Abstract
Measurements of inter- and intramolecular distances are important for monitoring structural changes and understanding protein interaction networks. Fluorescence resonance energy transfer and functionalized chemical spacers are the two predominantly used strategies to map short-range distances in living cells. Here, we describe the development of a hybrid approach that combines the key advantages of spectroscopic and chemical methods to estimate dynamic distance information from labeled proteins. Bifunctional spectroscopic probes were designed to make use of adaptable-anchor and length-varied spacers to estimate molecular distances by exploiting short-range collisional electron transfer. The spacers were calibrated using labeled polyproline peptides of defined lengths and validated by molecular simulations. This approach was extended to estimate distance restraints that enable us to evaluate the resting-state model of the Shaker potassium channel.
Introduction
New molecular insights have been gained in recent years by integrating an array of chemical approaches for study of proteins involved in several cellular and physiological processes. To study the dynamics of proteins, it is crucial to monitor inter- and intramolecular distances, often in real-time. Förster resonance energy transfer (FRET) is a spectroscopic method based on energy transfer between donor and acceptor chromophores (1) that has been widely adopted with the advent of genetically engineered probes (2). This technique allows us to probe dynamic distance changes between 10 and 100 Å and report on transient structures in a physiological milieu (1). Nonetheless, FRET estimates are very sensitive to orientation, size, and flexibility of the fluorescent groups, each of which may introduce errors when used for distance measurements. Lanthanide resonance energy transfer, a variant of FRET, significantly reduces errors due to the orientation factor. However, long lifetimes of the dye can systematically underestimate distances because measurements are weighted toward the distance of closest approach (3,4).
An alternative method uses chemical spacers capped by functional groups at both ends to measure point-to-point molecular distances (5–7). This chemical strategy is straightforward but has found limited application, because the readout is typically a single functional state and information about other conformations is not available. In some cases, streptavidin binding to a biotin-tagged spacer has been exploited, but this strategy may trap the protein in a nonnative state because the binding affinities are very high (8). Our goal here was to develop a method that combines spectroscopic probes with chemical spacers to overcome the inherent limitations of each of these techniques and provide a direct estimate of molecular distances within the 4–40 Å range.
Herein we report on the synthesis of a library of what we believe to be novel chemical probes and a subsequent approach based on a spectroscopic readout utilizing collisional quenching (1) to dynamically monitor distances between two sites. We envisioned that these hybridized probes would serve as heterobifunctional spectroscopic affinity labels for a desired target. We call the compounds “tethered quenchers”. We demonstrate that distance information can be inferred by measuring accessibility of a fluorescent tag on one site to a variable-length collisional quencher covalently affixed to a second site. Thus, the functionalized variable-length quenchers act as chemical calipers.
The feasibility of our approach was determined using a set of tethered quenchers to estimate distances in model polypeptides. The rigid polypeptides served as molecular rulers to calibrate the effective distance range for quenching afforded by our probes. Lengths of our chemical calipers, estimated from the experimental calibration studies, qualitatively matched those predicted from molecular-dynamics simulations. Biological application of the tethered-quencher approach was then defined by extending the method to monitor protein dynamics of an exemplar voltage-gated potassium (K+) channel, enabling dynamic distance estimates during voltage-dependent-state transitions. The studies reported here establish for the first time, to our knowledge, that tethered spectroscopic probes can be used as versatile chemical calipers to estimate dynamic molecular distances. This optical approach may prove useful for mapping subnanometer distance changes between both intra- and intermolecular sites in large protein complexes.
Materials and Methods
Peptide preparation
Polyproline peptides of defined lengths containing 6 or 10 proline residues (Pro6 or Pro10) were synthesized on an automated synthesizer (Prelude model; Protein Technologies, Tucson, AZ) by the University of Wisconsin-Madison Biotechnology Facility. The polyproline substrates were flanked with a fluorophore (tetramethylrhodamine (TAMRA)) on the N-terminal glycine cap and a C-terminal cysteine residue with a free sulfhydryl group that can be chemically modified. Peptides were stored as lyophilized powders.
Quencher preparation
Quenchers with varied-size polyethylene glycol (PEG) linkers were synthesized in approximately six steps for each compound. PEG linkers were chosen for their high solubility in aqueous solution. The nitroxy radical (NO•), or dibromo groups, and maleimide were introduced in the last two steps using an amide-coupling reaction and Click chemistry between alkyne and azide due to their high functional group compatibility. Synthesized compound were frozen and stored in dimethyl sulfoxide.
Cross-linking reaction
Tethered quenchers (10 mM, dimethyl sulfoxide) with a free maleimide group attached were reacted with polyproline substrates (2.5 mM, buffered water, pH 7.6) for 1 h and then frozen.
Purification, verification, and quantification of tethered quencher/polyproline complex
Compounds were purified on a reversed-phase HPLC (ProStar; Varian, McKinley Scientific, Sparta, NJ) equipped with a ProStar 210 delivery module and ProStar 335 photo-diode array detector (Varian, McKinley Scientific) using a C18 column (Zorbax SB-C18; Agilent Technologies, Santa Clara, CA) at a flow rate of 1 mL/min over 30–60 min using a linear gradient from 0.1% trifluoroacetic acid in 10% acrylonitrile/90% H2O to 90% acrylonitrile/10% H2O. Collected fractions were lyophilized and stored as a powder. Fractions were reconstituted in buffered water and analyzed using matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (Voyager DE Pro; Applied Biosystems, Life Technologies, Temecula, CA) to determine molecular weight. Concentrations were determined using data from a Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA) for each sample. Samples were diluted to a final concentration of 10 nM in tetrafluoroethylene.
