Abstract
We have recently demonstrated that intrarenal dopamine plays an important role in preventing the development of systemic hypertension. Similarly, renal cytochrome P-450 (CYP)-epoxygenase-derived arachidonic acid metabolites, epoxyeicosatrienoic acids (EETs), also are antihypertensive through inhibiting sodium reabsorption and vasodilation. The potential interaction between renal dopamine and epoxygenase systems was investigated. Catechol-O-methyl-transferase (COMT)−/− mice with increased intrarenal dopamine levels and proximal tubule deletion of aromatic amino acid decarboxylase (ptAADC−/−) mice with renal dopamine deficiency were treated with a low-salt diet or high-salt diet for 2 wk. Wild-type or Cyp2c44−/− mice were treated with gludopa, which selectively increased renal dopamine levels. In low salt-treated mice, urinary EET levels were related to renal dopamine levels, being highest in COMT−/− mice and lowest in ptAADC−/− mice. In high salt-treated mice, total EET and individual EET levels in both the kidney and urine were also highest in COMT−/− mice and lowest in ptAADC−/− mice. Selective increases in renal dopamine in response to gludopa administration led to marked increases in both total and all individual EET levels in the kidney without any changes in blood levels. qRT-PCR and immunoblotting indicated that gludopa increased renal Cyp2c44 mRNA and protein levels. Gludopa induced marked increases in urine volume and urinary sodium excretion in wild-type mice. In contrast, gludopa did not induce significant increases in urine volume or urinary sodium excretion in Cyp2c44−/− mice. These studies demonstrate that renal EET levels are maintained by intrarenal dopamine, and Cyp2c44-derived EETs play an important role in intrarenal dopamine-induced natriuresis and diuresis.
Keywords: dopamine, Cyp2c44, EET, natriuresis, AADC, COMT, gludopa
in the mammalian kidney, dopamine is a major regulator of net renal salt and water excretion and mediates 50–70% of renal sodium excretion under conditions of normal or excessive salt intake (2). Dopamine can inhibit net reabsorption in both the proximal and distal nephron by inhibiting activity of multiple transporters, including the type 3 Na/H exchanger (NHE3), type 2 Na-Pi cotransporter (NaPi-II), Na-bicarbonate cotransporter (NBC), and Na-K-ATPase in the proximal tubule; Na-K-2Cl (NKCC2) in the thick ascending limb of Henle (TAL); and the epithelial Na channel (ENaC) and aquaporin-2 (AQP2) in the collecting duct (1).
In regard to dopamine actions in the kidney, it is striking that the kidney possesses an intrarenal dopaminergic system, in which the circulating precursor, l-DOPA, is taken up in the proximal tubule by apical and basolateral transport proteins and converted to dopamine by amino acid decarboxylase (AADC). Intrarenal dopamine levels are also regulated by metabolism by carboxymethyl-O-transferase (COMT) and monoamine oxidase to inactive byproducts (2). In high salt-treated animals, there is increased proximal tubule dopamine production, which inhibits net salt and fluid reabsorption (4). In contrast, intrarenal dopamine production decreases in low salt-treated animals (3).
Alterations in intrarenal dopamine production and/or activity have been reported in essential hypertension. Although there have been studies indicating decreased dopamine production in some experimental models of hypertension such as deoxycorticosterone acetate/high-salt, other studies have indicated that dopamine signaling in the proximal tubule is also dysfunctional, due to abnormalities in GRK4 coupling to D1 receptors (10). In addition, in certain experimental models of hypertension, dopamine receptor expression is decreased (10).
The intracellular second messengers mediating dopamine's natriuretic effects have been the subject of previous studies but remain incompletely defined. Although there is good evidence for a role for cAMP as a mediator, there have also been studies implicating cytochrome P-450 arachidonic acid metabolites as second messengers of dopamine's actions (11, 18). These studies have concentrated upon a potential role of 20-hydroxyeicosatetraenoic acid (20-HETE), the arachidonic acid metabolite of the CYP4504A family, but have relied on the use of inhibitors rather than direct measurement or genetic manipulation.
In addition to 20-HETE, there is another family of CYP450 arachidonic acid metabolites, the epoxyeicosatrienoic acids (EETs), which are products of the CYP2C and CYP2J families of CYP450s. There is increasing evidence that alterations in renal EET production play a major role in the development of salt-sensitive hypertension. In normal animals and subjects, a high-salt diet significantly increases expression of renal epoxygenases (6, 16), suggesting that in states of chronic volume expansion, EETs maintain natriuresis (15, 25). Mice with genetic deletion of Cyp4a10 developed salt-sensitive hypertension and required higher systolic blood pressure to excrete a sodium load (20). Although Cyp4a10−/− mice do not have any defects in renal 20-HETE production, they have marked defects in renal EET production (20). Furthermore, mice with genetic deletion of the major renal epoxygenase enzyme, Cyp2c44, develop salt-sensitive hypertension (6, 24). Since the renal effects of EETs appear to mirror those of dopamine, the goal of the present studies was to determine whether EETs may serve as second messengers in the kidney to mediate dopamine's regulation of net salt and water excretion.
METHODS
Animals.
