Abstract
Since its inception in 19th-century Germany, the physiology laboratory has been a complex and expensive research enterprise involving experts in various fields of science and engineering. Physiology research has been critically dependent on cutting-edge technological support of mechanical, electrical, optical, and more recently computer engineers. Evolution of modern experimental equipment is constrained by lack of direct communication between the physiological community and industry producing this equipment. Fortunately, recent advances in open source technologies, including three-dimensional printing, open source hardware and software, present an exciting opportunity to bring the design and development of research instrumentation to the end user, i.e., life scientists. Here we provide an overview on how to develop customized, cost-effective experimental equipment for physiology laboratories.
Keywords: heart physiology, 3-D printing, open source manufacturing, optical mapping
in the 19th century, Carl Ludwig's Institute of Physiology at the University of Leipzig made more fundamental and lasting discoveries than any previous or current physiological laboratory (10). Throughout his career, Ludwig tried to cast physiology as an experimental hypothesis-driven science based on the principles of chemistry, physics, and engineering, rather than an empirical descriptive field (3, 10). Ludwig's exceptional impact is evident from his numerous disciples who are the founders of national physiology traditions in many countries on several continents.
One of his valuable contributions to life sciences was his recognition of the importance of engineering and technology in supporting a physiological inquiry. When Ludwig joined the Leipzig faculty in 1865, he was given the responsibility to design an institute devoted to teaching and researching physiology. The institute he developed had specialized facilities dedicated to science (chemistry, histology, and experimental physiology) and to engineering (machine, electric, and glass-blowing workshops), which promoted different disciplines to unite in the elucidation of organ system functions (3). This was in stark contrast to other world-renowned physiologists, who performed their studies isolated in one- or two-room laboratories (3).
Today's most productive physiology and biomedical institutions require not only expertise in the basic sciences but also adroit computer scientists to automate experiments, electrical engineers to build analog and digital hardware, and machinists to manufacture experimental setups. Individual laboratories are often ill equipped to overcome such diverse skill sets and consequently purchase equipment from industry at a high cost. Open source software and hardware have had an important impact on diminishing expenses, and here we highlight open source manufacturing as a new way to decrease cost and improve a laboratory's productivity.
Open source additive manufacturing uses three-dimensional (3-D) printing to create an object with high precision at micron resolution based on an open source digital design. Using digital 3-D designs gives researchers the flexibility to make quick custom modifications to previously designed parts, which can be shared in the public domain (11). Currently, our laboratory is using 3-D printing to improve the quality of experiments on a wide range of ex vivo perfused heart preparations from different species, including mouse, rabbit, canine, and human. We have designed tissue chambers, electrode holders, tissue restrainers, and optical components to optimize all aspects of an experiment: physiology, pharmacology, and measurements (electrical, mechanical, optical, and chemical). Thus we are no longer dependent on industry when requiring highly complex, low-volume parts. Here, we give an overview on how to go from a conceptual design to a 3-D-printed object and present several printed components that have benefited our research laboratory.
METHODS
Design, review, and print.
The first step in creating custom physiology laboratory equipment is turning an idea from a paper sketch into a digital design, which is illustrated here by a screw thread in Fig. 1. Digital designs can be made in any number of free computer-aided design programs, including SketchUp, Blender, and OpenSCAD. For those who are inexperienced with computer-aided design and are interested in creating their own designs, we refer you to the following Web sites: sites.google.com/site/3dprintingphysiolabefimov/ or http://efimov.wustl.edu/, where we provide links to tutorials. We recommend SketchUp for beginners as it takes less than 10 min to learn the basic functions.
Fig. 1.
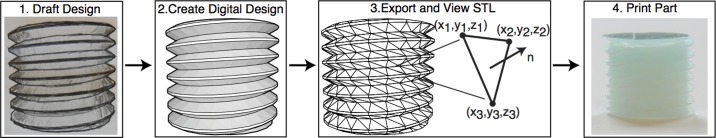
A digital design of a screw thread converted to a stereolithography (STL) file and printed. An STL file stores the three-dimensional (3-D) geometry as a collection coordinates (x,y,z) and surface normals (n).
