Abstract
Female sex predisposes individuals to poorer outcomes during respiratory disorders like cystic fibrosis and influenza-associated pneumonia. A common link between these disorders is dysregulation of alveolar fluid clearance via disruption of epithelial sodium channel (ENaC) activity. Recent evidence suggests that female sex hormones directly regulate expression and activity of alveolar ENaC. In our study, we identified the mechanism by which estradiol (E2) or progesterone (P4) independently regulates alveolar ENaC. Using cell-attached patch clamp, we measured ENaC single-channel activity in a rat alveolar cell line (L2) in response to overnight exposure to either E2 or P4. In contrast to P4, E2 increased ENaC channel activity (NPo) through an increase in channel open probability (Po) and an increased number of patches with observable channel activity. Apical plasma membrane abundance of the ENaC α-subunit (αENaC) more than doubled in response to E2 as determined by cell surface biotinylation. αENaC membrane abundance was approximately threefold greater in lungs from female rats in proestrus, when serum E2 is greatest, compared with diestrus, when it is lowest. Our results also revealed a significant role for the G protein-coupled estrogen receptor (Gper) to mediate E2's effects on ENaC. Overall, our results demonstrate that E2 signaling through Gper selectively activates alveolar ENaC through an effect on channel gating and channel density, the latter via greater trafficking of channels to the plasma membrane. The results presented herein implicate E2-mediated regulation of alveolar sodium channels in the sex differences observed in the pathogenesis of several pulmonary diseases.
Keywords: sex hormones, alveoli
sex steroid hormones have a very well described and essential role in sexual differentiation, maturation, and reproduction and have a more recently recognized role in cancer (21). In addition, sexual dimorphisms have been observed in many aspects of physiology, including pulmonary pathophysiology (5, 63). For example, females with cystic fibrosis present more often than males with bacterial lung infections and have greater rates of hospitalization (47, 58, 68). Also, epidemiological data collected during several influenza epidemics have revealed that, compared with young males, young adult females exhibit increased rates of hospitalization and greater mortality as a result of influenza-associated complications, such as pneumonia (32, 55, 67a). It is unclear whether the sexual dimorphisms in cystic fibrosis and influenza patient outcomes relate to genetic sex or to the effects of sex hormones alone.
A common link between these two diseases is that both cystic fibrosis and influenza infection alter alveolar fluid clearance (AFC) through dysregulation of the epithelial sodium channel (ENaC) in alveolar cells (8, 9). Alveolar ENaC has an essential role in regulation of AFC by regulating sodium transport, which is required to maintain a stable fluid layer across the alveolar epithelium (12). Sexual dimorphisms in AFC are apparent in both humans (2) and rats (33) and, at least in rats, are attributable to an amiloride-sensitive mechanism, suggesting a role for ENaC. Investigators have reported that mRNA expression of ENaC subunits in the lung changes both during the female estrous cycle (60) and in alveolar cells in response to exogenously administered female sex hormones (34).
In the latter study, Laube and colleagues showed that female sex hormones increased the number of cells with ENaC channel activity using a fetal distal lung epithelial cell line. However, this study did not address whether this effect was specific to β-estradiol (E2) or progesterone (P4) nor did the study address the identity of the steroid receptors and mechanisms responsible for this hormonal effect on alveolar ENaC. In addition, the study did not address whether the hormones increased ENaC activity through greater channel number or open probability (Po). Importantly, the cell model used in that study originates from fetal rats presenting a possible issue with respect to steroid receptor expression levels and steroid responses in these cells compared with those from postpubescent rats. Several studies show significant differences in sex steroid receptor expression and action during development (7, 50, 64). It is also not known whether these cells came from male or female rats, which can drastically affect responsiveness to sex steroid hormones, again in relation to steroid receptor expression differences between the sexes. Finally, the concentrations of hormones used were supraphysiological. In cycling female rats, the maximal circulating levels of E2 are <1 nM (16, 36) and for P4 are <100 nM (40).
Taken together, these studies suggest that female sex hormones might regulate alveolar ENaC expression and channel abundance, but, given the very high concentrations of hormones and sometimes inappropriate cell models in previous studies, it is unclear if these hormones regulate ENaC under physiological conditions. In the present study, we 1) determined whether the effect of physiological concentrations of female sex hormones on alveolar ENaC are specific to either E2 or P4, 2) identified whether the hormonal effect is specific to either the highly selective or nonselective ENaC present in alveolar cells, 3) characterized how the hormone(s) alters channel activity (i.e., Po or N, subunit expression, and localization, etc.), and 4) identified the steroid receptor responsible for the effect of this hormone.
MATERIALS AND METHODS
Cell culture and hormone treatments.
Rat (female) L2 cells (P29–P63) from ATCC (catalog no. CCL-149) were subcultured in Ham's F-12K (catalog no. 21127022; Invitrogen) medium supplemented with 2 mM l-glutamine (catalog no. 25030081; Invitrogen) and 1.5 g/l sodium bicarbonate supplemented with 10% fetal bovine serum (Invitrogen) and antibiotics. For experiments, these cells were seeded on 12 (0.5–1 × 104 cells/insert)- or 24 (2–4 × 105 cells/insert)-mm Corning Polyester Transwell permeable supports (catalog nos. 3460 and 3450, respectively) in a medium containing phenol red-free DMEM/F-12 (catalog no. 21041025; Invitrogen) supplemented with 10% charcoal-stripped fetal bovine serum (catalog no. F-6765; Sigma) and antibiotics. The top (apical side) medium was subsequently removed at 2–4 h postplating. In the next 24–48 h, the bottom (basolateral side) medium was replaced with fresh medium containing vehicle or hormones. For experiments assessing the acute effects of hormones on single channel activity, hormones were applied directly to the bath after a recording period of ∼5 min.
All hormones were dissolved in 100% ethanol such that the final concentration of ethanol used was ≤0.002% vol/vol (vehicle). E2 (catalog no. E2758) and P4 (catalog no. P8783) from Sigma-Aldrich (St. Louis, MO) were used at 0.73 nM (200 pg/ml) and 32–64 nM (10–20 ng/ml), respectively. 2,3-Bis(4-hydroxyphenyl)propionitrile (DPN), a selective agonist of estrogen receptor β (ERβ), and rel-1-[4-(6-bromo-1,3-benzodioxol-5-yl)-3aR,4S,5,9bS-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G-1), a selective agonist of the G protein-coupled estrogen receptor (Gper), were purchased from Cayman Chemical (Ann Arbor, MI) and used from 2 to 10 nM.
For knockdown of αENaC expression, we used a silencing vector [short-hairpin RNA (shRNA)] previously reported to reduce the expression of rat αENaC in vivo (38). Twenty-four hours postplating, we transfected the cells with 3 μg/cm2 of the pSilencer 3.0-H1 vector (Ambion) containing either a nontargeting shRNA sequence or one specific for rat αENaC using the Xfect Transfection Reagent (Clontech). We collected whole cell or cell surface proteins from cells 48–72 h posttransfection.
Single channel analyses.
The bath and electrode solutions consisted of 140 mM NaCl, 4 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES titrated to a pH of 7.4 with NaOH with a final osmolality of 290–310 mosmol/kgH2O. Filamented glass electrodes (TW-150F; World Precision Instruments) were pulled on a two-stage vertical puller to achieve a resistance of 5–10 MΩ. After the creation of a gigaohm seal on an individual cell, channel activity was recorded for 5–15 min at a pipette holding potential of 0 or 20 mV using a Dagan PC-One patch-clamp amplifier with a low-pass three-pole Bessel filter set at either 100 or 1,000 Hz. Recordings of activity at other holding potentials were used to generate current-voltage relationships. Data were digitally collected at a sampling frequency of 5 kHz (Digidata 1440a and pCLAMP10; Axon Instruments) and digitally filtered at 30 Hz to analyze single channel events. Channel density (N) in a given patch was calculated as the number of individual current transitions (levels) observed during the recording. The pCLAMP 10 software program calculated NPo using the equation:
where i denotes the number of open channels, ti the time the channels are open, and T the total recording time. NPo was multiplied by the fraction (f) of patches with observable channel openings and closures (Table 1) denoted as fNPo to correct for the presence of “empty” patches that exhibited no channel activity. Channel Po was calculated as the ratio of NPo/N. The slope of the current-voltage relationship was used to calculate channel conductance.
