Abstract
Diabetic nephropathy, the most common cause of progressive chronic renal failure and end-stage renal disease, has now reached global proportions. The only means to rescue diabetic patients on dialysis is renal transplantation, a very effective therapy but severely limited by the availability of donor kidneys. Hence, we tested the role of intravenous renal cell transplantation (IRCT) on obese/diabetic Zucker/SHHF F1 hybrid (ZS) female rats with severe ischemic and diabetic nephropathy. Renal ischemia was produced by bilateral renal clamping of the renal arteries at 10 wk of age, and IRCT with genetically modified normal ZS male tubular cells was given intravenously at 15 and 20 wk of age. Rats were euthanized at 34 wk of age. IRCT with cells expressing serum amyloid A had strong and long-lasting beneficial effects on renal function and structure, including tubules and glomeruli. However, donor cells were found engrafted only in renal tubules 14 wk after the second infusion. The results indicate that IRCT with serum amyloid A-positive cells is effective in preventing the progression of chronic kidney disease in rats with diabetic and ischemic nephropathy.
Keywords: chronic renal failure, diabetic nephropathies, organ regeneration, serum amyloid A
progressive chronic kidney disease (CKD) transitions to end-stage renal disease (ESRD) after reaching an undefined tipping point. This critical transition and the overall pathophysiology that drives the progression of CKD to that point are not well understood (31). Progressive CKD remains untreatable, and its conversion to ESRD is generally unstoppable. The most common cause of the progression of CKD to ESRD is diabetic nephropathy (DN) (13), a massive global problem with a prevalence of antecedent microalbuminuria and macroalbuminuria of 49% and impaired renal function of 22% in type 2 diabetics who had been unaware of having nephropathy (23). Our work with DN has revealed that acute renal injury, common in diabetic patients, is a major contributor to progressive CKD (13), a finding supported by epidemiological data (10, 28). In other words, very large numbers of diabetics are at high risk of progressive CKD, and many of those face the prospect of an irrevocable renal decline to ESRD. For some, renal transplantation is an effective way to treat ESRD; however, most ESRD patients encounter difficult barriers to reach transplantation: 20% are placed on waiting lists for kidney transplantation, and, after several years, only 10% eventually receive a kidney (33). The majority of ESRD patients remain on dialysis, and most patients ultimately succumb to painful complications.
One promising approach to CKD-ESRD is regenerative nephrology, and attempts have been made to restore renal function in animal models with stem cells, a logical sequel to reports (11, 29) demonstrating that endogenous stem cells are mobilized to the injured kidney, although the latter view was subsequently refuted (2, 21). Stem cell transplantation has been performed with bone marrow-derived mesenchymal stem cells (2), adipose tissue-derived stem cells (4), and embryonic stem cells (7). Unfortunately, renal engraftment of donor stem cells, or, for that matter, donor stem cell differentiation into renal cells, has not been verified in DN to our knowledge. We devised a completely different strategy and used adult renal cell transplantation in DN with CKD. We accomplished long-term kidney cell engraftment and successful renal regeneration with auto-transplants of adult primary kidneys cells reprogrammed to express the tubulogenic protein serum amyloid A1 (SAA) (19). We now report that allogeneic rat adult kidney cells, given intravenously, prevent long-standing progressive CKD caused by ischemia and DN in obese rats. The diabetic/ischemic model was designed to mimic DN in humans (12, 14), in which overt and/or subclinical episodes of acute kidney injury from ischemia and the resulting inflammation can contribute to the progression of CKD (13, 28, 32). The two separate doses of reprogrammed renal epithelial cells were given after the renal disease was well established, and engrafted donor cells were identified in recipient kidneys 14 wk after the last dose of cells. We also convey here that cell transplantation with SAA-expressing tubular cells restored damaged microvascular networks, assured the structural integrity of glomeruli and tubules, and prevented glomerular and interstitial fibrosis, the main pathological determinants of the progression of CKD in DN (5). We propose that intravenous renal cell transplantation (IRCT) is safe and very effective in preventing the progression of CKD in rats with advanced ischemic DN.
METHODS
Primary renal tubular cells.
Primary renal tubular cells were obtained from lean, age-matched male Zucker/SHHF F1 hybrid (ZS) rats (Charles River, Wilmington, MA), and renal cells from one lean rat were equally distributed to four obese rats, one in each of the four groups. Lean rats were euthanized by removal of both kidneys under general anesthesia. The kidney cortices were minced with scissors in S1 medium (described below) and digested with collagenase type IV (6 mg/dl, Worthington, Lakewood, NJ) at 37°C in 38% O2 and 5% CO2 for 50 min. Renal tubules were then separated by a Percoll gradient (19), divided into two sets, resuspended in 300 μl of transfection buffer (containing 20 mM HEPES, 142 mM KCl, 6 mM dextrose, 0.7 mM Na2HPO4, 5 mM MgATP, and 10 μM EGTA), and transfected by electroporation (40 V × 12 ms × 500 ms × 6 pulses). The isolated tubules were a mix of different tubule segments: approximately 25% proximal (positive for organic anion transporter 1), 20% thick ascending limb (positive for Tamm Horsfall protein), 15% collecting tubule (positive for aquaporin 2), and 5% distal convoluted tubule (positive for thiazide-sensitive cotransporter) (19). The remaining cells were not identified by these markers (19). Control or SAA1-negative (group A) tubules were cotransfected with empty vector pcDNA3.1 (30 μg), pAcGFP1-C1 [15 μg, green fluorescent protein (GFP) is the cytosolic label used to track cells in vivo, Clontech, Mountain View, CA], and pCruz HASIRT1 (15 μg, Addgene), which expresses sirtuin 1 (SIRT1), a NAD+-dependent deacetylase that may have a role in resistance to cellular stress and, thus, has been used in previous transplant protocols (19). For SAA1-positive cells (group B), pcDNA3.1 was replaced with pcDNA3.1-SAA1 plasmid (30 μg), which was manufactured and sequenced in our laboratory; its construction has been previously reported (15, 19). Transfection efficiencies were >70% (19).
