Abstract
The circadian clock plays an important role in the regulation of physiological processes, including renal function and blood pressure. We have previously shown that the circadian protein period (Per)1 regulates the expression of multiple Na+ transport genes in the collecting duct, including the α-subunit of the renal epithelial Na+ channel. Consistent with this finding, Per1 knockout mice exhibit dramatically lower blood pressure than wild-type mice. We have also recently demonstrated the potential opposing actions of cryptochrome (Cry)2 on Per1 target genes. Recent work by others has demonstrated that Cry1/2 regulates aldosterone production through increased expression of the adrenal gland-specific rate-limiting enzyme 3β-dehydrogenase isomerase (3β-HSD). Therefore, we tested the hypothesis that Per1 plays a role in the regulation of aldosterone levels and renal Na+ retention. Using RNA silencing and pharmacological blockade of Per1 nuclear entry in the NCI-H295R human adrenal cell line, we showed that Per1 regulates 3β-HSD expression in vitro. These results were confirmed in vivo: mice with reduced levels of Per1 had decreased levels of plasma aldosterone and decreased mRNA expression of 3β-HSD. We postulated that mice with reduced Per1 would have a renal Na+-retaining defect. Indeed, metabolic cage experiments demonstrated that Per1 heterozygotes excreted more urinary Na+ compared with wild-type mice. Taken together, these data support the hypothesis that Per1 regulates aldosterone levels and that Per1 plays an integral role in the regulation of Na+ retention.
Keywords: kidney, period 1, sodium transport, aldosterone, circadian clock
the circadian clock regulates many physiological functions, such as blood pressure (BP), metabolism, the immune response, and renal function (1, 7, 32, 35). Of note, Na+ excretion, plasma aldosterone levels, glomerular filtration rate, and renal blood flow exhibit rhythmic fluctuations (2, 25, 35, 39). At the molecular level, the circadian clock consists of four core proteins, which interact with each other to affect transcription of circadian target genes (11). These four canonical clock proteins are period (Per)1–3, cryptochrome (Cry)1–2, Bmal1, and Clock. Clock and Bmal1 form a heterodimer that interacts with E-boxes to transcriptionally upregulate clock-controlled genes, which include Per and Cry. Per and Cry dimerize and interact with Clock and Bmal1 to repress their transcriptional activity (3, 11). Per1 may not act as a canonical repressor, however, as increasing evidence suggests that Per1 may activate gene expression through an unknown mechanism, and this may occur in a tissue- and gene-specific manner (8, 16, 18, 31, 36). However, our recent work (30) demonstrated that this possible mechanism could be through repression of the circadian repressor Cry2. In this study, reduced expression of Per1 in vivo was associated with increased Cry2 expression and changes in Per1 target gene expression.
Increasing evidence links the circadian clock to the regulation of renal Na+ handling and overall BP control. Clock knockout (KO) mice are relatively hypotensive and exhibit dysregulated urinary Na+ excretion and mild diabetes insipidus (26, 40). Per2 KO mice exhibit decreased 24-h diastolic BP, increased heart rate, and reduced daytime dipping (18). Cry1/2 KO mice display salt-sensitive hypertension due to high aldosterone levels resulting from increased levels of 3β-dehydrogenase isomerase (mouse: Hsd3b6 and human: HSD3B1; hereafter referred to as 3β-HSD), an enzyme in the aldosterone synthesis pathway that is expressed exclusively in the zona glomerulosa of the adrenal glands (12). Surprisingly, no changes were observed in the expression of the aldosterone synthase gene (CYPB11), which encodes the rate-limiting step of aldosterone production. We (16, 18, 31, 36) have previously shown that Per1 regulates both the basal and aldosterone-mediated regulation of the α-subunit of the renal epithelial Na+ channel (α-ENaC). We (26) have also demonstrated that Per1 coordinately regulates the expression of multiple genes that contribute to the regulation of renal Na+ reabsorption. These include Per1-mediated positive regulation of Fxyd5, a positive regulator of Na+-K+-ATPase (22), and negative regulation of endothelin (ET)-1, a potent inhibitor of ENaC (9, 23). These findings predict that the loss of Per1 should result in decreased renal Na+ reabsorption, with subsequent decreased plasma volume and decreased BP. Indeed, we (36) have recently shown that Per1 KO mice have significantly lower BP compared with wild-type (WT) mice.
The renin-angiotensin-aldosterone system (RAAS) is a key hormonal regulator of Na+ balance and of overall fluid and BP control. Elevated aldosterone levels have been observed in Cry1/2 KO animals, and this effect was likely due to increased levels of 3β-HSD (12). Based on our observation that mice with reduced Per1 expression exhibit increased Cry2 (30), we hypothesized that a reduction of Per1 in vivo would result in the opposite phenotype. Thus, we investigated aldosterone levels in mice with reduced Per1 expression. Here, we show, for the first time, that reduced levels of Per1 in vivo result in reduced plasma aldosterone, decreased expression of 3β-HSD, and impaired renal Na+ conservation.
MATERIALS AND METHODS
Animals.
All animal use protocols were approved by the Institutional Animal Care and Use Committees of the University of Florida and North Florida/South Georgia Veterans Administration in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. WT and Per1 KO mice (129/sv) were originally provided by Dr. David Weaver (University of Massachusetts) (6). WT and Per1 heterozygous mice were bred in house by University of Florida Animal Care Services staff. Animals were maintained on a normal 12:12-h light-dark cycle. For plasma aldosterone experiments, mice were fed normal lab chow and given free access to water. Mice were anesthetized by inhaled isoflourane, and blood and tissues were collected and snap frozen in liquid nitrogen. Plasma and urinary levels of aldosterone were determined by ELISA (Enzo) per the manufacturer's instructions.
