Abstract
Our previous studies have shown that the anti-asthma traditional Chinese medicine herbal formula ASHMI (anti-asthma simplified herbal medicine intervention) inhibits acetylcholine-induced contractions of tracheal rings from ovalbumin-sensitized and naive mice in a β-adrenoceptor-independent manner. We sought to determine whether acute in vivo ASHMI administration inhibits airway hyperreactivity (AHR) in a murine model of allergic asthma and acetylcholine-induced tracheal ring constriction ex vivo and to elucidate the cellular mechanisms underlying these effects. Ovalbumin-sensitized mice received a single oral ASHMI dose 2 h before intravenous acetylcholine challenge. AHR was determined by invasive airway measurements. Myography was used to determine the effects of ASHMI on acetylcholine-induced constriction of tracheal rings from asthmatic mice with or without epithelial denudation. The effect of cyclooxygenase inhibition and EP2/EP4 receptor blockade on ASHMI attenuation of acetylcholine contractions was evaluated. Tracheal cAMP and PGE2 levels were measured by ELISA. A single acute oral dose of ASHMI dramatically reduced AHR in response to acetylcholine provocation in ovalbumin-sensitized mice (P < 0.001). In ex vivo experiments, ASHMI significantly and dose-dependently reduced tracheal ring constriction to acetylcholine (P < 0.05–0.001), which was epithelium independent and associated with elevated cAMP levels. This effect was abrogated by cyclooxygenase inhibition or EP2/EP4 receptor blockade. ASHMI also inhibited contraction to high K+ (P < 0.001). ASHMI increased tracheal ring PGE2 release in response to acetylcholine or high K+ (P < 0.05 for both). ASHMI produced direct and acute inhibition of AHR in vivo and blocked acetylcholine-induced tracheal ring constriction via the EP2/EP4 receptor pathway, identifying the mechanism by which ASHMI is an orally active bronchoprotective agent.
Keywords: ovalbumin sensitization, mice, tracheal rings, contraction, myography, PGE2
protection against bronchoconstriction is integral to successful asthma management. Treatment with long-acting β-adrenoceptor-agonists, in conjunction with adequate steroid therapy supplemented with short-acting-β-adrenoceptor agonists, has been shown to be an effective regimen for asthma therapy (1, 7). Inhaled β2-adrenoceptor agonists are the most widely used drugs for providing acute relief from airway constriction during asthma exacerbations (15, 29, 33, 35). Despite the general effectiveness of this treatment regimen, a subset of asthmatic patients respond poorly to β2-agonists due to various reasons, such as differences in disease phenotype, body weight, and perhaps pharmacogenomic variations (5, 10, 13, 39). Development of tolerance to β-agonists after chronic use has also been reported (10, 27). Waning efficacy of β2-agonists over time has been attributed to desensitization of the β2-adrenoceptor or upregulation of muscarinic receptors that promote increased airway hyperreactivity or loss of a protective effect (16, 22, 23). In addition, the long-term safety of β2-agonist therapy has been the subject of debate because of reports of increased cardiovascular risk, asthma exacerbation, and sudden death due to asthma associated with long-acting β-adrenoceptor-agonist therapy, especially as monotherapy (8, 14, 25). Thus non-β2-adrenoceptor therapeutic modalities that ameliorate airway hyperreactivity merit further investigation.
We developed the anti-asthma simplified herbal medicine intervention (ASHMI), a three-herb formula containing Ganoderma lucidum, Sophora flavescens, and Gylicirrhiz uralensis, and reported previously that chronic oral administration improved airway function and reduced inflammation in human asthmatic patients and was found to be safe and well tolerated (43). Chronic oral ASHMI administration prevented airway hyperreactivity (AHR) in murine asthma models and was associated with decreased inflammation and plasma histamine and leukotriene release following allergen challenge (37, 43, 45). We also demonstrated that acute ex vivo ASHMI treatment of tracheal rings from asthmatic and naive mice dramatically suppressed contractile responses to acetylcholine (ACh) provocation, indicating that ASHMI also directly affects airway smooth muscle (ASM) contraction. Importantly, this effect was independent of β2-adrenoceptor activation (45), but the mechanism for this direct effect on ASM was not further elucidated. The present study expands on these findings and includes assessment of oral ASHMI administration on acute in vivo airway constriction in response to an ACh challenge. We further employed pharmacological agents in ex vivo tracheal ring myography studies to determine the cellular mechanisms by which the acute addition of exogenous ASHMI blocked ACh-induced contractions.
METHODS
Mice
Six-week-old BALB/c were purchased from Jackson Laboratories and housed in a vivarium at The Icahn School of Medicine, Mount Sinai. Animals were cared for according to the guidelines, and all procedures were approved by Mount Sinai Institutional Animal Care and Use Committee (Mount Sinai IACUC).
Antigen Sensitization/Challenge and ASHMI Treatment
BALB/c mice were sensitized intraperitoneally with 200 μg ovalbumin (OVA) and 2 mg alum in 0.4 ml of phosphate-buffered saline on days 0 and 7, and then challenged intratracheally with 100 μg OVA in 50 μl phosphate-buffered saline on day 14. Four additional intratracheal challenges were administered following the schedules as shown in Fig. 1A to generate OVA-sensitized mice. This protocol induces AHR and pulmonary inflammation (45). To determine the acute effect of ASHMI on AHR, mice received 4.5 mg ASHMI dissolved in 0.5 ml drinking water by oral gavage 2 h before ACh provocation (Fig. 1A). Untreated sensitized were given water (vehicle). Mice that were not sensitized or treated served as naive controls for some experiments. The timing of ASHMI administration was chosen to determine the potential direct effect of ASHMI on airways, distinct from the well-characterized long-term suppression of AHR by ASHMI attributable to anti-inflammatory effects following 4–6 wk of ASHMI therapy (36, 45). As the effect of ASHMI on naive (nonsensitized) mice has been well characterized in our studies referenced above, ex vivo experiments described in the present study focuses on findings in OVA-sensitized mice. ASHMI was produced by Sino-Lion Pharmaceuticals (Weifang, China). Detailed quality control data for ASHMI have been published previously (26, 45).