Table 1.
Fraction of patches with observable channel openings/closings
| Group | No. of Patches | No. of Patches with Channels | Fraction of Patches with Channels |
|---|---|---|---|
| Vehicle | 21 | 13 | 0.62 |
| P4 (64 nM) | 11 | 6 | 0.55 |
| E2 (0.73 nM) | 18 | 17 | 0.94A |
P4, progesterone; E2, estradiol.
P < 0.05 vs. vehicle by z-test.
For patches with multiple channels of different types (highly selective vs. nonselective), point amplitude histograms were analyzed using the program PeakFit (SPSS, Chicago, IL), which is specifically designed to fit multiple Gaussian peaks. We fit the amplitude histograms, and, based on our observation of the records and the magnitude of the mean current for each peak, determined the type and number of channels (see Fig. 2). Once the assignments had been made, NPo could be calculated from a modification of the formula above:
in which N is the total number of observable levels for a specific channel type [e.g., highly selective channels (HSCs)], Ai is the area of the peak for the ith number of channels for a specific type (i.e., the area of the peak that corresponds to 1, 2, or 3 HSCs, etc.), and Atot is the sum of the area of all peaks (including the peak corresponding to all closed channels). This approach allowed us to resolve up to nine levels and assign them to either HSCs or nonselective channels (NSCs).
Fig. 2.
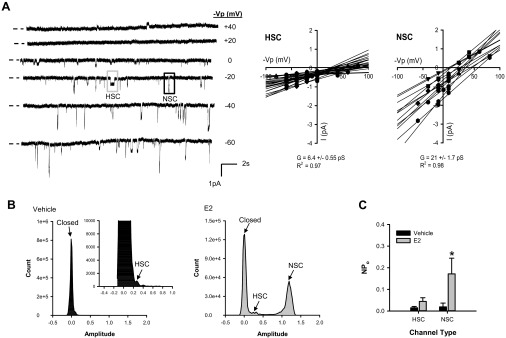
E2 selectively increased activity of nonselective ENaC in alveolar cells. We identified the HSC and NSC in single channel recordings from L2 cells using several different approaches. A: the first assessment used the differences in mean open time and current amplitude for the HSC and NSC over a range of holding potentials shown as minus the pipette holding potential (−Vp). Representative recordings of these channels are shown. The current-voltage relationship for a representative single channel record is shown as a scatter plot from −80 to + 80 mV (−Vp) for both the HSC (gray box) and NSC (black box) recorded from L2 cells. Linear regression analyses of current-voltage relationships for each individual patch revealed average conductances (slope of the line, G) of 6.4 ± 0.55 pS for the HSC (n = 24) and 21 ± 1.7 pS for the NSC (n = 12). B: two representative amplitude histograms are shown for single channel recordings from cells treated with vehicle (black) or E2 (gray) overnight. These histograms permit classification of individual peaks for the closed state and open states of the HSC and NSC based on differences in current amplitudes determined from the current-voltage relationship. C: we calculated NPo for the HSC and NSC from these amplitude histograms and pCLAMP analyses. Overnight exposure to E2 significantly increased NPo of the NSC. Data are shown as means ± SE. *P < 0.05 vs. vehicle of the same channel type by Mann-Whitney Rank Sum test.
Real-time PCR.
Cells were washed with ice-cold phosphate-buffered saline (PBS), and RNA was extracted using Trizol (Life Technologies) according to the manufacturer's protocol. The RNA was converted to cDNA using the QuantiTect Reverse Transcription Kit (Qiagen). Real-Time PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR instrument. For the reaction, we used 20 ng cDNA, validated probes specific for the rat genes Scnn1a or Actb (QuantiTect primer assay; Qiagen), and the QuantiTect Sybr Green PCR Kit according to the manufacturer's instructions. All reactions were run in triplicate and corrected for loading using Actb, calculated as the difference in cycle threshold (Ct), dCt = CtScnn1a − CtActb.
Cell surface biotinylation.
For biotinylation experiments, the cells (apical side only) were washed with ice-cold PBS two times. The PBS was replaced with a borate buffer containing 0.5 mg/ml biotin (EZ-Link Sulfo-NHS-SS-Biotin; ThermoScientific) and incubated at 4°C with rocking for 30 min. After removal of the biotin solution, the cells were incubated in medium containing 100 mM l-lysine monohydrochloride (Sigma-Aldrich) for 15 min at 4°C with rocking. The cells were washed two times with PBS and then lysed in RIPA buffer containing protease inhibitors and 2 mM EGTA. The protein concentration in each lysate was determined using the Pierce BCA Assay (ThermoScientific), and equal total protein amounts from each lysate were incubated with avidin beads (NeutraAvidin Agarose Resins; ThermoScientific) overnight or for 1 h at 4°C with rocking. The samples were centrifuged, and the supernatant was removed. The beads were washed with RIPA (as above) three times; and biotinylated proteins were eluted from the beads with incubation in Laemmli (SDS) sample buffer with 100 mM DTT at 95°C.
Animals.
Animal use was approved by the Emory University Institutional Animal Care and Use Committee. Female Wistar rats (192–265 g body wt) were maintained on a reverse light-dark cycle (14:10), fed standard laboratory chow (LabDiet 5001), and allowed free access to water. The stage of the estrous cycle for each rat was determined through microscopic assessment of vaginal smears over the course of 1–2 wk according to standard protocols (4, 22). Following death by live decapitation, tissues were collected, snap-frozen on dry ice, and stored at −80°C. Estrous cycle assessment was confirmed by measurement of uterine weight, which is greatest during proestrus (66).
Tissue membrane protein isolation.
A small portion (10–40 mg) of the lower tip of the left lung was washed in PBS containing protease inhibitors. The tissue was minced and homogenized using an Omni hand-held tissue grinder, and membrane proteins were extracted from the tissues using the Mem-PER kit (ThermoScientific). The Pierce SDS-PAGE Prep kit (ThermoScientific) was used to remove SDS-PAGE-incompatible detergents and concentrate the protein samples. Protein concentrations were measured using a BCA assay (ThermoScientific).
Western blotting.
Samples of equal total protein amounts were boiled in SDS sample buffer containing either 100 mM β-mercaptoethanol or DTT at 95°C. The samples were run on a precast polyacrylamide gel (Any kD; Bio-Rad) and transferred to polyvinylidene difluoride at 350 mA for 1.5–2 h. The blots were blocked in 2.5% Rodeo blocker (Affymetrix) in TBS with 0.05% Saddle Soap (Affymetrix) or in 5% nonfat dry milk (Carnation) in TBS with 0.05% Tween 20 for 1 h at room temperature. Blots were then incubated at 4°C in polyclonal primary antibodies (rabbit anti-rat αENaC 1:250) overnight with rocking. We generated the rabbit anti-rat αENaC antibody against a fusion protein of the rat αENaC epitope from K250-S347 present in the extracellular loop of the protein, using a method previously described by our laboratory to generate antibodies against Xenopus ENaC epitopes (39, 42, 43, 46, 59). Antibodies were extracted from rabbit serum using the Pierce Melon Gel IgG Purification Kit (catalog no. 45206; ThermoScientific). To determine specificity of the antibody, the immunizing peptide was extracted as a fusion protein tagged with maltose-binding protein (pMal vector, NEB), and antibodies were incubated for 1–2 h with this fusion protein before standard immunoblotting. After being washed, blots were incubated in 1:5,000 anti-rabbit IgG conjugated to horseradish peroxidase (HRP; Affymetrix) for 1 h. Alternatively, blots were incubated in 1:50,000 monoclonal mouse anti-β-actin-HRP (no. A3854; Sigma) at room temperature for 15–30 min. After exposure of blots to enhanced chemiluminescence reagent for 5 min, digital photos were taken with a Kodak Gel Logic 2200 imager. Densitometry was performed using ImageJ software (National Institutes of Health). Band intensity was corrected for background and normalized as a ratio to the loading control (actin). For each individual blot, the relative difference in band intensity was calculated compared with that in the vehicle or diestrous group (set to 1).