Cotransfected tubules were cultured in S1 medium. Two liters of S1 medium contained 10.7 g F-12 HAM, 8.32 g DMEM, 0.29 g l-glutamine, 4.78 g HEPES, 1.7 μg sodium selenite, 0.11 g sodium pyruvate and 3.2 ml phenol red, and pH was adjusted to 7.4 with sodium bicarbonate (Sigma). S1 medium was supplemented with 200 ng/ml hepatocyte growth factor and 400 ng/ml epidermal growth factor (R&D Systems, Minneapolis, MN). The medium also contained 100 μg/ml hydrocortisone, 35 μg/ml insulin, 32 μg/ml transferrin, and 42 ng/ml sodium selenite (Sigma), with 20% FCS. G-418 (75 μg/ml) was added after 48 h of culture for selection. In preparation for transplantation, male renal tubular cells were lightly trypsynized after 7–8 days in culture, washed in PBS, and injected intravenously in the tail vein of obese, diabetic female rats at 15 wk of age and again at 20 wk of age.
Animal protocols.
All experiments were conducted in conformity with the “Guiding Principles for Research Involving Animals and Human Beings,” and approved by the Institutional Care and Use Committee. Female ZS rats (Charles River) were acquired at 8 wk of age and fed Purina diet 5008 with 27% protein, 17% animal fat, and 56% carbohydrate. Weights, sera, and urine were collected biweekly, and chemistries were measured by the Indianapolis Veterans Affairs clinical laboratory. Urine protein was measured via ELISA according to the manufacturer's protocol (Exocell, Philadelphia, PA). At 10 wk of age, rats were anesthetized with inhaled isofluorane (0.5–1%) and placed on a homeothermic table to maintain the body core temperature at ∼37°C. In two groups of rats, both renal pedicles were occluded for 25 min with microaneurysm clamps as previously described (18). In two other groups, sham surgery consisted of the identical surgical procedure except that the renal pedicles were not clamped. Two groups of rats were later infused with donor cells: one sham-operated (sham) group (SA group; n = 6) received group A cells and another sham group (SB group; n = 6) received group B cells (see above). One ischemic group (IA group; n = 9) received group A cells, and the other ischemic group (IB group; n = 9) received group B cells (see above). The cell dose (106 cells/rat) was based on a previous study (19). A second infusion was administered given the chronicity of the injury.
Histology and immunohistochemistry.
Kidney sections were fixed in 3.8% paraformaldehyde and embedded in paraffin, and 5-μm sections obtained for Masson's trichrome to stain connective tissue, periodic acid-Schiff (PAS) to image cellular morphology, and Leder's stain to visualize neutrophils. Areas of glomerular and peritubular fibrosis were quantified using Metamorph imaging-processing software (Sunnyvale, CA) and expressed as fractional areas per ×200 microscope field, covering all available surfaces in all coded kidneys. Atrophic tubules were counted in blinded PAS-stained sections. Renal neutrophils were also counted in the kidney sections. Additional kidney sections were used to visualize the microvasculature by immunohistochemistry using an anti-Von Willebrand factor antibody (Autostainer procedure by Dako, Carpinteria, CA) (26). The microvasculature density was estimated from the pixel density representative of von Willebrand factor (Metamorph software). Immunohistochemistry for proliferating cell nuclear antigen (PCNA) was performed using anti-PCNA (sc-7907, Santa Cruz Biotechnology, Santa Cruz, CA) and Texas red secondary antibody. Quantification of PCNA-positive and apoptotic cells [determined by characteristic nuclear morphology of 4′,6-diamidino-2-phenylindole (DAPI)-labeled nuclei (14, 16)] was performed on blinded sections. Kidneys fixed in 4% paraformaldehyde were also sectioned into 100-μm slices with a Vibratome (Vibratome, St. Louis, MO). Sections were immersed in PBS with 0.2% Triton X-100 for 5 min, washed three times with PBS, blocked for 15 min in PBS with 0.2% BSA, incubated with the rabbit primary anti-SAA antibody (16, 19) in PBS for 30 min at 37°C, and then washed in PBS. Fluorescent secondary antibody, Texas red-conjugated donkey anti-rabbit (catalog no. 111-075-045, Jackson ImmunoResearch, West Grove, PA), was then applied for 30 min at 37°C followed by a wash. Nuclei were stained with the nuclear dye DAPI (Molecular Probes, Eugene, OR). Renal images of visualized intrinsic GFP and Texas red-labeled SAA were collected with a Leica DMI 3000B fluorescence microscope.
Fluorescent in situ hybridization of the Y chromosome.
Fluorescent in situ hybridization (FISH) was used to localize the Y chromosome in female kidneys months after IRCT with male renal cells as previously reported (19). In brief, at euthanization, recipient kidneys were immediately fixed in 10% neutral formalin, embedded in paraffin, cut along the sagittal main axis into 5-μm sections, and affixed to glass slides. Slides were sequentially placed in xylene for 15 min twice, 100% ethanol for 5 min twice, boiling saline-sodium citrate (SSC) buffer for 10 min, cooled down to room temperature, and washed with distilled water. Sections were then digested with 0.4% pepsin and 0.9% NaCl (pH 1.5) at 37°C for 50 min, washed with distilled water, immersed in 2× SSC for 10 min twice, and air dried. FISH was conducted with the fluorescent-labeled rat Y chromosome probe (Rat Idetet Chr Y Paint probe red, ID 556, catalog no. IDRR1070-0111, excitation: 548 nm and emission: 573 nm, ID Labs Biotechnology, London, ON, Canada) diluted 1:10, and 5 μl were applied to the prepared kidney section. The slide was covered, and kidney DNA and the probe were denatured at 69°C for 2 min and then hybridized overnight at 40°C. The slide was then uncovered and placed in warmed 0.4× SSC with 0.3% Nonidet P-40 (70°C for 2 min). The slide was incubated in 2× SSC solution at room temperature for 1 min. The section was then washed with distilled water, air dried, mounted with DAPI, covered, and imaged on a fluorescent microscope.
Renal SAA1 mRNA.
