Abstract
Hypothalamic-pituitary-gonadal (HPG) axis function fundamentally affects the physiology of eating. We review sex differences in the physiological and pathophysiological controls of amounts eaten in rats, mice, monkeys, and humans. These controls result from interactions among genetic effects, organizational effects of reproductive hormones (i.e., permanent early developmental effects), and activational effects of these hormones (i.e., effects dependent on hormone levels). Male-female sex differences in the physiology of eating involve both organizational and activational effects of androgens and estrogens. An activational effect of estrogens decreases eating 1) during the periovulatory period of the ovarian cycle in rats, mice, monkeys, and women and 2) tonically between puberty and reproductive senescence or ovariectomy in rats and monkeys, sometimes in mice, and possibly in women. Estrogens acting on estrogen receptor-α (ERα) in the caudal medial nucleus of the solitary tract appear to mediate these effects in rats. Androgens, prolactin, and other reproductive hormones also affect eating in rats. Sex differences in eating are mediated by alterations in orosensory capacity and hedonics, gastric mechanoreception, ghrelin, CCK, glucagon-like peptide-1 (GLP-1), glucagon, insulin, amylin, apolipoprotein A-IV, fatty-acid oxidation, and leptin. The control of eating by central neurochemical signaling via serotonin, MSH, neuropeptide Y, Agouti-related peptide (AgRP), melanin-concentrating hormone, and dopamine is modulated by HPG function. Finally, sex differences in the physiology of eating may contribute to human obesity, anorexia nervosa, and binge eating. The variety and physiological importance of what has been learned so far warrant intensifying basic, translational, and clinical research on sex differences in eating.
Keywords: neuroendocrinology, estrogens, testosterone, eating disorders, obesity
sex differences are pervasive in physiology and medicine (51, 64, 73, 109, 110, 466, 797, 826). The controls of eating and energy homeostasis are no exceptions. It was observed approximately 100 years ago that removal of the ovaries leads to marked accretion of adipose tissue in rats (697), that daily food intake expressed as kilocalories per gram body weight differs between male and female rats (778), and that food intake varies regularly through the ovarian cycle in intact female rats (674, 779). Sex differences in eating have been the subject of physiological research ever since. The clinical relevance of this work is increasingly evident. In the United States, women are approximately threefold more vulnerable than men to psychiatric eating disorders (346, 351) and approximately twofold more vulnerable to severe and morbid obesity (BMI ≥ 35 and 40 kg/m2, respectively, mass/height2) (226). Women also appear to suffer more from these disorders in terms of physical and psychological functioning and quality of life (24, 84, 273, 292, 465, 531, 762). The increased obesity burden suffered by women is reflected in the fact that >80% of bariatric surgery patients in the United States are women (568, 630). Obesity also decreases fertility and increases the risks of miscarriage and serious health problems for mother and child during pregnancy and after birth (357). In short, eating and weight management are special challenges for women's health. In light of this, our goals are to critically review present understanding of sex differences in the physiology of eating, to identify important gaps in current knowledge, and to highlight opportunities for basic and translational research. We focus on eating, that is, the controls of the “consummatory” behavior of meal taking and related measures of the total amount consumed. Except for a few instructive examples, we restrict our review to laboratory rats and mice and to anthropoid primates, i.e., monkeys, apes, and humans (infraorder Simiiformes or Anthropoidea).
We consider both male-female sex differences and sex-specific effects, i.e., effects that occur only in one sex, such as effects related to ovarian cycles, pregnancy, and lactation, as well as effects controlled by gonadal steroid hormones. As reflected in our review, there is much more work on females, especially ovarian-cycle effects and estrogen-mediated effects, than on male-female sex differences or androgen-mediated effects. We focus on biological sex differences, but emphasize at the outset that it is impossible to draw sharp lines between purely biological and nonbiological causes of sex differences in behavior (e.g., 48, 250, 797). We consider food choice only in the context of the total amount eaten. Although we review some subjective phenomena that are closely connected to eating per se, such as ratings of palatability, we do not review the wide range of subjective and behavioral phenomena integral to a full understanding of eating, for example, foraging and other “appetitive” behaviors (41), cognitive and social controls of eating, and stress- or immune-related controls.
Neuroendocrine Background
We begin with an overview of hypothalamic-pituitary-gonadal (HPG) axis function for several reasons. 1) A common source of error is the failure to recognize differences in HPG axis function among women, rats, mice, and other species, a potential problem that is compounded by the ever-increasing understanding of HPG axis physiology. 2) Most of the known HPG mechanisms underlying sex differences in eating involve gonadal steroid hormones. But because the mechanisms of many sex differences in eating remain unclear, it would be premature to assume that other HPG mechanisms are not involved. For example, changing levels of estrogens alone may fully explain changes in eating during the ovarian cycle in mice and rats, but do not do so in women. 3) The many metabolic feedbacks onto the hypothalamic controls of ovarian cycling and ovulation (187, 330, 646, 787, 812) suggest it is likely that hypothalamic reproductive physiology also controls eating, although such controls have not yet been identified. 4) Neuroendocrinology is a vibrant area, and many novel discoveries and concepts are likely to be relevant to the physiology of eating. We also discuss in this section some criteria that we used to select physiologically reasonable methods for hormone treatment and to distinguish apparently aphysiological results.
Gonadal steroid hormones.
Estrogens, androgens, and progestins (or progestagens) are groups of gondadal steroid hormones, each defined by its biological activity (74). In rats and anthropoid primates, 17β-estradiol (or estradiol) is the most potent estrogen and usually circulates in the highest concentrations (236, 556, 821). For example, rats' ovaries secrete ∼5–8-fold more estradiol than estrone (655), and exogenous estradiol inhibited eating ∼10-fold more potently than exogenous estrone in ovariectomized rats (766). Testosterone is the primary androgen, and progesterone is the primary progestin. Gonadal steroid hormones act on cognate receptors, i.e., estrogen receptors (ER), progestin receptors (PR), and androgen receptors (AR). Classical steroid receptors are nuclear receptors, although, as described below, the importance of membrane-mounted steroid receptors is increasingly apparent. For example, the principal ER, ERα, and ERβ, are expressed in tissue-specific patterns both in nuclei and on membranes (470, 479).
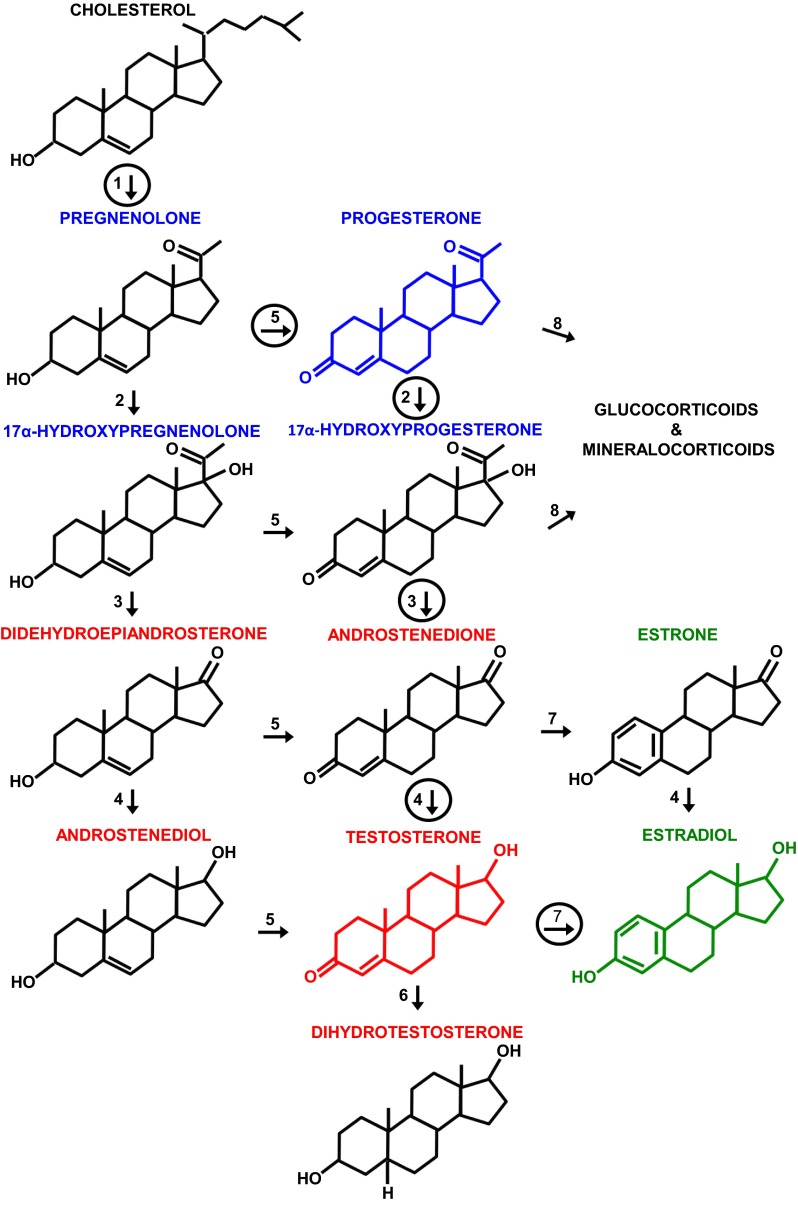
Figure 1 schematizes the principal pathways of human gonadal steroidogenesis (for a detailed review, see Ref. 484). Males and females produce both androgens and estrogens, and both have biological effects in each sex. The gonads are the source of almost all circulating androgens in men and of circulating estrogens and progestins in premenopausal women, and these molecules act as hormones. In contrast, most circulating estrogens and progestins in men, a significant amount of circulating androgens in premenopausal women, and all of the gonadal steroids in the plasma of postmenopausal women derive mainly from other tissues, and these molecules appear to have their main biological actions in those tissues before reaching the circulation; i.e., they do not act as classical hormones (411, 448, 669). This is sometimes referred to as intracrine function. Recognition of its importance has fundamentally changed endocrinology in recent decades. In the brain, locally produced steroids are called neurosteroids (240, 477, 478, 611). In anthropoid primates, the adrenal glands are a major source of precursor molecules for local steroid synthesis. In rats and mice, synthesis begins with cholesterol, which cannot pass the blood-brain barrier and is synthesized de novo in the brain, or with gonadal steroid hormones taken up from the circulation. For example, in rat hypothalamic neurons, circulating estrogens regulate the expression of 3β-hydroxysteroid dehydrogenase, which controls the synthesis of progesterone from cholesterol, and recent studies indicate that it is this neuroprogesterone, not endocrine progesterone, that initiates the LH surge and ovulation (477, 478). There is a report that another neurosteroid, 17α-estradiol, may affect eating (104). Finally, 2-hydroxyestradiol and 2-hydroxyestrone are catabolic products of estrogens that circulate in the blood and may act in the brain to affect food reward (29) (please see Physiological Sex Differences in Disordered Eating).
Fig. 1.
The principal pathways of human gonadal steroid hormone synthesis. Molecules are shown in standard line-angle diagrams, and enzymes are represented as numbered arrows, with the major pathway in adult gonads circled. Steroidogenesis begins with the cleavage of the 6 C side chain from cholesterol (C27H46O) by the mitochondrial cholesterol side-chain cleavage enzyme, otherwise known as P-450scc or CYP11A1 (arrow 1) to yield pregnenolone (C21H32O2). Note that it and other progestins (or progestagens; labeled in blue, with the structure of progesterone, the principal progestin, also in blue) are 21 C molecules. Subsequent steps occur on the smooth endoplasmic reticulum. Progestins are metabolized to androgens (labeled in red, with the principal androgen, testosterone, diagrammed in red), which are 19 C, and to mineralocorticoids and glucocorticoids (not shown), which are 21 C. Androgens are metabolized to estrogens (labeled in green, with the principal estrogen, estradiol, diagrammed in green), which are 18 C. Note that all of these steroids retain the basic 17 C “gonane” structure, consisting of three cyclohexane rings and one cyclopentane ring, but differ in the attached side groups and oxidation states of the rings. An additional estrogen, estriol (not shown), is synthesized in significant amounts only by the placenta and fetal liver. Other labeled enzymes: 2, 17α-hydroxylase; 3, 17, 20-lyase; 4, 17β-hydroxysteroid dehydrogenase;5, 3β-hydroxysteroid dehydrogenase; 6, 5α-reductase; 7, aromatase; 8, 21-hydroxylase.
Origins of sex differences in brain and behavior.
Biological sex differences derive from two evolutionary forces: natural selection, due to the different biological roles of males and females, and sexual selection, due to competition for mates. The proximal causes of biological sex differences comprise a complex interplay of genetic and endocrine mechanisms (9, 10, 32, 271, 425, 466, 471, 671, 807). In most mammals, the development of phenotypic sex differences is initiated by genes on the X and Y sex chromosomes, with females typically possessing the XX karyotype and males, the XY karyotype. There are relatively few genes on the sex chromosomes (∼0.15% of human genes are Y-linked and ∼4.5% are X-linked), and their functions are not yet fully understood. At the blastocyst stage of embryological development, corresponding to 70–100 cells in humans, most cells inactivate one or the other X chromosome, according to a random process. In these cells, only one of the two alleles of each gene is active. In humans, however, 15–25% of genes escapes X inactivation. A key sex-determination gene is Sry (Sex determining region on Y), whose presence causes the undifferentiated fetal gonad to develop into a testis; in the absence of Sry, autosomal or X genes induce ovarian differentiation. De Vries et al. (174) deleted Sry from the Y chromosome and inserted it in into an autosome, thus enabling dissociation of sex-chromosome effects from Sry-determined effects, i.e., mainly effects mediated by gonadal hormones. Analyses of this “four core-genotype” model indicate that while the majority sex differences in reproductive behaviors and the related brain structures in mice are controlled by Sry via gonadal hormones, a variety of other sex differences are controlled more by sex chromosomes than gonadal steroids (425). Two tests of eating in the four core-genotype model were recently reported (please see Sex differences in eating in rats and mice).
Relatively permanent effects of gonadal steroid hormones in early development or during puberty are called “organizational effects” (8, 551). Early masculinization and defeminization of male brains results from the surge in androgen secretion by the testes that occurs during the end of the embryonic period through the first postnatal day in rats and mice and between week 10 and 20 of pregnancy in humans (652). In mice and rats, these effects of androgens require their aromatization to estradiol, which combines with maternal estrogens prenatally; whether this is also so in humans is unclear. Female rat and mouse brains are protected from these processes because there is no perinatal gonadal androgen secretion and because the developing brain is protected from maternal estrogens by α-fetoprotein, which binds estrogens to create a complex that does not cross the placenta. The importance of α-fetoprotein is underscored by demonstrations by Bakker and her colleagues (33, 279) that 1) the brains and reproductive behavior of female transgenic mice that do not express α-fetoprotein were masculinized and defeminized with testosterone treatment, and 2) that the feminine phenotype was rescued by blocking embryonic metabolism of testosterone with an aromatase inhibitor. In humans, α-fetoprotein is abundant, but does not bind estrogens. Sex hormone-binding globulin, rather than α-fetoprotein, may protect the developing human female brain from estrogens (306).
Feminization begins during postnatal week 2 in rats and mice, when the infant ovary begins to secrete estrogens and α-fetoprotein secretion decreases. Further work by Bakker and Baum (32) on transgenic mice lacking aromatase clearly demonstrated the active role of estrogens in this process. They found, for example, that female mice lacking aromatase failed to develop normal female adult reproductive behavior (34) and that estradiol treatment between postnatal days 15 and 25, but not before day 15, was sufficient to reinstate normal adult behaviors (88).
In contrast to the permanent or “organizational” sex-differentiating effects of gonadal hormones early in development, effects at other life stages are often reversible and are called “activational effects” (8, 551). These occur only in the presence of the hormones involved and, therefore, wax and wane during reproductive life. Often activational sex differences require anatomic substrates generated in early development by sexually differentiated organizational processes.
HPG function in adults.
The fundamentals of HPG axis function in rats (and presumably in mice, but this has not been as extensively characterized) and humans (understood mainly from studies in monkeys) are similar (236, 556, 821) (134, 289, 290). Gonadotropin-releasing hormone (GnRH; formerly called luteinizing hormone-releasing hormone) is secreted pulsatilely from neurons located in the hypothalamic preoptic area in rats and mice and in the arcuate nucleus in monkeys and humans into the hypophyseal-portal circulation. This leads to secretion of follicle-stimulating hormone (or FSH) and luteinizing hormone (or LH) from the anterior pituitary into the general circulation. These stimulate the secretion of gonadal steroids. GnRH secretion is also regulated by a pulse generator, which has a constant period of about 2–3 h in men and 60–90 min in women. Both slower and faster frequencies fail to produce normal gonadal steroid levels. Gonadal steroids, LH, and other HPG-axis hormones provide feedback signals to both the pituitary and hypothalamic levels. Feedback is mainly negative in males and contributes to a relatively constant hormone secretion in adult males. The resulting plasma levels of testosterone are ∼2–3 ng/ml in mice, ∼1–3 ng/ml in rats, ∼8–15 ng/ml in cynomolgus monkeys, and ∼3–10 ng/ml in men (520). Both positive and negative feedbacks occur in females, leading to changing hormone secretion through the ovarian cycle, as described in the next sections.
SPONTANEOUS OVARIAN CYCLES.
HPG axis function and the control of ovulation vary widely across mammalian species. Many are seasonal ovulators (454), some are mating-induced ovulators (364), and a few, including rats, mice and anthropoid primates, display spontaneous cycles. In this latter group, ovulation occurs in spontaneous rhythms, or ovarian cycles, that occur regularly throughout the year between puberty and reproductive senescence, except during pregnancy and lactation. These are known as estrous cycles in rats, mice and several other species and as menstrual cycles in women, monkeys, apes, and a few other species in which the cycle ends with discharge of endometrial tissue.
Ovulation and behavioral estrus occur in 4–5-day cycles in rats and mice. Rats' sexual receptivity is maximal in the middle of the nocturnal phase of estrus and near zero during diestrus (estrous phases are defined in the next section) (72, 825). In contrast, women are sexually receptive throughout their ovarian cycle, although the degree of receptivity apparently varies (247, 295). There is little or no seasonal variation in reproductive function of mice and rats (421). In addition to reproductive behaviors, eating, locomotor activity, nest building, fluid intake, food hoarding, and other behaviors vary rhythmically during the estrous cycle (217, 224, 251, 727). The maxima of these cycles are not all in phase, and a variety of evidence indicates that they are separately controlled. For example, although facilitation of the copulatory behavior lordosis and decreased eating both occur during the night of estrus in rats, facilitation of lordosis requires progestins, as well as estrogens (549), but the estrous decrease in eating does not (15, 259, 766). Ovarian cycle effects are the most researched sex differences in eating, and we review them in detail.
OVARIAN CYCLE PHASE.