Statistical analyses.
We performed statistical analyses using SigmaPlot 12 (SysStat) or Microsoft Excel (z-test). For comparison of proportions, we evaluated statistical significance using a z-test or chi-squared test. For comparison of two normally distributed groups, we used a one- or two-sided t-test. For comparisons of samples that were not normally distributed or that had substantially different standard deviations, we used nonparametric tests, either a Mann-Whitney Rank Sum (two samples) or a Kruskal-Wallis One-Way ANOVA on Ranks (three samples) test. If significance was detected, we performed an appropriate post hoc test to detect differences between particular groups. For comparisons before and after treatment within the same group, we used a paired t-test. P < 0.05 was set as the threshold for significant difference between groups. Data are presented as means ± SE unless otherwise noted.
RESULTS
E2 stimulated alveolar ENaC single channel activity.
In studies examining the effect of female sex hormones on ENaC, investigators typically used supraphysiological hormone doses (4 nM-1 μM). To determine if female sex hormones affect ENaC activity in alveolar cells at physiological doses observed during the rodent estrous cycle (16, 36, 40), we treated L2 cells with a combination of 0.73 nM E2 and 32 nM P4 overnight or with E2 and P4 individually. The concentrations used are similar to the hormone concentrations observed during proestrous or estrous phases of the estrous cycle (16, 36, 40). Figure 1A shows representative channel recordings from cells treated with vehicle, E2–P4, E2, and P4 overnight. The recordings show both a HSC with a longer mean open time and smaller current amplitude than the NSC. Figure 1 assesses total ENaC activity as the combination of HSC and NSC. Combined E2-P4 treatment significantly increased ENaC fNPo in cell-attached patches from L2 cells (Fig. 1B).
Fig. 1.
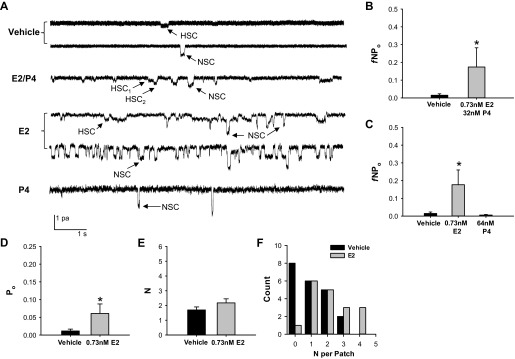
Estradiol (E2) stimulated epithelial sodium channel (ENaC) activity in rat alveolar cells. To examine the effect of female sex hormones on alveolar ENaC activity, we calculated the fraction of cell-attached patches with channel activity (f) and NPo of single channels recorded from L2 cells treated with vehicle (n = 21) or a combination of E2 and progesterone (P4) (n = 10) at concentrations similar to those observed during proestrus, E2 alone (n = 18), or P4 (n = 11) alone. A: representative single channel recordings are shown for each treatment group. HSC, highly selective channels; NSC, nonselective channels. B: exposure to combined E2–P4 significantly increased ENaC fNPo compared with vehicle. C: E2 alone increased ENaC fNPo, whereas P4 did not compared with vehicle. To determine the mechanism of E2's activation of ENaC in alveolar cells, we calculated the channel open probability (Po, D) and mean no. of channels in a patch (N, E) only for patches with observable channel openings and closings (vehicle n = 13 and E2 n = 17). E2 significantly increased ENaC Po compared with vehicle. F: the histogram shows the distribution of N detected in individual patches from cells treated overnight with vehicle (black bars) or E2 (gray bars). A N = 0 denotes a patch without observable channel openings or closures. E2 shifted the graph to the right. Data are displayed as means ± SE. *P < 0.05 vs. vehicle as determined by two-tailed t-test, one-way ANOVA with Student-Newman-Keuls post hoc test, or Mann-Whitney rank sum test.
Because investigators previously showed that P4 decreased ENaC Po when expressed in Xenopus oocytes (44), we hypothesized that E2 and P4 might have different effects on channel activity. Therefore, we next examined ENaC single channel activity in response to overnight exposure to 0.73 nM E2 or 64 nM P4, separately. Single channel recordings from patches of E2-treated cells showed a significantly greater sodium channel fNPo compared with those from vehicle-treated cells (Fig. 1C). There was no difference between P4 and vehicle-treated cells. We next assessed the effect of E2 on Po and N separately (Fig. 1, D and E, respectively). For these analyses, we only used patches with observable channel openings and closures, since we cannot tell if a patch with no distinct events occurs as a result of there being no channel in the patch or channels with a Po of zero. E2 significantly increased Po in patches with channel activity compared with vehicle. In contrast, E2 did not significantly affect N in those patches. However, we did observe that the fraction (f) of patches exhibiting channel activity was 94% in patches from cells treated with E2 overnight, whereas only 62 and 55% of patches from vehicle- or P4-treated cells exhibited channel activity, respectively (Table 1). Also, the distribution of the number of levels (channels) per patch shifted to the right (i.e., greater numbers of channels/patch) in the E2-treated group compared with vehicle (Fig. 1F). The data shown in Table 1 and Fig. 1F suggest that E2 increased the number of channels at the apical plasma membrane.
E2 selectively activated nonselective ENaC.
We next investigated whether the E2 effect on ENaC was specific to either the HSC or NSC. From current-voltage relationships, we calculated the conductance for channels present in all patches from the L2 cells. As shown in the representative recordings and graphs of the current-voltage relationships in Fig. 2A, there existed two predominant channel types: one with a small conductance (mean conductance of 6.4 ± 0.55 pS for all channels) and one with a large conductance (21 ± 1.7 pS for all channels). These results are consistent with those previously reported for the highly selective and nonselective ENaC present in alveolar cells (12). Using combined analyses of amplitude histograms and standard single channel analyses using ClampFit, we calculated NPo for HSCs and NSCs separately from cell-attached patches of vehicle- and E2-treated cells. Representative amplitude histograms are shown in Fig. 2B. Channel activity is low in the vehicle-treated groups as demonstrated by the small peak shown for the HSC in the amplitude histograms. NSCs exhibited a similar level of activity in vehicle-treated cells. However, with E2 treatment, activity of the NSC in particular increased as demonstrated by the larger peak in the E2 graph. Indeed, the mean NPo was significantly greater for the NSC after overnight exposure to E2 compared with vehicle (Fig. 2C). One data value was removed from the HSC-E2 group because of verification as an outlier by the Grubb's test. Table 2 shows the average current amplitudes, N, and Po of HSCs and NSCs from vehicle and E2-treated cells. E2 slightly increased Po of HSCs and dramatically for the NSCs compared with vehicle.
Table 2.