The Institutional Animal Care and Use Committee at Vanderbilt University approved all protocols. COMT−/− mice, which have increased intrarenal dopamine due to deletion of the major dopamine metabolism enzyme, and the corresponding wild-type mice on the 129J/sv background were obtained from Dr. Maria Karayiorgou at Rockefeller University (13). In the kidney, the dopamine precursor, l-DOPA, is converted to dopamine by AADC. ptAADC−/− mice on the 129/sv background were generated as previously reported (26). Cyp2c44−/− and wild-type (WT) mice on the 129/sv background were generated as previous reported (24). All mice were genotyped with PCR before use. Only male mice were used for experiments. Mice were fed normal pelleted rodent chow with 0.5% NaCl (Harlan). A subset of animals was placed on either a low-salt diet (0.02–0.03% NaCl, ICN Biochemicals, Irvine, CA) or a high-salt diet (8% NaCl, Research Diets, New Brunswick, NJ) for 2 wk. To collect overnight (16-h) urine, the animals were first acclimated individually in metabolic cages, and then a 16-h urine collection was instituted.
Since gludopa is not currently commercially available, it was synthesized at the Vanderbilt Chemical Biology Center. To confirm selective increases in dopamine levels in the kidney after gludopa administration, male 129J/sv mice (2 mo old) were given different doses of gludopa through subcutaneous osmotic minipumps (model 2001, Alzet) and euthanized after 7 days. For the acute effect of gludopa on natriuresis and diuresis, gludopa was given via intraperitoneal injection twice a day at a dose of 10 mg·kg−1·day−1 throughout the experiment. For all gludopa experiments, males were fed normal pelleted rodent chow with 0.5% NaCl, 16-h urine was collected, and urinary sodium excretion was determined using a flame photometer.
Measurement of tissue dopamine.
Dopamine was measured by high-pressure liquid chromatography coupled with electrochemical detection by the Neurochemistry Core Laboratory at the Vanderbilt University Center for Molecular Neuroscience Research (21).
Measurement of EETs.
The EETs and DHETs present in biological samples were characterized and quantified as follows: organ, plasma, or urine samples were collected and homogenized in two volumes of CHCl3/CH3OH (2:1, vol/vol) containing triphenylphosphine (0.1 mM final concentration) and equimolar mixtures of synthetic [2H2]8,9-, 11,12-, 14,15-EET; 5,6-, 8,9-, 11,12-, and 14,15-DHET (95–99 2H2 atom % enrichment at the C-20 position; 5–10 ng each) as internal standards. The combined organic phases were evaporated, saponified in the presence of 0.4 N KOH/CH3OH (2:8, vol/vol) and, after acidification (pH 4.0), the fatty acids were extracted into ethyl ether, purified by solid phase SiO2, dissolved in C2H5OH, and resolved and quantified by ultra high-pressure liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) using collision-induced fragmentation of the corresponding EET (m/z 318.5–319.5) and DHET (m/z 336.5–337.5) molecular ions. Selected product ion monitoring was done for diagnostic ions at m/z (biological samples) and m/z +2 (internal standard) at the following values: 8,9-EET, 69 and 71; 11,12-EET, 167 and 169; 14,15-EET, 113 and 115; 8,9-DHET, 127 and 129; 1,12-DHET, 167 and 169; and 14,15-DHET, 207 and 209. Quantifications were done by the isotope ratio method (5) using integrated ion current intensities for the 2H2-labeled and unlabeled eicosanoids.
Immunoblotting.
Kidney samples were homogenized with buffer containing 10 mM Tris·HCl (pH 7.4), 50 mM NaCl, 2 mM EGTA, 2 mM EDTA, 0.5% Nonidet P-40, 0.1% SDS, 100 μM Na3VO4, 100 mM NaF, 0.5% sodium deoxycholate, 10 mM sodium pyrophosphate, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. The homogenate was centrifuged at 15,000 g for 20 min at 4°C. An aliquot of supernatant was taken for protein measurement with a BCA protein assay kit (ThermoScientific, Rockford, IL). Immunoblotting and antibodies against different CYPs were described in a recent report (22).
RNA isolation and qRT-PCR.
Total RNA from the kidney or cultured proximal tubule epithelial cells were isolated using TRIzol reagents (Invitrogen). qRT-PCR was performed using TaqMan real-time PCR (7900HT; Applied Biosystems). The Master Mix and all gene probes were also purchased from Applied Biosystems. The probes used in the experiments included mouse S18 (Mm02601778), Cyp2c44 (Mm01197188), Cyp2j8 (Mm03412388), Cyp2j11 (Mm01268185), and Cyp2j13 (Mm01262154).
Measurement of urine creatinine.
Urinary creatinine concentration was determined using Albuwell-M kits (Exocell, Philadelphia, PA).
Cell culture.
Proximal tubule epithelial cells were generated from mice having the Immortomouse transgene (H-2Kb-tsA58) as described by Gewin et al. (12). The cells were cultured in DMEM/F12 media containing 2.5% FBS, 50 ng/ml hydrocortisone, 5 μg/ml insulin/transferrin/selenium, 6.5 ng/ml triiodothyronine, 92 μg/ml d-valine, and penicillin/streptomycin/amphotericin. The cells were incubated at 33°C with 10 ng/ml interferon-γ. Two weeks before the experiments, cells in passage 4 were transferred at 37°C and cultured in medium without interferon-γ. After 24-h starvation in medium without FBS, the cells were treated with dopamine (1 μM) and harvested at different time points for qRT-PCR.
Statistics.
Values are presented as means ± SE. ANOVA and Bonferroni two-tailed t-tests were used for statistical analysis, and differences were considered significant for P values <0.05.