Fluorescence spectroscopy
Fluorescent counts were measured using a QuantaMaster Model C-60/2000 Spectrofluorimeter (Photon Technologies International; Birmingham, NJ) with an excitation range 450–562 nm emitting at 572 nm to determine the relative excitation spectrum. The emission spectrum was assayed over a wavelength range from 525 to 700 nm, and excited at 500 nm. Peak emission wavelength of TAMRA was reliably observed at 565–572 nm. Excitation and emission spectra were obtained at 25°C.
Molecular biology and electrophysiology
Single-residue cysteines were engineered into select sites within the extracellular loops of a mutated (T449F, C245V, C301S, C308S, C462A) Shaker K+ channel by using a QuikChange mutagenesis kit (Stratagene, La Jolla, CA) and confirming by gene sequencing. For cRNA preparation, plasmids were linearized by Not I digestion. cRNA was generated by in vitro transcription using a T7 RNA polymerase kit (mMessage mMachine; Ambion, Grand Island, NY). Ionic conductance and fluorescent signals were obtained using a customized, cut-open, voltage-clamp fluorometry setup (CA-1B; Dagan Instruments, Minneapolis, MN. The cut-open setup was placed on a stage of an upright microscope (BX50WI; Olympus, Center Valley, PA). Light from a halogen-lamp source (Hamamatsu Photonics, Bridgewater, NJ) was filtered with a HQ535/50 bandpass filter and split using a Q565LP dichroic mirror (Chroma Technology, Bellows Falls, VT).
Emitted light was filtered with an HQ610/75 bandpass filter (Chroma Technology) and focused onto a PIN-020A Photodiode (OSI Optoelectronics, Hawthorne, CA) by a condenser lens. The photodiode was connected to the headstage of an integrating Axopatch 1B patch-clamp amplifier (MDS Analytical Technologies, Sunnyvale, CA). To ensure that the photocurrent was within the dynamic range of the amplifier, additional current was fed into the summing junction of the headstage. Ionic currents and fluorescent signals were obtained 24–36 h after cRNA injection into mature Xenopus laevis oocytes. Oocytes were incubated in hyperpolarizing or depolarizing solutions and labeled with trimethylrhodamine methyl ester perchlorate (5–50 μM) for fluorescence experiments. TEA and tethered quenching compounds (TEA-PEGn-NO•; n = 0–4) were prepared fresh for each experiment and bath-applied to yield a final concentration of 1–2 mM for fluorescence quenching and pharmacology experiments.
Simulations
Molecular-dynamics (MD) setups for the PEG and polyproline/PEG systems are described in the Supporting Material. For the PEG chains attached to the Shaker channel, Monte Carlo simulations were performed in the dihedral space only and energies were calculated with the software CHARMM 22 (http://www.charmm.org/) with the EEF1 solvation model. Ten million cycles, corresponding to 1.8–2.25 M of sampled conformations (the rest were rejected moves), were carried out at a constant temperature of 25°C.
Additional section available in the Supporting Material
Detailed Materials and Methods are available in the Supporting Material. To provide sufficient procedural details, however, an abbreviated Materials and Methods section is given here.
Results and Discussion
Rationale and synthesis of tethered spectroscopic probes
Tethered reactive groups offer a unique chemical strategy to obtain physical distance estimates by measuring the rate of reactivity as a function of spacer length. The reactivity of tethered functional groups depends on the distance of the attachment site from the reaction site and spacer length. Thus, by measuring the two variables, rate of reactivity and spacer length, the distance between the tether site and reactive site can be computed (7,9). Herein, to probe dynamic structural changes, we substituted quencher-fluorophore pairs where the fluorescence is quenched by a dynamic collisional quenching mechanism (Dexter energy transfer). This short-range (5–8 Å) mechanism of energy transfer, in contrast to FRET, requires the molecular orbitals of the fluorophore and quencher to overlap in space. Collisional frequency between the two molecules is influenced by diffusion rate and local concentration in solution (1). Short-range quenching of this type has been used to determine protein depth in lipid membranes (10), study folding (11) and accessibility (12,13), and to estimate intramolecular distances (14). Thus, we took advantage of the short-range requirement for collisional quenching of TAMRA fluorescence (1,14–16) and synthesized a library of spectroscopic probes (Fig. 1).
Figure 1.
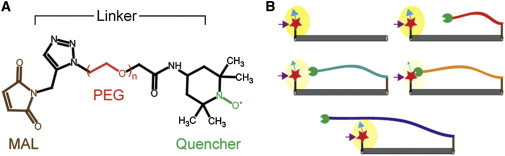
Strategy utilizing a tethered quencher approach to estimate distances. (A) Tethered quenchers are bifunctional spectroscopic probes that make use of a target-specific adaptable anchor and length-varied spacers to estimate molecular distances by exploiting short-range collisional electron transfer. Probes designed for initial characterization of the method using proline polypeptide substrates consist of a maleimide (MAL) anchor and a nitroxide radical quenching moiety separated by different-sized polyethylene-glycol (PEG) spacers. (B) N-terminal fluorescently-labeled (red star) substrate of known length with varied-length collisional quenchers (green circle) tethered to the C-terminus. The excited-state energy of a fluorescent probe (purple arrow) can be transferred, upon short-range overlap of donor and quencher orbitals, to a quenching group reducing the fluorescence emission (blue arrow).