For in vivo PF670462 experiments, weight-matched male WT 129/sv mice were given either vehicle (20% hydroxypropyl-β-cyclodextrin) or 30 mg/kg PF-670462 subcutaneously every 12 h for 2.5 days starting at noon and euthanized at midnight 12 h after the last injection. The dose and time have been previously published and described by others (27).
For normal and low- Na+ diet urinalysis experiments, mice were housed in metabolic cages, as previousy described (15), and fed a powdered diet (Teklad 99131, 0.2% Na+ and 0.6% K+) made as a gel (45% food, 1% agar, and 54% water) or a low-Na+ diet (Teklad 03582, 0.02% Na+ and 0.6% K+) also made as a gel. Mice had free access to a water bottle. Per1 heterozygous mice were fed what WT mice of a similar body weight ate the previous day. Fecal material was dried overnight at 200°C and digested in 0.75 N nitric acid overnight at 37°C, as previously described (15). Samples were then homogenized and filtered for analysis by flame photometry. Urine and feces were collected daily. Urine and fecal electrolytes were measured using a digital flame photometer (model 2655-00, Cole Parmer Instruments).
Cell culture.
NCI-H295R cells were purchased from the American Type Culture Collection (CRL-2128) and maintained as per American Type Culture Collection recommendations in DMEM-F-12 plus ITS+ premix (0.00625 mg/ml insulin, 0.00625 mg/ml transferrin, 6.25 ng/ml selenium, 1.25 mg/ml BSA, and 0.00535 mg/ml linoleic acid, BD Biosciences) and 2.5% Nu-Serum I (BD Biosciences).
For RNA silencing experiments, nontarget and SMARTpool small interfering (si)RNA for human Per1were purchased from Dharmacon. siRNA were used according to the manufacturer's instructions. Cells were transfected using Dharmafect 1.
Casein kinase (CK)1-δ/ε inhibitor experiments were performed as previously described (30, 31). For dose and time course, NCI-H295R cells were treated with either 0.1, 1, 10, or 100 μM of the CK1-δ/ε inhibitor PF-670462 or vehicle (water) for 24, 48, or 72 h. For nuclear entry experiments, NCI-H295R cells were treated with 0.1 μM PF-670462 or vehicle for 24 h.
RNA isolation and quantitative real-time PCR.
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. RNA (10 μg) was treated with DNA-free DNase I (Ambion). DNase I-treated RNA (2 μg) samples were used as the template for reverse transcription with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The resulting cDNAs (20 ng) were then used as the template in quantitative real-time PCRs (Applied Biosystems) to evaluate changes in Per1, HSD3B1, Hsd3b6, CYPB11, and actin mRNA levels. Cycle threshold (Ct) values were normalized against β-actin, and relative quantification was performed using the ΔΔCt method (21). Fold change values were calculated as the change in mRNA expression levels relative to the control level. TaqMan primer/probe sets were purchased from Applied Biosystems.
Nuclear protein isolation and Western blot analysis.
Nuclear extracts were isolated using the NE-PER kit (Pierce) according to the manufacturer's instructions. Protein concentrations were then quantified by BCA assay (Pierce). Proteins were separated on a 4–20% Tris-HCl Ready Gel (Bio-Rad) and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 2% nonfat dry milk in Tris-buffered saline plus 0.05% Rodeo Saddle Soap (TBSS; USB) and incubated overnight at 4°C with anti-Per1 (1:500, Pierce) or anti-β-actin (1:500, Santa Cruz Biotechnology) antibodies. β-Actin was used as a loading control. The membrane was washed with 2% nonfat dry milk in TBSS for 15 min, incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody, and then incubated in 2% nonfat dry milk in TBSS for 1 h at 4°C. After incubation, the blot was washed with TBSS for 15 min. Detection was performed using Novex ECL Chemiluminescent Substrate reagents (Invitrogen). Densitometry was performed using ImageJ (NIH; rsbweb.nih.gov/ij).
Statistical analysis.
All data are presented as means ± SE. Statistics were performed with Graphpad Prism (version 6). All graphs/plots were made with Graphpad Prism (version 6). The effects of time and treatment or time and genotype were analyzed by two-way ANOVA with a post hoc Student-Newman-Keuls test. An unpaired Student's t-test was used to compare differences between control and treated groups. All P values of <0.05 were considered significant.
RESULTS
Decreased levels of Per1 are associated with lower plasma aldosterone levels and reduced 3β-HSD expression in vivo.
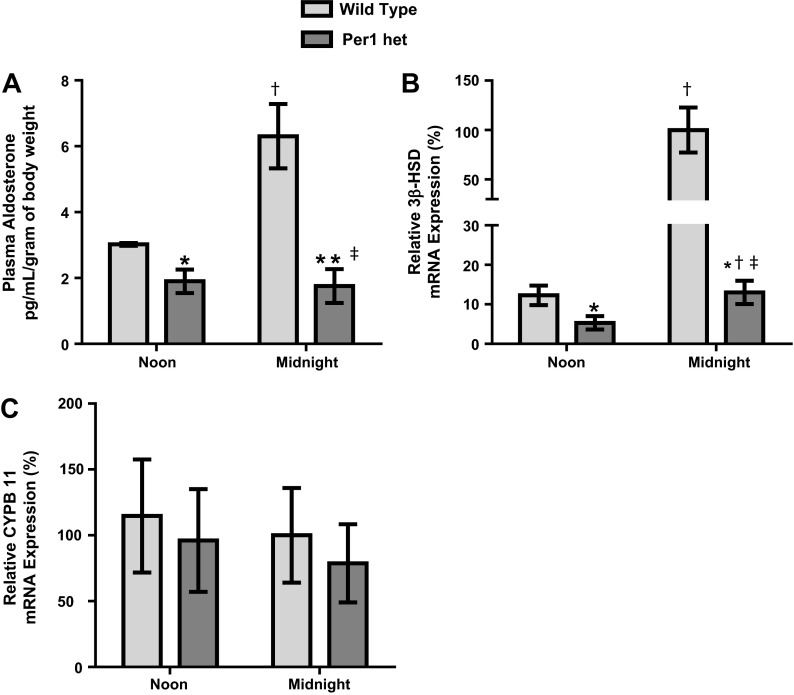
As mentioned above, it has been previously shown that Cry1/2 controls aldosterone synthesis through the regulation of 3β-HSD (12). We (30) have also previously shown that Per1 and Cry2 modulate opposing actions on Per1 target gene expression in the kidney and liver and that Per1 heterozygous mice have an approximate 50% reduction in Per1 protein expression in these tissues. Therefore, to determine if Per1 regulates aldosterone levels, plasma aldosterone was assessed in WT and Per1 heterozygous mice by ELISA at noon (middle of the inactive phase) and midnight (middle of the active phase) under normal dietary conditions. It is well established in humans and rodents that aldosterone increases during the active phase and decreases during the inactive phase (1, 26). In WT mice, plasma aldosterone levels were greater at midnight compared with noon, whereas Per1 heterozygous mice appeared to have a blunted circadian surge in plasma aldosterone levels (Fig. 1A). Additionally, Per1 heterozygous mice exhibited lower plasma aldosterone levels compared with WT mice at both time points tested.