RT-PCR was used for the amplification of SAA1 mRNA in recipient kidneys at euthanization. RNA was extracted from homogenized renal cortices in lysis buffer, isolated with a purification kit as recommended by vendor (catalog no. 12183-555, Invitrogen), and cleaned with an RNeasy Mini kit as recommended by vendor (catalog no. 74104, Qiagen, Valencia, CA). For RT-PCR, 2 μg of total RNA were used to synthesize cDNA with an AffinityScript QPCR cDNA Synthesis Kit as recommended by the vendor (catalog no. 600559, Aligent Technologies, Santa Clara, CA). The murine SAA1 mRNA was amplified using the following primers (15): forward 1, 5′-CGCCACCATGGAGGGTTTTTTTCATTTGTTCAC-3′; forward 2, 5′-TACAGGCTAGCGCCACCATGGAGGGTTT-3′; and reverse 1/2, 5′-TCAGGTGGATCCCTCAGTATTTGTCAG-3′.
Twenty-five microliters of PCR master solution contained 1× PCR buffer, 0.25 mM dNTP, 0.25 μM primers, and 0.25 μl Herculase II DNA polymerase (catalog no. 600675-51, Aligent). cDNA (0.5 μl) was added to each tube, and PCR amplification was conducted: 95°C at 5 min for 1 cycle, 95°C for 20 s, 57.5°C for 30 s, and 72°C for 30 s for 40 cycles, and then at 72°C for 7 min for 1 cycle (PCR System 2400, Perkin-Elmer, San Jose, CA). PCR products were separated on a 2.5% agarose gel.
Identification of DNA encoding the male sex-determining region on chromosome Y localized in female kidneys.
DNA was extracted from the recipient kidneys with the Wizard Genomic DNA Purification Kit as indicated by the manufacturer (Promega, Madison, WI). Specific sex-determining region on chromosome Y (SRY) DNA was then amplified from extracted kidney DNA using the following primers (25): forward 1 5′-AAGCGCCCCATGAATGC-3′; and reverse 1/2, 5′-AGCCAACTTGCGCCTCTCT-3′.
Twenty-five microliters of PCR master solution contained 1× PCR buffer, 0.25 mM dNTP, 0.25 μM primers, and 0.25 μl Herculase II DNA polymerase (catalog no. 600675-51, Aligent). Kidney DNA (0.5 μl) was added to each tube, and PCR amplification was conducted: 95°C for 5 min for 1 cycle, 95°C for 20 s, 57.5°C for 30 s, and 72°C for 20 s for 35 cycles, and then at 72°C for 7 min for 1 cycle (PCR System 2400, Perkin-Elmer). PCR products were separated on a 2.5% agarose gel.
Statistics.
Data are expressed as means ± SE. ANOVA was used to determine if differences among mean values reached statistical significance. Student's t-test (two tailed, two sample, unequal variance) was used for comparisons between groups (GraphPad Prism, La Jolla, CA). Tukey's test was used to correct for multiple comparisons. The null hypothesis was rejected at P < 0.05.
RESULTS
There were four major groups of female obese diabetic rats: two groups were subjected to sham surgery and two other groups were subjected to bilateral renal ischemia at 10 wk of age. Rats were cell transplanted at 15 and 20 wk of age, and all were euthanized at 34 wk of age. Sham rats were twice infused with SAA negative (group A) cells (control cells, SA group; n = 6) or with SAA-expressing (group B) cells (reprogrammed, SB group; n = 6). Rats subjected to bilateral renal ischemia were also twice infused with SAA-negative cells (control cells, IA group; n = 9) or with SAA-expressing (group B) cells (reprogrammed, IB group; n = 9). Two rats died in the group of nine rats with added renal ischemia and transplanted with SAA-negative cells; the rats died 2 and 8 wk after the second cell transplant. Animal weights and serum glucose values were comparable in all four diabetic groups with no statistically significances observed over the course of the study. (Mean blood glucose levels in the four diabetic groups were significantly higher than those in lean rats; Table 1.)
Table 1.
Mean weights and serum glucose for each group
| Weight, g |
Glucose, mg/dl |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | 10 wk | 16 wk | 20 wk | 34 wk | 10 wk | 16 wk | 20 wk | 34 wk |
| SA | 319 ± 9.2 | 426 ± 5.5 | 488 ± 7.9 | 566 ± 9.8 | 170 ± 18 | 173 ± 16 | 175 ± 15 | 158 ± 5.5 |
| SB | 317 ± 9.2 | 426 ± 6.8 | 487 ± 6.1 | 577 ± 4.2 | 166 ± 8.1 | 161 ± 9.7 | 180 ± 5.9 | 154 ± 3.2 |
| IA | 321 ± 9.5 | 426 ± 10 | 479 ± 11 | 552 ± 17 | 174 ± 8.8 | 175 ± 9.0 | 173 ± 14 | 150 ± 6.2 |
| IB | 311 ± 9.9 | 427 ± 11 | 481 ± 11 | 584 ± 28 | 174 ± 9.4 | 169 ± 16 | 182 ± 10 | 155 ± 5.1 |
| Lean | 314 ± 34 | 414 ± 10 | 478 ± 16 | 530 ± 12 | 122 ± 7.6 | 137 ± 5.2 | 124 ± 3.2 | 137 ± 1.3 |
Rats were divided into the following groups: rats subjected to sham operation and that received control, serum amyloid A (SAA)-negative tubular cells (SA group), rats subjected to sham operation and that received SAA-expressing tubular cells (SB group), rats subjected to renal ischemia and that received control, SAA-negative tubular cells (IA group), and rats subjected to renal ischemia and that received SAA-expressing tubular cells (IB group). Lean rats were lean, age-matched male Zucker/SHHF F1 hybrid rats. Mean blood glucose in the diabetic groups (SA, SB, IA, and IB groups) was significantly greater than in lean animals at each time point.