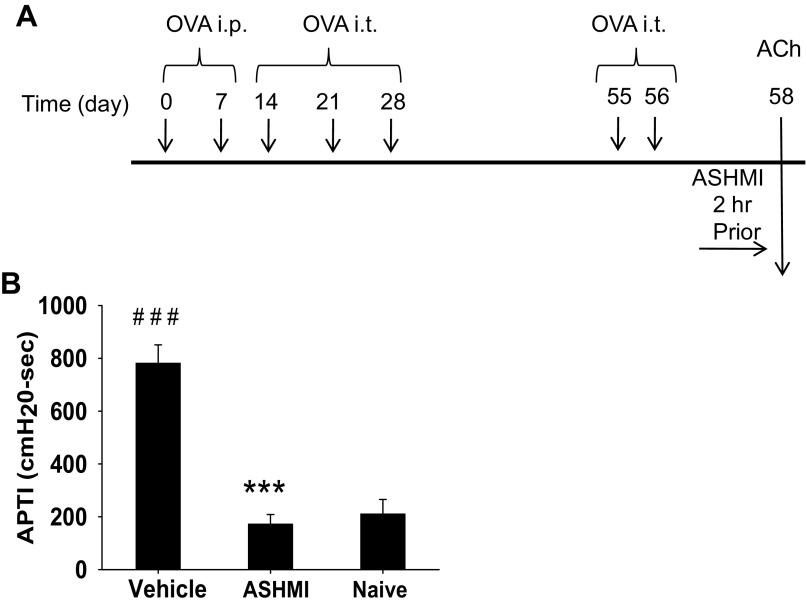
Fig. 1.
Anti-asthma simplified herbal medicine intervention (ASHMI) effects on in vivo airway hyperreactivity (AHR) to acetylcholine (ACh) provocation. A: ovalbumin (OVA)-sensitized mice were given 5 OVA intratracheal (it) challenges, and ASHMI was given 2 h before ACh exposure, which was given 48 h after the last OVA it. B: AHR to ACh exposure was determined by invasive measurement. APTI, airway pressure time index. Values are means ± SD; n = 4–5 mice/group. ***P < 0.001 vs. sham. ###P < 0.001 vs. naive.
Measurement of Airway Responses
An invasive technique was used to determine late-phase AHR to ACh provocation by measuring airway pressure changes following intravenous (iv) ACh, as reported previously in detail (28). Mice were anesthetized with a pentobarbital (80 mg/kg body wt)/xylazine (12 mg/kg body wt) injection given intraperitoneally and were ventilated via a tracheal cannula (18 gauge) at the rate of 120 breaths/min and a constant tidal volume of air (0.2 ml) with a RSP1002 Pressure Controlled Respirator System (Kent Scientific, Litchfield, CT). Muscle paralysis was induced by iv injection of decamethonium bromide (25 mg/kg). Airway pressure was measured with a pressure transducer via a port linked to tracheal cannula. Two minutes after establishing a stable airway pressure recording, ACh (100 mg/kg) was injected iv. The airway pressure changes were recorded for 4 min and calculated using VENTP software from the respiratory data-acquisition system (Kent Scientific). Airway responsiveness to ACh was expressed as time-integrated changes in peak airway pressure, referred to as airway pressure time index (APTI) (cmH2O/s).
Myography of Murine Tracheal Rings
Tracheas were excised from OVA-sensitized and challenged mice as shown in Fig. 1A, except that the fourth OVA challenge was given on day 29, and trachea was harvested on day 31. After removal of adventitious tissue, the trachea was cut into 3- to 4-mm rings, mounted on a Myodac 20 myography apparatus (ADInstruments, Denver, CO), and allowed to rest in physiological salt solution (PSS) (in mmol/l: 119 NaCl, 4.7 KCl, 1.17 MgSO4-7H2O, 1.18 KH2PO4, 2.5 CaCl2-2H2O, 25 NaHCO3, 0.027 EDTA, 5.5 glucose) with continuous oxygenation (95% O2/5% CO2) at 37°C for 30 min with no applied tension. Optimal passive tension (1 mN) was then applied to the rings. After stable baseline tension was achieved, the contractile potential of each ring was assessed by measuring contraction to 60 mM KCl before each experiment. After recording KCl responses, rings were washed four to six times and allowed to rest at baseline (1 mN) tension. In some experiments, tracheas were denuded by rubbing the lumen with flanged wire to remove epithelium. Contractile responses to ACh were evaluated using a submaximal concentration of 100 μM based on previous studies (45). For dose-response experiments, rings were pretreated with various doses of ASHMI (0–400 μg/ml) or PSS 30 min before ACh exposure. In some experiments, 5 μM indomethacin (Sigma Aldrich, St. Louis, MO), 5 μM nimesulide, or 0.5 μM SC-560 (both from Cayman Chemical, Ann Arbor, MI) was added simultaneously with ASHMI. In some experiments, the EP2 (AH6809) and EP4 (L161982) receptor antagonists (5 μM each, Cayman Chemicals, Ann Arbor, MI and Tocris Bioscience, Bristol, UK, respectively) were added 30 min before addition of ASHMI to organ baths and at concentrations determined in previous studies (4). In some experiments, rings were precontracted with 100 μM ACh before addition of ASHMI or PGE2. After completion of experiments, rings were washed and allowed to rest for 2 h. Contractile responses to 60 mM KCl were used to ascertain viability of tracheal rings after every experiment.
Measurement of PGE2.
We used methods described by Mehats et al. (30) with several modifications. Briefly, epithelium-intact or -denuded tracheal segments were exposed to ACh (100 μM) in the presence or absence of 200 μg/ml ASHMI in 250 μl of oxygenated PSS at 37°C. Samples were collected after 30 min and stored at −80°C until analyzed. Aliquots (50 μl) were assayed for PGE2 content by duplicate ELISA (Cayman Chemical, Ann Arbor, MI), according to the manufacturer's instructions.
Measurement of cAMP.
Tracheal tissue cAMP levels were measured using a commercial ELISA kit (R&D Systems, Minneapolis, MN). Briefly, epithelium-intact or -denuded tracheal tissue was snap frozen in liquid nitrogen and homogenized on dry ice and then was dissolved in medium provided in the kit. After centrifugation at 600 g for 10 min at 4°C, supernatants were assayed as per instructions provided by the manufacturer.
Statistical Methods
Data were analyzed using SigmaStat 2.03 (Systat, Chicago, IL). One-way ANOVA followed by Bonferroni adjustment was applied for all normally distributed data. Kruskal-Wallis one-way ANOVA on ranks, followed by Tukey's test, was used for data not normally distributed. Repeated-measures ANOVA on ranks was used for analysis of PGE2 dose-response data. P values < 0.05 were considered significant.