RESULTS
COMT−/− mice with high intrarenal dopamine (13), WT, and ptAADC−/− mice with intrarenal dopamine deficiency (26) were placed on a low-salt diet for 2 wk, and urine was collected for 2 consecutive days. Due to low urine volume and low urinary EET levels in low salt-treated mice, urine samples from three mice from each group were combined. Therefore, although there were six mice in each group, there were only two combined urine samples in each group. As indicated in Table 1, total urinary EET excretion was highest in low salt-treated COMT−/− mice, and lowest in low salt-treated ptAADC−/− mice (ng/mg creatinine: COMT−/−: 2.52; WT: 1.82; ptAADC−/−: 0.83), which correlated with renal dopamine levels. Urinary levels of 8,9-EET, 11,12-EET, and 14,15-EET were all lowest in ptAADC−/− mice. Similarly, kidney total EET levels were also highest in low salt-treated COMT−/− mice and lowest in ptAADC−/− mice (ng/g tissue: COMT−/−: 92.14 ± 3.00, P < 0.05 vs. WT; WT: 77.22 ± 3.30; ptAADC−/−: 66.36 ± 3.24, P < 0.05 vs. WT; n = 5 in each group).
Table 1.
Kidney dopamine levels and urinary EET excretion in low salt-treated mice
| Dopamine* | Total EET | 8,9-EET | 11,12-EET | 14,15-EET | N1** | N2** | |
|---|---|---|---|---|---|---|---|
| COMT−/− | 0.49 ± 0.01† | 2.52 | 0.62 | 0.64 | 1.27 | 6 | 2 |
| WT | 0.40 ± 0.02 | 1.82 | 0.50 | 0.52 | 0.79 | 6 | 2 |
| ptAADC−/− | 0.28 ± 0.03† | 0.83 | 0.23 | 0.19 | 0.41 | 6 | 2 |
Values are means ± SE. Mice were on a low-salt diet for 2 wk, kidneys were harvested for determination of dopamine, and 24-h urine was collected for determination of epoxyeicosatrienoic acid (EET).
COMT, catechol-O-methyl-transferase; AADC, amino acid decarboxylase.
Kidney dopamine level is expressed as ng/mg protein.
P < 0.05 vs. wild-type (WT) group; n = 3/group. Urinary EET excretion is expressed as ng/mg creatinine.
N1 is mouse number; N2 is combined urine sample number. The data are presented as the average of 2 combined urine samples.
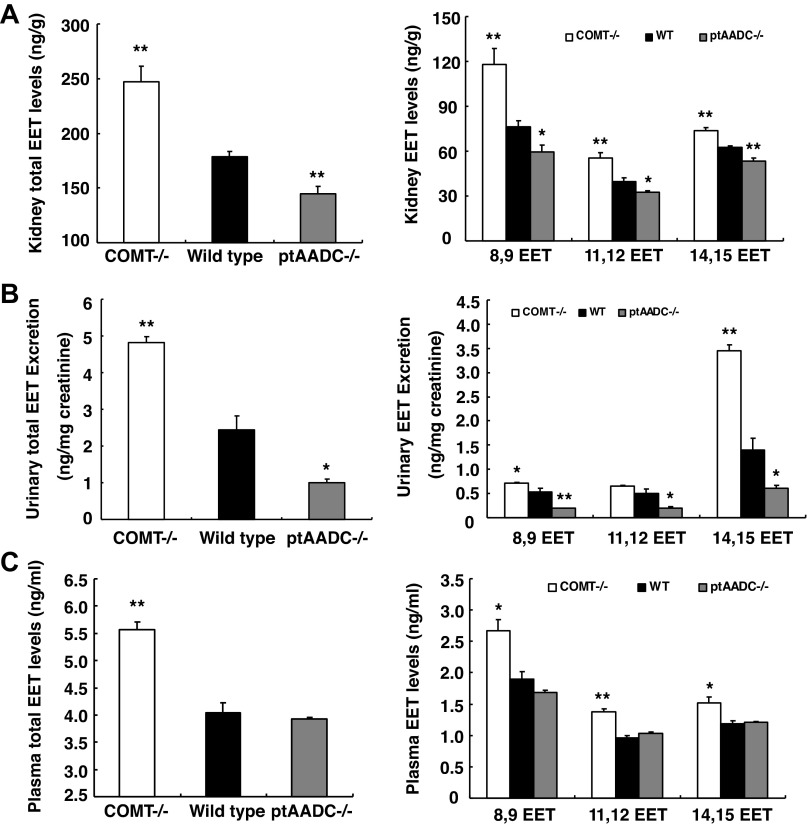
WT, COMT−/−, and ptAADC−/− mice were then placed on a high-salt diet for 2 wk, and EET levels in plasma, urine, and kidney were assayed. In response to volume expansion, total EET levels in the kidney increased in all experimental groups, which were highest in COMT−/− mice and lowest in ptAADC−/− mice (ng/g tissue: COMT−/−: 248.80 ± 14.33, P < 0.01 vs. WT; WT: 178.64 ± 5.01; ptAADC−/−: 145.05 ± 6.63, P < 0.01 vs. WT; n = 6/group). Further analysis indicated that renal levels of 8,9-EET, 11,12-EET, and 14,15-EET were all markedly higher in COMT−/− mice but were all markedly lower in ptAADC−/− mice compared with WT mice (Fig. 1A).
Fig. 1.