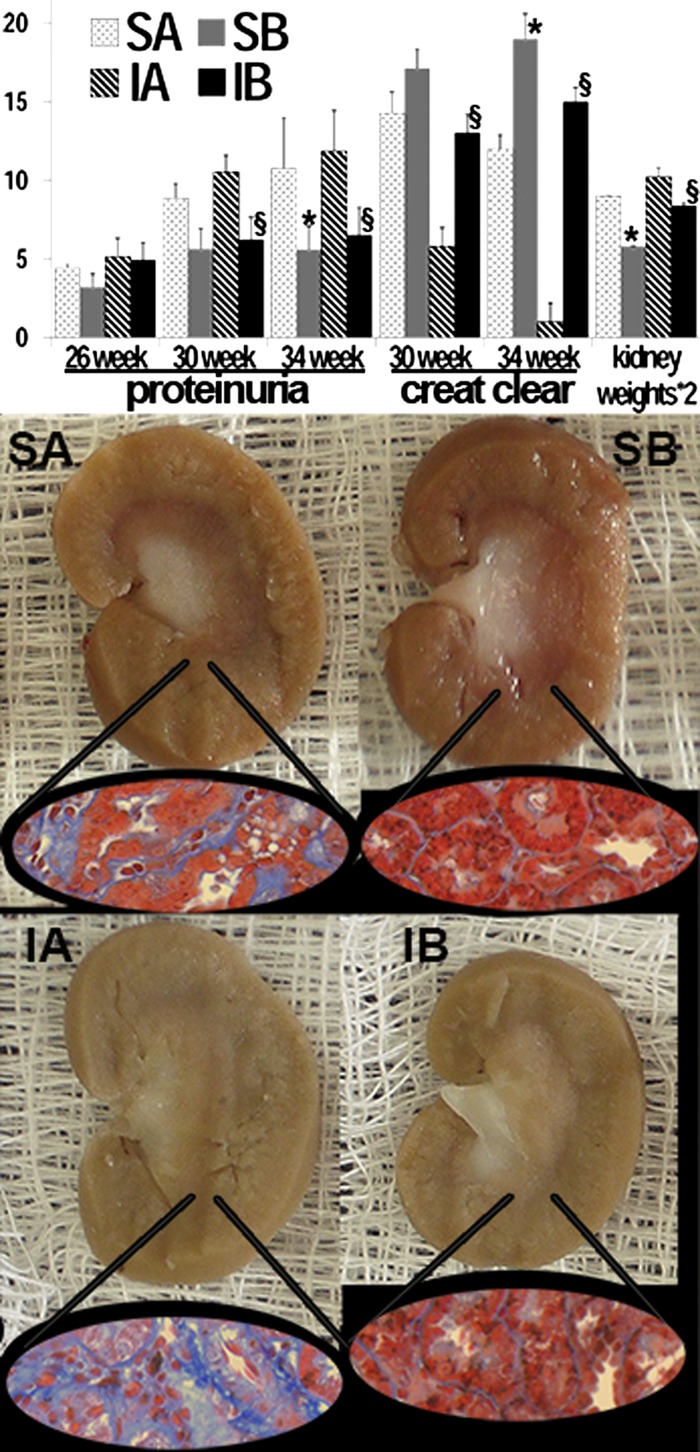
Urinary protein excretion was higher in rats from the sham and ischemic groups transplanted with control SAA-negative cells than in the two other respective groups transplanted with SAA-positive cells at 34 wk of age (Fig. 1). Unlike the control groups, proteinuria in the groups treated with SAA-positive cells did not worsen significantly from 26 to 34 wk. Renal function, as estimated from 24-h creatinine clearance, was significantly better in the ischemic group transplanted with SAA-positive cells than in the ischemic group transplanted with SAA-negative cells at 30 and 34 wk of age. Rat creatinine clearance data are affected by low creatinine production in rats and serum creatinine levels near detection limits. However, these results were complemented by histology (as described below). In addition, kidneys of sham and ischemic rats transplanted with SAA-negative cells were larger than kidneys of rats that received SAA-positive cells (Fig. 1). These large ischemic/diabetic kidneys from rats transplanted with SAA-negative cells were very pale and lacked corticomedullary differentiation, grossly consistent with advanced CKD, i.e., with widespread fibrosis and microvascular attenuation (Fig. 1). In marked contrast, the strikingly pallid appearance of ischemic/diabetic kidneys transplanted with SAA-negative cells was partly averted by cell transplantation with SAA-positive cells.
Fig. 1.
Effects of renal cell transplantation in diabetic nephropathy. Significant improvements in proteinuria (g albumin/g creatinine), creatinine clearance (ml/min ×10), and kidney weights (mg × 2) were seen in 34-wk-old rats that received serum amyloid A (SAA)-expressing cells at 15 and 20 wk of age (top). Better renal function was also seen in the SAA-positive postischemia group at 30 wk. Lack of progressive proteinuria from 26 to 34 wk was seen in the SAA-positive groups. Kidneys from obese/diabetic rats treated with control cells were pale and avascular with hemorrhage and fibrosis (visualized with trichrome stain; insets), in contrast to those from rats given SAA-expressing cells (bottom). Rats were divided into the following groups: rats subjected to sham operation and that received control, SAA-negative tubular cells (SA group; n = 6 rats), rats subjected to sham operation and that received SAA-expressing tubular cells (SB group; n = 6 rats), rats subjected to renal ischemia and that received control, SAA-negative tubular cells (IA group; n = 7 rats), and rats subjected to renal ischemia and that received SAA-expressing tubular cells (IB group; n = 9 rats). *P < 0.05 vs. the SA group; §P < 0.05 vs. the IA group.
