Abstract
We sought to investigate the effects of cockroach allergen (CRA) exposure on the lung macrophage population to determine how different macrophage phenotypes influence exacerbation of disease. CRA exposure caused significantly reduced expression of CD86 on lung macrophages. These effects were not systemic, as peritoneal macrophage CD86 expression was not altered. To investigate whether naïve macrophages could reduce asthma-like pulmonary inflammation, autologous peritoneal macrophages were instilled into the airways 24 h before the final CRA challenge. Pulmonary inflammation was assessed by measurement of airway hyperresponsiveness, mucin production, inflammatory cell recruitment, and cytokine production. Cell transfer did not have significant effects in control mice, nor did it affect pulmonary mucin production or airway hyperresponsiveness in control or CRA-exposed mice. However, there was significant reduction in the number of eosinophils recovered in the bronchoalveolar lavage (BAL) (5.8 × 105 vs. 0.88 × 105), and total cell recruitment to the airways of CRA-exposed mice was markedly reduced (1.1 × 106 vs. 0.57 × 106). The reduced eosinophil recruitment was reflected by substantially lower levels of eosinophil peroxidase in the lung and significantly lower concentrations of eotaxins in BAL (eotaxin 1: 3 pg/ml vs. undetectable; eotaxin 2: 2,383 vs. 131 pg/ml) and lung homogenate (eotaxin 1: 1,043 vs. 218 pg/ml; eotaxin 2: 10 vs. 1.5 ng/ml). We conclude that CRA decreases lung macrophage CD86 expression. Furthermore, supplementation of the lung cell population with peritoneal macrophages inhibits eosinophil recruitment, achieved through reduction of eotaxin production. These data demonstrate that transfer of naïve macrophages will reduce some aspects of asthma-like pulmonary inflammation in response to CRA.
Keywords: outbred, peritoneal, chemokine, neutrophil, mucin
asthma is one of the most common chronic inflammatory diseases in the world. It is characterized as reversible airway obstruction, inflammation, and hyperresponsiveness (AHR) (4). According to the Centers for Disease Control (CDC), there are ∼25 million asthma sufferers in the US alone, resulting in more than 3,000 deaths each year. Asthma-related health care costs are estimated at over $50 billion annually, and the prevalence is rising (33), demonstrating that asthma is a major public health problem.
The most widely used murine models for asthma use ovalbumin (OVA) as the sensitizing agent. These models typically use inbred or genetically modified mouse strains and require coadministration of adjuvants (usually alum) along with frequent high-dose exposures to achieve an asthma-like phenotype (25, 63). A more relevant model uses outbred mice exposed to cockroach allergen (CRA: a total body extract of the German cockroach Blatella Germanica) at a dose in the order of one hundred times lower than that used in many OVA models (29, 37, 59), including those that use a similar intratracheal (IT) instillation technique as used in this study (24). In the present model, all three exposures are via the physiologically relevant IT route, and, because CRA contains endogenous TLR ligands, such as LPS and chitin, there was no need for additional adjuvants. This, in addition to the fact that a significantly high percentage of children with asthma have a positive skin test reaction to CRA (49), makes a strong case for the relevance of this model in the study of human disease.
Many different types of immune cells have been implicated in asthma pathogenesis, including macrophages, eosinophils, lymphocytes, and mast cells (16). T cells and TH2-associated cytokines have been shown to play critical roles, as T cell-deficient mice do not develop lung inflammation and AHR (7), and the TH2 cytokines IL-4 and IL-13 are involved in many of the key features of asthma (60). In particular, IL-4Rα (which is required for both IL-4- and IL-13-mediated signaling) expression on macrophages, has a strong correlation with the severity of asthma parameters in an OVA model, including eosinophil influx (9). IL-4/13 stimulation of different types of cells (36, 53), including macrophages (58), has been shown to induce production of eotaxins 1 and 2, which are among the most important eosinophil chemoattractants (6, 18, 45).
Macrophages themselves are the most prevalent immune cell found in normal lung tissue and are involved in the functioning of both the innate and adaptive immune responses, forming an important bridge between the two (15, 30, 46). Several studies have shown that macrophages are involved in asthma disease progression, and their phenotype can be skewed toward an alternative activation pathway by IL-4 and IL-13, in contrast to the classical activation pathway induced by IFN-γ and LPS, among others (12). In addition, alternatively activated macrophages can themselves become potent sources of IL-4 (28, 48) and IL-13 (14, 22), thus providing a setting for chronic disease.
There is currently a lack of information on the effects of CRA exposure on the lung macrophage population. Based on the knowledge that, in our model, CRA induces significant pulmonary eosinophil infiltration, and that macrophage phenotypes are involved in recruitment of eosinophils during certain types of inflammation, the current study investigated the contribution of CRA-induced macrophage phenotypes in the exacerbation of disease.
MATERIALS AND METHODS
Animals.
Age-matched (9–12 wk old) female outbred HSD:ICR mice (Harlan Laboratories, Indianapolis, IN) were used in all experiments. Mice were housed in a dedicated temperature- and humidity-controlled room with 12-h:12-h light/dark cycles and had free access to food and water. All experimental protocols adhered to NIH guidelines and were reviewed and approved by the Institutional Animal Care and Use Committee at Boston University.
Allergen sensitization.
Lyophilized CRA was supplied by Greer Laboratories (Lenoir, NC) and diluted in an appropriate volume of sterile Hanks' balanced salt solution (HBSS). Mice were sensitized on day 0 with an IT instillation (as previously described; Ref. 56) of 50 μl containing CRA diluted 1:2 in sterile HBSS. Challenges on days 14 and 21 were carried out in exactly the same manner using a 1:4 dilution. In total, each mouse received ∼4 μg Blag1 and 2, and 1.2 μg LPS over the time course of the model. Control mice received 50 μl of sterile HBSS on equivalent days. The two groups of mice are referred to as HBSSx3 and CRAx3.
AHR.
Mice were placed in unrestrained whole body plethysmography chambers (Buxco Systems, Troy, NY) and allowed to acclimate. Baseline respiratory parameters were recorded for 5 min, before 2 min of exposure to aerosolized PBS or either 25 or 50 mg/ml methacholine (Sigma, St. Louis, MO), followed by a 5-min recording period, as previously described (54).
Sample collection.
Mice were killed at either 4 or 24 h following the final CRA challenge on day 21. The trachea was cannulated, and lungs were washed with 0.5 ml warm sterile HBSS multiple times for a total volume of 5 ml. The first 1 ml of fluid was centrifuged at 1,000 g for 5 min, and the cell-free supernatant was collected and retained for cytokine analysis. A Beckman-Coulter ZF particle counter (Coulter Electronics, Hialeah, FL) was used to obtain total cell counts. Then, 100,000 cells were spun onto a slide and stained with Diff-Quik (Siemens, Newark, DE), and a differential count under light microscopy was performed on 300 cells to identify the absolute numbers of different inflammatory cells.
The lungs were perfused with 2 ml sterile HBSS through the right ventricle, and the right lung was removed and placed in ice-cold protease inhibitor cocktail (Roche, Indianapolis, IN), containing 0.00005% Triton X-100 in PBS, before homogenization using a Brinkmann Polytron PT3000 (Kinematica, Bohemia, NY). The left lung was removed, and a portion was reserved for analysis by flow cytometry. The remainder was placed in 70% ethanol before preparation for histological analysis.
Mucin quantification.
Histology slides were stained by Periodic acid-Schiff (PAS) and quantified as previously described (54). Levels of mucin staining were determined using ImageJ v1.44 color deconvolution software. Values shown are the percentage area of each lung slice that stained PAS positive.
Flow cytometry.