High kidney dopamine levels were associated with high kidney epoxyeicosatrienoic acid (EET) production in response to high salt intake. A and B: 2 wk after a high-salt diet, kidney EET production (A) and urinary EET excretion (B) were higher in catechol-O-methyl-transferase (COMT)−/− mice but lower in amino acid decarboxylase (ptAADC)−/− mice, compared with wild-type (WT) mice. C: in response to a high-salt diet, plasma EET levels were higher in COMT−/− mice than WT mice and ptAADC−/− mice. *P < 0.05 vs. WT. **P < 0.01 vs. WT; n = 6/group.
As indicated in Fig. 1B, urinary excretion of total EETs was also increased in animals on a high-salt diet compared with a low-salt diet, with the greatest increase occurring in the COMT−/− mice, which have decreased intrarenal dopamine metabolism, and the smallest increase occurring in ptAADC−/−, which have defective intrarenal dopamine synthesis. As with renal EET levels, urinary EET levels were highest in COMT−/− mice and lowest in ptAADC−/− mice (ng/mg creatinine: COMT−/−: 4.82 ± 0.18, P < 0.01 vs. WT; WT: 2.44 ± 0.37; ptAADC−/−: 0.99 ± 0.10, P < 0.001 vs. WT; n = 6 in each group). Further analysis found that excretion of 8,9-EET, 11,12-EET, and 14,15-EET all increased in COMT−/− mice, but decreased in ptAADC−/− mice compared with WT mice (Fig. 1B). Although kidney levels of 8,9-EET, 11,12-EET, and 14,15-EET were roughly similar, 14,15-EET was the predominant EET in urine, consistent with the relative stability of 14,15-EET in urine.
As indicated in Fig. 1C, both total EET levels and individual EET levels in plasma were markedly elevated in high salt-treated COMT−/− mice compared with high salt-treated WT mice, while both total EET levels and individual EET levels in plasma were comparable between high salt-treated WT mice and high salt-treated ptAADC−/− mice.
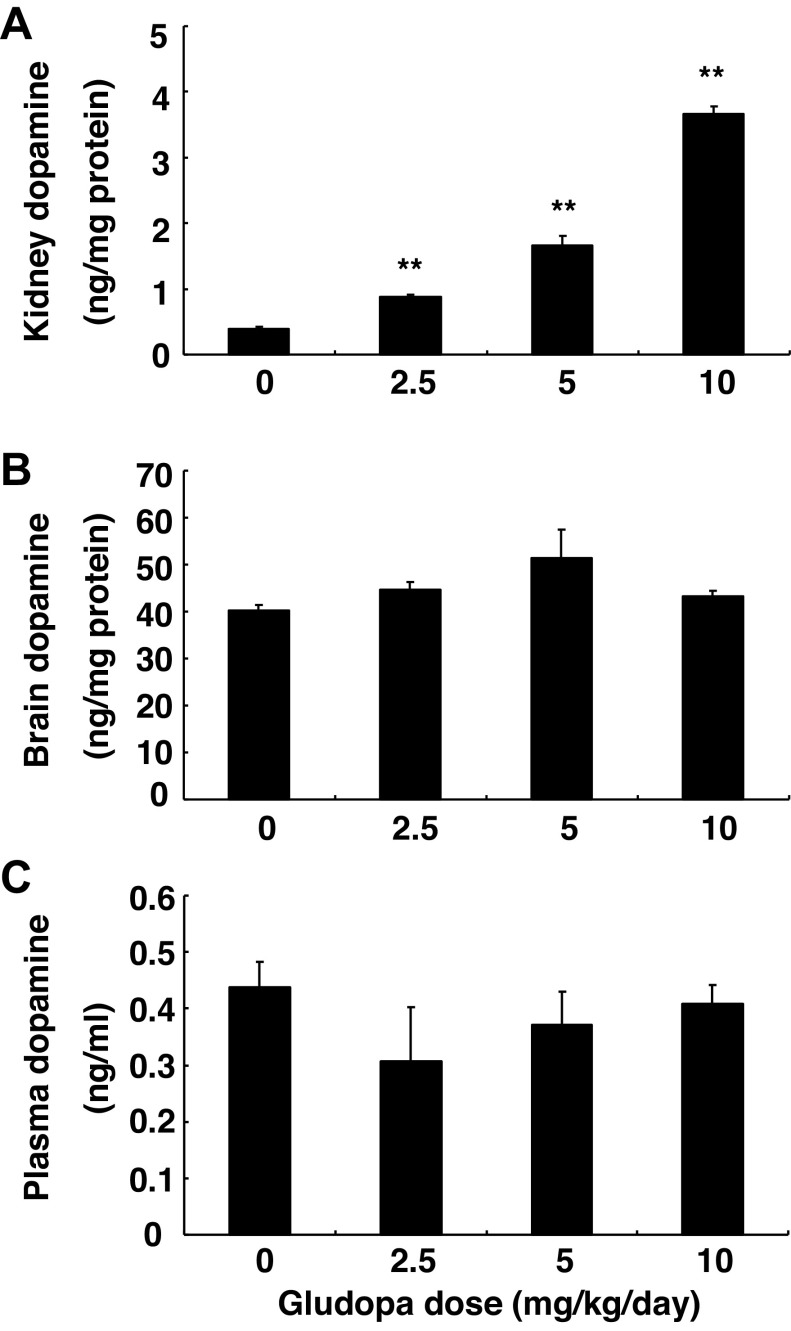
To exclude the potential effects of nonrenal dopamine and volume expansion due to high salt intake on renal EET production, we treated WT mice with gludopa, which selectively increases intrarenal dopamine levels. Male 129J/sv mice (2 mo old) were given different doses of gludopa via subcutaneous osmotic minipumps (model 2001, Alzet) and euthanized after 7 days. Tissue dopamine levels were determined using high-performance liquid chromatography coupled with electrochemical detection (21). As indicated in Fig. 2A, kidney dopamine levels increased as the dose of gludopa increased from 2.5 to 10 mg·kg−1·day−1. Treatment with 5 mg·kg−1·day−1 gludopa increased kidney dopamine levels from 0.39 ± 0.03 to 1.66 ± 0.18 ng/mg protein (P < 0.01, n = 3). As expected, dopamine levels in the brain and plasma were not affected by gludopa administration (Fig. 2, B and C).
