Abstract
Background
The efficacy of beta-blockers for treatment of patients with long QT syndrome type 3 (LQT3) has been repeatedly questioned, and it has been suggested that they might be detrimental for this genetic subgroup of patients with long QT syndrome (LQTS). The disquieting consequence has been that cardiologists confronted with LQT3 patients often do not even attempt pharmacologic therapy and implant cardioverter-defibrillators as first-choice treatment. However, the most recent clinical data indicate high efficacy of beta-blocker therapy in LQT3 patients.
Objective
The purpose of this study was to test the antiarrhythmic efficacy of beta-blockers in an established experimental model for LQT3.
Methods
After phenotypic validation of 65 ∆KPQ-SCN5A knock-in transgenic (TG) mice compared to 32 wild-type (WT) mice, we tested the effect of the arrhythmogenic cholinergic muscarinic agonist carbachol in 19 WT and 39 TG anesthetized mice, with and without pretreatment with propranolol given intraperitoneally.
Results
At the same heart rates, TG mice had a markedly longer QT interval than WT mice. Whereas carbachol had minor arrhythmic effects in the WT mice, it produced ventricular tachycardia (VT) and ventricular fibrillation (VF) in 55% of 20 TG mice. By contrast, in none of 19 TG mice pretreated with propranolol did VT/VF occur after carbachol injection.
Conclusion
These experimental data indicate that, contrary to previous reports, beta-blockade effectively prevents VT/VF in a validated LQT3 model. Together with the most recent clinical data, these findings indicate that there is no reason for not initiating protective therapy with beta-blockers in LQT3 patients.
Abbreviations: HR, heart rate; ICD, implantable cardioverter-defibrillator; IP, intraperitoneal; LQT3, long QT syndrome type 3; LQTS, long QT syndrome; TG, transgenic; VF, ventricular fibrillation; VPB, ventricular premature beat; VT, ventricular tachycardia; WT, wild type
Keywords: Long QT syndrome type 3, Beta-blocker, Transgenic mice, Sudden death
Among patients affected by long QT syndrome (LQTS) and who have been positively genotyped, those with gain-of-function mutations on the SCN5A gene, which causes an increase in the delayed Na+ inward current, represent approximately only 10% but constitute the most difficult subgroup to manage.1 Since the beginning of genotype–phenotype correlation studies, it was reported that the occurrence of sudden death was higher among patients with long QT syndrome type 3 (LQT3).2, 3 Moreover and of major concern, it was said that beta-blockers were largely ineffective in preventing their life-threatening arrhythmias4, 5, 6 and was at variance especially with LQT1 patients.4, 5, 7
A disquieting consequence of this concept was that too often the simple genetic diagnosis of LQT3 led to placement of an implantable cardioverter-defibrillator (ICD), even among patients who still were asymptomatic.8, 9 Also, beta-blocker therapy often was not even started in LQT3 patients based on the assumption that beta-blockers were ineffective. This view was based on rather small numbers of LQT3 patients but was also supported by experimental data obtained from cellular preparations10 and in transgenic (TG) mice.11
The concept of the lack of efficacy of beta-blockers for LQT3 patients was challenged when, based on a small population, it was pointed out that this apparent failure was the consequence of having lumped together all LQT3 patients, including those who had suffered a cardiac arrest in the first year of life.12 From this analysis it emerged that whereas LQT3 patients with events in infancy represent a subgroup at extremely high risk and largely unresponsive to therapy, those without early events appeared to be well protected by beta-blockers.12
We decided to reassess whether beta-blockers are ineffective against the arrhythmias that occur in the presence of an SCN5A mutation using an established whole animal model, the heterozygous ∆KPQ-SCN5A knock-in TG mice. Our findings indicate that, contrary to the prevailing opinion but in agreement with the most recent clinical data,13, 14 beta-blockers are very effective against the life-threatening arrhythmias of LQT3.
Methods
The murine model of LQT3 used in our study has been previously described in detail11, 15and was kindly donated by Peter Carmeliet. Heterozygous ΔKPQ-SCN5A knock-in TG mice were characterized and compared with wild-type (WT) littermate mice. All of the animals were adult males (average age 6 months, weight 40 g). The mice were anesthetized with Avertin 0.015 mL/g intraperitoneal (IP) injection,16 and the experiments were terminal.