Fig. 1.
Decreased period 1 (Per1) results in lower plasma aldosterone levels. Plasma aldosterone levels were determined by ELISA (ENZO). A: plasma aldosterone in wild-type (WT) and Per1 heterozygous (Per1 het) mice at noon and midnight. n = 4–9. B: relative 3β-dehydrogenase isomerase (3β-HSD) mRNA expression from adrenal glands harvested from WT and Per1 het mice at noon and midnight. n = 4–8. WT expression at midnight was set to 100%. C: relative CYPB11 mRNA expression from adrenal glands harvested from WT and Per1 het mice at noon and midnight. WT expression at midnight was set to 100%. n = 4–8. *P < 0.05 compared with WT mice; †P < 0.05 compared with time (noon vs. midnight); ‡P < 0.001, interaction via two-way ANOVA.
We hypothesized that the decreased plasma aldosterone levels seen in Per1 heterozygous mice could be due to decreased 3β-HSD levels. We measured 3β-HSD mRNA levels from WT and Per1 heterozygous adrenal glands at noon and midnight (Fig. 1B). In WT mice, 3β-HSD mRNA increased at midnight compared with noon, consistent with a role for 3β-HSD in the circadian pattern of aldosterone. Interestingly, Per1 heterozygous mice exhibited lower 3β-HSD levels at noon and midnight, with a significantly blunted circadian expression of this gene (Fig. 1B). Expression of the mRNA encoding the enzyme aldosterone synthase (CYPB11) did not change (Fig. 1C).
3β-HSD expression is decreased after Per1 knockdown in NCI-H295R cells in vitro.
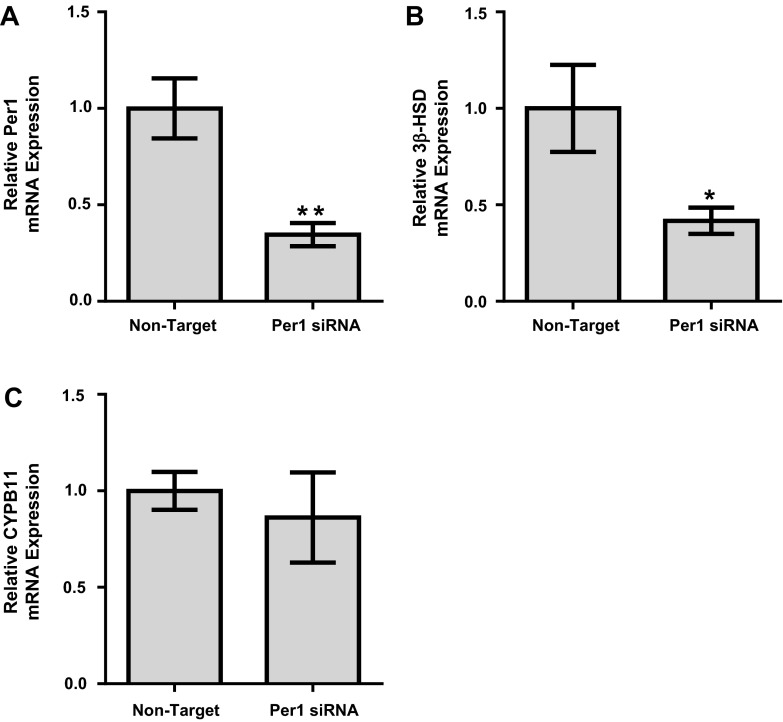
To further investigate the role of Per1 in the regulation of 3β-HSD, we treated human adrenal NCI-H295R cells (14, 28) with Per1 siRNA and evaluated the effect of Per1 knockdown on 3β-HSD mRNA expression using quantitative PCR. The NCI-H295R cell line was established from a primary adrenocortical carcinoma, expresses all the key enzymes for adrenal steroidogenesis, and has been demonstrated to secrete mineralocorticoids, glucocorticoids, and dehydroepiandrosterone (14). As expected, Per1 siRNA resulted in a significant decrease in Per1 mRNA expression (Fig. 2A). Importantly, Per1 siRNA resulted in a 58% decrease in 3β-HSD mRNA levels (Fig. 2B). Per1 siRNA resulted in no significant difference in CYPB11 mRNA expression (Fig. 2C). These results are consistent with our in vivo data (Fig. 1).