Lungs were diced and incubated in digestion buffer (2.4 U/ml Dispase, 0.1% Collagenase A, 30 μg/ml DNase; all supplied by Roche, Indianapolis, IN) in HEPES for 45 min at 37°C before being passed through a 100-μm cell strainer. Cells were resuspended in PBS containing 5% BSA and stained with appropriate fluorescent-coupled antibodies CD45, F4/80, CD80, CD86, and/or 7-AAD (BD Biosciences, Franklin Lakes, NJ). Macrophages were identified as CD45+, F4/80+. Fluorescence was read on a BD FACSCalibur (BD Biosciences). Data were analyzed using Flowjo 10 software (Treestar, Ashland, OR).
Peritoneal lavage and transfer.
Anesthesia was induced with 5% isofluorane and then reduced to 3.5% for the duration of the procedure. With the use of a 20-gauge catheterized needle, peritoneal cavity cells were recovered in 5 ml warm sterile HBSS containing 5 mM tripotassium EDTA. Cells were counted and resuspended at a concentration of 1 × 107/ml before IT instillation. A total volume of 50 μl was used, giving a total number of 500,000 transferred cells. Mice from both the HBSSx3 and CRAx3 groups that received the peritoneal macrophages on day 20 are referred to as the analogous transfer of macrophages (ATM) group. Mice that received the vehicle alone are referred to as the no-ATM group. To track the position of transferred cells, peritoneal cells were incubated for 30 min at 37°C in 1× HBSS containing 7 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) or 25 μg/ml FITC-Dextran (Sigma). Following incubation, cells were washed with HBSS containing 5 mM tripotassium EDTA and resuspended at 10 × 106 cells/ml before IT instillation into naïve mice. Mice were killed 48 h later, and the presence of fluorescently labeled cells in the lymph nodes, lung digest, and bronchoalveolar lavage (BAL) was assessed by flow cytometry.
Cell culture.
Digested whole lung and/or peritoneal wash (PW) cells were counted and resuspended at 1 × 106 cells/ml in cell culture media (RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 1% antibiotic-antimycotic solution; HyClone, Logan, UT). Cells were cultured in 24-well coated plates (BD Biosciences) at a density of 1 × 106 cells/well, either in media alone or in media plus 1% (by volume) stock CRA solution. After 72 h, the supernatant was collected, centrifuged at 1,000 g for 5 min, and analyzed by ELISA for cytokine concentrations.
ELISA.
Samples were diluted at either 1:2 (BAL) or 1:10 (lung homogenate supernatant), and the cytokine concentrations were analyzed using reagents supplied by R&D Systems (Minneapolis, MN) as previously described (43). All cytokines were assessed simultaneously to reduce interassay variability.
Myeloperoxidase and eosinophil peroxidase assays.
These assays were performed on whole lung homogenate as previously described (50), with minor modifications (42). The absorbance was read at 450 (myeloperoxidase, MPO) or 490 nm (eosinophil peroxidase, EPO), and data were expressed as the average absorbance of sample wells minus the average background absorbance (ΔOD).
Statistics.
Statistical analysis was carried out using Graphpad Prism 5.02 (GraphPad Software, La Jolla, CA). Data are presented as the means ± SE (unless otherwise stated), with statistically significant differences between groups determined by unpaired Student's t-test yielding a P value of <0.05 at the 95% confidence interval. Multiple groups were compared by one-way ANOVA with Dunnett's posttest used to compare to the control group, where applicable.
RESULTS
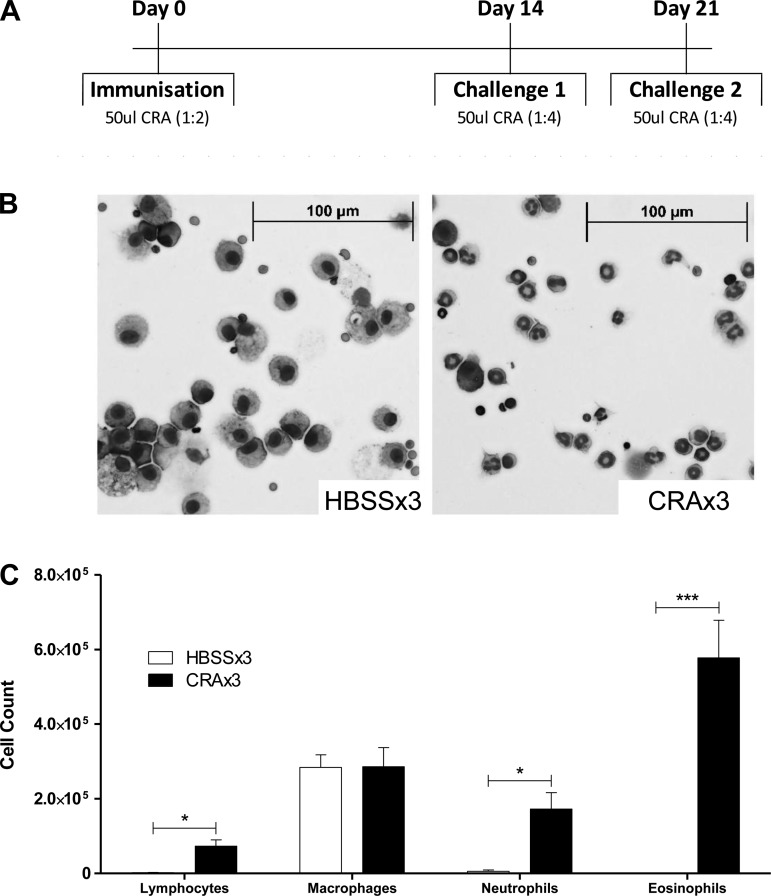
Our mouse model of CRA-induced asthma is well established and has been previously described (54–56). Mice were sensitized to CRA on day 0, followed by two challenges on days 14 and 21, as shown in Fig. 1A. Mice were killed either before the final exposure on day 21, or at 4, 16, or 24 h following the final CRA exposure. Following death, the lungs were lavaged, and the cell infiltrate was assessed. As anticipated based on prior work (54–56), there was a significant increase in the total number of cells recovered in the BAL of CRAx3 mice, compared with age-matched mice that received HBSS only in place of CRA (the HBSSx3 group). Differential counts of major cell subtypes revealed significant increases in the number of neutrophils, lymphocytes, and eosinophils 24 h after the final CRA exposure (Fig. 1, B and C). These data confirm that our model of CRA exposure elicits a strong pulmonary inflammatory response characterized by neutrophil and, in particular, eosinophil recruitment.
Fig. 1.
Cockroach allergen (CRA) exposure model. A: model schematic showing times and amounts of CRA delivered intratracheally. B: bronchoalveolar lavage (BAL) cells stained with Diff-Quick to differentiate lymphocytes, macrophages, neutrophils, and eosinophils. Shown are representative photomicrographs of control mice that received Hanks' balanced salt solution (HBSSx3) and mice that received CRA (CRAx3). C: quantification of data shown in B. Values are means ± SE; n = 5–6 mice per group; *P ≤ 0.05; ***P ≤ 0.005 comparing HBSSx3 vs. CRAx3.