Properties of ENaC channels in vehicle and E2-treated L2 cells
| Channel | Treatment | No. of Patches | Current Amplitude (pA) at 0 mV | N | Po |
|---|---|---|---|---|---|
| HSC | Vehicle | 10 | 0.26 ± 0.037 | 1.3 ± 0.15 | 0.0089 ± 0.0032 |
| E2 | 12 | 0.28 ± 0.028 | 1.3 ± 0.18 | 0.029 ± 0.0081A | |
| NSC | Vehicle | 8 | 0.66 ± 0.061 | 1.0 ± 0.00 | 0.019 ± 0.018 |
| E2 | 8 | 0.83 ± 0.085 | 1.5 ± 0.38 | 0.11 ± 0.053A |
Data shown as means ± SE. N, no. of channels; Po, open probability; HSC, highly selective channels; NSC, nonselective channels.
P < 0.05 vs. vehicle of same channel type by Mann-Whitney Rank Sum Test.
E2 did not affect gene expression but increased apical localization of αENaC.
Steroid hormones are most well-known for binding to cytosolic steroid receptors that translocate to the nucleus, bind hormone-responsive elements, and induce/repress transcription. Therefore, it is possible that E2, through a transcriptional mechanism, increased ENaC subunit mRNA and protein expression levels, resulting in a shift in the number of patches with observable channel activity. Because we detected greater activity of only the NSC with E2 treatment, we hypothesized that an effect of E2 on ENaC expression would be exclusive to αENaC because it is required for the NSC (29). To first assess this hypothesis, we examined whether overnight treatment with E2-P4 altered mRNA of αENaC in L2 cells using real-time PCR. We detected no dramatic differences in αENaC gene expression (mRNA) between vehicle (n = 3, dCt 14.8 ± 0.162 SD) and E2- and P4-treated (n = 3, dCt 14.5 ± 0.103 SD) cells.
To follow up these experiments, we determined if E2 changed αENaC protein expression or altered subcellular distribution of the subunit in L2 cells. We first characterized the newly generated αENaC antibody using antigen competition experiments. As shown in Fig. 3A, antigen competition demonstrated specificity of the antibody in whole cell lysates to proteins at ∼75 and 60 kDa, corresponding to full-length and cleaved αENaC, respectively. In cell surface lysates, the antigen competition experiments resolved specificity of the antibody to an ∼95-kDa band consistent with the full-length, glycosylated form of αENaC and to an ∼60-kDa band consistent with a known cleavage product of αENaC. To confirm specificity by an additional method, we reduced αENaC expression using silencing vectors. Knockdown of αENaC with shRNA eliminated or reduced the intensity of both the 75- and 60-kDa bands in whole cell lysates and of both the 95- and 60-kDa bands in cell surface lysates (Fig. 3B). The shRNA knockdown of αENaC produced a smaller size band detected by the antibody that is likely a result of translational repression, a common occurrence with RNA interference (65).
Fig. 3.
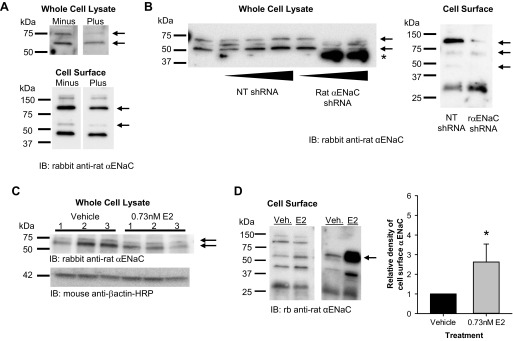
E2 increased cell surface abundance of cleaved αENaC in L2 cells. A: we first assessed the specificity of the αENaC antibody to proteins of interest in whole cell and cell surface lysates from L2 cells using antigen competition. In whole cell lysates, the antibody detected ∼75- and 60-kDa bands (−), both of which were decreased by the presence of the immunizing antigen (+). For cell surface lysates, the antibody detected 150-, 95-, 60-, and 47-kDa bands (−). The ∼95- and 60-kDa bands decreased in intensity in the presence of the immunizing antigen (+). B: to further confirm the specificity of the antibody, we transfected cells with a control nontargeting (NT) short-hairpin RNA (shRNA) or a rat αENaC shRNA to knock down protein levels of αENaC specifically. The αENaC shRNA eliminated the 75-kDa band and reduced the 60-kDa band in whole cell lysates and reduced the 95- and 60-kDa bands in cell surface extracts. C: we next examined the total protein abundance of αENaC in whole cell lysates from L2 cells treated with vehicle or E2 overnight. There were no significant differences in the 75- or 60-kDa bands. D: using cell surface biotinylation, we examined the apical plasma membrane abundance of αENaC in L2 cells treated with vehicle (Veh) or E2 overnight. Representative blots are shown with an arrow that denotes the band of interest. Densitometry revealed a significant increase in the density of the ∼60-kDa band of αENaC in E2-treated groups compared with vehicle (n = 5). Data are shown as means ± SE. *P < 0.05 vs. vehicle by z-test.
Using the validated antibody, we assessed the effect of E2 on αENaC expression in whole cell lysates from L2 cells. There were no significant differences in the levels of the 75- or 60-kDa αENaC bands in E2-treated L2 cells (Fig. 3C). Taken together with the mRNA data, these results indicate that, at physiological levels, E2 does not considerably affect expression of αENaC in alveolar cells.
In addition to their transcriptional effects, steroid hormones also rapidly activate signaling cascades involving protein kinases, secondary messengers, and transactivation of other receptors that affect trafficking of proteins to/from the plasma membrane (24). The steroid hormone, aldosterone, which is known to stimulate ENaC activity, both increases αENaC transcription and, through rapid signaling events, promotes greater apical membrane abundance of ENaC subunits (13). Thus, in the next experiment, we examined whether E2, like aldosterone, increases apical membrane localization of αENaC. Using cell surface biotinylation, we examined apical membrane abundance of αENaC in L2 cells after overnight exposure to vehicle or E2. We observed much greater apical membrane localization of the 60-kDa αENaC cleavage product in E2-treated cells compared with vehicle (Fig. 3D). These results are consistent with a predominant effect of E2 on αENaC localization and the nonselective ENaC channel.
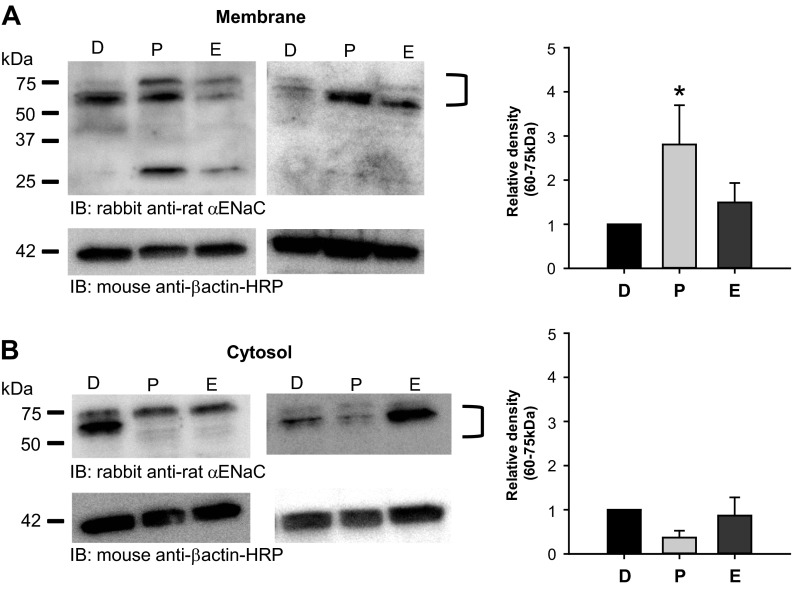
Membrane αENaC abundance is greatest in the female rat lung during proestrus.