Rat and mouse ovarian cycle phases or days are most frequently categorized by “vaginal cytology” (48, 236) based on Long and Evans's (440) classical description of the associations among ovulation, reproductive-tract histology, and reproductive behavior, and named as suggested by Heape (321). Vaginal estrus, marked by the cornification of vaginal epithelial cells, begins around the LH surge, which occurs near dark onset, and ends during the subsequent light phase. Thus, vaginal cytology is best sampled in the nocturnal phase or early in the diurnal phase, as indicated by the hatched bar at the bottom of Fig. 2. As mentioned above, behavioral estrus and the estrous decrease in eating are most prominent during the nocturnal phase after the LH surge. In order to have this nocturnal phase occur during the nominal day of estrus, cycle days should not begin at the midpoint of the dark phase, i.e., midnight, as ordinary clock-time days do. Beginning cycle days at the midpoint of the dark and using diurnal vaginal cytology to assign day names leads to the unfortunate consequence that estrous behaviors occur during the day labeled proestrus. This is a major a source of confusion in across-experiment comparisons. Therefore, here, we begin cycle days at dark onset and assign names based on early light-period vaginal cytology (15). The preovulatory phase of the cycle usually lasts 3 days, labeled diestrus 1 (or metestrus), diestrus 2 (or diestrus), and proestrus.
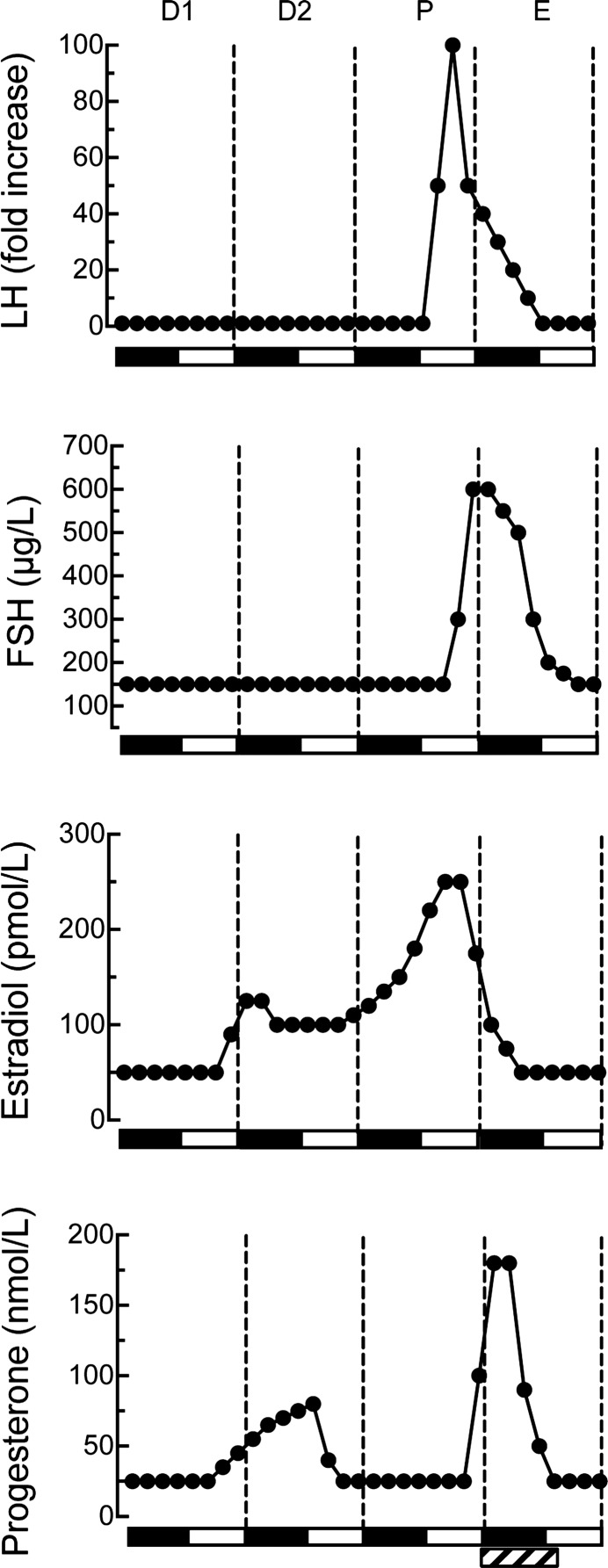
Fig. 2.
Plasma levels of LH, FSH, estradiol, and progesterone during the 4-day ovarian cycle of rats maintained under 12:12-h light-dark cycle. Ovarian cycle days, labeled on the basis of vaginal cytology and beginning at dark onset, are diestrus 1 (D1), diestrus 2 (D2), proestrus (P), and estrus (E). Values are smoothed averages based on several sources (101, 114, 444, 511, 684). Solid bars along x-axis indicate nocturnal periods. LH levels are presented as fold increases over basal (= 1) because published proestrous peak concentrations vary >20-fold. Estradiol's molecular weight is 272 and progesterone's is 314. Hormone concentrations during the additional day in 5 day-cycling rats are similar to those in diestrus 1 (282). The pattern may vary slightly in rats maintained under 14:10-h light-dark cycle (90). The hatched rectangle at the bottom right of the figure indicates the period during which estrous vaginal smears occur most regularly.
Women's cycle days are numbered either 1) forward from the first day of menses, which is the beginning of the follicular phase, and with day 14, the presumed day of ovulation, dividing the follicular and luteal phases, or 2) backward (follicular stage) and forward (luteal phase) from the LH peak. Detection of the LH peak by assaying plasma or urinary LH is considered the gold standard for ovarian-cycle research (55, 342). If LH is measured, the periovulatory phase is the 4 days around the LH peak (ovulation usually occurs within 1 day of the LH peak).
NEUROENDOCRINE CONTROL OF THE OVARIAN CYCLE.
The cyclic changes in LH, FSH, estradiol, and progesterone in 4 day-cycling rats and women are shown in Figs. 2 and 3, respectively. Some obvious differences are 1) cycle length is much longer in women (∼28 days) than in rats and mice (usually 4 or 5 days); 2) absolute levels of estradiol are much higher in women; 3) absolute levels of progesterone are lower in women; and 4) the pattern of hormone secretion after ovulation is very dissimilar in humans and rats (discussed below).
Fig. 3.
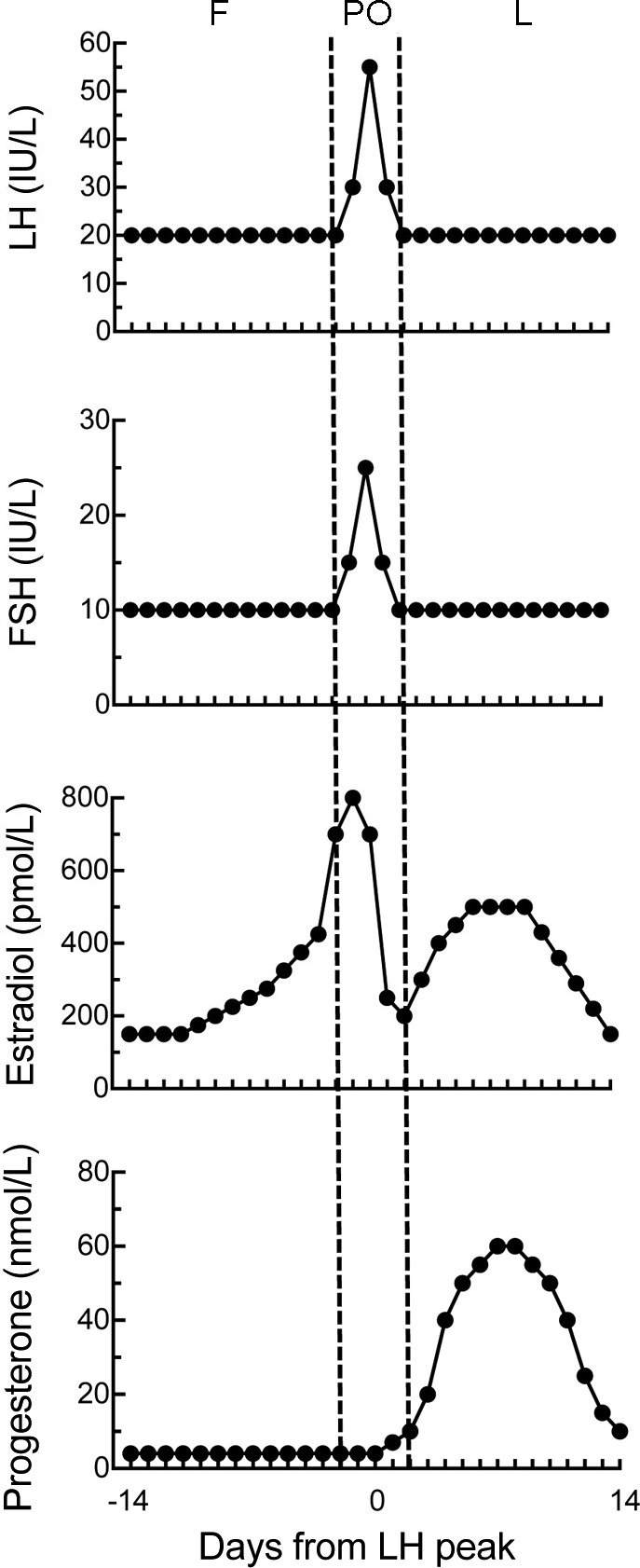
Plasma levels of LH, FSH, estradiol, and progesterone during the human ovarian cycle. Cycle phases, labeled with respect to the LH peak, are follicular (F), periovulatory (PO), and luteal (L). The follicular phase begins with menses, and the LH peak and ovulation occur ∼14 d later. Longer menstrual cycles are usually caused by prolonged follicular phases. Values are smoothed averages based on several sources (97, 617, 638, 735).
The patterns of LH, FSH, and estrogen secretion are similar in women and rats and mice during the preovulatory phase of the cycle, i.e., during the follicular phase in women and diestrus 1 through early estrus in rats and mice. The preovulatory levels of LH and FSH, although low, are required to increase follicular production of estrogens. LH stimulates production of androgens by the theca cells, which express LH receptors, cholesterol side-chain cleavage enzymes, and 17α-hydroxylase. FSH stimulates production of estrogens from these androgens by the granulosa cells, which express FSH receptors and aromatase. Estrogens act in the hypothalamus and pituitary to regulate cycle dynamics. During the preovulatory phase, estrogens exert mainly positive-feedback effects, causing progressive increases in the frequency and magnitude of GnRH pulses and, consequently, of LH pulses, culminating in a surge of LH that initiates ovulation. Ovulation occurs ∼36 h after the surge in women and ∼10 h after the LH surge in rats (i.e., late in the dark phase).
The pattern of progestin secretion during the preovulatory phase is different in women vs. rats and mice. There is virtually no progestin secretion during the human preovulatory phase, whereas in rats and mice, there is a small peak in progestin secretion during diestrus 2, originating from corpora lutea formed in the previous cycle (see below), and a larger peak just after the LH surge, originating from the granulosa cells of the preovulatory follicle. Plasma testosterone levels also vary through the menstrual cycle from ∼0.5 ng/ml during the follicular phase to ∼1.5 ng/ml during the luteal phase (524).
The postovulatory or luteal phase in anthropoid primates is marked by creation of the corpus luteum, which results from the transformation of granulosa and theca cells into carotenoid-concentrating, yellowish luteal cells after ovulation. Continuing LH secretion stimulates the corpus luteum to secrete estrogens and progestins; in women, this phase lasts ∼10–14 days. Progestins maintain the corpus luteum and stimulate angiogenesis and hypertrophy of the uterine endometrium (the decidual response). Estradiol levels are higher during most of the luteal phase than during the follicular phase, although lower than during the periovulatory phase. The human luteal phase ends with degeneration of the corpus luteum, shedding of the uterine decidua, and menstruation. The drop in secretion of progestins, estrogens, and inhibin releases the hypothalamus and pituitary from inhibition and increases GnRH pulse frequency, thus increasing LH and FSH secretion and initiating the follicular phase of a new cycle. If pregnancy occurs, secretion of chorionic gonadotropin maintains the corpus luteum.
Rats and mice do not provide an adequate model of the human luteal phase. Unlike women, rats and mice lack a prolonged postovulatory phase during which functional corpora lutea maintain high plasma estrogen and progestin levels (236, 313, 314). First, estrogen levels are basal by the time of ovulation and do not increase again until the next cycle. Second, rat corpora lutea begin to develop not after ovulation, as in women, but one or two cycles earlier; thus, two or three generations of corporal lutea are present simultaneously. These reach their maximal size and secretory potential during their final diestrus, which explains the small peak in progestin secretion during diestrus 2, and thereafter begin to degenerate. Another factor that decreases plasma progesterone levels and deciduation is that rat corpora lutea express 20α-dihydroxysteroid dehydrogenase, which converts progesterone to 20α-hydroxyprogesterone, a less biologically active progestin. A second, larger peak in progesterone occurs simultaneously with the LH surge and ends near ovulation. As already noted, this progesterone originates in the preovulatory follicles, not the corpora lutea. By a few hours after ovulation, progesterone levels are no longer sufficient to maintain the decidual response. Follicular estrogen secretion and the next cycle begin during the light phase after ovulation, after only 6–8 h of recovery of HPG axis and reproductive tract.
Stimulation of the rat uterine cervix during mating causes secretion of prolactin from the anterior pituitary, which maintains the corpora lutea for about the duration of the human luteal phase after nonfertile mating (“pseudopregnancy”) and throughout pregnancy (usually 21 days) after fertile mating. Pseudopregnancy also occurs spontaneously. Despite the maintenance of the corpora lutea and the similar durations of rat pseudopregnancy and the luteal phase, the endocrine profiles are different. Plasma levels of progesterone increase during pseudopregnancy as in the luteal phase, but plasma estradiol levels are minimal, as in normal pregnancy (249, 728). Inadvertent induction of pseudopregnancy can hamper studies of intact cycling rats.
Kisspeptin and gonadatropin-inhibitory peptide.
The recently discovered hypothalamic peptides kisspeptin (268, 283, 377, 552) and gonadatropin-inhibitory peptide (GnIH) or RFamide-related peptide-3 (127, 407, 745) play important roles in HPG axis function. In mice and rats, kisspeptin is expressed most densely in the preoptic area, anteroventral periventricular nucleus (AVPV), and arcuate nucleus (the latter is often called the infundibular nucleus in humans). GnRH neurons are a major target of kisspeptinergic fibers. Kisspeptin is vital for pubertal development. In mice or humans lacking the kisspeptin receptor Kiss1R, GnRH secretion is insufficient to support normal pubertal development (hypogonadotropic hypogonadism). An elegant recent study by Lomniczi et al. (439) indicates that the timing of puberty in female rats depends upon epigenetic silencing of two transcription-repressor genes that suppress kisspeptin expression.
After puberty, kisspeptin is involved in the feedback control of gonadal steroids on GnRH secretion. In both sexes, gonadectomy leads to increased kisspeptin mRNA in the arcuate nucleus and decreased kisspeptin mRNA in the AVPV, suggesting negative and positive feedbacks, respectively (in females, negative feedback predominates in the early and midfollicular phases, and positive feedback predominates in the late follicular and preovulatory phases; in males the two influences apparently are in tonic balance). Rometo et al. (610) showed that the estrogenic feedback effect also occurs in women by demonstrating 1) that the increases in GnRH secretion that occurs in postmenopausal women and in ovariectomized cynomolgus macaques (Macaca fascicularis) were associated with increased kisspeptin expression in the arcuate nucleus, and 2) that in ovariectomized monkeys estradiol treatment reversed both effects. In another interesting study (682), kisspeptin mRNA expression in the preoptic area and caudal arcuate nucleus and GnIH mRNA expression in the dorsal medial and paraventricular nuclei were higher in the follicular than the luteal phases of rhesus macaques (Macaca mulatta).
Estrogen signaling via ERα mediates kisspeptin function in a complex fashion. For example, conditional knockout of ERα in kisspeptin neurons both advances the onset of puberty and retards subsequent pubertal development in female mice (464). A recent study by Frazao et al. suggests that these disparate effects may be due in part to the different effects of estradiol acting via ERα on the electrophysiological activity of AVPV vs. arcuate kisspeptin neurons (233).
The synchrony of rat and mouse ovarian cycles with the circadian pacemaker, which results in cycle periods that are even multiples of days and which times the LH surge to occur at dusk, depends on kisspeptin neurons in the AVPV (236, 377, 793). In rats, estrogens stimulate these kisspeptin neurons, which, in turn, drive GnRH neurons in the preoptic area to produce the LH surge and ovulation; in contrast, in women, estrogenic positive feedback appears to be mediated by kisspeptin neurons in the arcuate nucleus that project to the mediobasal hypothalamus, with preoptic area and circadian inputs not required (236, 793).
GnIH is synthesized by neurons in the dorsomedial nucleus of the hypothalamus in rats and mice (127, 407, 408, 745, 747). In one study, GnIH was found in the arcuate nucleus in women and female cynomolgus macaques (610); in another study, it was found in the intermediate periventricular (which is adjacent to the dorsomedial nucleus) and paraventricular nuclei in female rhesus macaques (746); and in a third study, it was found in the arcuate, dorsomedial and intermediate periventricular nuclei of female rhesus macaques (682). Interestingly, in the latter study, kisspeptin mRNA expression in the preoptic area and arcuate nucleus and GnIH mRNA expression in the dorsomedial and paraventricular nuclei were higher in the follicular phase than in the luteal phase (682). GnIH fibers project to similar areas as the GnRH neurons, as well as several other brain areas (127, 407, 745). GnIH is thought to be involved in the negative feedback effects of estrogens early in the follicular phase and to sharpen the control of LH and FSH secretion by acting as a functional antagonist to GnRH.
Kisspeptin and GnIH both appear to affect eating. Central administration of kisspeptin inhibited eating in male mice (692), and central administration of GnIH stimulated eating in male mice, rats, and cynomolgus monkeys (127, 352, 498, 682). In addition, in female rats, knockdown of Arc kisspeptin neurons with saporin conjugated to the neurokinin-3 receptor, which the Arc kisspeptin neurons also express, reduced the effect of ovariectomy to increase body weight; unfortunately food intake was not measured (487). Fu and van der Pol (243) described a potential mechanism for the effects of kisspeptin and GnIH on eating. They found that kisspeptin fibers make excitatory synapses on arcuate nucleus neurons that express proopiomelanocortin, the precursor of the eating-inhibitory neuropeptide α-melanocortin-stimulating hormone (α-MSH), and indirectly inhibit arcuate neurons that express neuropeptide Y (NPY), which stimulates eating (sex differences in the effects of α-MSH and NPY are described below). Furthermore, GnIH inhibited the proopiomelanocortin neurons and reduced the effect of kisspeptin. Clearly, more work directed at analyzing sex differences in the eating effects of kisspeptin and GnIH is called for.
Reproductive senescence.
The loss of fertility in aging females has different causes in rats vs. anthropoid primates. In rats, the repeated exposure of the brain to estrogens through reproductive life leads to progressive degeneration and unresponsiveness of the arcuate nucleus, such that cycling ends well before the ovaries are depleted of ova (347). Older female rats retain potentially viable ovaries in a state of arrested follicular development for months, during which time they display vaginal estrus. In contrast to rats, although aging affects numerous facets of HPG function in women, cycling is maintained until the ovaries are depleted (98, 189, 235, 303, 420, 508, 638). Thus, from the perspective of hypothalamic-pituitary function, young ovariectomized rats provide a better model of human menopause than do older, reproductively senescent rats.