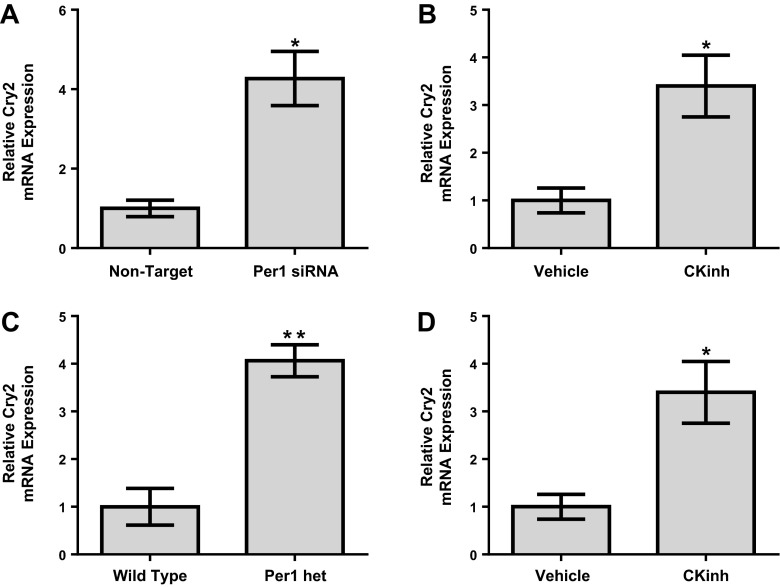
Fig. 2.
Per1 knockdown results in decreased 3β-HSD expression in adrenal cells in vitro. NCI-H295R cells were treated with nontarget or Per1 small interfering (si)RNA for 48 h. Quantitative PCR was used to evaluate changes in Per1 (A), 3β-HSD (B), or CYB11 (C) expression in Per1 siRNA versus the nontarget siRNA control. Values are presented as means ± SE; n = 3. *P < 0.05; **P < 0.01.
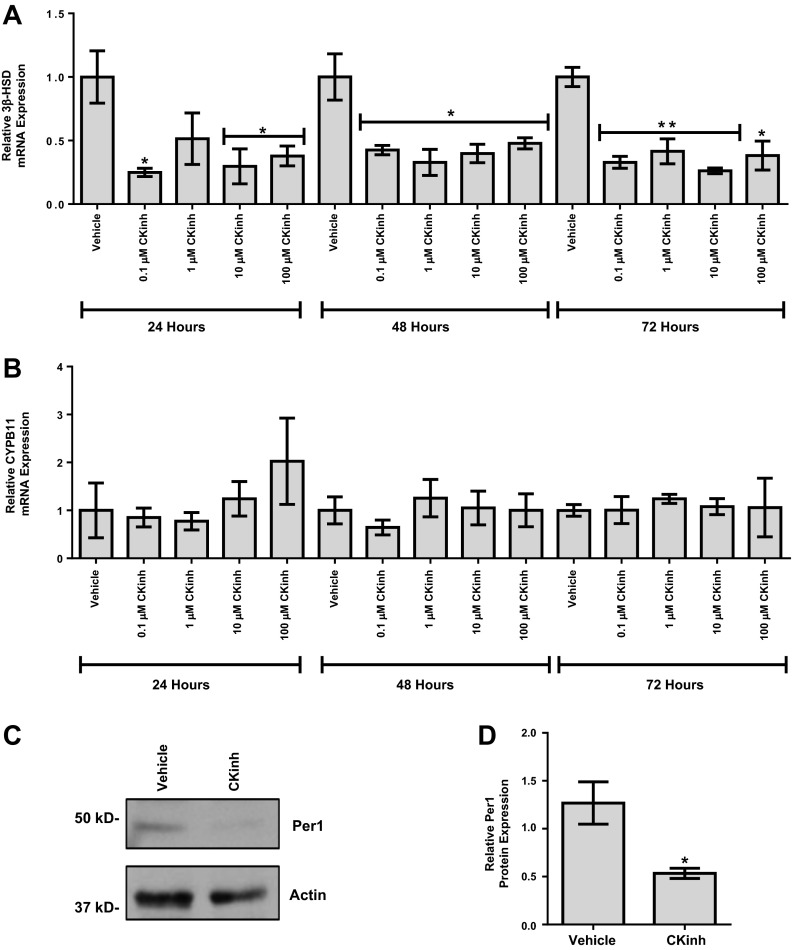
Per1 must be phosphorylated by CK1-δ/ε to enter the nucleus (20). We (30, 31) have previously shown that pharmacological inhibition of CK1-δ/ε using PF-670462 recapitulated the effects of Per1 knockdown, including increased levels of Cry2, decreased α-ENaC mRNA and protein expression, and, importantly, decreased ENaC channel activity. PF-670462 has been previously shown to have an IC50 for CK1-δ of 15 nM and 7.7 nM for CK1-ε (5). To provide further confirmation of the regulation of 3β-HSD, NCI-H295R cells were treated with PF-670462, and 3β-HSD mRNA levels were assessed by quantitative PCR. Confirming our siRNA results, 0.1 μM for 24 h was sufficient to achieve a significant reduction of 3β-HSD mRNA levels (Fig. 3A). CYPB11 was unchanged at all doses and times tested (Fig. 3B). To confirm that the reduction in 3β-HSD mRNA was due to decreased Per1 nuclear entry, NCI-H295R cells were treated with 0.1 μM PF-670462 for 24 h, nuclear extracts were collected, and Western blot analysis was performed to assess nuclear Per1 levels. Per1 nuclear levels were significantly decreased with PF-67042 treatment (Fig. 3, C and D).
Fig. 3.
Pharmacological blockade of Per1 nuclear entry results in decreased 3β-HSD expression in vitro. A and B: NCI-H295R cells were treated with the casein kinase (CK)1-δ/ε inhibitor (CKinh) PF-670462 at either 0.1, 1, 10, or 100 μM for 24, 48, or 72 h. Quantitative PCR was used to evaluate changes in 3β-HSD (A) and CYPB11 (B) gene expression in PF-670462-treated versus water-treated cells. Values are presented as means ± SE; n = 3. *P < 0.05; **P < 0.01. C: nuclear extracts were collected from NCI-H295R cells treated with 0.1 μM of the CK-1δ/ε inhibitor PF-670462 or water for 24 h. Western blot analysis was performed using anti-Per1 or anti-β-actin antibodies as a loading control. Data are representative of three independent experiments. D: densitometry analysis was used to quantitate the level of Per1 in C. Values are presented as means ± SE; n = 3. *P < 0.05.