The basic structure for the first series of compounds (see Fig. S1, Type 1, series a, in the Supporting Material) included a maleimide tethered with a tetramethylpiperidine oxide radical quencher separated by variable-length PEG spacers (see additional Methods in the Supporting Material). The maleimide group can react with an available nucleophile, such as a cysteine, by S-alkylation to anchor the compounds. PEG spacers were chosen for their high solubility in aqueous solution. Next, we investigated the feasibility of our method using these compounds as chemical calipers to estimate distances in model polypeptides.
Calibrating the tethered quencher approach
Polyprolines (n < 10) adopt a stiff all-trans type-II helix in aqueous solution, as shown by NMR (17) and single-molecule (18) fluorescence, and have been used to calibrate distances measurements for FRET studies (19,20). The relationship between the quenching efficiency and spacer-length of our synthetic compounds was calibrated with short polyprolines that form well-defined structures (Fig. 1 B). Polyprolines were functionalized by labeling an N-terminal glycine residue with a fluorescent reporter (TAMRA) and by adding a C-terminal cysteine for downstream chemical modification. Cysteine-reactive tailed quenchers of varying lengths were then attached at the C-terminal end and purified using HPLC. All products were verified by MALDI mass spectrometry.
We evaluated the quenching efficiency of the tethered probes using a proline 6 (Pro6) substrate. The substrate spacer for this experiment has been demonstrated to have a backbone contour length of ∼18 Å and act as a rigid rod (18). We measured the quenching efficiency of a range of variable-length NO• quenchers tethered to the C-terminal cysteine of Pro6 (Fig. 2, A and B; see Fig. S1, compounds A1–A6). Fig. 2 A shows the raw emission spectra of the labeled Pro6 in the presence and absence of the tethered quenchers.
Figure 2.

Relation between quenching efficiency and spacer length of PEG compounds affixed to proline polypeptides. Emission spectrum illustrating change in relative fluorescence intensity, in arbitrary units (Au), for (A) Pro6 and (C) Pro10 tethered to quenching compounds of varied PEG spacer lengths (see Fig. S1 in the Supporting Material; compound A1–A6). (Adjacent) Histograms of the percent change in fluorescence intensity as a function of the number of PEG spacers for (B) Pro6 and (D) Pro10 TEMPO is a cysteine-reactive compound with a nitroxide radical and no PEG spacers (PEG = 0).
The peak at ∼570 nm was measured for various conditions and normalized with respect to labeled Pro6 without attached quenchers and plotted as a function of PEG length (Fig. 2 B). The quenching efficiency exhibits a U-shaped (nonmonotonic) dependence with respect to the number of PEG units. This assay also appears to be exquisitely sensitive to small length changes. For example, increasing the tether by one PEG unit (∼3.62 Å; see Fig. S1, compounds A1–A2) reduced the fluorescence intensity ∼25%. Interestingly, this phenomenon appears to have a steep cutoff distance as illustrated by the quenching efficiency of A6 (eight PEG units), which suggests the quenching groups may have a directional preference or the spacers have a limited ability to bend backward.
Next, we investigated the quenching efficiency of A1–A6 on a longer substrate (Pro10, Fig. 2 C). The length of the Pro10 peptide has been estimated to be ∼30 Å by single molecule measurements (18). Our measurements of the emission spectra of the labeled Pro10 in the presence of various quenchers are consistent with the estimated length (Fig. 2 D). Attaching quenchers with spacers of up to four PEG units had no effect on the fluorescence of the peptide, but any subsequent increase in PEG length yielded a monotonic decrease in the peak intensity of the fluorescence. Due to the difficulty in synthesis of longer PEG spacers, we did not explore the possibility that a true fluorescence minimum may be observed for longer PEG lengths. Together, these data provide a calibration curve for tethered quenchers to provide distance estimates of up to 30 Å with increments of 4 Å.
Molecular simulations recapitulate experimental observations
To gain further insight into the behavior of the variable-length spacers, we examined various PEG spacers in the absence of any tethering using molecular simulations. MD simulations of the PEG tethers were carried out using an implicit solvent model (GBSW, see the Supporting Material). The distance between the first carbon and the last carbon in the PEG chain was calculated, allowing us to approximate the end-to-end distance for multiple PEG chains. As shown in Fig. 3 A, the distribution, for PEG chains containing <3 units, is narrow. This indicates that these molecules act as rigid units. However, addition of one PEG unit (PEG 4) noticeably broadens the distribution. We then carried out these simulations for longer PEG chains (n > 4), and observed the similar trend (Fig. 3 B). As the chain length increased, we observed two main features:
-
1.
The standard deviation increased, illustrating the wide range of configurations sampled as the chain becomes more flexible; and
-
2.
The end-to-end distance for chains containing <5 PEG units is a linear function of the number of PEG units, suggesting that the shorter chains may serve as rulers to approximate distances between two sites.
Figure 3.