Menopause occurs between 45 and 55 yr of age in ∼95% of women (276, 327, 629, 730). The menopausal transition is marked by increasing cycle-length variability, steady increases in basal FSH, and steady decreases in inhibin and anti-Müllerian hormone. Indeed, plasma levels of anti-Müllerian hormone in women 20–50 yr of age predicted the onset of menopause with an mean error of only 6 mo (730). In contrast, average plasma estradiol levels do not change much before menopause. Rather, they remain similar to those in younger women until menopause and then decrease over about a year to a fraction of the premenopausal basal level (98, 99, 303). Postmenopausal plasma estrogens derive mainly from the adipose tissue, and their levels are associated with adiposity (361). Obesity is also associated with slightly later age of menopause [i.e., a median delay of ∼1 yr in overweight and obese women in a recent careful study (492)]. The mechanisms for this are not understood (28, 424, 492). Aging also affects HPG function in men, with the result that bioavailable testosterone levels decrease by ∼2%/yr after age 40 (220, 308).
Hormone treatment regimens.
Gonadectomy and hormone replacement are classic endocrine methods. Because endogenous testosterone levels are relatively constant, mimicking them is simple. Endogenous testosterone levels in adult rats vary according to age, strain, etc., from ∼1–7 ng/ml, and near-physiological replacement is usually achieved with constant-release silicone-capsule implants (117, 118, 659). Because reproductive hormone levels cycle in female rats, however, constant-release pellets cannot be considered physiological. Indeed, daily or continuous peripheral administration of low estradiol doses or even single high doses of long-lasting estrogens such as estradiol valerate can disrupt ovarian cycling, induce pseudopregnancy, elicit progressive, aphysiological changes in several brain neurochemical receptor systems and in behavior, and greatly accelerate the degeneration of the arcuate nucleus (83, 179, 347, 467, 468, 528, 529, 647). In contrast, a weekly cyclic estradiol injection regimen in which 10 μg estradiol benzoate was injected on Tuesdays and Wednesdays and progesterone priming and sexual-receptivity tests were done on Fridays led to stable, normal levels of progestin and oxytocin receptors and in sexual receptivity (647). Plasma estradiol levels in rats maintained on this regimen, however, increased to more than 4 times the proestrous maximum for several days and never decreased below the proestrous maximum (804). Reducing the two estradiol benzoate doses to 2 μg led to a more normal magnitudes of plasma estradiol, but still maintained the proestrous level for an abnormal duration and did not fully reproduce normal patterns of food intake (255). In contrast to these weekly schedules, subcutaneous injection of 2 μg estradiol benzoate once each 4th day produced near-physiological 4-day cycles of plasma estradiol concentration (15, 476) (Fig. 4) and led to normal spontaneous meal patterns, food intake, and body weight (15). Note that because of the rapid rates of esterification of estradiol benzoate in the plasma and clearance of estradiol from the plasma in rats (417, 724), the durations of the estradiol increases in these studies are due mainly to the slow entry of subcutaneously injected estradiol into the circulation.
Fig. 4.
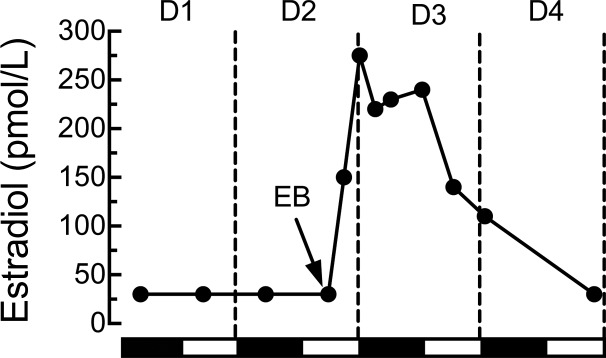
Plasma estradiol concentration during chronic, cyclic estradiol treatment. Plasma samples were taken in the 9th cycle of subcutaneous injection of 2 μg estradiol benzoate (EB) once each 4th day, at the middle of the light phase of day 2 (D2, arrow), which models diestrus 2 in intact rats. D4 of the treatment regimen modeled estrus based on maximally decreased eating behavior and increased sexual receptivity in progesterone-primed rats. Values below the detection threshold of our radioimmunoassay (30 pmol/l) are shown as 30 pmol/l. Reprinted from Hormones and Behavior, Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats, 42: 461–471, 2002; republished with permission from Elsevier; from Asarian and Geary (15).
Doses more than 20 μg estradiol consistently elicit signs of aversion in female rats, such as abnormal latency and duration of the eating-inhibitory effect, abnormal orofacial expressions, and the formation of conditioned taste aversions (253, 254, 333). We do not consider estradiol's aversive effects to be useful in the analysis of its physiological effects on eating and do not consider high-dose studies here.
Subcutaneous injection of 0.5 mg/rat progesterone or 0.5–2 mg/100 g body wt progesterone increased plasma progesterone concentration to about the estrous maximum, although the time course was prolonged compared with estrus (3). Less information is available concerning appropriate doses for mice or for other HPG hormones. Becker et al. (48) provide an excellent discussion of technical and interpretational issues surrounding peripheral gonadal steroid hormone treatment.
Dose is also crucial in interpreting central steroid hormone treatments. Implants of ∼3 ng 3H-labeled estradiol, prepared by filling the distal 1 mm of 28-gauge cannulas with 1:300 estradiol:cholesterol mixtures, into the ventromedial hypothalamic area (VMH) produced measurable label within only ∼500 μm of the implant site and, in combination with peripheral progesterone injections, were sufficient to increase sexual receptivity in ovariectomized rats (168, 169). A formal mapping study for the inhibition of eating has not been done. Tests performed by Butera and colleagues (103, 108) of intra-hypothalamic implants of ∼100 ng estradiol in the distal 1 mm of 28-gauge cannulas indicated that estradiol spread <1 mm in amounts sufficient to inhibit eating and did not produce peripheral estrogenic effects, such as increased uterine weight or cornification of vaginal epithelial cells. Intrahypothalamic implants of more concentrated estradiol mixtures, both in the study by Butera and Beikirch (103) and many earlier studies, were sufficient to produce peripheral effects. Because the threshold peripheral estradiol dose for the inhibition of eating seems to be less than that for cornification of vaginal epithelial cells (190), such large doses clearly cannot be used to identify local effects. We (732) demonstrated that doses of ∼200 ng 3H-labeled estradiol applied to the surface of the dorsal hindbrain just posterior to the area postrema in 1-mm2 pieces of absorbable surgical fabric produced measurable label only ∼200 μm caudally, ∼600 μm rostrally, and ∼500 μm ventrally and did not lead to detectable amounts of estradiol in the plasma.
Latency of estrogen's effect on eating.
A frequent source of confusion is that in rats and mice, most estrogen-dependent responses occur during the nocturnal phase of estrus, when plasma estrogen levels are low, not high (please see Fig. 2). This timing probably reflects the dependence of the behaviors on transcriptional effects of estrogens, whose downstream consequences require hours or days to complete. This reasoning suggests that events during the ovarian cycle that depend on gene expression are likely to be due to the increases in plasma estrogens during diestrus, i.e., 1–2 days prior to estrus, and not to the peak of estrogen concentration during proestrus. This has been shown to be the case both for the LH surge (236) and for lordosis, a reflexive proceptive behavior characteristic of estrus that depends on increased expression of progestin receptors (549). For example, acute antagonism of estrogenic function during diestrus blocked the proestrus surge of LH and ovulation, whereas the same treatment early in proestrus had no effect (221, 510).
The estrogenic inhibition of eating in ovariectomized mice and rats has a latency that suggests a similar interpretation. Physiological or modest pharmacological peripheral doses of estradiol in a lipid vehicle, such as sesame oil, decrease eating ∼24–48 h later in mice and rats, depending on the circadian time of administration (255, 287, 637, 731). Central administration of estradiol inhibited eating with a similar latency in rats (732). These data suggest that endogenous estrogens normally act in diestrus to initiate effects that result in reduced eating during estrus. We consider the typical ∼24–48 h latency of the estrogenic inhibition of eating to be a useful criterion for the physiological relevance of estrogenic treatments in rats and mice. That is, if an estrogen or estrogen agonist decreases eating in <24 h in mice or rats, it is unlikely to mimic the physiological action of endogenous estrogens (please see Refs. 203, 286, 333, 731 and Site of ER Controlling Eating for further discussion). Unfortunately, we know of no data on the time course of any estrogenic effect on eating or on HPG axis function in monkeys, apes, or women.
Sex Differences in Eating in Rats and Mice
Male-female differences.
Total daily energy intake in male rats exceeds that in females to an extent greater than predicted by their larger lean body mass and metabolic rate (790, 803). Normal “homeostatic” eating also contributes to the maintenance of significantly less body fat content in male than female rats (129). As described below, both organizational and activational effects of estrogens and androgens appear to contribute to these differences. There may be a species difference in how males' greater intake is expressed in spontaneous meal patterns: the greater total food intake of male than female Long-Evans rats maintained on a palatable liquid diet resulted mainly from larger meals (457), whereas the greater food intake of similarly maintained male than female C57BL/6J mice resulted entirely from more frequent meals (701).
Activational effects of estrogens and androgens contribute to the maintenance of normal levels of food intake in rats, but do so in opposite ways. With few exceptions, ovariectomy increases rats' daily food intake and body weight by increasing meal size, and estradiol treatment normalizes all three measures; in contrast, orchiectomy decreases daily food intake and body weight by decreasing meal frequency, and testosterone treatment normalizes them (15, 18, 77, 102, 115, 191, 202, 203, 267, 726, 764, 776). As we review below, the estrogenic control of eating in rats is the best understood of these phenomena. There are many species differences in the effects of gonadectomy on eating and weight. For example, as discussed below, ovariectomy often fails to elicit overeating in mice. In addition, in many species orchiectomy increases food intake and adiposity (341). This may be the case for monkeys and humans, as we also discuss below.
There is an interesting male-female sex difference in regulatory or homeostatic eating. Male mice that were acutely food-deprived for 24 h (513), chronically food-restricted until they lost about 15% body weight (660), or underwent partial lipectomy (660) all compensated by overeating, whereas similarly challenged female mice compensated by decreasing energy expenditure without overeating. A similar sex difference in postdeprivation eating occurred in both rats (751) and humans (820). The developmental origins of this sex difference are reviewed in the next section; whether activational effects also contribute is unknown.
There is also a sex difference in conditioned taste aversion learning in rats. In several tests, males and females acquired taste aversions to unconditioned stimuli such as LiCl similarly, but females' taste aversions extinguished faster after acquisition, i.e., began to ingest the conditioned stimulus, typically, a sweet solution, in normal amounts sooner when it was presented repeatedly in the absence of the unconditioned stimulus (160). Activational effects of both estrogens and androgens appear to contribute to this sex difference (116, 819). These findings merit further research because conditioned taste aversions are probably important in the control of eating in humans, especially in certain clinical populations, for example patients undergoing radiation or chemotherapy and patients with bulimia nervosa (65, 86, 641).
Development.
Work begun in the 1970s by Wade and colleagues (266, 764) and others (56, 507, 452) demonstrated that neonatal masculinization of female rat pups increased their food intake and decreased their sensitivity to the eating-inhibitory effects of estrogens as adults. The latter effect suggests that, as is the case for numerous sexually differentiated brain functions, activational effects of estrogens in adults require organizational programming of the developing neural substrate. Nohara et al. (513) recently discovered some of this substrate. They found that female mice that were masculinized with neonatal testosterone treatment ate like intact males in that 1) they ate more than intact females at 6 wk of age, as previously described, and 2), unlike intact females, they increased eating following a 24-h fast when tested as adults. Nohara et al. (513) also identified two changes in the physiology of hypothalamic proopiomelanocortin (POMC) circuits that may underlie the sex differences in eating (POMC is the precursor of the neurotransmitters α- and β-melanocyte-stimulating hormone, which are involved in energy homeostasis; please see Sex Differences in Central Controls of Eating). That is, both hypothalamic expression of the Pomc gene and the arborization of hypothalamic POMC neurons were reduced in neonatally masculinized females from the intact-female to the intact-male level. Comparison of neonatal treatment with estradiol and 5α-dihydrotestosterone, which cannot be converted to estradiol, verified that these effects were AR-mediated. Masculinization also led to hyperleptinemia and reduced the sensitivity of exogenous leptin to upregulate POMC, decrease eating and prevent adipose-tissue mass accumulation. These effects were estrogen dependent. The changes in plasma leptin concentrations and leptin sensitivity, however, lay outside the normal range, suggesting that the neonatal manipulations were not entirely physiological.
Chen et al. (122, 123) reported the first measurements of eating in the four core-genotype model of HPG axis development (described in The origins of sex differences in brain and behavior). They found that in C57BL/6J mice, both gonadal sex (testes or ovaries) and chromosomal sex (XX or XY) affected eating: 1) gonadal females (i.e., without Sry) ate more than gonadal males (with Sry) during the dark regardless of their chromosomal sex (XX or XY) (Fig. 5), and 2) gonadal and chromosomal females (XX with Sry) ate more than all other groups during the light and had an approximately twofold more fat mass (not shown). Estrogen and androgen treatments were not tested. Chen et al.'s (122, 123) and Nohara et al.'s (513) elegant studies, together with recent translational work on organizational influences on eating disorders (please see Physiological Sex Differences in Disordered Eating), should rekindle interest in the development of sex differences in eating (290).
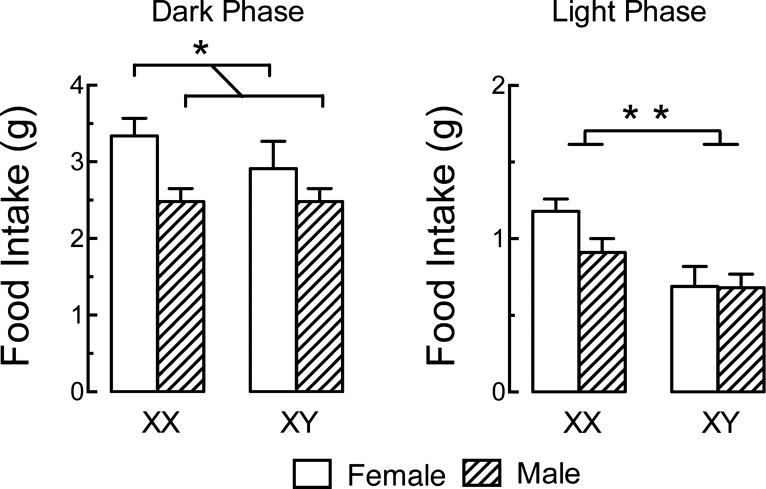
Fig. 5.
Developmental effects of both gonadal sex (testes or ovaries) and chromosomal sex (XX or XY) affect food intake. Mice were gonadectomized 4 wk before testing to eliminate the activational effects of sex hormones. Note that 1) during the dark phase (left), gonadal females ate more than gonadal males, regardless of their chromosomal sex, and 2) during the light phase (right; note altered scale), gonadal and chromosomal females (XX) ate more than all other groups; these mice also had an approximately twofold more fat mass (not shown). Tests (not shown) of mice with XO and XXY chromosomes indicated that these effects were due to X-gene dosage, not the presence of the Y chromosome. *P < 0.05; **P < 0.01. Republished with permission from Chen et al. (122).
Puberty involves changes in the secretion of HPG and other hormones, tissue sensitivity, and neuronal architecture (670, 671). Pubertal brain maturation appears to be necessary for the estrogenic inhibition of eating: 1) exogenous estradiol inhibited eating only in postpubertal rats (649, 765, 771), and 2) the amplification of endogenous cholecystokinin satiation by exogenous estradiol began at puberty (please see Cholecystokinin). In contrast, testosterone treatment did increase eating in prepubertal male rats (518), indicating that the maturation of the neural substrate for HPG-control of eating is sex-specific.
An organizational effect of estrogens also appears to be necessary for the stimulation of eating by prolactin treatment that occurs in adult female, but not male, rats (323). That is, prolactin stimulated eating in adult males whose brains were feminized by castration on postnatal day 1, but not in adult females masculinized by neonatal testosterone treatment. This organizational effect may be required for the increased eating that occurs in pregnancy and lactation described below.
Ovarian cycle.
The initial reports that rats eat least during the periovulatory (estrous) phase of the ovarian cycle and most during diestrus have been replicated countless times in rats (for reviews, see Refs. 18 and 764) and extended to mice (527, 547, 731), humans, and many other species. The estrous minimum in daily food intake is typically ∼20% less than the diestrous maximum. Rarely, food intake did not vary across the cycle. For example, Varma et al. (758) reported that Fischer 344 rats, a small, lean strain, did not eat less during estrus, and Petersen (547) reported that mice fed a honey-laced wheat-cereal diet ate more during estrus, although chow-fed fed mice ate less.
Importantly, the estrous decrease in eating in rats is due solely to a decrease in the size of spontaneous meals, with no contribution from a decrease in meal frequency (15, 77, 190, 207, 527, 547). Indeed, meal frequency usually increases during estrus [meal size was also reduced in the Fischer 344 rats mentioned above, but this was fully compensated for by the increase in meal frequency (758)]. These and data reviewed in the next section suggest that these two parameters of spontaneous feeding are controlled separately. The proximal physiological mechanisms for the estrous decrease in eating are discussed below. Fessler (222) has advanced an interesting hypothesis concerning its ultimate adaptive meaning.
There are several reports of altered macronutrient selection during the estrous cycle (42, 261, 358, 423, 808). The forms of macronutrients used in these studies and the specific changes in macronutrient selection observed varied widely, however, suggesting that food properties unrelated to macronutrient type caused the results. For example, as reviewed below, cyclic changes in the rewarding effect of sweet taste may contribute to cyclic changes in eating.
The estrous inhibition of eating in rats follows the diestrus increase in plasma levels of estrogens with the time lag discussed above (please see Latency of estrogen's effect on eating). In contrast, neither the smaller peak in plasma progestin levels during diestrus 2 nor the larger periovulatory peak is related to changed eating. The estrous inhibition of eating in rats is not secondary: 1) to stimulation of locomotor activity because the former is expressed as a decrease in meal size and the latter causes a decrease in meal frequency (207); 2) to increases in appetitive or consummatory reproductive behavior because both estradiol and progesterone are necessary to normalize most or all reproductive behaviors in ovariectomized rats (242, 549, 690); or 3) to estrogen-dependent changes in water intake (151, 224, 237, 355, 384, 406, 726, 727) because these are not synchronous in intact, cycling rats (maximum water intake occurs on diestrus 1 and decreases on diestrus 2) and because food intake increased ∼3 days before water intake increased after ovariectomy (727) [the estrogenic controls of eating and drinking also were dissociated in several tests in guinea pigs (155)]. Nevertheless, it would be useful to determine spontaneous meal patterns in a more naturalistic environment permitting social interactions, reproductive behavior, foraging for food, etc. (for example, Refs. 386 and 473).
Ovariectomy and hormone treatment.
Ovariectomy both abolishes the cyclicity of eating, as would be expected by disruption of the ovarian cycle, and increases daily food intake above the diestrous maximum for several weeks, leading to increased body weight and adiposity (15, 77, 130, 589, 662, 726, 764) (Fig. 6A). In contrast to the effect of menopause in women, however, the gain in adipose tissue in rats is mainly in the subcutaneous depots, not intra-abdominal depots, and lean body mass is increased, not decreased (274).
