Abstract
Tumor necrosis factor-α converting enzyme (TACE) is a cell membrane sheddase, expressed in both developmental lung epithelia and mesenchyme. Global abrogation of TACE results in neonatal lethality and multiple organ developmental abnormalities, including dysplastic lung. To further define the roles of TACE in regulating lung development, lung epithelial and/or mesenchymal specific TACE conditional knockout mice were generated. Blockade of TACE function in developing lung epithelial cells caused reduced saccular formation, decreased cell proliferation, and reduced mid-distal lung epithelial cell differentiation. In contrast, mesenchymal TACE knockout did not have any phenotypic change in developing lung. Simultaneous abrogation of TACE in both lung epithelial and mesenchymal cells did not result in a more severe lung abnormality. Interestingly, these lung-specific TACE conditional knockout mice were not neonatal lethal, and their lung structures were essentially normal after alveolarization. In addition, TACE conditional knockout in developing cardiomyocytes resulted in noncompaction of ventricular myocardium, as seen in TACE conventional knockout mice. However, these mice were also not neonatal lethal. In conclusion, lung epithelial TACE is essential for promoting fetal lung saccular formation, but not postnatal lung alveolarization in mice. Because the developmental abnormality of either lung or heart induced by TACE deficiency does not directly lead to neonatal lethality, the neonatal death of TACE conventional knockout mice is likely a result of synergistic effects of multiple organ abnormalities.
Keywords: tumor necrosis factor-α converting enzyme, lung development
tumor necrosis factor-α converting enzyme (TACE, also known as ADAM17) is one of the well-characterized cell membrane sheddases and belongs to the family of a disintegrin and metalloproteinase (ADAM). TACE contains a signal peptide, a zinc-dependent metalloprotease catalytic domain, a disintegrin domain, a transmembrane domain, and a short cytoplasmic tail (1, 9). TACE regulates protein activity through a mechanism called ectodomain shedding by which cell surface proteins are cleaved by the zinc-dependent metalloprotease of TACE. Up to now, 76 functionally diverse cell surface transmembrane proteins have been identified as substrates for TACE, including cytokines and their receptors [e.g., tumor necrosis factor (TNF)-α and tumor necrosis factor receptor], members of neuregulins and their receptors (e.g., transforming growth factor-α, amphiregulin, and ErbB4), and adhesion molecules (e.g., L-selection and intercellular adhesion molecule 1), etc. (11, 14, 18).
Conventional knockout of TACE by genetic deletion of the zinc-binding domain (TACEΔZn/ΔZn) results in neonatal lethality in mice, with abnormal developmental phenotypes in multiple organs (17), including lung and heart as previously reported by us (20, 26). However, it is still unknown which organ malformation directly causes the neonatal lethality of TACEΔZn/ΔZn mice and what are the primary defects and secondary defects. Therefore, to further define the roles of TACE protein in regulating fetal development in vivo, specifically in lung and heart, we employed a loxP/Cre genetic approach to generate lung epithelial-specific and/or lung mesenchymal-specific, or cardiomyocyte-specific conditional knockout mice. In this paper, we compare phenotypic changes in these mice with both wild-type and TACE conventional knockout mice.
MATERIALS AND METHODS
Targeting of the TACE genomic locus.
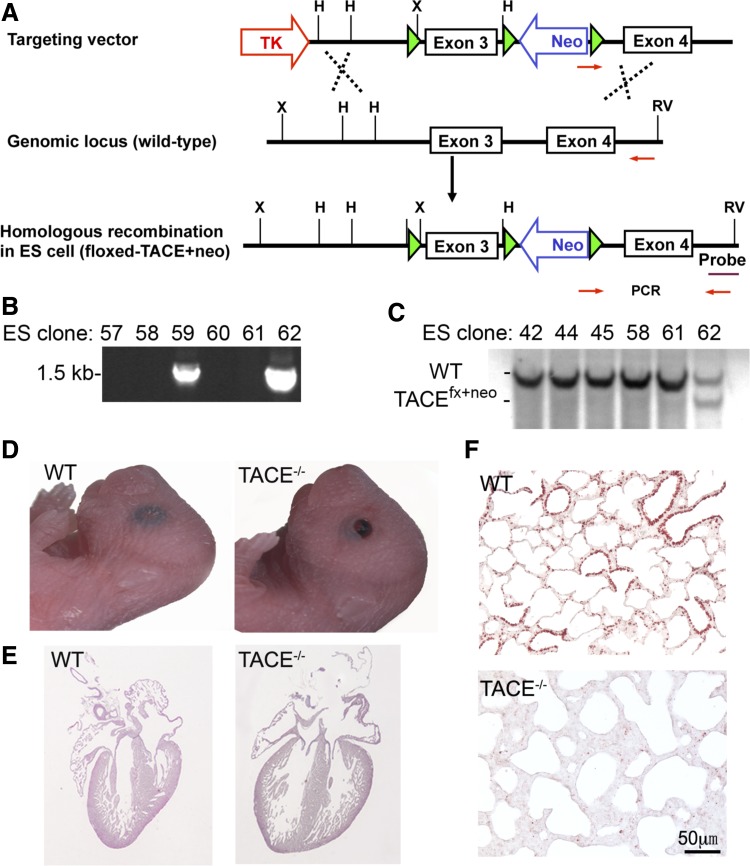
Floxed-TACE (TACEfx/fx) mice were generated in our laboratory. Briefly, a targeting vector was generated by subcloning TACE genomic DNA fragments into pNTKV-loxP vector, in which TACE exon 3 was flanked with loxP sites, in combination with an insertion of a floxed-PGK-neomycin cassette (loxP-exon3-loxP-PGKneo-loxP; Fig. 1A). Embryonic stem (ES) cells (derived from 129 mouse strain) were transfected with the targeting vector by electroporation and cultured under antibiotic selection for DNA homologous recombination at the National Heart, Lung, and Blood Institute Transgenic Facility. Several ES clones were positive for genomic DNA PCR screening with primers 5′-TGG AAT GTT TCA GAT TTG CAT ACC C-3′ and 5′-AAG AGC AAT CAG TGC TCT CAA CCG TTG-3′ (Fig. 1B). These ES clones were further verified for their DNA recombination by Southern blot. Briefly, genomic DNA was digested by EcoRV/HindIII and separated by agarose gel electrophoresis. After transfer to Nylon membrane, DNA was hybridized with a 3′-probe external to the targeting region to detect the allele with DNA homologous recombination (4.4 kb) compared with wild-type allele (6.2 kb) (Fig. 1C). The verified ES cells were then injected into C57BL/6J blastocysts to obtain chimeric mice of floxed-TACE. After further breeding, mice with germ line transmission of floxed-TACE were selected.
Fig. 1.
Generation of floxed-tumor necrosis factor-α converting enzyme (TACE) mice. A: schematic diagram of the floxed-TACE-neo vector and related embryonic stem (ES) cell genomic DNA structures before and after vector targeting. B: genotyping by genomic DNA PCR using the primers indicated in A. Clones 59 and 62 were positive for floxed-TACE-Neo homologous recombination. C: genotypic confirmation of the ES cell clones by Southern blot. Genomic DNA was digested by EcoRV (RV) and HindIII (H) enzyme, and the membrane was hybridized with a 3′-external probe indicated in A. WT, wild type. TACE conventional knockout (TACE−/−) mice were generated by crossing floxed-TACE and CMV-Cre mice. These newborn TACE−/− mice had phenotypes similar to those of TACEΔZn/ΔZn, including open eyelids (D), cardiac enlargement with deep intertrabecular recesses (E), and hypoplastic lung (F). Loss of TACE protein expression in TACE−/− mice was verified by TACE immunostaining (red color, F).