RESULTS
An Acute Single Dose of ASHMI Inhibited ACh-provoked AHR in OVA-sensitized Mice
To determine whether ASHMI produced a direct acute preventive effect on airways when given in vivo, a single dose of oral ASHMI was given to sensitized mice 2 h before iv ACh exposure, as indicated in Fig. 1A. AHR expressed as APTI values was significantly greater in vehicle-treated mice compared with naive animals, indicating successful establishment of AHR (P < 0.001, Fig. 1B). In contrast, APTI values of ASHMI-treated mice were dramatically lower than those of the vehicle group (P < 0.001) and essentially the same as those of naive mice. These results demonstrate that ASHMI acutely prevents AHR to ACh provocation in OVA-sensitized mice.
ASHMI Dose-dependently Suppressed ACh-induced Tracheal Ring Constriction
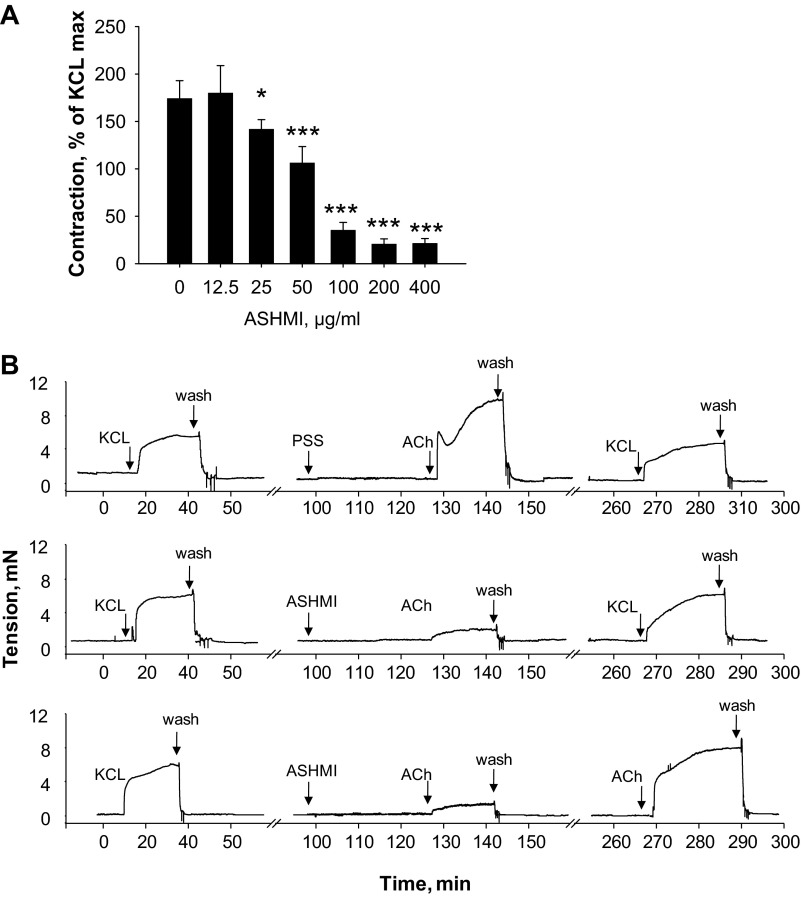
We next examined the dose dependence of ASHMI effects by pretreating tracheal rings from OVA-sensitized mice with various doses of ASHMI (0–400 μg/ml) ex vivo for 30 min. Addition of ASHMI to organ baths did not affect baseline tension at any dose studied. Upon stimulation with 10−4 M ACh, we found that significant inhibition of ACh constriction by ASHMI initially first occurred at 25 μg/ml (P < 0.05 vs. none, Fig. 2A). Maximal (∼80%) inhibition occurred at 200 μg/ml (P < 0.001 vs. none). ASHMI (400 μg/ml) produced no additional inhibition. Experiments with ASHMI at 200 μg/ml showed that abrogation of contractility was not due to toxicity, because responses of ASHMI-treated rings were not different from those of PSS-treated rings when KCl-induced contractility was tested 2 h after washout (Fig. 2B, middle), indicating that rings were still viable. ASHMI-induced suppression of ACh contractility was also relieved after washout (Fig. 2B, bottom), indicating reversibility of ASHMI effects.
Fig. 2.
Effect of ASHMI on mouse ex vivo tracheal ring contractility. A: tracheal contractility to 10−4 M ACh in the presence of various concentrations of ASHMI normalized to maximal constriction to 60 mM KCl. Values are means ± SD of 4–7 individual responses for each ASHMI concentration. *P < 0.05 and ***P < 0.001 compared with no ASHMI. B: representative real-time tracings of tracheal rings from OVA-sensitized mice stimulated with 10−4 M ACh after pretreatment with 200 μg/ml ASHMI (center panel, bottom trace) or physiological salt solution (PSS; vehicle; center panel, top trace). KCl (60 mM) was used to determine the maximum contractility of rings before ACh exposure and to confirm the viability of rings after experiments. In some experiments, 10−4 M ACh was used to determine reversibility of ASHMI effects (bottom panel).
Suppression of ACh-induced contraction to ASHMI is important, as ACh released from nerve terminals in murine and human studies has been shown to contribute to airway contractility and is thus physiologically relevant (2, 42). Murine airways do not respond to histamine (18), and we observed very weak responses to serotonin (data not shown). Moderate contraction to bradykinin was observed and was also significantly inhibited by ASHMI pretreatment (P < 0.001, data not shown). To determine whether ASHMI could relax a tracheal ring from OVA-sensitized mice after it was contracted with ACh, rings were precontracted with 100 μM ACh and then exposed to 200 μg/ml ASHMI or the vehicle PSS. In ACh-contracted rings treated with PSS, contraction was elevated for over 2 h, while rings treated with ASHMI after the ACh contraction relaxed over the same time period (data not shown).
