Abstract
The aim of this review was to evaluate whether eating vegetables before carbohydrates could reduce the postprandial glucose, insulin, and improve long-term glycemic control in Japanese patients with type 2 diabetes. We studied the effect of eating vegetables before carbohydrates on postprandial plasma glucose, insulin, and glycemic control for 2.5 y in patients with type 2 diabetes. The postprandial glucose and insulin levels decreased significantly when the patients ate vegetables before carbohydrates compared to the reverse regimen, and the improvement of glycemic control was observed for 2.5 y. We also compared the postprandial glucose and glucose fluctuations assessed by continuous glucose monitoring system for 72-h in patients with type 2 diabetes and subjects with normal glucose tolerance when subjects ate vegetables before carbohydrates and carbohydrates before vegetables in a randomized crossover design. The glycemic excursions and incremental glucose peak were significantly lower when the subjects ate vegetables before carbohydrates compared to the reverse regimen. This evidence supports the effectiveness of eating vegetables before carbohydrates on glucose excursions in the short-term and glycemic control in the long-term in patients with type 2 diabetes.
Keywords: type 2 diabetes, diet, eating order, postprandial glucose, glucose excursion
Introduction
A significant number of patients with diabetes remain poorly controlled, mainly as a result of poor diet compliance.(1–3) The important choices that affect blood glucose control in people with diabetes are made by themselves, and not by their physicians or other medical professionals. People with diabetes are advised to adopt an appropriate diet including dietary habits and meal patterns on a lifelong basis. Traditionally, recognition of the relationship between dietary constituents and glucose tolerance has contributed to the development of nutritional prescriptions. Such strategies aim to restrict energy intake and provide macronutrient balance. However, some diabetic patients may have difficulty understanding diets based on a restrict energy intake, as well as changing their daily food habits.
Hyperglycemia is associated with increased risk for atherosclerosis by suppressing endothelium-dependent vasodilation, activating thrombosis, and increasing oxidative stress in people with type 2 diabetes mellitus (T2D), with impaired glucose tolerance (IGT), and even in subjects with normal glucose tolerance (NGT).(4–8) A reduction in postprandial hyperglycemia by an α-glucosidase inhibitor delayed progression of carotid intima-media thickening in subjects with T2D.(9) In this article we reviewed our recent reports about the effects of eating “vegetables, including seaweed and mushrooms, before carbohydrates, including potatoes, pumpkin, and corn, etc.” on the reduction in postprandial glucose levels.
The Effect of Vegetables before Carbohydrates on Postprandial Glucose and Insulin
We reviewed the effect of eating vegetables before carbohydrates on postprandial plasma glucose and insulin in patients with T2D. We conducted a randomized crossover study in 15 outpatients with T2D controlled by diet (male/female; 7/8, age; 61.7 ± 11.6 y, duration of diabetes; 5.3 ± 8.8 y, body mass index (BMI); 24.7 ± 4.3 kg/m2, HbA1c; 6.4 ± 0.6%, fasting plasma glucose (FPG); 6.28 ± 1.12 mM). Patients ate test meals consisting of 150 g white rice and vegetable salad (sliced tomato and cabbage with olive oil dressing), eating either vegetables before carbohydrates or vice versa. Plasma glucose and serum insulin levels were evaluated at 0, 30, 60 and 120 min after each test meal. Plasma glucose and serum insulin levels between eating vegetables before carbohydrates and the reverse regimen were examined using the Student’s paired t test with SPSS 15.0 for Windows (SPSS Inc, Chicago, IL). All continuous variables are presented as the mean ± SD. P values <0.05 were considered statistically significant.