Fig. 2.
Gludopa selectively increased kidney dopamine production. The animals were treated with different doses of gludopa via minipump for 1 wk, and dopamine levels were measured. Gludopa increased kidney dopamine production in a dose-dependent pattern (A) but had no effect on dopamine levels in the brain (B) and plasma (C). **P < 0.01 vs. control; n = 3/group.
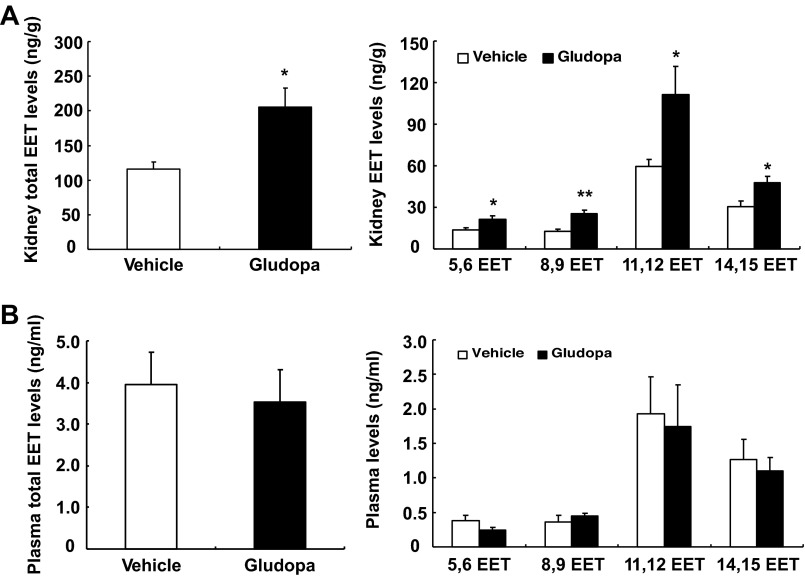
Gludopa treatment (5 mg·kg−1·day−1) for 7 days markedly increased kidney total EET levels (205.86 ± 26.81 vs. 116.24 ± 10.14 ng/g tissue treated with vehicle, P < 0.01, n = 4) (Fig. 3A). Gludopa treatment markedly increased all four individual EETs. 11,12-EET was the major EET under basal condition and after gludopa treatment, in accordance with previous reports (6, 7, 19, 20). In contrast, gludopa treatment had no effect on total EET levels (0.35 ± 0.08 vs. 0.39 ± 0.08 ng/ml of vehicle, P >0.05, n = 4) and individual EET levels in plasma (Fig. 3B).
Fig. 3.
Gludopa selectively increased EET levels in the kidney. The animals on a normal-salt diet were treated with gludopa (5 mg·kg−1·day−1) via minipump for 1 wk, and EET levels were measured. A: gludopa administration caused marked increases in total and individual EET production in the kidney. B. gludopa treatment had no effect on total and individual EET levels in plasma. *P < 0.05 vs. vehicle. **P < 0.01 vs. vehicle; n = 4/group.