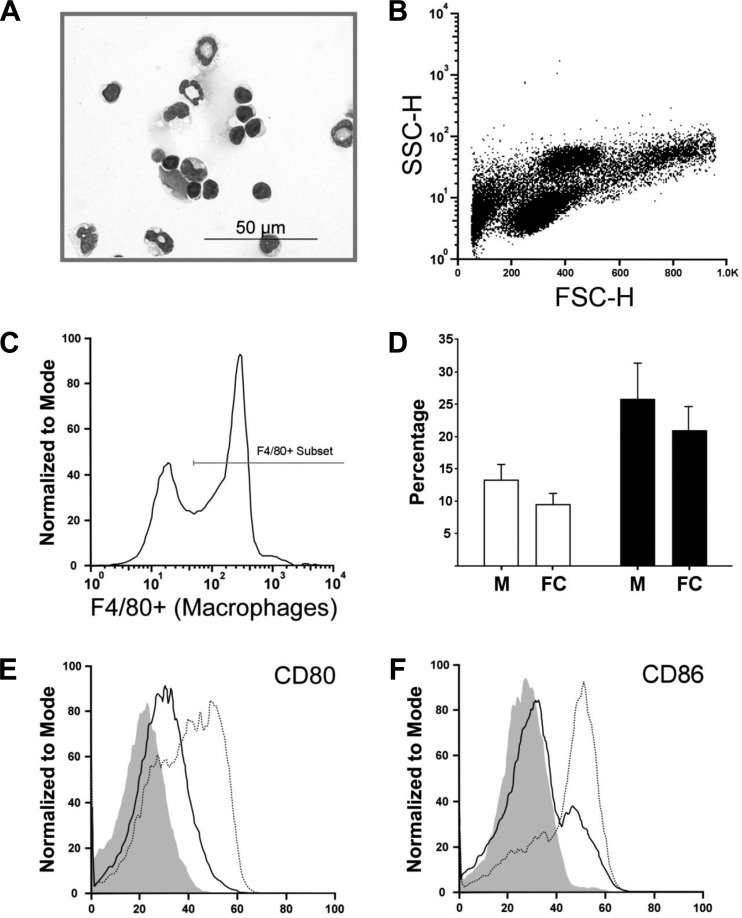
To further investigate the immune response, cells from the whole lung were also assessed. Lungs were enzymatically digested, and the cell populations were identified by microscopic inspection and flow cytometry. Figure 2A shows a typical lung digest (LD) preparation, demonstrating that cells remain intact following digestion, and Trypan blue staining revealed that over 80% of the cells were viable (data not shown). Figure 2B shows a representative forward/side scatter plot with readily identifiable populations of lymphocytes, granulocytes, etc. Figure 2C shows F4/80 expression used to identify macrophages; this strategy was used because it had good agreement with differential counts of LDs as assessed by light microscopy, shown in Fig. 2D. Macrophages could be further divided into two phenotypically distinct subsets based on their surface expression of CD80 and CD86, and it was found that macrophages from CRAx3 mice had lower expression levels of these two surface markers when compared with HBSSx3 mice (Fig. 2, E and F).
Fig. 2.
Identification of lung macrophages by flow cytometry. Mice were killed 24 h after final CRA exposure. Age-matched mice that were exposed to HBSS in place of CRA were used as controls. Whole lungs were excised and digested in Lung Digestion Buffer before being processed for FACS analysis. Shown are representative FACS plots from a series of experiments. A: representative photomicrograph of a lung digest cells stained with Diff-Quick. B: forward (FSC)/side (SSC) scatter dot-plot showing readily identifiable populations of putative lymphocyte, macrophage, and granulocyte cells. C: histogram of F4/80 expression on CD45+ cells, identified as macrophages. D: percentage size of the macrophage population as assessed by microscopy (M) or flow cytometry (FC). Open bars: HBSSx3; solid bars; CRAx3. E and F: CD80 and CD86 expression on macrophages (CD45+, F4/80+ cells) showing the reduction in expression following exposure to CRA. Solid line: CRAx3; dashed line: HBSSx3; shaded area: isotype control.
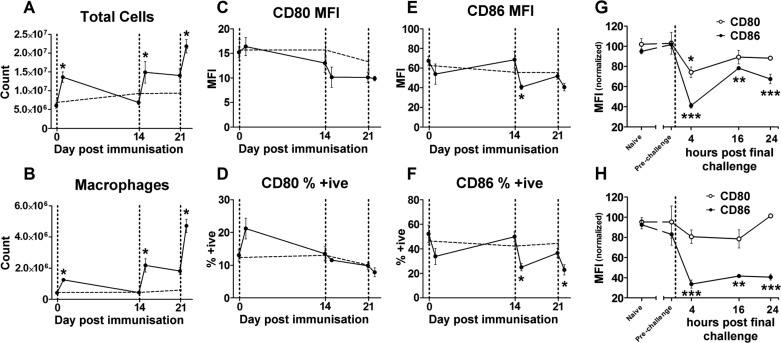
Macrophages are rapidly recruited to sites of inflammation, and their function can be readily manipulated by their environment. Therefore, the CD80 and CD86 change in lung macrophages over the 22-day time course of the model was assessed by flow cytometry (as depicted in Fig. 3). Mice were killed either immediately before or 24 h after each CRA exposure. Twenty-four hours after CRA instillation, there was a significant increase in the total number of cells, as well as the total number of macrophages (Fig. 3, A and B). To more fully determine the nature of the influence CRA exposure had over the macrophage phenotypes, surface expression of CD80 and CD86 were measured. These two molecules have important roles in the generation of adaptive immune responses (31, 57), but in this study their expression levels were assessed as markers of phenotypic change. In conjunction with well-established markers of the M2 phenotype such as Ym1 and Fizz1 (40), reduction in expression levels of CD80 and CD86 has been previously used in other studies as a marker of a reduction in M1 macrophage characteristics (19, 35, 39).
Fig. 3.
Lung macrophage changes over time. Mice were killed at different time points throughout the time course of the model, either before or 24 h after each CRA exposure (indicated by vertical dashed line). Mice exposed to HBSS in place of CRA were killed concurrently as age-matched controls, shown by the horizontal dashed line. Whole lungs were excised and digested before being processed for FACS analysis. Shown is the quantification of the flow cytometry data presented in Fig. 2. Cells were counted using a Coulter-counter, and percentage of the CD45+, F4/80+ populations used to calculate the total number of macrophages are shown. A: total lung cells recovered. B: absolute number of macrophages, showing significant increases in cell number following each CRA exposure. C–F: percent-positive and mean fluorescence intensity (MFI) expression of CD80 and CD86 on macrophages, showing altered expression of these surface molecules following CRA exposure. G and H: CD80 and CD86 expression levels from 4–24 h following the final CRA challenge. Values are normalized relative to HBSSx3 prefinal challenge levels. Values for all graphs are means ± SE; n = 4 mice per group; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005 compared with pre-CRA exposure for A–F, compared with “prechallenge” values for G and H.
Levels of expression were assessed by both the proportion of macrophages expressing the molecule on their surface (% positive) and the relative number of molecules expressed per macrophage (mean fluorescence intensity). Different patterns were seen for CD80 and CD86. Although no significant differences in CD80 expression were seen (Fig. 3, C and D), it was observed that, 24 h following every CRA challenge, there were reductions in the expression of CD86 on lung macrophages, particularly the last two challenges on days 14 and 21 (Fig. 3, E and F). This reduction was present as early as 4 h after exposure and remained at a reduced level up to 24 h after exposure (Fig. 3, G and H), demonstrating that the observed reduction at 24 h is not a compensatory late event. The expression levels before subsequent CRA challenges are not significantly different from those seen in HBSS-treated mice, indicating that the observed effect is temporary and reversible. These data show that CRA exposure has a significant effect on the lung macrophage population, as assessed by their surface expression levels of CD80 and CD86. Studies by other groups using these molecules as M1 markers (19, 35, 39) suggest that our observations represent genuine phenotypic changes. This provides evidence suggesting a role for macrophage phenotypes in the pathogenesis seen in our model.