ASHMI Suppresses Tracheal Ring Contraction in Response to High K+
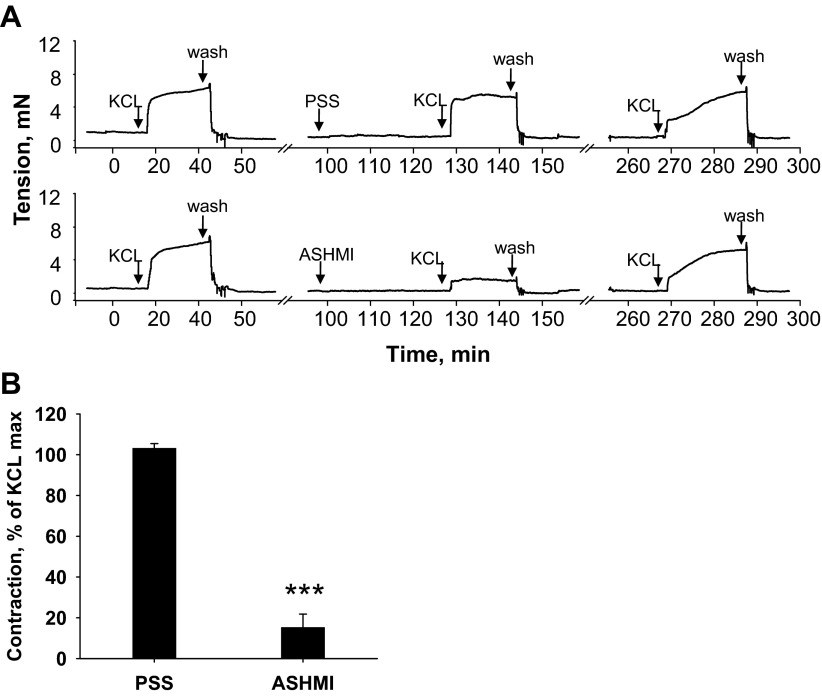
Unlike ACh, KCl-induced contractions do not rely on G protein-coupled receptor activation and occur due to plasma membrane depolarization and external calcium entry. Thirty-minute pretreatment with 200 μg/ml ASHMI attenuated KCl-induced contractions (Fig. 3; P < 0.001 compared with PSS pretreatment), indicating that the mechanism of ASHMI effects are not limited to the ACh-muscarinic receptor pathway.
Fig. 3.
Effect of ASHMI on high K+-induced contraction. Tracheal rings were stimulated with 60 mM KCl in the presence and absence of ASHMI. A: representative tracing of 4 separate experiments graphed in B. Values in B are means ± SD. ***P < 0.001 vs. none.
ASHMI Suppression of Tracheal Ring Constriction Is Epithelium-independent
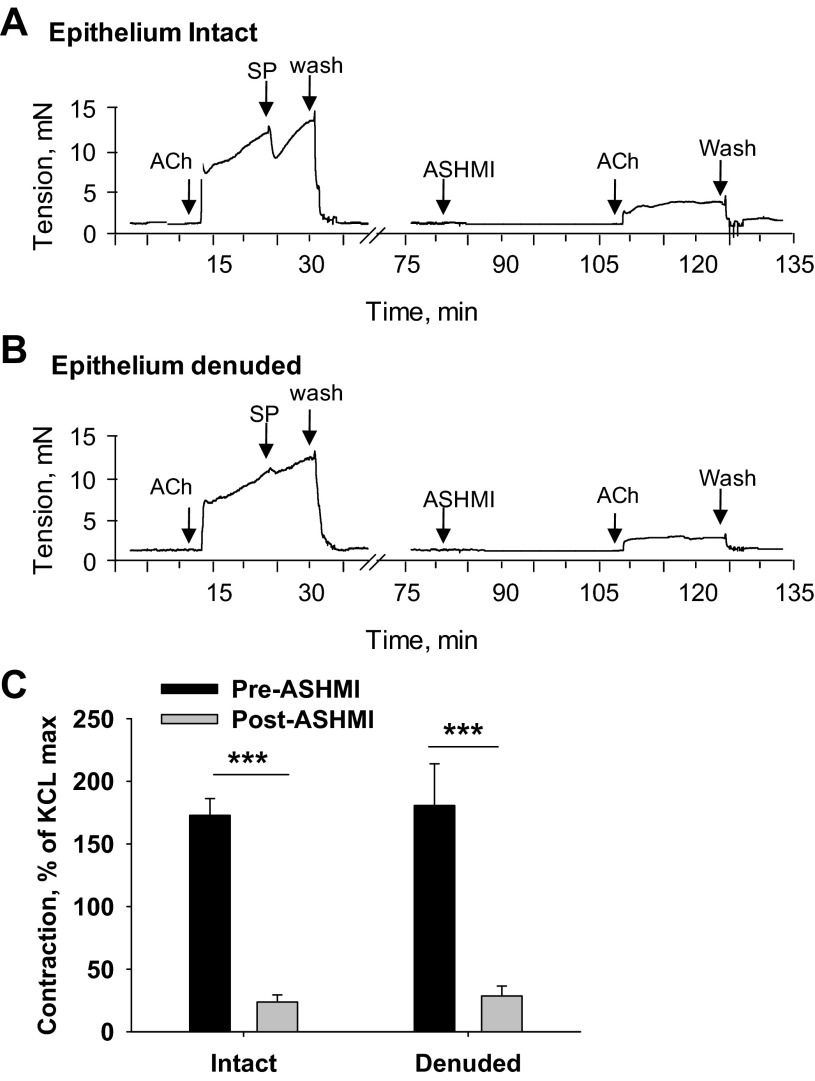
Airway epithelium possesses bronchoprotective functions, and its removal increases smooth muscle contractility (19). To determine whether the mechanism of ASHMI protection from ACh-induced contraction was epithelium dependent, we tested its effects on epithelium-denuded tracheal rings. Absence of epithelium was confirmed by testing ACh-induced contraction in the presence of substance P (SP), an epithelium-dependent bronchodilator (20). As expected, intact rings showed a rapid and transient relaxation response to 10 nM SP, whereas denuded rings showed little or no relaxation (Fig. 4, A and B). After washout of SP, rings were exposed to ASHMI or PSS for 30 min and subsequently constricted with 10−4 M ACh. Removal of airway epithelium did not affect ASHMI suppression of ACh-mediated constriction (Fig. 4B), which was the same as ASHMI-treated epithelium-intact rings (P < 0.01, Fig. 4C).
Fig. 4.
Effect of tracheal epithelium removal on ASHMI pretreatment effects on ex vivo ACh-induced contractions. A and B: representative real-time tracings of ACh-induced tracheal ring contractions with ASHMI pretreatment in rings in which the epithelium is intact (A) or denuded (B). Epithelium removal was confirmed by response to 10 nM substance P (SP). C: graphical data for multiple experiments shown in A and B normalized to maximal contraction with 60 mM KCl. Values are means ± SD of 4 separate experiments. ***P < 0.001.
