Abstract
Hypertension in the elderly substantially increases the risk of stroke and vascular cognitive impairment in part due to an impaired functional adaptation of aged cerebral arteries to high blood pressure. To elucidate the mechanisms underlying impaired autoregulatory protection in aging, hypertension was induced in young (3 mo) and aged (24 mo) C57BL/6 mice by chronic infusion of angiotensin II and pressure-induced changes in smooth muscle cell (SMC) intracellular Ca2+ concentration ([Ca2+]i) and myogenic constriction of middle cerebral arteries (MCA) were assessed. In MCAs from young hypertensive mice, pressure-induced increases in vascular SMC [Ca2+]i and myogenic tone were increased, and these adaptive responses were inhibited by the cytochrome P-450 ω-hydroxylase inhibitor HET0016 and the transient receptor potential (TRP) channel blocker SKF96365. Administration of 20- hydroxyeicosatetraenoic acid (HETE) increased SMC [Ca2+]i and constricted MCAs, and these responses were inhibited by SKF96365. MCAs from aged hypertensive mice did not show adaptive increases in pressure-induced calcium signal and myogenic tone and responses to HET0016 and SKF96365 were blunted. Inhibition of large-conductance Ca2+-activated K+ (BK) channels by iberiotoxin enhanced SMC [Ca2+]i and myogenic constriction in MCAs of young normotensive animals, whereas it was without effect in MCAs of young hypertensive mice. Iberiotoxin did not restore myogenic adaptation in MCAs of aged hypertensive mice. Thus functional maladaptation of aged cerebral arteries to hypertension is due to the dysregulation of pressure-induced 20-HETE and TRP channel-mediated SMC calcium signaling, whereas overactivation of BK channels is unlikely to play a role in this phenomenon.
Keywords: cerebral blood flow, 20-hydroxyeicosatetraenoic acid, autoregulation, canonical transient receptor potential, potassium channels, large-conductance Ca2+-activated K+ channels
there is growing evidence that hypertension, which affects ∼50 million Americans, exerts deleterious effects on the cerebral circulation and thereby causes accelerated brain aging (45). The elderly (≥65 years of age), representing the fastest-growing segment of the US population, have the highest prevalence of hypertension, and these individuals are more likely to develop cerebrovascular pathologies (5). Epidemiological studies provide evidence that in addition to the increased prevalence of hypertension in aging, the deleterious cerebrovascular effects of hypertension are also exacerbated in elderly patients, whereas younger subjects appear to be more protected from cerebromicrovascular and neurological damage induced by elevated blood pressure (10, 38, 54). There is strong evidence showing that in older patients hypertension-induced microvascular injury not only promotes the development of vascular cognitive impairment (24, 29, 45) but it also significantly increases the risk (risk ratio = ∼1.5) for sporadic Alzheimer's disease (25, 31), supporting the vascular hypothesis of the disease (11–13). Although the available human data suggest that advanced age and hypertension have synergistic effects (45), the specific age-related mechanisms through which aging increases the vulnerability of the cerebromicrovasculature to hypertension are not well documented in the literature.
Previous studies provide evidence that in young animals cerebral arteries exhibit functional and structural adaptation to hypertension, which protect the injury-prone distal portion of the cerebral microcirculation from pressure overload (53, 58, 60). Among these physiological adaptive responses the increased pressure-induced myogenic constriction of cerebral arteries is of great significance (27, 36, 51). In healthy individuals the cerebral circulation has significantly lower resistance than the vasculature of other tissues (e.g., skeletal muscle), which results in a substantially increased flow rate during diastole. As a result, flow and pressure pulsations can penetrate easier the distal, fragile portion of the cerebral arterial tree, especially if autoregulatory protection is impaired. Previous studies demonstrated that in young hypertensive animals increased pressure-induced myogenic constriction leads to an increased resistance at the level of the larger pial arteries (60), keeping pressure in the thin-walled, injury-prone arterioles and capillaries in the normal range. As this adaptive vascular response manifests, the range of cerebral blood flow autoregulation is extended to higher pressure values both in hypertensive patients and laboratory animals with experimentally induced hypertension (37, 53, 58, 60).
Clinical studies suggest that aging impairs autoregulatory protection to high blood pressure in the human brain (14). Our recent studies provide evidence that aged mice also exhibit pathological loss of cerebral autoregulatory protection, which contribute to an exacerbation of hypertension-induced cerebromicrovascular injury. Downstream consequences of cerebrovascular autoregulatory dysfunction in aged hypertensive mice include exacerbated disruption of the blood-brain barrier and neuroinflammation (microglia activation likely induced by leakage of plasma-derived factors through the damaged blood-brain barrier) (60, 69). There is increasing evidence that blood-brain barrier disruption and consequential neuroinflammation plays a causal role in neurocognitive decline associated with hypertension (24, 29, 60). Despite these advances, the cellular mechanisms underlying impaired functional adaptation of aged cerebral arteries to hypertension remain elusive.
The signaling mechanisms underlying the myogenic constriction of cerebral arteries include pressure-induced increases in smooth muscle cells (SMC) [Ca2+]i (8). Recent studies demonstrate that pressure-induced production of 20-HETE in SMCs and activation of transient receptor potential (TRP) channels play a key role in regulation of SMC [Ca2+]i and development of myogenic constriction of cerebral arteries (21, 26). Previous studies demonstrate that in young hypertensive rats (15) pressure-induced 20-HETE production in cerebral arteries is significantly increased. Furthermore, the available evidence suggests that in young animals several TRP channels are upregulated in hypertension (19, 41, 42). Yet, age-related alterations in pressure-induced, 20-HETE-, and TRP channel-dependent regulation of SMC [Ca2+]i have not been investigated.
Activation of large-conductance Ca2+-activated K+ (BKCa) channels represent another important mechanism modulating the magnitude of pressure-induced myogenic constriction (52). Previous studies demonstrate that BKCa channels act as negative feedback regulators of the myogenic response of cerebral arteries: Ca2+-dependent activation of these channels attenuates membrane depolarization-induced influx of extracellular Ca2+ through L-type Ca2+ channels, decreasing myogenic constriction (9, 35). Importantly, recent studies show that BKCa in SMCs in the arterial wall is predominantly activated by high pressure (52). Accordingly, in cerebral arteries isolated from young animals inhibition of BKCa channels was shown to increase myogenic constriction predominantly in the high pressure range, extending range of effective myogenic autoregulation (52). There are also studies suggesting that 20-HETE can inhibit BKCa channels in SMCs and that inhibition of BKCa channels may contribute to 20-HETE-mediated increases in arterial constriction (70). Yet, the role of BKCa channels in functional maladaptation of aged cerebral arteries to high pressure has not been elucidated.