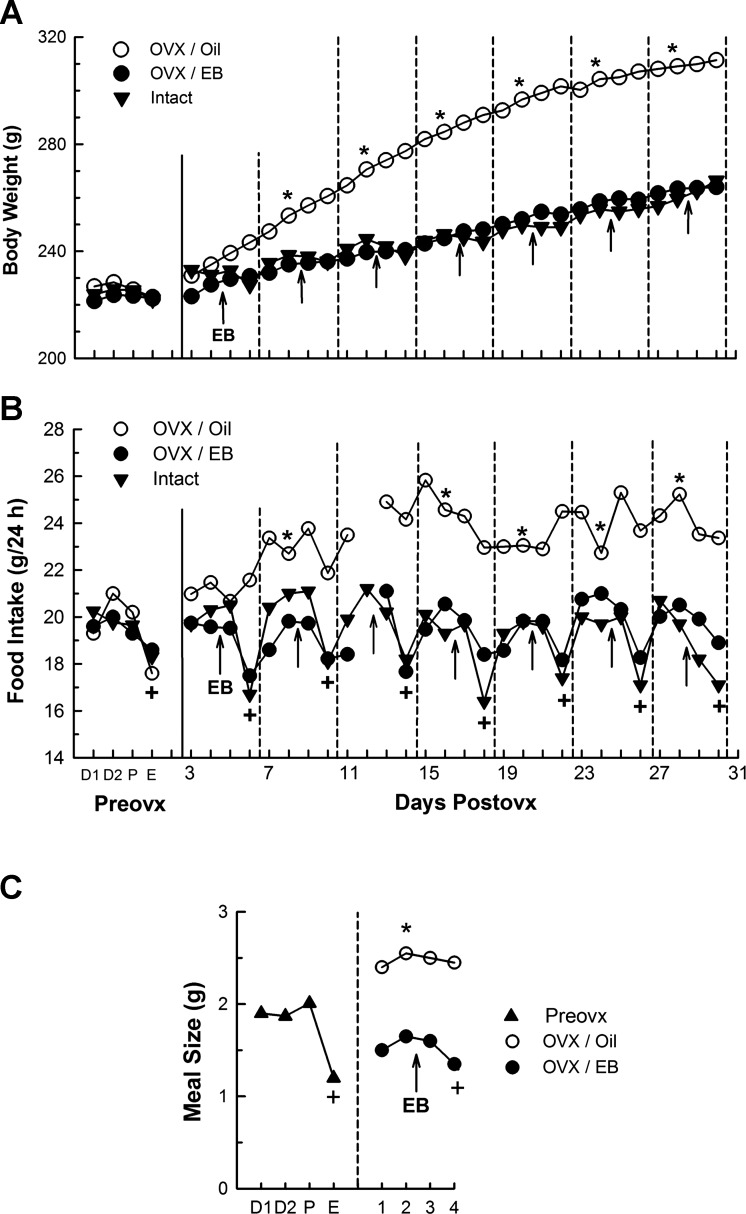
Fig. 6.
Cyclic estradiol benzoate (EB) treatment models the endogenous cycle and maintains normal patterns of body weight gain, daily food intake, and spontaneous meal size in ovariectomized (OVX) rats. A: OVX increased and cyclic estradiol treatment normalized body weight. Data to left of the solid vertical lines are from the last ovarian cycle before OVX (x-axis labels appear in panel B: Preovx; D1, diestrus 1, D2 diestrus 2; P, proestrus; E, estrus), and data to the right of the solid vertical lines are sham-operated intact rats (solid circles), OVX rats treated with EB (triangles), and OVX rats treated with the oil vehicle (open circles); dashed vertical lines divide the numbered 4-day treatment cycles (days 1–4), which are aligned so that the last day of each cycle is the second day after EB injection, the day that models estrus. B: OVX increased and cyclic estradiol treatment normalized daily food intake (x-axis labels explained above). Note 1) that OVX elevated, and EB normalized, the basal level of daily food intake (tonic estrogenic inhibition of eating; tested on day 2) and 2) that OVX eliminated, and EB reinstated, the drop in food intake during estrus in intact rats and on cycle day 4 in OVX rats (phasic or cyclic estrogenic inhibition of eating). C: OVX increased and cyclic estradiol treatment normalized nocturnal spontaneous meal size. Triangles indicate mean meal sizes during the last cycle Preovx (abbreviations as above); solid circles indicate mean Postovx meal sizes during cycles 2–7 of cyclic EB treatment (injection time indicated by arrow); and open circles indicate mean Postovx meal sizes in control rats treated with the oil vehicle. +Significantly different from intact rats and EB-treated rats on day 2; *Significantly different from diestrus 2 (intact group) or day 2 (OVX group). Meal frequency was not increased by OVX or decreased by EB (data not shown). Reprinted from Hormones and Behavior, Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats, 42: 461–471, 2002; republished with permission from Elsevier; from Asarian and Geary (15).
As Drewett (191) originally pointed out, the acyclicity and the increased overall level of eating after ovariectomy suggest that HPG-axis function normally exerts two influences on eating: 1) a tonic inhibition, whose loss after ovariectomy increases the basal level of eating, and 2) a phasic or cyclic inhibition, whose loss leads to acyclicity.
Early demonstrations that estradiol-treated ovariectomized rats ate normal amounts and maintained normal body weight indicated that estrogens are the crucial link between HPG-axis function and eating in rats (191, 589, 726, 764, 766, 771) (for reviews see Refs. 18, 102, 203, and 253). Most importantly, a near-physiological cyclic regimen of estradiol treatment was sufficient to maintain both the tonic and the phasic controls of eating, normal spontaneous meal patterns, and normal body weight in ovariectomized rats (15) (Fig. 6, B and C). In contrast, near-physiological doses of progesterone did not affect eating or body weight in rats, although pharmacological doses may do so (259, 294, 764, 766). The efficacy of estradiol treatment to normalize body weight may depend on when treatment is begun because estradiol was markedly less effective in reversing ovariectomy-induced weight gain than in preventing it (726).
As discussed in the previous section, the cyclic inhibition of eating during estrus is likely to result from the increase in estrogen secretion 24–48 h earlier during diestrus 2. This explanation, however, leaves unclear why the even higher estrogen level during proestrus fails to further decrease eating during diestrus 1. This unexpected pattern may result from the central processing of the estrogenic signal. It does not involve another ovarian control because it occurs in estradiol-treated ovariectomized rats as well (15).
The duration of exogenous estradiol's eating-inhibitory effect suggests that estrogen secretion during diestrus 2 and proestrus is sufficient to produce the tonic inhibition of eating throughout the cycle: 1) Asarian and Geary (16) observed that in ovariectomized rats maintained on weekly cyclic treatment with 2 μg estradiol benzoate, food intake returned to the level of untreated rats 5 days after estradiol treatment. Because of the rapid esterification of estradiol benzoate to estradiol and the rapid clearance of estradiol from the circulation (417, 724), estradiol levels return to basal within 2 days of injection. 2) Similarly, Gray and Greenwood (287) reported that ovariectomized rats' food intake was still decreased 7 days after injection of 2 μg estradiol benzoate. 3) Tarttelin and Gorski (727) reported that if rats spontaneously entered pseudopregnancy, during which there is little estrogen secretion, intake increased above the diestrous level after ∼5 days. These data suggest that the eating-inhibitory effect of estrogens secreted during diestrus and proestrus persists ∼4–7 days, more than long enough to explain the tonic inhibition of eating during the cycle.
GnRH, LH, FSH, and prolactin do not appear to mediate the effects of estradiol on eating because exogenous estradiol still inhibited eating in hypophysectomized rats (771). Hypophysectomy also decreases the secretion of estrogens, however, so the observation that hypophysectomy did not increase eating or body weight in the same study (771) appears paradoxical. It may be that capacity of hypophysectomized rats to gain weight is impaired. Consistent with this idea, a more selective lesion, transgenic deletion of FSH receptors, prevented ovarian follicle development and produced a typical estrogen-deficiency syndrome in female mice, including increased body weight and adiposity; unfortunately eating was not measured (162).
The effects of ovariectomy and of estradiol treatment on eating in rats, like the estrous decrease in eating, are expressed solely as changes in spontaneous meal size; i.e., increases and decreases, respectively (15, 77, 107, 376). Meal frequency usually decreases slowly after ovariectomy and increases with estradiol treatment, but not enough to balance the meal size effects, at least for several weeks after ovariectomy. Female mice also decrease meal size during estrus (547). As reviewed below, this specificity has been a useful clue for investigations of the underlying mechanisms. In contrast, the mechanism for the slowly developing, apparently compensatory decrease in meal frequency that limits the effect of ovariectomy on body adiposity has received no attention.
Finally, there appears to be a species difference in the effect of ovariectomy on eating in mice and rats. In mice, ovariectomy usually (350, 604, 796), but not always (75, 130), increases body weight and adiposity without affecting eating. This is presumably due to the many effects of estrogens on physical and metabolic energy expenditure, energy metabolism, and adipose tissue physiology (40, 361, 462, 463, 729, 767). It would be interesting to determine whether this species difference is related to one or more of the neural mechanisms underlying the divergent controls of eating and energy expenditure described in male rats (e.g., Refs. 36, 500, 673).
Pregnancy and lactation.
As described in Wade and Schneider's expert reviews (645, 770), animals respond in a variety of ways to the energetic challenges of pregnancy and lactation. Rats and mice eat more and select different micronutrients and macronutrients during pregnancy and lactation (26, 81, 133, 197, 214, 639, 691, 779). The underlying neuroendocrine controls are not well understood. Part of the cause may be simply the release from the estrogenic inhibition of eating, as suggested by the pseudopregnancy and ovariectomy data reviewed above. Other factors must also be involved, however, because rats eat more during the later stages of pregnancy and during lactation than after ovariectomy. Both oral and postingestive factors may contribute. Bowen (80) found an increase in the intake of a sweet food in pregnant rats, suggesting that the phenomenon in pregnant women described below is, at least in part, physiological. The increase in intake of sweet foods may be specific because, in another study (691), pregnant rats increased intakes of a 55% high-fat diet and of chow similarly. Potential roles for CCK and leptin signaling in the increase in eating during pregnancy are reviewed below (please see subsections Cholecystokinin and Leptin).
Lactational hyperphagia again highlights the primacy of meal size in the HPG control of eating in rats discussed above. That is, rat dams increase spontaneous meal size early in lactation and increase meal frequency only later on and if nursing larger litters (227, 702). Lactational hyperphagia is not dependent on ovarian function because it was not affected by postpartum ovariectomy (227). It may result from increases in NPY in the dorsomedial hypothalamus driven by increased prolactin secretion and by downregulation of an inhibitory αMSH input (119, 802). The reductions in basal insulin and leptin that accompany lactation do not cause the hyperphagia because normalization of insulin and leptin levels did not affect it (811).
Contemporary investigators have not pursued a classical observation by Curt Richter on dietary self-selection by pregnant and lactating rats (251). Richter (590) showed that if rats could obtain certain micronutrients, such as sodium and calcium, from sources other than the source of dietary energy, energy intake during pregnancy and lactation was markedly reduced. Thus, the appetites for micronutrients, not energy, drive much of the hyperphagia in chow-fed rats during pregnancy and lactation. Clearly, the mechanisms underlying these effects should be investigated in situations that permit the rats to regulate micronutrient homeostasis and energy homeostasis separately.
Androgens.
Androgens have activational effects on eating in rats in addition to the organizational effects described above. Adult orchiectomy decreases rats' daily food intake and body weight, and androgen treatment, usually with testosterone propionate, normalizes both (115, 266, 402, 516, 518, 622, 764, 767, 776). Testosterone increased eating similarly in one study in mice (546), but not another (495). In both species, the eating effects were due to changes in meal frequencies, with meal size moving in the opposite direction (115, 546). The mechanisms through which orchiectomy and androgen treatment affect eating have been studied far less than those of ovariectomy and estrogen treatment. Androgens, like estrogens, have many metabolic effects that can lead to changes in body weight and composition in the absence of changes in eating (40, 361, 462, 463, 625, 729, 767), and orchiectomy and androgen treatment seem to affect body weight and body composition more reliably than they do eating. Transgenic mice lacking androgen receptors also increased adiposity without increasing eating (216).
The effects of androgens on eating may be related in part to aromatization to estrogens. In some (288, 519, 664), but not all (199, 266, 622), studies, treatment with relatively high doses of testosterone propionate, which can be aromatized to estrogens, increased eating more potently than similar doses of nonaromatizable androgens, such as of 5-alpha-dihydrotestosterone propionate. It remains unclear, however, whether physiological androgen doses would produce such an effect.
Sex Differences in Eating in Anthropoid Primates
Male-female differences.
Although males and females may eat differently from a very young age, most sex differences in human eating do not appear to be physiologically based (171, 188, 234, 565, 605, 783, 788). For example, Wardle et al. (783), in an analysis of data from 23 countries, found that women chose fewer high-fat foods and more fruits and high-fiber foods than men, but that health beliefs explained ∼50% of the effects and dieting status as much as 20%. Nevertheless, there are at least four apparently physiological sex differences in human eating. 1) Men, who are generally larger than women, eat more than women and, as in rats, increases in meal size rather than in meal frequency produced this difference (172). 2) Men were more responsive than women to the negative-feedback effects of oral nutrient loads on eating in several situations (170, 544, 605). 3) Men were more responsive than women to the eating-stimulatory effect of food deprivation (820), paralleling the rat and mouse phenomena described above. 4) Finally, again as in rats and mice, normal homeostatic eating maintains a significantly higher body adiposity in women than in men; at a “normal” BMI of 22–23 kg/m2, women had 26% fat as a percent of body weight vs. 13% in men (245). Understanding the mechanisms underlying this sex difference may have important ramifications for the general understanding of energy homeostasis.
A number of epidemiological studies done in several Western societies that involved different ethnicities and social strata, included adults and children as young as 2–5 yr of age, and assessed intakes of solid foods, carbonated beverages, and fruit juices failed to detect male-female differences in sugar intake, expressed as a percentage of total energy intake (78, 539, 733, 782). Sex differences in food selection did appear, however, in surveys of obese persons. Drewnowski et al. (194) reported that obese men identified high-fat, high-protein foods among their favorites, whereas obese women identified, high-fat, high-carbohydrate foods, especially high-sugar foods, among their favorites. Macdiarmid et al. (451) showed that this difference was reflected in food intake: obese women ate more high-sugar, high-fat foods (median intake ∼146 g/day of cakes, chocolate, etc.) than did obese men (∼103 g/day), leading to a higher sugar intake (21 vs. 17% of daily energy); nonobese persons did not show this difference. As discussed below, these differences may result from sex differences in flavor hedonics. It is important to determine whether they develop prior to obesity or are consequences of obesity (e.g., Ref. 696). Finally, recent data suggest that the trend toward overeating in the United States is stronger in women, who increased daily energy intake 22% between 1971 and 2004, than men, who increased only 10% (437). In that overeating appears to be the primary cause of the obesity epidemic (195, 496, 716), this difference could contribute to the sex differences in obesity prevalence mentioned above (226).
Three findings support the view that a physiological sex difference affects sweet preference in anthropoid primates: 1) Among the Hadza of Tanzania, hunter-gatherers who derive >90% of their energy from wild food, although honey was the most preferred food in both sexes, women preferred sweet berries more than meat, whereas men preferred meat more than berries (61). 2) A field study of wild Borneo orangutans (Pongo pygmaeus) indicated that when sweet fruits were in season, males increased their intake about twofold (from 3,800 to 8,400 kcal/day), whereas females increased their intake about four-fold (from 1,800 to 7,400 kcal/day) (401). 3) In a laboratory study in which savanna baboons (Papio cynocephalus) were offered 75% sucrose fruit-flavored candy and chow pellets, females ate relatively more sugar than males (228).
Ovarian cycle.
CYCLIC CHANGES IN EATING.
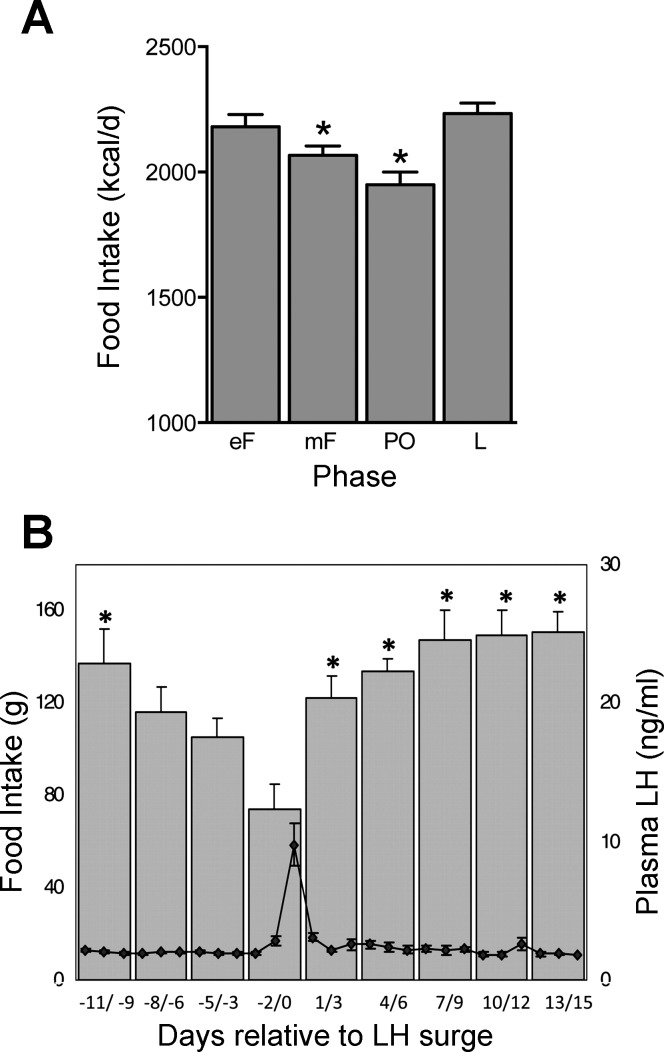
We know of three within-subjects studies of women's eating throughout the ovarian cycle (229, 278, 450). These involved a total of 53 women. Food intake was measured by weighing the food and converting these data to energy equivalents, and cycling was characterized by urinary LH assays and reports of menses. As shown in Fig. 7A, these studies demonstrated that eating decreases through the follicular phase to a minimum during the periovulatory phase and is similarly high during the early-follicular and luteal phases. The weighted mean differences in food intake between the luteal and periovulatory phases was 275 kcal/day, and that between the mid-follicular and luteal phases was 228 kcal/day. Significant differences in food intake between the midfollicular and midluteal phases were detected in 9 of 10 similarly designed studies in which only those two cycle phases were tested (n = 192, mean effect, 218 kcal/day) (38, 353, 431, 436, 458, 544, 558, 561, 725, 809); the negative result occurred in the smallest study (n = 9) (809). Similar cyclic changes in food intake also were found in many studies reviewed by Buffenstein et al. (92) and by Dye and Blundell (198), which used less sensitive methods, such as use of body temperature to monitor cycling and use of food diaries to measure eating (789). Those studies also provided evidence that the cyclic changes in eating do not occur during anovulatory cycles (38, 596).
Fig. 7.