Inhibition of CK1-δ/ε in vivo results in the reduction of 3β-HSD mRNA.
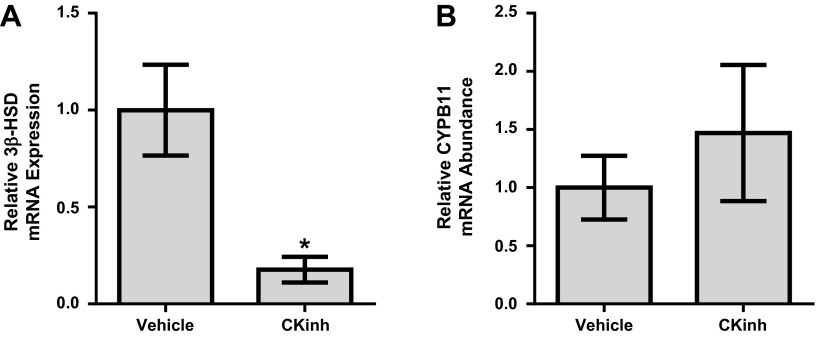
To further confirm the regulation of 3β-HSD by Per1, WT mice were either given vehicle or PF-670462 as previously published and described by others (27). Adrenal glands were harvested, and 3β-HSD mRNA levels were assessed by quantitative PCR. Confirming our in vitro results, PF-670462 treatment resulted in significantly decreased levels of 3β-HSD mRNA (Fig. 4A). CYPB11 mRNA expression was unchanged (Fig. 4B).
Fig. 4.
Pharmacological blockade of Per1 nuclear entry results in decreased 3β-HSD in vivo. Weight-matched male WT 129/sv mice were given either vehicle (20% hydroxypropyl-β-cyclodextrin) or 30 mg/kg PF-670462 subcutaneously every 12 h for 2.5 days starting at noon and euthanized at midnight 12 h after the last injection, as previously published and described (27). Adrenal glands were harvested, and 3β-HSD (A) and CYPB11 (B) mRNA expression was measured by quantitative PCR. Values are presented as means ± SE; n = 4. *P < 0.05 compared with WT mice.
Loss or reduction of Per1 results in increased levels of Cry2 in adrenal glands and NCI-H295R cells.
It has been previously demonstrated that Cry1/2 KO mice have increased aldosterone synthesis due to increased levels of 3β-HSD (12). We (30) have recently shown that Per1 and Cry2 mediate opposing actions on Per1 target gene expression in the liver and kidney and that mice with reduced levels of Per1 have increased levels of Cry2. To test the effect of reduced Per1 on Cry2 adrenal gland cells and tissue, Cry2 mRNA levels were determined by quantitative PCR. siRNA-mediated knockdown of Per1 in NCI-H29R cells resulted in increased mRNA expression of Cry2 (Fig. 5A). Pharmacological blockade of nuclear Per1 entry also resulted in increased levels of Cry2 in vitro (Fig. 5B). Adrenal gland Cry2 mRNA expression was elevated in Per1 heterozygous mice euthanized at midnight relative to WT control mice (Fig. 5C). Consistent with these results, PF-670462 treatment in vivo also resulted in increased levels of Cry2 mRNA in the adrenal glands of WT mice relative to vehicle-treated control mice (Fig. 5D).
Fig. 5.
Reduction of Per1 expression in vitro and in vivo results in increased cryptochrome (Cry)2 mRNA in adrenal cells and tissue. NCI-H295R cells were treated with nontarget or Per1 siRNA for 48 h. Quantitative PCR was used to evaluate changes in Cry2 expression in Per1 siRNA versus the nontarget siRNA control. Values are presented as means ± SE; n = 3. *P < 0.05. B: NCI-H295R cells were treated with 0.1 μM of the CK1-δ/ε inhibitor PF-670462 for 24 h. Quantitative PCR was used to evaluate changes in Cry2 gene expression in PF-670462-treated versus water-treated cells. Values are presented as means ± SE; n = 3. *P < 0.05. C: relative Cry2 mRNA expression from adrenal glands harvested from WT and Per1 het mice at midnight. n = 4–8. **P < 0.01 compared with WT mice. D: weight-matched male WT 129/sv mice were given vehicle (20% hydroxypropyl-β-cyclodextrin) or 30 mg/kg PF-670462 subcutaneously every 12 h for 2.5 days starting at noon and euthanized at midnight 12 h after the last injection as previously described (27). Adrenal glands were harvested, and Cry2 expression was measured by quantitative PCR. Values are presented as means ± SE; n = 4. *P < 0.05 compared with WT mice.
Mice with reduced levels of Per1 exhibit impaired Na+ retention.