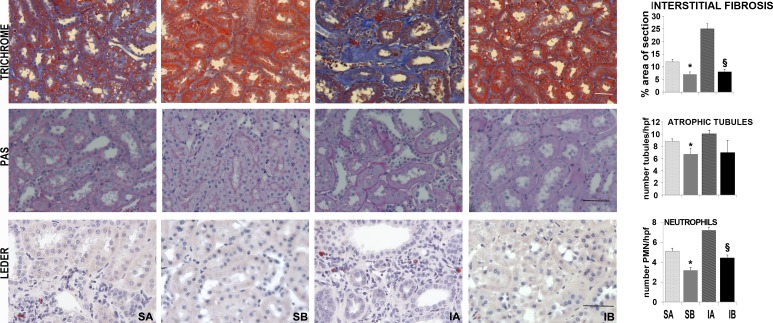
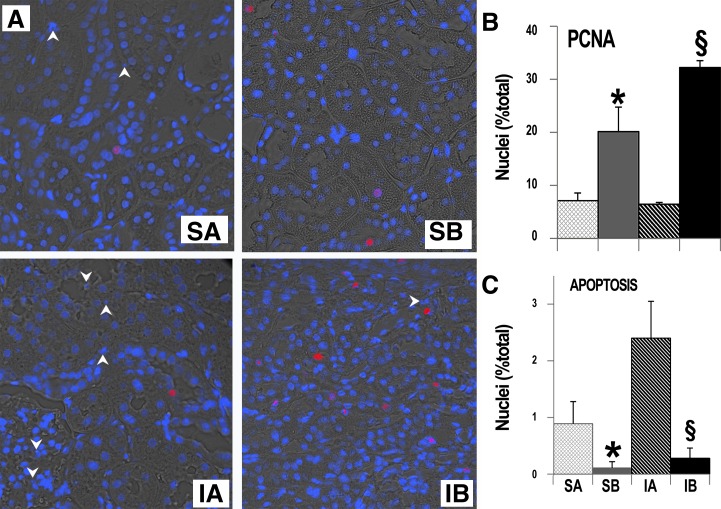
Three fundamental features of renal histology were then delineated with specific stains, as shown in Fig. 2. Masson's trichrome stain showed extensive peritubular fibrosis in sham rats transplanted with SAA-negative cells, a feature even more pronounced in ischemic rats transplanted with SAA-negative cells. In contrast, peritubular or interstitial renal fibrosis was restricted to very small areas in sham and ischemic rats transplanted with SAA-positive cells. PAS stain, which was used to characterize the cellular integrity in tubules and glomeruli, revealed significant tubular and glomerular damage, with profound cellular loss in tubules and glomeruli of sham and ischemic rats transplanted with SAA-negative cells. Finally, Leder's stain exposed large numbers of peritubular neutrophils in sham and ischemic rats transplanted with SAA-negative cells, whereas the renal neutrophil number was much lower in rats transplanted with SAA-positive cells. As in human DN (13), the structural abnormalities in the SAA-positive groups may be more severe than the functional impairments. Nevertheless, these three pathological features of DN were dramatically reduced in rats transplanted with SAA-positive cells. In addition, we found that repeated SAA-positive cell transplants promoted cell proliferation, or DNA repair, as exemplified by the higher proportion of tubular nuclei expressing PCNA. Some, but not all, of the PCNA-positive cells were also GFP positive. In conjunction with the higher rates of tubular cell proliferation, we found lower rates of renal cell apoptosis among rats transplanted with SAA-positive cells. These findings were demonstrated weeks after the last cell transplant (Fig. 3).
Fig. 2.
Effects of renal cell transplantation on renal histology. Decreased fibrosis (blue, top), tubular atrophy (middle), and neutrophil infiltration (red cells, bottom) were observed in obese/diabetic rats treated with SAA-expressing cells both after sham surgery or renal ischemia. Representative trichrome-stained sections of cortex (top) as well as periodic acid-Schiff (PAS)-stained (middle) and Leder-stained (bottom) sections are included. The quantification of ×200 microscope fields/group is shown in the graphs. For interstitial fibrosis, n = 85 for the SA group, 92 for the SB group, 63 for the IA group, and 92 for the IB group. For atrophic tubules, n = 85 for the SA group, 92 for the SB group, 63 for the IA group, and 92 for the IB group. For neutrophil number, n = 45 for the SA group, 45 for the SB group, 79 for the IA group, and 47 for the IB group. Scale bar = 50 μm. *P < 0.05 vs. SA; §P < 0.05 vs. IA.
Fig. 3.
Effects of renal cell transplantation on cell proliferation. Significantly higher expression of proliferating cell nuclear antigen (PCNA) and less apoptosis were seen in kidneys from rats that received SAA-expressing (vs. control) cells. A: representative images stained with anti-PCNA, Texas red secondary antibody, and 4′,6-diamidino-2-phenylindole to label all nuclei. B and C: quantification of PCNA-positive (B) and apoptotic (C) nuclei. Forty-three images were evaluated. *P < 0.05 vs. SA; §P < 0.05 vs. IA.
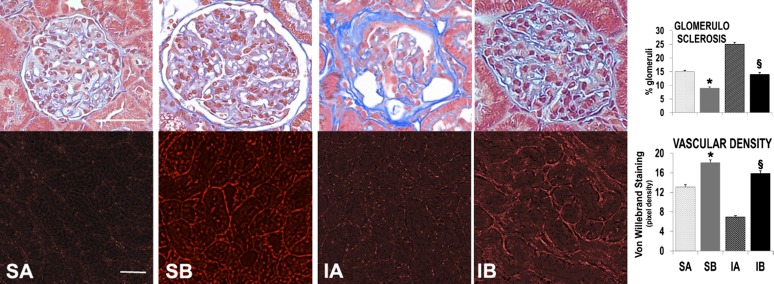
Glomerular sclerosis is characteristic in progressive CKD (5, 13), and it was extensive in ischemic/diabetic rats treated with SAA-negative cells and, although present, was significantly less in ischemic rats injected with SAA-positive cells. Whereas glomerular fibrosis was relatively minor in the two sham groups, the average fraction of glomerular fibrosis was still lower in sham rats injected with SAA-positive cells than in sham rats injected with SAA-negative cells. Therefore, transplantation with SAA-positive cells supported tubular and glomerular integrity. We deduced from these results that adequate nutrient delivery to those tissues required a competent microvasculature, specifically the maintenance of vulnerable peritubular capillaries (22). Accordingly, we labeled the renal peritubular microvasculature with an anti-Von Willebrand factor antibody (26) and evaluated the role of cell transplantation (Fig. 4). The images shown in Fig. 4 illustrate severe microvascular attenuation in the renal peritubular capillaries of ischemic/diabetic rats transplanted with SAA-negative cells. In contrast, this distinctive vascular pathology of progressive CKD in DN (22) was less in the groups treated with SAA-positive cells.