The present study was designed to test the hypothesis that hypertension in aging is associated with impaired myogenic constriction of cerebral arteries due to dysregulation of pressure-induced, 20-HETE-, and TRP channel-dependent changes in SMC [Ca2+]i. To test our hypothesis, we induced hypertension in young and aged C57BL/6 mice by chronic infusion of angiotensin II. In isolated middle cerebral arteries (MCAs), parallel changes in pressure-induced SMC [Ca2+]i and myogenic constriction in response to inhibition of 20-HETE synthesis, TRP channels, and BKCa activation were assessed.
METHODS
Animals.
Young (3 mo; n = 40) and aged (24 mo; n = 40) male C57BL/6 mice were purchased from the National Institute on Aging. All mice were maintained under specific pathogen-free barrier conditions. Water and normal laboratory diet were available ad libitum. All procedures were approved by the Institutional Animal Use and Care Committees of the participating institutions.
Infusion of angiotensin II.
Alzet osmotic mini-pumps (Model 2006; 0.15 μl/h, 42 days; Durect, Cupertino, CA) were implanted into young and aged mice. Pumps were filled either with saline vehicle or solutions of ANG II (Sigma Chemical, St. Louis, MO) that delivered (subcutaneously) 1,000 ng·min−1·kg−1 of ANG II for 28 days (60, 65). Pumps were placed into the subcutaneous space of ketamine-xylazine anesthetized mice through a small incision in the back of the neck that was closed with surgical sutures. All incision sites healed rapidly without the need for any medication.
Blood pressure measurements.
Systolic blood pressure of mice was measured by the tail cuff method (CODA Non-Invasive Blood Pressure System; Kent Scientific, Torrington, CT) before and 4 wk after the minipump implantation.
Simultaneous measurement of diameter and smooth muscle [Ca2+]i in pressurized isolated middle cerebral arteries.
On day 28 postimplantation mice were decapitated, the brains were removed, and segments of the MCAs were isolated using microsurgery instruments for functional studies, as reported (59, 64). Segments of MCAs were mounted onto two glass micropipettes in an organ chamber in oxygenated (21% O2-5% CO2-75% N2) Krebs′ buffer (pH = 7.4) and pressurized to 60 mmHg. The hydrodynamic resistance of the micropipettes was matched. Inflow and outflow pressures were controlled and measured by a pressure servo-control system (Living Systems Instrumentation, Burlington, VE). Inner vascular diameter was measured with a custom-built videomicroscope system and continuously recorded using a computerized data acquisition system (62). To assess changes in [Ca2+]i, the arterial smooth muscle was loaded with fura 2 [1 μM fura 2-acetoxymethyl ester; loading time: 15 min, at room temperature]. After the chamber was loaded it was washed out five times, and the vessels were allowed to equilibrate at 60 mmHg for another 30 min for complete de-esterification of fura-2 AM (46). Changes in Ca2+ fluorescence ratio (R340:380) were measured by the ratiometric fluorescence method (23, 63) using the Ionoptix Microfluorimeter System (Ionoptix, Milton, MA).
After the equilibration period, changes in SMC [Ca2+]i and vasoconstrictor responses to depolarizing concentrations (60 mmol/l) of KCl were obtained, followed by washout. Changes in SMC [Ca2+]i and myogenic constriction were then assessed in response to stepwise increases in intraluminal pressure (from 20 to 80 and 160 mmHg, each step for 5 min). To assess the role of 20-HETE in age- and hypertension-induced changes in the myogenic response and SMC [Ca2+]i, MCAs were incubated with the cytochrome P-450 ω-hydroxylase inhibitor HET0016 (10−6 mol/l, for 30 min, purchased from Cayman Chemical, Ann Arbor, MI). Vascular responses to stepwise increases in intraluminal pressure were then re-assessed. HET0016 was previously reported to selectively inhibit the formation of 20-HETE by inhibiting CYP4A and CYP4F isoforms in renal microsomes isolated from spontaneously hypertensive rats (IC50: 35.2 nM) and in human kidney (IC50: 8.9 nM) (48).
To assess the role of canonical TRP (TRPC) channels in aging- and hypertension-induced changes in myogenic constriction, in additional experiments pressure-induced vasomotor and calcium responses were obtained before and after incubation of MCAs with SKF96365 (5 × 10−6 mol/l, for 15 min), a potent and specific blocker of TRPC channels. To further substantiate the role of 20-HETE in regulation of SMC [Ca2+]I, vascular responses to exogenous administration of 20-HETE (from 10−8 to 3 × 10−6 mol/l) were also assessed in the absence and presence of SKF96365.
To determine the role of large-conductance Ca2+-activated K+ (BKCa) channels in aging- and hypertension-induced alterations in myogenic constriction of MCAs, in separate experiments pressure-induced vasomotor and calcium responses were obtained in the absence and presence of the selective BK channel blocker iberiotoxin (10 nmol/l). To determine the role of voltage-dependent K+ channels, MCAs were co-incubated with 4-aminopyridine (1 mmol/l; a blocker of members of Kv1 [Shaker, KCNA] family of voltage-activated K+ channels) and pressure-induced vasomotor and calcium responses were re-assessed. At the conclusion of each experiment the passive pressure-diameter curves were obtained (pressure range: 0 to 180 mmHg) in the presence of Ca2+-free Krebs′ buffer containing the L-type calcium channel inhibitor nifedipine (10−5 mol/l) to achieve maximal vasodilatation.
Quantitative real-time RT-PCR.
A quantitative real-time RT-PCR technique was used to analyze mRNA expression of genes that are involved in the regulation of pressure-induced myogenic constriction (Table 1) using a Strategen MX3000 platform, as previously reported (7). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (7). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes Hprt, Ywhaz, B2m, and Actb were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Table 1.
Oligonucleotides for real-time RT-PCR
| mRNA Targets | Description | Sense | Antisense |
|---|---|---|---|
| Trpc1 | Transient receptor potential canonical channel1 | ATCCTCCTCGTCGCCCAA | TCATTCAAGGTGTTCTCCTCCT |
| Trpc3 | Transient receptor potential canonical channel3 | AAGCGACGGAGGAATTAG | ACTCACATCTCAGCACAC |
| Trpc4 | Transient receptor potential canonical channel4 | GAGTGTTTACCATACCTATAC | GGTTCATATTACTTCTGCTT |
| Trpc5 | Transient receptor potential canonical channel5 | CATCTGCTCCTCAAATTC | GTTGTAACTTGTTCTTCCT |
| Trpc6 | Transient receptor potential canonical channel6 | TCTCCACTTGAAGCCATA | TTGTTAGCCTCAGCAATG |
| Trpc7 | Transient receptor potential canonical channel7 | CAGGATTGTGAAACTAAGC | GTCTCGTGTATCTGATGG |
| Trpm4 | Transient receptor potential cation channel subfamily m4 | AAACCATTTGATGAGGATTT | GAAGGACAGATTCCCAAC |
| Trpv4 | Transient receptor vanilloid channel 4 | CACCTAAGCCAGCACAAG | TGAGGAGAGCAATAAATAATACAC |
| Cav1 | Caveolin 1 | GTGGTCAAGATTGACTTTG | GTAGACAACAAGCGGTAA |
| Cav2 | Caveolin 2 | CTGGTCATTATTATAGGTCATT | GATGCTTGTGGCTATTAC |
| Hprt | Hypoxanthine phosphoribosyltransferase 1 | TGCTGCGTCCCCAGACTTTTG | AGATAAGCGACAATCTACCAGAGG |
| Actb | β-Actin | AATAAGTGGTTACAGGAAGTC | ATGAAGTATTAAGGCGGAAG |
| B2m | β-2-Microglobin | CGGTCGCTTCAGTCGTCAG | CAGTTCAGTATGTTCGGCTTCC |
| Ywhaz | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, ζ-polypeptide 1 | ACTGTCTTGTCACCAACCATTC | GGGCTGTAGAGAGGATGAGG |
Statistical analysis.
