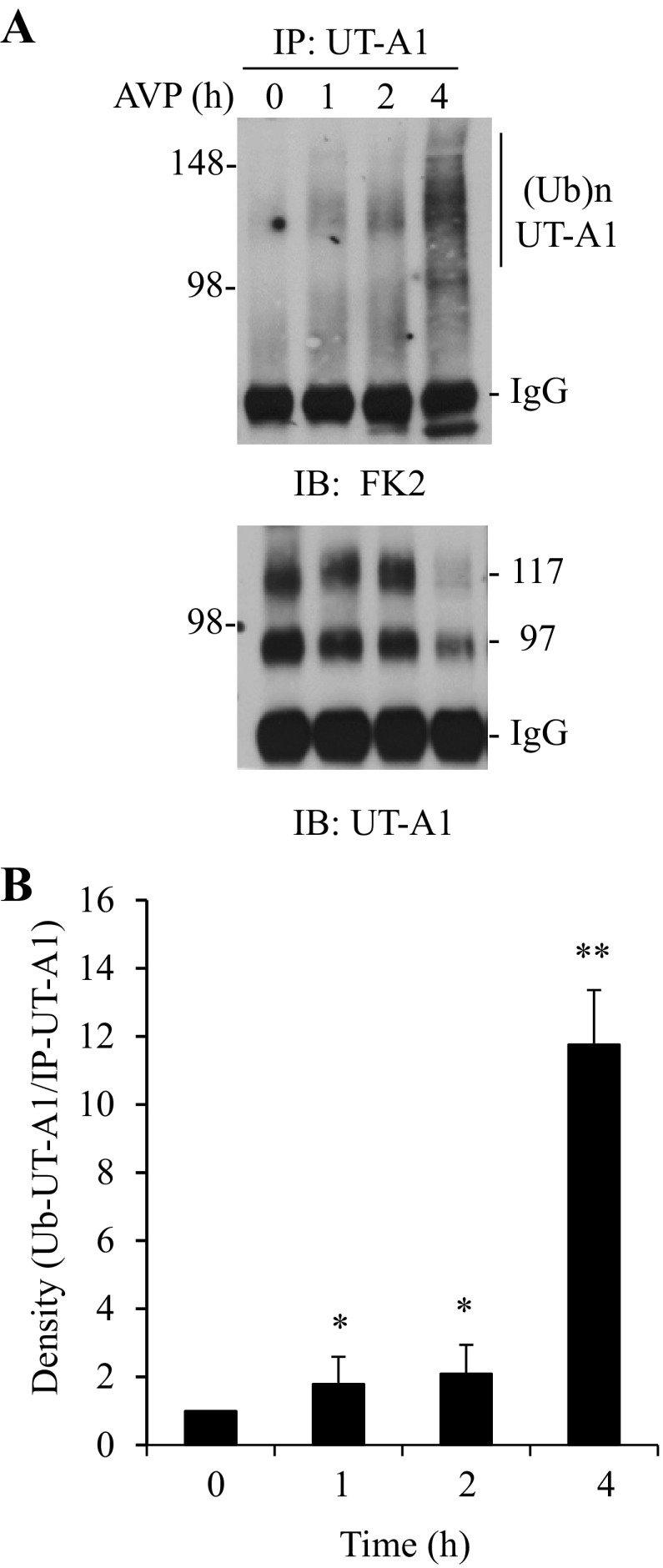
To examine whether activation of UT-A1 also induces UT-A1 ubiquitination in vivo, rat IMCD suspensions were prepared and treated with vasopressin for different time periods. Ubiquitinated UT-A1 was examined by immunoprecipitation with UT-A1 antibody followed by immunoblotting with ubiquitin antibody. Vasopressin treatment-induced UT-A1 ubiquitination is largely detected by FK2 (Fig. 7) but not by FK1 (data not shown). This is consistent with the in vitro data in Fig. 1 showing that activation of the cAMP/PKA pathway by FSK in cells, or vasopressin in rat IMCD suspensions, causes UT-A1 monoubiquitination. The effect of vasopressin treatment on UT-A1 ubiquitination is observed at 1 h, is increased at 2 h, and is markedly increased at 4 h. The same membrane was reprobed with UT-A1. At 4 h of treatment, UT-A1 was significantly decreased, corresponding with increased UT-A1 ubiquitination and protein degradation.
Fig. 7.
Ex vivo study of vasopressin on UT-A1 ubiquitination. A: rat kidney IMCD suspensions were incubated with 0.1 μM vasopression (AVP) at 37°C for the indicated times. UT-A1 ubiquitination was evaluated by immunoprecipitation with UT-A1 and subsequently immunoblotted with FK2 ubiquitin antibody. The same membrane was stripped and reprobed with UT-A1 antibody. B: densitometry analysis of ubiquitinated UT-A1 from three experiments (n = 3). The relative intensity of time 0 was set as 1 (*P < 0.05; **P < 0.01).