The PGKNeo cassette was then removed by crossing the floxed-TACE mice with EIIa-Cre mice by random loxP DNA recombination (24), generating mice with loxP sites only on both sides of TACE exon 3 (TACEfx). TACE knockout allele (TACE−) derived from TACEfx was also generated by crossing TACEfx/fx and X-linked CMV-Cre mice (19). Upon Cre-mediated deletion of TACE exon 3, a stop codon (TGA) was created immediately in the TACE protein open-reading frame to terminate TACE protein translation prematurely in the prodomain region, resulting in deficiency of TACE function.
Mouse strains and breeding.
Inducible lung epithelial-specific Tet-On Cre transgenic mice (SPC-reverse tetracycline transactivator, SPC-rtTA/TetO-Cre) were provided by Dr. Jeffrey Whitsett (16). Mesoderm-specific Dermo1-Cre heterozygous knockin mice (Dermo1-Cre) were obtained from Dr. David Ornitz (4, 25). NKX2.5-Cre transgenic mice were provided by Dr. Robert Schwartz (13). All mice were bred in the C57BL/6 strain background and genotyped by genomic DNA PCR. Mice used in this study were housed in a pathogen-free conditions and were reviewed and approved by the Institutional Animal Care and Use Committee at the Saban Research Institute of Children's Hospital Los Angeles.
Lung epithelial-specific TACE conditional knockout (TACE Ep-CKO) mice were generated by crossing TACEfx/fx and TACE+/−/SPC-rtTA/TetO-Cre mice, with doxycycline (Dox) induction [625 mg/kg in food (TestDiet, Richmond, IN) and 0.5 mg/ml in drinking water (Sigma-Aldrich, St. Louis, MO)] from embryonic day (E) 6.5. Time-pregnant TACE Ep-CKO (TACEfx/−/SPC-rtTA/TetO-Cre) and control (TACEfx/+, TACEfx/−, TACEfx/+/SPC-rtTA, TACEfx/+/TetO-Cre, TACEfx/−/SPC-rtTA, or TACEfx/−/TetO-Cre) mice were compared for their lung development. Mesoderm-specific TACE conditional knockout (Me-CKO) and myocardium-specific TACE conditional knockout (My-CKO) were generated by crossing TACEfx/fx and TACE+/−/Dermo1-Cre and TACE+/−/NKX2.5-Cre mice, respectively. Mice with TACE Me-CKO carried genotypes of TACEfx/−/Dermo1-Cre, and TACE My-CKO had genotypes of TACEfx/−/NKX2.5-Cre.
Histology and morphometric analysis.
Lung or heart tissues were fixed in 4% paraformaldehyde at 4°C overnight, then dehydrated and embedded in paraffin. Hematoxylin and eosin (H&E)-stained tissue sections were used for histological examination. For lung morphometric analysis, intratracheal instillation with fixation solution under 25 cmH2O pressure was applied before harvesting lung specimens. Five tissue sections from the same embedded lobe of each sample were randomly chosen at ∼250-μm intervals and stained with H&E. The mean linear intercept (MLI) was measured according to established methods. Briefly, an image of each examined section was digitally captured at ×100 magnification. The horizontal and vertical lines of a rectangle grid at 0.9-mm intervals were then used to count alveolar surface intersections. The MLI was calculated by the equation: the sum of the length of all counting lines divided by total number of counted intercepts of alveolar septa (3). The morphometric data were analyzed with Student's t-test to compare the differences between mean values. P values ≤0.05 were considered statistically significant.
Western blot.
TACE and other proteins in lung tissue lysate were detected by Western blot as described in our previous publication (22). Briefly, fresh lung tissues were lysed on ice in RIPA buffer containing 1 mmol/l phenylmethylsulfonyl fluoride, Halt protease and phosphatase inhibitor cocktail (Thermo Scientific), and 1 mmol/l sodium orthovanadate. Protein concentration was measured by the Bradford method using reagents purchased from Bio-Rad Laboratories (Hercules, CA). Equal amounts (40 μg) of total tissue lysate proteins were separated in 4–12% gradient NuPAGE gels using a MOP buffering system (Invitrogen). The proteins were transferred onto PVDF membrane, and proteins of interest were detected by Western blot using appropriate antibodies.
Protein immunostaining.
Antigen retrieval was performed by boiling lung tissue sections either in Tris-EDTA buffer (10 mM Tris·HCl, 1 mM EDTA, 0.05% Tween 20, pH 9.0) for immunofluorescence staining or in citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for immunohistochemistry. The tissue sections were blocked by 10% donkey or goat serum for 1 h at room temperature, followed by incubation with primary antibody overnight at 4°C. AlexaFluor-labeled donkey secondary antibodies or biotin-labeled goat secondary antibody (Invitrogen) was used for detection. The primary antibodies were rabbit anti-TACE (AB930; R&D Systems), rabbit anti-Pro-surfactant protein C (SP-C) (WRAB-9337; Seven Hills Bioreagents, Cincinnati, OH), goat anti-club cell-specific protein (CCSP) (SC-9772; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-α-smooth muscle actin (SMA) (A2547; Sigma-Aldrich), mouse anti-β-tubulin IV (MU178-UC; BioGenex, San Ramon, CA), and rabbit anti-platelet endothelial cell adhesion molecule 1 (PECAM-1) (LS-B1932; Lifespan Biosciences, Seattle, WA).
Quantitative RT-PCR.
Total RNA was isolated from snap-frozen lung tissues using the RNeasy kit (Qiagen, Valencia, CA) following the manufacturer's protocol. cDNAs were synthesized using the iScript kit (Bio-Rad). Real-time quantitative PCR were performed using SsoFast EvaGreen Supermix and detected by an iCycler-iQ system (Bio-Rad), as reported previously (23). The PCR primers for SP-C, CCSP, aquaporin 5 (AQP5), β-tubulin IV, and GAPDH have been described in our previous publication (21). The following primer sequences were used for vascular endothelial growth factor α (VEGFα): 5′-CTG GAC CCT GGC TTT ACT GC-3′ and 5′-TGA ACT TGA TCA CTT CAT GGG ACT-3′.
Cell proliferation.
Cell proliferation was analyzed by measuring proliferating cell nuclear antigen (PCNA)-positive cells. Briefly, PCNA immunofluorescence staining was performed using mouse anti-PCNA antibody (13-3900; Invitrogen) following the procedures described above. Six images of PCNA-stained tissue section were randomly captured at ×200 magnification. The numbers of PCNA-positive cells and total cells in each image were automatically counted using Image-J software. The proliferation rate was estimated by calculating the percentages of PCNA-positive cells.
Data presentation and statistical analysis.
At least five pairs of TACE conditional knockout mice and wild-type littermate control mice from different dams were analyzed in each experimental subgroup. All quantitative data were expressed as means ± SE. Statistical difference between two independent groups was assessed by an independent-sample t-test; P values ≤0.05 were considered statistically significant.