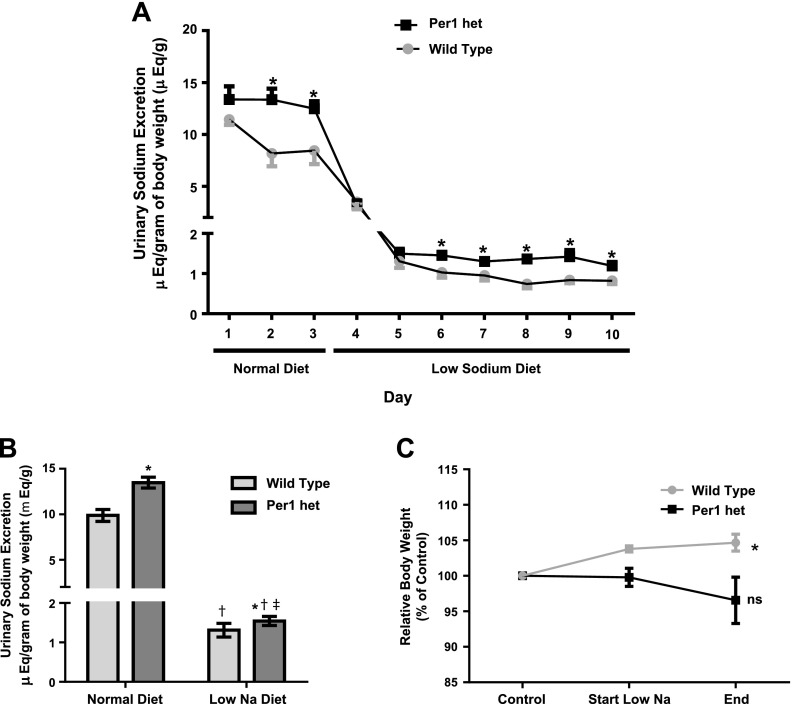
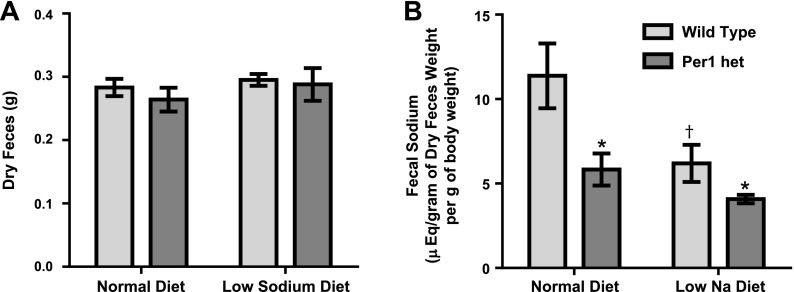
The mineralocorticoid hormone aldosterone, a component of the RAAS system, is a key regulator of Na+ reabsorption in the kidney (29, 34). Per1 heterozygous mice exhibited lower plasma aldosterone at noon and midnight compared with control mice (Fig. 1). We (16, 17, 31, 36) have previously demonstrated that Per1 regulates multiple genes involved in Na+ reabsorption in the collecting duct, including the regulation of α-ENaC. These observations led us to hypothesize that mice with reduced levels of Per1 would have increased urinary Na+ excretion. To determine if Per1 heterozygous mice excrete more Na+, weight-matched male WT 129/sv and Per1 heterozygous mice were placed in metabolic cages, as previously described (15), and allowed to acclimate for 3 days. Per1 heterozygous mice were fed the same amount of food as the WT mice ate the previous day. Mice were fed a normal gel diet (0.2% Na+) with free access to water. After 3 days, mice were switched to a low-Na+ diet (0.02%) for 7 days. Urine and feces were collected daily. WT and Per1 heterozygous urinary Na+ excretion decreased dramatically in response to the low-Na+ diet (Fig. 6A). Per1 heterozygous mice exhibited higher urinary Na+ excretion relative to WT mice, and this effect was observed on the normal diet and on the low-Na+ diet (Fig. 6A). When compared by genotype and time, Per1 heterozygous mice demonstrated increased urinary Na+ excretion on both normal and low-Na+ diets (Fig. 6B). These results are consistent with our previous report (18) demonstrating higher levels of Na+ in the urine of Per1 KO mice compared with WT mice. Over the course of the experiment, WT mice gained 4.6% body weight compared with starting values. In contrast, Per1 heterozygous mice had no significant change in weight compared with control values (Fig. 6C). Fecal Na+ levels were significantly lower in Per1 heterozygous mice than compared with WT mice, with no apparent difference in fecal output (Fig. 7, A and B). This result may reflect a compensatory response to the increased urinary Na+ excretion.
Fig. 6.
Decreased Per1 results in impaired renal Na+ conservation. Weight-matched male WT 129/sv and Per1 het mice were placed in metabolic cages and allowed to acclimate for 3 days. Per1 het mice were fed the same amount of food as the WT mice ate the previous day. Mice were fed a normal gel diet (0.2% Na+) with free access to water. After 3 days, mice were switched to a low-Na+ diet (0.02%) for 7 days. Urine samples were collected daily. Flame photometry was performed to determine urinary Na+ concentrations. A: urinary Na+ retention over time with the average urinary Na+ retention during the normal diet and low-Na+ diet. n = 5. *P < 0.05 compared with WT mice on given day. B: data shown by genotype and diet, averaged over the course of the normal diet (days 1–3) versus the low-Na+ diet (days 4–10). n = 5. *P < 0.05 compared with WT mice; †P < 0.05 compared with time (noon vs. midnight); ‡P < 0.01, significant interaction by two-way ANOVA. C: body weight relative to starting weight (day 3 of the acclimation period). n = 5. *P < 0.05 compared with control.
Fig. 7.
Per1 het mice excrete less Na+ in their feces on normal and low-Na+ diets. Weight-matched male WT 129/sv and Per1 het mice were placed in metabolic cages and allowed to acclimate for 3 days. Per1 het mice were fed the same amount of food as the WT mice ate the previous day. Mice were fed a normal gel diet (0.2% Na+) with free access to water. After 3 days, mice were switched to a low-Na+ diet (0.02%) for 7 days. Feces were collected daily. Flame photometry was performed to determine fecal Na+ concentrations on day 3 (normal diet) and day 10 (low-Na+ diet). Fecal Na+ excretion from day 3 (normal diet) and day 10 (low-Na+ diet) is shown. n = 6. *P < 0.05 compared with WT mice; †P < 0.05 compared with time (noon vs. midnight).
Decreased Per1 results in decreased urinary aldosterone levels.