After the digital design has been finalized, it needs to be exported as an stereolithography (STL) file format and visualized with an STL viewer. An STL file approximates a 3-D geometry as a collection of triangles by storing a list of both the xyz vertices and unit normal of every triangle. Errors can occur converting to STL, and thus it is prudent to examine objects with an STL viewer (Netfabb Studio Basic) that specializes in detecting and repairing damages in the digital mesh. Once the mesh is confirmed to have no damage, the STL file can be sent to a commercial 3-D printing company, a purchased 3-D printer, or a personally constructed 3-D printer such as open source Fab@Home (7) or RepRap (4). For further information on this process, including all our digital designs, software, and troubleshooting tips, please refer to the above Web sites.
Our physiology equipment.
We created our digital designs in AutoCAD ($99.00), Google SketchUp 7, 8, 2013 (free), or Google SketchUp Pro (various academic licenses available for free or at reduced pricing). Digital designs were viewed in Netfabb Studio Basic (free; Netfabb, Parsberg, Germany) to ensure printer-ready meshes. To date, we have printed numerous customized tissue chambers (Fig. 2A), electrode holders (Fig. 2B), tissue restrainers (Fig. 2C), and many other experimental laboratory components (i.e., luer connectors, bubble traps, electronic holders).
Fig. 2.
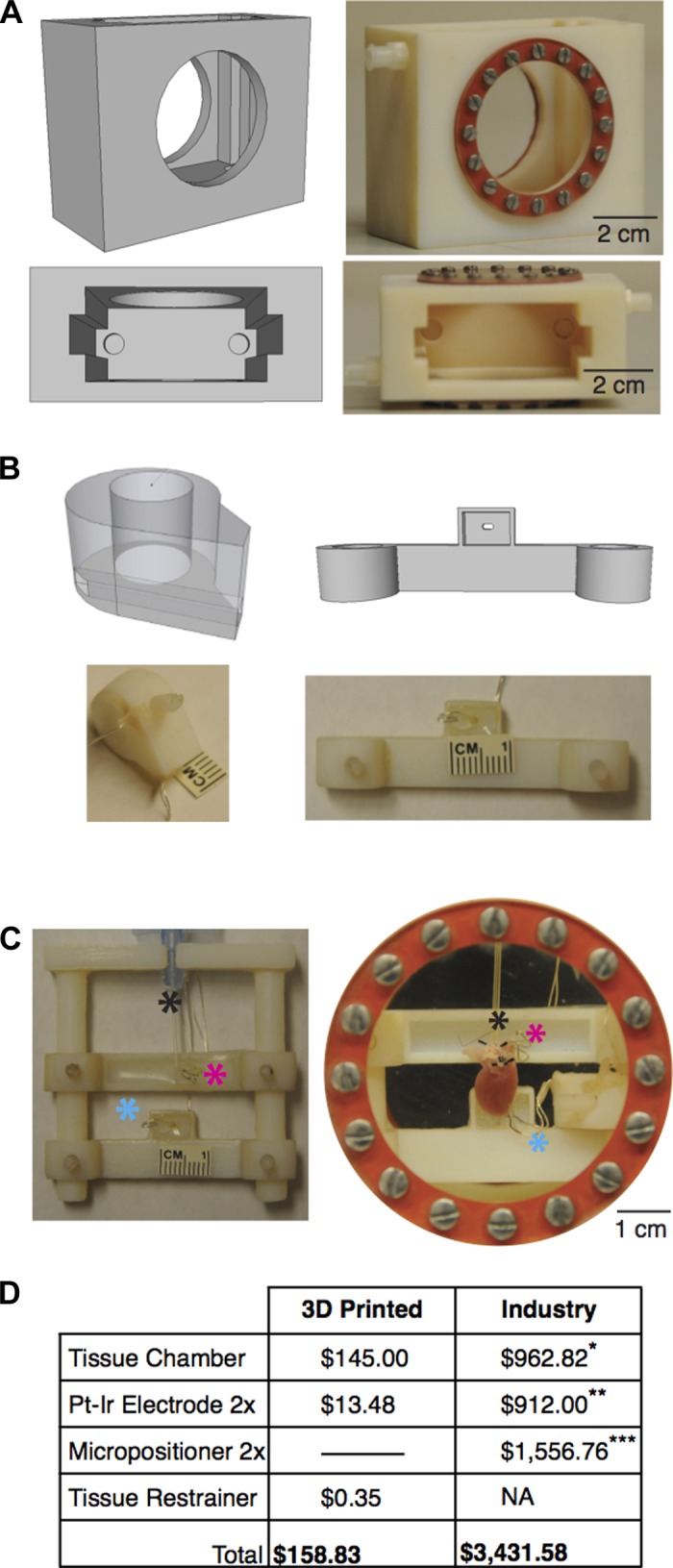
Digital designs and 3-D printed objects for physiology experiments. A: mouse tissue chamber. B: example electrode holders encapsulating platinum-iridium wire. C: mouse heart restrainer. D: cost analysis between printed and industry bought products. Black asterisk, cannula; pink asterisk, atrial pacing electrode; teal asterisk, ventricular pacing electrode. *Radnoti Tissue Camber, No. 166060, 200 ml; **Harvard Apparatus, Coaxial Stimulation Electrode, No. 730219; ***Tritech Research, Narishige Magnet-Mount Three Axis Coarse Micromanipulator, MB-PP2. NA, not applicable.