RESULTS
Conditional knockout of TACE in lung epithelial cells vs. mesenchymal cells during fetal mouse lung development.
TACEΔZn/ΔZn mice are conventional knockout, in which TACE is inactivated by deleting the zinc-binding domain in all cells. To further dissect TACE function in vivo, we then generated a mouse line in which the exon 3 of TACE gene was flanked with loxP DNA elements (Fig. 1, A–C) so that the exon 3 can be deleted (TACE−/−) upon Cre-mediated loxP DNA recombination. Deletion of exon 3 in TACE transcript shifts its open-reading frame, and introduces a STOP codon in the prodomain region, resulting in premature termination of TACE protein translation. Therefore, the expressed NH2-terminal 77-amino acid peptide does not contain TACE functional domains ranging from metalloprotease to cytoplasmic tail. This has been verified in vivo by generating TACE−/− conventional knockout mice through crossbreeding TACEfx/fx and X-linked CMV-Cre mice. Loss of TACE protein expression in TACE−/− mice was verified by TACE immunostaining of the lung tissue (Fig. 1F). These TACE−/− mice had the same phenotypes as observed in TACEΔZn/ΔZn knockout mice (17, 20, 26), including neonatal lethality, open eyelids, enlargement of cardiac ventricle with deep intertrabecular recesses, and neonatal pulmonary hypoplasia (Fig. 1, D–F).
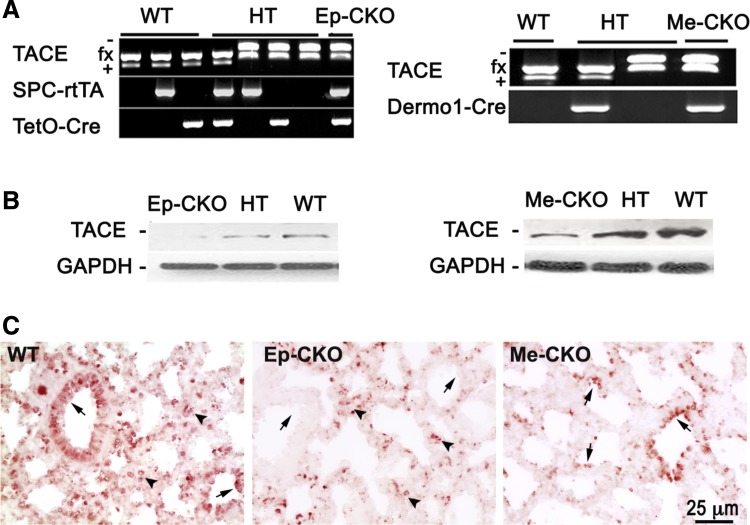
As shown in our previous publication, TACE is expressed in both fetal lung epithelial and mesenchymal cells depending on the developmental stages (26). To understand the cell type-specific role of TACE in regulating lung development, we then generated TACE conditional knockout specifically in lung epithelial cells or in mesenchymal cells of developing lungs. Lung epithelial cell-specific TACE conditional knockout mice (TACE Ep-CKO) were obtained by crossing TACEfx/fx and SPC-rtTA/TetO-Cre driver line, with Dox induction starting from E6.5, while mesenchymal cell-specific TACE conditional knockout (TACE Me-CKO) was achieved by crossing TACEfx/fx and the Dermo1-Cre driver line. In addition to DNA genotyping, TACE conditional knockouts were confirmed at the protein level by Western blot and immunohistochemistry (Fig. 2). As mentioned above, TACE is expressed in both epithelial and mesenchymal cells of perinatal lungs, and homozygous knockout of TACE in one cell compartment only resulted in significant reduction, but not full abrogation, of TACE protein as measured in whole lung tissue lysate, shown in Fig. 2B. The level of TACE protein in heterozygous knockout lung is between that of wild-type and homozygous knockout. Furthermore, lack of TACE protein expression in lung epithelium or mesenchyme of TACE Ep-CKO or TACE Me-CKO mice was verified by TACE protein immunostaining of lung tissue sections (Fig. 2C).
Fig. 2.
Generation of lung epithelial-specific (Ep) or mesenchymal-specific (Me) TACE conditional knockout mice. A: PCR genotyping of offspring from crossbreeding between TACEfx/fx and TACE+/−/SPC-rtTA/TetO-Cre, or between TACEfx/fx and TACE+/−Dermo1-Cre mice. B: significant reductions of TACE protein in the whole lung tissue lysates of related homo- or heterozygous knockout mice at postnatal (P) day 1 were detected. C: TACE deletions in lung epithelia vs. mesenchyme were further verified by TACE protein immunostaining. TACE protein expression in lung epithelium (arrows) or mesenchyme (arrowheads) was absent in TACE Ep-CKO and TACE Me-CKO lungs, respectively, compared with that in wild-type control.
Conditional knockout of TACE gene specifically in lung epithelium resulted in abnormal lung development but no neonatal lethality.
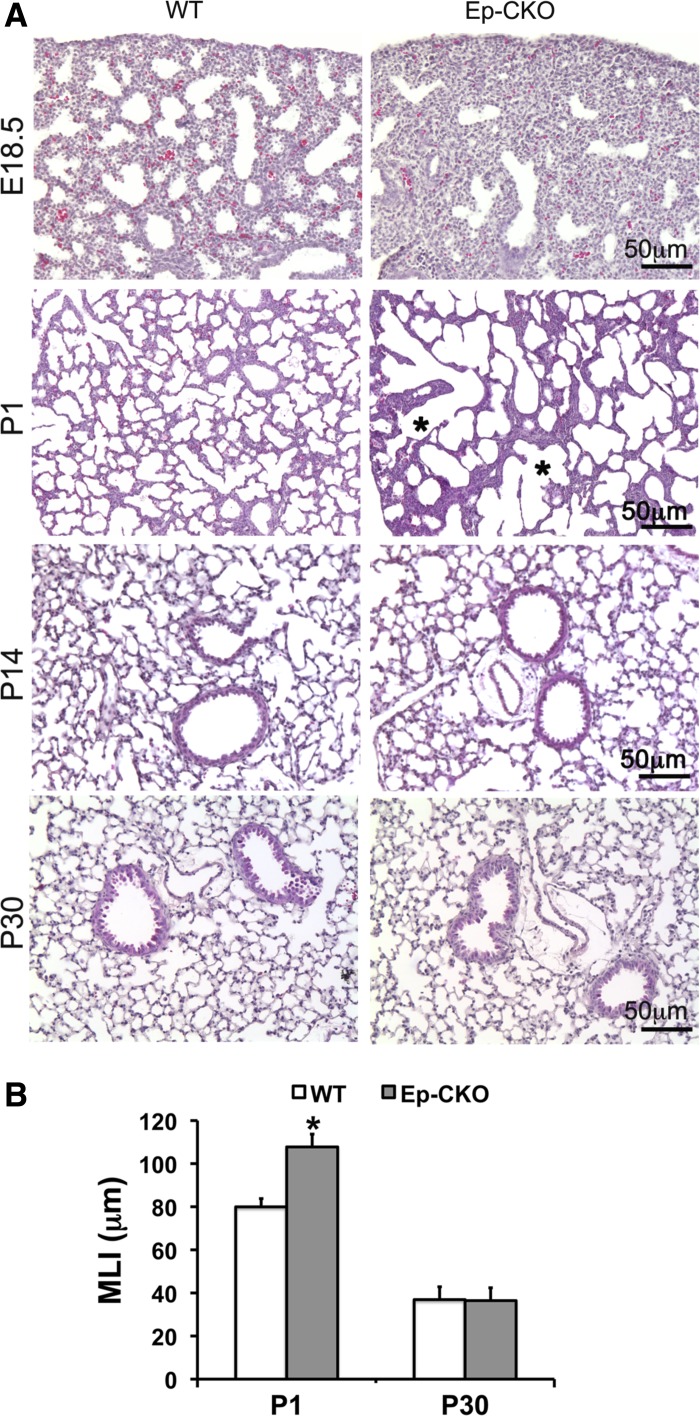
In contrast to the phenotype of conventional TACE−/− mice in which both respiratory distress and neonatal lethality were observed, neonatal TACE Ep-CKO mice survived without obvious respiratory distress. Lung tissue specimens were then harvested at prenatal E18.5 and postnatal day (P) 1, 14, and 30 for further analyses. Interestingly, marked reduction of terminal sacs and thickened surrounding mesenchyme in peripheral TACE Ep-CKO lung, compared with wild-type littermate controls, were detected at prenatal E18.5 (Fig. 3). Upon air inflation after birth, larger but fewer air sacs were observed at P1 TACE Ep-CKO lungs, as quantitatively verified by increased MLI (107.77 ± 5.95 μm in TACE Ep-CKO lungs vs. 79.95 ± 3.84 μm in wild-type controls, P < 0.01). Surprisingly, the abnormal morphological changes of TACE Ep-CKO lungs observed at E18.5 and P1 were no long detected after alveolarization was initiated (P14 and P30; Fig. 3), suggesting that TACE deficiency in lung epithelial cells only affects saccular formation, but has no effect on alveogenesis.