Daily food intake during the ovarian cycle in women (A) and rhesus macaques (B). Note the progressive decreases in food intake during the follicular phase in both women and monkeys and the high, constant levels of food intake during most of the luteal phase in monkeys (women's data were averaged across the entire luteal phase). Women's data (kilocalories eaten per day; values are expressed as means ± SE) are calculated from three studies in which food intake was measured by weighing, and the cycle phase was monitored with urinary LH and reports of menses in a total of 34 women. In each study, data were averaged across the early-follicular (eF; 4 day), midfollicular (mF; ∼9 day), periovulatory (PO; 4 day), and luteal (L; ∼11 day) phases. *Significantly different from luteal phase. Adapted from Am. J. Clin. Nutr. (1993; 57: 43–46), American Society for Nutrition (229); Am. J. Clin. Nutr. (1989; 49: 252–258), American Society for Nutrition (278); and Am. J. Clin. Nutr. (1989; 49: 1164–1168), American Society for Nutrition (450). Monkey data are plasma LH concentrations (open circles, means ± SE) and daily food intakes (solid bars, means ± SE) averaged across consecutive 3-day intervals relative to the LH peak (day 0) in 7 monkeys. *Significantly different from day −2/0. Reprinted with permission from Human Reproduction Update, Brain imaging studies of appetite in the context of obesity and the menstrual cycle, Dean A. Van Vugt, 16: 276–292, 2010 (756).
None of the studies reviewed above determined whether the cyclic changes observed were due to changes in spontaneous meal size, as in rats, or changes in meal number. In two studies involving test meals, however, meal size was significantly less in the midfollicular phase than in the luteal phase (85, 561), consistent with the idea that in women as in rats, cyclic changes in eating are expressed as changes in spontaneous meal size.
Cyclic changes in eating across ovarian cycle similar to that seen in women were found in several studies of rhesus macaques (153, 154, 156, 373, 615) (Fig. 7B) and in both captive and wild chacma baboons (Papio ursinus) (70). The monkey studies demonstrate that eating decreases continuously during the follicular phase and is maintained at a relatively constant high level during the luteal phase. In wild baboons, the cyclic effect was similar in females that were in consort with males and those that were not in consort, indicating that reproductive behavior did not influence eating.
All of these data, together with the apparently similar periovulatory decrease in eating in rats and mice, indicate that cyclic changes in women's eating are biological sex differences under the control of HPG axis function. The magnitudes of the effects are more than large enough to be relevant to body weight regulation; recent estimates suggest that consistent imbalances of only 50–100 kcal/day are sufficient to cause the gradual development of obesity (437, 496).
HPG-AXIS MEDIATION.
Eating during the follicular phase in anthropoid primates is closely associated with changes in estrogen levels. From a neuroendocrine perspective, the phase of maximal intake in rats, diestrus, better parallels the early-follicular phase than the luteal phase. Thus, if estrogens do inhibit eating during the follicular phase, then food intake should be maximal during the early follicular phase, when estrogen levels are lowest, and then decrease progressively until the periovulatory phase, when estrogen levels are highest. Although this hypothesis has not been investigated quantitatively, the available data in women (229, 278, 450) and rhesus monkeys (154, 156, 756) are consistent with it (Fig. 7).
In contrast, the contribution of gonadal steroids to the control of eating during the luteal phase is problematic. Estrogen levels are higher in the mid-luteal phase than the mid-follicular phase, although food intake is less during the mid-follicular phase. This pattern is inconsistent with a simple estrogenic inhibitory effect. Progestins do not appear to provide a solution. Progestins are secreted during the luteal phase, and exogenous progesterone can reduce the eating-inhibitory effect of estrogens, but this appears to be a pharmacological rather than a physiological effect in rats (259, 764, 766) and monkeys (70, 154, 372). For example, using estradiol and progesterone doses that led to changes in plasma levels that were within the range occurring during the menstrual cycle, Czaja (154) failed to detect any effect of either acute or chronic progesterone treatment on eating in ovariectomized rhesus macaques. The effect of progestins in the absence of endogenous gonadal steroids has not been studied in women. Pelkman et al. (544), however, failed to detect any effect of depot medroxyprogesterone on food intake in an adequately powered, prospective, placebo-controlled study in cycling women. That result extends an earlier study in which a low-dose estrogen (35 μg/day ethinyl estradiol) together with escalating progestin doses (0.5–1.0 mg/day norethindrone) failed to detectably affect food intake measured with diet records in cycling women (201). These studies suggest, but do not prove, that some factor other than estrogens and progestins controls eating during the luteal phase. Further work is required to identify that factor.
MACRONUTRIENT INTAKE.
Dye and Blundell (198) reviewed 15 studies in which macronutrient intakes were tabulated across the ovarian cycle; none found a significant effect. Nevertheless, there may be a cyclic change in the intake of sweet foods. Bowen and Grunberg (82) found that women ate more of three sweet test foods during the luteal phase than during the mid-follicular phase, whereas intakes of salty and of bland foods did not vary. Similarly, Fong and Kretch (229) reported that women consumed significantly more sugar-containing carbonated drinks (typically about 0.3 M sucrose) during the luteal phase (417 ± 27 ml/day; mean ± SE) than during the midfollicular, periovulatory, or menses phases (335 ± 31, 359 ± 26, and 370 ± 26 ml/day, respectively). Kemnitz et al. (373), however, failed to find a cyclic change in rhesus macaques' intake of 0.5 M sucrose that was offered 3 days/wk, 2 h/day. The possible contributions of cyclic changes in sensory and hedonic processing of sweets is discussed below.
INTERACTION WITH COGNITIVE CONTROLS OF EATING.
The follicular and periovulatory decrease in eating can be modulated by non biological factors. For example, the decrease is apparently blunted or absent in women with high levels of dietary restraint (92, 198), a psychological trait related to the ability to limit eating cognitively that is an important control of eating in many young women (322, 328, 794). In an interesting study, Li et al. (431) found that the difference in energy intake between the follicular and luteal phases in a group of university students was ∼10% when weekday data were used and ∼23% when weekend data were used. This weekend effect was not attributable to changes in alcohol intake, in frequency of restaurant visits, or in the daily level of energy intake, which did not vary significantly across the week. One possibility is that it was due to the relaxation of cognitive restraint on weekends, but this was not measured.
Food cravings, i.e., recurring intense desires for specific foods, are a common and stable aspect of human eating, distinct from the palatability [i.e., craved foods are not simply those an individual finds most palatable (786, 813)]. In both the United States and Spain, women crave sweet foods most frequently, whereas men crave mainly savory foods (543, 623, 823). This sex difference may be related to the sex difference in sweet hedonics discussed below.
Chocolate is the single most craved food among women in the United States, United Kingdom, Canada, and Spain, and many women report cyclic changes in craving, especially for chocolate, with a maximum in the late luteal and menses phases (738, 822). This cyclic effect does not seem to be hormonally based: 1) neither frequency of cravings nor the type of food craved were significantly related to circulating estrogen levels (597); 2) progesterone administration did not affect cravings (480); 3) premenopausal and postmenopausal women who were chocolate cravers had similar frequencies of chocolate craving (338); and 4) there was no cyclic change in chocolate cravings in Spanish women whose chocolate cravings were as intense as those reported by U.S. American women who did crave cyclically (822).
Carbohydrate craving may represent a form of self-medication for dysphoria (137). Early reports linked carbohydrate craving to serotonergic (640) and opioidergic (192, 193) function, but neither lead has been pursued. Carbohydrate craving is often discussed in relation to premenstrual dysphoria and bulimia nervosa, but whether they are, indeed, linked remains controversial (132, 296, 752, 813).
Loss of ovarian function and hormone treatment.
Menopause increases adiposity independent of aging (336, 429, 535, 550, 714, 739, 741), and some data indicate that high-estrogen hormone-replacement treatment (HRT) prevents this (297, 312, 419, 628, 686). Whether ovariectomy (or oophorectomy), menopause, or exogenous estrogens affect eating in women, however, remains in doubt, perhaps due to the inadequacies in the designs used so far. For example, Lovejoy et al. (443) had subjects complete 4-day food diaries annually and failed to detect any change in total energy intake between 3 yr premenopause and 2 yr postmenopause. The power of this study, however, was such that changes of >30% were required for significance. We know of three reports in which HRT failed to affect eating (585, 587, 672). None of these studies, however, was strongly designed. For example, in one (587), only a single day's food intake was estimated by interview, and in another (585), a between-subjects design with only 6 and 9 women was used. In the third study (672), normal-weight women who had had natural menopause 0.5–5 yr previously were randomly assigned either an HRT regimen or to placebo in a double-blind design (n = 14 each), and eating was estimated by food diaries for 3-day periods at the onset and after 2 yr of treatment. HRT was associated with a decrease in daily intake of 212 kcal/day (12%), compared with no change in untreated women, but none of the differences was statistically significant. The power of the food diary method to detect differences and the potential influence of the time since menopause were not assessed. In addition, HRT was a relatively high-progestin regimen (6.25 mg/day oral conjugated estrogens plus 2.5 mg/day medroxyprogesterone), which may have masked an effect of estrogens on eating, as occurs in rats (766).
In contrast, experiments with monkeys provide stronger support for the hypothesis that loss of ovarian function increases eating due to release from estrogenic inhibition. Sullivan et al. (705) showed rhesus macaques' food intake increased 29% and body weight 3% during the first 2 mo after ovariectomy compared with preovariectomy baselines. In a follow-up study (707), they found that 3 mo of treatment with the selective estrogen-receptor modulator GSK232802A decreased body weight 4.6% in a group of ovariectomized rhesus macaques whose body weights previously increased 4.8% after ovariectomy. GSK232802A decreased food intake ∼5–10% during the treatment period, and food intake also correlated negatively (r = 0.52) with weight loss during this period. Finally, GSK232802A increased spontaneous physical activity, measured with triaxial accelerometry. Interestingly, the fattest, least active monkeys showed the largest activity increases and weight losses. They also previously reported that spontaneous activity levels, but not food intake levels, were inversely correlated with weight gain over a 3-mo period in intact female rhesus macaques (706). These data, together with Lovejoy et al.'s (443) report that spontaneous activity decreases across the menopausal transition, identify physical activity and eating as important targets for future menopause research.
Pregnancy and lactation.
Women increase energy intake during pregnancy and lactation in a way that appears to meet their increased energetic costs (47, 71, 569). Part of the mechanism appears to be related to altered food preferences. Most women increase intake of craved foods during the third through the seventh month of pregnancy (58, 81, 337, 564). Sweet foods, including fruits and fruit juices, dairy foods, and chocolate are most frequently craved. Increased food cravings during pregnancy are also thought to contribute to excessive gestational weight gain, which occurs in about one third of pregnant women and increases a variety of health risks both for mother and child (144, 526, 642, 654). For this reason and because the results of current intervention strategies are inconsistent (721), the physiological controls of increased eating during pregnancy are an important research challenge.
Some women also develop aversions, often to foods that are usually liked. Because food aversions and nausea develop most frequently around the third month of pregnancy, it is possible that they are produced by the increases in human chorionic gonadotropin that occur at this time (244, 540). A moderate degree of nausea and vomiting is considered normal during pregnancy and may improve pregnancy outcomes (244, 540).
Androgens.
The role of androgens in eating in anthropoid primates is unknown. In rhesus monkeys, testosterone treatment failed to significantly affect eating despite producing large (∼50%) increases in body weight and lean body mass in 7–9 yr-old monkeys that had been orchiectomized at 0–3 mo of age (375). The potential influences of the early orchiectomy and the long orchiectomy-treatment delay on the results were not assessed. We know of no studies of androgens and eating in healthy men. Male hypogonadism has a variety of causes, but often appears as men's testosterone levels decrease during aging (161, 553, 711). It is associated with increased adiposity. Changes in appetite have not been reported to our knowledge. Hypogonadism is treated with testosterone, which suppresses the HPG axis and leads to infertility, or, if fertility is desired, by stimulating the HPG axis with either gonadotropins or the estrogen-receptor modulator clomiphene citrate, which decrease estrogenic negative feedback (161, 553, 711). Thus, hypogonadal men seem to present a potentially interesting model for investigation of androgenic influences on eating.
ER Mechanisms Controlling Eating
Two types of nuclear ER, ERα, and ERβ, were identified in the 1990s (178, 512). These are encoded by separate genes, ESR1 and ESR2 in humans and Erα and Erβ in mice. The two genes have overlapping but distinct expression patterns in the brain and periphery, and Erα and Erβ knockout mice display distinctive phenotypes. The classical mode of action of nuclear ER involves binding to estrogen-response elements (ERE), which, in turn, bind to DNA and affect gene transcription, usually over the course of hours to days (125, 326). More recently, it has been found that ERα and ERβ, as well as a variety of novel ER, are expressed outside the nucleus, usually on cell membranes (428, 475, 548, 602, 759). In addition to genomic effects via non-ERE transcription promotors, membrane ER have rapid (i.e., within seconds) nongenomic effects. These rapid effects are increasingly recognized to be crucial for many neural actions of estrogens.
ERα in rats and mice.
The phenotypes of transgenic mice with null mutations affecting estrogen signaling via ERα demonstrate a necessary role for ERα role in weight regulation, but do not clearly disclose their role in the control of eating. Heine et al. (324) characterized the obesity phenotype of the original line of Erα−/− mice. Both male and female Erα−/− mice had excess body weight and body fat, especially intra-abdominal fat, signs of metabolic syndrome, and decreased energy expenditure. Food intake, tested continuously beginning at 60 days of age, was unchanged in male Erα−/− mice; again females were not tested. Ohlsson et al. (523) found a similar adiposity phenotype in another line of Erα−/− mice, but did not measure eating. Jones et al. (356) tested transgenic aromatase-deficient mice, which cannot synthesize estrogens, and found that food intake was reduced in females beginning before 5 wk of age; males were not tested. Xu et al. (812) reported that female transgenic mice lacking ERα only in the brain ate more than wild-type mice at 7 wk of age, but they did not test other ages. Finally, Park et al. (537) described a strain of Erα−/− mice in which non-ERE estrogen signaling was transgenically rescued, Erα−/AA mice. Eating tests beginning at 3 wk of age failed to reveal any difference between female wild-type mice and either Erα−/− or Erα−/AA mice in intake of chow or of a 45% high-fat diet. We conclude that there is no apparent reason to expect disruption of peripheral ERα signaling to obscure an effect of brain ERα lesion on eating and, therefore, that until Xu et al.'s (812) solitary report of increased eating in transgenic mice with disrupted ERα is extended, the Erα−/− phenotype fails to provide clear evidence that ERα signaling is necessary for the normal control of eating. Given the difficulty in interpreting negative transgenic null-mutation data and the many positive indications that ERα is involved in the control of eating that we review below, however, we do not consider this a crucial failure.
To better establish whether activational effects of estrogens control eating and adiposity, Geary et al. (257) tested the effects of estradiol treatment in Erα−/− mice that were ovariectomized after puberty. Estradiol reduced daily food intake, weight gain, and fat gain in wild-type, but not Erα−/− mice (Fig. 8). Estradiol also increased CCK satiation in wild-type, but not Erα−/−, mice (please see Cholecystokinin). Because CCK is involved in the phasic control of eating by estrogens, these data indicate that an activational effect of estrogen signaling via ERα is involved in both the phasic and the tonic estrogenic control of eating. This does not preclude the possibility that organizational effects mediated by ERα are also important for these controls.
Fig. 8.
Estradiol treatment reduced daily food intake (A) and body weight gain (B) in ovariectomized wild-type (WT) mice, but not in Erα−/− mice. Constant-release estradiol pellets that produced plasma estradiol levels near the proestrous maximum or control vehicle pellets were subcutaneously implanted during ovariectomy. Data are from days 8–25 postoperatively because mice regained their presurgical weights by day 8. *Significantly less than control WT mice. Republished with permission from Geary at al. (257).
In one study of Erα−/− mice, there was a suggestion of a non-ERα contribution to the estrogenic inhibition of eating: Naaz et al. (502) found that food intake was lower in ovariectomized than in nonovariectomized Erα−/− mice during one of three weeks tested. For the following reasons, this report requires replication to be considered as substantial evidence: 1) circulating estrogen levels are ∼10-fold elevated in ERα−/− mice (140), so that ovariectomized Erα−/− mice receiving a physiological estrogen treatment would be a better comparison; 2) the difference in food intake between ovariectomized and nonovariectomized mice over the 3-wk test was not significant; and 3) the three individual weeks were tested independently, and the significance level for at least one positive result in three trials is only P < 0.14.
Pharmacological studies buttress the conclusion that ERα mediates the activational eating-inhibitory actions of estrogens. There are several reports that the selective ERα agonist 4,4,4-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol inhibits eating in ovariectomized rats and mice (203, 309, 603, 637). This compound, however, inhibits eating much faster than estradiol and activates corticotropin-releasing hormone neurons in the paraventricular nucleus, which estradiol does not, suggesting that it has nonspecific, possibly stressful, effects (731). More convincing is Santollo et al.'s (635) report that the ERα antagonist methyl-piperidinopropyl pyrazole blocked the eating-inhibitory actions of exogenous estradiol in ovariectomized rats and prevented the estrous decrease in eating in intact rats. These data indicate that ERα mediates both the cyclic and the tonic activational, eating-inhibitory effects of estrogens.
ERα in humans.
An epidemiological study of allelic variation at locus rs7757956 in intron 4 of ESR1, which encodes human ERα, provides compelling evidence that an activational effect of estrogen signaling via ERα is related to adiposity in girls. Tobias et al. (737) classified a sample of children who were on average 11.8 yr old according to pubertal development, using the Tanner scale (Tanner scores are based on the development of pubic hair and breasts in girls and pubic hair, penis, and testicles in boys; 1 is prepubertal, 2, early, 3 mid-, 4, late, and 5 postpubertal or adult). They found that girls with TT genotype at locus rs7757956 who were in Tanner stages 3–5 had 9% more body fat, as measured by DEXA, than girls with TA or AA genotypes. No such effect was detected in boys. Girls with TT genotype who were in Tanner stages 3–5 also had 18% more height-adjusted body fat than girls with TT genotype who were in Tanner stages 1–2; the comparable difference in girls with TA or AA genotype was significantly less, 7%. As a result, girls with TT genotype who were in Tanner stages 3–5 had a 36% increase in the risk of being overweight, according to UK pediatric norms, whereas girls with TT genotype who were in Tanner stages 1–2 had no increased risk. Interestingly, the TT genotype was the most frequent, occurring in ∼75% of boys and girls, suggesting that increased adiposity in women was once adaptive. How this ESR1 polymorphism affected adiposity is unknown. From our perspective, a crucial question is whether it affected fat mass by reducing estrogens' eating-inhibitory effect or, for example, by affecting adipose-tissue metabolism directly (178). Other ESR1 polymorphisms have also been related to increased adiposity (525, 763), but in each case, the findings are inconsistent across populations studied (737, 763).
ERβ.