PEG chain distributions and contact models from MD simulations. Data shown in panels A and B are for isolated PEG chains, and those for panels C–H are for PEG chains attached to polyproline peptides, with the chromophore (TAMRA) and quencher groups attached to the PEG chain and polyproline as in the experimental setup. (A) Distribution of end-to-end distances of PEG chains (n = 1–4). The distance between the first carbon and the last carbon in PEG chain was measured. (B) Average end-to-end distances with standard deviations over the simulations as the error bars. Points were fit using a spline function. (C and D) (White ribbon) Polyproline backbone (Pro6); (pink sticks) PEG. (Ball-and-stick representation with oxygen in red, nitrogen in blue, and carbon in cyan) TAMRA and NO•; all hydrogens are hidden. Direct contact is observed in panels C and D for the PEG 3 and PEG 5 simulations, respectively. In contrast, a wrapping contact is observed for the PEG 7 (E) and PEG 9 (F) simulations. (G and H) Simulated fluorescence intensity estimates as a function of PEG unit. Fluorescence intensity was calculated by integrating the normalized OO distance distribution from 0 to 7.5 Å (peak), for (G) Pro6 and (H) Pro10. OO distance is measured between oxygen in TAMRA group and oxygen in NO• group.
To further investigate the contact probability between two sites as a function of chain flexibility, we ran simulations with PEG chains of different lengths attached to a polyproline (Pro6 or Pro10). The chromophore and quencher were attached to the polyproline and PEG as in the experimental setup; care was exercised to treat the interactions that involve the chromophore and quencher (see Fig. S2 and Fig. S3). To simulate quenching as a function of PEG spacers, we derived a quenching probability by monitoring the OO distance between oxygen in the TAMRA aromatic ring and oxygen in the quencher group (NO•). Our analysis shows that regardless of the lengths of the polyproline and the PEG chain, the OO distance distribution peaks at ∼7.5 Å, which clearly reflects the attraction between TAMRA and NO•. Therefore, a cutoff value of 7.5 Å was used to estimate the quenching probability by counting the fraction of conformations in which the OO distance is shorter than the cutoff (see the Supporting Material ).
Analysis of the trajectories from different simulations revealed two different contact modes between TAMRA and NO•. When the PEG chain is short (m = 3–5, see the Supporting Material), more-extended structures are observed (Fig. 3, C and D)—referred to as the direct contact mode. With a longer PEG chain (m = 7, Fig. 3 E), it wraps around TAMRA. This is referred to as the wrapping contact mode. Formation of the wrapping contact mode reflects favorable van der Waals interactions between the PEG chain and the large aromatic surface of TAMRA. This interaction is reminiscent of the fact that PEG 400 increases the solubility of benzoic acid (21). However, with even longer PEG chains (m = 9), the entropy cost for a compact chain configuration becomes too large and the degree of wrapping loosens up (Fig. 3 F). Owing to the stabilizing effect of the compact wrapping conformation, the fluorescence intensity minimum (peak quenching probability) occurs at a PEG length favoring the compact wrapping contact. This corresponds to a PEG length of 6–7 for the Pro6 systems and qualitatively explains the nonmonotonic behavior of the fluorescence intensity (compare Figs. 3 G and 2 B).
For Pro10, formation of the direct contact mode becomes increasingly easier as the PEG length increases from m = 3–9. Thus, no wrapping contact is observed for PEG chains up to m = 9. These observations are qualitatively consistent with the nonmonotonic dependence of the fluorescence intensity on the PEG length (compare Figs. 3 H and 2 D). Because Pro10 is substantially longer than Pro6, it is presumed that longer PEG chains are required to form the wrapping contact mode and therefore reach a minimum in the fluorescence intensity. In short, the MD simulations are able to qualitatively capture the dependence of the quenching profile on the PEG chain length for both Pro6 and Pro10, lending support to the qualitative correlation between features of quenching profile and distance.
Toolset for distance mapping in a biological system
To validate the utility of the tethered quenchers as an approach to obtain distance measurements in living cells, we targeted the voltage-dependent Shaker K+ channel. Among the numerous biological targets we considered, voltage-gated ion channels (VGICs) were an attractive target owing to their essential role in cellular excitability (22). Several high-resolution structures of VGICs have been solved (23–27) but, in all instances, the voltage-sensor is in an activated conformation. To understand the detailed workings of VGICs, it is necessary to obtain information about the structure of the channel in resting conformation, which is still a matter of debate (28). Spectroscopic methods (3,29–34) suggest a small movement of the voltage sensor, whereas chemical (8,35–42) spacer data are consistent with large-scale rearrangement of the voltage sensor. Thus, we postulated that the tethered quencher approach would complement the previous measurements and may shed new light on voltage-sensor movements. This strategy allows for examination of both conducting (activated/open) and nonconducting (resting) functional states.
To map the conformations of the voltage sensor in resting and activated states, environment-sensitive fluorescent tags were attached to engineered cysteines in the voltage-sensing or pore domain of the Shaker K+ channel. In response to depolarizing pulses from a resting membrane potential, these fluorescent probes typically generate a quenching or a dequenching signal (29,31) due to a change in microenvironment. Addition of an extrinsic quencher can modify this fluorescence signal by preferentially quenching the fluorescent probe in one state relative to the other. As shown in Fig. 4 C, if the fluorescence signal decreases upon channel activation, then a resting-state-specific quencher will further decrease the amplitude of the fluorescence signal. If, on the other hand, the quenching group prefers the activated state, then the fluorescence signal will increase. No change in the amplitude of the fluorescence signal indicates that either none of the states are quenched, or that the quenching is state-independent.
Figure 4.