Fig. 4.
Effects of renal cell transplantation on glomerulosclerosis and vascular density. Significantly decreased glomerulosclerosis and improved microvascular density were observed in rats that received SAA-expressing cell transplants. Glomerulosclerosis was evaluated in trichrome-stained sections and vascular density by immunostaining with anti-von Willebrand factor antibody. Quantification is shown in the graphs. For numbers of glomeruli/group measured, n = 1,054 for the SA group, 888 for the SB group, 1,050 for the IA group, and 1,363 for the IB group. For numbers of ×200 microscope fields/group, n = 8 for the SA group, 15 for the SB group, 32 for the IA group, and 16 for the IB group. Scale bar = 50 μm. *P < 0.05 vs. SA; §P < 0.05 vs. IA.
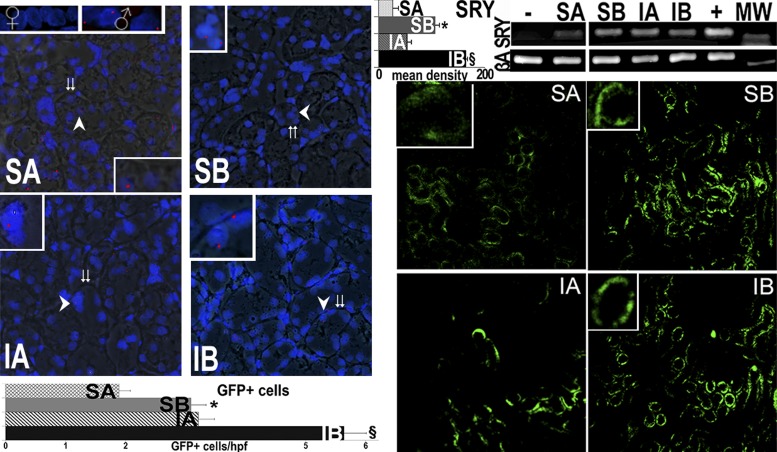
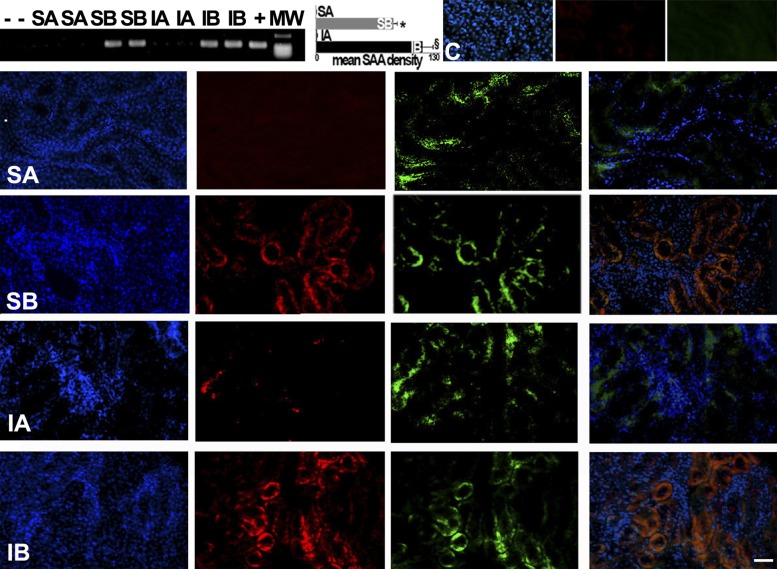
We then tested the possibility that sustained donor cell engraftment occurred during the long-term improvements in progressive CKD by cell transplantation. Four independent methods were used to identify male donor cells in female recipient kidneys, as shown in Figs. 5 and 6. FISH with a probe specific to the rat Y chromosome revealed its unmistakable presence in female recipient kidneys transplanted with male kidney cells, largely in cortical tubules. In addition, the presence of the SRY male determinant gene was confirmed by PCR in the female recipient kidneys and not in female kidneys that did not receive male cells (Fig. 5). Moreover, fluorescence microscopy of recipient kidneys showed that GFP-expressing donor cells were incorporated into the tubules, but not in glomeruli, of the host kidneys. GFP-positive cells were usually found in clusters of “grafted” tubules (Fig. 5). Furthermore, RT-PCR was used to demonstrate the expression of SAA mRNA in recipient kidneys. SAA mRNA was only expressed in the kidneys that received SAA-positive cells (Fig. 6). Finally, SAA protein expression in transplanted cells was confirmed by the colocalization of SAA immunofluorescence and GFP fluorescence only in sham and ischemic kidneys from rats that received SAA-positive cells (Fig. 6).
Fig. 5.
Male tubular cell engraftment in female kidneys. Engraftment of infused cells is shown via fluorescence in situ hybridization (FISH; red) for the Y chromosome. Merged fluorescence and bright images show FISH-positive cells from male donors in tubules of female kidneys 14 wk after the final cytotherapy treatment (age: 34 wk) as well as in normal males but not in female kidneys (insets show the areas at the arrows). Double arrows show tubular basement membranes. In addition, specific DNA encoding the male determinant gene sex-determining region on the Y chromosome (SRY) was found in female kidneys that received male tubular cells but not in female kidneys that did not receive cells (negative control). The positive controls correspond to DNA extracted from the normal liver of male Sprague-Dawley rats. Mean band density for the SRY PCR is presented in top graph. Right: GFP-positive cells within kidneys of rats treated with control (A) or SAA-positive (B) cells with quantification of GFP-positive cells in a total of 212 high-power fields (hpf). In contrast, GFP-positive cells were <1 cells/hpf in kidneys of normal rats without renal injury (16). GFP-positive cells were very rarely seen in other organs (Table 2). MW, molecular weight marker; βA, β-actin (to insure the equivalent DNA substrate for PCR). *P < 0.05 vs. SA; §P < 0.05 vs. IA.
Fig. 6.