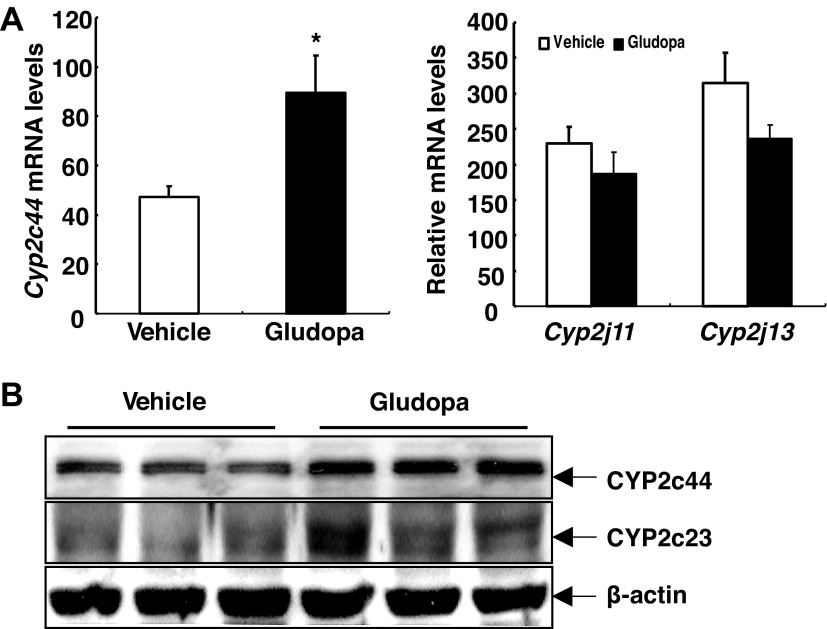
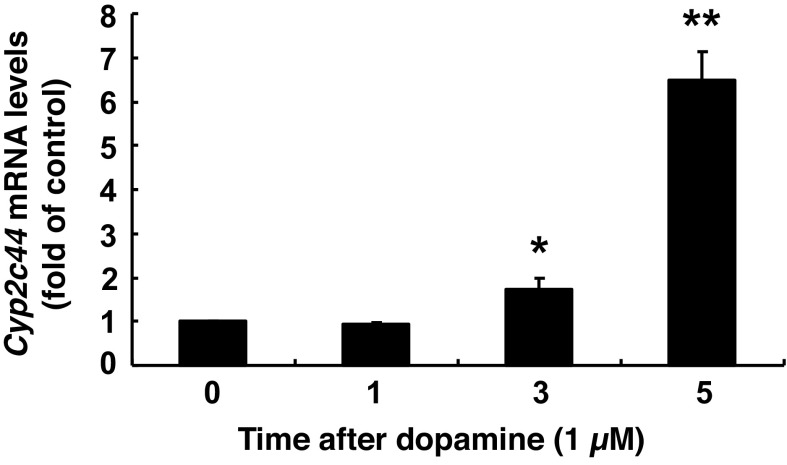
To investigate which CYP epoxygenase might mediate the increased renal EET production in response to gludopa treatment, qPCR analysis for murine CYP epoxygenases was carried out. As indicated in Fig. 4A, gludopa treatment led to marked increases in Cyp2c44 mRNA levels, but had no effect on the mRNA levels of Cyp2j11 and Cyp2j13, consistent with studies indicating that mouse Cyp2c44 is the predominant and functionally relevant kidney epoxygenase, which is mainly responsible for the generation of kidney EETs (6, 24). The mRNA levels of Cyp2j8 in the kidney were minimal. Immunoblotting indicated that Cyp2c44 protein levels were also increased with gludopa treatment (Fig. 4B). As confirmation, an antibody against Cyp2c23, the major rat kidney CYP epoxygenase with nearly 90% amino acid sequence identity with Cyp2c44, also showed similarly increased expression (Fig. 4B) (6, 8). To investigate whether dopamine directly regulates Cyp expression, cultured mouse proximal tubule epithelial cells were treated with dopamine and different Cyp mRNA levels were determined. As indicated in Fig. 5, dopamine increased Cyp2c44 mRNA levels as early as 3 h but had no effect on other Cyps (data not shown).
Fig. 4.
Gludopa administration selectively increased kidney Cyp2c44 expression. The animals on a normal-salt diet (0.5% NaCl) were treated with gludopa (5 mg·kg−1·day−1) via minipump for 1 wk and EET levels were measured. A: gludopa increased renal Cyp2c44 mRNA levels but had no effect on mRNA levels of Cyp2j11 and Cyp2j13. *P < 0.05 vs. vehicle; n = 4/group. B: gludopa increased kidney Cyp2c44 protein levels as indicated by immunoblotting detected with a specific Cyp2c44 antibody as well as an antibody against CYP2c23, the major rat kidney CYP epoxygenase with nearly 90% amino acid sequence identity with Cyp2c44.
Fig. 5.
Dopamine increased Cyp2c44 mRNA levels in cultured mouse proximal tubule cells. Dopamine (1 μM) increased Cyp2c44 mRNA levels in mouse proximal tubule cells as early as 3 h after dopamine administration. *P < 0.05 vs. vehicle. **P < 0.01 vs. vehicle; n = 3/group.
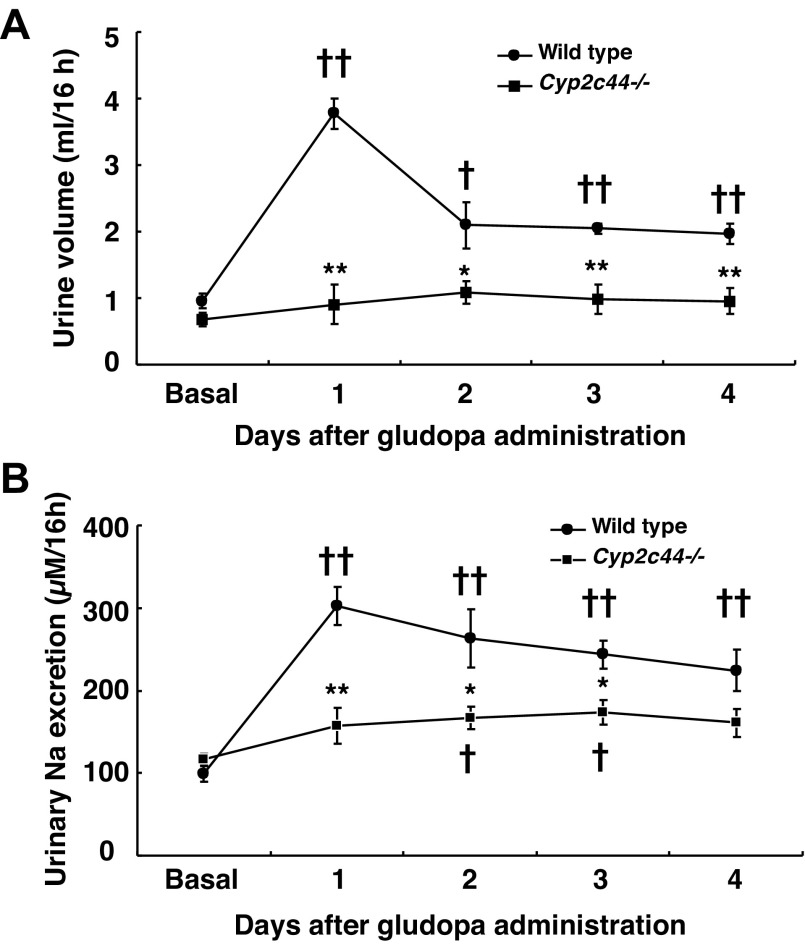
We utilized Cyp2c44−/− mice to determine the potential role of Cyp2c44 in intrarenal dopamine-mediated natriuresis and diuresis. Gludopa was given via intraperitoneal (ip) injection at a dose of 10 mg·kg−1·day−1m (ip injection, twice a day, 9:00 a.m. and 4:00 p.m., respectively). Sixteen-hour overnight urine from individual mice was collected. In WT mice, gludopa treatment led to marked increases in urine volume, with the greatest effect seen on day 1 [ml/16 h: control: 0.95 ± 0.11; gludopa (day 1): 3.77 ± 0.23, P < 0.001, n = 4]. In contrast, in Cyp2c44−/− mice urine volume did not reach statistical differences compared with pretreatment at any time point after gludopa treatment (Fig. 6A), indicating that gludopa-induced diuresis was significantly attenuated in Cyp2c44−/− mice. Similarly, gludopa treatment led to marked increases in urinary sodium excretion in WT mice at all time points [μm/16 h: control: 99.65 ± 9.93; gludopa (day 1): 302.08 ± 23.43, P < 0.001, n = 4]. In contrast, gludopa induced only modest increases in natriuresis, which only reached statistical significance on the days 2 and 3 [μm/16 h: control: 116.04 ± 7. 83; gludopa (day 2): 166.90 ± 13.79, P < 0.05, n = 4 (Fig. 6B)].
Fig. 6.