The mouse chamber, electrode holders, and tissue restrainer were printed with Objet 24 printer using VeroWhitePlus material (Stratasys, Eden Praire, MN). The rabbit torso chamber (Fig. 4) was printed with Projet 3000 printer and EX2000 material (Engineering and Manufacturing Services, Tampa, FL). The CLARITY electrophoretic tissue clearing (ETC) chambers (Fig. 5) were printed on a Stratasys Dimension printer using acrylonitrile butadiene styrene (ABS) plastic (Print to 3-D, Tunkhannock, PA), with selective laser sintering using nylon 12 material (ZoomRP, Valencia, CA), or on a MakerGear M2 printer using ABS plastic (Beachwood, OH). Additional pieces, such as optical glass, conductive material, O-rings, and Sylgard were added postprinting. Sylgard is a silicone elastomer that is deposited in designed slits of tissue restrainers for tissue pinning.
Fig. 4.
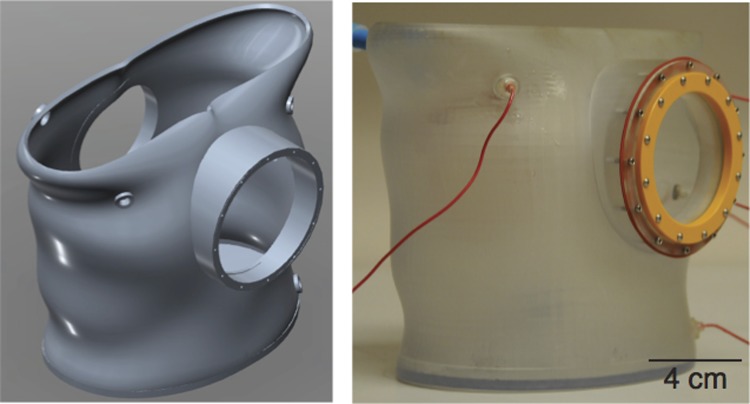
Digital design and 3-D printed tissue chamber in the shape of a human torso and scaled proportionally for a rabbit heart.
Fig. 5.
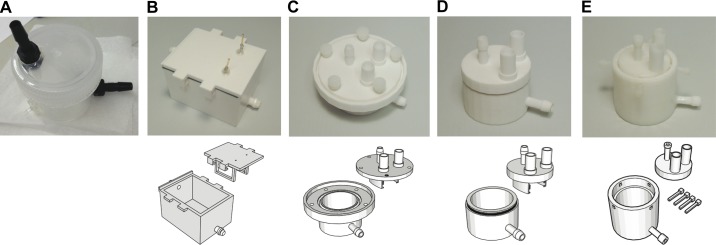
Prototyping a CLARITY chamber. A: photograph of the original CLARITY chamber that was not printed. B–E: sequential 3-D printed (top) CLARITY chambers and digital designs (bottom).
Tissue preparation.