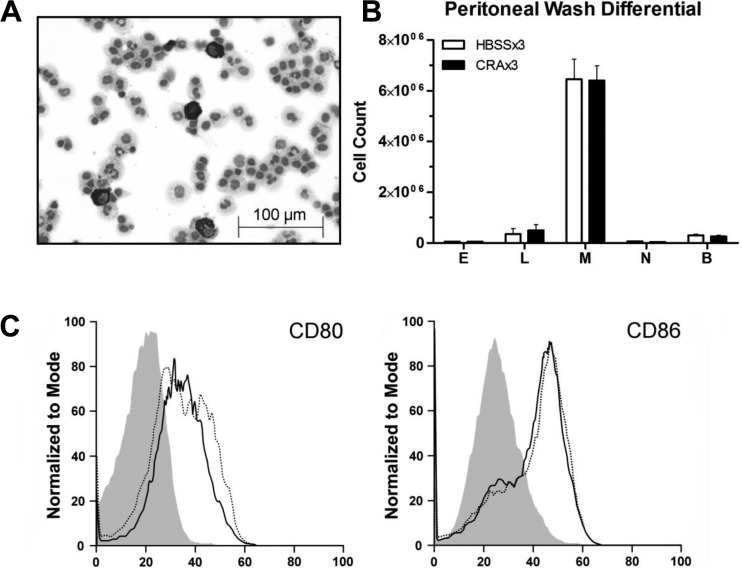
We sought to determine whether the observed CRA-induced response was limited to the lungs or was systemic. Assessment of the peritoneal cavity cell population, using the same methodology as used for the lungs, revealed that there were no discernible differences between HBSSx3 and CRAx3 mice with regard to number of cells recovered, differential counts (Fig. 4, A and B), or macrophage surface expression of CD80 and CD86 (Fig. 4C). Based on these results, we concluded that the peritoneal cells remained at a state equivalent to that seen in HBSSx3 mice, unaffected by pulmonary CRA exposure throughout the 22-day time course of the model.
Fig. 4.
Peritoneal cavity cells are unaffected by pulmonary CRA exposure. Peritoneal cells were collected by lavage with 5 ml warm HBSS from mice 24 h after final CRA exposure or age-matched HBSS-exposed controls. Using the same criteria for the identification of lung cells, no significant differences were observed in cells from the peritoneal cavity between CRAx3 and HBSSx3 mice. A: representative photomicrograph of peritoneal lavage cells stained with Diff-Quick. B: quantification of data shown in A. Values are mean ± SE; n = 5–6 mice per group. E, eosinophils; L, lymphocytes; M, macrophages; N, neutrophils; B, basophils. C: representative FACS histograms showing surface expression of CD80 and CD86 on peritoneal macrophages (CD45+, F4/80+ cells). Solid line: CRAx3. Dashed line: HBSSx3. Shaded area: isotype control.
Because the model uses outbred mice to more accurately simulate the human population, we are faced with challenges not present when using inbred strains. Notably, the use of adoptive transfer techniques to assess the contribution of macrophage populations cannot be performed due to the potential confounding factor of incompatible major histocompatibility complex expression between the donor and recipient. However, as shown in Fig. 4, the peritoneal cavity cells have the potential to be used in an autologous transfer because, unlike pulmonary macrophages, their quantity and phenotype (as measured by levels of CD80 and CD86 expression) have not been altered by the CRA-induced lung inflammation. Also, as shown in Fig. 4B, a live PW yields a substantial number of cells (∼6 million) that are ∼90% macrophages, and therefore these cells can be used in an adoptive transfer with minimal manipulation that might otherwise affect the activation status of the macrophages, i.e., cell purification and ex vivo culture.
We sought to determine whether peritoneal macrophages could be employed to alter the lung immune response in CRA-exposed mice. Therefore, we developed an assay to measure cytokine production by cells exposed to CRA in culture. A single-cell suspension, obtained from enzymatically digested whole lungs, was cultured at a concentration of 1 × 106 cells/ml in the presence of CRA for 72 h. Cytokine concentrations in the cell-free supernatant were measured by ELISA.
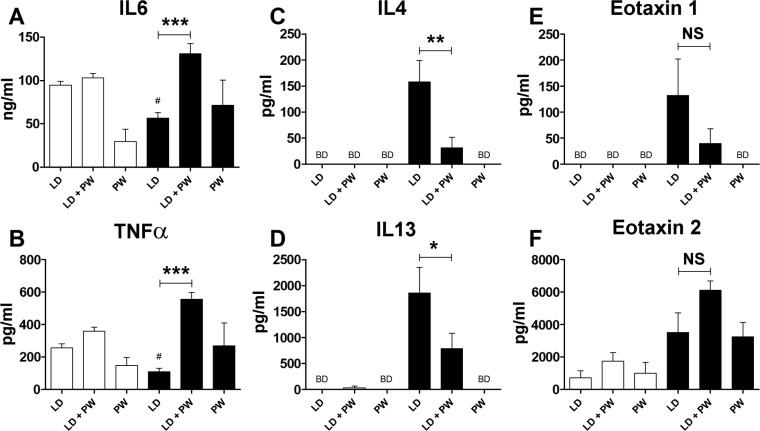
As macrophages can be proinflammatory, we measured the concentrations of IL-6 and TNF-α, two cytokines associated with acute inflammation. LD cells from CRAx3 mice produced lower quantities of these cytokines compared with cells from HBSSx3 mice. When lung and peritoneal cells from CRAx3 mice are cocultured, there is a significant increase in cytokine production compared with stimulated LD cells. PW cells from both HBSSx3 and CRAx3 mice produced similar levels of these two cytokines (Fig. 5, A and B). IL-4 and IL-13 are two cytokines strongly associated with TH2 responses and are considered to be key components of asthma pathogenesis (60). As shown in Fig. 5, C and D, LD cells from HBSSx3 mice and PW cells from both HBSSx3 and CRAx3 mice did not produce detectable levels of these cytokines. However, LD cells from CRA exposure produced high concentrations of both IL-4 and IL-13, and this was significantly reduced when they were cocultured with PW cells. Significant eosinophil influx is observed in the CRA-induced asthma model (Fig. 1, B and C), and both IL-4 and -13 are known to positively influence eotaxin production (34, 36, 58). In vitro production of eotaxin 1 and 2 is observed, but the picture is complex (Fig. 5, E and F). Eotaxin 1 follows the same pattern as seen with IL-4 and -13: LD cells from HBSSx3 mice and PW cells from HBSSx3 and CRAx3 mice do not produce detectable amounts of eotaxin 1. LD cells from CRAx3 mice produced large quantities of eotaxin 1, and this was not significantly reduced when cocultured with PW cells (Fig. 5E). With eotaxin 2, the pattern is closer to that seen with IL-6 and TNF-α: both lung and peritoneal cells from both HBSSx3 and CRAx3 mice produced appreciable quantities of the cytokine when cultured alone. When cocultured, the production is enhanced although the effect is not significant (Fig. 5F). The findings from this ex vivo culture assay suggest that peritoneal cells have the potential to alter the nature of the lung response to CRA. In particular, the direction of the alteration is a reduction in TH2-associated cytokines (IL-4 and -13), and an increase in cytokines associated with acute inflammation (IL-6 and TNF-α).
Fig. 5.
Cytokine production by lung and peritoneal cells exposed to CRA. Lung and peritoneal cells were harvested from either fully sensitized mice (CRAx3) or age-matched controls (HBSSx3). Lung digest (LD) and peritoneal wash (PW) cells were cultured in the presence of CRA, and the concentration of cytokines in the supernatant was assessed after 72 h. A and B: proinflammatory cytokines IL-6 and TNF-α. C and D: TH2-associated cytokines IL-4 and IL-13. E and F: eosinophil chemoattractants eotaxin 1 and 2. Open bars: HBSSx3; solid bars: CRAx3. BD, below detection limit. Values are means ± SE; n = 4–12 mice per group; #P ≤ 0.05 compared with corresponding HBSSx3 LD value; NS: not significant (P > 0.05). Comparing bars underneath horizontal lines: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005.