Data were analyzed by two-way ANOVA or one-way ANOVA followed by Tukey's post hoc tests, as appropriate. The relationship between changes in SMC [Ca2+]i and arterial constriction was analyzed using Pearson's correlation analysis. A P value less than 0.05 was considered statistically significant. Data are expressed as means ± SE. Myogenic tone is calculated as follows: ((DP − DA)/DP) × 100, where DP is passive diameter (obtained in the absence of Ca2+) and DA is active diameter of the vessels at a given intraluminal pressure value.
RESULTS
Role of SMC calcium signaling in functional maladaptation of aged cerebral arteries to hypertension.
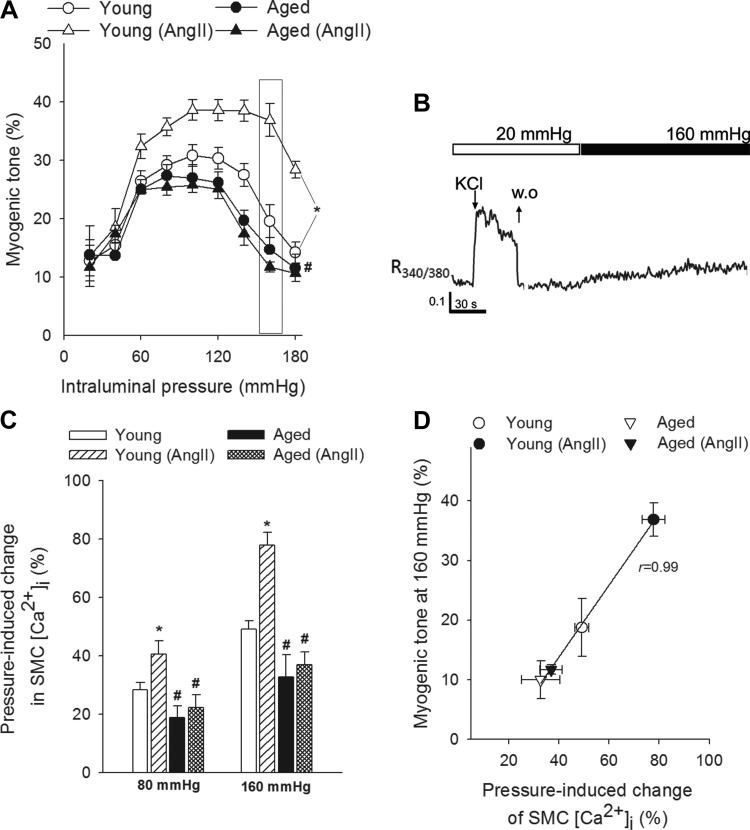
Blood pressure was significantly increased in both young and aged mice receiving ANG II infusion (in mmHg; young control: 100 ± 4, young + ANG II: 150 ± 3, Aged: 97 ± 4, Aged + ANG II: 154 ± 3). Figure 1A shows myogenic tone developed in isolated MCAs at intraluminal pressures of 20–180 mmHg. In MCAs of young control mice, increases in intravascular pressure increased myogenic constriction up to 60 mmHg, and myogenic tone was maintained at almost the same level at up to ∼120 mmHg. Myogenic tone then tended to decrease at higher pressures, and MCAs dilated gradually (Fig. 1A). In MCAs from young hypertensive mice, pressure-induced constriction was significantly enhanced and the myogenic tone was maintained at almost the same level up to ∼160 mmHg. MCAs of aged mice developed a slightly decreased myogenic constriction and did not exhibit a similar hypertension-induced adaptive increase in myogenic tone, which was observed in young mice (Fig. 1A). The pressure-passive diameter curves were similar in MCAs from each group (not shown).
Fig. 1.
Aging impairs adaptation of cerebrovascular myogenic autoregulation and smooth muscle cell (SMC) calcium signaling to hypertension. A: myogenic tone of middle cerebral arteries (MCAs) of young control, young hypertensive (young + ANG II), aged control, and aged hypertensive (aged + ANG II) mice. Data are means ± SE; n = 8 in each group. *P < 0.05 vs. young; #P < 0.05 vs. young + ANG II. Myogenic tone is expressed as percentage of the maximally dilated passive diameter of each vessel at the same intraluminal pressure. B: representative tracing of fura-2 340/380 fluorescence ratio in an isolated MCA showing increases in SMC intracellular Ca2+ concentration ([Ca2+]i) in response to depolarizing concentrations of KCl [60 mM; washout (WO)] and increased intraluminal pressure (160 mmHg). C: summary data showing increases in SMC [Ca2+]i in response to increases in intraluminal pressure to 80 and 160 mmHg. Data are expressed as percentage of KCl (60 mM)-induced calcium signals. Data are means ± SE; n = 8 in each group (*P < 0.05 vs. young; #P < 0.05 vs. young + ANG II). D: relationship between pressure (160 mmHg)-induced changes in SMC [Ca2+]i and myogenic tone of MCAs. Data are means ± SE; n = 8 in each group.
In isolated MCAs depolarizing concentrations of KCl elicited rapid increases in SMC [Ca2+]i, which were readily reversible upon washout (Fig. 1B). Exposure of young MCAs to stepwise increases in intraluminal pressure (from 20 mmHg to 80 and then to 160 mmHg) resulted in significant increases in SMC [Ca2+]i (Fig. 1, B and C). In MCAs of aged mice pressure-induced increases in SMC [Ca2+]i did not differ significantly from the pressure-induced calcium signal observed in young MCAs (Fig. 1C). In MCAs from young hypertensive mice pressure-induced increases in SMC [Ca2+]i were significantly enhanced (Fig. 1C). In contrast, aged MCAs did not exhibit a similar hypertension-induced adaptive increase in pressure-induced calcium signal, which was observed in MCAs of young hypertensive mice (Fig. 1C). The magnitude of pressure-induced SMC calcium signal and the magnitude of pressure-induced myogenic tone observed in MCAs from the different experimental groups showed a significant positive correlation (Fig. 1D).
Role of decreased 20-HETE production in dysregulation of pressure-induced SMC calcium signaling and myogenic constriction in MCAs of aged hypertensive mice.