The failure of several selective ERβ agonists (309, 603, 637, 731) and one selective ERβ antagonist (635) to affect eating in rats or mice and the failure of null mutation of ERβ to affect the eating and body weight responses to ovariectomy and estradiol treatment in mice (257) indicate that ERβ is not involved in the estrogenic control of eating or energy homeostasis in rats or mice. Two studies, however, suggest that this issue may merit further research. First, Liang et al. (432) reported that the eating-inhibitory effect of intracerebroventricular infusion of estradiol was reduced by simultaneous intracerebroventricular infusion of ERβ-antisense oligonucleotides, but not by ERα-antisense oligonucleotides. Both of these results are anomalous, and the paradigm has not been further tested. It may have been important that a water-soluble estradiol compound was used because water-soluble estradiol produced unusual results in several tests (please see Hypothalamus). Second, Yepuru et al. (814) reported that high-fat diet-fed ovariectomized mice chronically treated with a novel selective ERβ agonist ate less and did not gain body weight or fat. Their agonist appeared to function by activating uncoupling protein-1 and blocking peroxisome proliferator-activated receptor-γ in the adipose tissue. No evidence was presented, however, to indicate whether these effects were physiological or purely pharmacological.
Novel estrogen receptors.
Two novel G protein-coupled ERs may be involved in the estrogenic control of eating. One, GPR30, is expressed on the endoplasmic reticulum of a variety of cell types (503, 588). Haas et al. (298) reported that male and female mice with null mutations of GPR30 (GPR30−/− mice) developed marked intra-abdominal obesity, as well as a number of other abnormalities. The effects of ovariectomy and estradiol treatment, however, did not differ between wild-type mice and a different strain of GPR30−/− mice (795). A second novel ER, Gq-mER, is expressed on the cell membranes of proopiomelanocortin (POMC), dopamine, and GABA neurons in the arcuate nucleus of the hypothalamus (576, 601, 602). Gq-mER reduces the activation of potassium channels on GABAB and μ-opioid receptors and affects the expression of NPY and other neuropeptides involved in the control of energy homeostasis. The Gq-mER-selective agonist STX reduced food intake and body weight gain in ovariectomized guinea pigs under several conditions (576, 599, 600). Importantly, however, doses of STX and estradiol that had similar effects on total food intake had opposite effects on spontaneous meal patterns: estradiol decreased meal frequency, whereas STX decreased mainly meal size (600). This suggests that STX is unlikely to mimic the tonic effects of endogenous estrogens on eating in guinea pigs. Unfortunately, whether the estrous decrease in eating in guinea pigs (156) is produced by changes in meal size or meal frequency is unknown.
More generally, the apparent necessity of ERα for the estrogenic inhibition of eating in rats and mice and the ∼2-day latency of this effect both indicate that the rapid actions of novel membrane ER are unlikely to be sufficient for the estrogenic inhibition of eating in these species (12, 731). They may, however, interact with slower, ERα-mediated effects to control eating. Kow and Pfaff (404) described just such an interaction in the control of the lordosis reflex. They found that maximal lordosis was induced by two brief pulses of estradiol, separated by 5 h, delivered into the VMH, and that this occurred even if only one pulse was estradiol and the other pulse was a complex of estradiol bound to albumin complex, which does not penetrate cell membranes and, therefore, activated membrane ER.
Site of ER Controlling Eating
Eckel (203) and Rivera and Eckel (592) provided pharmacological evidence that activation of ER in the brain is necessary and sufficient for the estrogenic inhibition of eating by comparing the eating-inhibitory effects of peripheral and central injections of ICI-182,780, a pure antiestrogen that apparently does not cross the blood-brain barrier. Infusion of ICI-182,780 into the lateral ventricle reversed the eating-inhibitory effect of peripheral estradiol injection in ovariectomized rats, but subcutaneous ICI-182,780 injections did not. Conversely, peripheral, but not central, ICI-182,780 treatment blocked estradiol's effects on uterine weight and vaginal cytology. Xu et al.'s (812) report that transgenic mice with a brain-specific deletion of ERα are hyperphagic and obese is also consistent with Rivera and Eckel's (592) data, although a developmental defect appeared to account for most of their effect (i.e., hyperphagia seemed to appear before puberty), and the effects of ovariectomy and estrogenic stimulation were not tested.
Investigations of brain sites of ER that control eating using local estradiol administration and brain lesions are summarized in Table 1 (studies using central doses greater than ∼300 ng are not included for the reasons described in Hormone treatment regimens). As Table 1 and the discussion below indicate, there is no consistent evidence for a role of any hypothalamic ER population in the estrogenic control of eating in rats or mice. In contrast, several approaches implicate ERα in the caudal medial nucleus of the solitary tract (cmNTS) in the estrogenic inhibition of eating.
Table 1.
Status of central ER populations in the control of eating
| Site | Sufficient? | Necessary? | Comment |
|---|---|---|---|
| Brain stem | |||
| cmNTS | Yes (732) | Yes (19, 20) | ERα/c-Fos colocalization |
| DR | Yes (636) | Latency | |
| Hypothalamus | |||
| VMH | Yes (636) | Latency | |
| No (103, 533) | No (75, 374, 499, 734, 812) | ||
| MPOA | Yes (158, 636) | Latency | |
| No (103, 343) | No (381) | ||
| PVN | Yes (103, 104, 108) | Yes (106) | |
| No (343) | No (159) |
Sufficiency is indicated by a decrease in eating following administration of small amounts of estradiol in the brain area indicated in ovariectomized rats; necessity is indicated by a diminished or absent effect of peripheral estradiol on eating or weight gain following electrolytic, chemical, or molecular lesion of the brain area indicated in ovariectomized rats or, in the case of the ventromedial hypothalamus (VMH) in mice. VMH data include both the ventromedial nucleus (VMN) and the arcuate nucleus (Arc). References are shown in parentheses.
DR, dorsal raphe nucleus; cmNTS, caudal medial nucleus of the solitary tract; MPOA, medial preoptic area; PVN, paraventricular nucleus.
“Latency” indicates that the eating-inhibitory effect was much faster (i.e., a few hours) than that of peripherally administered estradiol (i.e., ∼2 days), as described in text. “ERα/c-Fos colocalization” indicates that food stimuli elicited neural activity, as indicated by c-Fos immunocytochemistry, in ERα-expressing neurons.
cmNTS.
The first suggestion that the nucleus of the solitary tract (NTS) might be involved in the estrogenic control of eating came from our finding that the NTS was one of the brain areas in which estradiol treatment increased the number of neurons expressing c-Fos after food ingestion or CCK injection in ovariectomized rats and mice (205, 206, 257, 688) (the effects of estrogens on CCK satiation are reviewed in Cholecystokinin). The next c-Fos finding was more interesting. Estradiol increased the numbers of cells expressing c-Fos in response to intraduodenal infusions of fat only in one brain area, the cmNTS just posterior to the area postrema. The effect was CCK-dependent, and, most importantly, many of the neurons expressing c-Fos also expressed ERα (17). The cmNTS has the densest population of ERα-expressing neurons in the NTS (17, 643, 663, 668, 688, 689, 757).
We then showed that local administration of estradiol to the surface of the hindbrain over the cmNTS was sufficient to reduce eating and to increase the number of ERα-expressing cmNTS neurons that expressed c-Fos after CCK injections (732). Finally, we tested the effects of administration of anti-ERα siRNA into the cmNTS of ovariectomized rats. cmNTS anti-ERα siRNA treatment prevented estradiol treatment 1) from tonically or cyclically decreasing meal size and food intake, 2) from increasing the eating-stimulatory effect of CCK-1 receptor (CCK-1R) antagonism, 3) from increasing CCK-induced c-Fos expression in the NTS or hypothalamus, and 4) from restraining body-weight gain (Fig. 9) (19, 20). These data indicate that cmNTS ERα are necessary for much or all of estrogens' effects on meal size, food intake, and body weight.
Fig. 9.
Estradiol treatment reduced nocturnal spontaneous meal size (A) and body weight gain (B) in ovariectomized control rats, but not in ovariectomized rats that received bilateral injections of adenovirus-vectored anti-ERα siRNA in the caudal medial nucleus of the solitary tract (cmNTS) (ERαKD; control rats received cmNTS injections of antiluciferase siRNA). Rats received subcutaneous injections of 2 μg estradiol benzoate (EB) or the oil vehicle (Oil) each 4th day. Meal sizes are averages of data from the cycle day of the estradiol treatment regimen that modeled estrus, from cycles 5–9; this day should reflect both the cyclic and the tonic effects of estradiol. Body weight gains are from surgery through cycle 9. *Significantly less than oil-treated rats that received the control siRNA (19, 20).
Hypothalamus.
Surprisingly, hypothalamic ER and hypothalamic neural networks do not appear to be crucial for the tonic estrogenic inhibition of eating. Some positive results have been reported, but in each case, these are countered by convincing negatives or procedural questions. For example, Butera and colleagues (102–104, 106, 108) reported that bilateral implants of ∼100–300 ng estradiol into the paraventricular nuclei of ovariectomized rats modestly (in comparison to peripheral estradiol injection) reduced food intake in ovariectomized rats and that subcutaneous injections of estradiol did not inhibit eating in ovariectomized rats with bilateral lesions of the paraventricular nuclei, but, for reasons that remain unclear, others failed to replicate these results (159, 343). For example, Dagnault and Richard (159) found that peripheral estradiol implants inhibited eating through a 26-day test as much in ovariectomized rats with bilateral lesions of the paraventricular nuclei as sham-lesioned rats. This indicates that neither ER in the paraventricular nuclei nor paraventricular neural networks are necessary for the tonic estrogenic inhibition of eating.
The situation is similar for other hypothalamic areas. Dagnault and Richard (158) and Santollo et al. (636) reported that implants of estradiol into the medial preoptic area inhibited eating in ovariectomized rats, but neither Butera and Beikirch (103) nor Hrupka et al. (343) could replicate this. It may have been important that both positive results were obtained with a water-soluble estradiol preparation containing ∼5% estradiol and 95% 2-hydroxypropyl-β-cyclodextrin that inhibited eating rapidly, within 2 h (158) or 24 h (636). This is much faster than the normal latency of peripheral estradiol's eating-inhibitory effect, 24–48 h (please see Latency of estrogen's effect on eating). These rapid effects may have been mediated by membrane ER, as suggested by reports that estradiol immediately affects electrophysiological activity in the medial preoptic area (370, 371). In addition, peripheral estradiol still reduced eating and body weight in ovariectomized rats with lesions of the medial preoptic area (381). Implants of modest amounts of estradiol into the ventromedial hypothalamus, which would stimulate ER in both the ventromedial and arcuate hypothalamic nuclei, did not inhibit eating (103, 533), and peripheral estradiol still inhibited eating in ovariectomized rats (374, 734) or mice (75) with ventromedial hypothalamic lesions. In addition, administration of anti-ERα siRNA into the ventromedial hypothalamus did not affect the estrogenic inhibition of eating in ovariectomized mice, although it decreased energy expenditure, increased body weight, and prevented the estrogenic stimulation of locomotor activity (499). Similarly, transgenic female mice lacking ERα specifically in neurons expressing steroidogenic-factor 1, which are found only in the VMH, were not hyperphagic, although they were hypometabolic and had massive hypertrophy of the gonadal fat pads (812). Santollo et al. (636) recently reported that administration of 250 ng of a water-soluble estradiol preparation into the arcuate nucleus inhibited eating in ovariectomized rats. Again, the inhibition occurred unusually rapidly, in <24 h, suggesting that it did not reflect a normal estrogenic effect. The same is true of their report (636) that showed that administration of water-soluble estradiol into the dorsal raphe nucleus inhibited eating in ovariectomized rats.
Sex Differences in Peripheral Controls of Eating
Eating is controlled by a cascade of peripheral positive- and negative-feedback signals that are sequentially activated by food stimuli from the oropharynx, gastrointestinal system, and postabsorptive compartment (67, 416, 678, 799). Neural feedback signals from the oropharynx follow bites or licks of food by seconds and are present more or less continuously throughout the meal. Neural and endocrine signals arising from gastrointestinal and metabolic food stimuli begin during the meal and are maintained for minutes to hours afterward. Other signals related to postprandial metabolism or to delayed and integrated consequences of eating, notably changes in adiposity, may act tonically. Overall, dozens of peripheral feedback signals are thought to be involved in the control of eating. Eleven of these signals have been reported to display sex differences (Table 2).
Table 2.
Summary of sex differences in peripheral controls of eating
| Rat, mouse | Human | |
|---|---|---|
| Orosensory capacity | + | +++ |
| Gastric mechanoreception | − | + |
| Ghrelin | + | − |
| CCK | +++ | + |
| GLP-1 | + | − |
| Apolipoprotein A-IV | + | − |
| Glucagon | + | − |
| Insulin | + | + |
| Amylin | + | − |
| Fatty acid oxidation | + | − |
| Leptin | + | − |
+++, strong evidence of physiological role in mediating a sex difference in eating; + some evidence; −, no data. Please see text for details.
Gustation.
Sex differences in the contribution of orosensory stimuli to the control of eating can arise from 1) differences in gustatory sensation, reviewed in this section, or 2) differences in central processing of peripheral gustatory and other oral stimuli, in particular, hedonic evaluations made by the brain, reviewed in Orosensory hedonics.
Sex differences in electrophysiological responses to sapid stimuli have been reported at three levels of the gustatory pathway. Stratford et al. (699) found that whole-nerve chorda tympani responses to lingual application of various concentrations of monosodium glutamate (umami taste) and to combinations of linoleic acid and monosodium glutamate were greater in male than in female rats, whereas responses to NaCl and NH4Cl were similar in males and females. These data matched the ingestive responses to the solutions. Di Lorenzo and Monroe (181, 182) investigated the response of third-order gustatory neurons in the parabrachial nucleus to sweet, sour, bitter, and salt stimuli. Single-neuron activity elicited by lingual application of sucrose was increased more in intact and ovariectomized female rats than in male rats, and females had more “sweet-best” neurons. In addition, quinine elicited more activity in ovariectomized females than in intact females or males. An apparently contradictory result was reported by Verhagen et al. (760), who found that sweet and salt stimuli elicited greater increases in activity in male rats than in female rats in fourth-order gustatory neurons in the thalamic taste nucleus. These data indicate that there are sex differences in electrophysiological responses of the gustatory system, but do not yet provide a firm basis for the interpretation of behavioral sex differences.
There are sex differences in human gustatory capacity at the most peripheral level—the taste receptors. Women have more fungiform taste papillae on the anterior tongue than men (Fig. 10), and their papillae tend to have more pores leading to the taste buds (46, 573). The density of fungiform papillae is associated with the density of afferent innervation of the tongue by the chorda tympani and trigeminal nerves; in addition, it is significantly correlated with 1) the sensory intensity (independent of hedonic valence) of sweet solutions and foods, 2) tactile acuity on the tongue surface, and 3) the sensory intensity of creaminess of fatty foods (213, 316, 317). As a result, because women generally have more fungiform taste papillae than men, they perceive foods as sweeter and creamier than men. Thus, to paraphrase Linda Bartoshuk (43), men and women live in different taste worlds. There is also evidence that pregnancy alters gustatory sensation (573). For example, in one study (196), women perceived bitter taste stimuli as more intense early in pregnancy, but less intense later in pregnancy, compared with nonpregnant women. The effects of such sensory differences on eating, however, have not yet been extensively investigated. Furthermore, the changes in bitter sensitivity during pregnancy do not offer an obvious potential explanation for the changes in eating observed during pregnancy because the two do not occur at the same time (please see Pregnancy and Lactation).
Fig. 10.
Frequency histograms of the density of fungiform papillae on the anterior tongue in 23 men and 25 women. A 2 × 2 χ2-test indicated that the sex difference in distributions was significant; note that ∼40% of the women sampled had more than 100 FP/cm2 vs. only ∼5% of men. Reprinted from Physiology and Behavior, PTC/PROP tasting: anatomy, psychophysics, and sex effects, Linda M. Bartoshuk, Valerie B. Duffy, Inglis J. Miller, 56: 1165–1171, 2002; republished with permission from Elsevier; from Bartoshuk et al. (46).
Gastric mechanoreception.
Signals related to gastric mechanoreception, motility, volume, and emptying contribute to the control of eating (166, 167, 339, 360). Although there are sex differences in gastric emptying, there is no direct evidence that these underlie sex differences in the control of eating. We are not aware of reports of male-female sex differences in gastric emptying in rats. Gangula et al. (248) reported a trend for slower emptying in female than male rats 4 h after a meal, suggesting that there may be reliable differences at earlier times after meals.
In female rats, 15 min emptying of an intragastric load was increased by ovariectomy and normalized by estradiol or estradiol-progesterone treatment, but not by progesterone alone (120). CCK contributed to the inhibitory effect of estradiol on gastric emptying because 1) estradiol increased plasma CCK levels after the intragastric load and 2) the CCK1 receptor antagonist devazepide blocked the effect (806). Blaustein and Wade (76) did not see an effect of estradiol on gastric emptying; whether this was because they tested anesthetized rats or another procedural difference is not clear. Gastric emptying was also increased in lactating rats (121). In one study, neither orchiectomy nor testosterone treatment affected gastric emptying in male rats (120).
In contrast to the lack of male-female sex differences in gastric emptying in rats, women empty both solids and liquids from the stomach significantly more slowly than men (60, 163, 349, 399). Differences often appear soon enough (i.e., <30 min) to potentially affect within-meal control of eating. In one study (349), a similar male-female sex difference in gastric emptying of solid food was found for postmenopausal women who took estrogen-progestin hormone treatments, but not for postmenopausal women who did not. Whether the ovarian cycle affects emptying is not clear: 1) Gill et al. (270) found that solid food, but not liquid food, emptied from the stomach faster during follicular phase than the luteal phase; 2) Brennan et al. (85) found that liquid food emptied from the stomach faster during the follicular phase; and 3) Horowitz et al. (340) failed to find cycle differences in emptying of either solid or liquid food. Eating was measured in only one of these tests. Brennan et al. (85) reported that oral loads of 50 g glucose in 300 ml water emptied from the stomach ∼15–20% more slowly and that intake during a meal 90 min later was ∼700 kJ less during the midfollicular phase than the midluteal phase in normal-weight women. If the food ingested during these meals also emptied more slowly, the decrease in meal size is unlikely to have resulted from feedback effects of postgastric food stimuli. Thus, these data suggest that the potency of satiation signals related to gastric mechanoreception varies during the ovarian cycle in women.
Ghrelin.
Ghrelin is unique among gut peptides in that its secretion increases during intermeal intervals and that ghrelin administration increases eating, apparently by acting centrally (383). Clegg et al. (130) reported that both intraperitoneal and intraventricular (third ventricle) ghrelin administration increased intake significantly more in intact male rats and untreated ovariectomized female rats than intact female rats or estradiol-treated ovariectomized rats. Furthermore, the eating-stimulatory effect of both peripheral and central ghrelin administration varied cyclically in both intact, cycling rats and ovariectomized rats cyclically treated with estradiol, such that ghrelin was more efficacious during diestrus 1 and 2 than during proestrus or estrus (Fig. 11). Butera (102) found similar changes in the eating-stimulatory potency of peripheral ghrelin. These data suggest that ghrelin may be necessary for both the tonic and the cyclic estrogenic controls of eating. One aspect of the ghrelin's effect in ovariectomized rats, however, is inconsistent with this conclusion and requires resolution. Ghrelin's effects in ovariectomized rats were due to changes in meal frequency, not in meal size (102, 130), whereas estrogens affect meal size, not meal frequency (please see Ovariectomy and hormone treatment).
Fig. 11.
