Abstract
The stoichiometry of the side-chain cleavage of cholesterol (cholest-5-en-3β-ol), 20α-hydroxycholesterol (cholest-5-ene-3β,20α-diol), and 20α,22-dihydroxycholesterol (cholest-5-ene-3β,20α,22-triol) has been examined with respect to TPNH, oxygen and H+. Side-chain cleavage of cholesterol can be described by the following equation:
Cholesterol + 3TPNH + 3H+ + 3O2 → Pregnenolone (3β-hydroxypregn-5-ene-20-one) + isocapraldehyde + 3 TPN+ 4H2O
Stoichiometry of 20 α-hydroxycholesterol is 2TPNH and 2O2 per mole of cleavage and with 20α, 22-dihydroxycholesterol the values are 1:1:1. In addition 1 mole of H+ is consumed per mole of TPNH oxidized in each of these reactions. These observations are in keeping with a mechanism previously proposed in the literature, namely:
Cholesterol → 20α-OH-Cholesterol → 20α, 22-di-OH-Cholesterol → pregnenolone
Discordant observations are reviewed and it is concluded that insufficient data are available to sustain objections to this pathway.
Keywords: mitochondria, adrenal cortex, steroidogenesis
Full text
PDF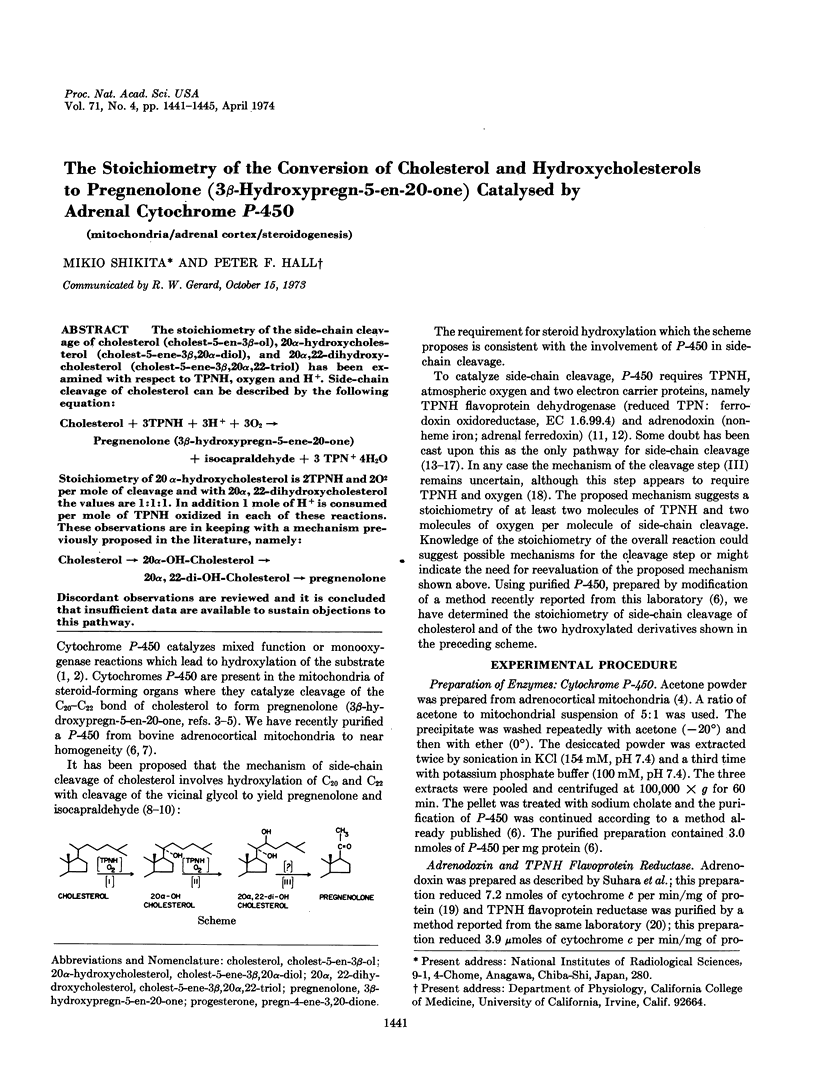
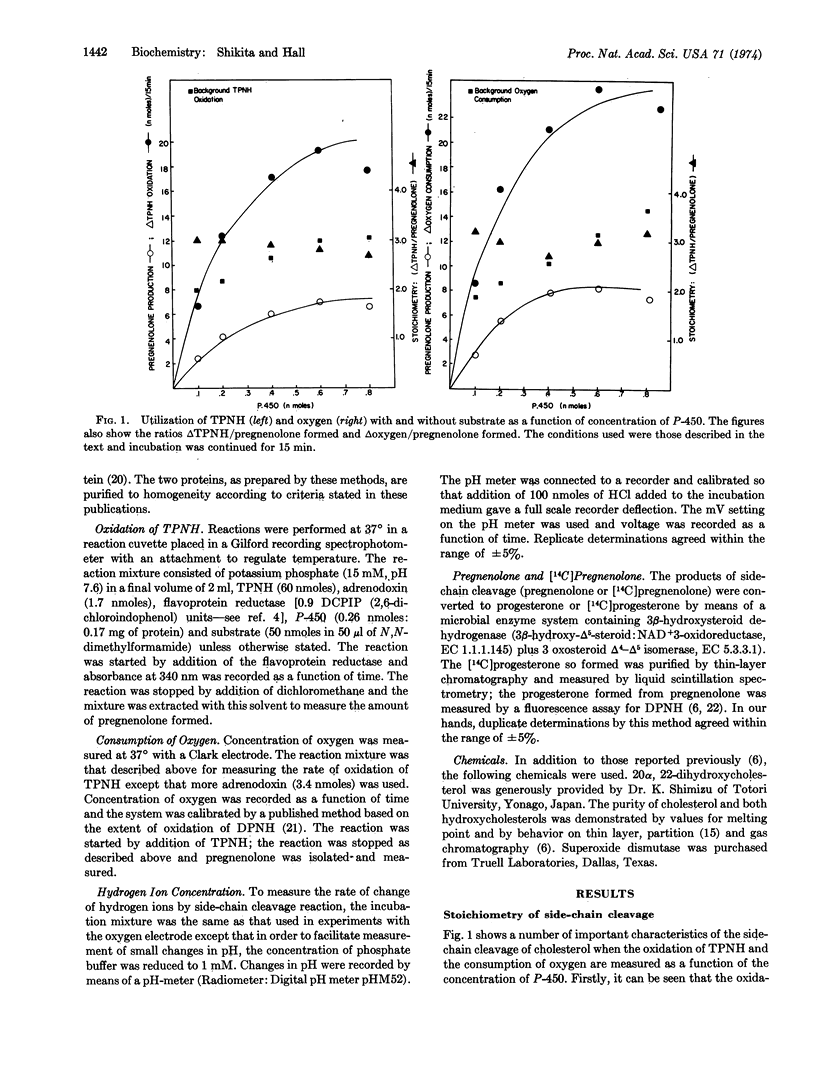
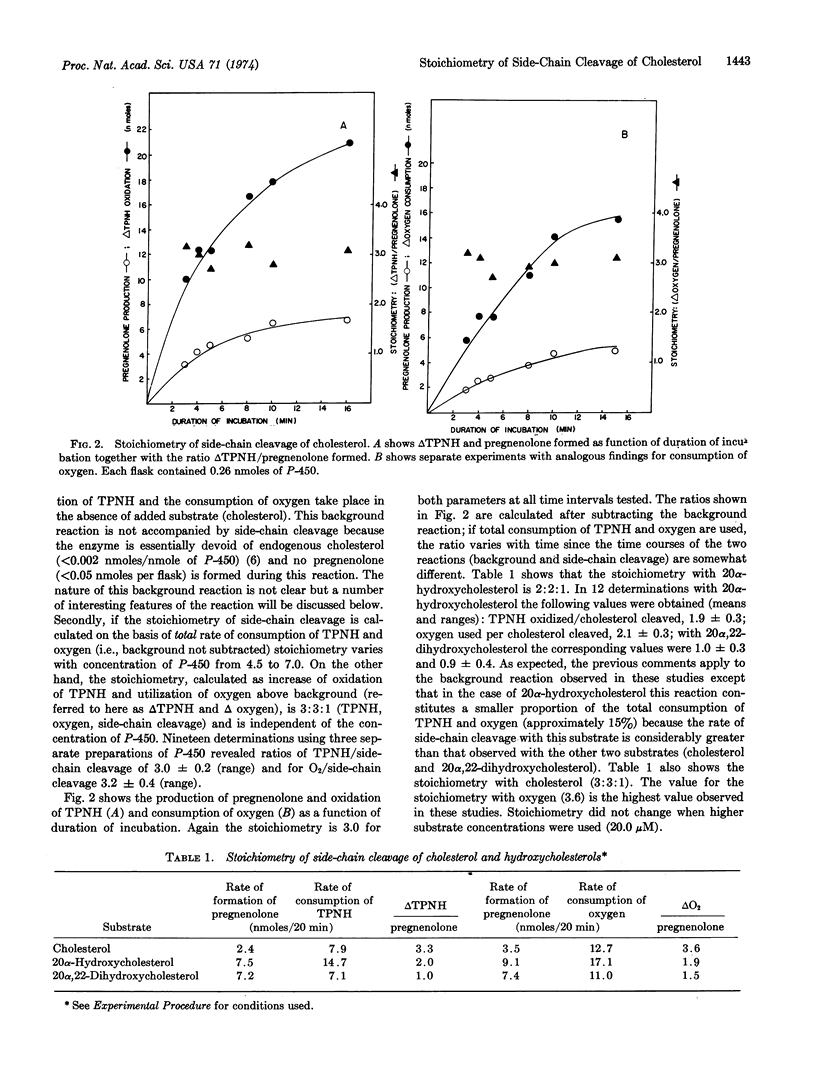


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein S., Zamoscianyk H., Kimball H. L., Chaudhuri N. K., Gut M. Transformation of labeled cholesterol, 20-alpha-hydroxycholesterol, (22R)-22-hydroxycholesterol, and (22R)-20-alpha, 22-dihydroxycholesterol by adrenal acetone-dried preparations from guinea pigs, cattle and man. Steroids. 1970 Jan;15(1):13–60. doi: 10.1016/s0039-128x(70)80003-4. [DOI] [PubMed] [Google Scholar]