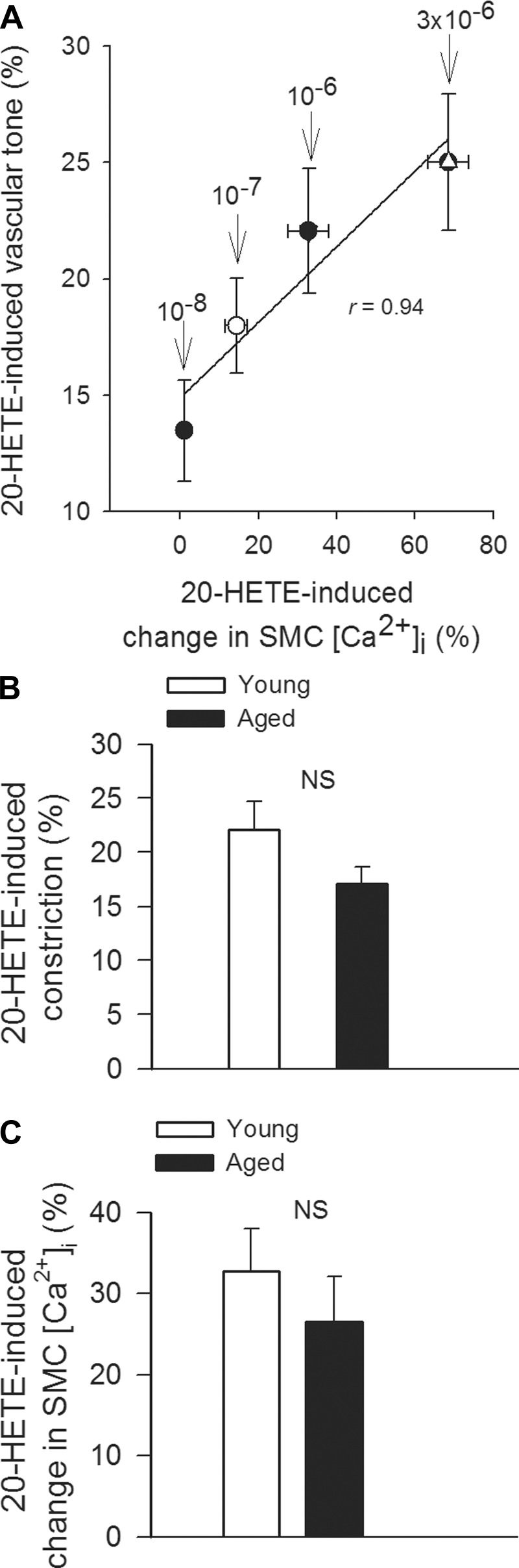
To assess the role of 20-HETE in pressure-induced SMC calcium signaling and functional adaptation of cerebral arteries to hypertension, we tested the effect of HET0016 on SMC [Ca2+]i and myogenic constriction at pathophysiologically relevant pressures (160 mmHg; Fig. 2). We found that in MCAs of young hypertensive mice increased pressure-induced calcium signal (Fig. 2A) and myogenic tone (Fig. 2B) were significantly inhibited by HET0016, eliminating the difference between the four groups, whereas HET0016 did not significantly affect SMC [Ca2+]i and myogenic tone in MCAs of aged hypertensive mice. In MCAs of young hypertensive mice the magnitude of HET0016-induced decreases in pressure-induced calcium signal and myogenic tone were proportional (Fig. 2B), suggesting that 20-HETE mediates myogenic vasoconstriction predominantly via increasing SMC [Ca2+]i. To further substantiate the role of 20-HETE in regulation of SMC [Ca2+]i, vascular responses to exogenous administration of 20-HETE were assessed. We found that in control MCAs 20-HETE elicited dose-dependent increases in SMC [Ca2+]i, which were accompanied by proportional increases in arterial tone (Fig. 3A). These findings support the view that 20-HETE promotes constriction of cerebral arteries predominantly by increasing SMC [Ca2+]i,. 20-HETE elicited comparable increases in arterial tone (Fig. 3B) and SMC [Ca2+]i (Fig. 3C) in MCAs isolated from young and aged animals, showing that 20-HETE-induced intracellular signaling pathways are not affected by aging.
Fig. 2.
Role of 20-hydroxyeicosatetraenoic acid (HETE) in functional adaptation of MCAs to hypertension. A: increases in SMC [Ca2+]i in MCAs in response to increases in intraluminal pressure (from 20 mmHg to 160 mmHg) in the absence and presence of the specific cytochrome P-450 ω-hydroxylase inhibitor HET0016. Data are expressed as percentage of KCl (60 mM)-induced calcium signals. Data are means ± SE; n = 8 in each group (*P < 0.05 vs. young; #P < 0.05 vs. young + ANG II). B: relationship between pressure (160 mmHg)-induced changes in SMC [Ca2+]i and myogenic tone of MCAs of young and aged hypertensive mice in the absence and presence of HET0016. Data are means ± SE; n = 8 in each group.
Fig. 3.
A: relationship between 20-HETE-induced changes in SMC [Ca2+]i and tone of control MCAs. Data are expressed as percentage of KCl (60 mM)-induced responses. Data are means ± SE; n = 8 in each group. B and C: 20-HETE-induced constrictions (B) and changes in SMC [Ca2+]i in MCAs isolated from young and aged mice. Data are means ± SE; n = 8 in each group. NS, nonsignificant.
Role of impaired TRP channel activity in dysregulation of pressure-induced SMC calcium signaling and myogenic constriction in MCAs of aged hypertensive mice.