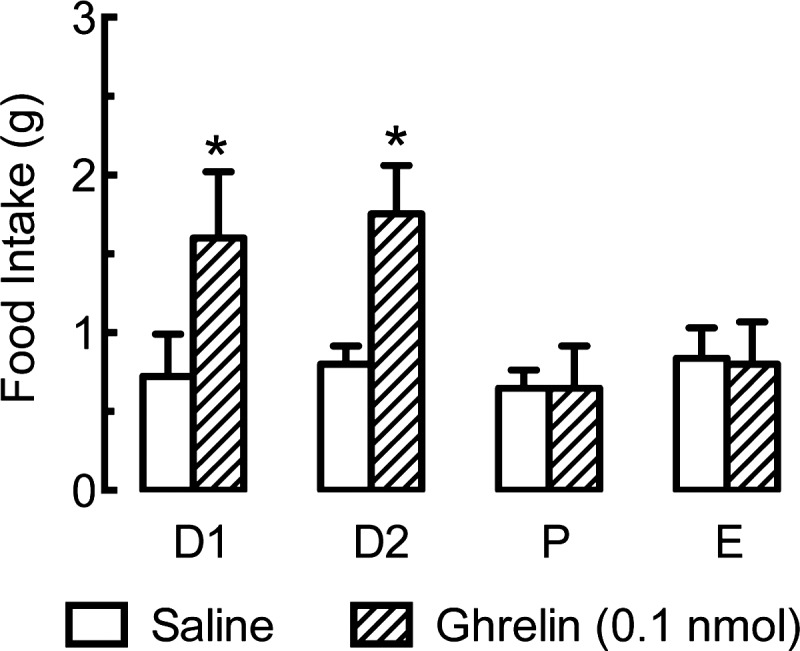
Cyclic changes in the eating-stimulatory effect of ghrelin in rats. The estrous cycle was monitored in intact rats (D1, diestrus 1; D2, diestrus 2; P, proestrus; E, estrus), and third cerebroventricular injections of 0.1 nmol of ghrelin or saline were tested on each cycle day. Ghrelin stimulated eating only on D1 and D2. *Significantly different from saline, same cycle day. Reprinted with permission from the American Diabetes Association from Diabetes, Deborah J. Clegg, Lynda M. Brown, Jeffrey M. Zigman, Christopher J. Kemp, April D. Strader, Stephen C. Benoit, Stephen C. Woods, Michela Mangiaracina, and Nori Geary, 56: April 2007; from Clegg et al. (130).
Sex differences in ghrelin's eating-stimulatory effect may be mediated by changes in ghrelin secretion, as well as changes in ghrelin sensitivity. Female rat gastric mucosal cells that are immunopositive for ghrelin also express ERα, and ovariectomy temporarily increases both the number of cells immunopositive for ghrelin and plasma ghrelin level (130, 461, 626). The time courses of the postovariectomy increases in plasma ghrelin level and in eating were strikingly similar (130), again consistent with a tonic estrogenic effect of ghrelin on eating in rats. Ghrelin levels have not been determined through the estrous cycle, and no changes in plasma levels of active ghrelin were detected in normally cycling women (157).
How estrogens influence ghrelin's eating-stimulatory effect in mice is not clear. Clegg et al. (130) reported that ovariectomy did not increase food intake or body weight in transgenic mice with null mutations of the ghrelin receptor, suggesting a necessary role for estrogens. Maletínská et al. (453), however, failed to detect any influence of estradiol treatment on the eating-inhibitory and weight-reducing effects of twice daily subcutaneous injections of the ghrelin antagonist [d-Lys3]GHRP-6 in ovariectomized mice fed chow or high-fat diet. A parsimonious potential resolution of these data is that estrogens act during early development to reduce the eating-stimulatory effect of ghrelin in adult mice.
Ghrelin levels are positively associated with testosterone levels in men and estrogen levels in women, and ghrelin levels increased both in hypogonadal men treated with testosterone (532) and postmenopausal women treated with estrogens (369, 541). These findings, however, have not yet been linked to the control of eating.
Cholecystokinin.
CCK is an important controller of meal-ending satiation in animals and humans (52, 416, 591, 677). CCK secretion increases during meals, and premeal administration of CCK-1R antagonists increased meal size in both male rats and men. CCK-1R antagonism also reduces or eliminates the satiating action of intraduodenal infusions of several nutrients, including, in male rats, fats, proteins, and some carbohydrates and, in humans, fats. CCK acts locally in the abdomen to initiate a vagal afferent signal that is transmitted to the NTS.
RATS AND MICE.
There is extensive evidence that an activational effect of estrogens increases CCK satiation in female rats (16–18, 102, 204, 345, 435, 455, 774). 1) In intact female rats, intraperitoneal CCK injection inhibited eating during proestrus or estrus, but not during diestrus, and the desatiating effect of CCK-1R antagonism was larger during estrus than during diestrus. 2) The de-satiating effect of CCK-1R antagonism was evident only after puberty in intact females. In ovariectomized rats, estradiol treatment 3) increased the satiating effect of intraperitoneal CCK injection and 4) increased the desatiating effect of CCK-1R antagonism, with the effect in each case larger on the day modeling estrus than on the day modeling diestrus. 5) Estradiol treatment also increased the satiating effect of intraduodenal infusions of small amounts of fat in ovariectomized rats, and this was reversed by CCK-1R antagonism. Some of these data are shown in Fig. 12. [Wager-Srdar et al. (774) failed to see some of these effects, perhaps because of their use of high doses of CCK and estradiol.]
Fig. 12.
Sex differences in the satiating effect of endogenous CCK in rats, assessed with the CCK-1 receptor antagonist devazepide. A and B: intact female rats were maintained in cages permitting recording of spontaneous meal patterns and were undisturbed except for daily injections of vehicle (Veh) or 1 mg/kg devazepide (Dev) 1 h before dark onset on the day of diestrus 2 or estrus. Note that devazepide significantly increased meal size during estrus (*P < 0.05), but not during diestrus, and that devazepide did not alter nocturnal meal frequency. Further tests indicated that the effect of devazepide did not depend on the smaller control meal sizes during estrus than diestrus 2 (data not shown). Reprinted from Peptides, Endogenous cholecystokinin's satiating action increases during estrus in female rats, Lisa A. Eckel and Nori Geary, 20: 451–456, 1999; republished with permission from Elsevier; from Eckel and Geary (204). C: intakes of 0.8 M sucrose during minute 5–45 in chronically estradiol-treated and control, oil-treated ovariectomized rats that sham fed with open gastric cannulas, were acutely pretreated with devazepide (Dev) or saline and received intraduodenal infusions of 10% Intralipid (0.44 ml/min) or saline (SAL) from minute 5 to 15. Note that estradiol increased the eating-inhibitory effect of Intralipid (*P < 0.05) and that this was completely reversed by Dev (+P < 0.05). From Asarian and Geary (17). D: devazepide-induced increases in nocturnal food intake (means ± SE) in female rats that were injected with 1 mg/kg devazepide every second day, according to a random schedule, and were tested for vaginal opening (puberty) daily, starting at 22 days of age. Puberty occurred at 30 ± 1 day of age, and rats cycled regularly thereafter, as indicated by observation of vaginal estrus every 4th or 5th day. Data are shown as mean intakes prior to puberty and on days of estrus in each week after puberty (devazepide had no effects on other days; data not shown). Note that devazepide increased food intake only after puberty. (*Significantly different from saline on matched test days, P < 0.05; **P < 0.01) (455).
Estrogens act via ERα to increase CCK satiation. Estradiol treatment did not increase the desatiating effect of CCK-1R antagonism in ovariectomized Erα−/− mice (257) or in ovariectomized rats in which ERα in the NTS were silenced by treatment with anti-ERα siRNA (20).
Modulation of CCK satiation may also contribute to the hyperphagia of pregnancy and lactation. The eating-inhibitory potency of intraperitoneal injection of CCK was less on day 14 of pregnancy than in rats tested during diestrus and decreased progressively during lactation (775). The mediating mechanism is unknown, but apparently does not require elevated prolactin (775).
In contrast to the involvement of CCK in the estrogenic control of eating, CCK does not appear to be involved in either the organizational or activational effects of androgens on eating. Shimizu et al. (661) reported that the effects of orchiectomy and testosterone treatment tended to be increased, not decreased, in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, in which meal size, daily food intake, and body weight are increased due to a CCK-1R-null mutation (719). It is important to note that both peripheral and central CCK-1R signaling are disrupted in OLETF rats and that as a result of the latter, they also overexpress neuropeptide Y in the dorsomedial hypothalamus (490). Thus, Shimizu et al.'s (661) data suggest that androgens do not interact with any of these eating-control mechanisms.
HUMANS.
The evidence for a sex difference in the satiating effect of CCK in humans is mixed. Burton-Freeman et al. (100) reported that there were close relationships between subjective hunger and fullness, rated on 100-mm visual-analog scales, and plasma CCK concentration after various 30% fat test meals in both men and women, but that the relationships differed between the sexes. For example, in women, but not in men, meals with almonds as the main fat source produced less CCK secretion and less fullness than the other meals. Overall, subjective fullness increased more in women, ∼3.7 mm for each 1 pmol/l change in plasma CCK concentration vs. only ∼1.5 mm/pmol/l CCK in men. Under different conditions, however, Nolan et al. (514) detected no sex differences in hunger or satiety ratings despite that postprandial CCK concentrations were elevated markedly more in women than in men 5 min after a test meal and were consistently, although nonsignificantly, elevated more in women throughout the following hour. Kissileff et al. (385) found that intravenous infusion of 112 ng/min CCK-8 significantly reduced meal size in women (∼120 g), but not in men (∼70 g) but, again, the sex difference was not significant. It is possible that the apparent sex difference in this study was due to differential effects of the CCK infusions on plasma CCK levels because identical doses were given to all subjects, although women weighed less (59 vs. 72 kg). Unfortunately, none of these three studies monitored menstrual cycling, which, as the studies discussed below suggest, may have added significant variability to the results.
Two attempts to detect cyclic changes in endogenous CCK satiation in women produced encouraging data. Pohle-Krauza et al. (561) had women ingest 10 g l-phenylalanine, which is a potent CCK secretogogue in humans (although not in rats), 20 min prior to lunch and dinner buffet meals in the midfollicular and the midluteal phases. In 11 subjects with low rigid-restraint [i.e., scores of 0 or 1 in the rigid-restraint subscale of the three-factor eating questionnaire (703)], l-phenylalanine reduced total daily food intake significantly more in the follicular phase than in the luteal phase. Surprisingly, just the opposite occurred in the 9 women with high rigid-restraint scores. These data suggest that CCK, ovarian cycling, and cognitive restraint may interact in the control of eating in women. Whether these effects were related to differences in CCK secretion was not determined. Brennan et al. (85), in the study reviewed just above (please see Gastric mechanoreception), found no cyclic change in postprandial CCK levels after a test meal. As gastric emptying was significantly reduced during the follicular phase, however, it is possible that intraintestinal nutrient stimuli stimulated CCK secretion more effectively during the follicular phase.
VAGAL MECHANISMS.
That estradiol increases the satiating potency of exogenous CCK in ovariectomized rats (105, 259) indicates that estrogens change the neural processing of CCK afferent signaling to modulate CCK satiation rather than increasing CCK secretion. Such altered processing could occur due to changes in CCK-1 receptor function in vagal afferent terminals in the abdomen. Because most CCK receptors in the NTS are expressed on the central terminals of vagal afferents (136, 491), we estimated changes in abdominal vagal CCK-1 receptor function by measuring estradiol's effects on CCK-1 receptor function in the NTS (258). Neither CCK-1 receptor number nor affinity was altered. Thus, estrogen signaling apparently either 1) increases transduction of CCK-1 receptor activation into a vagal afferent signal (hypothesis 1) or 2) modulates processing of this signal in the NTS or further downstream (hypothesis 2). The findings that female rats have ∼50% more myelinated vagal afferent fibers than males due to the presence of a specific low-threshold fiber type and that the excitability of these fibers is estrogen-dependent (430, 575) are consistent with hypothesis 1. On the other hand, the necessity of ERα expression in the NTS for the estrogenic modulation of CCK satiation (please see cmNTS) strongly supports hypothesis 2. Perhaps both mechanisms contribute.
Because lesion of abdominal vagal afferents eliminates the satiating actions of exogenous and endogenous CCK in male rats, vagotomy should also interfere with the estrogenic inhibition of eating. This has been tested once, and the result was negative. That is, ovariectomy still increased body weight, and subsequent estradiol treatment still decreased body weight, in rats with total subdiaphragmatic vagotomies (212) The reason for this apparent discrepancy is unclear. It may have been important that neither meal patterns nor food intake was measured in this study or that, because of the debilitating effects of total vagotomy (405), all the vagotomized rats maintained body weights well below those of control rats. It is also possible that the apparent discrepancy is due to compensatory effects of other estrogen-related but vagally independent signals.
Glucagon-like peptide-1.
Glucagon-like peptide-1 (GLP-1) acting in peripheral paracrine or endocrine and central neurocrine modes appears to contribute to the physiological control of meal size in rats and humans (39, 53, 318, 319, 791). Estradiol increased both the satiating potency of intraperitoneal injections of GLP-1 (Asarian, L, unpublished data) and the desatiating potency of intraperitoneal injections of the GLP-1 receptor-antagonist exendin-9 in ovariectomized rats (13). We assume that this is related to the satiating action of peripheral GLP-1 because, although central GLP-1 neurons are located in the cmNTS, they do not express ERα and do not express c-Fos in response to intraduodenal infusions of Intralipid (Asarian, L, unpublished data). As GLP-1 agonists are attractive candidates for obesity therapy (21, 798), these initial findings certainly merit pursuit.
Apolipoprotein A-IV.
Apolipoprotein A-IV is synthesized in the brain, as well as in the intestines, and appears to act physiologically to inhibit eating (744). A study by Shen et al. (657) indicates that apolipoprotein A-IV signaling in the NTS may contribute to the estrogenic inhibition of eating: 1) ovariectomy increased eating and weight gain more in mice with null mutations of the apolipoprotein A-IV gene than in wild-type mice; 2) cyclic estradiol treatment increased the eating-inhibitory effect of an acute injection of apolipoprotein A-IV into the fourth cerebral ventricle of ovariectomized rats; and 3) ovariectomy decreased expression of apolipoprotein A-IV mRNA in the NTS of rats. In addition, apolipoprotein A-IV appeared to interact with CCK signaling to produce satiation in male rats (438), suggesting the interesting hypothesis that the same NTS estrogen-signaling pathway may modulate both CCK and apolipoprotein A-IV satiation.
Glucagon.
Glucagon is released from the pancreas during meals, apparently largely due to cephalic phase reflexes, and contributes to the physiological control of meal size in rats and perhaps in humans (300, 801). In ovariectomized rats, estradiol treatment increased both the satiating potency of intrameal hepatic portal infusions of glucagon and the desatiating potency of antagonism of endogenous glucagon by hepatic portal infusion of glucagon antibodies (256). Whether this reflected a cyclic or a tonic effect of estradiol, and whether similar phenomena occur in cycling rats was not determined. There is also a sex difference in glucagon secretion in humans that may be relevant to eating. Both basal plasma glucagon concentration and prandial glucagon concentrations were approximately twofold higher in normal-weight or obese men than in normal-weight or obese women (112). These data indicate that the renewed interest in glucagon as a pharmacological tool in the treatment of obesity (300) should include studies of sex differences in its eating-inhibitory actions.
Insulin.
Basal plasma levels of insulin correlate with body fat mass, and tonic insulin signaling is hypothesized to act as a negative-feedback signal linking adiposity to the central nervous system control of energy homeostasis, i.e., to be an adiposity signal (91, 801), although the hypothesis is increasingly controversial (275, 311, 331, 799).
RATS AND MICE.
Clegg et al. (129, 131) reported a male-female sex difference in the eating-inhibitory effect of insulin; i.e., that injection of 1 or 4 mIU insulin into the third cerebral ventricle inhibited eating more in male rats than in age- or weight-matched females. Keen-Rhinehart et al. (368), however, saw no such sex difference in tests of peripubertal male and female rats, which they speculated may have been due to lower endogenous estrogen levels in the young rats. To add to the uncertainty, Brüning et al. (91) reported that female, but not male, transgenic mice with brain-specific-null mutations of the insulin receptor were hyperphagic. This effect may have been secondary to different degrees of disruptions of the HPG axis: female knockout mice had a ∼90% reduction in plasma LH concentration, vs. a ∼40% reduction in males.
Clegg et al. (129) also reported that both central (20 ng estradiol benzoate once each 4th day into the third ventricle) and subcutaneous (2 μg estradiol benzoate once each 4th day) estradiol treatments decreased the inhibition of eating produced by central injection of 8 mIU insulin in ovariectomized rats. These surprising data suggest that an activational influence of estrogens dampens insulin's anorectic potency; i.e., that insulin opposes the physiological estrogenic inhibition of eating. Where in the brain the injected hormones acted was not determined.
HUMANS.
Hallschmid and colleagues (59, 305) reported a male-female sex difference in insulin's effects in humans similar to that reported by Clegg et al. (129, 131) in rats. In one study (59), normal-weight (mean BMI, ∼21–23) subjects self-administered 160 IU regular human insulin or the vehicle intranasally before eating a buffet breakfast; women used estrogen-dominant contraceptives and were tested with an interval of 28 days to control for cyclic effects on eating. Insulin significantly decreased food intake in men, from 1,351 ± 105 to 1,159 ± 96 kcal, but not in women, increasing from 769 ± 44 to 787 ± 41 kcal, and the sex difference was significant. Interestingly, performance in a memory task was improved by insulin in women but not in men. This was followed by an 8 wk, placebo-controlled trial of four daily intranasal insulin treatments, once before each meal and again before bedtime (305). Insulin significantly reduced body fat mass, estimated by bioelectric impedance, ∼1.4 kg in men and had no detectable effect in women. A variety of neuroendocrine, cardiovascular, metabolic, and subjective measures suggested that these effects were not secondary to deleterious side effects, that the subjects could not detect whether they had received insulin or placebo, and that insulin selectively reduced premeal hunger. A similarly designed trial in obese men indicated that although chronic insulin treatment had the same cognitive effects in obese men as had been found in the normal-weight men, it failed to reduce body weight, suggesting that obesity may induce a kind of insulin resistance in the neural networks regulating body weight (304).
Amylin.
Amylin, a peptide cosecreted with insulin from the pancreatic β-cells, has a physiological eating-inhibitory action, and amylin agonists are promising antiobesity agents (446, 447, 618, 619, 801). Two tests of the estrogenic modulation of amylin's eating-inhibitory effects produced opposite results. Trevaskis et al. (742) reported that cyclic estradiol treatment markedly reduced the potency of chronic subcutaneous infusion of 50 μg·kg−1·day−1 amylin to reduce eating and body weight in ovariectomized rats. On the other hand, Asarian et al. (14) found that in acute eating tests, estradiol cyclically increased the satiating potency of exogenous amylin and the desatiating potency of amylin antagonism. Estradiol also increased the number of dopamine β-hydroxylase-positive cells in the area postrema that expressed c-Fos after amylin injection, consistent with the catecholaminergic mediation of amylin's satiating effect. Especially in view of amylin's physiological role and therapeutic potential, it is important to resolve the contrasting outcomes of these studies.