There are three stages of the rodent estrous cycle: diestrus, proestrus, and estrus (22). The total cycle length is 4–5 days long with typically 2 days of diestrus followed by 1 day each of proestrus and estrus. The proestrous phase of the rodent cycle corresponds to the follicular phase of the human female, in which circulating E2 levels are maximal (0.37–0.73 nM in rodents). During diestrus and estrus, E2 levels are considerably less (0.073–0.15 nM in rodents). To assess if E2 altered αENaC membrane abundance in vivo, we compared the abundance of αENaC membrane protein from the lungs of female rats at the diestrous, proestrous, and estrous phases of the estrous cycle. We detected ∼75-, 60-, and 30-kDa bands for αENaC in rat lung protein samples consistent with the full-length, nonglycosylated form of rat αENaC (predicted 77 kDa full length) along with two distinct cleavage products described previously in the literature. We also identified these cleavage products in cell surface samples from the L2 rat alveolar cells. Membrane αENaC levels (75- and 60-kDa band) in the lung appeared greatest in female rats during proestrus and, possibly, estrus compared with diestrus (Fig. 4A). Densitometry revealed that the membrane abundance of the 75- and 60-kDa αENaC proteins was almost three times greater in lungs from proestrous rats compared with those from diestrous rats. In addition, cytosolic abundance of αENaC decreased in lungs from proestrous rats, although this effect was not significant compared with those in diestrus (Fig. 4B). These data are consistent with an effect of E2 on alveolar αENaC membrane localization in vivo.
Fig. 4.
Membrane αENaC protein levels are greatest in the female rat lung during proestrus. A: representative blots of αENaC membrane protein (hydrophobic fraction) from lungs of female rats at the diestrous (D), proestrous (P), and estrous (E) stages of the estrous cycle are shown. Densitometry was performed to measure the relative difference in combined intensity of the ∼60 and 75-kDa bands detected for membrane αENaC between diestrus (n = 5) (set to 1) and proestrus (n = 6) or estrus (n = 5). The relative density of membrane αENaC was ∼3 times greater in rat lungs during proestrus compared with diestrus. B: representative blots of αENaC cytosolic protein (hydrophilic fraction) from lungs of female rats at the diestrous, proestrous, and estrous stages of the estrous cycle are shown. Densitometry was performed to measure the relative difference in combined intensity of the ∼60- and 75-kDa bands detected for cytosolic αENaC between diestrus (n = 3) (set to 1) and proestrus (n = 3) or estrus (n = 3). There were no significant differences between the groups. All data points are shown as means ± SE. *P < 0.05 vs. diestrus as determined by z-test.
E2 rapidly activated ENaC through the Gper.
Our observation that E2 significantly increased ENaC Po suggested that E2 has a direct effect on channel gating. The nongenomic effects of steroid hormones on channel gating occur rapidly (within minutes) (15, 24, 51), so we tested this hypothesis by assessing the rapid effects of E2 on ENaC activity in L2 cells. We recorded single sodium channel events in response to an acute application of E2. Representative recordings are shown in Fig. 5A. Within ∼5–10 min after application of E2, ENaC NPo increased significantly from baseline activity (Fig. 5B). These data confirm a rapid effect of E2 on ENaC single channel function.
Fig. 5.
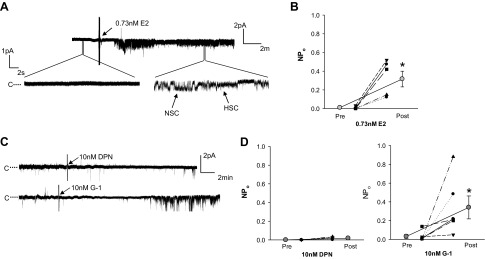
E2 rapidly stimulated ENaC NPo in alveolar cells through G protein-coupled estrogen receptor (Gper). To determine if and how E2 exhibited rapid effects on ENaC activity, we measured ENaC single channel activity immediately preceding (Pre) and after application of E2 or the estrogen receptor agonists, 2,3-bis(4-hydroxyphenyl)propionitrile (DPN) and rel-1-[4-(6-bromo-1,3-benzodioxol-5-yl)-3aR,4S,5,9bS-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G-1) (Post). Black symbols represent data from an individual patch before and after treatment, connected by a dotted line. Gray circles represent the mean ± SE of these points pre- and posttreatment, connected by a solid line. A: a representative recording of channel activity before and after E2 treatment is shown. B: E2 significantly increased ENaC NPo within minutes after application to the bath. C: representative single channel recordings are shown from L2 cells before and after treatment with 10 nM DPN (ERβ agonist) or 10 nM G-1 (Gper agonist). D: NPo did not change with application of DPN (n = 4) but was significantly increased with G-1 (n = 6) treatment. *P < 0.05 vs. Pre as determined by one-sided paired t-test.
E2, like other steroid hormones, acts through binding and activation of steroid hormone receptors, of which there exist three well-known estrogen receptors: ERα, ERβ, and Gper (15, 62). Both ERβ and Gper are expressed in the lung (3, 7, 57). To determine if either of these receptors mediated the rapid effect of E2 to increase ENaC NPo, we performed cell-attached patch clamp in L2 cells and recorded single channel activity before and after application of the ERβ agonist, DPN, or the Gper agonist, G-1. Representative recordings are shown in Fig. 5C. DPN did not significantly affect channel NPo (Fig. 5D). In contrast, within 10–15 min, G-1 significantly increased ENaC NPo from a baseline of 0.0316 ± 0.0217 to 0.341 ± 0.122, an effect comparable to that observed for E2.
Estrogen receptors alter cell surface abundance of full-length and cleaved αENaC.
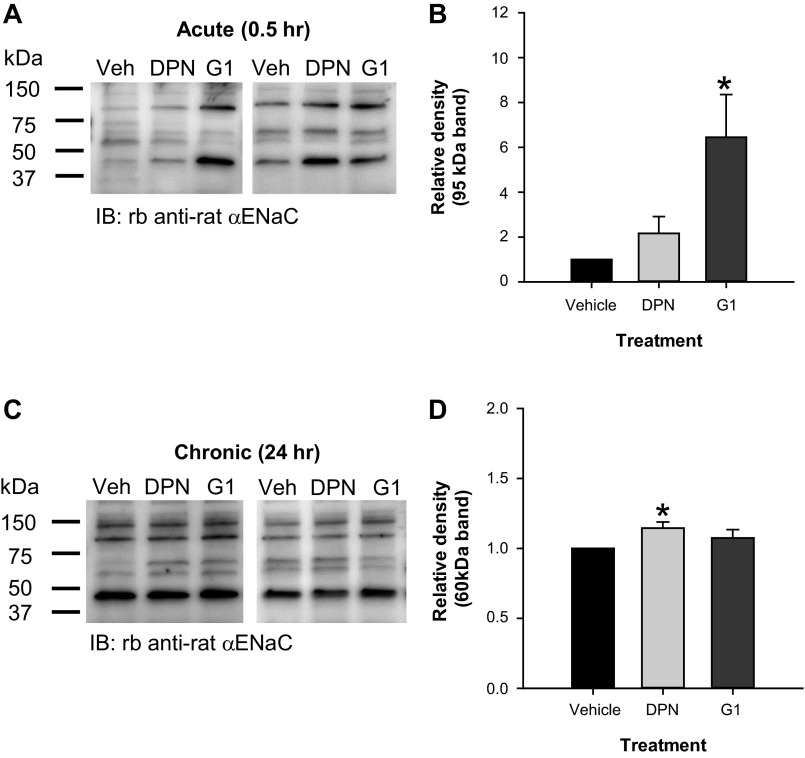
To determine the mechanisms of the acute and chronic effect of E2 on ENaC activity, we examined differences in cell surface abundance of full-length and cleaved αENaC in L2 cells treated for 30 min or overnight with vehicle, 2 nM DPN, or 10 nM G-1. Using cell surface biotinylation, we observed differences in cell surface αENaC abundance within 30 min after treatment with G-1 compared with DPN or vehicle. A representative blot is shown in Fig. 6A. Densitometric analysis revealed that 30 min application of G-1 increased cell surface abundance of the 95-kDa band detected for rat αENaC (Fig. 6B). These data are consistent with our observation that G-1 rapidly induced ENaC activity in L2 cells and suggest that the mechanism involves changes in αENaC membrane localization.