Fig. 3.
Lung epithelial-specific deletion of TACE resulted in abnormal perinatal lung development. A: hematoxylin and eosin (H&E)-stained lung tissue section of TACE Ep-CKO mice and WT littermates at different developmental stages. The diameters (shown by asterisk) of pulmonary alveoli in P1 TACE Ep-CKO lung were significantly increased compared with WT controls. B: morphometric analysis of TACE Ep-CKO lung by mean linear intercept (MLI) at different development stages. The MLI was significantly larger in P1 TACE Ep-CKO lungs (107.77 ± 5.95 μm) than that of wild-type controls (79.95 ± 3.84 μm, P < 0.01). After postnatal lung alveolarization (P14 and P30), the morphological difference between TACE Ep-CKO and wild-type control was no longer detected (MLI: 36.44 ± 0.91 μm in TACE Ep-CKO lung vs. 36.89 ± 0.17 μm in WT control at P30). *P < 0.05 (n = 5 for each genotype at the specified age).
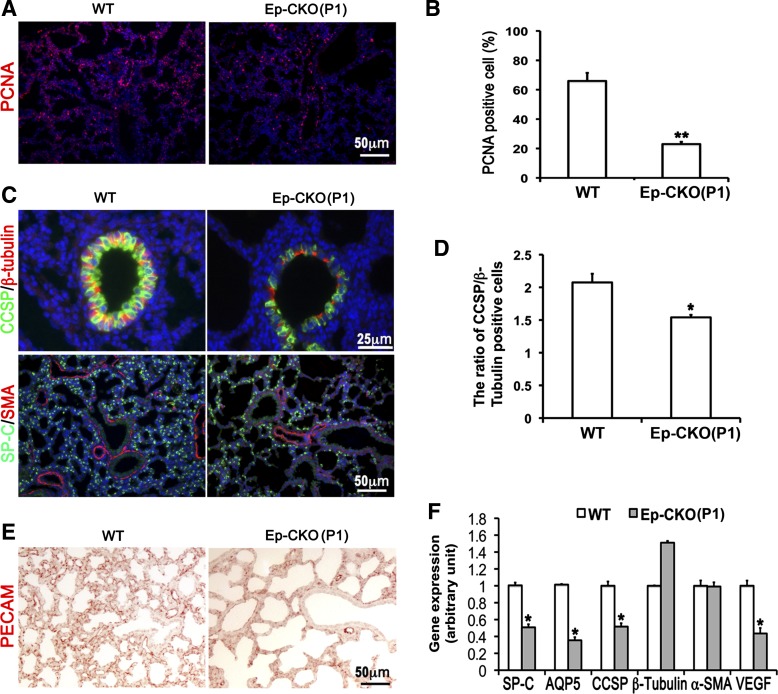
Consistently, reduced cell proliferation of TACE Ep-CKO lung at P1 (Fig. 4, A and B), but not at the end of alveolarization (P30; Fig. 5), was detected by PCNA immunostaining (23.0 ± 1.5% in P1 TACE Ep-CKO compared with 66.0 ± 5.5% in P1 wild type, P = 0.002). Furthermore, cell differentiation in TACE Ep-CKO lung was evaluated by measuring expression of specific epithelial and mesenchymal cell markers using real-time PCR at the mRNA level and immunostaining at the protein level. As shown in Fig. 4F, mRNA expression of genes marking distal differentiated lung alveolar epithelial cells (SP-C and AQP5) and middle/proximal airway club cells (CCSP) was significantly reduced in the P1 TACE Ep-CKO lung compared with wild-type controls. Immunofluorescence staining of the related proteins further verified these changes at the protein level (Fig. 4C). Interestingly, in addition to the reduction of CCSP-positive club cells (Fig. 4, C and D), shown by reduction in the ratio of CCSP/β-tubulin IV-positive cells (1.54 ± 0.13 in Ep-CKO lung vs. 2.08 ± 0.13 in wild-type control, P = 0.013), a flattened cell shape was also observed for TACE Ep-CKO club cells. Expression level and distribution pattern of SMA (a marker for smooth muscle cells and myofibroblasts) were not altered in TACE Ep-CKO lung compared with the wild-type control (Fig. 4, C and F). However, expression of VEGFα was significantly decreased in P1 TACE Ep-CKO lung (Fig. 4F), and was accompanied by a reduction in pulmonary capillary formation, as shown by endothelial cell marker PECAM-1 staining (Fig. 4E). These data suggest that neonatal lung epithelial TACE is important in regulating cell proliferation and differentiation as well as pulmonary capillary formation, which promote lung maturation at birth.
Fig. 4.