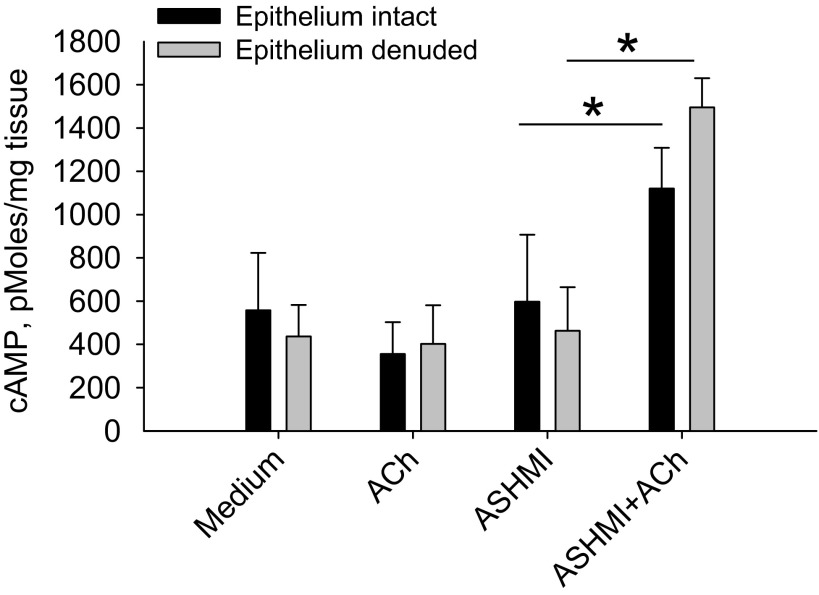
ASHMI Prevention of Tracheal Ring Constriction Is Associated with Elevated cAMP Production
cAMP is a critical second messenger in ASM anti-contractile signaling pathways (3). We found elevated cAMP levels in ACh-stimulated tracheal tissues treated with ASHMI compared with tissue exposed to ASHMI alone, ACh alone, or medium alone (P < 0.05, Fig. 5). Consistent with epithelium-independent effects of ASHMI shown in Fig. 3, the profile of cAMP enhancement in ACh-triggered tracheal tissue was unaffected by epithelium removal.
Fig. 5.
Evaluation of cAMP in response to ASHMI. cAMP levels in epithelium-intact and epithelium-denuded tracheal ring tissue were measured by ELISA. Values are means ± SD of 4 separate experiments. *P < 0.05.
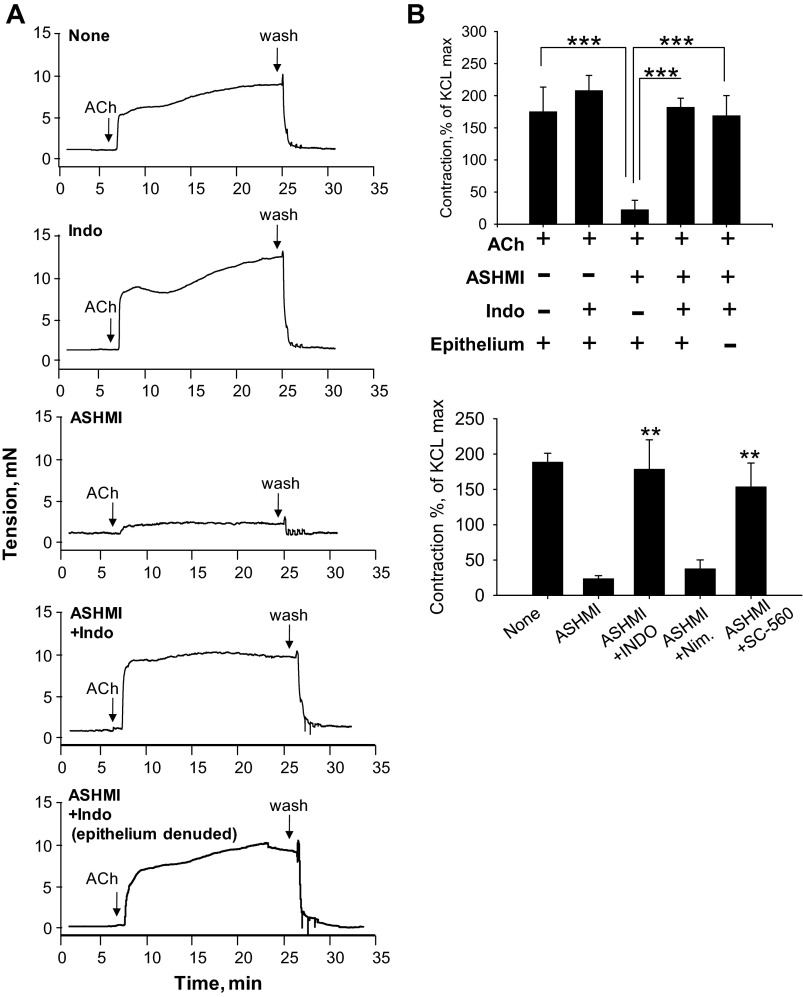
Inhibition of Tracheal Constriction by ASHMI Is Dependent on Cyclooxygenase-1 Activity
Having demonstrated previously that ASHMI function is independent of β2-adrenoceptor agonist activity (45), we questioned whether bronchodilatory prostaglandins synthesized by the cyclooxygenase (COX) pathway (17), also known to increase cAMP levels, potentially contributed to ASHMI effects. ASHMI-pretreated rings were stimulated with 10−4 M ACh in the presence or absence of the nonselective COX inhibitor indomethacin. ASHMI reduction of ACh-induced constriction of tracheal rings was completely abrogated by indomethacin (P < 0.001), demonstrating a major role for COX products in ASHMI function (Fig. 6). Indomethacin had no significant effect on constriction of rings in the absence of ASHMI. Furthermore, we found that ASHMI effects were not altered by the COX-2-selective antagonist nimesulide, but pretreatment with indomethacin or the COX-1-selective inhibitor SC-560 blocked ASHMI-induced suppression of contraction, implying participation of COX-1 pathway in ASHMI function (Fig. 6B).
Fig. 6.