Cyp2c44 deficiency attenuated gludopa-mediated natriuresis and diuresis. WT and Cyp2c44−/− mice (on rodent chow with 0.5% NaCl) were treated with gludopa (10 mg·kg−1·day−1, intraperitoneal injection, twice a day) and 16-h urine was collected for measurement of urine volume and electrolyte excretion. A: gludopa induced markedly increases in urine volume in WT mice, which were blunted in Cyp2c44−/− mice. B: gludopa induced markedly increases in urine Na excretion in WT mice, which were blunted in Cyp2c44−/− mice. †P < 0.05 vs. control. ††P < 0.01 vs. control. *P < 0.05 vs. corresponding WT. **P < 0.01 vs. corresponding WT; n = 4/group.
DISCUSSION
A Guytonian view of hypertension posits that dysfunctional salt and water excretion by the kidney ultimately underlies the development and maintenance of hypertension. Studies by us and others have determined that intrarenal dopamine mediates natriuresis and diuresis and that abnormalities in intrarenal dopamine production or signaling can lead to development of salt-sensitive hypertension (14). The current results indicate that intrarenal dopamine increases production of renal EET production, and EETs are significant mediators of the natriuresis and diuresis induced by dopamine signaling.
The kidney is a major target for extraneural dopamine actions. Dopamine's cellular actions are mediated by signaling through G protein-coupled, seven-transmembrane receptors. There are five known renal dopamine receptors, which are divided into two subclasses: D1-like and D2-like receptors. D1-like receptors (D1 and D5) are coupled to Gs and stimulate adenylate cyclase. D2-like receptors (D2, D3, and D4) are coupled predominantly to Gi. Dopamine receptors are expressed in distinct subtype-specific distributions in the kidney, and D1-like and D2-like receptors appear to interact to mediate biological signaling (10). In renal tubules, dopamine receptors have been localized to the proximal tubule, thick ascending limb of Henle, and collecting duct and may inhibit sodium transport in each of these segments (2).
We utilized mice with increased (COMT−/−) and decreased (ptAADC−/−) kidney dopamine levels. In addition, we administered the synthetic dipeptide γ-l-glutamyl-l-DOPA (gludopa) a prodrug of l-DOPA that is filtered by the glomerulus, taken up in the proximal tubule, and enzymatically converted to l-DOPA by γ-l-glutamyltranspeptidase in the brush-border membrane of proximal tubules. Renal and urinary EET levels were lower with decreased intrarenal dopamine and higher with increased intrarenal dopamine (COMT−/− mice and gludopa administration). Also of note, in both WT and COMT−/− mice, renal and urinary EET levels increased with a high-salt diet, consistent with increased intrarenal dopamine production. Furthermore, although plasma levels of EETs were increased in COMT−/− mice, which have systemic inhibition of dopamine metabolism, the alterations in EET levels seen in ptAADC−/− mice and with gludopa administration were confined to the kidney and urine, confirming that renal dopamine can directly modulate renal EET production.
In high salt-treated animals, 8,9-EET levels were highest among EETs in both kidney and plasma, while 14,15-EET levels were highest in urine, consistent with its relative stability in urine. Of note, in gludopa-treated animals, 11,12-EET levels were highest among EETs in the kidney. The underlying mechanism for the difference of the ratio of renal EET regioisomers between high salt-treated and gludopa-treated mice will require further investigation.