To demonstrate the feasibility of using 3-D printed objects in the physiology laboratory, we show experimental results from a mouse heart preparation, which was approved by the Institutional Animal Care and Use Committee of Washington University in St. Louis. A single explanted mouse heart was obtained from a C57BL/6 mouse. The mouse was given an intraperitoneal injection of 100 units of heparin followed by an anesthetic mixture of ketamine-xylazine (ketamine, 80 mg/kg; and xylazine, 10 mg/kg). The heart was rapidly excised through a midsternal incision and placed on a Langendorff apparatus where it was retrogradely perfused with oxygenated (95% O2-5% CO2) Tyrode solution, consisting of (in mM) 128.2 NaCl, 1.3 CaCl, 4.7 KCl, 1.05 MgCl2, 1.19 NaH2PO4, 20.0 NaHCO3, and 11.1 glucose. Tissue was perfused under constant pressure (60–80 mmHg) with perfusate pH continuously monitored (Oakton Instruments, Vernon Hills, IL). Perfusate temperature was maintained at 37 ± 1°C using a heated recirculating bath (NESLAB EX7, Thermo Scientific). Preplaced Ag/AgCl pellet electrodes (WPI, Sarasota, FL) monitored (PowerLab 26T; ADInstruments, Colorado Springs, CO) far-field ECG throughout experimentation.
Optical mapping and data analysis.
Optical mapping of transmembrane potential (Vm) and calcium transients (CaT) was done as previously described (5, 9). Briefly, tissue was stained with voltage-sensitive dye (RH-237; 5 μl in 1 mg/ml DMSO; Life Technologies, Carlsbad, CA) and immobilized by addition of blebbistatin (10 μM; Tocris Bioscience, Ellisville, MO) to reduce motion artifact. Administration of calcium indicator rhod 2-AM (Life Technologies) enabled imaging of CaT. Optical mapping data were recorded from a MiCAM ULTIMA (SciMedia, Costa Mesa, CA) imaging system at high-spatial (100 × 100 pixel) and -temporal (1,000 frames/s) resolution. Optical signals were processed using a freely available MATLAB program described in Laughner et al. (6). All optical data were filtered using a 3 × 3 pixel spatial filter, a 0–100-Hz finite impulse response filter, and normalized. Activation times were defined as the maximum first derivative of the fluorescence signal upstroke.
Results and Discussion
To improve the quality of experimentation, we designed and printed species-specific equipment. Figure 2A shows the digital design and printed chamber for a vertical Langendorff-perfused mouse heart, where optical imaging is done through the side glass window. Unlike imaging a horizontally oriented heart from the surface (5), optical mapping of a vertically submerged preparation reduces artifact of the rippling fluid created by a superfusion pump (9). Figure 2B displays two examples of electrode holders that slide over the columns of the tissue restrainer (Fig. 2C) and are secured to the restrainer by screws. After tissue dissection and aortic cannulation, the preplaced stimulating electrodes are easily positioned onto the right atrial appendage and apex. Previously, we had to tediously place stimulating electrodes onto the surface of the heart using micromanipulators, delaying experimentation.
Figure 2C shows an example tissue restrainer that keeps all hearts in the same orientation and slides into the mouse tissue chamber. Consistent orientation between experiments is vital for calculating electrical intervals from a far-field ECG that is dependent on heart-lead orientation. In contrast to glass tissue chambers, the printed mouse chamber does not require a heated water jacket to maintain temperature because of the material's low thermal conductivity. Overall, this setup reduces the time of tissue preparation by 30 min and thus enables the completion of longer physiological protocols. Furthermore, there is a striking volumetric difference between the printed mouse chamber (80 ml; Fig. 2A) and our previous commercially available chamber (200 ml, No. 166060, Radnoti, Monrovia, CA). Consequently, the quantity of both dyes and pharmacological agents per experiment can be reduced. A cost analysis of the 3-D printed equipment versus industry-purchased equipment is summarized in Fig. 2D. The overall savings of printing equipment is extraordinary: ∼$3,200. Two printed electrode holders have a material cost of $0.14, and the addition of platinum-iridium wire (No. 778000, A-M systems, Sequim WA) postprinting is $13.34. By 3-D printing our custom designs, we can avoid the necessity of using expensive equipment such as micromanipulators, and the savings is enough to purchase the newest generation 3-D printer.
Figure 3 demonstrates the high-quality data collected from the 3-D printed mouse setup. Vm and CaT were simultaneously imaged on the posterior surface of the ex vivo Langendorff heart preparation. When the preparation was being paced from the apex, Vm spread across the epicardial surface in 13 ms (Fig. 3B). Figure 3C displays representative Vm (black) and CaT (teal) signals during S1–S1 (180 ms) pacing with Vm preceding CaT. Ventricular fibrillation was initiated with S1–S2 (140–55 ms) pacing and uncouples Vm from CaT.