Based on the implications of the data presented in Figs. 4 and 5, we developed a protocol to harvest cells from the peritoneal cavity of live mice using a minimally invasive sterile process that does not require postprocedure antibiotics or analgesics. On day 21 of the model (24 h before the final CRA exposure), the peritoneal cavity was lavaged, the recovered cells were immediately counted, and 5 × 105 cells were intratracheally instilled into the mice using exactly the same procedure as for CRA exposure.
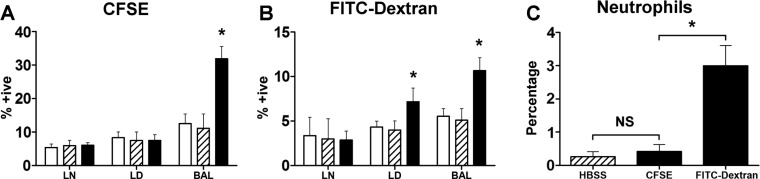
To determine the localization of transferred cells, fluorescently labeled peritoneal cells were instilled into the lungs of a single group of naïve mice. Forty-eight hours later, the presence of labeled cells was evaluated by flow cytometry. As shown in Fig. 6A, CFSE-labeled (i.e., transferred) cells remain largely within the airways and are detectable in the BAL only. Cells stained with FITC-Dextran were also seen in the lung digest (Fig. 6B). It is possible that exposure to dextran activated the macrophages and enabled their migration out of the airways (26). Furthermore, a significant number of neutrophils were seen in the BAL of mice transferred with FITC-Dextran-labeled cells (again indicating activation), but not in the BAL of mice that received CFSE-labeled cells or cells incubated in HBSS alone (Fig. 6C). This observation indicates that the peritoneal cell harvest and transfer procedure does not by itself cause undue activation of the macrophages. The viability of recovered fluorescently labeled cells in the BAL was assessed by 7-AAD staining and was found to be ∼80% (data not shown).
Fig. 6.
Location of peritoneal macrophages following transfer. Peritoneal macrophages were labeled with either carboxyfluorescein diacetate succinimidyl ester (CFSE) (A) or FITC-conjugated dextran (B) and intratracheally instilled into the lungs of mice. 48 h later, BAL, lung digest (LD), and lymph nodes (LN) were processed and analyzed by flow cytometry (solid bars). As controls, unlabeled cells (hatched bars) and mice that did not receive any transferred cells (open bars) were examined. C: number of neutrophils in the BAL of mice that received either unlabeled (hatched bar), CFSE, or FITC-dextran-stained (solid bars) peritoneal cells was assessed. Values are means ± SE; n = 4 mice per group; *P ≤ 0.05 compared with unstained cell transfer (A and B), or compared with CFSE group (C). NS, not significant (P > 0.05).
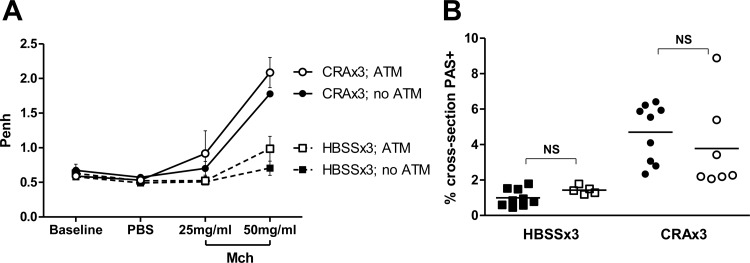
ATM in vivo had no significant effects on either AHR or mucin production (Fig. 7, A and B) in either HBSSx3 or CRAx3 mice. As shown in Fig. 8, with regards to the HBSSx3 mice, there were very few significant observable differences between mice that received the ATM (solid bars) and mice that only received HBSS on day 20 (no ATM: open bars). This shows that the introduction of peritoneal macrophages per se into the airways of mice has minimal proinflammatory effects.
Fig. 7.
Airway hyperresponsiveness (AHR) and mucin production following autologous macrophage transfer (ATM). Mice were given either intratracheal HBSS (no ATM) or autologous cells (ATM) 24 h before the final challenge. A: 24 h following the final challenge, AHR was assessed by exposure to increasing concentrations of methylcholine (Mch). CRAx3 mice had significantly higher enhanced pause (Penh) than HBSSx3 mice (P = 0.0123). ATM had no significant effect on AHR within groups. B: lung sections were stained with PAS, and mucin production was assessed. CRAx3 mice had significantly higher levels of mucin than HBSSx3 mice (P < 0.0001). ATM had no significant effect on mucin production within groups. ○, CRAx3; ATM. ●, CRAx3; no ATM. □, HBSSx3; ATM. ■, HBSSx3; no ATM. Values are mean ± SE (A) or absolute value with mean represented as a horizontal black line (B); n = 4–9 mice per group; NS, not significant (P > 0.05).
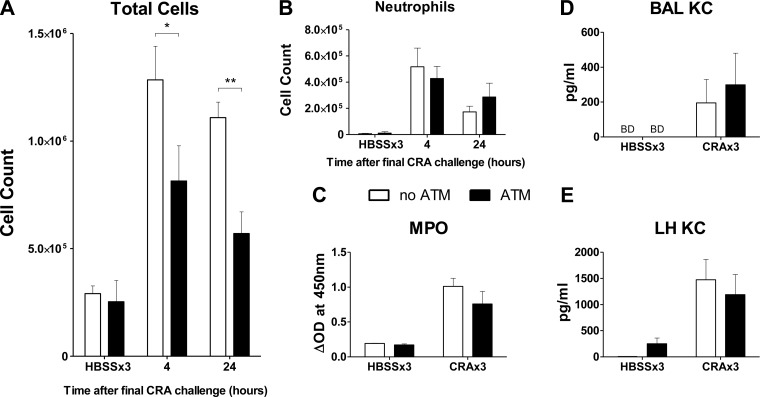
Fig. 8.
ATM significantly reduces pulmonary inflammatory cell recruitment. Peritoneal cells were collected on day 20 and reintroduced into the lungs intratracheally. On day 21 mice received the final exposure to CRA and were killed 4 or 24 h later. Lungs were lavaged, and the recovered cells were stained with Diff-Quick. A: total cells recovered in BAL, showing reduction in number of cells recruited at 4 and 24 h following final CRA exposure. No effect was seen in the lungs of HBSSx3 mice (killed 24 h after final HBSS exposure). B: total neutrophils recovered in BAL, showing no significant changes in mice that received ATM compared with no ATM. C: lavaged lungs were excised and processed for myeloperoxidase (MPO) assay to determine the numbers of neutrophils sequestered in whole lung tissue. No significant reduction in MPO activity was observed in mice that received the ATM compared with no ATM. The concentrations of neutrophil chemotactic cytokines were measured in the BAL (D) and whole lung homogenate (LH) (E); no significant effects of ATM were seen. KC, keratinocyte-derived chemokine. Open bars: no ATM group; solid bars: ATM group. Values are means ± SE; n = 4–6 mice per group; *P ≤ 0.05; **P ≤ 0.01 comparing the indicated groups.