ECG recordings and measurements
ECG recording started 2 minutes after onset of anesthesia while the mice were placed on a heating pad with continuous monitoring of body temperature. Five needle electrodes (one electrode implanted subcutaneously in each limb and one placed in the precordial position) connected to AD Instruments Ltd, (Oxford, UK) amplifiers set with bandpass filtering between 0.03 and 1 kHz were used for six-lead ECG recording.
ECG parameters (heart rate [HR], RR and QT intervals) were analyzed blindly to genotype. Measurements were performed using the signal-averaged ECG (mouse SAECG v1.2 program, AD Instruments) through a template-matching algorithm. QT interval (from onset of QRS complex to return to baseline of T wave) was measured in lead I. The remaining leads were mainly used to validate what was observed in lead I (i.e., ventricular arrhythmias, prolonged QT, etc.).
Cardiac arrhythmias were classified by the most severe episode as absent, minor (isolated ventricular premature beats [VPBs], couples of VPBs, ventricular bigeminy), or major (ventricular tachycardia [VT], ventricular fibrillation [VF]).
Study protocol
Our investigation follows the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Ethics Review Board of the Italian Ministry of Health. All procedures were performed in accordance with the animal care guidelines of the Federation of Laboratory Animals Science Associations. After baseline ECG variables were recorded, all mice, still under anesthesia, were assigned to one of the two arms of the study. The mice either were injected with the cholinergic agonist carbachol or were pretreated with propranolol followed 2 to 3 minutes later by carbachol injection. All animals were monitored continuously by ECG for up to 30 minutes after the administration of carbachol until the end of the experiment. All drugs used were provided by Sigma-Aldrich Srl, Milan, Italy and AstraZeneca SpA, Basiglio, Italy.
Pharmacologic interventions
The cholinergic agonist carbachol (carbamylcholine chloride 0.5 mg/kg) was administered IP in all mice. Within 1 to 2 minutes of injection, HR decreased from baseline. We selected a 2-minute good-quality ECG tracing to obtain HR and QT measurements following carbachol. ECG monitoring continued up to 30 minutes to assess the potential occurrence of arrhythmic events.
After the baseline ECG variables had been recorded, propranolol 0.1 mg/kg was injected IP Two to three minutes after the injection, a 6% to 9% decrement in HR was achieved, and HR and QT measurements on propranolol were made. Carbachol was then injected, and ECG monitoring continued for up to 30 minutes to observe arrhythmic events.
Genotyping was performed using a specific custom TaqMan assay (Life Technologies Italia, Monza, Italy) to discriminate between WT and mutant SCN5A sequence on a 7900 HT fast real-time polymerase chain reaction instrument (Life Technologies).
Statistical analysis
Continuous variables are given as mean ± SD and were compared among groups defined by genetic status and treatment protocol using the unpaired Student t test or analysis of variance, as appropriate. Whenever assumptions of normality and homogeneity of variance were questionable, the equivalent nonparametric tests (Mann-Whitney or Kruskal-Wallis test for independent samples) were used. Similarly, changes of basal ECG parameters after drug exposure within genetic groups were analyzed with t test or Wilcoxon signed rank test for paired samples. Categorical variables are expressed as absolute and relative frequencies and were analyzed by χ2 or Fisher exact test. Post-treatment survival to the primary endpoint of VT/VF was described by Kaplan-Meier cumulative estimates, with comparison performed by the log rank test. P <.05 (two-sided) was considered significant. All analyses were made using SPSS Statistics (version 19, IBM Italia SpA, Italy).