Effect of cyclooxygenase inhibition on ASHMI pretreatment effects on ACh ex vivo tracheal contractions. A: representative real-time tracing of tracheal ring contraction in ASHMI (200 μg/ml) or PSS pretreated rings in the presence or absence of 5 μM indomethacin (Indo) and presence or absence or epithelium. B, top: graphic representation of experiments shown in A normalized to maximal contraction with 60 mM KCl. Bottom: ASHMI function in the presence and absence of COX-1/2 selective antagonists [0.5 μM SC-560 or 5 μM nimesulide (nim), respectively]. Values are means ± SD of 3–4 separate experiments. **P < 0.01. ***P < 0.001.
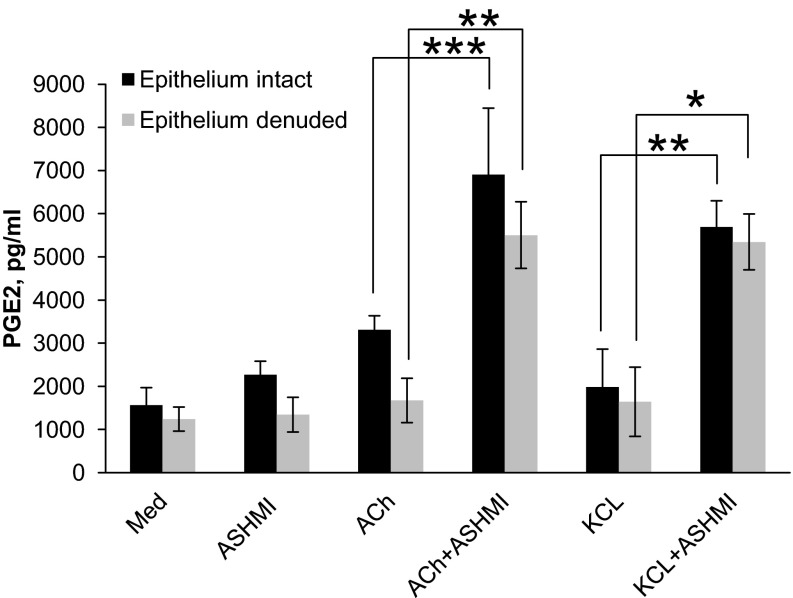
ASHMI Increased Murine Tracheal Ring PGE2 Levels
The COX product PGE2 is a well-established bronchodilatory prostaglandin (20, 40, 41). We investigated whether ASHMI treatment affected PGE2 production in tracheal tissues. ASHMI- and PSS-pretreated tracheal rings were stimulated with ACh, KCl, or PSS, and PGE2 levels in the incubation medium were determined by ELISA. ASHMI pretreatment caused a significant increase in PGE2 levels in ACh or KCl triggered rings that was significantly higher than those in media from ACh alone or KCl alone stimulated rings (P < 0.001 and P < 0.05, respectively, Fig. 7). ASHMI-only treatment did not significantly affect PGE2 levels. Furthermore, activity of ASHMI on PGE2 levels was not affected by the absence of epithelium.
Fig. 7.
Effect ASHMI on PGE2 levels. PGE2 levels in medium conditioned by intact- or epithelium-denuded tracheal rings pretreated with ASHMI (200 μg/ml) or medium alone (Med), and then subsequently stimulated with 10−4 M ACh or medium alone for 20 min, were measured by ELISA. Values are means ± SD of 4 separate experiments. *P < 0.05. **P < 0.01. ***P < 0.001.
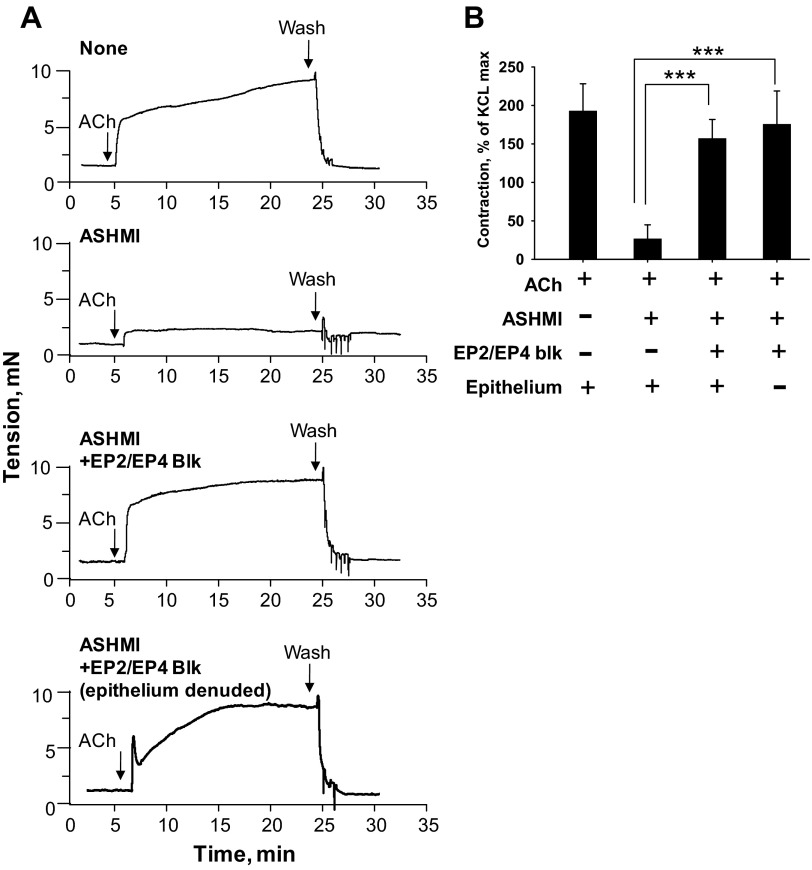
ASHMI Prevention of Airway Tracheal Ring Contractility Was Abrogated By Blocking PGE2 Receptors
To determine the functional contribution of PGE2 to ASHMI inhibition of constriction, we evaluated ACh responses of ASHMI-treated tracheal rings in the presence and absence of pharmacological inhibitors of EP2/EP4 receptors. Murine tracheal relaxation responses to PGE2 have been shown to be mediated by the combined activity of EP2 and EP4 receptors (4). As shown in Fig. 8, ASHMI inhibition of contraction was abrogated by the combined presence of 5 μM each of EP2/EP4 blockers AH6809 and L161982 (P < 0.001 vs. ASHMI). Furthermore, the presence of epithelium was not required for this activity, as the effect of EP2/EP4 block in epithelium-denuded rings was similar to responses in epithelium-intact rings. These results suggest that ASHMI inhibition of tracheal contractility is dependent on activation of EP2/EP4 receptors.
