Abstract
The purpose of the study was to evaluate the effects of Lactobacillus pentosus strain b240 (b240) intake and appropriate physical training on salivary secretory immunoglobulin A secretion in elderly adults with low physical fitness. Elderly adults with low physical fitness (daily step count below 3,500 steps) were divided into 2 groups: a b240 intake + exercise group (b240 group) and a placebo intake + exercise group (placebo group). Each subject continued intake of b240 or placebo and moderate-intensity resistance exercise for 12 weeks. Before and 4, 8, and 12 weeks after the start of intervention, each subject underwent saliva sampling. Before and after intervention, physical fitness tests and step count were measured. Our results showed that secretory immunoglobulin A secretion in 57 subjects during the b240/placebo intake period was significantly greater in the b240 group than in the placebo group (p<0.05). There were no significant changes in physical fitness tests before and after intervention in the 2 groups. The daily amount of walking increased significantly after intervention in both groups (p<0.05). These results suggest that in elderly adults with low physical activity and fitness, intake of b240 with appropriate physical exercise elevate salivary secretory immunoglobulin A secretion.
Keywords: Lactobacillus pentosus, salivary SIgA, mucosal immunity, elderly, low physical fitness
Introduction
Aging compromises the immune system, potentially leading to an increase in the incidence of infection, cancer, malignant neoplasm, and autoimmune disease.(1) Respiratory tract infection (RTI) is a major disease for elderly people, and individuals over 70 years of age have shown high incidences of influenza or pneumonia and a high death rate from these diseases.(2,3)
Secretory immunoglobulin A (SIgA) contained in saliva serves as a major effector in mucosal immunity by preventing submucosal invasion of pathogens. These pathogens enter through the oral cavity, specifically binding to the bacteria, viruses, etc.(4) Decreased SIgA secretion is thought to elevate the incidence of RTI.(5,6) Salivary SIgA secretion has been reported to decrease with age.(7) Aging-related decrease in salivary SIgA secretion is thought to facilitate submucosal invasion of pathogens, leading to an increased incidence of infection. Thus, potentiation of mucosal immune function in elderly people may lead to prevention of infection and thus seems to be very important in maintaining and facilitating the health of elderly people.
Recently, lactobacilli were shown to reinforce immune function, and that intake of lactobacilli by elderly adults resulted in an increase in blood T-helper (Th) cell count and natural killer (NK) cell count and enhancement of NK cell/phagocytic activity.(8) Lactobacillus pentosus strain b240 (b240) is an anaerobic, non-sporulating, gram-positive bacterium originally isolated from fermented tea leaves.(9) Oral administration of b240 to mice resulted in increased synthesis of IgA from mucosal tissue and increased serum IgG level.(10) Intake of b240 increases the secretion of salivary SIgA in healthy adult women and healthy elderly adults.(11,12) These findings suggest that intake of lactobacilli may enhance the mucosal immune function in elderly people, leading to a reduced risk of infection. Oral intake of b240 for 20 weeks significantly reduced the incidence rate of the common cold in elderly adults.(13)
Moderate physical exercise also elevates immune function.(14) In a study of young adults, appropriate physical exercise elevated salivary SIgA secretion, accompanied by a reduced incidence of RTI.(6) In our previous study in elderly people, salivary SIgA secretion was higher in individuals who walked approximately 7,000 steps per day than in those who walked approximately 3,000 steps per day.(15) Additionally, salivary SIgA secretion in elderly people walking approximately 7,000 steps per day was further elevated by moderate physical training (endurance and resistance training).(16) Therefore, maintaining appropriate physical activity and physical training is expected to elevate mucosal immune function in elderly people.
According to Spirduso et al.,(17) the physical fitness level among elderly adults is not uniform, but varies from individual to individual, allowing elderly adults to be stratified according to physical fitness level, ranging from individuals with high to low fitness levels. Elderly adults with low fitness levels may also exhibit low daily physical activity, and salivary SIgA secretion in relatively inactive elderly adults may be lower than that in more active elderly adults.(16) Long-term continuation of moderate physical exercise is necessary to reinforce immune function.(14) However, it is not always easy for individuals with low physical fitness to practice such moderate physical exercise because of the high intensity of exercise and inability to practice some kinds of exercise. Potentiation of immune function may be achieved more efficiently if the effects of nutrient intake and other factors are combined with the effects of physical training. Intake of lactobacilli in addition to physical exercise is expected to manifest additional effects in elevating the immune function. However, no report has been published evaluating the influence of lactobacillus intake on immune responses in elderly people practicing physical exercise. This study was designed to test whether a combination of lactobacillus intake and physical training can elevate salivary SIgA secretion more markedly in elderly adults with low physical fitness than physical training without lactobacillus intake.