Lung epithelium-specific deletion of TACE resulted in decreased cell proliferation, impaired epithelial cell differentiation, and reduced pulmonary capillary formation. A and B: cell proliferation was detected by proliferating cell nuclear antigen (PCNA) immunostaining and analyzed as a percentage of PCNA-positive cells. Cell proliferation was significantly reduced in P1 TACE Ep-CKO lung (23.0 ± 1.5%, **P < 0.01) compared with wild-type controls (66.0 ± 5.5%). C and D: immunostaining of molecular markers of differentiated lung cells in TACE Ep-CKO lungs. Alveolar epithelial cells were significantly reduced in the P1 TACE Ep-CKO lung. Moreover, club cells in the airways were also significantly reduced and flattened in shape in TACE Ep-CKO compared with the controls. The reduction of club cell in bronchiole was quantified by calculating the ratio of club cell-specific protein (CCSP)-positive to β-tubulin IV-positive cells (2.08 ± 0.13 in wild-type controls vs. 1.54 ± 0.13 in Ep-CKO lung, *P = 0.013). Expression level and distribution pattern of α-smooth muscle actin (SMA), a marker of smooth muscle cells and myofibroblasts, were similar between TACE Ep-CKO and normal control lungs. Cell nuclei were counterstained by DAPI (blue). E: reduced platelet endothelial cell adhesion molecule 1 (PECAM)-positive cells demonstrate impaired capillary formation in Ep-CKO lung. F: gene expression of surfactant protein C (SP-C), aquaporin 5 (AQP5), CCSP, and vascular endothelial growth factor α (VEGFα) at the mRNA level was significantly decreased in TACE Ep-CKO lung as measured by real-time PCR. *P < 0.05.
Fig. 5.
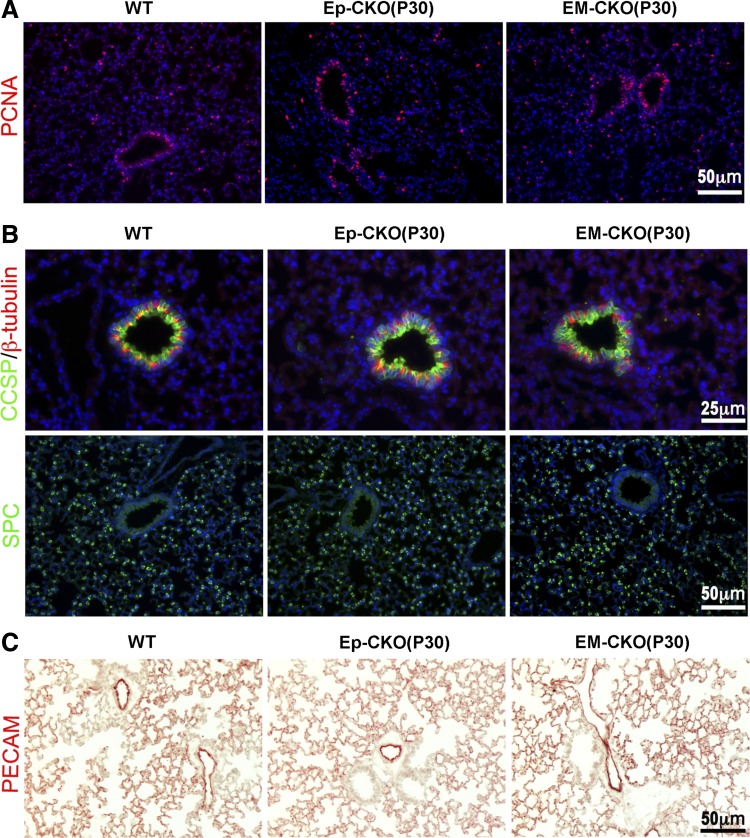
No phenotypic changes were detected in P30 TACE Ep-CKO or EM-CKO lung. A: cell proliferation was examined by PCNA immunostaining. No significant difference was observed for PCNA-positive cells among the lungs of TACE Ep-CKO, EM-CKO, and wild-type mice. B and C: immunostaining of molecular markers for differentiated lung cells, including type II alveolar epithelial cells (SP-C), club cells (CCSP), ciliated airway epithelial cells (β-tubulin IV), and endothelial cells (PECAM). No significant differences were observed among the lung with indicated different TACE genotypes. Cell nuclei were counterstained by DAPI (blue in A and B).
Abrogation of TACE in developing mouse lung mesenchyme had no impact on lung development.
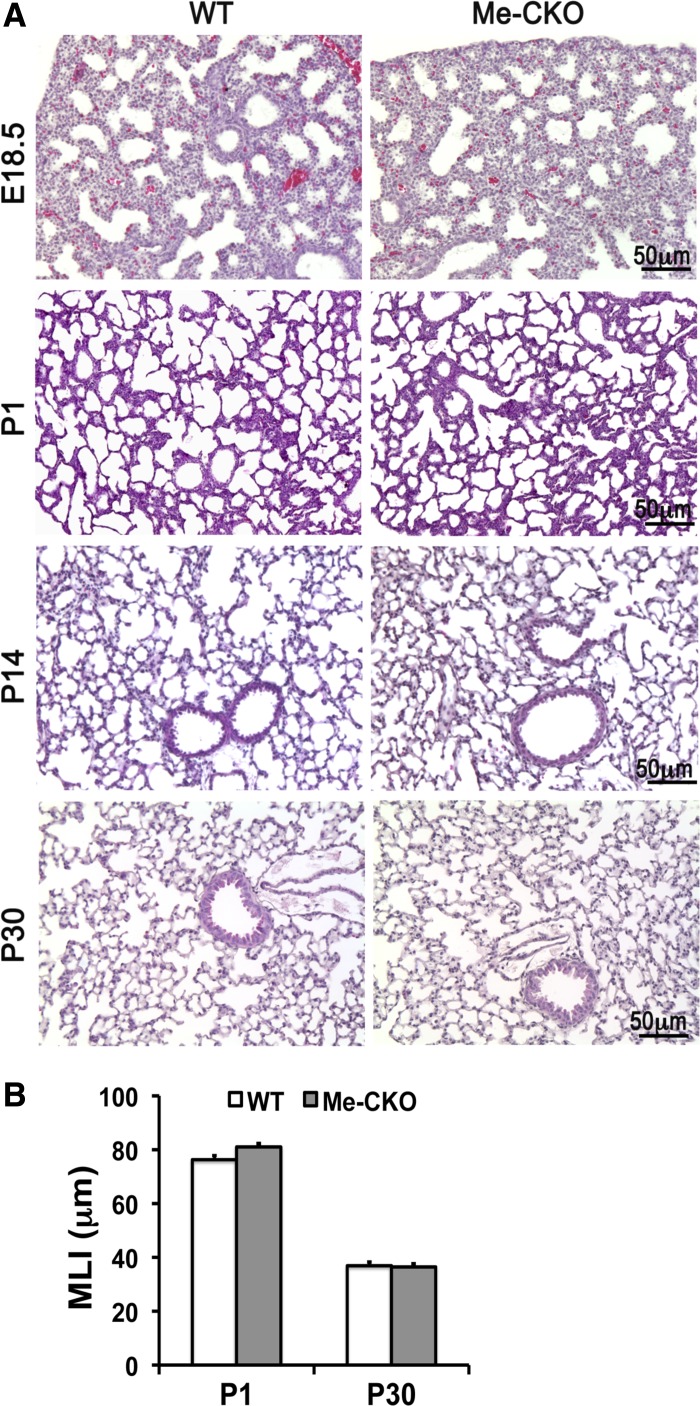
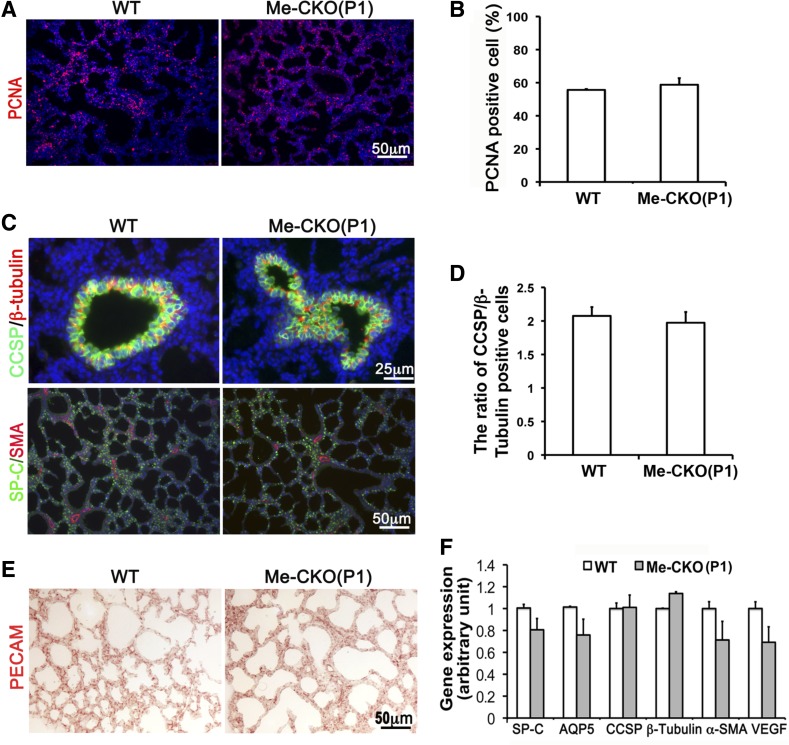
Because TACE expression is also detected in the mesenchyme of fetal and adult lung, it is possible that TACE expressed in lung mesenchymal cells may also play a role in regulating lung development. In this study, the mesenchyme-specific TACE conditional knockout (TACE Me-CKO) mice were generated by crossing TACEfx/fx with Dermo1-Cre mice (Fig. 2, A and B), as described above. Cre recombinase activity in the Dermo1-Cre mouse driver line is detected in mesoderm-derived tissues, including lung and tracheal mesenchyme, branchial arches, somites, and condensed mesenchyme-derived chondrocytes and osteoblasts, from E9.5 (5, 10, 25). TACE Me-CKO mice had no signs of respiratory distress or other observable phenotypes. Further lung morphological analyses did not show changes in overall lung structures from neonatal saccular formation to postnatal secondary alveolarization (E18.5-P30) in TACE Me-CKO mice (Fig. 6). Neither altered cell proliferation nor abnormal proximal and distal lung epithelial cell differentiation was detected in TACE Me-CKO lung (Fig. 7). In addition, smooth muscle cells of both airways and vasculatures and pulmonary capillary formation were not affected. Therefore, TACE expression in lung mesenchymal cells does not have an indispensable role in lung development.