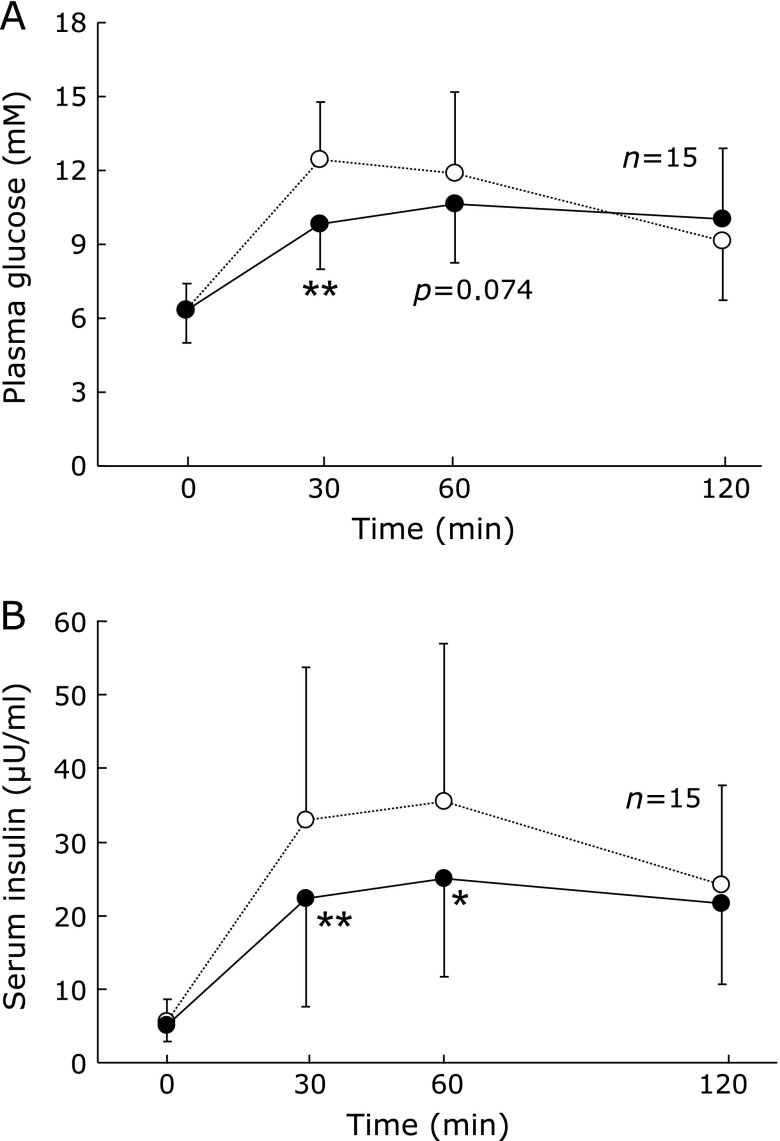
Postprandial plasma glucose levels in those following the vegetables before carbohydrates regimen were reduced at 30 and 60 min compared to the reverse regimen (Fig. 1A). Postprandial serum insulin decreased significantly at 30 and 60 min in the vegetables before carbohydrates regimen (Fig. 1B).(10)
Fig. 1.
Time course of (A) plasma glucose or (B) serum insulin at 0, 30, 60 and 120 min after eating rice before vegetables (open circle) or the reverse regimen (closed circle) (n = 15). Data are expressed as mean ± SD. Carbohydrates first vs vegetables first, *p<0.05, **p<0.01.
Cited from Imai S et al.J Japan Diab Soc, 2010; 53: 112–115.
Eating Vegetables before Carbohydrates Improves Postprandial Glucose Excursions
A continuous glucose monitoring system (CGMS) is capable of detecting hypoglycemia and hyperglycemia that may be undetectable by self monitoring blood glucose and glycated hemoglobin.(11) Particularly, the mean amplitude of glycemic excursions (MAGE) is a significant determinant of overall metabolic control, as well as increased risk of diabetes complications.(12) We examined whether eating vegetables before carbohydrates could reduce the daily postprandial glucose excursions assessed by CGMS in Japanese patients with T2D and subjects with NGT. All participants were assigned to CGMS (Medtronic Minimed Gold, Northridge, CA) for 72-h by eating test meals of vegetables before carbohydrates and carbohydrates before vegetables on the 2nd and the 3rd day in a randomized crossover design. All study protocols were approved by the Ethics Committee of the School of Comprehensive Rehabilitation at Osaka Prefecture University, and each subject provided written informed consent before enrollment in the studies. All experiments were carried out in accordance with the Declaration of Helsinki.
The test meals consisted of rice/bread, meat/fish, 500 g of vegetables (tomato, spinach, broccoli, and radish, etc.), and contained 21 g of dietary fiber and 125.6 kJ kg−1 per day. Energy intake was adjusted by the amount of rice and bread for each participant and the rest of the meals were identical. The energy ratio of protein, fat, and carbohydrates was 17%, 25%, and 58%, respectively. The subjects ate the first dishes of vegetables for 5 min, then the main dish for 5 min, and consumed rice or bread for 5 min successively within a 15 to 20 min total eating time for each meal, and vice versa.