Fig. 8.
Functional contribution of EP2/EP4 receptors to ASHMI effects. A: representative real-time tracings of epithelium-intact and -denuded tracheal ring contraction to 10−4 M ACh with pretreatment of PSS (first panel), 200 μg/ml ASHMI (second panel), or 200 μg/ml ASHMI plus EP2/EP4 receptor antagonists (AH6809/L161982, 5 μM each, third and fourth panels). B: graphic representation of experiments as shown in A normalized to maximal contraction to 60 mM KCl. Values are means ± SD. ***P < 0.001.
DISCUSSION
The primary findings of the present study are that the anti-asthma traditional Chinese medicine herbal formula ASHMI inhibits airway constriction when given orally to OVA-sensitized mice or by ex vivo exposure of tracheal rings from the same mice. These effects of ASHMI were dose dependent, and, in ex vivo experiments, reversible after washout. In addition to protection from AHR following ACh provocation, we have found that ASHMI also inhibited KCl- and bradykinin-induced airway constriction, indicating that ASHMI blockade of airway constriction is not limited to suppression of muscarinic signaling.
ASHMI by itself did not reduce baseline tension of tracheal rings, or increase cAMP levels or PGE2 production; however, PGE2 levels were enhanced by ASHMI after stimulation with ACh or KCl. There is previous evidence demonstrating PGE2 release in response to ACh stimulation (9, 32, 38). The rate-limiting step of PGE2 synthesis is arachidonic acid release via activation of phospholipase A2, which has been shown to have a calcium-dependent isoform-cytosolic phospholipase A2, in addition to calcium-independent isoforms (12). Since calcium increase follows stimulation with ACh or KCl, the requirement of these triggers for ASHMI function suggests the possibility that calcium-dependent cytosolic phospholipase A2 may be involved in our system. We speculate that ASHMI potentiates PGE2 release in isolated airway rings, although the precise mechanisms are still under investigation.
In mechanistic experiments, we found that ASHMI effects were dependent on COX-1 activity and EP2/EP4 receptor activation, independent of the presence of the epithelium, and was accompanied by increases in PGE2 and cyclic AMP. Our data suggest that ASHMI stimulates synthesis of PGE2 in a cell other than the epithelium, which, in turn, activates EP2/EP4 receptors on ASM cells to increase cyclic AMP, which inhibits contraction or facilitates relaxation. PGE2 is a well-established bronchodilator (17, 40, 41), but, unlike previous reports where PGE2 function was epithelium-dependent (4, 20), ASHMI-mediated suppression of airway constriction was epithelium independent. Our present hypothesis is that ASHMI effects are mediated by regulation of PGE2 levels and activity in airway cells other than epithelium, such as fibroblasts, smooth muscle cells, or cartilage [all known to produce PGE2 (32, 43, 27, 31, 33)], and it is possible that multiple cell types participate to bring about effects seen at the organ level. In agreement with our present study, a recent report by Kaufman et al. (24) also described an epithelium-independent prostaglandin-mediated bronchodilatory pathway in guinea pig trachea, although the exact identity and cellular source of the prostaglandin were not reported.
Anti-bronchoconstrictive effects of PGE2 in human asthmatic patients have been demonstrated previously. Gauvreau et al. (21) found that inhaled PGE2 reduced allergen-induced bronchoconstriction and methacholine responsiveness, and Sestini et al. (34) and others found near complete protection from aspirin-induced bronchoconstriction in aspirin sensitive asthmatics (31). Currently, there is renewed interest in targeting EP4 receptors for bronchodilator therapy in humans (6, 11). Thus ASHMI treatment may provide an opportunity to maximize the bronchoprotective effects of PGE2 by enhancing levels and activity of endogenous PGE2. As described in previous reports, ASHMI is a complex formula containing several classes of compounds, including alkaloids, flavonoids, and triterpenoids; thus ASHMI modulation of ASM contraction may involve multiple compounds (45). Recently, we (44) showed that trifolirhizin, a flavanoid component of ASHMI, inhibited ACh-induced constriction of murine tracheal rings via a non-β-adrenergic agonist pathway. The extent to which trifolirhizin contributes to whole ASHMI effects described in this study is unknown. Identification of other active components of ASHMI is actively being pursued.
Our study results suggest that ASHMI may be a valuable oral asthma drug that could potentially provide relief from bronchoconstriction, especially in patients responding poorly to β-adrenoceptor agonists due to genetic reasons or those who have become tolerant after chronic use. Future clarification of the mechanism of action of ASHMI on human airway function is ongoing.
GRANTS
This work was supported by the National Institutes of Health Grants PO1 AT002647 to X.-M. Li. K. D. Srivastava was supported by Ruth L. Kirchstein Predoctoral Fellowship Award F31AT003769 and KL2 Faculty Scholar Award KL2TR000069 from Mount Sinai Clinical and Translational Science Award.
DISCLOSURES
X.-M. Li and H. A. Sampson hold US Patent PCT/US05/08600 for ASHMI and are shareholders of Herb Springs LLC.
AUTHOR CONTRIBUTIONS
Author contributions: K.D.S. and X.-M.L. conception and design of research; K.D.S. performed experiments; K.D.S. analyzed data; K.D.S. and X.-M.L. interpreted results of experiments; K.D.S. prepared figures; K.D.S. drafted manuscript; K.D.S., H.A.S., and C.W.E. edited and revised manuscript; K.D.S., H.A.S., C.W.E., and X.-M.L. approved final version of manuscript.