- CONSTANTOPOULOS G., TCHEN T. T. Cleavage of cholesterol side chain by adrenal cortex. I. Cofactor requirement and product of clevage. J Biol Chem. 1961 Jan;236:65–67. [PubMed] [Google Scholar]
- Constantopoulos G., Carpenter A., Satoh P., Tchen T. T. Formation of isocaproaldehyde in the enzymatic cleavage of cholesterol side chain by adrenal extract. Biochemistry. 1966 May;5(5):1650–1652. doi: 10.1021/bi00869a029. [DOI] [PubMed] [Google Scholar]
- HALL P. F., KORITZ S. B. INHIBITION OF THE BIOSYNTHESIS OF PREGNENOLONE BY 20-ALPHA-HYDROXYCHOLESTEROL. Biochim Biophys Acta. 1964 Nov 8;93:441–444. doi: 10.1016/0304-4165(64)90404-0. [DOI] [PubMed] [Google Scholar]
- HARDING B. W., WONG S. H., NELSON D. H. CARBON MONOXIDE-COMBINING SUBSTANCES IN RAT ADRENAL. Biochim Biophys Acta. 1964 Nov 22;92:415–417. doi: 10.1016/0926-6569(64)90206-8. [DOI] [PubMed] [Google Scholar]
- Hall P. F. Electron transport in relation to steroid biosynthesis. Inhibition of side-chain cleavage of cholesterol by hyperbaric oxygen. Biochemistry. 1967 Sep;6(9):2794–2802. doi: 10.1021/bi00861a021. [DOI] [PubMed] [Google Scholar]
- Koritz S. B., Moustafa A. M. Determination of small amounts of pregnenolone. Anal Biochem. 1971 Sep;43(1):134–138. doi: 10.1016/0003-2697(71)90117-5. [DOI] [PubMed] [Google Scholar]