Scenarios for fluorescence quenching and data from voltage-clamp fluorometry experiments. (A) A general chemical structure of the pore-anchored potassium-channel-specific tethered quencher backbone. (B) Percent inhibition of ionic potassium current (IK) after addition of vehicle, commercial TEA, or tethered quenchers (C2) with a TEA moiety (T.Q.). Additional controls were tested including freely diffusing (F.D.) conditions where the TEA moiety is absent from the T.Q. compound and no anchor where the high-affinity TEA site within the channel pore is removed. (C) Panel of hypothetical schemes for tethered quenching of fluorescently labeled residues. (Left) Voltage sensors (blue) of labeled channels in a resting state when no currents pass through the pore (yellow) yield a baseline fluorescent signal, F, from an unquenched state. Upon depolarization (+Vm) voltage sensors activate, which results in fluorescence quenching because the probe is exposed to the solvent. Next, consider a tethered quencher that quenches the fluorophore in the resting state to some degree. Upon depolarization, the signal goes down further and is equal to the activated-state signal. The net result is that the change in fluorescence is reduced (ΔF2 < ΔF1) when the quencher interacts with the fluorophore in resting state. If, on the other hand, the quencher interacts with the probe in the activated state of the voltage sensor, then the fluorescence signals from the activated state will be further reduced. As a result, the difference between the resting and activated fluorescence signals will increase (ΔF1 > ΔF3) and the ΔF will be higher in the presence of quencher. In some cases, the fluorescence increases upon depolarization in the absence of quenchers. In those instances, if the ΔF increases upon addition of the quencher, then the label and quencher are interacting much more effectively in the resting state. In contrast, a reduced ΔF signal indicates that the label and quencher are in close proximity in the activated state. (D) Representative voltage-dependent fluorescence signals in the presence and absence of tethered quenchers of varying PEG lengths (0–4). (Black traces) Absence of quenchers. (Colored traces) Variable-length quenchers added during the recordings. (Traces) Baseline-corrected for background fluorescence before initial recording. (Top columns) Mutant constructs; (left) quenching conditions. Scale bar represents 20 ms; depolarization voltage step = +40 mV.
For these experiments, we synthesized another set of tethered quenchers (see Fig. S1; compounds C0–C4) with a pore-blocking moiety (TEA) to anchor our calipers to the channel (Fig. 4 A and Fig. S1, Type 2, series a). Cysteine residues were then engineered into extracellular regions (S1–S2, S3–S4, and S5–S6) of a cysteine-removed (Δ4Cys) channel background (31). Initially, several extracellular target sites for cysteine insertion were scanned. The criteria for choosing ideal sites was based on the ability to fluorescently report a change in environment as a function of voltage, and estimated distance from the channel pore determined from crystal structures.
We then generated a high-affinity TEA site (T449F) within the pore (43). The phenylalanine residues contributed by each of the four subunits coordinate and tightly bind to the quaternary ammonium ion via a cation-π interaction (44). This substitution significantly increases the blocker affinity and effectively reduces the Ki by ∼100-fold (∼20–0.5 mM) compared to wild-type channels (43).
This point becomes critically important because at sufficiently high concentrations, free quenchers can directly quench fluorescence of the label on the channel. To this end, we showed that a working concentration <10 mM for the NO• quenchers does not reduce the fluorescence intensity (see Fig. S4). Thus, utilizing the high-affinity clone allowed the use of a lower concentration for the tethering studies and eliminated the possibility of contamination from freely diffusing/nonanchored molecules. Potency of C0–C4 was determined based on blocking of K+ current under voltage-clamp and comparison to the commercially available TEA. Ionic currents were significantly reduced by 78.5 ± 6.2% (p = 0.00014; n = 4) and 78.1 ± 8.6% (p = 0.0014; n = 3) with 1 mM of the commercial TEA and C0–C4, respectively, compared to vehicle (Fig. 4 B). Other controls such as quenching groups lacking a TEA moiety did not significantly reduce ionic currents (97.7 ± 2.1%, p = 0.67, n = 3 and 93.8 ± 2.4%, p = 0.23, n = 7), indicating that the observed current reduction is due to specific binding of the TEA to the K+ channel pore.
Tethered quenchers as chemical calipers for Shaker K+ channels
We obtained distance constraints for four different sites on the Shaker K+ channel using voltage-clamp fluorometry. As shown in Fig. 4 D (black traces), fluorescence intensity increases during depolarization for the E422C site, whereas the intensity decreases for the other three sites. The polarity of the signals allows us to obtain distance restraints in both activated (upward deflection, E422C) and resting (downward deflection, A359C, M356C, and T276C) states (45). The voltage-dependent fluorescent signals for each site are shown in Fig. 4 D in the presence and absence of the variable-length tethered quenchers. Control experiments using 1 mM commercially available TEA or S-C1 did not alter the fluorescent signal intensity. In contrast, addition of the pore-anchored quenchers significantly modified the signals in a site- and spacer-specific manner.
Using the structural coordinates for TEA-bound KcsA (46). we placed TEA in the putative resting state of the Kv 1.2/2.1 paddle chimera (47). Upon inspection of the resting-state model (Fig. 5, A and B), we observed that one monomer appeared disconnected and lacked several molecular contacts conserved within the other monomers. Therefore, this monomer was not included in the average distance calculations.
Figure 5.