However, there are significant effects seen in CRA-sensitized and -challenged mice that received the ATM compared with no ATM (i.e., CRA-exposed mice that received IT HBSS instead of the ATM on day 20). As shown in Fig. 8A, significantly fewer cells are recovered in the BAL of ATM mice at both 4 and 24 h following the final CRA challenge. Differential counts of BAL cells revealed no significant differences in the numbers of lymphocytes or macrophages (data not shown); despite the prior transfer of macrophages into the lung, this was the expected result, as the cells recovered in the BAL represent an incomplete sampling of the total cell population within the airways.
Neutrophil influx was also unaffected at both early (4 h) and late (24 h) time points following the final CRA challenge (Fig. 8B). MPO levels in whole lung homogenates show that the CRA challenge protocol increases MPO compared with HBSSx3 mice, but the ATM had no effect in either HBSSx3 or CRAx3 mice, indicating that it did not induce neutrophil sequestration in the lung parenchyma (Fig. 8C). Concentrations of the neutrophil chemoattractant keratinocyte-derived chemokine were also measured in the BAL fluid and whole lung homogenate, and no significant differences were observed (Fig. 8, D and E). In total, the ATM had minimal impact on pulmonary neutrophil recruitment or induction of neutrophil chemokines by CRA.
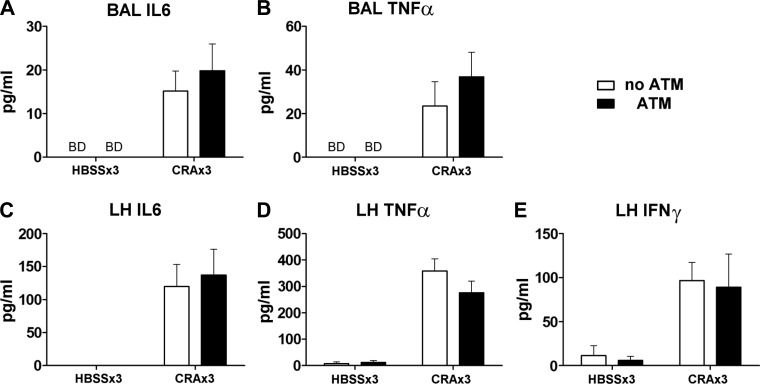
In vitro experiments showed an increase in the production of the proinflammatory cytokines IL-6 and TNF-α when lung and peritoneal cells were cocultured in the presence of CRA (Fig. 5, A and B, solid bars). As these cytokines are particularly involved in the acute phase response, in vivo pulmonary neutrophil influx following ATM was assessed. No significant differences in IL-6, TNF-α, or IFN-γ were detected in vivo lung homogenate or BAL. IFN-γ concentration was below detection limits in the BAL (Fig. 9).
Fig. 9.
Cytokine concentrations in the BAL and lung homogenate (LH). Peritoneal cells were collected on day 20 and reintroduced into the lungs intratracheally. On day 21 mice received the final exposure to CRA and were killed 4 or 24 h later. Cytokine concentrations in the BAL (A and B) and LH (C–E) were assessed by ELISA. No significant effects of the ATM procedure were observed. Open bars: no ATM group; solid bars: ATM group. Values are mean ± SE; n = 4–6 mice per group.
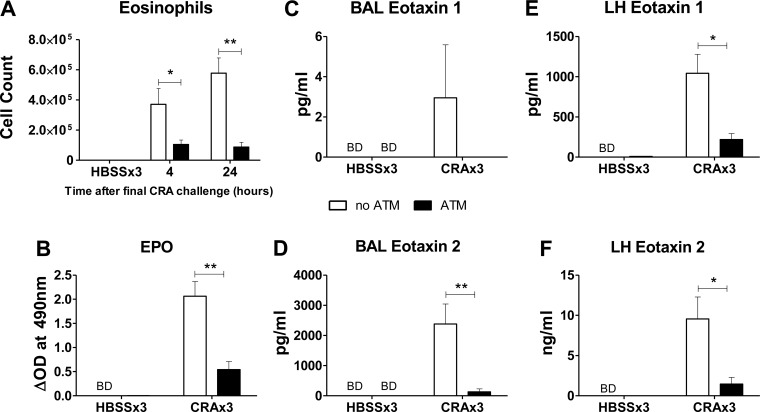
Pulmonary eosinophil recruitment is a critical feature of the asthmatic response (61), and the ATM procedure did significantly decrease the recruitment of eosinophils. Again, there were no observed effects of the ATM procedure in HBSSx3 mice with regard to presence of eosinophils in the BAL (Fig. 10A), sequestration of eosinophils in the lung (as measured by EPO assay, Fig. 10B), or production of eosinophil chemoattractants eotaxin 1 and 2 (Fig. 10, C–F). Levels of eotaxin 1 and 2 in HBSSx3 mice were either below detection or at concentrations less than 1% of those seen in CRAx3 mice. In CRAx3 mice that received the ATM, the number of eosinophils recovered in BAL fluid was significantly reduced at both 4 and 24 h after the final CRA exposure (Fig. 10A). Eosinophil sequestration in the lung tissue was also significantly reduced, as measured by EPO levels (Fig. 10B). In addition, at 24 h following the final CRA exposure, BAL levels of the eosinophil chemotactic cytokines eotaxin 1 and 2 were reduced in mice that received the ATM compared with the no-ATM group (Fig. 10, C and D), and a similar situation was observed in the whole lung homogenate (Fig. 10, E and F). This reduction of eosinophil chemokines represents a possible mechanism for the observed reduction in eosinophil recruitment.
Fig. 10.
ATM significantly reduces pulmonary eosinophil recruitment. Peritoneal cells were collected on day 20 and reintroduced into the lungs intratracheally. On day 21 mice received the final exposure to CRA and were killed 4 or 24 h later. Lungs were lavaged and the recovered cells stained with Diff-Quick. A: Total eosinophils recovered in BAL showed a significant reduction in number in mice that received ATM compared with no ATM. No effect was seen in the lungs of HBSSx3 mice (killed 24 h after final HBSS exposure). B: lavaged lungs were excised and processed for eosinophil peroxidase (EPO) assay to obtain a correlate of eosinophil number in whole lung tissue. A significant reduction in EPO activity was observed in the whole lung homogenate of mice that received the ATM at 24 h after final CRA challenge. BAL supernatant was reserved, and the concentration of eotaxins measured by ELISA. Significant reductions in the concentration of eosinophil chemoattractants eotaxin 1 (C) and 2 (D) were seen in the BAL fluid of mice that received the ATM. No significant effects were seen in HBSSx3 mice. Whole lung homogenate (LH) supernatant was also analyzed by ELISA; significant reductions in both eotaxin 1 (E) and 2 (F) were observed in the ATM groups. No significant effects were seen in HBSSx3 mice. Open bars: no ATM group; solid bars: ATM group. Values are means ± SE; n = 4–6 mice per group; *P ≤ 0.05; **P ≤ 0.01.
DISCUSSION
There is considerable evidence to suggest that macrophages play a key role in the pathogenesis of pulmonary allergic disease; they also represent one of the most abundant cell types in the airways of both naïve and sensitized mice (15, 30, 46). In this study, we have shown that exposure to CRA has a significant effect on macrophage phenotypes, increasing the number of macrophages in the lung and reducing their surface expression of CD80 and CD86, molecules, which, among others, have been previously established as useful in assessing the phenotype of macrophages (1, 10, 19, 20, 35, 39, 51). We have also developed a novel procedure to supplement the lung macrophage population in our outbred mouse model; to our knowledge the use of an ATM has never been investigated in the CRA-induced asthma model.
Our present data show that, within the CRA-induced asthma model, expression of both CD80 and CD86 on lung macrophages is reduced following allergen exposure. Although costimulatory molecule expression on macrophages and other cells has been previously studied in asthma (2, 21, 31, 57), this is the first report using a CRA-induced mouse model.