Results
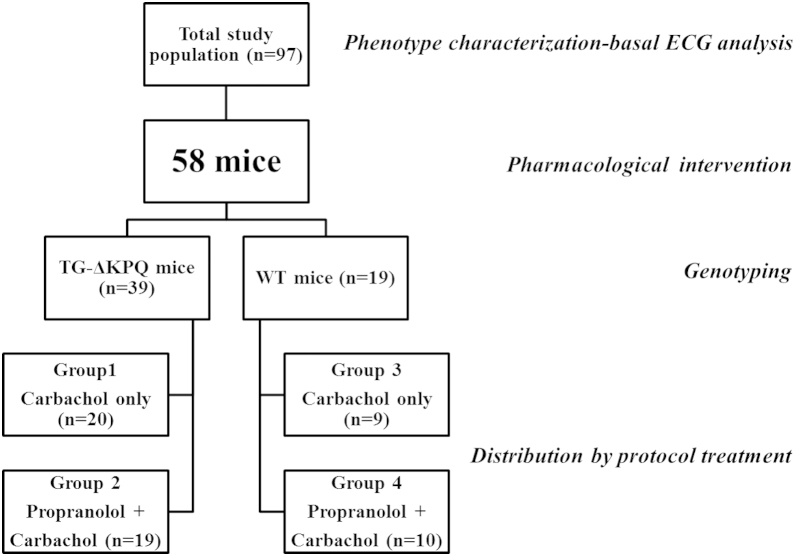
The study population, divided into subgroups by pharmacologic intervention and genotype, is shown in Figure 1.
Figure 1.
Outline of the study population subdivided into subgroups according to intervention protocol and genotyping. TG = transgenic; WT = wild type.
Basal ECG
Basal ECG was recorded in 97 mice (65 TG and 32 WT). Phenotypic characterization confirmed that, compared to their WT littermates, TG mice had a significantly more prolonged QT interval (77 ± 12 ms vs 61 ± 6 ms, P <.001) and a significantly lower mean basal HR (340 ± 52 bpm vs 371 ± 47 bpm, P = .005). Given the observed difference in basal HR between TG and WT animals and the established relationship between RR and QT intervals, we also compared the QT at fixed ranges of RR intervals (140–159 ms, 160–179 ms, 180–199 ms, and 200–220 ms) to control for QT adaptation, as it is done in infants.17 As expected, at all predefined RR ranges, TG mice showed a significantly (P <.001 for all comparisons) longer QT interval compared to WT mice (69 ± 8 ms, 76 ± 9 ms, 79 ± 10 ms, and 83 ± 10 ms vs 58 ± 5 ms, 60 ± 6 ms, 65 ± 5 ms, and 68 ± 8 ms, respectively; Table 1 and Figure 2).
Table 1.
Comparison of basal QT at different RR ranges between TG-ΔKPQ and WT-mice
| RR range (ms) | Basal QT in TG-ΔKPQ mice | Basal QT in WT mice | P value |
|---|---|---|---|
| 140–159 | 69 ± 8 (37) | 58 ± 5 (25) | <.001 |
| 160–179 | 76 ± 9 (47) | 60 ± 6 (22) | <.001 |
| 180–199 | 79 ± 10 (50) | 65 ± 5 (20) | <.001 |
| 200–220 | 83 ± 10 (42) | 68 ± 8 (14) | <.001 |
Number of mice with available measure is reported in parentheses.TG = transgenic; WT = wild type.
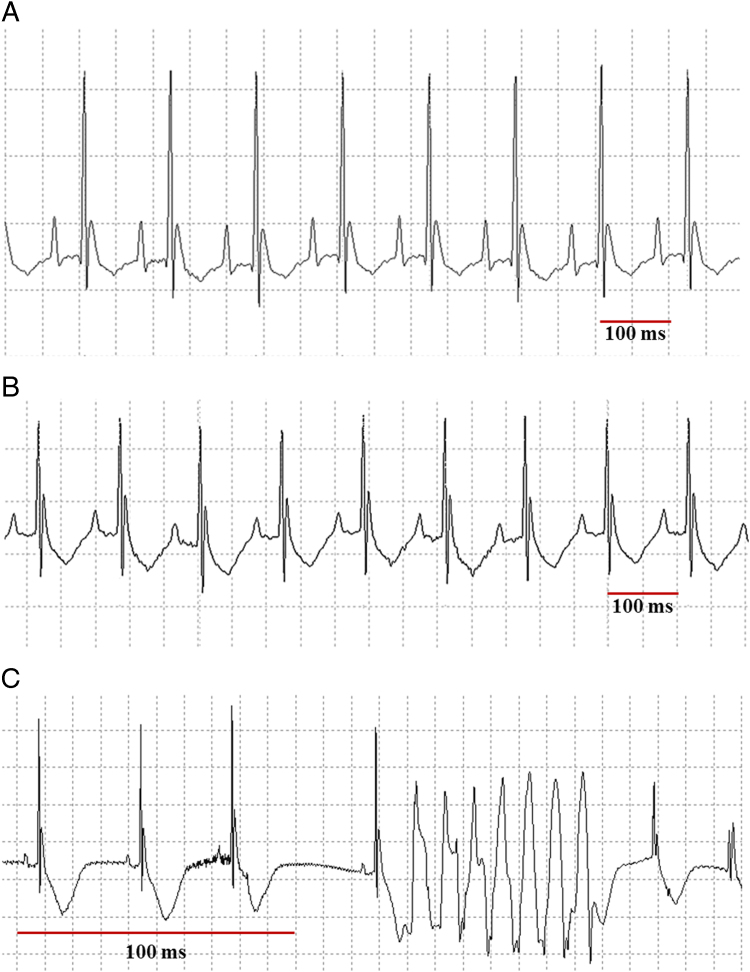
Figure 2.