Nineteen outpatients with T2D (male/female; 6/13, age; 65.5 ± 9.4 y, duration of diabetes; 16.4 ± 10.2 y, BMI; 22.5 ± 3.1 kg/m2, HbA1c; 7.2 ± 1.0%, FPG; 8.06 ± 2.67 mM, diet/oral hypoglycemic agents/insulin treatment; 3/3/13: mean ± SD or n) and 21 subjects with NGT (male/female; 2/19, age; 29.8 ± 11.3 y, BMI; 20.8 ± 3.0 kg/m2, HbA1c; 5.4 ± 0.6%, FPG; 4.89 ± 0.50 mM) were enrolled in the study. Glycemic parameters between the day of eating vegetables before carbohydrates and the day of eating carbohydrates before vegetables were examined using the Student’s paired t test with SPSS 15.0 for Windows. All continuous variables are presented as the mean ± SD. P values <0.05 were considered statistically significant.
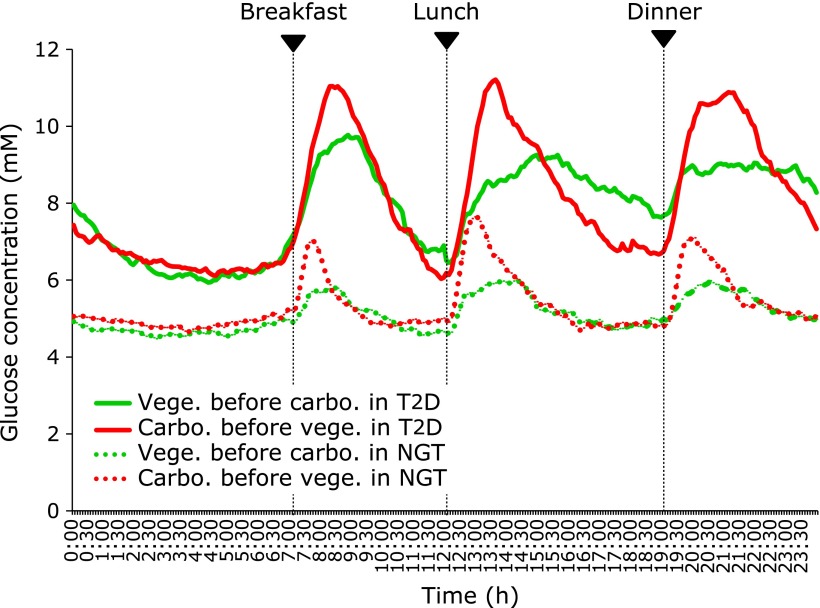
The mean of the daily glucose values was plotted to show the reduction in glucose excursions of eating vegetables before carbohydrates compared to the reverse regimen in subjects with T2D and NGT (Fig. 2). The levels of SD, MAGE, large amplitude of glycemic excursions (LAGE), mean 1-h postprandial glucose (PPG), mean incremental area under the glucose curve0-3h (IAUC0-3h), and mean incremental glucose peak (IGP) were all significantly reduced when the participants ate vegetables before carbohydrates compared to the reverse regimen in both subjects with T2D and NGT (Table 1). The mean blood glucose levels did not show any difference between the two treatments because the hyperglycemia and hypoglycemia were suppressed when subjects ate vegetables before carbohydrates.(13)
Fig. 2.
The mean of the daily glucose values were plotted to show the reduction in glucose excursions by eating vegetables before carbohydrates compared to the reverse regimen in both subjects with type 2 diabetes (n = 19) and normal glucose tolerance (n = 21).
Table 1.