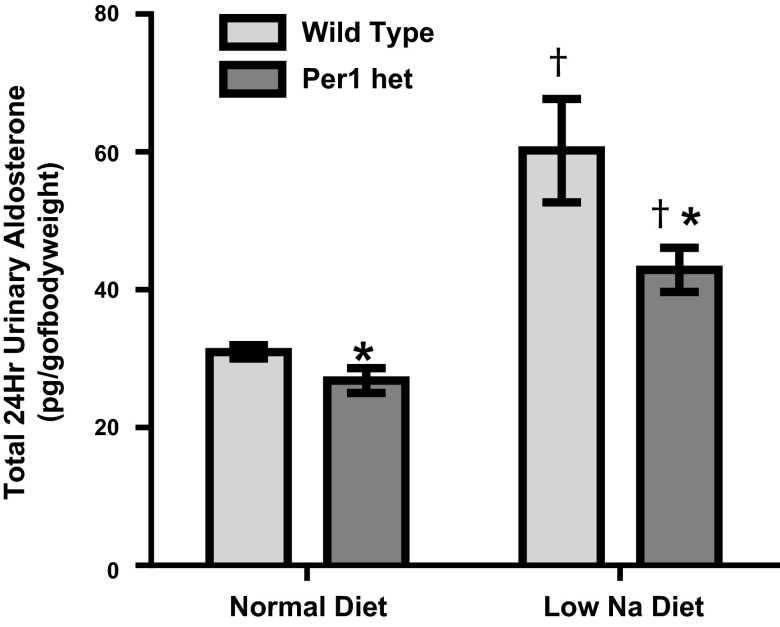
A low-Na+ diet results in an increase in aldosterone synthesis, termed an “aldosterone response” (38). Per1 appears to regulate aldosterone levels, at least partially through decreased expression of 3β-HSD. To test if Per1 heterozygous mice had a defect in the aldosterone response, urinary aldosterone measurements were made using samples from the metabolic cage experiments shown in Fig. 6. Measurements were made using urine samples from the last day of the normal diet and the last day of the low-Na+ diet (days 3 and 10, respectively). As expected, WT mice exhibited an increase in urinary aldosterone in response to the low-Na+ diet (Fig. 8). Importantly, urinary aldosterone levels were lower in Per1 heterozygous mice compared with WT mice on the normal diet and on the low-Na+ diet. However, Per1 heterozygous mice underwent an increase in urinary aldosterone in response to the low-Na+ diet, suggesting that the RAAS system is intact in these mice (Fig. 8).
Fig. 8.
Decreased Per1 results in decreased urinary aldosterone levels. Weight-matched male WT 129/sv and Per1 het mice were placed in metabolic cages and allowed to acclimate for 3 days. Per1 het mice were fed the same amount of food as the WT mice ate the previous day. Mice were fed a normal gel diet (0.2% Na+) with free access to water. After 3 days, mice were switched to a low-Na+ diet (0.02%) for 7 days. Urinary aldosterone levels were determined by ELISA (ENZO) in WT and Per1 het mice on normal (day 3) and low-Na+ (day 10) diets. n =5. *P < 0.05 compared with WT mice; †P < 0.05 compared with diet (normal vs. low Na+).
DISCUSSION
The purpose of this study was to investigate the role of Per1 in the regulation of plasma aldosterone and renal Na+ retention. We showed that Per1 regulated 3β-HSD expression in vitro through the use of RNA silencing and pharmacological blockade of Per1 nuclear entry in the NCI-H295R human adrenal cell line. These results were confirmed in vivo. Mice with reduced levels of Per1 had decreased levels of plasma aldosterone and decreased mRNA expression of 3β-HSD. Decreased Per1 in vivo was associated with increased urinary Na+ excretion. These data support a novel role for Per1 in the regulation of serum aldosterone levels and renal Na+ retention.
It is well established that the RAAS and renal Na+ retention play a critical role in BP control. Multiple circadian mutant mice have an established BP phenotype. Clock KO mice are hypotensive, potentially due to decreased levels of the preglomerular vasoconstrictor 20-HETE (40). Bmal1 KO mice exhibit reduced BP during the active phase (10). Per2 KO mice exhibit decreased 24-h diastolic BP, increased heart rate, and reduced daytime dipping (18). Cry1/2 KO mice exhibit salt-sensitive hypertension, proposed to be due to increased synthesis of aldosterone (12). We (36) have previously demonstrated that Per1 KO mice have significantly reduced BP compared with WT control mice. It is likely that multiple other factors contribute to the BP phenotype of these circadian mutant mice, including sympathetic activity (33, 37), the heart (13), nitric oxide (19), and the vasculature (4). Interestingly, a recent report (24) using gene expression profiling in normotensive and hypertensive humans of white European ancestry discovered that Per1 was overexpressed in the renal medulla of hypertensive patients.
Together with our previous report (36), the data presented here suggest that loss or reduction of Per1 in vivo results in a phenotype that closely resembles that of Clock KO mice but is opposite that of mice lacking Cry1/2. Clock KO mice display a hypotensive phenotype and decreased urinary Na+ retention (26, 40), whereas Cry1/2 KO mice exhibit salt-sensitive hypertension and increased aldosterone production (12). Per1 KO mice have significantly lower BP than WT control mice (36), and the present study demonstrates that Per1 heterozygous mice exhibit reduced serum aldosterone levels and increased urinary Na+ excretion. Consistent with these apparently opposite roles for Per1 and Cry in the regulation of BP and renal Na+ handling, Cry2 was upregulated in vitro in adrenal gland cells and in vivo in adrenal gland tissue after reduction of Per1 expression. We (30) have previously shown that Cry2 is upregulated in the renal cortex of Per1 heterozygous mice relative to WT control mice. Considering the canonical circadian clock mechanism in which Per and Cry are described as transcriptional repressors, it might seem surprising that loss of Per1 and Cry1/2 in vivo results in opposite phenotypes. However, the results presented here support our recent finding that Per1 and Cry2 appear to mediate opposing actions on Per1 target genes in the kidney and liver (30).