- Luttrell B., Hochberg R. B., Dixon W. R., McDonald P. D., Lieberman S. Studies on the biosynthetic conversion of cholesterol into pregnenolone. Side chain cleavage of a t-butyl analog of 20 -hydroxycholesterol, (20R(-t-butyl-5-pregnene-3 ,20-diol, a compound completely substituted at C-22. J Biol Chem. 1972 Mar 10;247(5):1462–1472. [PubMed] [Google Scholar]
- McIntosh E. N., Uzgiris V. I., Alonso C., Salhanick H. A. Spectral properties, respiratory activity, and enzyme systems of bovine corpus luteum mitochondria. Biochemistry. 1971 Jul 20;10(15):2909–2916. doi: 10.1021/bi00791a018. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- SHIMIZU K., GUT M., DORFMAN R. I. 20alpha,22x-Dihydroxycholesterol, an intermediate in the biosynthesis of pregnenolone (3beta-hydroxypregn-5-en-20-one) from cholesterol. J Biol Chem. 1962 Mar;237:699–702. [PubMed] [Google Scholar]
- Shikita M., Hall P. F. Cytochrome P-450 from bovine adrenocortical mitochondria: an enzyme for the side chain cleavage of cholesterol. I. Purification and properties. J Biol Chem. 1973 Aug 25;248(16):5598–5604. [PubMed] [Google Scholar]
- Shikita M., Hall P. F. Cytochrome P-450 from bovine adrenocortical mitochondria: an enzyme for the side chain cleavage of cholesterol. II. Subunit structure. J Biol Chem. 1973 Aug 25;248(16):5605–5609. [PubMed] [Google Scholar]
- Shimizu K. Requirement of NADPH and molecular oxygen for the side-chain cleavage of 20-alpha, 22-xi-dihydroxycholesterol. Arch Biochem Biophys. 1968 Jun;125(3):1016–1017. doi: 10.1016/0003-9861(68)90539-0. [DOI] [PubMed] [Google Scholar]