Materials and Methods
Participants
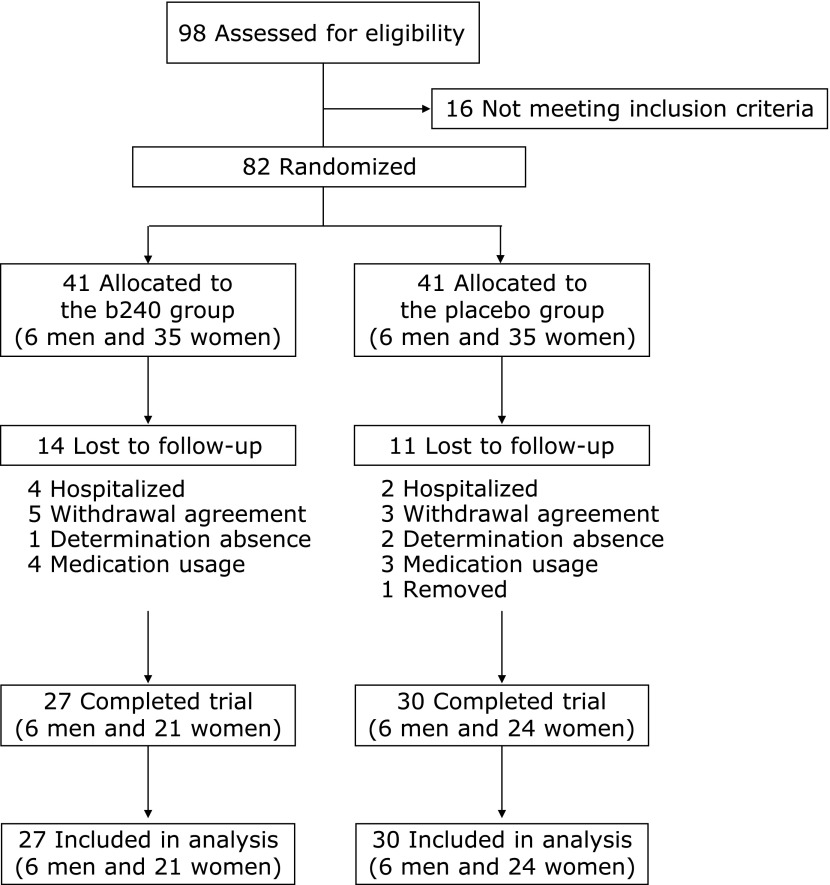
The research protocol was approved by the Ethics Committee of University of Tsukuba. Sedentary low active (⩽3,500 steps/day) elderly volunteers were recruited from a local nursing home. A total of 98 medical screenings were conducted (Fig. 1). Individuals who were smokers, consuming medicines (e.g., antibiotics, antiflatulent agents, antidiarrheals, gastrointestinal prokinetic agents, steroids, and immunosuppressive agents), or had a disease (e.g., hepatitis, cancer, inflammatory bowel disease, rheumatism, and dementia) were excluded from the study. Eligible participants completed a health questionnaire and wore an electrical pedometer to establish sufficient inactivity before being randomly allocated to 2 groups. Eighty-two participants were randomly assigned to the b240 group (b240) and the placebo group (placebo) on the basis of age, sex, physical activity (step count per day), and salivary SIgA secretion rate in a double-blind manner (Fig. 1). Potential participants were given a detailed explanation of the risks, stress, and potential benefits of the study before signing an informed consent form. The study conformed to the principles outlined in the Declaration of Helsinki in 2008.
Fig. 1.
Flow diagram for the study.
Experimental design
All subjects participated in an exercise training session. Subjects in the b240 group took 125 mL of lactobacillus beverage (heat-killed b240, 2 × 109 cells containing sterile water) in the every morning after breakfast for 12 weeks. Subjects in the placebo group consumed placebo beverage (125 mL of water without b240) for the same duration. Both the b240 and placebo beverage were identical in appearance. Assessments (anthropometric measurements and saliva and blood samples) were performed in the morning (6:00 a.m. to 9:30 a.m.) before (0 week) and after 4 weeks, 8 weeks, and 12 weeks of beverage intake and exercise training and intake. The status of test beverage intake was assessed daily by the nursing staff and was recorded in a diary.
Composition of b240 beverage
The b240 beverages used in this study were provided by the Otsuka Pharmaceutical Co., Ltd (Tokyo, Japan). Each beverage contained heat-killed b240, 2 × 109 cells in 125 ml of sterile water. Placebo beverages were the same, except these beverages did not contain lactobacillus.
