Abstract
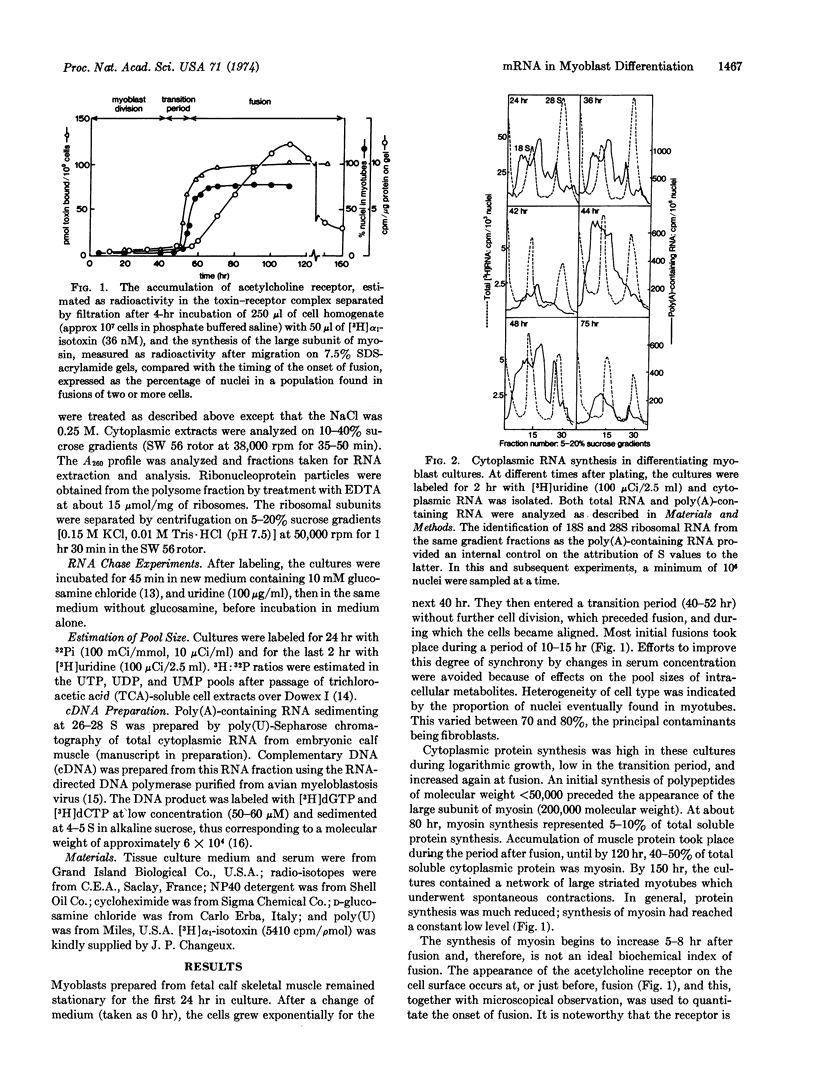
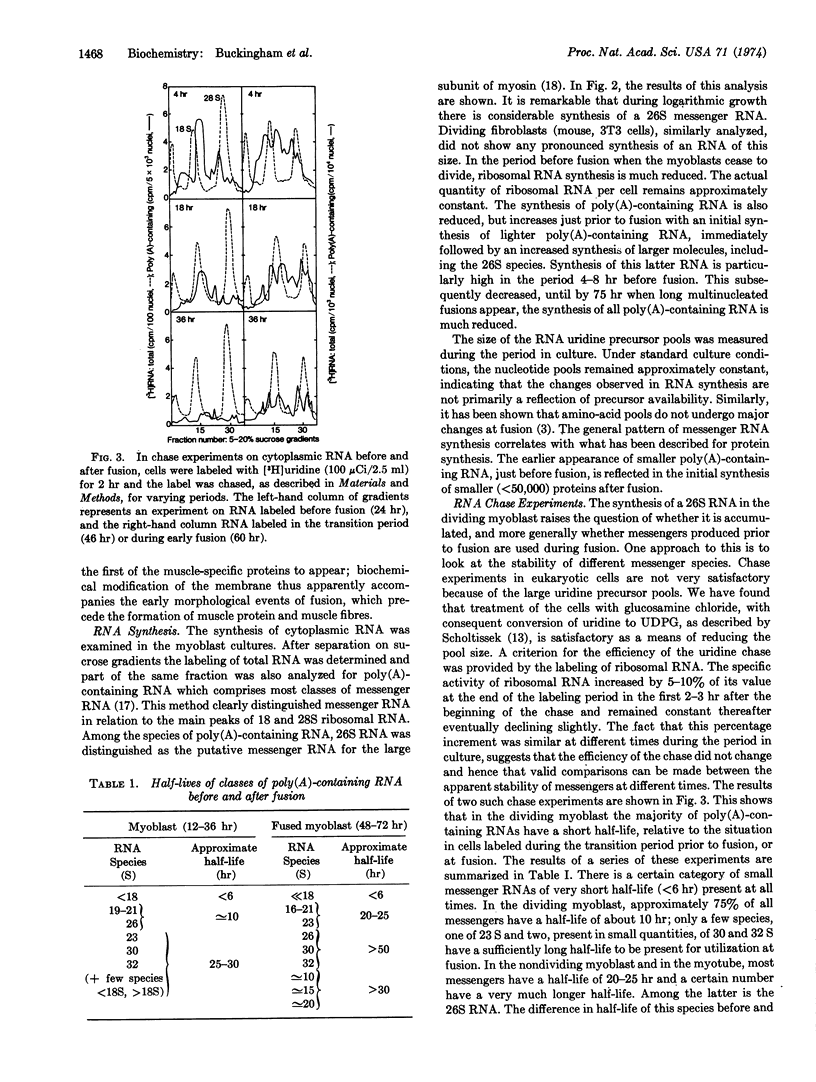
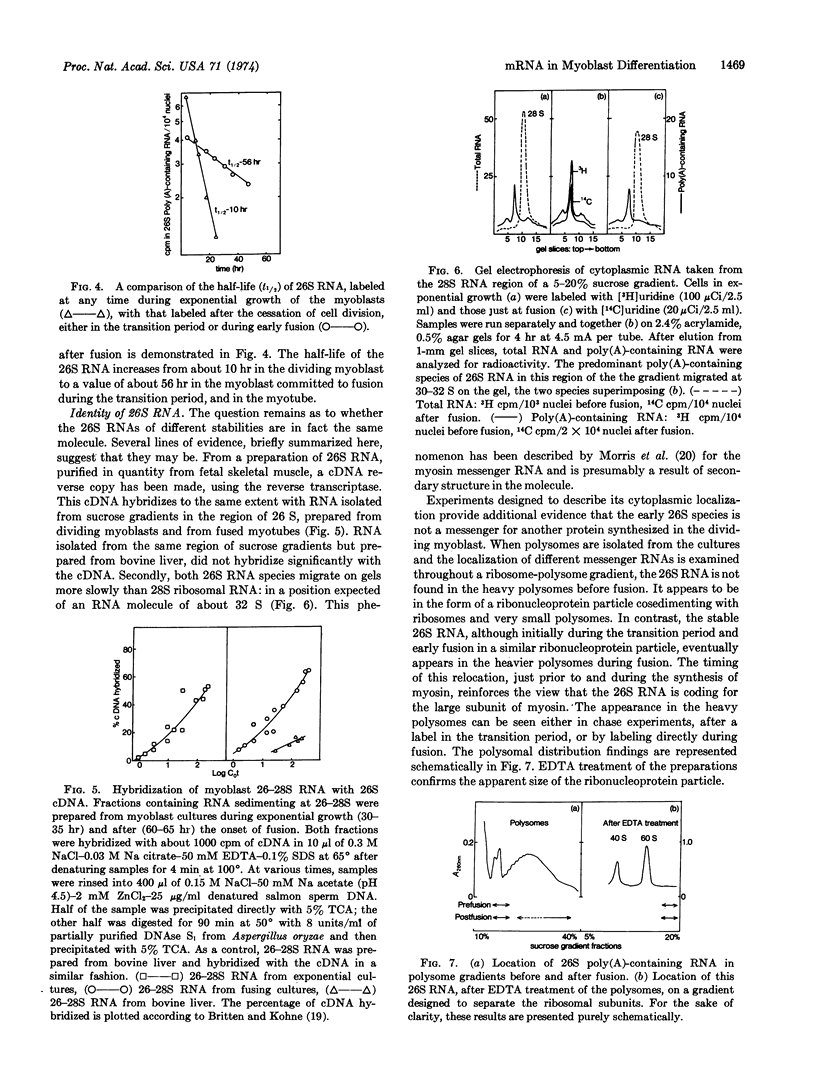
The synthesis of poly(A)-containing cytoplasmic RNA was examined in primary myoblast cultures prepared from skeletal muscle of fetal calves. After a period of cell division, these cells undergo fusion, with concomitant appearance of acetylcholine receptor and subsequent myosin synthesis. In the dividing myoblast there is a high level of messenger RNA synthesis, including a 26S RNA, the size of a putative messenger for the large subunit of myosin. In the transition period prior to fusion, there are quantitative changes in RNA synthesis. At this time, there is a pronounced production of 26S RNA, which diminishes during fusion. The possibility that 26S RNA is accumulated in the dividing myoblast was investigated by chase experiments. At fusion, there is a marked increase in the half-lives of a number of messenger RNA species, including 26 S, which increases from about 10 hr in the dividing cell to a value of more than 50 hr. The identity of the more rapidly turning over 26 S in the myoblasts, compared to that of the 26 S at fusion, was examined in terms of polysomal distribution, migration on gels, and hybridization with complementary DNA for the myosin message. The results of these analyses suggest that the 26S species are identical. Thus, it would appear that in a predetermined cell like the myoblast, the