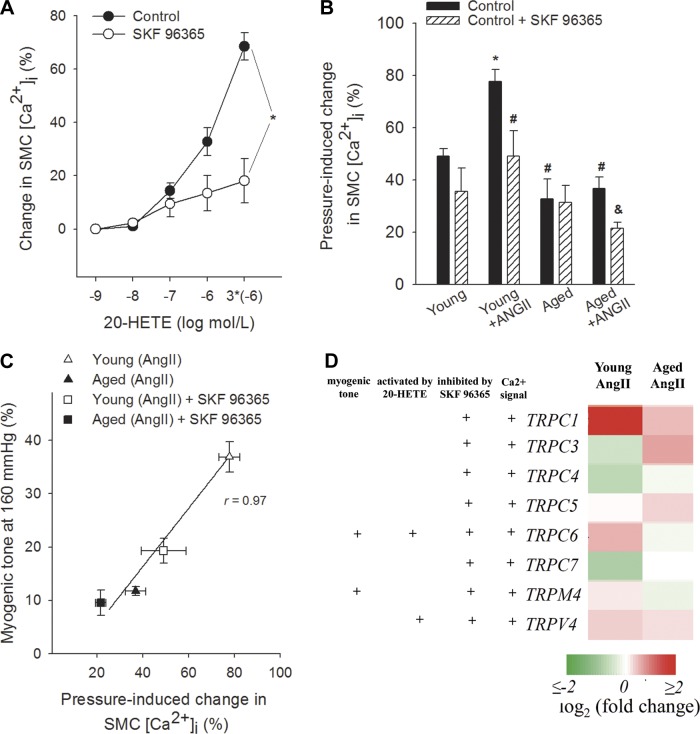
Because previously we found that in mouse aortas and cerebral arteries the vasoconstrictor effect of 20-HETE is significantly inhibited by the TRP channel inhibitor SKF96365, in the present study we also assessed the role of TRP channels in 20-HETE-dependent functional adaptation of cerebral arteries to hypertension using SKF96365. We found that in control MCAs 20-HETE-induced dose-dependent increases in SMC [Ca2+]i were significantly attenuated by SKF96365 (Fig. 4A). In MCAs of young hypertensive mice increased pressure-induced calcium signal (Fig. 4B) and myogenic tone (Fig. 4C) were significantly attenuated by SKF96365. In contrast, in MCAs of aged hypertensive mice SKF96365 elicited only a slight decrease in pressure-induced calcium signal (Fig. 4B) and myogenic tone (Fig. 4C). Analysis of the SMC [Ca2+]i-myogenic tone relation showed that in MCAs of young hypertensive mice inhibition of both TRP channel activation (Fig. 4C) and 20-HETE production (Fig. 2B) resulted in similar parallel decline in VSMC [Ca2+]i and myogenic constriction, suggesting that 20-HETE mediates myogenic vasoconstriction predominantly via increasing SMC [Ca2+]i through activation of TRP channels. Hypertension in young mice was associated with upregulated cerebrovascular expression of TRPC6 and TRPC1 channels (Fig. 4D) and also tended to increase the expression of TRPM4, although this response did not reach statistical significance (P = 0.077). The aforementioned adaptive responses were significantly impaired or absent in MCAs of aged hypertensive mice (Fig. 4D).
Fig. 4.
Role of transient receptor potential (TRP) channels in 20-HETE-induced vascular responses and functional adaptation of MCAs to hypertension. A: canonical TRP (TRPC) channel blocker SKF96365 inhibits 20-HETE-induced increases in SMC [Ca2+]i in control MCAs. Data are expressed as percentage of KCl (60 mM)-induced calcium signals. Data are means ± SE; *P < 0.05 vs. untreated control. B: pressure (160 mmHg)-induced increases in SMC [Ca2+]i in MCAs in the absence and presence of SKF96365. Data are means ± SE; n = 8 in each group. *P < 0.05 vs. young control; #P < 0.05 vs. young + ANG II; &P < 0.05 vs. aged + ANG II. C: relationship between pressure (160 mmHg)-induced changes in SMC [Ca2+]i and myogenic tone of MCAs of young and aged hypertensive mice in the absence and presence of SKF96365. Data are means ± SE; n = 8 in each group. D: heat map is a graphic representation of hypertension-induced changes in mRNA expression of TRP channels in MCAs of young and aged mice, depicted by color intensity, from highest (bright red) to lowest (bright green) expression (n = 6 in each group). +TRP channels, which were shown to play a role in arterial myogenic constriction and pressure-induced modulation of SMC [Ca2+]i and which can be activated by 20-HETE and inhibited by SKF96365. These classifications are based on data from the literature (17, 30, 40, 57). Note that MCAs of young hypertensive mice show significant upregulation of TRPC6, whereas this adaptive response is significantly impaired in MCAs of aged hypertensive mice.
TRP channels have been found to colocalize with caveolae, which were shown to decline with age (1–3, 20, 33). Interestingly, knocking out of caveolin 1 results in decreased myogenic constriction of cerebral arteries as well (2). Here we found that expression of Cav1 is significantly upregulated in MCAs of young hypertensive mice (young: 1 ± 0.09, young + ANG II: 1.31 ± 0.11; P < 0.05), whereas hypertension did not increase its expression in MCAs of aged mice (aged: 0.69 ± 0.24, aged + ANG II: 0.88 ± 0.06; not significant). Expression of Cav2 was also decreased with advanced age and was unaffected by hypertension in mouse MCAs (young: 1 ± 0.06, young + ANG II: 1.06 ± 0.06, aged: 0.70 ± 0.08, aged + ANG II: 0.65 ± 0.04).
Role of BKCa channels in regulation of pressure-induced SMC calcium signaling and myogenic constriction in MCAs.
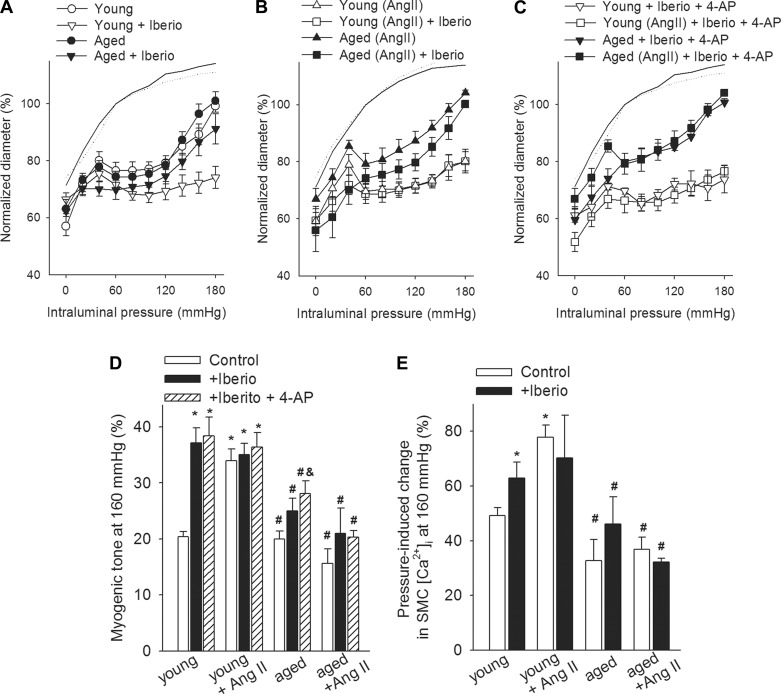
Regulation of large-conductance Ca2+-activated K+ (BKCa) channel activity represents an important mechanism modulating the magnitude of pressure-induced myogenic constriction (32, 52). To assess the role of BKCa channels in functional maladaptation of cerebral arteries to hypertension in aging, we tested the effect of iberiotoxin on myogenic constriction (Fig. 5, A–C). We found that in MCAs of young normotensive mice, myogenic tone (Fig. 5D) and pressure-induced SMC calcium signals (Fig. 5E) were significantly increased by iberiotoxin, especially in the pathophysiologically relevant high pressure range. In MCAs of young hypertensive mice, myogenic tone (Fig. 5D) and pressure-induced SMC calcium signals (Fig. 5E) were unaffected by iberiotoxin, suggesting that inactivation of BKCa channels contribute to functional adaptation of MCAs to hypertension. Although in MCAs of aged normotensive mice iberiotoxin increased slightly, the myogenic constriction (Fig. 5, A and D) tended to increase pressure-induced SMC calcium signals (Fig. 5E), and these effects were largely absent in MCAs of aged hypertensive mice (Fig. 5, D and E). Importantly, inhibition of BKCa channels did not eliminate the differences in myogenic constriction (Fig. 5, C and D) and pressure-induced increases in SMC [Ca2+]i (Fig. 5E) between the four groups. Co-administration of 4-AP (to inhibit voltage-dependent K+ channels) did not augment myogenic constriction in MCAs of aged hypertensive mice, and the difference between the myogenic tone in iberiotoxin and 4-AP-treated MCAs of young and aged hypertensive mice was still discernible (Fig. 5, C and D).