Exercise program
Subjects in the both groups participated in an exercise program 5 days per week for 12 weeks. The exercise program was supervised and conducted by experienced instructors. The training program involved stretching and resistance training. Resistance training included 6 exercises (sit-up, leg-extension, hip-extension, trunk flexion, hip extension, and getting out of a chair) without the use of weights.
Anthropometric measurements
Body mass, percent of fat mass, and percent of muscle mass were recorded using a digital scale (bioelectrical impedance analysis, HBF-354-IT2; Omron Co., Ltd., Kyoto, Japan) with each subject wearing light clothing and no footwear.
Measurement of daily physical activity
To assess physical activity, we used an electrical pedometer (HJ-720IT; Omron Co., Ltd.). Participants were instructed to wear an electrical pedometer for 14 consecutive days during all walking hours, except during bathing. Participants were instructed to go about their normal lives unrestricted and were asked to not look at the electrical pedometer to observe how many steps they had taken each day. Electrical pedometer placement was standardized on the belt or waistband according to the manufacturer’s recommendation.
Assessment of nutritional intake
Values for nutritional intake were obtained from a straight 3-day food record kept beginning at 0 week. Subjects were asked to be as accurate as possible in recording the amounts and types of food and fluid consumed. Values of daily nutritional intake were calculated using dietary assessment software (Excel Nutrition ver. 2.3; Kenpakusha, Tokyo, Japan). Additionally, participants were asked to maintain their normal dietary patterns during the study period.
Physical fitness tests
Subjects performed physical fitness tests, which included 5 tests (isometric grip strength using a handgrip dynamometer, muscle endurance using sit-up test during 30 s, body balance using one leg balance with eyes open, flexibility of the body using the sit-and-reach test, and agility using 10-m obstacle walking time) at 0 week and 12 weeks, as described in “Physical Fitness Test” by the Japan Ministry of Education, Culture, Sports, Science, and Technology.(18)
Saliva analysis
The subject was instructed to avoid alcohol consumption on the day before the test and fasted from 9:00 p.m. on the day before the test. Saliva samples were obtained between 6:00 a.m. and 9:30 a.m. after overnight fasting. Each subject ingested 1 bottle of prescribed food (Calorie Mate Jelly, Otsuka Pharmaceutical Co., Ltd.) 1 h before saliva collection, after which the oral cavity was rinsed with tap water. Next, 5 min before saliva collection, each subject rinsed his/her oral cavity 3 times with mineral water. Immediately before saliva collection, saliva remaining in the oral cavity was swallowed, and the head was immediately inclined slightly forward, followed by gentle fixation of posterior teeth using the tip of the tracheal tube connected to a low pressure suction pump (low-pressure serial suction pump, plug socket type for installment, Seastar Corporation, Tokyo, Japan) to begin saliva collection. Immediately after the start of saliva collection, the subject closed his/her mouth, and saliva was collected into a tube for 5 min, with care taken to avoid contact of the tracheal tube inlet with the cheek and tongue. Collected saliva was stored on ice. Fifteen minutes after completing saliva collection, saliva was again collected for 5 min using the same procedure. The subject remained stationary during the period from 5 min before the start of saliva collection to the end of the second session of saliva collection. SIgA concentration was measured using an enzyme-linked immunosorbent assay (ELISA). SIgA concentration (µg/ml) was multiplied by the amount of saliva flow for 5 min (ml/5 min) to yield the amount of SIgA secretion rate (µg/5 min). The mean of the 2 measurements represents the amount of saliva flow.
Blood analysis
After saliva sampling, blood samples were collected in vacutainers containing sodium ethylenediaminetetraacetic acid (EDTA). We quantified total leukocytes, lymphocytes, and monocytes from whole blood samples using a multichannel hemocyte analysis system (SE-9000; Sysmex Corp., Hyogo, Japan).
Data analysis and statistics
Descriptive data are presented as means ± SD. For all analysis, p<0.05 was considered statistically significant. Comparison between the Placebo and b240 groups for the baseline criterion measures was made by unpaired-Student t test. Data were subjected to repeated measures 2-way analysis of variance (ANOVA). The Tukey-Kramer test was employed for the post hoc test. Time effect of intervention within each group was analyzed by a paired-Student’s t test, in relation to changes in descriptive data, physical fitness test, and hematological measurements. Changes in SIgA-related parameters (amount of saliva flow rate, SIgA concentration, amount of SIgA secretion rate) were subjected to ANOVA (0–12 weeks) based on a mixed model.