transition to the differentiated state of myotube that is synthesizing muscle specific proteins is effected by the stabilization of messenger already being actively transcribed: terminal differentiation, with respect to myosin synthesis, is preceded by the stabilization of 26S RNA.
Keywords: poly(A)-containing RNA, poly(U)filters, chase experiments, polysome distribution, complementary DNA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Caput D., Luzzati D., Gros F. Characterization of messenger RNA from embryonic muscle. Biochimie. 1972;54(2):187–194. doi: 10.1016/s0300-9084(72)80103-2. [DOI] [PubMed] [Google Scholar]
- Digglemann H., Faust C. H., Jr, Mach B. Enzymatic synthesis of DNA complementary to purified 14S messenger RNA of immunoglobulin light chain. Proc Natl Acad Sci U S A. 1973 Mar;70(3):693–696. doi: 10.1073/pnas.70.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- Heywood S. M., Nwagwu M. Partial characterization of presumptive myosin messenger ribonucleic acid. Biochemistry. 1969 Sep;8(9):3839–3845. doi: 10.1021/bi00837a050. [DOI] [PubMed] [Google Scholar]
- Luzzati D., Drugeon G. The effect of actinomycin D on RNA and protein synthesis during differentiation of myoblasts of line L 6 E: the half-life of functional myosin messenger. Biochimie. 1972;54(9):1157–1167. doi: 10.1016/s0300-9084(72)80020-8. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Rourke A. W., Heywood S. M. Myosin synthesis and specificity of eukaryotic initiation factors. Biochemistry. 