DISCUSSION
Eukaryotic cells contain two major proteolytic systems, the lysosome and the 26S proteasome system, that mediate protein degradation. We (7) and another group (28) reported that inhibition of proteasome activity, but not lysosome activity, stabilizes the UT-A1 proteins, indicating that the UT-A1 degradation pathway involves ubiquitination and degradation by the 26S proteasome rather than by the lysosome pathway. In this study we provided new evidence that activation of the cAMP/PKA pathway by FSK stimulates UT-A1 to undergo monoubiquitination and lysosome mediated-protein degradation, which is distinct from that of the unstimulated condition. We made several observations: 1) FSK stimulation promotes cell plasma membrane UT-A1 monoubiquitination; 2) the ubiquitin E1 enzyme inhibitor PYR-41 inhibits FSK-induced UT-A1 endocytosis and degradation; 3) degradation of UT-A1 induced by FSK is blocked by lysosomal but not proteasomal inhibitors; 4) FSK-stimulated endocytic UT-A1 is mainly localized in endosomes and lysosomes; 5) mutation of PKA phosphorylation sites Ser486 and Ser499 ameliorates FSK-induced UT-A1 ubiquitination; and 6) vasopressin treatment ex vivo induces kidney inner medulla UT-A1 ubiquitination.
Protein ubiquitination is a much more sophisticated process than other posttranslational modification such as phosphorylation and other processes. This is mainly due to the ability of ubiquitin to form various and different linkages. Multiple ubiquitin moieties transferred to target proteins generates a polyubiquitin chain. A ubiquitin molecule has seven lysine residues (K6, K11, K27, K29, K33, K48, and K63), and all can serve as a base for ubiquitin chain elongation (33). Monoubiquitination transfers one single ubiquitin to one single lysine or several monoubiquitin molecules to multiple lysine residues. The ubiquitinated proteins can be degraded by either the proteasome or lysosome system. In general, polyubiquitination targets proteins for degradation by the 26S proteasome, and monoubiquitination facilitates the internalization and degradation of membrane proteins by the endosome-lysosome pathway. Some membrane proteins are capable of being degraded by proteasomal and lysosomal pathways (1), depending on the type of ubiquitination (monoubiquitin vs. polyubiquitin) and the state of the protein complex (phosphorylation, etc). Endogenous ENaC in renal A6 cells is degraded by the proteasome complex, but heterologously expressed ENaC subunits are degraded by both lysosomal and proteasomal systems in MDCK cells (22). In addition, to be modified by polyubiquitin and subsequently degraded in the proteasome, as reported earlier (7, 28), in the current study, for the first time we found that UT-A1 protein undergoes monoubiquitin modification and a lysosome-dependent degradation when it is activated/phosphorylated. Thus UT-A1 can be polyubiquitinated and degraded through a proteasome pathway and can also be monoubiquitinated and degraded in a lysosome system. The two different pathways of UT-A1 ubiquitination and degradation depend on the state of the protein and play important roles under different physiological conditions.
Although some membrane protein endocytosis is mediated by polyubiquitination (32, 37) or even in a ubiquitination-independent manner (11, 35), accumulating evidence has shown that monoubiquitin can serve as an efficient sorting signal for both membrane protein internalization and endososomal-lysosomal targeting, such as for GPCR, RTKs, hERG, and skAE1 (15, 24, 30, 31). We previously reported that UT-A1 internalization can occur through both caveolae and clathrin-coated pits (CCP) under nonstimulation conditions (20). FSK stimulation promotes UT-A1 ubiquitination and endocytosis. The increased UT-A1 internalization is predominantly through a clathrin-mediated route (29). Here, we demonstrated that FSK-induced UT-A1 ubiquitination occurring mainly on the cell membrane is monoubiquitination. We propose that after activation by vasopressin in vivo (or FSK in vitro), UT-A1 is monoubiquitinated and that this monoubiquitination triggers cell membrane UT-A1 endocytosis to early endosome, and subsequently targets UT-A1 to the lysosome for degradation.
As shown in the current study (Fig. 4), FSK stimulation causes UT-A1 ubiquitination, rapid internalization, and protein transfer to early endosomes. In early endosomes, not all proteins are destined to the lysosomes for degradation; some could be sorted to recycle back to the plasma membrane (18). FSK stimulation increases internalized UT-A1 from endosomes to lysosomes for degradation. We also believe that some UT-A1 could be deubiquitinated and then recycled back to the plasma membrane. However, the current study is not able to answer whether FSK stimulation increases or decreases UT-A1 recycling back to the cell membrane. Further experiments will be required to address this interesting issue.