In summary, here we present in vivo evidence that is consistent with our in vitro observations of the regulation of α-ENaC (16, 18) and ENaC activity (31) by Per1. Together with our previously published report (36) regarding a role for Per1 in the coordinate regulation of multiple genes involved in the regulation of renal Na+ reabsorption, the data presented here support a role for Per1 in the regulation of renal Na+ reabsorption through a potential mechanism involving the modulation of serum aldosterone levels.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-085193 and DK-098460 and the ASN Foundation for Kidney Research (to M. L. Gumz) and American Heart Association Predoctoral fellowship 13PRE16910096 (to J. Richards).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R. and M.L.G. conception and design of research; J.R., K.-Y.C., S.C.A., G.S., L.A.J., and I.J.L. performed experiments; J.R., S.C.A., and M.L.G. analyzed data; J.R., S.C.A., I.J.L., C.S.W., and M.L.G. interpreted results of experiments; J.R. prepared figures; J.R. drafted manuscript; J.R., S.C.A., I.J.L., C.S.W., and M.L.G. edited and revised manuscript; M.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of L. Jeffers: Emory Univ. School of Medicine, Atlanta, GA.
REFERENCES
- 1.Agarwal R. Regulation of circadian blood pressure: from mice to astronauts. Curr Opin Nephrol Hypertens 19: 51–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Light RP. The effect of measuring ambulatory blood pressure on nighttime sleep and daytime activity–implications for dipping. Clin J Am Soc Nephrol 5: 281–285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht U. The mammalian circadian clock: a network of gene expression. Front Biosci 9: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Anea CB, Cheng B, Sharma S, Kumar S, Caldwell RW, Yao L, Ali MI, Merloiu AM, Stepp DW, Black SM, Fulton DJ, Rudic RD. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res 111: 1157–1165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L, St Germain K, Schaeffer E, Tate B, Sprouse J. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther 322: 730–738, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bollinger T, Bollinger A, Oster H, Solbach W. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology 56: 574–580, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Bose S, Boockfor FR. Episodes of prolactin gene expression in GH3 cells are dependent on selective promoter binding of multiple circadian elements. Endocrinology 151: 2287–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA 104: 3450–3455, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16: 67–74, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res 106: 647–658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazdar AF, Oie HK, Shackleton CH, Chen TR, Triche TJ, Myers CE, Chrousos GP, Brennan MF, Stein CA, La Rocca RV. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res 50: 5488–5496, 1990 [PubMed] [Google Scholar]
- 15.Greenlee MM, Lynch IJ, Gumz ML, Cain BD, Wingo CS. Mineralocorticoids stimulate the activity and expression of renal H+,K+-ATPases. J Am Soc Nephrol 22: 49–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of αENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochim Biophys Acta 1799: 622–629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunieda T, Minamino T, Miura K, Katsuno T, Tateno K, Miyauchi H, Kaneko S, Bradfield CA, FitzGerald GA, Komuro I. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res 102: 607–614, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci USA 108: 16451–16456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem 280: 37717–37724, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lynch IJ, Welch AK, Kohan DE, Cain BD, Wingo CS. Endothelin-1 inhibits sodium reabsorption by ETA and ETB receptors in the mouse cortical collecting duct. Am J Physiol Renal Physiol 305: F568–F573, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, Morris BJ. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 58: 1093–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Moore-Ede MC. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol Regul Integr Comp Physiol 250: R737–R752, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 23: 1019–1026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perreau-Lenz S, Vengeliene V, Noori HR, Merlo-Pich EV, Corsi MA, Corti C, Spanagel R. Inhibition of the casein-kinase-1-ε/δ prevents relapse-like alcohol drinking. Neuropsychopharmacology 37: 2121–2131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainey WE, Bird IM, Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol 100: 45–50, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Reid IA, Morris BJ, Ganong WF. The renin-angiotensin system. Annu Rev Physiol 40: 377–410, 1978 [DOI] [PubMed] [Google Scholar]
- 30.Richards J, All SC, Skopis G, Cheng KY, Compton B, Srialluri N, Stow LR, Jeffers LA, Gumz ML. Opposing actions of Per1 and Cry2 in the regulation of Per1 target gene expression in the liver and kidney. Am J Physiol Regul Integr Comp Physiol 305: R735–R747, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, Gumz ML. Inhibition of αENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CKIδ/ε. Am J Physiol Renal Physiol 303: F918–F927, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeder A, Loh DH, Jordan MC, Roos KP, Colwell CS. Circadian regulation of cardiovascular function: a role for vasoactive intestinal peptide. Am J Physiol Heart Circ Physiol 300: H241–H250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheward WJ, Naylor E, Knowles-Barley S, Armstrong JD, Brooker GA, Seckl JR, Turek FW, Holmes MC, Zee PC, Harmar AJ. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: behavioral effects are more significant than direct outputs. PLos One 5: e9783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simoes ESAC, Flynn JT. The renin-angiotensin-aldosterone system in 2011: role in hypertension and chronic kidney disease. Pediatr Nephrol 27: 1835–1845, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol 22: 598–604, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59: 1151–1156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki J, Ogawa M, Tamura N, Maejima Y, Takayama K, Maemura K, Honda K, Hirata Y, Nagai R, Isobe M. A critical role of sympathetic nerve regulation for the treatment of impaired daily rhythm in hypertensive Dahl rats. Hypertens Res 33: 1060–1065, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Thomas W, Harvey BJ. Mechanisms underlying rapid aldosterone effects in the kidney. Annu Rev Physiol 73: 335–357, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Vagnucci AH, Shapiro AP, McDonald RH., Jr Effects of upright posture on renal electrolyte cycles. J Appl Physiol 26: 720–731, 1969 [DOI] [PubMed] [Google Scholar]
- 40.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA 106: 16523–16528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]