Results
Subject characteristics
During the study period, there were no physical ailments or abnormalities resulting from b240 beverage intake. All subjects were compliant with the procedure (non-ingestion rate was 10% or more during all periods). Moreover, no subjects were injured during the exercise program.
Physical composition of participants is summarized in Table 1. Physical composition at 0 week showed no difference between the b240 group and the placebo group. Body weight decreased significantly in both the b240 group and the placebo group (p<0.05). The b240 group showed a significant decrease in BMI and body fat ratio and a significant increase in muscular ratio (p<0.05).
Table 1.
Descriptive data for placebo and b240 groups before and after 12 weeks
| Placebo |
b240 |
|||
|---|---|---|---|---|
| 0 week | 12 weeks | 0 week | 12 weeks | |
| n | 30 (6 men, 24 women) | 27 (7 men, 20 women) | ||
| Age (yr) | 82.8 ± 9.4 | 80.3 ± 7.6 | ||
| Height (cm) | 145.9 ± 9.6 | 147.2 ± 8.8 | ||
| Body mass (kg) | 52.0 ± 12.3 | 51.1 ± 12.7* | 52.9 ± 6.8 | 51.5 ± 6.8* |
| BMI (kg/m2) | 24.2 ± 4.7 | 24.3 ± 4.9 | 23.6 ± 3.1 | 22.9 ± 2.9* |
| Fat mass (%) | 37.0 ± 4.8 | 34.6 ± 6.2 | 37.6 ± 4.9 | 36.3 ± 4.7* |
| Muscle mass (%) | 19.8 ± 1.6 | 21.0 ± 2.0 | 20.3 ± 2.6 | 20.8 ± 2.5* |
Values are expressed as means ± SD. BMI: body mass index. *Values different from 0 week within group, p<0.05.
Daily physical activity and physical fitness tests
The number of steps walked was used as an indicator of daily physical activity; results of physical fitness tests are shown in Table 2. Significant difference between the b240 group and the placebo group in the number of steps at 12 weeks was noted (p<0.05). In both groups, the number of steps walked increased significantly after 12 weeks compared to baseline levels (p<0.05), and the increase was greater in the placebo group. There was no significant inter-group difference in the results of any physical fitness test. Both groups showed no significant changes in the results of any test.
Table 2.
Physical fitness tests for placebo and b240 groups before and after 12 weeks
| Placebo |
b240 |
|||
|---|---|---|---|---|
| 0 week | 12 weeks | 0 week | 12 weeks | |
| Step count (steps/day)† | 1,510 ± 1,083 | 3,203 ± 2,054* | 1,463 ± 1,153 | 2,171 ± 2,112* |
| Right grip strength (kg) | 14.8 ± 5.5 | 15.1 ± 6.0 | 15.9 ± 4.2 | 16.0 ± 5.0 |
| Left grip strength (kg) | 14.4 ± 4.5 | 14.7 ± 5.0 | 17.0 ± 6.9 | 17.4 ± 7.6 |
| Sit-up (times/30 s) | 6.2 ± 1.9 | 4.4 ± 4.0 | 4.2 ± 3.1 | 2.8 ± 2.4 |
| Sit-and-reach (cm) | 25.3 ± 7.9 | 22.8 ± 8.3 | 26.8 ± 9.9 | 26.4 ± 8.4 |
| One-leg balance (s) | 6.7 ± 6.5 | 6.7 ± 9.6 | 19.0 ± 17.2 | 21.6 ± 21.5 |
| 10-m obstacle walking (s) | 19.3 ± 16.6 | 19.0 ± 13.4 | 15.4 ± 10.9 | 15.2 ± 11.7 |
Values are expressed as means ± SD. *Values different from 0 week within group, p<0.05. †Treatment × time interaction, p<0.05.
Nutritional intake
Table 3 shows the results of the nutritional survey before the start of intervention. There was no significant inter-group difference in any nutritional parameter analyzed. In both groups, the amount of each nutrient ingested varied little from the recommended level.(19)
Table 3.
Nutritional intake for placebo and b240 groups before intervention
| Placebo | b240 | Reference values | |
|---|---|---|---|
| Carolies (kcal/day) | 1,500 ± 181 | 1,495 ± 224 | 1,350–1,600 |
| Protein (g/day) | 52.6 ± 8.6 | 52.3 ± 8.5 | 50–60 |
| Fat (g/day) | 39.8 ± 9.4 | 40.9 ± 8.0 | 23–44 |
| Carbohydrate (g/day) | 225.9 ± 36.0 | 224.4 ± 38.3 | 169–236 |
| Retinol (µg/day) | 582.1 ± 180.1 | 516.0 ± 216.4 | 450–650 |
| Vitamin C (mg/day) | 91.5 ± 30.7 | 91.6 ± 33.3 | 100 |
Values are expressed as means ± SD. The reference values were taken from Dietary Reference Intakes for Japanese (The Japan Ministry of Health, Labour and Welfare, 2004).