Macrophages, as antigen-presenting cells, play a key role in the bridge between innate and adaptive immunity. Costimulation during antigen presentation is essential for the formation of an effective adaptive immune response, as has been shown in murine studies using CD80/CD86 knockout mice (31, 57). Furthermore, studies in mice and humans have shown that, following airway exposure to allergen, there is increased expression of CD80 and/or CD86 on macrophages (2, 5).
As our data contradicts these findings, it is worth reemphasizing the differences between our model and the more widely used OVA-induced asthma models. The CRA used in our model is identical to that used in clinical skin testing, and all of the CRA exposures are via the physiologically relevant IT route and do not use any exogenous adjuvants. In addition to the complex nature of CRA (being a whole body extract containing LPS, chitin, and other uncharacterized components), these observations suggest that differences are to be expected between the two models.
Several studies in both mice (19, 35) and humans (1, 20) have shown an association between reduction of CD80 and CD86 and the reduction of M1 characteristics. Additional studies have shown the association of low expression levels of CD80 and CD86 with the M2 phenotype (10, 39, 51). The purpose of this study was not to characterize the macrophage phenotype induced by CRA exposure but rather to demonstrate that CRA exposure induces a change in phenotype and that addition of naive macrophages will reduce eosinophil recruitment. In this regard, CD80 and CD86 are evaluated here as biomarkers, as opposed to functional molecules influencing the mechanistic behavior of macrophages. Therefore, the conclusions drawn here relate to the changes in the expression levels of these molecules rather than their biological function. Although we do not go so far as to assert that CRA induces an M2 phenotype, a clear phenotypic change is occurring, and, judging by the observed reduced expression levels of CD80 and CD86, this change has certain characteristics of a reduction in the M1 phenotype.
As it is relatively easy to activate macrophages ex vivo, it is important to note that the ATM procedure had no significant effects in mice not exposed to CRA, and peritoneal cells from both HBSSx3 and CRAx3 mice produced similar levels of cytokines when stimulated in culture (Fig. 5). These observations suggest that the ATM procedure does not exert its effects simply by passive induction of opposing or atypical inflammation but rather by specifically acting within the environment already set up in the CRA-exposed lung. There is no obvious set of conditions to control for the addition of peritoneal macrophages, other than the vehicle (HBSS alone). An irrelevant cell population would have the confounding effects of cytokine production and cell-cell interactions that may be induced by any live cell. The idea of using killed or inactivated peritoneal cells is also flawed, as it has previously been shown that adoptive transfer of apoptotic or necrotic cells can dramatically influence the immune response in distinct ways (17).
Macrophages have been shown to be involved in the generation of tolerance to nonspecific stimuli such as LPS (23, 44, 62), and this process could be one possible mechanism behind our observations. However, based on the fact that no reduction in the proinflammatory cytokines IL-6 and TNF-α following the ATM procedure was observed, our data suggest that tolerance is not playing a significant role in our model. BAL concentration of the TH1-associated cytokine IFN-γ was also unaffected following macrophage transfer.
We have used the ATM procedure to demonstrate that macrophages can influence the immune response to CRA, in particular causing a significant reduction in eotaxin 1 and 2 levels, with a concomitant reduction in eosinophil recruitment to, and sequestration in, the lung.
As the presence of eosinophils has been correlated with severity of asthma (3), and there is evidence that these cells play a role in the induction of AHR (11), the fact that in our autologous transfer model we observed no reduction in AHR requires explanation. In OVA-based murine models of asthma, the glucocorticoid dexamethasone has been shown to reduce both AHR and pulmonary eosinophil recruitment (including the reduction of eotaxins and IL-5). However, specific inhibition of eosinophilia by monoclonal antibodies against eotaxin and/or IL-5 has been shown to have no effect on AHR (8). This indicates that distinct and separate mechanisms are involved in the generation of AHR and eosinophil recruitment and that dexamethasone treatment does not necessarily reduce AHR by inhibiting eosinophilia. These observations also lend weight to the data presented here, which suggests that the ATM procedure exerts its effects on eosinophils through a specific mechanism rather than nonspecific immunosuppression or the induction of tolerance.
Multiple different cells types can act as sources of eotaxin, including epithelial cells, fibroblasts, and eosinophils (27, 36, 41). It has been shown that macrophages themselves are able to produce appreciable quantities of eosinophil chemoattractants (including eotaxin 1 and 2; Ref. 47) following activation via IL-4Rα (58), which is also known to be involved in the development of an alternatively activated phenotype via signals mediated by both IL-4 and IL-13 (13, 32, 38, 52). As we have shown, CRA induces significant production of IL-4 and IL-13 by lung cells, and, although the precise cellular source of these cytokines remains undefined, it is known that they both play a role in upregulating eotaxin production (34, 36, 58). Whereas the reduction of IL-4 and IL-13 was not accompanied by a corresponding decrease in eotaxin 2 levels in vitro, reduction in both eotaxin 1 and 2 was seen in the BAL fluid and whole lung homogenate in vivo; this observation serves to expose the limitations of a solely in vitro approach.
The results presented here highlight the fact that the phenotype of macrophages can alter an established immune response, without the need for ex vivo manipulation or the addition of exogenous substances. The fact that the ATM is a rapid procedure with very little associated trauma, and the potential ease with which the transferred macrophages might be deliberately manipulated, opens up a variety of avenues for future investigation of the mechanisms behind our observations. This approach has obvious implications in furthering the study of mechanisms behind macrophage-mediated pathogenesis in the CRA-induced asthma model, as well as a variety of disease models, where the use of outbred mice is preferable.
GRANTS
This work was supported in part by NIH grants ES 013528 and HL 07501.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.R.B., D.M.S., S.N., and D.G.R. conception and design of research; D.R.B. and D.M.S. performed experiments; D.R.B. analyzed data; D.R.B. and D.G.R. interpreted results of experiments; D.R.B. prepared Figs.; D.R.B. drafted manuscript; D.R.B., S.N., J.K., and D.G.R. edited and revised manuscript; D.R.B., D.M.S., S.N., J.K., and D.G.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would thank Elizabeth Duffy for providing technical assistance and expertise.