SAA expression in recipient kidneys. The presence of recipient kidney SAA mRNA was detected by RT-PCR. Quantification of band density in multiple blots is shown in the graph. SAA mRNA was found in kidneys after reprogrammed cell infusions, demonstrating transcriptional activity. The representative fluorescence images show nuclei (blue), immunoreactive SAA (red), and GFP-positive cells (green) in each group [including normal controls (C) without renal injury]. In the right images, images of nuclei, SAA, and GFP are merged to demonstrate colocalization (orange) of SAA and GFP in SB and IB groups, consistent with expression in the same cells. Scale bar = 50 μm. *P < 0.05 vs. SA; §P < 0.05 vs. IA.
Table 2.
Mean number of green fluorescent protein-positive cells by organ removed at 34 wk
| Group | Lung | Liver | Spleen |
|---|---|---|---|
| SA | 0.06 ± 0.04 | 0.14 ± 0.04 | 0.20 ± 0.14 |
| SB | 0.14 ± 0.08 | 0.09 ± 0.09 | 0.05 ± 0.05 |
| IA | 0.09 ± 0.05 | 0.04 ± 0.04 | 0.07 ± 0.07 |
| IB | 0.11 ± 0.08 | 0.07 ± 0.05 | 0.13 ± 0.10 |
DISCUSSION
There is no effective way to arrest progressive CKD in DN where vasculopathy (22, 27, 31) and renal ischemia can advance the progression of CKD toward ESRD (32), constituting the main problem in nephrology today (24). Once a patient develops ESRD, renal transplantation is the only effective means of freedom from dialysis dependency. Unfortunately, the need for kidneys vastly exceeds availability, and while kidney transplantation is an excellent option, it is feasible only in a small minority of dialysis patients (33). Therefore, the numbers of diabetic CKD and ESRD patients continue to climb and, with that, the enormous economic cost required for their care (22a). Accordingly, new tactics are badly needed to limit the progression of CKD to ESRD in DN. In this report, we describe the role of IRCT (19) in obese and diabetic rats with an episode of ischemic acute kidney injury followed by progressive CKD. We termed this morbid sequence of events the postischemic inflammatory syndrome and have previously described its eventual outcome toward ESRD (12, 14). The reported ischemic/diabetic rat nephropathy model was designed to mimic progressive CKD from DN, a condition driven by subclinical episodes of renal ischemia and tubular necrosis (13). Cell transplants were performed on obese/diabetic rats with severe and progressive CKD at 15 and 20 wk of age, and rats were then followed for 14 wk more, to 34 wk of age. There were no attempts to modify dietary or metabolic factors on any of the groups. IRCT was performed with normal adult male ZS rat renal tubular cells reprogrammed to express a murine isoform of SAA1 (15) before transplantation. These reprogrammed primary renal cells grow out of harvested renal tubules transfected with the SAA gene and, after 1 wk, form de novo tubules in vitro, the basic structure of renal epithelial redifferentiation and a presumed requirement for their regenerative supporting effects (15, 16). SAA expression was chosen to reprogram and redifferentiate cultured renal cells because of its strong tubulogenic property (15), an idea supported by our current and previous work (15, 16, 19). For example, we found that even poorly differentiated rat NRK52E cells dramatically improved renal function in multiple models of acute kidney injury only when these cells expressed SAA (16). In these studies, no effect was seen in the absence of renal failure (16). The original experiments with SAA-positive and SAA-negative NRK52E cells were followed by successful transplantation experiments with primary renal epithelial cells (19). In these experiments, renal tubular cells were harvested from a rat donor with advanced CKD, reprogrammed and expanded ex vivo, and then given back to the donor. SAA-positive renal cells reversed the donor CKD when auto-transplanted intravenously (19). In addition, improvements in renal function and histological injury and expression of SAA1 were seen in nondiabetic postischemic animals (19).
We now report on another advancement derived from our earlier cell transplant work: the long-term prevention of progressive CKD in diabetes and ischemia by transplanted renal cells. We achieved near-complete renal protection with IRCT using SAA-expressing primary adult renal cells. These results corroborate the great potential of primary renal cell transplants in devastating clinical situations. The two sequential cell transplants were given to rats with severe chronic renal injury from diabetes and ischemia (12, 14). We then followed the progression of CKD until obese/diabetic rats were 34 wk of age and found that cell transplants, given at 15 and 20 wk of age, dramatically prevented the renal damage caused by diabetes and by added renal ischemia sustained at 10 wk of age. Moreover, higher rates of renal tubular cell proliferation and lower rates of cell apoptosis were observed 14 wk after the last transplant with SAA-positive cells. In contrast, at this late stage, posttransplant glomerular proliferation and apoptosis were not detected. Furthermore, at termination, we verified that male SAA-expressing renal cells were still present in recipient kidneys 19 and 14 wk after the two cell transplants, respectively. In a previous study (16), colocalization of transporters (organic anion transporter 1 and Na+/H+ exchanger 3) with GFP was found in kidneys from rats treated with SAA-positive cells, suggesting that the engrafted cells are functional. We assume that persistent engraftment of donor cells was responsible for the long-standing renal protection, although we do not know how donor renal cells limited nephropathy. While finding aggregate distributions of donor cells suggested replication in host kidneys, the transplanted cells could only represent a minority of the final and total tubular cell mass. Moreover, it is very unlikely that adult donor epithelial cells acted as progenitors, capable of regenerating damaged microvascular networks or glomeruli. We suggest that anchored donor tubular cells positively influenced (via paracrine or endocrine signaling) recipient renal cells to repopulate the injured kidney. In a previous study (16) in other models of renal failure, we have found increased expression of PCNA in kidneys from rats that received SAA-positive cells compared with control cells. In addition, the transplanted cells may have (e.g., via secreted VEGF (19)] attracted renal cell endothelial precursors, circulating cells that are thought to contribute to restoration of vascular function after injury (3). Better microvascular function (Fig. 4) results in better local renal perfusion and the delivery of O2 and nutrients, and this has been postulated to result in less tissue injury and inflammation (Fig. 2) as well as better function (Fig. 1). The mechanism of renal regeneration is at the forefront of cell therapy, and previous designs were focused on attempts to replace diverse cell populations of the injured kidney with pluripotent cells (2, 4, 7, 11, 29). However, progenitor cells do not become functional renal cells and do not restore a normal renal