Fig. 5.
Role of potassium channels in regulation of cerebrovascular myogenic tone in aged hypertensive mice. A–C: steady-state changes in diameter of MCAs isolated from young control, young + ANG II, aged control, and aged + ANG II mice in response to increases in intraluminal pressure in the absence and presence of iberiotoxin (Iberio) and 4-AP (which inhibit BKCa and voltage-activated K+ channels, respectively). Vascular diameters are expressed as percentage of the maximally dilated passive diameter of each vessel at 80 mmHg. Bar graphs show the effects of K+ channel blockers on myogenic tone (D) and pressure (160 mmHg)-induced increases in SMC [Ca2+]i in MCAs (E). Data are means ± SE (n = 8 in each group). *P < 0.05 vs. young control; #P < 0.05 vs. young + ANG II; &P < 0.05 vs. aged + ANG II.
DISCUSSION
The results of this study suggest that functional maladaptation of aged cerebral arteries to hypertension is due to the dysregulation of pressure-induced, 20-HETE, and TRP channel-mediated SMC calcium signaling.
Since the pioneering work of Kontos et al. (36) and Faraci and Heistad (18), it has been established that in the cerebral circulation larger pial arteries have a significant role in regulation of cerebrovascular resistance, thus myogenic constriction of proximal branches of the cerebrovascular tree, including the MCA, is uniquely important for protection of the cerebral microcirculation. There is strong evidence that pressure-induced myogenic constriction of the cerebral arteries in healthy young animals (Fig. 1A) acts as a critical homeostatic mechanism that assures that increased systemic arterial pressure cannot penetrate the distal portion of the cerebral microcirculation and cause damage to the thin-walled arteriolar and capillary microvessels (27, 36). In normotensive animals the autoregulatory pressure range tended to be wider in MCAs of young mice than in aged arteries (Fig. 1A). Studies showing that myogenic constriction of isolated rat skeletal muscle arteries (32) decreases with age and that the dynamic component of the myogenic response decreases in the brachial artery of elderly humans (43) suggest that aging exerts general effect on local regulation of vascular resistance.
In cerebral arteries isolated from hypertensive young mice (Fig. 1A) and rats (49, 51), the myogenic constriction at high pressures is augmented, suggesting that the pressure range for autoregulatory cerebrovascular protection is extended. This functional adaptation of cerebral arteries to higher systemic blood pressure is believed to protect the cerebral microcirculation (27, 36, 51, 53, 58). Here we provide evidence that cerebral arteries of aged mice do not exhibit a hypertension-induced adaptive increase in myogenic tone observed in young mice (Fig. 1A), extending our recent findings (60). The functional maladaptation of aged cerebral arteries to hypertension is likely responsible for the loss of autoregulatory protection in the brain observed in vivo, which likely allows high blood pressure to penetrate the distal, injury-prone portion of the cerebral microcirculation, leading to significant disruption of the blood-brain barrier and cerebromicrovascular damage in aged hypertensive mice (60). Our findings potentially have important clinical significance, since exacerbation of microvascular damage in aged hypertensive mice is likely causally linked to increased neuroinflammation and cognitive decline. In the present study we confirm that in cerebral arteries stepwise increases in intraluminal pressure are associated with proportional increases in vascular SMC (VSMC) [Ca2+]i (Fig. 1, B–D), which have a central role in myogenic constriction of cerebral arteries (34). In MCAs of young hypertensive mice, augmented myogenic tone was associated with an increased VSMC [Ca2+]i (Fig. 1, C and D), suggesting that upregulation of pressure-induced calcium signaling underlies the functional adaptation of cerebral arteries to high blood pressure. The findings that hypertension in aged mice did not enhance VSMC [Ca2+]i suggest that pathways regulating pressure-induced Ca2+ signaling are responsible for functional maladaptation of aged cerebral arteries to hypertension.
Previous studies provide strong evidence that generation of 20-HETE plays an important role in pressure-induced constriction of cerebral arteries (21, 26). Here we show for the first time that inhibition of 20-HETE production attenuates pressure-induced Ca2+ signals in VSMCs and decreases myogenic constriction of MCAs from young hypertensive animals, whereas it exerts no significant effect on pressure-induced responses of MCAs from aged hypertensive animals (Fig. 2). These findings support the concept that in young animals activation of a 20-HETE-dependent pathway underlies functional adaptation of cerebral arteries to hypertension and that this adaptive response is dysfunctional in aging. Further evidence in support of this hypothesis are provided by previous studies showing that in young hypertensive rats pressure-induced 20-HETE production in cerebral arteries is significantly increased (15) and by our very recent work that hypertension in young mice (but not in aged mice) is associated with adaptive upregulation of cytochrome P-450 4A ω-hydroxylases capable to produce 20-HETE (Cyp4A12, 10, 14) (60). Moreover, administration of exogenous 20-HETE significantly increases VSMC [Ca2+]i, which augments myogenic constriction of MCAs (Fig. 3).