1972 May 23;11(11):2061–2066. doi: 10.1021/bi00761a010. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Detection of an unstable RNA in chick fibroblasts after reduction of the UTP pool by glucosamine. Eur J Biochem. 1971 Dec;24(2):358–365. doi: 10.1111/j.1432-1033.1971.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Gage L. P., Brown D. D. The genes for silk fibroin in Bombyx mori. J Mol Biol. 1972 Oct 14;70(3):637–649. doi: 10.1016/0022-2836(72)90563-3. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Galibert F., Lepetit A., Auger M. A. L'électrophorèse des acides ribonucléiques en gel de polyacrylamide. Biochimie. 1972;54(3):339–354. doi: 10.1016/s0300-9084(72)80213-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yaffe D. Developmental changes preceding cell fusion during muscle differentiation in vitro. Exp Cell Res. 1971 May;66(1):33–48. doi: 10.1016/s0014-4827(71)80008-3. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Fuchs S. Autoradiographic study of the incorporation of uridine-3H during myogenesis in tissue culture. Dev Biol. 1967 Jan;15(1):33–50. doi: 10.1016/0012-1606(67)90004-8. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kato A. Crystallin synthesis by chicken lens. 3. mRNA stabilization under in vitro culture conditions. Exp Cell Res. 1972;71(2):361–371. doi: 10.1016/0014-4827(72)90305-9. [DOI] [PubMed] [Google Scholar]