Characteristics of glycemic excursion in subjects with T2D and NGT
| Patients with T2D (n = 19) | Subjects with NGT (n = 21) | |||||
|---|---|---|---|---|---|---|
| Vegetables before carbohydrates | Carbohydrates before vegetables | p | Vegetables before carbohydrates | Carbohydrates before vegetables | p | |
| MPG (mM) | 8.01 ± 1.97 | 8.16 ± 1.90 | ns | 5.04 ± 0.37 | 5.22 ± 0.49 | ns |
| SD (mM) | 1.69 ± 0.67 | 2.38 ± 1.13 | <0.01 | 0.69 ± 0.19 | 0.91 ± 0.38 | <0.01 |
| MAGE (mM) | 4.36 ± 1.86 | 6.52 ± 3.17 | <0.01 | 1.56 ± 0.74 | 2.44 ± 1.09 | <0.01 |
| LAGE (mM) | 6.82 ± 2.24 | 9.43 ± 3.98 | <0.01 | 3.10 ± 0.74 | 4.53 ± 1.66 | <0.01 |
| Mean 1-h PPG (mM) | 9.17 ± 2.52 | 10.22 ± 3.50 | <0.05 | 5.77 ± 0.87 | 6.83 ± 1.55 | <0.001 |
| Mean 2-h PPG (mM) | 9.22 ± 2.74 | 10.61 ± 3.86 | <0.01 | 5.61 ± 0.77 | 5.83 ± 1.28 | ns |
| Mean IAUC0-3h (mM) | 334 ± 254 | 546 ± 356 | <0.05 | 132 ± 85 | 191 ± 138 | <0.01 |
| Mean IGP (mM) | 2.99 ± 1.82 | 5.50 ± 3.34 | <0.001 | 1.56 ± 0.73 | 2.50 ± 1.33 | <0.001 |
Data are expressed as mean ± SD. Vegetables before carbohydrates vs carbohydrates before vegetables. MPG, mean plasma glucose; SD, standard deviation; MAGE, mean amplitude of glycemic excursions; LAGE, largest amplitude of glycemic excursions. PPG; postprandial plasma glucose; IAUC, incremental area under the curve; IGP, incremental glucose peak.
The Effect of Eating Vegetables before Carbohydrates on Long-term Glycemic Control
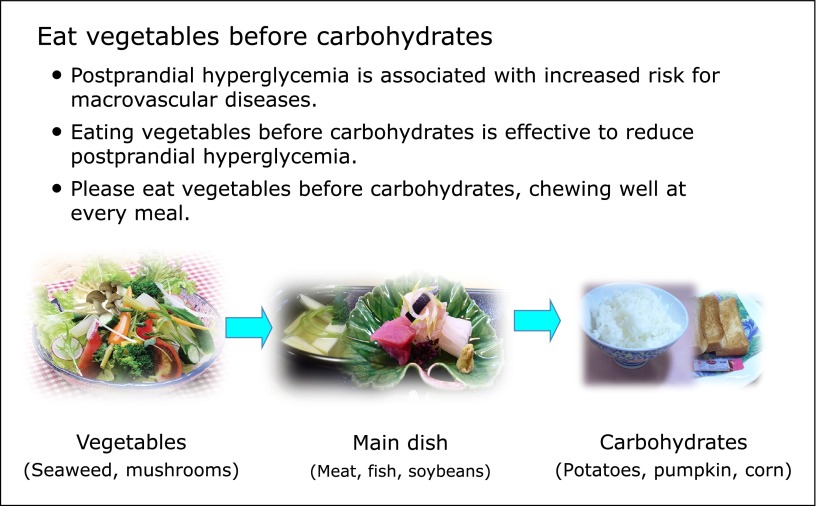
In addition to lowering acute postprandial glucose levels, we studied whether educating diabetic patients to eat vegetables before carbohydrates was effective on long-term glycemic control. To test this hypothesis, we carried out a retrospective study in patients with T2D that compared changes in HbA1c as the primary outcome and changes in weight, serum lipids, and blood pressure as the secondary outcomes. A total of 333 outpatients were divided into two groups to receive instructions about eating vegetables before carbohydrates (educational group, n = 196) or a control group (n = 137) who underwent a medical examination by a doctor. All patients were scheduled for return visits every 4 weeks with a physical examination and patients in the educational group were routinely scheduled to see dietitians at every visit. Depending on the patient’s current dietary intake, intervention aimed to encourage increased consumption of vegetables, including seaweed and mushrooms, and eating them first for 5 min, then the main dishes (meat, fish, soybeans, etc.) for 5 min, and rice or bread, including potatoes, pumpkin, corn, etc., successively within a 15 to 20 min eating time using an original educational brochure in the educational group (Fig. 3). The patients in the control group were given general information about lifestyle and diabetes risk by either the doctor or nurses at every visit. Student’s t tests were used to assess the difference between the clinical parameters in the two study groups and paired t tests were performed to analyze them within groups over time. All analyses were performed using SPSS 15.0 for Windows.
Fig. 3.
The educational brochure about eating vegetables before carbohydrates for the patients with type 2 diabetes.