Fig. 3.
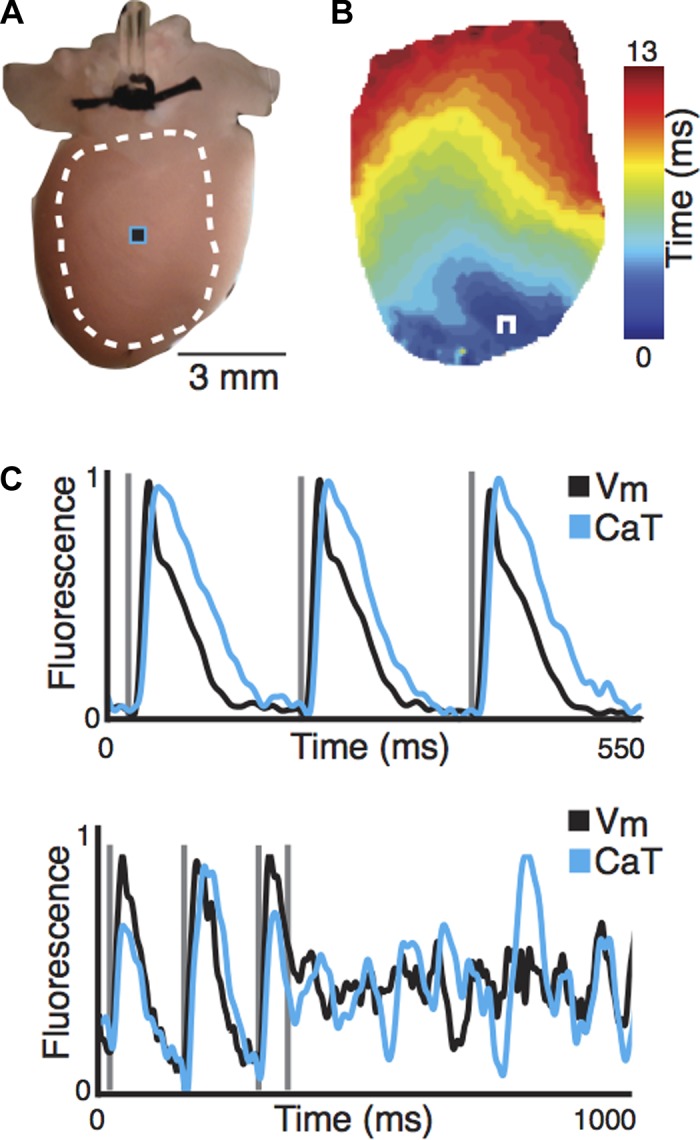
Physiological experiment of a Langendorff-perfused mouse heart. A: posterior surface of mouse heart, where white dotted line and black/teal square indicate location of activation map (B) and optical signals (C), respectively. B: activation map of the transmembrane potential across the epicardial surface of mouse heart. C: representative transmembrane potential (Vm) and calcium transient (CaT) signals during pacing (top) and induction of ventricular fibrillation (bottom). White square pulse, location of pacing electrode.
For a specific set of experiments, our laboratory created a tissue chamber in the shape of a human torso derived from a computed tomography scan of a patient and scaled proportionally to a rabbit heart (Fig. 4). Twelve holders were made in the digital design to accommodate electrodes in the configuration of a standard 12-lead ECG. Collecting a 12-lead ECG is rare in ex vivo experimentation due to lengthy instrumentation setup and low signal-to-noise recordings. Multiple leads are important because they mitigate spurious findings of a single lead and are capable of identifying anatomical structures of cardiomyopathy. Having stable, high signal-to-noise 12-lead ECGs makes signal analysis straightforward and identification of disease reliable.
In addition to improving the quality of experimental data and decreasing cost, using digital designs has given our laboratory the flexibility to make quick modifications to experimental setups without assistance from industry. In Fig. 5, we demonstrate the prototyping process for custom-designed laboratory equipment. Figure 5, A–E, are various iterations of an ETC chamber designed for CLARITY, which is a method to render tissue optically transparent while preserving 3-D molecular structure (2). This is achieved by first transforming the tissue into a hydrogel-hybridized form and then subjecting it to the ETC process in a detergent buffer. Figure 5A is the original chamber constructed based on the CLARITY protocol, without assistance from 3-D printing, whereas Fig. 5, B–E, are printed chambers (top) and their digital designs (bottom). Given the detergent buffer and conditions for ETC, the flexibility to continually redesign, prototype, and test new chambers has been critical to developing a stable, leak-resistant chamber for this process.