Fig. 6.
Estrogen receptor agonists, DPN and G-1, exhibited time-dependent effects on cell surface abundance of αENaC in L2 cells. Using cell surface biotinylation, we examined the cell surface abundance of αENaC in L2 cells treated with vehicle, 2 nM DPN, or 10 nM G-1 for 30 min (acute) or 24 h (chronic). A: representative blots for the acute experiments are shown. Densitometry was performed to detect relative differences in cleavage products of αENaC at the cell surface (n = 4). B: data for the relative density of the 95-kDa band are shown as means ± SE with vehicle set to 1. G-1 significantly increased the intensity of the 95-kDa band at the apical membrane. C: representative blots for the chronic experiments are shown. Densitometry was performed to detect relative differences in cleavage products of αENaC at the cell surface (n = 4–5). D: data for the relative density of the 60-kDa band are shown as means ± SE with vehicle set to 1. DPN significantly increased apical membrane abundance of the 60-kDa band after 24 h. Differences were analyzed by one-sided z-test. *P < 0.05 vs. vehicle.
Our previous results showed between a two- and threefold increase in the 60-kDa cleavage product of αENaC at the cell surface in L2 cells treated overnight with E2. Thus, we examined whether the effect of E2 originated via ERβ or Gper. Overnight treatment with 2 nM DPN or 10 nM G-1 did not appear to have a dramatic effect on αENaC cell surface abundance (Fig. 6C). However, there was a modest but significant increase in the relative density of the 60-kDa band with DPN treatment (Fig. 6D), suggesting that ERβ signaling also affects ENaC apical localization with chronic exposure to E2.
DISCUSSION
Several studies now indicate that sex steroid hormones alter ENaC expression and/or activity in several tissues, including the lung. In this study, we showed that the female sex hormone, β-estradiol (E2), increases ENaC NPo when rat alveolar cells are exposed to the hormone chronically (overnight) or acutely (within minutes) and that E2 primarily regulates the activity of the nonselective ENaC channel. We further demonstrated that chronic E2 exposure produces an increase in channel density and Po through what appears to be largely a result of greater membrane abundance of the mature form of αENaC, the ∼60-kDa cleavage product. Consistent with this effect, we observed approximately three times greater membrane abundance of full-length and cleaved αENaC in lung homogenates from female rats in proestrus compared with those in diestrus, suggesting a role of E2 in vivo. Moreover, we discovered that the estrogen receptor, Gper, mediates the acute effect of E2 on ENaC activity and rapidly increases αENaC cell surface levels. The more chronic effect of E2 to alter αENaC cell surface abundance at least partially involves ERβ. The results presented herein demonstrate for the first time that E2 specifically increases alveolar ENaC activity and membrane abundance both in vitro and in vivo through the estrogen receptor, Gper. Overall, these results strongly support a role of ENaC in the sex differences observed in many pulmonary diseases.
In 1998, Sweezey and colleagues showed that lung mRNA expression of αENaC was greater in female rats compared with males and that combined E2 and P4 (not individual) treatment of alveolar epithelial cells increased amiloride-sensitive short-circuit current (60). Over the next decade, sex steroid regulation of alveolar ENaC was largely unstudied. However, several papers were published regarding sex differences and sex hormone regulation of ENaC in the kidney (1, 19, 25, 30, 52). In the past year, two groups published data showing that female sex hormones (E2 and P4 in combination) increase amiloride-sensitive current and the number of responsive patches in lung epithelial cells (34) and that female rats exhibit greater amiloride-sensitive AFC than males (33). Neither of these studies addressed the role of E2 and P4 separately, and the in vitro study used supraphysiological concentrations of these sex hormones that led to questions about the physiological role of these hormones to alter ENaC activity. Our results show, for the first time, that, at physiological relevant concentrations, the sex hormone responsible for enhanced ENaC activity in the female lung is E2, not P4, and that this effect involves mechanisms outside of the traditional role of steroid hormones to regulate transcription.
There exist several possibilities for the mechanism of E2's effect on alveolar ENaC. Steroid hormones are commonly assumed to act through a transcriptional mechanism mediated by cytosolic hormone receptors; despite this assumption, much literature now exists showing rapid, nongenomic actions of steroid hormones. Our results suggest that E2 stimulates ENaC activity through a nongenomic mechanism because we observed no effect of E2 on αENaC gene expression or total protein levels.
For both their genomic and nongenomic effects, estrogens are most well-known to act through the estrogen receptors, ERα and ERβ. Reports indicate that the fetal and adult lung only express ERβ (7, 57). However, in 2005, Revankar and colleagues described a novel estrogen receptor, GPR30 (51). The investigators described this receptor as a transmembrane G protein-coupled estrogen receptor (also known as Gper) predominantly found in the endoplasmic reticulum. Previous to that discovery, other investigators cloned the Gper gene from rat lung and originally named it GPR41 (3). Since that time, Gper in the lung has largely been left unstudied other than in lung tumors (56). For a review of the role of Gper in cancer and normal renal/vascular physiology, please refer to the article by Filardo and Thomas (15).
Our results indicate that the acute effect of E2 on ENaC activity is predominately mediated through Gper. This effect likely occurs either as a result of insertion of new channels in the plasma membrane or decreased retrieval of old channels out of the plasma membrane as indicated by increased cell surface localization of αENaC within 0.5 h after treatment with the Gper agonist, G-1. Gper's regulation of ENaC plasma membrane insertion and/or retrieval is a completely novel mechanism of regulation of ENaC. Future studies should address the intracellular mechanisms (e.g., cAMP/PKA pathway) that mediate this effect and whether it is specific to females or also occurs in males.
Our results also demonstrate that E2 predominantly activates the nonselective ENaC present in L2 alveolar cells. Previous studies from our laboratory demonstrated that the αENaC subunit is required for activity of the NSC in isolated alveolar type II cells (29). Thus, our result showing that E2 specifically increases αENaC membrane abundance in L2 cells and in lungs from proestrous rats supports our conclusion that E2 selectively increases NSC activity in type II alveolar cells. However, NSCs are present in both alveolar type I and type II cells (12), and the L2 cell line used in this study predominantly has an alveolar type II-like phenotype (10). Thus, future studies should focus on whether E2 activates NSCs and maybe even HSCs in type I alveolar cells.
Our results demonstrate that E2 and the estrogen receptor agonists affected cell surface abundance of different forms of the αENaC subunit. Since 2003, there have been several publications reporting different cleavage products for αENaC as well as for the β- and γENaC subunits (11, 14, 17, 18, 20, 26–28, 48, 61). Most of these studies focused on the different products present in kidney tissue. However, a few addressed the products in lung tissues (11, 20, 61). Based on these studies, we conclude that the specific cleavage products detected depend upon the specific tissue (cells and organs), species, glycosylation, and the antibody epitope used in the experiments.