Improvements in glycemic control were observed in patients in the educational group after the intervention and the levels of blood pressure, total cholesterol, and LDL cholesterol decreased significantly in both groups after 1 and 2.5 y (Table 2). After the intervention, dietary energy intake, protein, fat, and carbohydrates decreased in the educational group. These reductions in dietary nutrients were due to a decrease in the consumption of grain (rice), fruits, oil, sweets, and beverages. On the other hand, consumption of vegetables increased after intervention in the educational groups.(14)
Table 2.
Clinical parameters of patients in the two study groups
| Educational group (n = 196) | Control group (n = 137) | |||||
|---|---|---|---|---|---|---|
| Baseline | 1 y | 2.5 y | Baseline | 1 y | 2.5 y | |
| HbA1c (%) | 8.6 ± 1.8 | 7.7 ± 1.8*** ††† | 7.5 ± 1.7*** ††† | 8.2 ± 1.5 | 8.3 ± 1.6 | 8.1 ± 1.7 |
| BMI (kg/m2) | 24.2 ± 4.9 | 24.1 ± 5.3 | 22.9 ± 7.2** | 24.2 ± 4.0 | 24.4 ± 3.8 | 23.6 ± 5.3 |
| SBP (mmHg) | 132 ± 17 | 125 ± 11*** † | 127 ± 12*** | 138 ± 16 | 128 ± 12*** | 127 ± 10*** |
| DBP (mmHg) | 76 ± 11 | 72 ± 9*** | 71 ± 8*** | 75 ± 10 | 70 ± 8*** | 70 ± 8*** ††† |
| Total cholesterol (mg/dl) | 215 ± 37 | 202 ± 36*** | 200 ± 36*** | 210 ± 34 | 201 ± 36* | 195 ± 38*** |
| LDL cholesterol (mg/dl) | 131 ± 33 | 121 ± 32*** | 117 ± 33*** | 124 ± 29 | 117 ± 26* | 113 ± 30** |
| HDL cholesterol (mg/dl) | 58 ± 16 | 59 ± 15 | 60 ± 17* †† | 57 ± 14 | 57 ± 13 | 55 ± 14* |
| Triglyceride (mg/dl) | 142 ± 83 | 132 ± 76 | 127 ± 74* | 140 ± 89 | 153 ± 113 | 148 ± 117 |
Data are expressed as mean ± SD. Baseline vs 1 y or 2.5 y;*p<0.05, **p<0.01, ***p<0.001. Educational group vs. control group; †p<0.05, ††p<0.01, †††p<0.001. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure. Cited from Imai S et al. J Japan Dietetic Asso, 2010; 53: 16–23.
What to Eat First and How to Eat to Reduce Postprandial Blood Glucose and Insulin
This evidence supports the effectiveness of eating vegetables before carbohydrates on glucose excursions in the short-term and glycemic control in the long-term in patients with T2D. Dietary carbohydrates consumed after vegetables were digested slowly and required less insulin for subsequent metabolic disposal by the dietary fiber in the vegetables.(15–18) Other factors may influence the glycemic response and digestion of carbohydrates in the small intestine, including the rate of digestion, cooking method, transit time, and rate of intestinal absorption.(19–21) Similar to our study, Horowitz et al.(22,23) reported that whey protein and olive oil consumed before meals resulted in a substantial reduction in postprandial glycemia in patients with diabetes. Protein and oil given before carbohydrates stimulate glucagon-like peptide 1 (GLP-1) secretion, and delay gastric emptying which leads to a reduction in glycemic excursions. However, in contrast to the reduced insulin secretion observed after eating vegetables, whey protein augmented insulin secretion, possibly by a combination of the incretin effect and the direct stimulation of β-cells by absorbed amino acids.(24) Effects of eating vegetables before carbohydrates are probably similar to the gut peptide-based therapies for diabetes which may act predominantly by slowing gastric emptying, particularly when eating vegetables and protein before the carbohydrates.
Dietary intervention should aim not only to suppress hyperglycemia and hypoglycemia, but also glucose excursions in order to reduce vascular damage. The approach of eating vegetables before carbohydrates supports the concept of emphasizing food choices, what to eat first, how to eat and not just to concentrate on energy intake. The method was simple and easy to teach for physicians and other medical professionals. The most important point was that the method made it easy to make the appropriate behavioral changes, to increase the consumption of vegetables and to reduce the consumption of rice and sweets for patients. As one aspect of nutritional education, eating vegetables before carbohydrates should be advised to patients with T2D and this advice could even be applicable to healthy people in order to prevent future cardiovascular events.