The intracellular signaling pathways activated by 20-HETE are multifaceted (22, 44, 50, 68). Findings of the present study suggest 20-HETE-induced activation of TRP channels mediates 20-HETE-induced increases in VSMC [Ca2+]i in mouse MCAs (Fig. 4A). Importantly, although in cerebral arteries of young mice hypertension upregulates TRP channel-dependent mediation of pressure-induced = increases in VSMC [Ca2+]i (Fig. 4B) and myogenic constriction (Fig. 4C), this adaptive response is impaired in aged angiotensin II-infused hypertensive mice. Analysis of the VSMC [Ca2+]i-myogenic tone relation (Fig. 2B and Fig. 4C) confirms that both inhibition of 20-HETE production and TRP channel activation attenuate myogenic constriction in MCAs of young hypertensive mice in a similar manner (primarily by decreasing VSMC [Ca2+]i) and that loss of 20-HETE/TRP channel-dependent component of myogenic tone is responsible for the decreased pressure-induced constriction in MCAs of aged hypertensive mice. At present it is unclear which TRP channel(s) are responsible for the observed phenomena, but several lines of evidence suggest that TRPC6 may play a key role in the functional maladaptation of aged arteries to high blood pressure. First, previous studies demonstrate that predominantly activation of TRPC6 channels mediates 20-HETE-induced increases in intracellular Ca2+ levels in VSMCs (30) and that it contributes to autoregulation and myogenic constriction of young cerebral arteries (66). TRPC6 channels are activated by membrane stretch (57), via a 20-HETE (30), G protein, and phospholipase C-dependent pathway (47). In the present study we used a concentration of SKF96365, which significantly inhibits TRPC6 as demonstrated by electrophysiological studies (30). Furthermore, although in cerebral arteries of young mice hypertension upregulates TRPC6 expression, this adaptive response fails to manifest in aged hypertensive mice (Fig. 4D). It should be noted, however, that vascular expression of other TRP channels also appears to be affected by hypertension (Fig. 4D). Although TRPC1, whose expression increases in MCAs of young hypertensive mice, contributes to agonist-induced constriction (67), it does not appear to contribute to myogenic constriction (16, 56). TRPC3 and TRPC7 form heterotrimeric channels with TRPC6; however, the pattern of expression of these channels is inconsistent with their involvement in adaptive increases in myogenic tone in young hypertensive animals. Previous studies suggest that TRPM4 channels may contribute to myogenic constriction (17, 30, 40, 57). Therefore, future studies are warranted to elucidate the role of TRPM4 channels in age-related arterial maladaptation to hypertension. TRPV4 channels are thought to counter-regulate myogenic tone (6, 8). Thus the observed hypertension-induced increases in TRPV4 expression in MCAs of young mice are inconsistent with the involvement of these channels in adaptive increases in myogenic tone.
The age-related mechanism(s) that are responsible for dysregulation of 20-HETE/TRPC-dependent pathways in hypertensive mice are presently unknown and may include an age-related IGF-1 deficiency (61). This concept is supported by our recent data showing that IGF-1 deficiency in mice (Igf1f/f + MUP-iCre-AAV8) results in impaired hypertension-induced adaptive changes in expression of TRPC6 and cytochrome P-450–4A ω-hydroxylases and decreased cerebral arterial myogenic tone, mimicking the aging phenotype (Toth and Ungvari, unpublished observation).
Because inhibition of the 20-HETE/TRP channel pathway does not completely abolish myogenic constriction in cerebral arteries of young hypertensive mice, we cannot exclude a potential role for other mechanisms in functional maladaptation to hypertension in aging as well. In addition to activation of TRP channels, regulation of BKCa channel activity represents another important mechanism modulating the magnitude of pressure-induced myogenic constriction (32, 52). Importantly, previous studies demonstrate that high pressure is associated with significant activation of BKCa channels leading to forced dilation of cerebral arteries (autoregulatory breakthrough) (52). In support of the aforementioned concept, here we demonstrate that pharmacological inhibition of BKCa channels prevents forced dilation of young MCAs (Fig. 5A and 6A) increasing VSMC [Ca2+]i (Fig. 6B). In contrast, in MCAs of young hypertensive mice inhibition of BKCa channels did not increase further myogenic tone (Fig. 5B and 6A) and VSMC [Ca2+]i (Fig. 6B). 20-HETE was shown to inhibit BKCa channels (22, 39, 50, 70). Our model predicts that increased 20-HETE production in young hypertensive arteries is associated with a decreased BKCa activity (enhancing myogenic constriction), which would explain the lack of further iberiotoxin-induced increases in myogenic tone and VSMC [Ca2+]i in these vessels (Fig. 6B). The findings that in arteries of aged hypertensive mice inhibition of BKCa channels did not increase significantly myogenic tone (Fig. 5B and 6A) and VSMC [Ca2+]i (Fig. 6B) suggest that BKCa channels do not play a key role in functional maladaptation of aged cerebral arteries to high pressure. Previous studies reported that with advanced age the activity of KV channels may increase in skeletal muscle arteries (32) and that disruption of KV channel function may contribute to the adaptation of young rat cerebral arteries to hypertension (4). Yet data obtained with inhibitors of KV channels (Fig. 5, C and D) suggest that these channels play a negligible role in modulation of myogenic constriction in mouse MCAs and do not contribute to age-related decreases in myogenic tone in hypertensive mice. There is growing evidence that caveolae formation colocalizes TRPC channels and BKCa channels with membrane microdomains, which improves efficiency of mechanotransduction in VSMC (1, 55). Accordingly, decreased expression of the scaffolding protein caveolin-1, which is the main component of the caveolae plasma membranes, was reported to attenuate the myogenic response in murine cerebral arteries (2). Because expression of caveolin-1 declines with age (20, 33), the role of caveolae in dysregulation of myogenic adaptation to hypertension in aging should be considered in future studies (28).
In conclusion, the functional adaptation of cerebral arteries to high pressure in angiotensin II-infused aged mice is impaired due to dysregulation of pressure-induced, 20-HETE, and TRPC channel-mediated changes in VSMC [Ca2+]i. Future studies should elucidate the specific age-related mechanism that contributes to the aforementioned alterations in the VSMCs, including the role of age-related changes in membrane microdomains and endocrine factors (61).
GRANTS
This work was supported by grants from the American Heart Association (to P. Toth, A. Csiszar, and Z. Ungvari), the American Federation for Aging Research (to A. Csiszar), the Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar, Z. Ungvari, and W. E. Sonntag), the Nemzeti Fejlesztési ügynökség (SROP-4.2.2.a-11/1/KONV-2012-0024 and -0017 to A. Koller; SPOR-4.2.1/b-10/2/KONV-2010–0012 to A. Koller and Z. Ungvari), the Hungarian Scientific Research Fund (OTKA; K 108444), the National Center for Complementary and Alternative Medicine (R01-AT006526 to Z. Ungvari); the National Institute on Aging (AG031085 to A. Csiszar; AG038747 to W. E. Sonntag), and the Ellison Medical Foundation (to W. E. Sonntag).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.T., A.C., W.E.S., and Z.I.U. conception and design of research; P.T., A.C., Z.T., D.S., T.G., and M.L.S. performed experiments; P.T., A.C., Z.T., D.S., T.G., M.L.S., and Z.I.U. analyzed data; P.T., A.C., Z.T., D.S., A.K., W.E.S., and Z.I.U. interpreted results of experiments; P.T. and Z.I.U. prepared figures; P.T., A.K., and Z.I.U. drafted manuscript; P.T., A.C., Z.T., A.K., M.L.S., W.E.S., and Z.I.U. edited and revised manuscript; P.T., A.C., Z.T., D.S., T.G., A.K., M.L.S., W.E.S., and Z.I.U. approved final version of manuscript.