Although open source manufacturing offers many advantages, progress in 3-D printing technology is essential for open source manufacturing to be used to create fully functional experimental setups. Currently, the materials available for printing physiological setups are somewhat limited. Two plastics used by our laboratory with inexpensive 3-D printers (cost, $400–2,500) include ABS (∼$35.00/kg) and polylactic acid (∼$35.00/kg). Both plastics have melting points well beyond the physiological range (>100°C), making them ideal for physiology equipment. When compared with polylactic acid, ABS has improved mechanical strength and flexibility but is more prone to warping during the printing process. Polycarbonate is gaining prevalence for 3-D printing in industrial and desktop settings, because of its strength, heat resistance, and optical transparency. In addition, professional selective laser-sintering printers are able to print using nylon plastic, which offers high heat and chemical resistance. Printers capable of printing metal parts are currently expensive ($15,000–1,000,000+), and all conductive material shown here was added postprinting. In the future a single printer that is capable of incorporating both structural components as well as electrical circuits (1, 7, 8) will enable researchers to create fully function experimental setups for a wide range of biomedical investigations customized for each individual experiment.
Overall, open source manufacturing combined with open source hardware and software offers life scientists a cost-effective technique that will improve both the quality and productivity of biomedical research.
GRANTS
This research and development was supported by National Institutes of Health Grants R21-HL-112278, R01-HL-114395, R21-HL-108617, R01-HL-095010, and R01-EB-008999.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S.S. and I.R.E. conception and design of research; M.S.S., E.W., C.C.S., and K.M.H. performed experiments; M.S.S., E.W., C.C.S., K.M.H., C.G., S.R.G., and I.R.E. analyzed data; M.S.S., E.W., C.C.S., K.M.H., C.G., S.R.G., and I.R.E. interpreted results of experiments; M.S.S. prepared figures; M.S.S. and I.R.E. drafted manuscript; M.S.S., K.M.H., and I.R.E. edited and revised manuscript; M.S.S. and I.R.E. approved final version of manuscript.
REFERENCES
- 1.Calvert P. Inkjet printing for materials and devices. Chem Mater 13: 3299–3305, 2001 [Google Scholar]
- 2.Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature 497: 332–337, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fye WB. Carl Ludwig and the Leipzig Physiological Institute: ‘a factory of new knowledge’. Circulation 74: 920–8, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Jones R, Haufe P, Sells E, Iravani P, Olliver V, Palmer C, Bowyer A. RepRap—the replicating rapid prototyper. Robotica 29: 177–191, 2011 [Google Scholar]
- 5.Lang D, Sulkin MS, Lou Q, Efimov IR. Optical mapping of action potentials and calcium transients in the mouse heart. J Vis Exp 55: e3275, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laughner JI, Ng FS, Sulkin MS, Arthur RM, Efimov IR. Processing and analysis of cardiac optical mapping data obtained with potentiometric dyes. Am J Physiol Heart Circ Physiol 303: H753–H765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipson H. Homemade: the future of functional rapid prototyping. IEEE Spectrum 42: 24–31, 2005 [Google Scholar]
- 8.Lopes AJ, MacDonald E, Wicker RB. Integrating stereolithography and direct print technologies for 3D structural electronics fabrication. Rapid Prototyping J 18: 129–143, 2012 [Google Scholar]
- 9.Pietka TA, Sulkin MS, Kuda O, Wang W, Zhou D, Yamada KA, Yang K, Su X, Gross RW, Nerbonne JM, Efimov IR, Abumrad NA. CD36 protein influences myocardial Ca2+ homeostasis and phospholipid metabolism: conduction anomalies in CD36-deficient mice during fasting. J Biol Chem 287: 38901–38912, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothschuh KE. History of Physiology. Huntington, New York: R. E. Krieger, 1973, p. 204–212, 248–258 [Google Scholar]
- 11.Zhang C, Anzalone NC, Faria RP, Pearce JM. Open-source 3D-printable optics equipment. PLoS One 8: e59840, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]