Acknowledgments
This study was supported in part by a Grant-in Aid for Scientific Research from the Ministry of Education, Science and Culture (23500809) and Osaka Prefecture University.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bloomgarden ZT, Karmally W, Metzger MJ, et al. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care. 1987;10:263–272. doi: 10.2337/diacare.10.3.263. [DOI] [PubMed] [Google Scholar]
- 2.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006;144:465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 3.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22:1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 4.Cederberg H, Saukkonen T, Laakso M, et al. Postchallenge glucose, A1C, and fasting glucose as predictors of type 2 diabetes and cardiovascular disease: a 10-year prospective cohort study. Diabetes Care. 2010;33:2077–2283. doi: 10.2337/dc10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Flaviani A, Picconi F, Di Stefano P, et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care. 2011;34:1605–1609. doi: 10.2337/dc11-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng F, Lu W, Jia C, Li H, Wang Z, Jia W. Relationships between glucose excursion and the activation of oxidative stress in patients with newly diagnosed type 2 diabetes or impaired glucose regulation. Endocrine. 2010;37:201–208. doi: 10.1007/s12020-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 7.Succurro E, Marini MA, Arturi F, et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis. 2009;207:245–249. doi: 10.1016/j.atherosclerosis.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Shiozaki M, Matsuura K, Tanaka T, Yokoyama J, Utsunomiya K. Evaluation of efficacy of acarbose on glucose fluctuation and postprandial glucose using continuous glucose monitoring in type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13:467–470. doi: 10.1089/dia.2010.0153. [DOI] [PubMed] [Google Scholar]
- 10.Imai S, Matsuda M, Fujimoto S, et al. Crossover study of the effect of “vegetables before carbohydrates” on reducing postprandial glucose and insulin in Japanese subjects with type 2 diabetes mellitus. J Japan Diab Soc. 2010;53:112–115. [Google Scholar]
- 11.Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28:1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 12.Rizzo MR, Marfella R, Barbieri M, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33:2169–2174. doi: 10.2337/dc10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai S, Fukui M, Ozasa N, et al. Eating vegetables before carbohydrates improves postprandial glucose excursions. Diabet Med. 2013;30:370–372. doi: 10.1111/dme.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai S, Matsuda M, Togawa C, Oyabu K, Kajiyama S. Effect of eating ‘vegetables before carbohydrates’ on glycemic control in Japanese outpatients with type 2 diabetes. J Japan Dietetic Asso. 2010;53:1084–1091. [Google Scholar]
- 15.Wong JM, Jenkins DJ. Carbohydrates digestibility and metabolic effects. J Nutr. 2007;137:S2539–S2546. doi: 10.1093/jn/137.11.2539S. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh M, Miller C. A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr Rev. 2001;59:52–55. doi: 10.1111/j.1753-4887.2001.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrates exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 18.Sheard NF, Clark NG, Brand-Miller JC, et al. Dietary carbohydrates (amount and type) in the prevention and management of diabetes: a statement by the American Diabetes Association. Diabetes Care. 2004;27:2266–2271. doi: 10.2337/diacare.27.9.2266. [DOI] [PubMed] [Google Scholar]
- 19.Thorne MJ, Thompson LU, Jenkins DJ. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am J Clin Nutr. 1983;38:481–488. doi: 10.1093/ajcn/38.3.481. [DOI] [PubMed] [Google Scholar]
- 20.Holt S, Heading RC, Carter DC, Prescott LF, Tothill P. Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol. Lancet. 1979;1:636–639. doi: 10.1016/s0140-6736(79)91079-1. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz SE, Levine GD. Effects of dietary fiber on intestinal glucose absorption and glucose tolerance in rats. Gastroenterology. 1980;79:833–836. [PubMed] [Google Scholar]
- 22.Ma J, Stevens JE, Cukier K, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrates meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32:1600–1602. doi: 10.2337/dc09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentilcore D, Chaikomin R, Jones KL, et al. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab. 2006;91:2062–2067. doi: 10.1210/jc.2005-2644. [DOI] [PubMed] [Google Scholar]
- 24.Fieseler P, Bridenbaugh S, Nustede R, et al. Physiological augmentation of amino acid-induced insulin secretion by GIP and GLP-I but not by CCK-8. Am J Physiol. 1995;268:E949–E955. doi: 10.1152/ajpendo.1995.268.5.E949. [DOI] [PubMed] [Google Scholar]