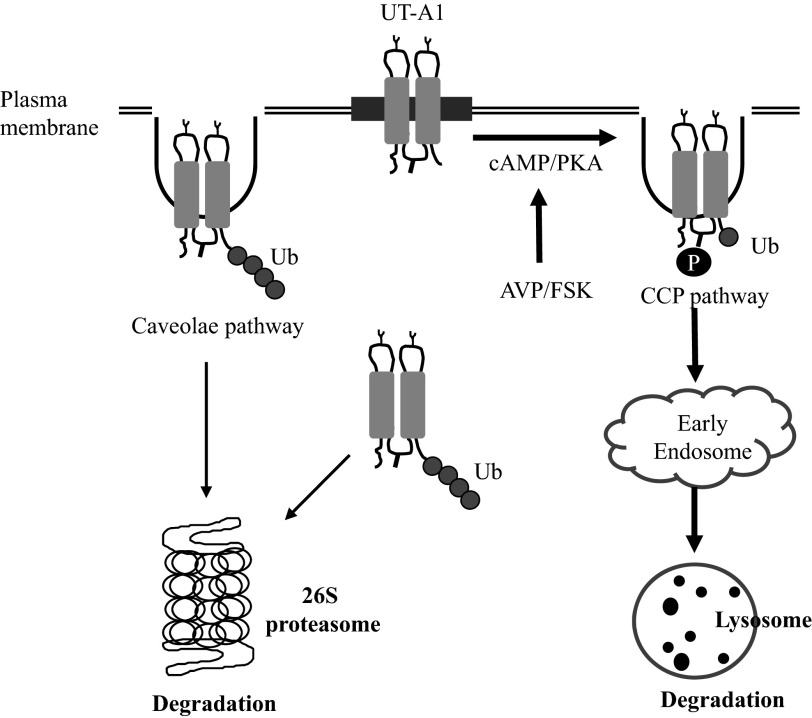
In summary, the major finding of this study is that activation of cAMP/PKA by FSK promotes UT-A1 monoubiquitination, ubiquitination-dependent internalization, and lysosome-mediated protein degradation. This is an important amendment to the early finding by our group (7) and another group (28) that UT-A1 undergoes polyubiquitination and is degraded in the proteasome system independently of any stimulation. Fig. 8 illustrates UT-A1 ubiquitination, endocytosis, and protein degradation under basal and stimulated conditions. UT-A1 has two endocytic pathways (20) and two protein degradation pathways [current study and (7, 28)]. The detailed regulatory mechanisms of how the UT-A1 route to these two different endocytic pathways and the two different degradation systems could be very complicated; however, a key regulator of the sorting, trafficking, and turnover of UT-A1 is ubiquitination. We presume that the caveolin pathway is responsible for constitutive UT-A1 internalization, whereas the clathrin-coated pit pathway may mediate the regulated endocytosis of UT-A1 stimulated by vasopressin/FSK, and that this pathway is accelerated by monoubiquitination. The monoubiquitinated UT-A1 is trafficked to the lysosome for degradation. In contrast, cytosolic UT-A1, misfolded UT-A1 from the endoplasmic reticulum, and constitutively internalized cell surface UT-A1 (mostly from the caveolae-mediated endocytic pathway) is polyubiquitinated and targeted to the proteasome for degradation. Needless to say, both endocytic pathways, both ubiquitination processes, and both protein degradation systems are important and required for proper cell functions. They cooperate in concert to maintain the cell in perfect homeostasis under both normal and stimulated conditions.
Fig. 8.
Illustration of UT-A1 ubiquitination, internalization, and degradation. In normal conditions, cytosolic UT-A1 (including misfolded UT-A1 from endoplasmic reticulum) and constitutively internalized cell surface UT-A1 (mostly from the caveolae-mediated endocytic pathway) is polyubiquitinated and degraded in a 26S proteasome system. However, upon AVP/FSK stimulation, UT-A1 is phosphorylated and processed for monoubiquitination at the cell surface and internalized via the clathrin-coated pits (CCP) pathway. The internalized monoubiquitinated UT-A1 is trafficked to early endosome, then targeted to the lysosome for degradation. AVP, arginine vasopressin; PKA, protein kinase A.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-087838 to G. Chen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.S. and G.C. conception and design of research; H.S. and M.C. performed experiments; H.S., M.C., J.M.S., and G.C. analyzed data; H.S., M.C., J.M.S., and G.C. interpreted results of experiments; H.S., M.C., and G.C. prepared figures; H.S. and G.C. drafted manuscript; J.M.S. and G.C. edited and revised manuscript; G.C. approved final version of manuscript.