Quenching summary at multiple sites within the Shaker potassium channel. General open-state structure of the rat Kv1.2/2.1 paddle chimera (A, side-in; B, top-down) with TEA docked in the pore (blue arrow) and the four engineered cysteine sites in color. Structure derived from the Protein Data Bank (PDB:2BOC). Scale bar (bottom right, panel A) is equal to one PEG unit. Histograms illustrating averaged responses (n = 3–10) for tethered quenching experiments for each cysteine construct (C) E422C, (D) A359C, (E) M356C, and (F) T276C with the varied-length PEG spacers (0–4). Normalized ΔΔF was calculated by taking the difference in ΔF before and after addition of the quencher and normalizing it with respect to ΔF before addition. Maximal responses were fit using a spline function to generate an approximate PEG-unit quenching response curve. The response curve is right-shifted when comparing the maximal response upon moving N-terminal in the protein with respect to the pore. Colors are conserved throughout the figure.
The average measured distance from the center nitrogen of TEA to residues corresponding to the sites labeled in the Shaker K+ channel (based on sequence homology (24)) were 27 ± 2 Å for E422, 30 ± 2 Å for A359, 30 ± 2 Å for M356, and 50 ± 3 Å and T276 in the resting state. Fig. 5, C–E, shows that the fluorophore at E422C is maximally quenched by PEG2 whereas those at positions A359C and M356C were quenched most efficiently by PEG3. Among the compounds tested, PEG4 appears to quench the fluorophore on T276C most efficiently, but it is unclear whether this compound is the optimal quencher because we lack data for longer-length quenchers. Interestingly, the average point-to-point distances between the center nitrogen on TEA and oxygen in the quenching moiety, in an extended conformation, for PEG-2 is ∼30 Å, PEG-3 is ∼33 Å, and PEG-4 is ∼36 Å. Thus, the quenching efficiency of the various length quenchers appears to be in good qualitative agreement with the positions of these sites relative to the TEA in the resting-state structural model (47).
The assumption that these PEG spacers are in an extended conformation is not unreasonable, because when there are fewer than four PEG units these linkers do not have sufficient conformational flexibility to bend back, as evidenced in our molecular simulations (Fig. 3). Although the size and orientation of the fluorophore and the molecular contacts between the caliper and the protein are important for determining the quenching efficiency, our assay appears to be surprisingly sensitive and can discriminate between sites separated by only a few Ångstroms, suggesting this method may be suitable for sub-nanometer-distance measurements. Furthermore, our experimental distance estimates suggest that the resting-state model of the voltage-gated K+ channel proposed by Jensen et al. (47) based on constrained MD simulations is within reason and likely to resemble the functional state of the channel.
Concluding Remarks
In summary, we have developed what we believe to be a novel method to dynamically measure molecular distances by fusing chemical and spectroscopic approaches. This versatile strategy resulted in a synthesis of an apparently new class of probes called tethered quenchers. Efficiency and sensitivity was evaluated using model polypeptides to calibrate our tethered quenchers, then validated in a biological setting using the multimeric Shaker K+ channel. By taking advantage of a short-range quenching mechanism and chemical tethering, we demonstrate that we can reliably probe dynamic distances <30 Å while resolving length differences of <4 Å. Critical to the utility of our method for measuring point-to-point distances is the observation that an optimal point of quenching can be obtained. This unique feature of our assay is illustrated by the fact that the point of optimal quenching is directly dependent on the number of PEG units, where linkers too short or too long do not result in maximal quenching.
It is notable that Cha and Bezanilla (12) showed that pore-blocking molecules such as TEA and agitoxin can affect the fluorescence signal arising from a modulation of the conformational changes associated with pore opening. However, this would not be predicted to affect our quenching measurements because all recordings were done in presence of TEA, allowing us to examine the trend in quenching from TEA-NO• to TEA-(PEG)4- NO•. As such, a reliable estimate between the two points can be determined using the end-to-end distances of the specific quenching compound. These data are consistent with the hypothesis that shorter PEG chains can act as stiff, rigid units where the extended conformation closely resembles the average end-to-end distance. Indeed, our MD simulations qualitatively reproduce these results, demonstrating that shorter PEG chains (<7 PEG units) allow a direct contact between the fluorophore and the quencher to form.
As a result, quenching efficiency is predicted to increase as a function of the number of PEG units where optimal quenching occurs when the PEG chain flexibility allows for increased probability of direct contacts to form. However, with longer PEG chains, chain entropy favors a loose configuration, decreasing the contact probability Thus, at a qualitative level, it is appropriate to correlate the PEG length at which the fluorescence intensity reaches a minimum with the distance that separates the tethering points of the fluorophore and the PEG chain. However, the interaction between the PEG chain and other components in the system is critical. Therefore, it is conceivable that the interaction between the PEG chain and the environment can affect the observed behavior.
Given the structural similarity between VGICs and the available chemical anchors, the strategy established here can be extended to examine more-complex proteins such as the heteromultimeric voltage-gated Na+ or Ca+ channels. As such, this method can be used to complement chemical and spectroscopic approaches based on the target, desired readout, and system constrains. Furthermore, with the recent progress of unnatural amino acid (UAA) incorporation in chemical biology, one can easily adapt a tethered quencher strategy to investigate protein-protein interactions and estimate distances without the limitations of the fluorophore attachments. Such an application may utilize a combination of chemistry, pharmacology, and toxicology, and fluorescence spectroscopy in addition to UAA incorporation. For example, PEG units can be added to a target molecule (48,49), Click chemistry can be used to attach an array of bioconjugates, and fluorescent UAA derivatives (with turn-on, turn-off properties) can be incorporated into the protein backbone along with target-selective toxins in a site-specific manner (2,50).
