Abstract
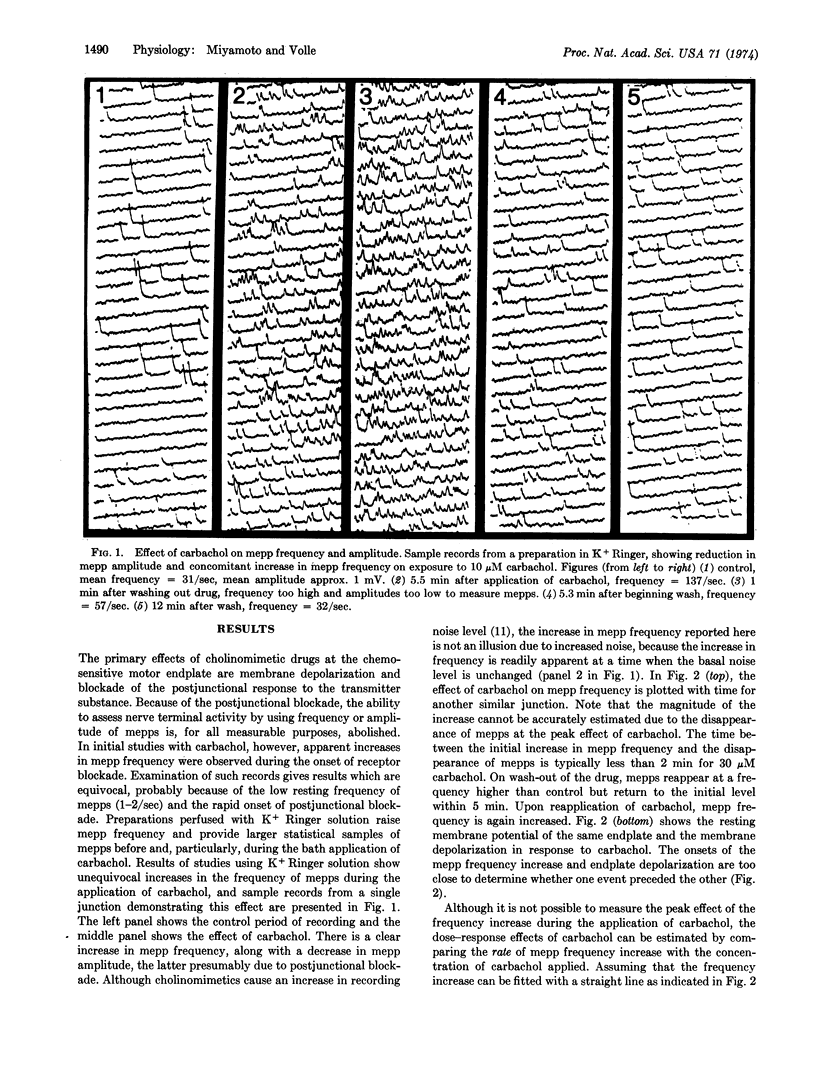
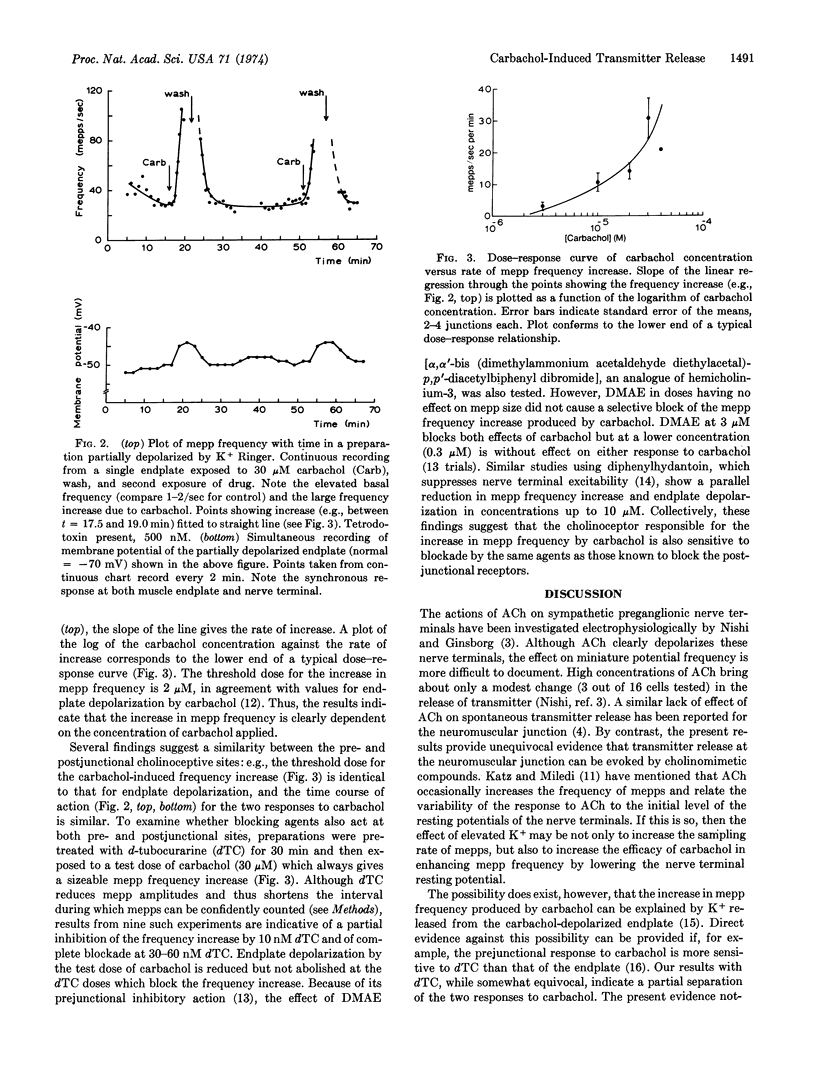
In the endplates of rat phrenic nerve-diaphragm, application of the acetylcholine-like compound, carbachol, causes a marked increase in transmitter release, as measured electrophysiologically using miniature endplate potential frequency. Washing out of carbachol reverses the increase in frequency. The ability of carbachol to increase transmitter release is greatly enhanced by perfusion of the preparation with Ringer solution containing elevated K+. At concentrations of carbachol greater than 30 μM, the onset of the postjunctional blocking action of carbachol is too rapid and obscures the increase in miniature potential frequency. The rate of increase in transmitter release is dependent on the concentration of carbachol applied and can be antagonized by d-tubocurarine (10-60 nM) and other blocking compounds. These findings, in contrast to previous reports, indicate that cholinergic nerve endings, like adrenergic nerve endings, respond to applied acetylcholine-like drugs with measurable increases in transmitter output.
Keywords: miniature endplate potential frequency, cholinomimetics, nerve terminal depolarization, prejunctional cholinoceptive sites, cholinergic neurosecretion
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine and some related substances on conduction in mammalian non-myelinated nerve fibres. J Physiol. 1961 Feb;155:372–384. doi: 10.1113/jphysiol.1961.sp006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. ACETYLCHOLINE IN ADRENERGIC TRANSMISSION. Annu Rev Pharmacol. 1965;5:163–182. doi: 10.1146/annurev.pa.05.040165.001115. [DOI] [PubMed] [Google Scholar]
- Blaer L. C. The effect of facilitatory concentrations of decamethonium on the storage and release of transmitter at the neuromuscular junction of the cat. J Pharmacol Exp Ther. 1970 Dec;175(3):664–672. [PubMed] [Google Scholar]
- Brown D. A., Jones K. B., Halliwell J. V., Quilliam J. P. Evidence against a presynaptic action of acetylcholine during ganglionic transmission. Nature. 1970 Jun 6;226(5249):958–959. doi: 10.1038/226958a0. [DOI] [PubMed] [Google Scholar]