The antibody used will have a significant effect on the bands detected for αENaC because of the presence of protease cleavage sites in the extracellular loop of the protein. Indeed, furin cleavage sites exist immediately adjacent to the NH2-terminal side of the epitope of the antibody that we designed. Hughey and colleagues previously demonstrated that, at the plasma membrane, the protease furin cleaves the full-length glycosylated form of αENaC (“immature”) with a molecular mass of 95 kDa (26, 27). This results in the production of 65- and 30-kDa bands that correspond to the “mature” or more active form of the protein. In contrast to the other cited studies, Hughey and colleagues used epitope tags of the NH2- and COOH-termini to isolate the individual cleavage products of the subunit, making nonspecific antibody effects unlikely. Ergonul and colleagues also detected these bands in rat kidney and found that aldosterone regulated the levels of the glycosylated (∼85 kDa) and the cleaved product (30 kDa) of αENaC (33).
Consistent with both of those observations, we detected ∼95- and 60-kDa bands for αENaC at the apical plasma membrane of L2 rat alveolar cells. In addition to these bands, we also detected a band of ∼47 kDa for αENaC at the plasma membrane of L2 cells. No other publication has explicitly described the presence of this cleavage product for αENaC. However, antigen preadsorption of our antibody reduced the intensity of this band, and this product increased with G-1 treatment of the L2 cells along with ENaC activity. Future studies should address the identity of this band and whether it represents a specific cleavage product of αENaC.
Investigators examining αENaC in the lung detected a 75-kDa version of αENaC that likely corresponds to the nonglycosylated form of the protein, a doublet at 60 and 65 kDa, and a 30-kDa cleavage product (9, 16, 61). We detected both the 75- and ∼60-kDa bands in rat lung membrane samples. We do not yet know whether the 30-kDa band detected in the rat lung membrane homogenates is a specific cleavage product of ENaC. However, there exist several potential trypsin cleavage sites within our antibody epitope, many of which would explain the presence of the 30-kDa band and even the 47-kDa band present in L2 cells. In addition, results from several of the publications above show the presence of a 30-kDa band for αENaC in the kidney and lung. Taken all together, these publications and our results suggest that the 30-kDa band is a specific αENaC cleavage product. Although the role of glycosylation on ENaC function is not clear, it is clear from previous studies that proteases regulate the function of ENaC in the plasma membrane. In effect, the cleavage products of αENaC have significant meaning with respect to activity of the channel.
Our observations that E2 activates alveolar ENaC activity may have implications in diseases like cystic fibrosis and influenza. After the onset of puberty, female patients exhibit more severe symptoms and a worse prognosis than males for both of these diseases. Cystic fibrosis is a genetic disease in which affected individuals have an inactivating mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) leading to both respiratory and digestive disorders. Many studies have provided evidence that CFTR controls AFC through its negative regulation of sodium and fluid reabsorption by the ENaC in alveolar cells (35, 37, 41, 45, 49, 54, 69). Thus inactivation of CFTR in cystic fibrosis leads to dehydrated airways that produce thick mucus with resulting persistent cough and greater chance of infection. In light of our data showing that E2 increases alveolar ENaC activity, we speculate that the worsened symptoms present in females with cystic fibrosis relate to an additional increase in alveolar ENaC activity that correlates with circulating E2 levels. Whether female cystic fibrosis patients exhibit worse symptoms during certain stages of the menstrual cycle and whether certain types of hormonal contraceptives worsen or improve these symptoms would be quite interesting and important to examine.
In contrast to cystic fibrosis, influenza infection decreases AFC in mice (23, 67) and directly inhibits activity of ENaC in alveolar cells (8). Interestingly, human and rodent females exhibit worsened prognosis with influenza infection (31, 32, 53, 55, 67a) and, at least in humans, these sex differences in mortality and morbidity relate to influenza-associated pneumonia. In addition, influenza causes a state of constant diestrus in female rodents resulting in low circulating levels of E2, and treatment of female rodents with E2 reduces the rate of influenza-associated mortality (53). Taken together, we propose a double-hit hypothesis in which influenza directly inhibits alveolar ENaC activity and reduces circulating E2 levels. This, in turn, produces a drastic reduction in AFC in females. We also propose that this is the mechanism of influenza-associated pneumonia and the observed greater mortality rate in females. However, this theory remains to be tested.
In conclusion, herein we demonstrated that the female sex steroid, E2, activates alveolar ENaC, and more specifically nonselective ENaC, through both an increase in channel Po and density of the αENaC subunit at the apical membrane of rat alveolar cells. Our results also show that this effect likely occurs in a time-dependent fashion via two distinct estrogen receptors, Gper and ERβ. Future investigation should focus on further understanding the intracellular mechanisms (e.g., Gper, ERβ, cAMP/PKA, etc.) by which E2 changes ENaC activity and on the applicability of these findings to treat pulmonary diseases that disproportionately affect females, with particular emphasis on cystic fibrosis and influenza.
GRANTS
M. M. Greenlee received training funds from National Institutes of Health Grants 5K12-GM-000680-12 and 5T32-HL-076118-08. D. C. Eaton received grant funding from NIH Grants 5R37-DK-037963-25 and 5R01-HL-063306-09.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.M.G., B.J.D., G.N.N., and D.C.E. conception and design of research; M.M.G., J.D.M., L.Y., Q.Y., B.J.D., and C.S.H. performed experiments; M.M.G., J.D.M., and D.C.E. analyzed data; M.M.G., J.D.M., G.N.N., and D.C.E. interpreted results of experiments; M.M.G. prepared figures; M.M.G. drafted manuscript; M.M.G., J.D.M., L.Y., B.J.D., C.S.H., and D.C.E. edited and revised manuscript; M.M.G., J.D.M., and D.C.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bernice B. Gallego and Otor Al-Khalili for technical assistance.
REFERENCES
- 1.Arias-Loza PA, Muehlfelder M, Elmore SA, Maronpot R, Hu K, Blode H, Hegele-Hartung C, Fritzemeier KH, Ertl G, Pelzer T. Differential effects of 17beta-estradiol and of synthetic progestins on aldosterone-salt-induced kidney disease. Toxicol Pathol 37: 969–982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastarache JA, Ong T, Matthay MA, Ware LB. Alveolar fluid clearance is faster in women with acute lung injury compared to men. J Crit Care 26: 249–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonini JA, Anderson SM, Steiner DF. Molecular cloning and tissue expression of a novel orphan G protein-coupled receptor from rat lung. Biochem Biophys Res Commun 234: 190–193, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4: Appendix 4I, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol 293: L272–L278, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Carvalho O, Goncalves C. Expression of oestrogen receptors in foetal lung tissue of mice. Anat Histol Embryol 41: 1–6, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Chen XJ, Seth S, Yue G, Kamat P, Compans RW, Guidot D, Brown LA, Eaton DC, Jain L. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol 287: L366–L373, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302: L1141–L1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas WH, Chapple PJ. Characterization of monolayer cultures of type II alveolar pneumonocytes that produce pulmonary surfactant in vitro. Dev Biol Stand 37: 71–76, 1976 [PubMed] [Google Scholar]