Fig. 6.
Lung mesenchymal-specific deletion of TACE did not result in lung morphological change. A: histological comparison of lungs between TACE Me-CKO and WT mice at different developmental stages. E18.5, embryonic day 18.5. B: morphometric analysis (MLI) of TACE Me-CKO lungs at P1 and P30. There is no significant difference between TACE Me-CKO lungs and wild-type controls.
Fig. 7.
Lung mesenchymal-specific deletion of TACE results in no detectable changes in lung cell proliferation and differentiation. A and B: cell proliferation was detected by PCNA immunostaining. No significant differences were observed between TACE Me-CKO lungs (60.4 ± 4.5%) and wild-type controls (58.0 ± 2.1%). C and D: immunostaining of molecular markers of differentiated lung cells in TACE Me-CKO lungs. No significant changes were observed in Me-CKO lung. DAPI (blue) was used for cell nuclei counterstaining. E: capillary endothelial cells were detected by PECAM immunohistochemistry. No significant change was observed in TACE Me-CKO compared with wild-type control. F: gene expression at the mRNA level was quantified by real-time RT-PCR.
Simultaneous TACE conditional knockout in both lung epithelia and mesenchyme resulted in perinatal lung abnormalities similar to those of TACE Ep-CKO mice.
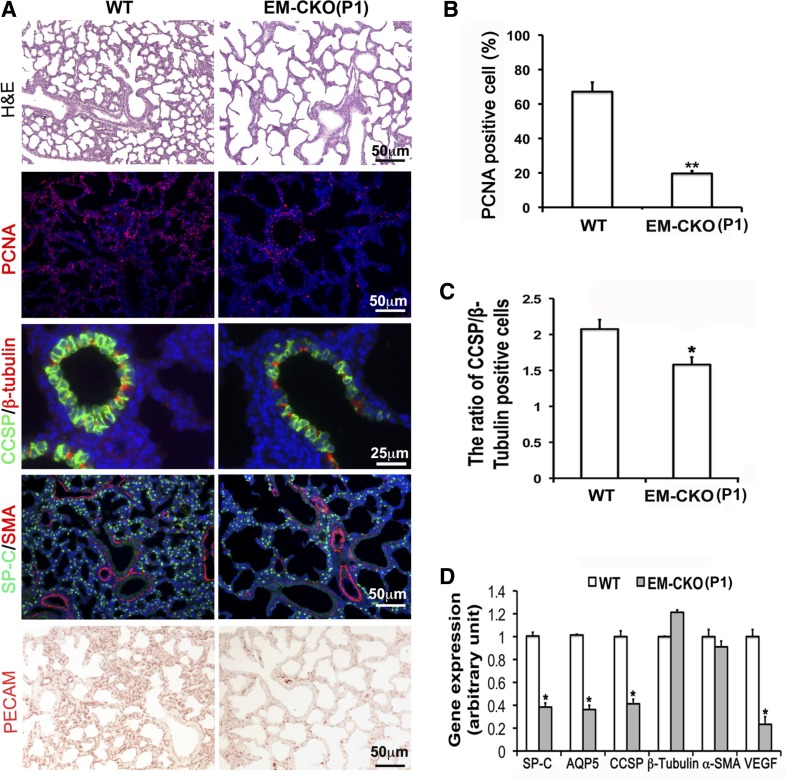
Without additional environmental insult, adult TACE Ep-CKO or TACE Me-CKO mice did not show any structural and functional abnormality in lung, although altered lung developmental cell biology was detected perinatally. To determine whether there is functional redundancy or compensation between lung epithelial and mesenchymal TACE protein, we then generated TACE conditional knockout in both epithelial and mesenchymal cells (TACE EM-CKO) by crossing TACE+/−/SPC-rtTA/TetO-Cre mice with TACEfx/fx/Dermo1-Cre mice with Dox induction from E6.5 to P1. The changes of lung histopathology of neonatal TACE EM-CKO mice were similar to those seen in TACE Ep-CKO lung (Fig. 8), including enlarged airspaces with fewer number of saccules (MLI: 111.36 ± 6.84 μm in EM-CKO lung vs. 83.89 ± 3.65 μm in wild-type controls, P < 0.001), reduced cell proliferation (19.67 ± 1.35% in EM-CKO lung vs. 67.18 ± 4.2% in wild type, P < 0.005), reduced distal lung epithelial cell differentiation, decreased club cell number in middle/proximal airway accompanied by flattened club cell shape (the ratio of CCSP-positive cells to β-tubulin IV-positive cells: 1.58 ± 0.10 in EM-CKO lung vs. 2.08 ± 0.13 in wild-type controls, P < 0.05), and impaired capillary formation. However, the phenotypic changes caused by TACE abrogation in both epithelial and mesenchymal cells of perinatal lung were not so severe as to cause neonatal lethality, as seen in TACE−/− whole body conventional knockout mice. Interestingly, despite neonatal abnormalities, normal lung morphology of TACE EM-CKO mice was restored after alveolarization (Fig. 5).