- CIANI S., EDWARDS C. THE EFFECT OF ACETYLCHOLINE ON NEUROMUSCULAR TRANSMISSION IN THE FROG. J Pharmacol Exp Ther. 1963 Oct;142:21–23. [PubMed] [Google Scholar]
- Cabrera R., Torrance R. W., Viveros H. The action of acetyl choline and other drugs upon the terminal parts of the postganglionic sympathetic fibre. Br J Pharmacol Chemother. 1966 May;27(1):51–63. doi: 10.1111/j.1476-5381.1966.tb01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B., Katz H. S. The release of acetylcholine by acetylcholine in the cat's superior cervical ganglion. Br J Pharmacol. 1970 Jun;39(2):428–438. doi: 10.1111/j.1476-5381.1970.tb12905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. The specific effect of potassium on transmitter release by motor nerve terminals and its inhibition by calcium. J Physiol. 1973 Jan;228(2):435–458. doi: 10.1113/jphysiol.1973.sp010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., GRAY J. A. B. The excitant action of acetylcholine and other substances on cutaneous sensory pathways and its prevention by hexamethonium and D-tubocurarine. J Physiol. 1953 Jan;119(1):118–128. doi: 10.1113/jphysiol.1953.sp004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Spontaneous activity at a mammalian neuromuscular junction in tetrodotoxin. Acta Physiol Scand. 1965 Aug;64(4):475–476. doi: 10.1111/j.1748-1716.1965.tb04206.x. [DOI] [PubMed] [Google Scholar]
- FERRY C. B. The synpathomimetic effect of acetylcholine on the spleen of the cat. J Physiol. 1963 Jul;167:487–504. doi: 10.1113/jphysiol.1963.sp007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B. L., GUERRERO S. ON THE ACTION OF DEPOLARIZING DRUGS ON SYMPATHETIC GANGLION CELLS OF THE FROG. J Physiol. 1964 Aug;172:189–206. doi: 10.1113/jphysiol.1964.sp007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Depolarizing neuromuscular block. J Pharmacol Exp Ther. 1971 Aug;178(2):339–349. [PubMed] [Google Scholar]