ECG tracings of one wild-type (WT) and two transgenic (TG) mice. A: WT mouse, RR 117 ms, basal heart rate (HR) 513 bpm, QT 60 ms. B: TG Mouse, RR 119 ms, basal HR 506 bpm, QT 74 ms. C: TG mouse, 6 minutes after 0.5 mg/kg carbachol intraperitoneal. A and B illustrate the longer QT interval, for the same HR, present in TG vs WT mice. C shows the occurrence of what looks like an episode of torsades de pointes ventricular tachycardia in a TG mouse exposed to carbachol.
Effect of drugs on ECG
Fifty-eight mice (39 TG and 19 WT) underwent pharmacologic experiments to evaluate the proarrhythmic effect of carbachol and the potential protective role of propranolol pretreatment. Specifically, 20 TG and 9 WT mice were treated with carbachol alone (groups 1 and 3), whereas 19 TG and 10 WT mice were pretreated with propranolol and then injected with carbachol (groups 2 and 4; Figure 1).
Whereas mean basal HR was confirmed to be significantly lower in the 39 TG mice compared to the 19 WT animals (352 ± 52 bpm vs 383 ± 49 bpm, P <.05), no significant difference in HR was observed across the four groups (P = .10; Table 2). Mean values of basal QT interval were almost identical within both TG subgroups (76 ± 15 ms in group 1 and 76 ± 12 ms in group 2) and within both WT groups (60 ± 6 ms in group 3 vs 62 ± 5 ms in group 4). As expected, each TG subgroup had a significantly (P <.05) longer QT interval compared to its corresponding WT counterpart.
Table 2.
Heart rate and QT interval before and after propranolol and/or carbachol injections in TG and WT mice
| TG |
WT |
P value** | |||
|---|---|---|---|---|---|
| Group 1* (n = 20) | Group 2* (n = 19) | Group 3* (n = 9) | Group 4* (n = 10) | ||
| Pre-treatment measurements | |||||
| Basal HR (bpm) | 360 ± 61 | 344 ± 39 | 395 ± 65 | 372 ± 29 | .10 |
| Basal QT (ms) | 76 ± 15† | 76 ± 12† | 60 ± 6 | 62 ± 5 | <.001 |
| Post-treatment measurements | |||||
| HR postpropranolol (bpm) | 313 ± 42 | 351 ± 30 | .02 | ||
| QT postpropranolol (ms) | 81 ± 12 | 66 ± 9 | .002 | ||
| HR postcarbachol (bpm) | 187 ± 35‡ | 159 ± 48‡ | 177 ± 36‡ | 153 ± 40‡ | .09 |
| QT postcarbachol (ms) | 106 ± 35‡ | 116 ± 32‡ | 100 ± 22‡ | 100 ± 20‡ | .48 |
HR = heart rate.
Group 1 = ΔKPQ transgenic mice treated with carbachol; group 2 = ΔKPQ transgenic mice treated with propranolol and carbachol; group 3 = wild-type (WT) mice treated with carbachol; group 4 = WT mice treated with propranolol and carbachol.
P values from analysis of variance or unpaired t test.
P <.05 TG mice groups 1 and 2 vs corresponding groups 3 and 4 WT counterparts, after post hoc Bonferroni test for multiple comparisons.
P <.001 vs corresponding baseline measurement.