- Sih C. J. Enzymatic mechanism of steroid hydroxylation. Science. 1969 Mar 21;163(3873):1297–1300. doi: 10.1126/science.163.3873.1297. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Boyd G. S. The cholesterol side-chain cleavage system of bovine adrenal cortex. Eur J Biochem. 1967 Oct;2(3):275–285. doi: 10.1111/j.1432-1033.1967.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Boyd G. S. The cholesterol side-chain cleavage system of the adrenal cortex: a mixed-function oxidase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):10–17. doi: 10.1016/0006-291x(66)90402-5. [DOI] [PubMed] [Google Scholar]
- Suhara K., Ikeda Y., Takemori S., Katagiri M. The purification and properties of NADPH-adrenodoxin reductase from bovine adrenocortical mitochondria. FEBS Lett. 1972 Nov 15;28(1):45–47. doi: 10.1016/0014-5793(72)80673-2. [DOI] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. Improved purification of bovine adrenal iron-sulfur protein. Biochim Biophys Acta. 1972 Apr 15;263(2):272–278. doi: 10.1016/0005-2795(72)90079-7. [DOI] [PubMed] [Google Scholar]
- Takemoto C., Nakano H., Sato H., Tamaoki B. I. Fate of molecular oxygen required by endocrine enzymes for the side-chain cleavage of cholesterol. Biochim Biophys Acta. 1968 Jul 1;152(4):749–757. [PubMed] [Google Scholar]
- Van Lier J. E., Smith L. L. Sterol metabolism. XVI. Cholesterol 20-alpha-hydroperoxide as an intermediate in pregnenolone biosynthesis from cholesterol. Biochem Biophys Res Commun. 1970 Aug 11;40(3):510–516. doi: 10.1016/0006-291x(70)90931-9. [DOI] [PubMed] [Google Scholar]