Saliva analysis
Table 4 shows the changes in saliva flow rate, salivary SIgA concentrations and salivary SIgA secretion rate. Significant difference was noted on saliva flow rate in both groups (p<0.05). However, saliva flow rate in both groups did not show significant change during study period. No significant difference was revealed by analysis of salivary SIgA concentrations in any of the 2 groups. Salivary SIgA concentrations in both groups also did not change significantly during study period. Changes in the amount of salivary SIgA secretion rates showed a significant difference noted in both groups (p<0.05). On the other hand, during the period from 0 week to 12 weeks, the amount of salivary SIgA secreted was significantly greater in the b240 group than in the placebo group (p<0.05).
Table 4.
Changes in saliva flow rate, salivary secretory immunoglobulin A concentration, and salivary secretory immunoglobulin A secretion rate during the study period
| 0 week | 4 weeks | 8 weeks | 12 weeks | |
|---|---|---|---|---|
| Saliva flow rate (ml/5 min)† | ||||
| Placebo | 1.1 ± 0.8 | 1.2 ± 0.8 | 1.2 ± 0.8 | 1.2 ± 0.7 |
| b240 | 1.5 ± 0.9 | 1.5 ± 0.9 | 1.7 ± 0.9 | 1.7 ± 0.9 |
| SIgA concentration (µg/ml) | ||||
| Placebo | 29.3 ± 11.2 | 29.7 ± 16.8 | 31.8 ± 17.1 | 28.0 ± 16.6 |
| b240 | 26.6 ± 11.4 | 30.3 ± 16.6 | 29.4 ± 23.1 | 27.6 ± 19.0 |
| SIgA secretion rate (µg/5 min)† | ||||
| Placebo | 27.1 ± 19.0 | 32.2 ± 29.4 | 39.2 ± 41.2 | 30.8 ± 22.3 |
| b240 | 35.1 ± 21.3 | 42.2 ± 31.0 | 47.0 ± 42.4 | 44.5 ± 32.0 |
Values are presented as means ± SD. †Treatment × time interaction, p<0.05.
Leukocyte subpopulations
Hematological data is presented in Table 5. In the analysis of changes in leukocyte count, no significant difference between b240/placebo intake and physical exercise was noted between the 2 groups, but the b240 group showed a significant reduction in leukocyte count at 12 weeks (p<0.05). In the placebo group, leukocyte count generally decreased after 12 weeks although this change was not statistically significant (p = 0.06). In analyzing changes in monocyte count, significant difference was noted in both groups (p<0.05). Monocyte count decreased significantly after 12 weeks in the b240 group (p<0.05), while this value showed no significant change in the placebo group. Neutrophil and lymphocyte counts did not significantly change between the 2 groups.
Table 5.
Hematological measurements for placebo and b240 groups before and after 12 weeks
| Placebo |
b240 |
|||
|---|---|---|---|---|
| 0 week | 12 weeks | 0 week | 12 weeks | |
| Leukocyte (cells/µl) | 5,585 ± 1,328 | 5,115 ± 1,089 | 5,928 ± 1,766 | 5,248 ± 1,313* |
| Neutrophil (cells/µl) | 3,316 ± 1,219 | 2,957 ± 912 | 3,554 ± 1,467 | 3,007 ± 1,072 |
| Monocyte (cells/µl)† | 288.5 ± 92.6 | 272.0 ± 66.7 | 330.9 ± 138.2 | 270.9 ± 81.8* |
| Lymphocyte (cells/µl) | 1,777 ± 550 | 1,696 ± 548 | 1,867 ± 688 | 1,807 ± 658 |
Values are expressed as means ± SD. *Values different from 0 week within group, p<0.05. †Treatment × time interaction, p<0.05.
Discussion
The present study was designed to evaluate the effects of lactobacillus intake with physical exercise on salivary SIgA secretion in low active elderly adults and fitness. The amount of salivary SIgA secreted was greater in the b240 group than in the placebo group, suggesting that b240 intake during physical training may be involved in reinforcing mucosal immune function.
This study was conducted in elderly adults with low physical activity (walking 3,500 steps or less per day on average). The average rating on each physical fitness test for elderly subjects was lower than the average for the nationwide population aged between 75 and 79 [The Japan Ministry of Education, Culture, Sports, Science and Technology (http://www.mext.go.jp/)], allowing the subjects of this study to be categorized as elderly adults with low physical fitness.