Compared to baseline values, carbachol treatment significantly decreased HR and prolonged QT interval in all mice (Table 2). The relative (%) change from baseline of both HR and QT following carbachol was rather similar across the four subgroups, independent of genotype or propranolol pre-treatment (HR: 47% ± 11%, 53% ± 16%, 54% ± 12%, 59% ± 10%, P = .07; QT: 39% ± 29%, 54% ± 34%, 69% ± 37%, 64% ± 31%, P = .1).
Arrhythmic events
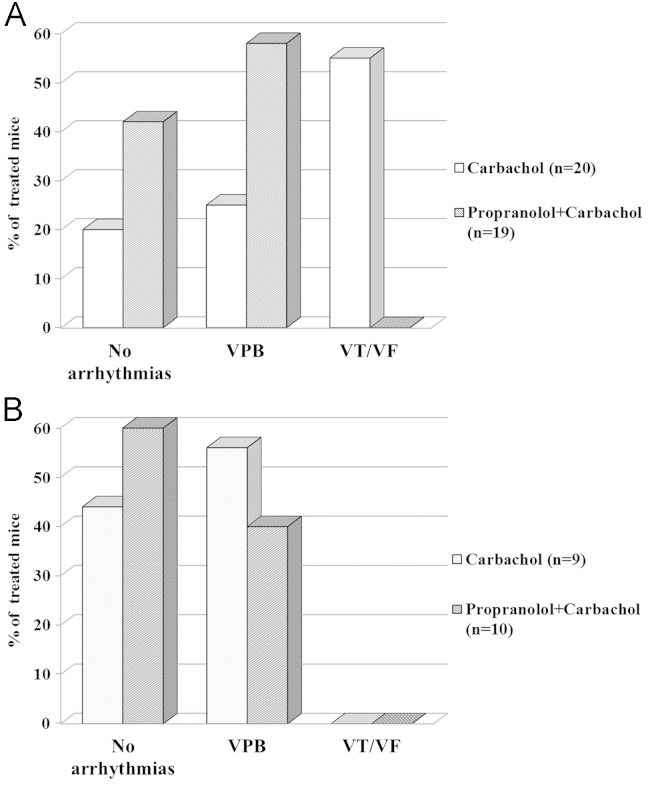
The occurrence of cardiac arrhythmias according to genotype and drug exposure is summarized in Figure 3.
Figure 3.
Carbachol-induced arrhythmias in transgenic (TG; A) and wild-type (WT; B) mice with and without pretreatment with propranolol. VF = ventricular fibrillation; VPB = ventricular premature beat; VT = ventricular tachycardia.
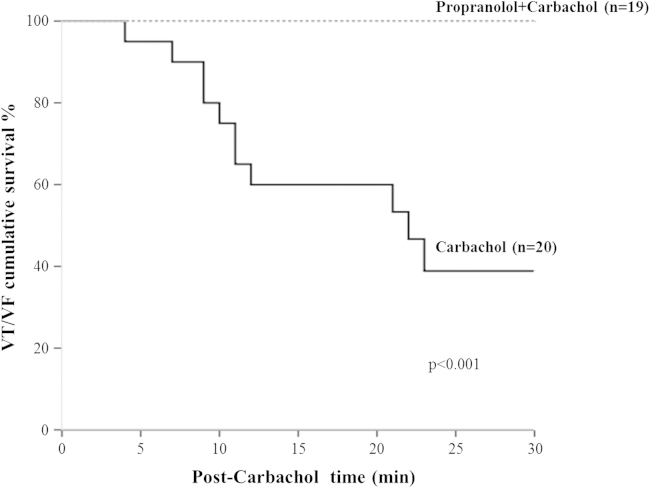
In the TG ΔKPQ-SCN5A mice, the overall incidence of drug-induced arrhythmias was high (27/39 [69%]) but with striking differences between groups 1 and 2. Specifically, in group 1, of the 20 TG mice injected only with carbachol, 4 (20%) had no arrhythmias, 5 (25%) showed minor events (VPBs), and 11 (55%) developed major arrhythmias (VT and/or VF), which occurred at a median time of 11 minutes (interquartile range 9–21) after carbachol injection. By contrast, in group 2, among the 19 TG mice pretreated with propranolol and then injected with carbachol, none developed major arrhythmias; isolated VPBs were observed in 11 mice (58%), and 8 (42%) had no cardiac arrhythmias (Figure 3A). Therefore, pre-treatment with propranolol had a marked and significant (P <.001) effect on the occurrence of major arrhythmias among TG mice as it was associated with the total absence of VT/VF compared to TG mice not pretreated with propranolol (i.e., with 100% cumulative survival to VT/VF compared to 39%; Figure 4).