REFERENCES
- 1.Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW, Knawy BA. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev 9: 620–641, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Balbo P, Silvestri M, Rossi GA, Crimi E, Burastero SE. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy 31: 625–636, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel FB. Eosinophilic inflammation in asthma. N Engl J Med 323: 1033–1039, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med 344: 350–362, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chen CL, Lee CT, Liu YC, Wang JY, Lei HY, Yu CK. House dust mite Dermatophagoides farinae augments proinflammatory mediator productions and accessory function of alveolar macrophages: implications for allergic sensitization and inflammation. J Immunol 170: 528–536, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med 182: 1169–1174, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med 4: 344–355, 1998 [PMC free article] [PubMed] [Google Scholar]
- 8.Eum SY, Maghni K, Hamid Q, Eidelman DH, Campbell H, Isogai S, Martin JG. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol 111: 1049–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Ford AQ, Dasgupta P, Mikhailenko I, Smith EM, Noben-Trauth N, Keegan AD. Adoptive transfer of IL-4Ralpha+ macrophages is sufficient to enhance eosinophilic inflammation in a mouse model of allergic lung inflammation. BMC Immunol 13: 6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. Antagonistic effects of GM-CSF and IFNgamma. PLoS One 8: e56045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol 123: 2925–2927, 1979 [PubMed] [Google Scholar]
- 12.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 18: 60–65, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Hocking WG, Golde DW. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med 301: 580–587, 1979 [DOI] [PubMed] [Google Scholar]
- 16.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 18: 673–683, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci USA 100: 6724–6729, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humbles AA, Conroy DM, Marleau S, Rankin SM, Palframan RT, Proudfoot AE, Wells TN, Li D, Jeffery PK, Griffiths-Johnson DA, Williams TJ, Jose PJ. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo. J Exp Med 186: 601–612, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizuka EK, Ferreira MJ, Grund LZ, Coutinho EM, Komegae EN, Cassado AA, Bortoluci KR, Lopes-Ferreira M, Lima C. Role of interplay between IL-4 and IFN-gamma in the in regulating M1 macrophage polarization induced by Nattectin. Int Immunopharmacol 14: 513–522, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Jaguin M, Houlbert N, Fardel O, Lecureur V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol 281: 51–61, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Janssen EM, Wauben MH, Nijkamp FP, van Eden W, van Oosterhout AJ. Immunomodulatory effects of antigen-pulsed macrophages in a murine model of allergic asthma. Am J Respir Cell Mol Biol 27: 257–264, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, Rhim TY, Park CS. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol 33: 290–296, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Karp CL, Wysocka M, Ma X, Marovich M, Factor RE, Nutman T, Armant M, Wahl L, Cuomo P, Trinchieri G. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur J Immunol 28: 3128–3136, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Keane-Myers AM, Gause WC, Finkelman FD, Xhou XD, Wills-Karp M. Development of murine allergic asthma is dependent upon B7–2 costimulation. J Immunol 160: 1036–1043, 1998 [PubMed] [Google Scholar]
- 25.Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, Kemeny DM, Lotvall J, Pauwels RA, Plopper CG, Schmidt D, Sterk PJ, Van Oosterhout AJ, Vargaftig BB, Chung KF. Murine models of asthma. Eur Respir J 22: 374–382, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol 183: 1983–1989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komiya A, Nagase H, Yamada H, Sekiya T, Yamaguchi M, Sano Y, Hanai N, Furuya A, Ohta K, Matsushima K, Yoshie O, Yamamoto K, Hirai K. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol 225: 91–100, 2003 [DOI] [PubMed] [Google Scholar]
- 28.La Flamme AC, Kharkrang M, Stone S, Mirmoeini S, Chuluundorj D, Kyle R. Type II-activated murine macrophages produce IL-4. PLoS One 7: e46989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Duan XH, Wu JF, Liu BJ, Luo QL, Jin HL, Du YJ, Zhang HY, Cao YX, Dong JC. Impact of psychosocial stress on airway inflammation and its mechanism in a murine model of allergic asthma. Chin Med J 126: 325–334, 2013 [PubMed] [Google Scholar]
- 30.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J 7: 1678–1689, 1994 [PubMed] [Google Scholar]
- 31.Mark DA, Donovan CE, De Sanctis GT, He HZ, Cernadas M, Kobzik L, Perkins DL, Sharpe A, Finn PW. B7-1 (CD80) and B7-2 (CD86) have complementary roles in mediating allergic pulmonary inflammation and airway hyperresponsiveness. Am J Respir Cell Mol Biol 22: 265–271, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27: 451–483, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59: 469–478, 2004 [DOI] [PubMed] [Google Scholar]
- 34.May RD, Monk PD, Cohen ES, Manuel D, Dempsey F, Davis NH, Dodd AJ, Corkill DJ, Woods J, Joberty-Candotti C, Conroy LA, Koentgen F, Martin EC, Wilson R, Brennan N, Powell J, Anderson IK. Preclinical development of CAT-354, an IL-13 neutralizing antibody, for the treatment of severe uncontrolled asthma. Br J Pharmacol 166: 177–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of the macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49: 503–510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mochizuki M, Bartels J, Mallet AI, Christophers E, Schroder JM. IL-4 induces eotaxin: a possible mechanism of selective eosinophil recruitment in helminth infection and atopy. J Immunol 160: 60–68, 1998 [PubMed] [Google Scholar]
- 37.Moon HG, Kang CS, Choi JP, Choi DS, Choi HI, Choi YW, Jeon SG, Yoo JY, Jang MH, Gho YS, Kim YK. Acetyl salicylic acid inhibits Th17 airway inflammation via blockade of IL-6 and IL-17 positive feedback. Exp Mol Med 45: e6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosser DM. The many faces of macrophage activation. J Leukoc Biol 73: 209–212, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Na YR, Yoon YN, Son DI, Seok SH. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS One 8: e63451, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett 85: 173–180, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Nakajima T, Yamada H, Iikura M, Miyamasu M, Izumi S, Shida H, Ohta K, Imai T, Yoshie O, Mochizuki M, Schroder JM, Morita Y, Yamamoto K, Hirai K. Intracellular localization and release of eotaxin from normal eosinophils. FEBS Lett 434: 226–230, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Natarajan S, Kim J, Bouchard J, Cruikshank W, Remick DG. Reducing LPS content in cockroach allergens increases pulmonary cytokine production without increasing inflammation: a randomized laboratory study. BMC Pulm Med 11: 12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods 255: 149–157, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol 164: 3476–3479, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood 91: 2240–2248, 1998 [PubMed] [Google Scholar]
- 46.Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 31: 3–7, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol 175: 5341–5350, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Pouliot P, Turmel V, Gelinas E, Laviolette M, Bissonnette EY. Interleukin-4 production by human alveolar macrophages. Clin Exp Allergy 35: 804–810, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 336: 1356–1363, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Schneider T, Issekutz AC. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods 198: 1–14, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Schraufstatter IU, Zhao M, Khaldoyanidi SK, Discipio RG. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 135: 287–298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teran LM, Mochizuki M, Bartels J, Valencia EL, Nakajima T, Hirai K, Schroder JM. Th1- and Th2-type cytokines regulate the expression and production of eotaxin and RANTES by human lung fibroblasts. Am J Respir Cell Mol Biol 20: 777–786, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Vaickus LJ, Bouchard J, Kim J, Natarajan S, Remick DG. Assessing pulmonary pathology by detailed examination of respiratory function. Am J Pathol 177: 1861–1869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaickus LJ, Bouchard J, Kim J, Natarajan S, Remick DG. Inbred and outbred mice have equivalent variability in a cockroach allergen-induced model of asthma. Comp Med 60: 420–426, 2010 [PMC free article] [PubMed] [Google Scholar]
- 56.Vaickus LJ, Bouchard J, Kim J, Natarajan S, Remick DG. Oral tolerance inhibits pulmonary eosinophilia in a cockroach allergen induced model of asthma: a randomized laboratory study. Respir Res 11: 160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Rijt LS, Vos N, Willart M, Kleinjan A, Coyle AJ, Hoogsteden HC, Lambrecht BN. Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of TH2 effector responses in a mouse model of asthma. J Allergy Clin Immunol 114: 166–173, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Watanabe K, Jose PJ, Rankin SM. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J Immunol 168: 1911–1918, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Wei M, Chu X, Guan M, Yang X, Xie X, Liu F, Chen C, Deng X. Protocatechuic acid suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Int Immunopharmacol 15: 780–788, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17: 255–281, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Woodruff PG, Khashayar R, Lazarus SC, Janson S, Avila P, Boushey HA, Segal M, Fahy JV. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol 108: 753–758, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Ziegler-Heitbrock HW, Wedel A, Schraut W, Strobel M, Wendelgass P, Sternsdorf T, Bauerle PA, Haas JG, Riethmuller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem 269: 17001–17004, 1994 [PubMed] [Google Scholar]
- 63.Zosky GR, Sly PD. Animal models of asthma. Clin Exp Allergy 37: 973–988, 2007 [DOI] [PubMed] [Google Scholar]