REFERENCES
- 1.Adebiyi A, Thomas-Gatewood CM, Leo MD, Kidd MW, Neeb ZP, Jaggar JH. An elevation in physical coupling of type 1 inositol 1,4,5-trisphosphate (IP3) receptors to transient receptor potential 3 (TRPC3) channels constricts mesenteric arteries in genetic hypertension. Hypertension 60: 1213–1219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adebiyi A, Zhao G, Cheranov SY, Ahmed A, Jaggar JH. Caveolin-1 abolishment attenuates the myogenic response in murine cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1584–H1592, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res 106: 1603–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol 291: C348–C356, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ, Harrington RA. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 123: 2434–2506, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci 67: 313–329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol 35: 1116–1120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 532–535, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 67: 564–569, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnevale D, Lembo G. ′Alzheimer-like′ pathology in a murine model of arterial hypertension. Biochem Soc Trans 39: 939–944, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Carnevale D, Mascio G, Ajmone-Cat MA, D′Andrea I, Cifelli G, Madonna M, Cocozza G, Frati A, Carullo P, Carnevale L, Alleva E, Branchi I, Lembo G, Minghetti L. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging 33: 205 e219–e229, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Carnevale D, Mascio G, D′Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60: 188–197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellani S, Bacci M, Ungar A, Prati P, Di Serio C, Geppetti P, Masotti G, Neri Serneri GG, Gensini GF. Abnormal pressure passive dilatation of cerebral arterioles in the elderly with isolated systolic hypertension. Hypertension 48: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H2455–H2465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond) 119: 19–36, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 66: 8–17, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta 1772: 895–906, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridolfsson HN, Patel HH. Caveolin and caveolae in age associated cardiovascular disease. Journal of geriatric cardiology 10: 66–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Gebremedhin D, Yamaura K, Harder DR. Role of 20-HETE in the hypoxia-induced activation of Ca2+-activated K+ channel currents in rat cerebral arterial muscle cells. Am J Physiol Heart Circ Physiol 294: H107–H120, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Gokina NI, Knot HJ, Nelson MT, Osol G. Increased Ca2+ sensitivity as a key mechanism of PKC-induced constriction in pressurized cerebral arteries. Am J Physiol Heart Circ Physiol 277: H1178–H1188, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Z, Qiu C, Viitanen M, Fastbom J, Winblad B, Fratiglioni L. Blood pressure and dementia in persons 75+ years old: 3-year follow-up results from the Kungsholmen Project. J Alzheimers Dis 3: 585–591, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol 300: H1557–H1565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension 6: 408–419, 1984 [DOI] [PubMed] [Google Scholar]
- 28.Hausman N, Martin J, Taggart MJ, Austin C. Age-related changes in the contractile and passive arterial properties of murine mesenteric small arteries are altered by caveolin-1 knockout. J Cell Mol Med 16: 1720–1730, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke 40: S40–S44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res 104: 1399–1409, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Israeli-Korn SD, Masarwa M, Schechtman E, Abuful A, Strugatsky R, Avni S, Farrer LA, Friedland RP, Inzelberg R. Hypertension increases the probability of Alzheimer's disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology 34: 99–105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang LS, Kim S, Dominguez JM, 2nd, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol 107: 389–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawabe JI, Grant BS, Yamamoto M, Schwencke C, Okumura S, Ishikawa Y. Changes in caveolin subtype protein expression in aging rat organs. Molecular and cellular endocrinology 176: 91–95, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol 269: H348–H355, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol Heart Circ Physiol 234: H371–H383, 1978 [DOI] [PubMed] [Google Scholar]
- 37.Kontos HA, Wei EP, Raper AJ, Rosenblum WI, Navari RM, Patterson JL., Jr Role of tissue hypoxia in local regulation of cerebral microcirculation. Am J Physiol Heart Circ Physiol 234: H582–H591, 1978 [DOI] [PubMed] [Google Scholar]
- 38.Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, Kawas C, Breitner JC, Fitzpatrick A, Dulberg C. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 64: 1548–1552, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem 272: 27345–27352, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Liedtke W. TRPV channels′ role in osmotransduction and mechanotransduction. Handb Exp Pharmacol: 473–487, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Linde CI, Karashima E, Raina H, Zulian A, Wier WG, Hamlyn JM, Ferrari P, Blaustein MP, Golovina VA. Increased arterial smooth muscle Ca2+ signaling, vasoconstriction, and myogenic reactivity in Milan hypertensive rats. Am J Physiol Heart Circ Physiol 302: H611–H620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D, Yang D, He H, Chen X, Cao T, Feng X, Ma L, Luo Z, Wang L, Yan Z, Zhu Z, Tepel M. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension 53: 70–76, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Lott ME, Herr MD, Sinoway LI. Effects of age on brachial artery myogenic responses in humans. Am J Physiol Regul Integr Comp Physiol 287: R586–R591, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res 72: 126–136, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 11: 1039–1047, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrelli SP. Selective measurement of endothelial or smooth muscle [Ca2+]i in pressurized/perfused cerebral arteries with fura-2. J Neurosci Methods 97: 145–155, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27: 3092–3103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.New DI, Chesser AM, Thuraisingham RC, Yaqoob MM. Cerebral artery responses to pressure and flow in uremic hypertensive and spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 284: H1212–H1216, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKC alpha-mediated inhibition of KCa channel. Br J Pharmacol 137: 1362–1370, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol 249: H914–H921, 1985 [DOI] [PubMed] [Google Scholar]
- 52.Paterno R, Heistad DD, Faraci FM. Potassium channels modulate cerebral autoregulation during acute hypertension. Am J Physiol Heart Circ Physiol 278: H2003–H2007, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990 [PubMed] [Google Scholar]
- 54.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 64: 1734–1740, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riddle MA, Hughes JM, Walker BR. Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am J Physiol Cell Physiol 301: C1404–C1414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt K, Dubrovska G, Nielsen G, Fesus G, Uhrenholt TR, Hansen PB, Gudermann T, Dietrich A, Gollasch M, de Wit C, Kohler R. Amplification of EDHF-type vasodilatations in TRPC1-deficient mice. Br J Pharmacol 161: 1722–1733, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strandgaard S, Jones JV, MacKenzie ET, Harper AM. Upper limit of cerebral blood flow autoregulation in experimental renovascular hypertension in the baboon. Circ Res 37: 164–167, 1975 [DOI] [PubMed] [Google Scholar]
- 59.Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab 31: 2096–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 10.1038/jcbfm.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci 67: 599–610, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ungvari Z, Koller A. Endothelin and PGH2/TXA2 enhances myogenic constricton in hypertension by increasing Ca2+ sensitivity of arteriolar smooth muscle. Hypertension 36: 856–861, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Ungvari Z, Koller A. Selected contribution: NO released to flow reduces myogenic tone of skeletal muscle arterioles by decreasing smooth muscle Ca2+ sensitivity. J Appl Physiol 91: 522–527, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Ungvari Z, Pacher P, Kecskemeti V, Koller A. Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage-dependent Ca2+ channel opener. Stroke 30: 1949–1954, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab 30: 56–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Xie A, Aihara Y, Bouryi VA, Nikitina E, Jahromi BS, Zhang ZD, Takahashi M, Macdonald RL. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 27: 1692–1701, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol 486: 297–306, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178–201, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol Regul Integr Comp Physiol 270: R228–R237, 1996 [DOI] [PubMed] [Google Scholar]