In addition to increased EET production, we also determined that increased intrarenal dopamine can induce expression of Cyp2c44, the major renal epoxygenase. We did not detect alterations in Cyp2j8, Cyp2j12, or Cyp2j13, which have also been reported to produce EETs in the kidney (23). Gludopa administration also led to a small increase in Cyp4a12, the major omega hydroxylase in the kidney (not shown). Since dopamine production in the kidney is confined to the proximal tubule, the site of AADC expression, and the highest concentration of Cyp450 expression in the kidney is also in the proximal tubule (9, 17), we utilized conditionally immortalized mouse proximal tubule cells and confirmed that dopamine will directly increase Cyp2c44 expression. Further in vivo and in vitro studies will be required to determine whether the effect of dopamine to increase proximal tubule EET production is completely dependent on increased expression of Cyp2c44 or if it will also increase EET production by increasing free arachidonic acid and/or activity of existing Cyp2c44 enzyme.
To determine whether EETs are involved in mediating the renal effects of dopamine, we compared the natriuretic effect of gludopa in WT and Cyp2c44−/− mice. The significant attenuation of gludopa-mediated natriuresis and diuresis in Cyp2c44−/− mice indicates that EETs may serve as a second messenger for dopamine in the mammalian nephron. Therefore, these studies provide a new link between the intrarenal dopaminergic and cytochrome P-450 systems in the regulation of natriuresis and diuresis.
GRANTS
These studies were supported in part by grants from the National Institutes of Health (DK38226, DK51265, DK62794, CA122620), by the Vanderbilt O'Brien Center (DK79341), and funds from the Veterans Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.-Z.Z. and R.C.H. provided conception and design of research; M.-Z.Z., Y.W., B.Y., L.G., and S.W. performed experiments; M.-Z.Z., Y.W., J.H.C., and R.C.H. analyzed data; M.-Z.Z., J.H.C., and R.C.H. interpreted results of experiments; M.-Z.Z. and R.C.H. prepared figures; M.-Z.Z. and R.C.H. drafted manuscript; M.-Z.Z. and R.C.H. edited and revised manuscript; M.-Z.Z., J.H.C., and R.C.H. approved final version of manuscript.
REFERENCES
- 1.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol 1: 1075–1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines AD. Effects of salt intake and renal denervation on catecholamine catabolism and excretion. Kidney Int 21: 316–322, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Bertorello A, Hokfelt T, Goldstein M, Aperia A. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol Renal Fluid Electrolyte Physiol 254: F795–F801, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Capdevila J, Dishman E, Karara A, Falck JR. Cytochrome P-450 arachidonic acid epoxygenase: Sterochemical characterization of epoxyeicosatrienoic acids. Methods Enzymol 206: 441–453, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Capdevila J, Wang W. Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension. Curr Opin Nephrol Hypertens 22: 163–169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capdevila JH. Regulation of ion transport and blood pressure by cytochrome p450 monooxygenases. Curr Opin Nephrol Hypertens 16: 465–470, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int 72: 683–689, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem 52: 447–454, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol 2: 637–650, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez MM, Gonzalez D, Williams JM, Roman RJ, Nowicki S. Inhibitors of 20-hydroxyeicosatetraenoic acid (20-HETE) formation attenuate the natriuretic effect of dopamine. Eur J Pharmacol 686: 97–103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gewin L, Vadivelu S, Neelisetty S, Srichai MB, Paisit P, Pozzi A, Harris RC, Zent R. Deleting the TGF-β type II receptor in the proximal tubule attenuates acute kidney injury. J Am Soc Nephrol 23: 2001–2011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95: 9991–9996, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RC, Zhang MZ. Dopamine, the kidney, and hypertension. Curr Hypertens Rep 14: 138–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He FJ, MacGregor GA. Salt, blood pressure and the renin-angiotensin system. J Renin Angiotensin Aldosterone Syst 4: 11–16, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Hoagland KM, Flasch AK, Roman RJ. Inhibitors of 20-HETE formation promote salt-sensitive hypertension in rats. Hypertension 42: 669–673, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Kaergel E, Muller DN, Honeck H, Theuer J, Shagdarsuren E, Mullally A, Luft FC, Schunck WH. P450-dependent arachidonic acid metabolism and angiotensin II-induced renal damage. Hypertension 40: 273–279, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kirchheimer C, Mendez CF, Acquier A, Nowicki S. Role of 20-HETE in D1/D2 dopamine receptor synergism resulting in the inhibition of Na+-K+-ATPase activity in the proximal tubule. Am J Physiol Renal Physiol 292: F1435–F1442, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or genetically controlled alterations of the renal microsomal cytochrome P450 epoxygenase induce hypertension in rats fed a high salt diet. J Clin Invest 94: 2414–2420, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Jr, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest 116: 1696–1702, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA 102: 2174–2179, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidkovka N, Rao R, Mei S, Gong Y, Harris RC, Wang WH, Capdevila JH. Epoxyeicosatrienoic acids (EETs) regulate epithelial sodium channel activity by extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated phosphorylation. J Biol Chem 288: 5223–5231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarborough PE, Ma J, Qu W, Zeldin DC. P450 subfamily CYP2J and their role in the bioactivation of arachidonic acid in extrahepatic tissues. Drug Metab Rev 31: 205–234, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Sun P, Antoun J, Lin DH, Yue P, Gotlinger KH, Capdevila J, Wang WH. Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake. Hypertension 59: 339–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol 124: 719–727, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]