- Ginsborg B. L. On the presynaptic acetylcholine receptors in sympathetic ganglia of the frog. J Physiol. 1971 Jul;216(1):237–246. doi: 10.1113/jphysiol.1971.sp009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Schmidt R. F., Yokota T. The effect of acetylcholine upon mammalian motor nerve terminals. J Physiol. 1965 Dec;181(4):810–829. doi: 10.1113/jphysiol.1965.sp007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Parsons R. L. Characteristics of postjunctional carbamylcholine receptor activation and inhibition. Am J Physiol. 1972 Mar;222(3):793–799. doi: 10.1152/ajplegacy.1972.222.3.793. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOELLE G. B. A new general concept of the neurohumoral functions of acetylcholine and acetylcholinesterase. J Pharm Pharmacol. 1962 Feb;14:65–90. doi: 10.1111/j.2042-7158.1962.tb11057.x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K., Nishi S. Cholinergic receptors at sympathetic preganglionic nerve terminals. J Physiol. 1968 May;196(2):293–310. doi: 10.1113/jphysiol.1968.sp008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M. The interaction of presynaptic polarization with calcium and magnesium in modifying spontaneous transmitter release from mammalian motor nerve terminals. J Physiol. 1969 Aug;203(2):281–299. doi: 10.1113/jphysiol.1969.sp008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S. Cholinergic and adrenergic receptors at sympathetic preganglionic nerve terminals. Fed Proc. 1970 Nov-Dec;29(6):1957–1965. [PubMed] [Google Scholar]
- Parsons R. L. Changes in postjunctional receptors with decamethonium and carbamylcholine. Am J Physiol. 1969 Sep;217(3):805–811. doi: 10.1152/ajplegacy.1969.217.3.805. [DOI] [PubMed] [Google Scholar]
- Riker W. F., Jr, Standaert F. G. The action of facilitatory drugs and acetylcholine on neuromuscular transmission. Ann N Y Acad Sci. 1966 Jan 26;135(1):163–176. doi: 10.1111/j.1749-6632.1966.tb45470.x. [DOI] [PubMed] [Google Scholar]
- STANDAERT F. G. THE ACTION OF D-TUBOCURARINE ON THE MOTOR NERVE TERMINAL. J Pharmacol Exp Ther. 1964 Feb;143:181–186. [PubMed] [Google Scholar]
- Volle R. L. Frequency dependent decrease of quantal content in a drug-treated neuromuscular junction. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(3):271–284. doi: 10.1007/BF00500288. [DOI] [PubMed] [Google Scholar]