Fig. 8.
TACE conditional knockout simultaneously in both lung epithelium and mesenchyme resulted in abnormal lung development at P1. A–C: changes of lung morphology, cell proliferation, and differentiation in TACE EM-CKO mice. Pulmonary alveoli in TACE EM-CKO lung were significantly enlarged compared with WT controls (H&E). Reduced cell proliferation, shown by PCNA immunostaining, was detected in TACE EM-CKO lung (19.67 ± 1.35 vs. 67.18 ± 4.2% in WT control, **P < 0.005). Molecular markers for differentiated cells were also examined by immunohistochemistry. Cells positively stained for SP-C or CCSP were significantly decreased in TACE EM-CKO lung, whereas SMA-positive or β-tubulin IV-positive cells remained at the same level as in WT controls. The ratio of CCSP-positive cells to β-tubulin IV-positive cells is reduced (1.58 ± 0.10 in EM-CKO lung vs. 2.08 ± 0.13 in WT control, *P < 0.05). Impaired capillary formation in EM-CKO lung is demonstrated by reduced PECAM-positive cells. DAPI (blue) was used for cell nuclei counterstaining. D: significant reduction of gene expression of SP-C, AQP5, CCSP, and VEGF at the mRNA level was verified in P1 TACE EM-CKO lung tissue by real-time PCR. *P < 0.05.
Abnormal myocardial development caused by TACE cardiomyocyte-specific conditional knockout did not directly result in neonatal lethality.
As demonstrated above, the lung developmental abnormality did not result in neonatal lethality, which was observed in TACE conventional knockout mice (20). We then wondered whether other developmental abnormalities, particularly the pathology of noncompaction of ventricular myocardium, were the direct cause for the neonatal lethality in TACE conventional knockout mice. Myocardium-specific TACE conditional knockout (TACE My-CKO) mice were then generated by crossing TACEfx/fx mice with NKX2.5-Cre mice. Not surprisingly, cardiac enlargement with reduced myocardial compaction was observed in E18.5 TACE My-CKO mice (Fig. 9), which was similar to that seen in TACE−/− conventional knockout heart (Fig. 1E). However, the neonatal TACE My-CKO mice survived without any observable phenotype by gross view. This suggests that the neonatal lethality in TACE−/− conventional knockout mice is not directly caused by single developmental abnormality of lung or heart, but rather from the synergistic effects of multiple organ dysfunctions.
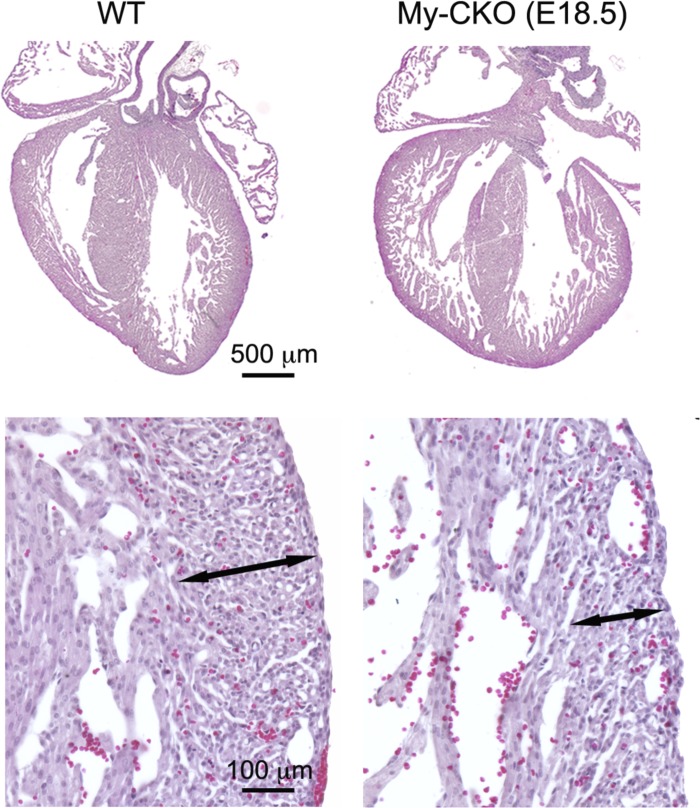
Fig. 9.
Abnormal myocardial development in TACE cardiomyocyte-specific conditional knockout. In E18.5 TACE My-CKO mouse, the heart was significantly enlarged, accompanied by a thickened trabecular layer with deep intertrabecular recesses and a thinner outer compact layer of myocardium (shown in high magnification).
DISCUSSION
Proteolytic processing of transmembrane proteins can serve as a posttranslational regulation of their functions. Indeed, 2–4% of the proteins on the cell surface are subjected to ectodomain shedding (2). TACE is a central component that mediates this process to regulate protein function, such as increasing free forms of TGF-α and TNF-α by releasing them from the cell surface and activating ErbB4 nuclear signaling by releasing its intracellular domain from the cell membrane (15).
Studies by our group and others have demonstrated that global abrogation of TACE function by genetically deleting zinc-binding domain (TACEΔZn/ΔZn) results in neonatal lethality with multiple developmental defects (1, 20), including defects in lung and heart. However, the direct effects of TACE deficiency on lung development and maturation and the related outcomes are not clear. To study cell- and organ-specific TACE function in vivo, we first generated a TACEfx/fx mouse line in which the exon 3 of TACE gene is flanked with inserted loxP DNA sequences. To confirm that deletion of floxed-exon 3 of TACE gene caused loss of TACE function, TACE−/− conventional knockout mice were then generated from our new TACEfx/fx mice by crossing them with X-linked CMV-Cre driver line. Those TACE−/− mice had the same phenotypes as reported in TACEΔZn/ΔZn knockout mice, including neonatal lethality, open eyelids, enlargement of cardiac ventricle with deep intertrabecular recesses, and neonatal pulmonary hypoplasia. Next, we generated the lung epithelial cell- or/and mesenchymal cell-specific TACE conditional knockout mice to determine whether a pulmonary defect is the primary cause for the neonatal lethality of TACE knockout mice. Interestingly, lung epithelial-specific TACE conditional knockout alone did not result in severe respiratory distress and neonatal lethality, although reduced saccular structure formation and altered lung cell proliferation and epithelial cell differentiation were observed. To exclude the possibility that mesenchymal TACE may partially compensate for the loss of epithelial TACE function, lung mesenchymal-specific TACE knockout was achieved. Surprisingly, no phenotypic abnormality was observed in lung mesenchymal TACE conditional knockout mice. Furthermore, combined TACE knockout in both lung epithelial and mesenchymal cells did not result in more severe pulmonary phenotypes than those obtained in TACE epithelial knockout alone. No neonatal lethality was seen in these mice. Thus, these data suggest that abnormal lung development directly caused by lung-specific TACE deficiency is relatively mild and does not result in respiratory distress. Particularly, postnatal alveolarization is not affected, and the lung structure is essentially restored to normal by the end of alveolarization, when loss of TACE function is restricted to lung tissue. The specific mechanisms underlying the transient lung phenotypes in lung epithelial cell-specific conditional knockout mice are not clear. One possibility is that TACE-mediated protein ectodomain shedding in lung epithelial cells under normal conditions is not critical to the alveolarization process, and that the growth factors and cytokines required for alveolarization can be released from other unaffected organs/systems to compensate for the deficit of these factors in lung. Future studies in which TACE global knockout will be induced from the postnatal stage will address this possibility. The other possibility is that the function of TACE in postnatal lung epithelial cells could be achieved by other cell surface sheddases. About 37 ADAM family members have been found in the mouse genome, with functional overlap among some of them (6, 9). Identification of the common targets of TACE and other ADAM family members in postnatal lung epithelial cells may provide the clues to test this possibility.