It is also notable that the fundamental characteristics of the tethered quencher probes can be tailored based on the biological system using caged groups or profluorescent derivatives, various quenching molecules, and anchoring pairs. For example, we successfully substituted the nitroxy radical for a brominated moiety and consistently achieved quenching (∼70% relative to NO•) of our fluorescent probe. The flexibility afforded by our approach allows one to develop a strategy suitable for not only their protein of interest but within the expression system. Taken together, such multifaceted strategies may play a critical role in understanding protein dynamics and elucidating regulatory mechanisms involved in pathology with the aim of developing better therapeutics to treat the disorders.
Acknowledgments
We thank the University of Wisconsin-Madison Peptide Synthesis Facility, Dr. Senes, and L. LaPointe for HPLC and lyophilizer access, and Dr. Juneja and Prof. Nilsson for sharing modified CHARMM source code for simulations with scaled van der Waals attractions. We appreciate the valuable comments from S. Chowdhury and Dr. McCaslin.
Verification of peptide crosslinking and spectrophotometric data were obtained at the University of Wisconsin-Madison Biophysics Instrumentation Facility, established with support from the University of Wisconsin-Madison and grants No. BIR-9512577 (National Science Foundation) and No. S10 RR13790 (National Institutes of Health). This work was funded by National Institutes of Health grants No. 5T32HL007936-09 to B.W.J., Nos. GM084140 and NS081293 to B.C., and by Department of Energy grant No. DEFG02-04ER25627 to L.Z.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons-Attribution Noncommercial License (http://creativecommons.org/licenses/by-nc/2.0/), which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Suqing Zheng, Leili Zhang, and Xiaoxun Li contributed equally to this article.
Supporting Material
References
- 1.Lakowicz J.R. Springer; New York: 2006. Principles of Fluorescence Spectroscopy. [Google Scholar]
- 2.de Graaf A.J., Kooijman M., Mastrobattista E. Nonnatural amino acids for site-specific protein conjugation. Bioconjug. Chem. 2009;20:1281–1295. doi: 10.1021/bc800294a. [DOI] [PubMed] [Google Scholar]
- 3.Posson D.J., Ge P., Selvin P.R. Small vertical movement of a K+ channel voltage sensor measured with luminescence energy transfer. Nature. 2005;436:848–851. doi: 10.1038/nature03819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posson D.J., Selvin P.R. Extent of voltage sensor movement during gating of Shaker K+ channels. Neuron. 2008;59:98–109. doi: 10.1016/j.neuron.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlin A., Akabas M.H. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 6.Singer S.J. Covalent labeling of active sites. Adv. Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- 7.Wofsy L., Metzger H., Singer S.J. Affinity labeling—a general method for labeling the active sites of antibody and enzyme molecules. Biochemistry. 1962;1:1031–1039. doi: 10.1021/bi00912a013. [DOI] [PubMed] [Google Scholar]
- 8.Ruta V., Chen J., MacKinnon R. Calibrated measurement of gating-charge arginine displacement in the KvAP voltage-dependent K+ channel. Cell. 2005;123:463–475. doi: 10.1016/j.cell.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Karlin A. Chemical modification of the active site of the acetylcholine receptor. J. Gen. Physiol. 1969;54:245–264. doi: 10.1085/jgp.54.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay A., London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987;26:39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- 11.Eftink M.R. The use of fluorescence methods to monitor unfolding transitions in proteins. Biophys. J. 1994;66:482–501. doi: 10.1016/s0006-3495(94)80799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha A., Bezanilla F. Structural implications of fluorescence quenching in the Shaker K+ channel. J. Gen. Physiol. 1998;112:391–408. doi: 10.1085/jgp.112.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arias H.R., Valenzuela C.F., Johnson D.A. Transverse localization of the quinacrine binding site on the Torpedo acetylcholine receptor. J. Biol. Chem. 1993;268:6348–6355. [PubMed] [Google Scholar]
- 14.Zhu P., Clamme J.P., Deniz A.A. Fluorescence quenching by TEMPO: a sub-30 Å single-molecule ruler. Biophys. J. 2005;89:L37–L39. doi: 10.1529/biophysj.105.071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.London E. Investigation of membrane structure using fluorescence quenching by spin-labels. A review of recent studies. Mol. Cell. Biochem. 1982;45:181–188. doi: 10.1007/BF00230086. [DOI] [PubMed] [Google Scholar]
- 16.Abrams F.S., London E. Calibration of the parallax fluorescence quenching method for determination of membrane penetration depth: refinement and comparison of quenching by spin-labeled and brominated lipids. Biochemistry. 1992;31:5312–5322. doi: 10.1021/bi00138a010. [DOI] [PubMed] [Google Scholar]