Figure 4.
Cumulative survival to major arrhythmias (ventricular tachycardia/ventricular fibrillation [VT/VF]) in transgenic mice according to protocol treatment.
Among the WT mice overall, only minor arrhythmias were observed after the pharmacologic interventions, and this occurred in 9 of 19 (47%). Specifically, in group 3, of the nine mice treated with carbachol, 5 (56%) had isolated VPBs whereas none developed major arrhythmias (VT/VF; Figure 3B). Similarly, in group 4 none of the 10 WT mice pretreated with propranolol and then injected with carbachol had major arrhythmias, whereas four developed isolated VPBs and six had no arrhythmias at all (Figure 3B). Thus, among the WT mice, all carbachol-induced arrhythmias were minor and unaffected by propranolol (P = .66; Figure 3).
Discussion
The present study demonstrates that propranolol effectively prevents carbachol-induced life-threatening arrhythmias in an established animal model for LQT3. The cholinergic stimulation produced by carbachol causes bradycardia and cardiac arrhythmias in vivo in this reliable experimental preparation, which presents the classic features of LQT3 in the clinic.11, 15, 18 The striking protection afforded by propranolol mirrors the latest clinical observations in LQT3 patients and forces a reassessment of the previous assumptions that have caused so much harm to some of these patients.
Previous clinical data
Identification of the major LQTS genes in the mid-1990s1 has had a major impact on clinical management.19 A negative consequence has been the pressure on all investigators (present ones included) to publish, as soon as possible, their own data on genotype–phenotype correlation. Genotyped patients providing data on the effect of therapy initially were scanty, and this resulted in publications with very limited numbers but nonetheless conveying important messages. In 2000, Moss et al6 reported the effect of beta-blockers in 869 LQTS patients, of whom 139 had been genotyped. Of the 28 LQT3 patients, only four had cardiac events before therapy, and the conclusion was “Although the number of patients with LQT3 is quite small and the event rates in this genotype are quite low, no beneficial beta-blocker effect was evident in LQT3.”6 This was followed by two larger studies that conveyed the same message. In 2001, Schwartz et al4 reported on 670 genotyped patients, all with cardiac events; 65 (9.7%) were LQT3 patients. Among those on beta-blocker therapy, mortality was 4% for both LQT1 and LQT2 patients but reached 17% for LQT3 patients. Even though only 18 patients were in this group, it was concluded that “LQT3 patients are at higher risk at longer cycle lengths. This raises concerns, not yet supported by evidence, regarding the use of beta-blockers because of the attendant reduction in heart rate.”4 In 2004, Priori et al5 reported on 335 genotyped LQTS patients, of whom 28 (8.3%) were LQT3. Cardiac arrests on beta-blocker therapy were more frequent (14%) in LQT3 than LQT1 (1%) and LQT2 patients (7%). They suggested that “prophylactic defibrillator therapy may be a reasonable addition to beta-blockers in patients with LQT2 or LQT3 genotypes.”5 These convergent messages, despite all being based on limited numbers and expressed cautiously, led many cardiologists to the unwarranted conclusion that beta-blockers were not protecting LQT3 patients. The unfortunate consequence has been the high number of LQT3 patients who received a prophylactic ICD despite being asymptomatic and without having ever been treated with beta-blockers.9
Eventually, we realized that this concept was not fitting with our personal clinical experience, and the analysis of individual outcomes unmasked an important and striking difference: whereas patients with cardiac events in the first year of life had a very poor prognosis and died soon, those who were asymptomatic in their first year were very well protected by beta-blockers.12 Our breakthrough has just been confirmed by the largest study ever performed in LQT3 patients (n = 403), which demonstrates that among those without events in the first year of life mortality on beta-blockers is just close to 3%.13 Thus, the available clinical data indicate clearly that beta-blockers are very effective for LQT3 patients as well, with the exception of the small subgroup of symptomatic infants who need an ICD and/or left cardiac sympathetic denervation.12, 20, 21, 22
Previous experimental data
While rumors about the lack of efficacy of beta-blockers for LQT3 patients were spreading in the cardiology community, two experimental studies appeared and claimed to “closely mirror the clinical experience”10 and to “confirm the clinical observation that LQT3 patients do not benefit from beta-blocker therapy.”11