Based on our above data, it appears likely that neonatal lethality in conventional TACE knockout mice is caused by a synergistic effect of developmental defects of multiple organs. In our previous studies, we have noticed that TACEΔZn/ΔZn mice exhibit markedly enlarged fetal hearts with increased myocardial trabeculation and reduced cell compaction, mimicking the pathology of noncompaction of ventricular myocardium observed in human patients (20). To determine whether this cardiac abnormality results in neonatal lethality of TACE conventional knockout mice, we then generated cardiomyocyte-specific TACE conditional knockout mice. Although these mice had an abnormal cardiac phenotype similar to that observed in TACEΔZn/ΔZn mice, neonatal lethality was still not detected, and the abnormal cardiac structure persisted to adulthood (data not included). Therefore, cardiac abnormality alone in TACE conventional knockout mice is not sufficient to cause neonatal lethality. The neonatal respiratory distress that is prevalent in TACE conventional knockout mice may result from combined cardiac and pulmonary abnormalities that act synergistically to potentiate dysfunction (14, 22). For example, a reduced pulmonary capillary network may increase myocardial afterload, and reduced cardiac output may further decrease gas-exchange efficiency in the already hypoplastic lung. However, further experiments with combined TACE knockout in lung and heart are required to confirm this hypothesis.
The molecular mechanisms by which deficiency of TACE function causes retardation of peripheral lung development and maturation may be explored in future experiments. One potential candidate that is cleaved by TACE is ErbB4. One of the ErbB4 isoforms is subjected to sequential TACE and Presenilin-1 cleavage (7). The released intracellular domain of ErbB4 has been shown to act as a cofactor for Sftpb transcription and type II cell differentiation (12, 27). In addition, ErbB4 has been shown to be essential to mediate stretch-induced fetal lung cell differentiation (8). Therefore, further study on ErbB4 cleavage and activation in the different cell-specific TACE knockout lungs will help to understand how TACE-ErbB4 plays a role in promoting perinatal lung development and maturation.
In conclusion, blockade of TACE-mediated protein ectodomain shedding in lung epithelium results in a retardation of fetal lung development at late gestation, yet has no negative impact on postnatal lung alveolarization in mice. Although loss of TACE function in both lung and heart leads to significant pathological changes in their development, the malformation of each organ alone induced by TACE deficiency does not directly lead to lethality. This suggests that the neonatal lethality observed in TACE conventional knockout mice results from the synergistic effects of multiple organ abnormalities.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-109932 and HL-068597 (W. Shi), an American Heart Association Grant in Aid (W. Shi), and a California Institute of Regenerative Medicine Training Grant (W. Xu and Y. Luo).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.X., C.L., and W.S. conception and design of research; W.X., C.L., V.K., H.C., CH.L., and W.Z. performed experiments; W.X., C.L., V.K., H.C., Y.L., and W.S. analyzed data; W.X., C.L., V.K., and W.S. interpreted results of experiments; W.X. and W.S. prepared figures; W.X. and W.S. drafted manuscript; W.S. edited and revised manuscript; W.S. approved final version of manuscript.
REFERENCES
- 1.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385: 729–733, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6: 32–43, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Sun J, Buckley S, Chen C, Warburton D, Wang XF, Shi W. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol 288: L683–L691, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Zhuang F, Liu YH, Xu B, Del MP, Deng W, Chai Y, Kolb M, Gauldie J, Warburton D, Moses HL, Shi W. TGF-β receptor II in epithelia versus mesenchyme plays distinct role in developing lung. Eur Respir J 32: 285–295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PloS one 3: e1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med 29: 258–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeing K, Zscheppang K, Mujahid S, Murray S, Volpe MV, Dammann CE, Nielsen HC. Presenilin-1 processing of ErbB4 in fetal type II cells is necessary for control of fetal lung maturation. Biochim Biophys Acta 1813: 480–491, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Z, Wang Y, Nayak PS, Dammann CE, Sanchez-Esteban J. Stretch-induced fetal type II cell differentiation is mediated via ErbB1-ErbB4 interactions. J Biol Chem 287: 18091–18102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci 30: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Li L, Cserjesi P, Olson EN. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev Biol 172: 280–292, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Li N, Boyd K, Dempsey PJ, Vignali DA. Non-cell autonomous expression of TNF-alpha-converting enzyme ADAM17 is required for normal lymphocyte development. J Immunol 178: 4214–4221, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Purevdorj E, Zscheppang K, von Mayersbach D, Behrens J, Brinkhaus MJ, Nielsen HC, Schmiedl A, Dammann CE. ErbB4 regulates the timely progression of late fetal lung development. Biochim Biophys Acta 1803: 832–839, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2–5/Cre gene using ROSA26 reporter mice. Genesis 31: 176–180, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Moss ML, Sklair-Tavron L, Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Practice Rheumatol 4: 300–309, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294: 2179–2181, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Perl AK, Zhang L, Whitsett JA. Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol 40: 1–3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol 7: 380–387, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080–5081, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi W, Chen H, Sun J, Buckley S, Zhao J, Anderson KD, Williams RG, Warburton D. TACE is required for fetal murine cardiac development and modeling. Dev Biol 261: 371–380, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P, Jr, Ma JC, Warburton D, Shi W. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am J Pathol 172: 571–582, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Chen M, Ge N, Xu J. Hemagglutinin from the H5N1 virus activates Janus kinase 3 to dysregulate innate immunity. PloS one 7: e31721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Lan Q, Chen M, Chen H, Zhu N, Zhou X, Wang J, Fan H, Yan CS, Kuang JL, Warburton D, Togbe D, Ryffel B, Zheng SG, Shi W. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PloS one 7: e40314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Li C, Garrett-Beal L, Larson D, Wynshaw-Boris A, Deng CX. Direct removal in the mouse of a floxed neo gene from a three-loxP conditional knockout allele by two novel approaches. Genesis 30: 1–6, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130: 3063–3074, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Chen H, Peschon JJ, Shi W, Zhang Y, Frank SJ, Warburton D. Pulmonary hypoplasia in mice lacking tumor necrosis factor-alpha converting enzyme indicates an indispensable role for cell surface protein shedding during embryonic lung branching morphogenesis. Dev Biol 232: 204–218, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Zscheppang K, Konrad M, Zischka M, Huhn V, Dammann CE. Estrogen-induced upregulation of Sftpb requires transcriptional control of neuregulin receptor ErbB4 in mouse lung type II epithelial cells. Biochim Biophys Acta 1813: 1717–1727, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]