Salivary SIgA serves as a major effector in mucosal immune function, playing the role of preventing submucosal invasion of pathogens.(4) The amount of salivary SIgA secreted differed significantly between the b240 group and the placebo group, and this parameter during study period was higher in the b240 group than in the placebo group, indicating that b240 intake increases salivary SIgA secretion. This result supports the findings from previous studies that b240 intake increases salivary SIgA secretion in young women(11) and healthy elderly subjects,(12) and that b240 elevates SIgA secretion in mouse Peyer’s patch cells ex vivo.(9) There has been no study examining the effects of lactobacillus intake on salivary SIgA in response to exercise training in elderly adults. Gleeson et al.(20) suggested that in athletes, lactobacillus intake may be related to better maintenance of salivary SIgA secretion and reduction of RTI frequency during periods of training and competition. Therefore, lactobacillus may substantially upregulate salivary SIgA secretion under the effect of exercise training. The effect of lactobacilli as well as the influence of nutrient ingestion has been reported in relation to SIgA secretion.(21,22) The present study, however, incorporated a nutritional survey immediately before the start of intervention and did not analyze changes in nutrients ingested during intervention. We can rule out biases in diet between the b240 group and the placebo group because the elderly adults lived in the same facility and ate the same diets. Furthermore, the nursing staff ensured that the dietary styles were unchanged during the intervention period. Thus, the influence of varying nutrients during the intervention period was avoided. In future studies, changes in nutrient intake during intervention can be monitored.
It has been shown that the influence of physical exercise on the immune system is closely associated with the intensity, frequency, and duration of exercise and that long-term continuation of moderate exercise is necessary to reinforce the immune system.(14) In our previous study, salivary SIgA secretion increased markedly following continuation of moderate composite training (durability and resistance training) 5 times per week for 6 months, accompanied by improvement in some physical fitness tests (sit-up test, sit-and-reach test, and agility in 10-m obstacle walking). (16) In the present study, however, neither the b240 group nor the placebo group showed significant changes in ratings for physical fitness tests. This discrepancy between the previous and present studies may be attributable to the following factors: (1) physical training was performed for 12 weeks in the present study, i.e., for a period shorter than that in the previous study; and (2) the intensity of exercise was not equivalent to moderate exercise in the present study since the subjects were individuals with low physical fitness. It therefore seems likely that the intensity and duration of exercise in the present study were not high or long enough to stimulate elevation in salivary SIgA levels. We reported that 8 months of low-intensity endurance and resistance exercise training did not change immune parameters, such as lymphocyte subpopulations, in frail elderly nursing home residents.(23) It has been reported that resistance training does not alter the immune system, while durability training appears more likely to affect the immune system.(24) Walking with an intensity of 80% ventilatory threshold for 12 weeks elevated salivary SIgA secretion in elderly adults.(25) Further studies analyzing the style, intensity, frequency, and duration of physical exercise on salivary SIgA secretion should be conducted. Active walking during daily life was shown in our previous study to elevate salivary SIgA secretion.(15) In the present study, the mean number of steps walked per day increased from 12 weeks in both groups.
The mechanism of immune system regulation by lactobacilli involves many unresolved questions. In this connection, it has been reported that lactobacillus DNA binds to the Toll-like receptor 9 of phagocytes to activate signal transduction(26,27) and that the sugar chain structure in the lactobacillus cell wall plays the role in its biological activity.(28–30) It is known that IgA is produced by plasma cells, which are mature IgA-producing B cells produced in the salivary gland; IgA is secreted into the salivary gland duct as SIgA and then binds to the polymeric immunoglobulin receptor (pIgR) expressed on mucosal epithelial cells.(31) IgA formation and pIgR expression in epithelial cells are regulated by cytokines secreted by Th cells.(32) Some investigators reported that the capability to produce antibodies by blood B cells decreased with age(33) and that pIgR expression in mouse small intestine decreased with age.(34) Antigen presenting cells, activated by lactobacillus, may activate Th cells and stimulate antibody-producing cells mediated by cytokines such as IL-6. Furthermore pIgR expression activated by lactobacillus, potentially contribute to enhance SIgA secretion. This hypothesis requires further investigation. The responses of these systems to physical exercise appears to affect SIgA secretion on the basis of previous reports that excessive exercise reduced salivary SIgA secretion and pIgR mRNA expression in rats(35) or reduced Th cells in the mouse submandibular gland.(36) The relationship between lactobacillus intake with moderate physical exercise and the processes of SIgA secretion has not been studied. If these relationships are demonstrated clearly, it would contribute to institute conditioning program for elderly adults.