- 17.Best R.B., Merchant K.A., Eaton W.A. Effect of flexibility and cis residues in single-molecule FRET studies of polyproline. Proc. Natl. Acad. Sci. USA. 2007;104:18964–18969. doi: 10.1073/pnas.0709567104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuler B., Lipman E.A., Eaton W.A. Polyproline and the “spectroscopic ruler” revisited with single-molecule fluorescence. Proc. Natl. Acad. Sci. USA. 2005;102:2754–2759. doi: 10.1073/pnas.0408164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stryer L., Haugland R.P. Energy transfer: a spectroscopic ruler. Proc. Natl. Acad. Sci. USA. 1967;58:719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schimmel P.R., Flory P.J. Conformational energy and configurational statistics of poly-L-proline. Proc. Natl. Acad. Sci. USA. 1967;58:52–59. doi: 10.1073/pnas.58.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rytting E., Lentz K.A., Vakatesh S. Aqueous and cosolvent solubility data for drug-like organic compounds. AAPS J. 2005;7:E78–E105. doi: 10.1208/aapsj070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hille B. Sinauer; Sunderland, MA: 2001. Ion Channels of Excitable Membranes. [Google Scholar]
- 23.Jiang Y., Lee A., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 24.Long S.B., Tao X., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 25.Payandeh J., Gamal El-Din T.M., Catterall W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payandeh J., Scheuer T., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X., Ren W., Yan N. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swartz K.J. Sensing voltage across lipid membranes. Nature. 2008;456:891–897. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cha A., Bezanilla F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron. 1997;19:1127–1140. doi: 10.1016/s0896-6273(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 30.Chanda B., Asamoah O.K., Bezanilla F. Gating charge displacement in voltage-gated ion channels involves limited transmembrane movement. Nature. 2005;436:852–856. doi: 10.1038/nature03888. [DOI] [PubMed] [Google Scholar]
- 31.Mannuzzu L.M., Moronne M.M., Isacoff E.Y. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- 32.Chanda B., Bezanilla F. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J. Gen. Physiol. 2002;120:629–645. doi: 10.1085/jgp.20028679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha A., Snyder G.E., Bezanilla F. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature. 1999;402:809–813. doi: 10.1038/45552. [DOI] [PubMed] [Google Scholar]
- 34.Glauner K.S., Mannuzzu L.M., Isacoff E.Y. Spectroscopic mapping of voltage sensor movement in the Shaker potassium channel. Nature. 1999;402:813–817. doi: 10.1038/45561. [DOI] [PubMed] [Google Scholar]
- 35.Larsson H.P., Baker O.S., Isacoff E.Y. Transmembrane movement of the Shaker K+ channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 36.Ahern C.A., Horn R. Focused electric field across the voltage sensor of potassium channels. Neuron. 2005;48:25–29. doi: 10.1016/j.neuron.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Blaustein R.O., Cole P.A., Miller C. Tethered blockers as molecular ‘tape measures’ for a voltage-gated K+ channel. Nat. Struct. Biol. 2000;7:309–311. doi: 10.1038/74076. [DOI] [PubMed] [Google Scholar]
- 38.DeCaen P.G., Yarov-Yarovoy V., Catterall W.A. Disulfide locking a sodium channel voltage sensor reveals ion pair formation during activation. Proc. Natl. Acad. Sci. USA. 2008;105:15142–15147. doi: 10.1073/pnas.0806486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henrion U., Renhorn J., Elinder F. Tracking a complete voltage-sensor cycle with metal-ion bridges. Proc. Natl. Acad. Sci. USA. 2012;109:8552–8557. doi: 10.1073/pnas.1116938109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lainé M., Lin M.C., Papazian D.M. Atomic proximity between S4 segment and pore domain in Shaker potassium channels. Neuron. 2003;39:467–481. doi: 10.1016/s0896-6273(03)00468-9. [DOI] [PubMed] [Google Scholar]
- 41.Aziz Q.H., Partridge C.J., Sivaprasadarao A. Depolarization induces intersubunit cross-linking in a S4 cysteine mutant of the Shaker potassium channel. J. Biol. Chem. 2002;277:42719–42725. doi: 10.1074/jbc.M207258200. [DOI] [PubMed] [Google Scholar]
- 42.Elinder F., Männikkö R., Larsson H.P. S4 charges move close to residues in the pore domain during activation in a K channel. J. Gen. Physiol. 2001;118:1–10. doi: 10.1085/jgp.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992;8:483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- 44.Ahern C.A., Eastwood A.L., Horn R. A cation-π interaction between extracellular TEA and an aromatic residue in potassium channels. J. Gen. Physiol. 2006;128:649–657. doi: 10.1085/jgp.200609654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathak M.M., Yarov-Yarovoy V., Isacoff E.Y. Closing in on the resting state of the Shaker K+ channel. Neuron. 2007;56:124–140. doi: 10.1016/j.neuron.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Lenaeus M.J., Vamvouka M., Gross A. Structural basis of TEA blockade in a model potassium channel. Nat. Struct. Mol. Biol. 2005;12:454–459. doi: 10.1038/nsmb929. [DOI] [PubMed] [Google Scholar]
- 47.Jensen M.O., Jogini V., Shaw D.E. Mechanism of voltage gating in potassium channels. Science. 2012;336:229–233. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- 48.Shozen N., Iijima I., Hohsaka T. Site-specific incorporation of PEGylated amino acids into proteins using nonnatural amino acid mutagenesis. Bioorg. Med. Chem. Lett. 2009;19:4909–4911. doi: 10.1016/j.bmcl.2009.07.105. [DOI] [PubMed] [Google Scholar]
- 49.Deiters A., Cropp T.A., Schultz P.G. Site-specific PEGylation of proteins containing unnatural amino acids. Bioorg. Med. Chem. Lett. 2004;14:5743–5745. doi: 10.1016/j.bmcl.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 50.Liu C.C., Schultz P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