- 11.Downs CA, Kriener LH, Yu L, Eaton DC, Jain L, Helms MN. beta-Adrenergic agonists differentially regulate highly selective and nonselective epithelial sodium channels to promote alveolar fluid clearance in vivo. Am J Physiol Lung Cell Mol Physiol 302: L1167–L1178, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol 71: 403–423, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Eaton DC, Malik B, Saxena NC, Al-Khalili OK, Yue G. Mechanisms of aldosterone's action on epithelial Na+ transport. J Membr Biol 184: 313–319, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153: 2953–2962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores A, Velasco J, Gallegos AI, Mendoza FD, Everardo PM, Cruz ME, Dominguez R. Acute effects of unilateral sectioning the superior ovarian nerve of rats with unilateral ovariectomy on ovarian hormones (progesterone, testosterone and estradiol) levels vary during the estrous cycle (Abstract). Reprod Biol Endocrinol 9: 34, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frindt G, Ergonul Z, Palmer LG. Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frindt G, Palmer LG. Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium. Am J Physiol Renal Physiol 297: F1249–F1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambling L, Dunford S, Wilson CA, McArdle HJ, Baines DL. Estrogen and progesterone regulate alpha, beta, and gammaENaC subunit mRNA levels in female rat kidney. Kidney Int 65: 1774–1781, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Caballero A, Rasmussen JE, Gaillard E, Watson MJ, Olsen JC, Donaldson SH, Stutts MJ, Tarran R. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA 106: 11412–11417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannelli P, Di Donato M, Giraldi T, Migliaccio A, Castoria G, Auricchio F. Targeting rapid action of sex-steroid receptors in breast and prostate cancers. Front Biosci (Elite Ed) 4: 453–461, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80: 84–97, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Gu X, Li P, Liu H, Li N, Li S, Sakuma T. The effect of influenza virus A on th1/th2 balance and alveolar fluid clearance in pregnant rats. Exp Lung Res 37: 445–451, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology 152: 4489–4495, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heo NJ, Son MJ, Lee JW, Jung JY, Kim S, Oh YK, Na KY, Yoon HJ, Joo KW, Han JS. Effect of estradiol on the expression of renal sodium transporters in rats. Climacteric 16: 265–273, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279: 48491–48494, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 280: L646–L658, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Kienitz T, Allolio B, Strasburger CJ, Quinkler M. Sex-specific regulation of ENaC and androgen receptor in female rat kidney. Horm Metab Res 41: 356–362, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Klein SL, Hodgson A, Robinson DP. Mechanisms of sex disparities in influenza pathogenesis. J Leukoc Biol 92: 67–73, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein SL, Passaretti C, Anker M, Olukoya P, Pekosz A. The impact of sex, gender and pregnancy on 2009 H1N1 disease (Abstract). Biol Sex Differ 1: 5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kooijman EE, Kuzenko SR, Gong D, Best MD, Folkesson HG. Phosphatidylinositol 4,5-bisphosphate stimulates alveolar epithelial fluid clearance in male and female adult rats. Am J Physiol Lung Cell Mol Physiol 301: L804–L811, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Laube M, Kuppers E, Thome UH. Modulation of sodium transport in alveolar epithelial cells by estradiol and progesterone. Pediatr Res 69: 200–205, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SY, Oh SJ, Yun KU, Kim HM, Kim BH, Lee K, Kim SK. Expression of hepatic and ovarian cytochrome P450 during estrous cycle in rats. Arch Toxicol 86: 75–85, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Leroy C, Prive A, Bourret JC, Berthiaume Y, Ferraro P, Brochiero E. Regulation of ENaC and CFTR expression with K+ channel modulators and effect on fluid absorption across alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L1207–L1219, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Li T, Folkesson HG. RNA interference for alpha-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol 290: L649–L660, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Duke BJ, Malik B, Yue Q, Eaton DC. Biphasic regulation of ENaC by TGF-α and EGF in renal epithelial cells. Am J Physiol Renal Physiol 296: F1417–F1427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Fontana CM, Maselli ME, de Di Nasso FE, Telleria CM, Caron RW. Regulation of prolactin secretion during the estrus in rats: possible role of glucocorticoids. Reproduction 142: 477–485, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Lu C, Jiang C, Pribanic S, Rotin D. CFTR stabilizes ENaC at the plasma membrane. J Cyst Fibros 6: 419–422, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Malik B, Schlanger L, Al-Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC. Enac degradation in A6 cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Malik B, Yue Q, Yue G, Chen XJ, Price SR, Mitch WE, Eaton DC. Role of Nedd4–2 and polyubiquitination in epithelial sodium channel degradation in untransfected renal A6 cells expressing endogenous ENaC subunits. Am J Physiol Renal Physiol 289: F107–F116, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Michlig S, Harris M, Loffing J, Rossier BC, Firsov D. Progesterone down-regulates the open probability of the amiloride-sensitive epithelial sodium channel via a Nedd4–2-dependent mechanism. J Biol Chem 280: 38264–38270, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, Balagani R, Traver G, Sznajder JI, Factor P. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res 96: 999–1005, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Niisato N, Ohta M, Eaton DC, Marunaka Y. Hypotonic stress upregulates beta- and gamma-ENaC expression through suppression of ERK by inducing MKP-1. Am J Physiol Renal Physiol 303: F240–F252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olesen HV, Pressler T, Hjelte L, Mared L, Lindblad A, Knudsen PK, Laerum BN, Johannesson M. Gender differences in the Scandinavian cystic fibrosis population. Pediatr Pulmonol 45: 959–965, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Picard N, Eladari D, El Moghrabi S, Planes C, Bourgeois S, Houillier P, Wang Q, Burnier M, Deschenes G, Knepper MA, Meneton P, Chambrey R. Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem 283: 4602–4611, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Qadri YJ, Cormet-Boyaka E, Benos DJ, Berdiev BK. CFTR regulation of epithelial sodium channel. Methods Mol Biol 742: 35–50, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Quadros PS, Wagner CK. Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology 149: 3054–3061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Riazi S, Maric C, Ecelbarger CA. 17-beta Estradiol attenuates streptozotocin-induced diabetes and regulates the expression of renal sodium transporters. Kidney Int 69: 471–480, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubenstein RC, Lockwood SR, Lide E, Bauer R, Suaud L, Grumbach Y. Regulation of endogenous ENaC functional expression by CFTR and DeltaF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L88–L101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serfling RE, Sherman IL, Houseworth WJ. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. Am J Epidemiol 86: 433–441, 1967 [DOI] [PubMed] [Google Scholar]
- 56.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol 36: 524–531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simoes DC, Psarra AM, Mauad T, Pantou I, Roussos C, Sekeris CE, Gratziou C. Glucocorticoid and estrogen receptors are reduced in mitochondria of lung epithelial cells in asthma. PLoS One 7: e39183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stephenson A, Hux J, Tullis E, Austin PC, Corey M, Ray J. Higher risk of hospitalization among females with cystic fibrosis. J Cyst Fibros 10: 93–99, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 275: 25760–25765, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Sweezey N, Tchepichev S, Gagnon S, Fertuck K, O'Brodovich H. Female gender hormones regulate mRNA levels and function of the rat lung epithelial Na channel. Am J Physiol Cell Physiol 274: C379–C386, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Tan CD, Selvanathar IA, Baines DL. Cleavage of endogenous gammaENaC and elevated abundance of alphaENaC are associated with increased Na(+) transport in response to apical fluid volume expansion in human H441 airway epithelial cells. Pflugers Arch 462: 431–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 11: 597–608, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 33: 1–47, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood. Horm Behav 59: 353–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys 42: 217–239, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wisel MS, Datta JK, Saxena RN. Changes in the levels of protein and steroid hormones in the plasma and steroid hormone receptors in the uterus of normal cycling guinea pigs. Steroids 56: 148–153, 1991 [DOI] [PubMed] [Google Scholar]
- 67.Wolk KE, Lazarowski ER, Traylor ZP, Yu EN, Jewell NA, Durbin RK, Durbin JE, Davis IC. Influenza A virus inhibits alveolar fluid clearance in BALB/c mice. Am J Respir Crit Care Med 178: 969–976, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67a.World Health Organization Update: WHO confirmed human cases of avian influenza A. (H5N1) infection, November 2003-May 2008. Wkly Epidemiol Rec 83: 415–420, 2008 [PubMed] [Google Scholar]
- 68.Zeitlin PL. Cystic fibrosis and estrogens: a perfect storm. J Clin Invest 118: 3841–3844, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Z, Duerr J, Johannesson B, Schubert SC, Treis D, Harm M, Graeber SY, Dalpke A, Schultz C, Mall MA. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros 10, Suppl 2: S172–S182, 2011 [DOI] [PubMed] [Google Scholar]