Shimizu and Antzelevitch10 used their arterially perfused wedge of canine ventricle and, having modeled LQT3, reported beneficial effects of sympathetic stimulation (by isoproterenol) that were abolished by propranolol. Their conclusion that “β-blockade might be contraindicated in LQT3” had a significant impact on the clinical decisions of the subsequent years. The problem with their study was the assumption that isoproterenol, a nonbiologic substance, would mimic what happens in real life when there is a sudden increase in sympathetic activity. Reality is different. During progressive sympathetic activation, as occurs during exercise, blood-borne epinephrine has a dominant effect and, because of its simultaneous effect on all cardiac cells, reduces electrical heterogeneity and increases electrical stability. What happens experimentally with isoproterenol is similar, but stimulation is limited to the beta-receptors. By contrast, sudden centrally mediated sympathetic activation elicits a mostly localized release of norepinephrine from neural terminals, which increases both regional heterogeneity and cardiac electrical instability. This explains the profound difference in the correlation between sudden death and HR responses during exercise and mental stress.23 Thus, it had to be expected that uniform beta-adrenergic stimulation by isoproterenol perfusion would reduce the dispersion of repolarization caused by anthopleurin (which augments late INa), and that this “beneficial” effect produced by stimulation of beta-adrenergic receptors would be prevented by propranolol, a drug whose function is blocking the beta-receptors. We do not believe that the interaction between isoproterenol and propranolol in isolated tissue preparations justifies assumptions on the antiarrhythmic efficacy of propranolol in patients exposed to sudden neurally mediated release of norepinephrine.
Fabritz et11 al used the same model of the present study and reported that propranolol did not prevent the arrhythmias induced by carbachol. We do not have a ready explanation for these different results, as sometimes occurs among laboratories. We can only point to some experimental difference, such as propranolol assumed by drinking water vs injected IP and an ambulatory vs anesthetized state. However, a major and potentially important difference is the number of TG animals tested with carbachol plus propranolol, which was 19 mice in the present experiments and only four in those by Fabritz et al.11
When experimental results differ, it is an old and good rule to verify what happens in the patients. It is now evident, based on adequate numbers, that beta-blockers are very effective in protecting LQT3 patients from life-threatening arrhythmias.12, 13, 14 The exception represented by infants with cardiac arrest in the first year of life does not impact on the concept.12
The present experiments
The present experiments have been rather straightforward. We used a well-validated animal model for LQT3,11, 15, 18 confirmed its characteristic QT prolongation, reproduced VT/VF by the muscarinic agonist carbachol, and observed that propranolol-pretreated mice did not develop VT/VF after carbachol. We repeated these interventions in WT mice and observed modest effects of carbachol essentially unmodified by propranolol. The efficacy of propranolol likely reflects the combination of beta-adrenergic blockade with its well-known “membrane stabilizing” effect, which includes significant reductions in both peak and late Na+ current. This effect is present but modest for nadolol and is completely absent for metoprolol.24 Indeed, we believe that for LQT3 patients, propranolol should be regarded the beta-blocker of choice.
The decrease in HR produced by propranolol and its modest effect on QT interval were as expected given that we looked at the QT as measured and not at the QTc to avoid the overcorrection associated with very short RR intervals. When carbachol produced VT/VF in the TG mice, most of the arrhythmias occurred within 15 min from the injection. The Kaplan–Meier curve provides a graphic evidence of the protective effect of propranolol in these LQT3 animals.
Study limitations
Even though the IP dose of propranolol per kilogram is the same as that given intravenously in man, it is fair to remember that beta-blocker therapy in LQTS patients is administered orally. Therefore, some difference in the magnitude of the effects cannot be ruled out. Also, we studied the effect of propranolol in one specific, albeit fairly representative, SCN5A mutation; accordingly, we cannot exclude that with different mutations one could observe different degrees of protection, as we already reported.25
Clinical implications
These experimental results carry clinical implications. They should help dispel, once and for all, the concern that beta-blockade provides no protection against VT/VF occurring in experimental models for LQT3 and thereby also in patients. Furthermore, when taken together with the most recent clinical data, these observations send a clear and unequivocal message to practicing cardiologists that LQT3 patients should be placed on beta-blocker therapy as first choice without hesitation,14 with preference given to propranolol.