phenotype (6). What is more, in some cases, transplanted stem cells may even acquire a totally undesirable and uncontrolled phenotype in recipient kidneys with CKD (20). Our results indicate that in progressive CKD from DN, improved renal function and structure, inclusive of glomeruli, tubules, and microvessels, can be accomplished by SAA-positive renal cell transplants, a discovery that addresses the conundrum of trying to replace exceedingly diverse complex cell groups (8). In other words, tubular cell transplants positively affect most, if not all, renal cell types in the recipient. The broad benefit of cell transplants points to a general action that can be best explained by an improved vasculature with better delivery of O2/nutrients. Furthermore, this vascular hypothesis is also supported by our comprehensive genomic analysis of DN, which showed that renal ischemia was the driving force behind most alterations to the renal transcriptomes in DN (17). There have been reports of stem cell therapy in various models of CKD. However, unequivocal evidence of stem cell differentiation is lacking, and there is no real consistency in the effects among various groups (1). In conclusion, we propose that in CKD, adult renal epithelial cells offer a physiological and effective means to effect the preservation of renal function.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5-R01-DK-082739 and the Paul Teschan Research Fund of Dialysis Clinics, Incorporated (to K. J. Kelly), and the Department of Defense and the Veterans Affairs Merit Review program (to J. H. Dominguez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.J.K. and J.H.D. conception and design of research; K.J.K., J.Z., L.H., M.W., S.Z., and J.H.D. performed experiments; K.J.K., J.Z., L.H., M.W., S.Z., and J.H.D. analyzed data; K.J.K., J.Z., M.W., S.Z., and J.H.D. interpreted results of experiments; K.J.K. prepared figures; K.J.K. and J.H.D. edited and revised manuscript; K.J.K., J.Z., and J.H.D. approved final version of manuscript; J.H.D. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Barbara Kluve-Beckerman for the SAA antibody.
REFERENCES
- 1.Caldas HC, Hayashi AP, Abbud-Filho M. Repairing the chronic damaged kidney: the role of regenerative medicine. Transplant Proc 43: 3573–3576, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebrahimi B, Li Z, Eirin A, Zhu XY, Textor SC, Lerman LO. Addition of endothelial progenitor cells to renal revascularization restores medullary tubular oxygen consumption in swine renal artery stenosis. Am J Physiol Renal Physiol 302: F1478–F1485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, Kuo HC, Pinkernell K. Fresh and cryopreserved, uncultured adipose tissue-derived and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant 25: 3874–3884, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic renal disease: more than an aftermath of glomerular injury? Kidney Int 56: 1627–1637, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Guo JK, Cantley LG. Cellular maintenance and repair of the kidney. Annu Rev Physiol 72: 357–376, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Hauser PV, De Fazio R, Bruno S, Sdei S, Grange C, Bussolati B, Benedetto C, Camussi G. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol 177: 2011–2021, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendry CE, Little MH. Reprogramming the kidney: a novel approach for regeneration. Kidney Int 82: 138–146, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med 363: 1833–1845, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY. Where is the epidemic in kidney disease? J Am Soc Nephrol 21: 1607–1611, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly KJ, Burford JL, Dominguez JH. Post-ischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol 297: F923–F931, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Kelly KJ, Dominguez JH. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol 32: 469–475, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Kelly KJ, Dominguez JH. Treatment of the post-ischemic inflammatory syndrome of diabetic nephropathy. Nephrol Dial Transplant 25: 3204–3212, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Kelly KJ, Kluve-Beckerman B, Dominguez JH. Acute-phase response protein serum amyloid A stimulates renal tubule formation: studies in vitro and in vivo. Am J Physiol Renal Physiol 296: F1355–F1363, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Kelly KJ, Kluve-Beckerman B, Zhang J, Dominguez JH. Intravenous cell therapy for acute renal failure with serum amyloid A protein-reprogrammed cells. Am J Physiol Renal Physiol 299: F453–F464, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Kelly KJ, Liu Y, Zhang J, Goswami C, Lin H, Dominguez JH. Comprehensive genomic profiling in diabetic nephropathy reveals predominance of proinflammatory pathways. Physiol Genomics 45: 710–719, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intracellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci USA 91: 812–816, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly KJ, Zhang J, Wang M, Zhang S, Dominguez JH. Intravenous renal cell transplantation for rats with acute and chronic renal failure. Am J Physiol Renal Physiol 303: F357–F365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 18: 1754–1764, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Erichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlondorff D, Cohen CD. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18: 1765–1776, 2007 [DOI] [PubMed] [Google Scholar]
- 22a.National Institute of Diabetes and Digestive and Kidney Diseases United States Renal Data System. 2010 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, 2010, vol. 1 [Google Scholar]
- 23.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG; DEMAND Investigators Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 69: 2057–2063, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Ritz E, Zeng XX, Rychlik I. Clinical manifestations and natural history of diabetic nephropathy. Contrib Nephrol 170: 19–27, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Seckert CK, Renzaho A, Tervo HM, Krause C, Deegen P, Kuhnapfel B, Reddehase MJ, Grzimek NK. Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J Virol 83: 8869–8884, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiel AO, Gilbert JC, Jilma B. von Williebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation 117: 1449–1459, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Temm C, Dominguez JH. Microcirculation: nexus of comorbidities in diabetes. Am J Physiol Renal Physiol 293: F486–F493, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 6: 2567–2572, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Uzu T, Kida Y, Shirahashi N, Harada T, Yamauchi A, Nomura M, Isshiki K, Araki S, Sugimoto T, Koya D, Haneda M, Kashiwagi A, Kikkawa R. Cerebral microvascular disease predicts renal failure in type 2 diabetes. J Am Soc Nephrol 21: 520–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LYC, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1993 [DOI] [PubMed] [Google Scholar]