REFERENCES
- 1.Ancot F, Leroy C, Muharram G, Lefebvre J, Vicogne J, Lemiere A, Kherrouche Z, Foveau B, Pourtier A, Melnyk O, Giordano S, Chotteau-Lelievre A, Tulasne D. Shedding-generated Met receptor fragments can be routed to either the proteasomal or the lysosomal degradation pathway. Traffic 13: 1261–1272, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Beal R, Deveraux Q, Xia G, Rechsteiner M, Pickart C. Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc Natl Acad Sci USA 93: 861–866, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blount MA, Mistry AC, Fröhlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem 282, 37885–37893, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Fröhlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem 281: 27436–27442, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Howe AG, Xu G, Fröhlich O, Klein JD, Sands JM. Mature N-linked glycans facilitate UT-A1 urea transporter lipid raft compartmentalization. FASEB J 25: 4531–4539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Huang H, Fröhlich O, Yang Y, Klein JD, Price SR, Sands JM. MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am J Physiol Renal Physiol 295: F1528–F1534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem 78: 857–902, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Eden ER, Huang F, Sorkin A, Futter CE. The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic 13: 329–337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksen J, Bjørn-Yoshimoto WE, Jørgensen TN, Newman AH, Gether U. Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons. J Biol Chem 285: 27289–27301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Urea transport in MDCK cells that are stably transfected with UT-A1. Am J Physiol Cell Physiol 286: C1264–C1270, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Fujimuro M, Sawada H, Yokosawa H. Production and characterisation of monoclonal antibodies specific to multiubiquitin chains of polyubiquitinated proteins. FEBS Lett 349: 173–180, 1994 [DOI] [PubMed] [Google Scholar]
- 14a.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol 5: 461–466, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Han SO, Xiao K, Kim J, Wu JH, Wisler JW, Nakamura N, Freedman NJ, Shenoy SK. MARCH2 promotes endocytosis and lysosomal sorting of carvedilol-bound β(2)- adrenergic receptors. J Cell Biol 199: 817–830, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry AG, Hislop JN, Grove J, Thorn K, Marsh M, von Zastrow M. Regulation of endocytic clathrin dynamics by cargo ubiquitination. Dev Cell 23: 519–532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicke L. Gettin' down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol 9: 107–112, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19: 141–172, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Feng X, Zhuang J, Fröhlich O, Klein JD, Cai H, Sands JM, Chen G. Internalization of UT-A1 urea transporter is dynamin dependent and mediated by both caveolae- and clathrin-coated pit pathways. Am J Physiol Renal Physiol 299: F1389–F1395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans 37: 937–953, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Leon S, Haguenauer-Tsapis R. Ubiquitin ligase adaptors: regulators of ubiquitylation and endocytosis of plasma membrane proteins. Exp Cell Res 315: 1574–1583, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Malik B, Price SR, Mitch WE, Yue Q, Eaton DC. Regulation of epithelial sodium channels by the ubiquitin-proteasome proteolytic pathway. Am J Physiol Renal Physiol 290: F1285–F1294, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Musch MW, Puffer AB, Goldstein L. Volume expansion stimulates monoubiquitination and endocytosis of surface-expressed skate anion-exchanger isoform. Am J Physiol Regul Integr Comp Physiol 294: R1657–R1665, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci USA 93: 5495–5500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickart CM, Fushman D. Endosomal transport via ubiquitination. Trends Cell Biol 21: 647–655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sands JM. Molecular mechanisms of urea transport. J Membr Biol 191: 149–163, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Stewart GS, O'Brien JH, Smith CP. Ubiquitination regulates the plasma membrane expression of renal UT-A urea transporters. Am J Physiol Cell Physiol 295: C121–C129, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Su H, Carter BC, Laur O, Sands JM, Chen G. Forskolin stimulation promotes urea transporter UT-A1 ubiquitination, endocytosis, and degradation. Am J Physiol Renal Physiol 303: F1325–F1332, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun T, Guo J, Shallow H, Yang T, Xu J, Li W, Hanson C, Wu JG, Li X, Massaeli H, Zhang S. The role of monoubiquitination in endocytic degradation of human Ether-a-go-go-related Gene (hERG) channels under low K+ condition. J Biol Chem 286: 6751–6759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell 1: 193–202, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Varghese B, Barriere H, Carbone CJ, Banerjee A, Swaminathan G, Plotnikov A, Xu P, Peng J, Goffin V, Lukacs GL, Fuchs SY. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol Cell Biol 28: 5275–5287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vernace VA, Schmidt-Glenewinkel T, Figueiredo-Pereira ME. Aging and regulated protein degradation: who has the UPPer hand. Aging Cell 6: 599–606, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wall SM, Han JS, Chou CL, Knepper MA. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 262: F989–F998, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Ye S, Cihil K, Stolz DB, Pilewski JM, Stanton BA, Swiatecka-Urban A. c-Cbl facilitates endocytosis and lysosomal degradation of cystic fibrosis transmembrane conductance regulator in human airway epithelial cells. J Biol Chem 285: 27008–27018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Li S, Patterson C, You G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol Pharmacol 83: 217–224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]