Acknowledgments
We are grateful to Federica Greco and Elisa Marelli for help in the preparation and conduction of the experiments and to Pinuccia De Tomasi for expert editorial support.
Footnotes
The financial support of Telethon–Italy (Grant No. GGP 09247) is gratefully acknowledged.
References
- 1.Schwartz P.J., Crotti L., Insolia R. Long QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–877. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zareba W., Moss A.J., Schwartz P.J., for the International Long-QT Syndrome Registry Research Group Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 3.Priori S.G., Schwartz P.J., Napolitano C. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz P.J., Priori S.G., Spazzolini C. Genotype-phenotype correlation in the long QT syndrome. Gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Priori S.G., Napolitano C., Schwartz P.J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 6.Moss A.J., Zareba W., Hall W.J. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 7.Vincent G.M., Schwartz P.J., Denjoy I. High efficacy of beta-blockers in long QT syndrome type 1: contribution of non-compliance and QT prolonging drugs to the occurrence of beta-blocker treatment “failures.”. Circulation. 2009;119:215–221. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 8.Etheridge S.P., Sanatani S., Cohen M.I. Long QT syndrome in children in the era of implantable defibrillators. J Am Coll Cardiol. 2007;50:1335–1340. doi: 10.1016/j.jacc.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz P.J., Spazzolini C., Priori S.G. Who are the long-QT syndrome patients who receive an implantable cardioverter defibrillator and what happens to them? Data from the European long-QT syndrome implantable cardioverter-defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu W., Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- 11.Fabritz L., Damke D., Emmerich M. Autonomic modulation and antiarrhythmic therapy in a model of long QT syndrome type 3. Cardiovasc Res. 2010;87:60–72. doi: 10.1093/cvr/cvq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz P.J., Spazzolini C., Crotti L. All LQT3 patients need an ICD. True or false? Heart Rhythm. 2009;6:113–120. doi: 10.1016/j.hrthm.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Wilde A.A., Kaufman E.S., Shimizu W. Sodium channel mutations, risk of cardiac events, and efficacy of beta-blocker therapy in type 3 long QT syndrome. Heart Rhythm. 2012;9(Suppl):S321. [Google Scholar]
- 14.Schwartz P.J., Ackerman M.J. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34:3109–3116. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 15.Nuyens D., Stengl M., Dugarmaa S. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–2007. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- 16.Hart C.Y., Burnett J.C., Jr, Redfield M.M. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol. 2001;281:H1938–H1945. doi: 10.1152/ajpheart.2001.281.5.H1938. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz P.J., Stramba-Badiale M., Segantini A. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 18.Fabritz L., Kirchhof P., Franz M.R. Effect of pacing and mexiletine on dispersion of repolarisation and arrhythmias in ΔKPQ SCN5A (long QT3) mice. Cardiovasc Res. 2003;57:1085–1093. doi: 10.1016/s0008-6363(02)00839-8. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz P.J., Ackerman M.J., George A.L., Jr, Wilde A.M. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz P.J. Cutting nerves and saving lives. Heart Rhythm. 2009;6:760–763. doi: 10.1016/j.hrthm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz P.J., Priori S.G., Cerrone M. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 22.Collura C.A., Johnson J.N., Moir C., Ackerman M.J. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–759. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Jouven X., Schwartz P.J., Escolano S. Excessive heart rate increase during mild mental stress in preparation for exercise predicts sudden death in the general population. Eur Heart J. 2009;30:1703–1710. doi: 10.1093/eurheartj/ehp160. [DOI] [PubMed] [Google Scholar]
- 24.Besana A., Wang D.W., George A.L., Jr, Schwartz P.J. Nadolol block of Nav1.5 does not explain its efficacy in the long QT syndrome. J Cardiovasc Pharmacol. 2012;59:249–253. doi: 10.1097/FJC.0b013e31823d2fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D.W., Crotti L., Shimizu W. Malignant perinatal variant of long QT syndrome caused by a profoundly dysfunctional cardiac sodium channel. Circ Arrhythm Electrophysiol. 2008;1:370–378. doi: 10.1161/CIRCEP.108.788349. [DOI] [PMC free article] [PubMed] [Google Scholar]