The study suggested that salivary SIgA level, an indicator of mucosal immune function, is elevated in the individuals who ingest lactobacillus in addition to physical exercise on the immune systems of elderly adults with low physical fitness. The enhanced mucosal immune function observed in the b240 group may be primarily attributable to lactobacillus intake since the b240 group showed no change in physical fitness test ratings and that the magnitude of daily physical activity increase was greater in the placebo group.
Acknowledgments
We thank Prof. Sanae Okada, Faculty of Applied Bioscience, Tokyo University of Agriculture for providing us with lactic acid bacteria. This study was supported by aid from Otsuka pharmaceutical Co., Ltd. and a grant from a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22300235 to I. K.).
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- NK
natural killer
- pIgR
polymeric immunoglobulin receptor
- RTI
respiratory tract infection
- SIgA
secretory immunoglobulin A
- Th
T-helper
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Shephard RJ, Shek NP. Exercise, aging and immune function. Int J Sports Med. 1995;16:1–6. doi: 10.1055/s-2007-972954. [DOI] [PubMed] [Google Scholar]
- 2.Gutiérrez F, Masiá M, Mirete C, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. 2006;53:166–174. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 3.The Japan Ministry of Health, Labour and Welfare . Vital Statistics of Japan 2004. Tokyo: Health and Welfare Statistics Association; 2006. pp. 238–239. [Google Scholar]
- 4.Lamm ME, Nedrud JG, Kaetzel CS, Mazanec MB. IgA and mucosal defense. APMIS. 1995;103:241–246. doi: 10.1111/j.1699-0463.1995.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 5.Gleeson M, McDonald WA, Pyne DB, et al. Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc. 1999;31:67–73. doi: 10.1097/00005768-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol. 2002;87:153–158. doi: 10.1007/s00421-002-0609-1. [DOI] [PubMed] [Google Scholar]
- 7.Tanida T, Ueta E, Tobiume A, Hamada T, Rao F, Osaki T. Influence of aging on candidal growth and adhesion regulatory agents in saliva. J Oral Pathol Med. 2001;30:328–335. doi: 10.1034/j.1600-0714.2001.300602.x. [DOI] [PubMed] [Google Scholar]
- 8.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 9.Okada S, Daengsubha W, Uchimura T, Ohara N, Kozaki M. Flora of lactic acid bacteria in Miang produced in northern Thailand. J Gen Appl Microbiol . 1986;32:57–65. [Google Scholar]
- 10.Yamahira S, Toba M, Kishi K, Okamatsu H. Stimulation of mucosal immune system by lactic acid bacteria originating in tea. Jpn J Lactic Acid Bact. 2006;17:57–60. [Google Scholar]
- 11.Kishi K, Kotani Y, Yamahira S, et al. Lactobacillus plantarum ONRIC b0240 enhanced salivary IgA in healthy adult volunteers. Jpn J Lactic Acid Bact. 2006;17:132–137. [Google Scholar]
- 12.Kotani Y, Shinkai S, Okamatsu H, et al. Oral intake of Lactobacillus pentosus strain b240 accelerates salivary immunoglobulin A secretion in the elderly: a randomized, placebo-controlled, double-blind trial. Immun Ageing. 2010;26:7–11. doi: 10.1186/1742-4933-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinkai S, Toba M, Saito T, et al. Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2013;109:1856–1865. doi: 10.1017/S0007114512003753. [DOI] [PubMed] [Google Scholar]
- 14.Nieman DC. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994;26:128–139. doi: 10.1249/00005768-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu K, Kimura F, Akimoto T, Akama T, Kuno S, Kono I. Effect of free-living daily physical activity on salivary secretory IgA in elderly. Med Sci Sports Exerc. 2007;39:593–598. doi: 10.1249/mss.0b013e318031306d. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu K, Kimura F, Akimoto T, et al. Effects of exercise, age and gender on salivary secretory immunoglobulin A in elderly individuals. Exerc Immunol Rev. 2007;13:55–66. [PubMed] [Google Scholar]
- 17.Spirduso WW, Francis KL, MacRae PG. Physical dimensions of aging (2nd ed) Champaign, IL: Human Kinetics; 2005. pp. 1–374. [Google Scholar]
- 18.The Japan Ministry of Education, Culture, Sports, Science and Technology . Physical Fitness Test. Tokyo: Gyosei; 2000. pp. 1–135. [Google Scholar]