REFERENCES
- 1.Agarwal R, Khan A, Aggarwal AN, Gupta D. Is the SMART approach better than other treatment approaches for prevention of asthma exacerbations? A meta-analysis. Monaldi Arch Chest Dis 71: 161–169, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Aizawa H, Inoue H, Miyazaki N, Hara N. Histamine-induced increase in isometric tension of smooth muscle is mediated by local vagus nerve in human bronchus. Respiration 67: 652–656, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir Res 7: 34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzary RW, Cocks TM. Lipopolysaccharide induces epithelium- and prostaglandin E(2)-dependent relaxation of mouse isolated trachea through activation of cyclooxygenase (COX)-1 and COX-2. J Pharmacol Exp Ther 317: 806–812, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. New drugs for asthma. Semin Respir Crit Care Med 33: 685–694, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leseche G, Longrois D, Norel X. PGE(2) receptor [EP(4)] agonists: potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther 25: 115–118, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Berger WE, Noonan MJ. Treatment of persistent asthma with Symbicort (budesonide/formoterol inhalation aerosol): an inhaled corticosteroid and long-acting beta2-adrenergic agonist in one pressurized metered-dose inhaler. J Asthma 47: 447–459, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Bian B, Kelton CM, Wigle PR, Guo JJ. Evaluating safety of long-acting beta agonists (LABAs) in patients with asthma. Curr Drug Saf 5: 245–250, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Borda E, Furlan C, Orman B, Reina S, Sterin-Borda L. Nitric oxide synthase and PGE2 reciprocal interactions in rat dental pulp: cholinoceptor modulation. J Endod 33: 142–147, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Broadley KJ. Beta-adrenoceptor responses of the airways: for better or worse? Eur J Pharmacol 533: 15–27, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Buckley J, Birrell MA, Maher SA, Nials AT, Clarke DL, Belvisi MG. EP4 receptor as a new target for bronchodilator therapy. Thorax 66: 1029–1035, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50, Suppl: S237–S242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carolan BJ, Sutherland ER. Clinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advances. J Allergy Clin Immunol 131: 627–634, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Cazzola M, Matera MG. Safety of long-acting beta2-agonists in the treatment of asthma. Ther Adv Respir Dis 1: 35–46, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med 327: 1198–1203, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Chong LK, Suvarna K, Chess-Williams R, Peachell PT. Desensitization of beta2-adrenoceptor-mediated responses by short-acting beta2-adrenoceptor agonists in human lung mast cells. Br J Pharmacol 138: 512–520, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke DL, Dakshinamurti S, Larsson AK, Ward JE, Yamasaki A. Lipid metabolites as regulators of airway smooth muscle function. Pulm Pharmacol Ther 22: 426–435, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Rodriguez S, Broadley KJ, Ford WR, Kidd EJ. Increased muscarinic receptor activity of airway smooth muscle isolated from a mouse model of allergic asthma. Pulm Pharmacol Ther 23: 300–307, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Folkerts G, Nijkamp FP. Airway epithelium: more than just a barrier! Trends Pharmacol Sci 19: 334–341, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Fortner CN, Breyer RM, Paul RJ. EP2 receptors mediate airway relaxation to substance P, ATP, and PGE2. Am J Physiol Lung Cell Mol Physiol 281: L469–L474, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Gauvreau GM, Watson RM, O'Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med 159: 31–36, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Hancox RJ, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, Taylor DR. Bronchodilator tolerance and rebound bronchoconstriction during regular inhaled beta-agonist treatment. Respir Med 94: 767–771, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Haney S, Hancox RJ. Tolerance to bronchodilation during treatment with long-acting beta-agonists, a randomised controlled trial. Respir Res 6: 107, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman EH, Fryer AD, Jacoby DB. Toll-like receptor 7 agonists are potent and rapid bronchodilators in guinea pigs. J Allergy Clin Immunol 127: 462–469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly HW. Risk versus benefit considerations for the beta(2)-agonists. Pharmacotherapy 26: 164S–174S, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Wisnivesky J, Li XM, Kattan M. Safety and tolerability of an antiasthma herbal formula (ASHMITM) in adult asthmatics: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. J Altern Complement Med 15: 735–743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Ye YM, Hur GY, Lee HY, Jee YK, Lee SH, Holloway JW, Park HS. Effect of beta2-adrenergic receptor polymorphism in asthma control of patients receiving combination treatment. Yonsei Med J 50: 182–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XM, Huang CK, Zhang TF, Teper AA, Srivastava K, Schofield BH, Sampson HA. The Chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol 106: 660–668, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Martinez FD. Managing childhood asthma: challenge of preventing exacerbations. Pediatrics 123, Suppl 3: S146–S150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehats C, Jin SL, Wahlstrom J, Law E, Umetsu DT, Conti M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J 17: 1831–1841, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Melillo E, Woolley KL, Manning PJ, Watson RM, O'Byrne PM. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am J Respir Crit Care Med 149: 1138–1141, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Nile CJ, Gillespie JI. Interactions between cholinergic and prostaglandin signaling elements in the urothelium: role for muscarinic type 2 receptors. Urology 79: 240–23, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Ramshaw HS, Woodcock JM, Bagley CJ, McClure BJ, Hercus TR, Lopez AF. New approaches in the treatment of asthma. Immunol Cell Biol 79: 154–159, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, Vaghi A, Folco GC, Bianco S, Robuschi M. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med 153: 572–575, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Shore SA, Moore PE. Regulation of beta-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol 137: 179–195, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Srivastava K, Sampson H, Li XM. The anti-asthma Chinese herbal formula ASHMI provides more persistent benefits than dexamethasone in a murine asthma model (Abstract). J Allergy Clin Immunol 127: AB261, 2011 [Google Scholar]
- 37.Srivastava K, Zhang T, Yang N, Sampson H, Li XM. Anti-asthma simplified herbal medicine intervention-induced long-lasting tolerance to allergen exposure in an asthma model is interferon-gamma, but not transforming growth factor-beta dependent. Clin Exp Allergy 40: 1678–1688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J 15: 2057–2072, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Thomas M, Price D. Impact of comorbidities on asthma. Expert Rev Clin Immunol 4: 731–742, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, Stock J, McNeisch J, Valancius C, Panettieri RA, Jr, Penn RB, Koller BH. Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am J Physiol Lung Cell Mol Physiol 284: L599–L606, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol 25: 40–46, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Weigand LA, Myers AC, Meeker S, Undem BJ. Mast cell-cholinergic nerve interaction in mouse airways. J Physiol 587: 3355–3362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, Mu DZ, Du JB, Li GH, Wallenstein S, Sampson H, Kattan M, Li XM. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol 116: 517–524, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yang N, Liang B, Srivastava K, Zeng J, Zhan J, Brown L, Sampson H, Goldfarb J, Emala C, Li XM. The Sophora flavescens flavonoid compound trifolirhizin inhibits acetylcholine induced airway smooth muscle contraction. Phytochemistry 95C: 259–267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang T, Srivastava K, Wen MC, Yang N, Cao J, Busse P, Birmingham N, Goldfarb J, Li XM. Pharmacology and immunological actions of a herbal medicine ASHMI on allergic asthma. Phytother Res 24: 1047–1055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]