- 19.The Japan Ministry of Health, Labour and Welfare . Dietary Reference Intakes for Japanese. Tokyo: National Institute of Health and Nutrition; 2004. pp. 1–94. [Google Scholar]
- 20.Gleeson M, Bishop NC, Oliveira M, Tauler P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab. 2011;21:55–64. doi: 10.1123/ijsnem.21.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Gangopadhyay NN, Moldoveanu Z, Stephensen CB. Vitamin A deficiency has different effects on immunoglobulin A production and transport during influenza A infection in BALB/c mice. J Nutr. 1996;126:2960–2967. doi: 10.1093/jn/126.12.2960. [DOI] [PubMed] [Google Scholar]
- 22.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 23.Kapasi ZF, Ouslander JG, Schnelle JF, Kutner M, Fahey JL. Effects of an exercise intervention on immunologic parameters in frail elderly nursing home residents. J Gerontol A Biol Sci Med Sci. 2003;58:636–643. doi: 10.1093/gerona/58.7.m636. [DOI] [PubMed] [Google Scholar]
- 24.Flynn MG, Fahlman M, Braun WA, et al. Effects of resistance training on selected indexes of immune function in elderly women. J Appl Physiol. 1999;86:1905–1913. doi: 10.1152/jappl.1999.86.6.1905. [DOI] [PubMed] [Google Scholar]
- 25.Kimura F, Shimizu K, Akama T, Akimoto T, Kuno S, Kono I. The effects of walking exercise training on immune response in elderly. Int J Sport Health Sci. 2006;4:508–514. [Google Scholar]
- 26.Shimosato T, Kitazawa H, Katoh S, et al. Augmentation of T(H)-1 type response by immunoactive AT oligonucleotide from lactic acid bacteria via Toll-like receptor 9 signaling. Biochem Biophys Res Commun. 2005;326:782–787. doi: 10.1016/j.bbrc.2004.11.119. [DOI] [PubMed] [Google Scholar]
- 27.Shimosato T, Tohno M, Kitazawa H, et al. Toll-like receptor 9 is expressed on follicle-associated epithelia containing M cells in swine Peyer’s patches. Immunol Lett. 2005;98:83–89. doi: 10.1016/j.imlet.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Hosono A, Lee J, Ametani A, et al. Characterization of a water-soluble polysaccharide fraction with immunopotentiating activity from Bifidobacterium adolescentis M101-4. Biosci Biotechnol Biochem. 1997;61:312–316. doi: 10.1271/bbb.61.312. [DOI] [PubMed] [Google Scholar]
- 29.Yoshizawa Y, Ametani A, Tsunehiro J, et al. Macrophage stimulation activity of the polysaccharide fraction from a marine alga (Porphyra yezoensis): structure-function relationships and improved solubility. Biosci Biotechnol Biochem. 1995;59:1933–1937. doi: 10.1271/bbb.59.1933. [DOI] [PubMed] [Google Scholar]
- 30.Delneste Y, Donnet-Hughes A, Schiffrin EJ. Functional foods: mechanisms of action on immunocompetent cells. Nutr Rev. 1998;56:S93–S98. doi: 10.1111/j.1753-4887.1998.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 31.Tomasi TB, Plaut AG. Humoral aspects of mucosal immunity. In: Gallin JI, Fauci AS, editors. Advances in Host Defense Mechanisms. New York: Raven Press; 1985. pp. 31–61. [Google Scholar]
- 32.Loman S, Jansen HM, Out TA, Lutter R. Interleukin-4 and interferon-gamma synergistically increase secretory component gene expression, but are additive in stimulating secretory immunoglobulin A release by Calu-3 airway epithelial cells. Immunology. 1999;96:537–543. doi: 10.1046/j.1365-2567.1999.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frasca D, Riley RL, Blomberg BB. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin Immunol. 2005;17:378–384. doi: 10.1016/j.smim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Yanagihara T, Kumagai Y, Norose Y, et al. Age-dependent decrease of polymeric Ig receptor expression and IgA elevation in ddY mice: a possible cause of IgA nephropathy. Lab Invest. 2004;84:63–70. doi: 10.1038/labinvest.3700012. [DOI] [PubMed] [Google Scholar]
- 35.Kimura F, Aizawa K, Tanabe K, et al. A rat model of saliva secretory immunoglobulin: a suppression caused by intense exercise. Scand J Med Sci Sports. 2008;18:367–372. doi: 10.1111/j.1600-0838.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- 36.Boudreau J, Hoffman-Goetz L. Long-duration freewheel funning and submandibular lymphocyte response to forced exercise in older mice. Can J Physiol Pharmacol. 2006;84:565–572. doi: 10.1139/y06-011. [DOI] [PubMed] [Google Scholar]