Metabolic signals.
A distributed network of metabolite- and hormone-sensing neurons in the brain and periphery contributes to energy homeostasis, in part via the control of eating (69, 416, 427). A relatively small amount of data suggests that there are sex differences in the metabolic control of eating. Wade and Gray (767) proposed in 1979 that estrogens control eating indirectly by increasing the flux of energy metabolites from the adipose tissue into the circulation, from which they reach hepatic or brain metabolic receptors. Attempts to provide experimental support for this hypothesis failed (287, 517, 533, 582, 583), however, and they soon abandoned it (768). More recent experiments with metabolic inhibitors have produced inconsistent results. Swithers et al. (717, 718) found that mercaptoacetate, which antagonizes fatty-acid oxidation, increased eating in male rats, but not females, whereas Sandoval et al. (632) found that mercaptoacetate was more effective in females than males. The cause for this discrepancy is unclear. Sandoval et al. (632) also found a sex difference related to glucose metabolism: 2-deoxy-d-glucose (2DG), which inhibits glycolysis, elicited more eating in male than female rats. Unexpectedly, both estradiol-treated and untreated ovariectomized females were more sensitive to 2DG than intact females (632), which contrasts with an earlier report (469) that estradiol treatment decreased the eating-stimulatory effect of 2DG in ovariectomized rats. Thus, more research is required to determine whether there are sex differences in metabolic controls of eating, and if so, whether they are estrogen-regulated.
Leptin.
Basal plasma levels of leptin, like those of insulin, are tightly correlated with body fat mass and are thought to signal adiposity status to the brain and to initiate compensatory changes in eating and energy expenditure, especially in the case of reduced adiposity (218, 239, 422, 501). Dissecting the normal role of endogenous leptin in energy homeostasis has been far more difficult than was expected on the basis of the exaggerated obesity phenotype exhibited by mice, rats or humans with null mutations in the genes encoding leptin or its signaling receptor, leptin receptor-b (252, 331, 799). Nevertheless, there are now several lines of evidence that leptin tonically inhibits eating in mice, rats, and humans (1, 301, 310, 400, 418, 459, 614, 755, 824). It does not appear that this control is sexually differentiated. Although there are clear sex differences in leptin secretion, there is little evidence that these are related to sex differences in eating.
LEPTIN SECRETION.
Basal plasma leptin levels are linearly related to fat mass in both men and women, with obese and never-obese persons falling on the same line (612, 613). Men and women have different relationships, however, with plasma concentrations of leptin per kilogram body fat mass markedly higher in women. Postmenopausal women have less basal plasma leptin per kilogram fat mass than premenopausal women, but more than men. Genetic differences, differences in androgen and estrogen levels, and regional fat distribution all appear to contribute to these sex differences in basal plasma leptin levels, with no single factor alone sufficient (488, 598, 612, 613).
Basal plasma leptin concentration is correlated with fat mass in rats as well, but in contrast to humans, postpubertal male rats have higher basal plasma leptin levels than postpubertal females (415, 497, 683). The implication of this for leptin signaling is not clear, however, because plasma concentration of the soluble leptin receptor-e, which acts as a plasma binding protein that reduces leptin signaling, also is much higher in postpubertal male rats than postpubertal female rats (681).
LEPTIN AND SEX DIFFERENCES IN EATING.
No role for the sex differences in basal plasma leptin concentration described above in eating has been established. Acute injections of leptin into the third cerebral ventricle, however, inhibited eating more in female rats than male rats (2, 129, 131). This sex difference may be related to activational effects of gonadal steroids. In ovariectomized rats, estradiol treatment increased leptin's eating-inhibitory potency (2, 129, 131) and upregulated leptin receptor-b, the signaling form, in the hypothalamus (474, 595). The physiological relevance of these effects for leptin's normal tonic eating-inhibitory effect, however, is uncertain. 1) Plasma estradiol levels did not influence the effect of chronic leptin treatment on body fat mass in female mice (545) or rats (124). 2) The eating-inhibitory potency of chronic leptin treatment did not vary over the ovarian cycle in intact rats (208). 3) Normal-weight women ate less during the periovulatory phase than during the luteal phase, but leptin levels were higher in the luteal phase and were not correlated with eating during any phase (536).
Androgens may affect leptin's eating-inhibitory potency in the opposite manner as estrogens. This is because exogenous leptin had smaller effects on eating and on nuclear translocation of leptin-signal transducer and activator of transcription 3 in Arc neurons in transgenic mice lacking AR than in male wild-type mice (215). Basal levels of food intake in the AR-knockout and in wild-type mice, however, were not different (215).
Pregnant and lactating rats are both hyperleptinemic and, in terms of the inhibition of eating, leptin resistant. This resistance may be due to increased prolactin levels (412, 743); i.e., release from the tonic inhibitory effect of leptin on eating may increase food intake during pregnancy and lactation. In support of this, Grattan and colleagues (25, 26, 285, 412, 413) showed that 1) pregnancy-induced leptin resistance was associated with elevated neuropeptide Y mRNA and decreased POMC mRNA in the arcuate nucleus during pregnancy, 2) leptin receptor mRNA and leptin-induced phosphorylated-signal transducer and activator of transcription-3 protein decreased in the VMH during pregnancy, and 3) injection of leptin into the third cerebral ventricle that reduced daily food intake by ∼20% in nonpregnant rats had no effect in pregnant rats or in pseudopregnant rats after intracerebroventricular infusions of prolactin that were designed to mimic the pattern of placental lactogen secretion that occurs during pregnancy. The development of leptin resistance during lactation and lack of effect of leptin on eating during lactation were recently replicated by Suzuki et al. (713). A useful further step would be to verify that progressive decreases in endogenous leptin signaling are necessary for the progressively increased food intake during pregnancy and lactation, as could be done, for example, using leptin antagonism (459, 824). Changes in NPY and melanocortin signaling in the dorsomedial hypothalamus were also implicated in pregnancy-induced hyperphagia (119).
Finally, the many physiological interactions between leptin and gonadal steroids, for example, in the early development of neural architecture controlling adult behavior (493, 667, 805), in pubertal development (187, 563), and in nutritional infertility (645, 769, 787), certainly justify further investigation of their interactions in the control of normal eating and adiposity (see Ref. 567 for further discussion).
Sex Differences in Central Mechanisms Controlling Eating
Neurochemical controls of eating.
Neuropharmacological studies, including molecular genetic manipulations of neurochemicals, implicate several brain signaling molecules in sex differences in eating. We review some of the most interesting data. An unfortunate aspect of this literature is that, as yet, no concrete links have been forged between the neurochemical findings reviewed here and the progress in identifying either the sites of ER or the peripheral mechanisms mediating sex differences in eating reviewed above.
SEROTONIN (5-HYDROXYTRYPTAMINE, 5HT).
Serotonergic neurotransmission plays integral roles in both satiation and food reward (302, 315, 414, 416). Whether there are male-female differences in the serotonergic control of eating is not clear. In one study, fenfluramine inhibited eating in female rats more than in male rats (209), whereas in another study, it did not (150). In contrast, there are several indications that estrogens affect serotonin-induced eating. Eckel et al. (209) found that the eating-inhibitory effect of the 5HT agonist fenfluramine was 1) larger in intact, cycling rats during estrus than during diestrus and 2) increased by estradiol treatment in ovariectomized rats (593). In addition, they found that intraperitoneal and lateral cerebroventricular injections of meta-chlorophenylpiperazine, a selective antagonist of 5HT2C receptors, increased eating more in estradiol-treated than control ovariectomized rats (594). Souquet and Rowland (687), however, found no difference in the effect of chronic fenfluramine treatment in estradiol-treated than control ovariectomized rats. Taken together, these data suggest that 5HT-5HT2C signaling is involved in estrogens' cyclic, periovulatory eating-inhibitory effect, but not its tonic eating-inhibitory effect. The role of 5HT in the estrogenic control of eating may also be diet-specific because cyclic variations in the eating-inhibitory effect of 5HT were not detected when rats were fed more palatable foods or offered different macronutrient sources (325, 538).
5HT1A autoreceptors also may be involved in the periovulatory decrease in eating because 8-hydroxy-2–9(di-n-propylamino)tetralin (8-OH-DPAT), which decreases 5HT signaling by stimulating 5HT1A autoreceptors, stimulated eating less 1) in estradiol-treated than in control ovariectomized rats (627), 2) in intact females rats than in ovariectomized rats (150), and 3) during proestrus and estrus than during diestrus in cycling rats (749).
Some of this estrogen-serotonin interaction may involve the amygdala. This is because 1) Eckel et al. (205, 206) found that estradiol increased eating and CCK-induced expression of c-Fos in the central nucleus of the amygdala (205, 206), and 2) Parker et al. (538) reported there were cyclic changes in the eating-stimulatory effect of injections of the 5HT2A/2C receptor-antagonist metergoline into the posterior basolateral amygdala. King et al. (379, 380) reported that female rats ate more than male rats following amygdala lesions, but because ovariectomy has similar effects in intact and amygdala-lesioned rats, these data are difficult to relate to those of Eckel et al. (205, 206) and Parker et al. (538).
Rivera et al. (594) reported that estradiol treatment increased 5HT2C receptor content in the dorsal one-third of the caudal brain stem (∼10–14.6 mm caudal to bregma), but not in the hypothalamus, suggesting that estradiol increases 5HT signaling by increasing the numbers of 5HT2C receptors in the caudal brain stem. It is important to determine the relationship between these neurons and the cmNTS ERα neurons mediating some of estradiol's eating-inhibitory and weight-regulatory effects (please see cmNTS). In addition, because the satiating effect of CCK in male rats and mice is mediated in part by 5HT-5HT2C signaling (11, 559, 560), it is important to determine whether 5HT-5HT2C signaling mediates the sex differences in CCK satiation (please see CCK).
MELANOCYTE-STIMULATING HORMONES AND MELANOCORTIN RECEPTORS.
α- and β-MSH signaling via MC3 and MC4 receptors contribute crucially to the control of energy homeostasis, with MC4 receptor mechanisms apparently more linked to the control of eating and MC3 receptor mechanisms to the control of metabolism (54, 722, 723). Although MC4 receptors are widely expressed in the brain, populations in the arcuate and paraventricular nuclei of the hypothalamus and caudal brain stem have been most strongly implicated in its eating effects.
There is a striking male-female sex difference in the effect of transgenic null mutations of the MC4 receptor gene in mice (Mc4r−/− mice): female Mc4r−/− mice increased food intake and body weight vs. female wild-type mice markedly more than male Mc4r−/− mice (348, 712) (Fig. 13). In one study (348), excess body weight began to accumulate around puberty in both sexes, suggesting activational effects of gonadal steroid hormones influence MSH-MC4 receptor signaling. There is also a male-female sex difference in the effects of loss of function polymorphisms of the human MC4 receptor gene, such that affected women accrete about twice as much excess BMI as men, ∼8–9 kg/m2 vs. ∼4–5 kg/m2 (177, 704). In addition, in a laboratory test-meal study, children of either sex with MC4 receptor polymorphisms ate more than twice as much as control children (219). MC4 receptor defects comprise the commonest form of human monogenic obesity, with a prevalence of ∼2–5% in obese Europeans (521, 722, 723). Thus, mutations of the MC4 receptor represent an important sex difference in human eating and weight regulation that is accessible to study in mice.
Fig. 13.
Male-female sex differences in the effects of transgenic deletion of the melanocortin 4 receptor gene (Mc4r−/−) on eating and weight gain in mice. Male and female Mc4r−/− and wild-type (WT) mice were fed a low-fat diet (LFD) from weaning to age 12 wk and then a high-fat diet (HFD) until week 22. Data are means of food intakes (kJ/day) ± SE and were measured for low-fat diet (LFD) from age 7 to 12 wk (top) and a high-fat diet (HFD) from age 12 to 22 wk (middle). Bottom: body weight gains (g, means ± SE) between week 7, when all mice had similar weights, to week 22. Note that female Mc4r−/− apparently increased food intake relative to female WT mice more than male Mc4r−/− mice on both diets and gained more weight than males (although the authors reported significant main effects of sex in their ANOVA, they did not test these relative differences for significance). Republished with permission of the Endocrine Society, from Endocrinology, Gregory M. Sutton, James L. Trevaskis, Matthew W. Hulver, Ryan P. McMillan, Nathan J. Markward, M. Josphine Babin, Emily A. Meyer, and Andrew A. Butler, 147: 2186–2006, 2006; permission conveyed through Copyright Clearance Center, Inc.; from Sutton et al. (712).
In contrast to Mc4r−/− mice, transgenic mice with null mutations of the MC3 receptor did not increase intake of either low- or high-fat diet (712). Mutants of both sexes gained excess weight, apparently due to a variety of metabolic defects.
Neuropharmacological studies to date have not recapitulated the sex difference in eating shown by Mc4r−/− mice. 1) Lateral ventricular injections of the broad-spectrum MC receptor agonist MTII affected eating similarly in male and female rats (131). 2) Lateral ventricular injections of MTII affected eating similarly in estradiol-treated and control ovariectomized rats (562). 3) Lateral ventricular injections of the MC3/4 receptor antagonist SHU9119 affected eating similarly in estradiol-treated and control ovariectomized rats (562). Some have claimed that following SHU9119 treatment in this study (562), the estrogenic inhibition of eating was “not detected” [(599), p 7] or estradiol was “unable to induce anorexia” [(463), p 5]. This is incorrect. SHU9119 did not change the effect of estradiol in tests when it occurred. For example, estradiol decreased 24-h food intake ∼5 g both in rats treated with 500 pmol SHU9119 and in untreated rats [Fig. 1 in (562)].
One possible explanation for the apparent discrepancy between the transgenic and neuropharmacological data is that Mc4r−/− mutation might disrupt early development of the HPG axis and that females may be relatively more sensitive to the disruption than males. A recent study, however, seems inconsistent with this hypothesis. That is, Xu et al. (812) found that female transgenic mice in which ERα was deleted from neurons expressing Pomc, the gene encoding MSH, became hyperphagic beginning around puberty, suggestive of disruption of an activational rather than an organizational effect of estrogens. In this study, however, the weight gain was limited to lean body mass, with no effect on fat mass. Because ovariectomy increases fat mass more that lean mass, the significance of these data for the normal estrogenic regulation of eating and energy homeostasis is unclear.
Agouti-related peptide and neuropeptide Y.
Neurons expressing Agouti-related peptide (AgRP), an inverse agonist of MC4 receptors, coexpress neuropeptide Y (NPY) and are found solely in the arcuate nucleus of the hypothalamus; NPY neurons are more widespread (87, 416). NPY and AgRP/NPY neurons project to a variety of forebrain and brain stem sites involved in the control of eating and body weight, and central administration of either peptide potently stimulates eating (22, 87, 416, 693). Although transgenic deletion of the Npy gene or the Agrp gene or silencing them in early development did not affect adult eating or body weight, silencing them in adulthood led to pronounced anorexia (293, 445, 534, 574). Whether there were sex differences in these effects was not reported. Central injections of AgRP elicited similar effects on food intake and body weight in intact and gonadectomized male and female rats (131, 281), however, suggesting that the male-female sex difference in the effect of MC4 receptor mutations described above is not related to AgRP function.
A study by Olofsson et al. (527) indicates that AgRP/NPY neurons play a selective role in the periovulatory decrease in eating in mice. They reported that 1) hypothalamic Agrp and Npy gene expression decreased during estrus and 2) mice with massive degeneration of AgRP/NPY neurons due to transgenic deletion of mitochondrial transcription factor-A in AgRP neurons did not reduce food intake during estrus (Fig. 14), although they were fertile and vaginal estrous cycling was normal. AgRP/NPY neuron-deficient mice displayed normal average levels of food intake and normal body weight, indicating that these neurons selectively control the cyclic, but not the tonic, eating-inhibitory effect of estrogens. This effect was indirect in that hypothalamic AgRP/NPY neurons did not express ERα. The authors also investigated the effects of estradiol treatment, but these data are difficult to interpret physiologically because of the high doses used (2 μg centrally and 150 μg peripherally, please see Hormone treatment regimens). Whether AgRP/NPY neurons are involved in the periovulatory decrease in eating in other species has not been tested, although ovariectomy increased hypothalamic NPY and AgRP mRNA contents in rats (130) and estradiol reduced the concentration of AgRP in the cerebrospinal fluid of ovariectomized rhesus monkeys (810). Central injections of AgRP had similar effects in estradiol-treated and control ovariectomized rats (562, 633), which, together with Olofsson et al.'s (527) data, suggests that estrogens may modulate AgRP synthesis and release rather than MC4 receptor function or downstream mechanisms. In contrast to the lack of effect of estradiol on AgRP's eating-stimulatory effect, estradiol did increase NPY-induced eating in one study (633), suggesting that estrogens may modulate NPY receptor function.
Fig. 14.
Agouti-related peptide/neuropeptide Y (AgRP/NPY) neurons are necessary for the periovulatory decrease in eating in mice. Mice in which AgRP and NPY neurons were transgenically deleted (solid squares) did not show an estrous decrease in daily food intake under conditions in which wild-type control mice did (open symbols). M, diestrus 1; D, diestrus 2; P, proestrus; E estrus. From Olofsson et al. (527).
MELANIN-CONCENTRATING HORMONE.
Melanin-concentrating hormone (MCH) is a hypothalamic neuropeptide that stimulates eating and may contribute to sex differences in eating. Santollo and Eckel (634) reported that the eating-stimulatory effect of lateral cerebroventricular injections of MCH was 1) greater during diestrus than during estrus, 2) reduced by estradiol treatment in ovariectomized rats, and 3) greater in male rats than in estradiol-treated ovariectomized females.
Orosensory hedonics.
Orosensory hedonic or palatability responses result from evaluative processes in the brain that produce subjective experiences (pleasure, surfeit, disgust, etc.) and controls of eating related to them (66, 68, 416, 607). A variety of data suggest that there are important physiological sex differences in these processes.
RATS.
The hedonic control of eating can be assessed behaviorally in animals in situations that minimize the postoropharyngeal effects of food. This can be done by measuring lick rates or intake at just the onset of the meal, in brief-access tests, or in sham-eating tests, during which open gastric cannulas prevent ingested food from accumulating in the stomach or entering the intestines (679). An elegant apparatus for brief-access tests offers animals repeated 10-s exposures to as many as eight different test fluids in random order in the same session, so that the postoropharyngeal food stimuli that develop during the session do not differ for the different test fluids (680). Using this method, Curtis et al. (152) found that 1) male rats licked approximately twofold more 0.025 or 0.05 M sucrose than did females, 2) males and females licked similarly during exposure to 0.1–0.4 M sucrose (Fig. 15A) and 3) male rats licked less than females during exposure to mixtures of 0.05 M sucrose and 0.05–0.5 M NaCl. The relative preference of male rats for dilute sucrose may be an organizational effect because licking was similar in untreated and estradiol-treated ovariectomized rats. This palatability difference appeared to be sufficient to control total amount eaten because when offered 0.025 M sucrose and water overnight, males drank approximately twofold more sucrose per gram body weight than either estradiol-treated or untreated ovariectomized females (Fig. 15B). This interpretation is tentative, however, because the postoropharyngeal effects of sucrose were not controlled. The salt preference appears to mirror a sex difference in “need-free” intake of 3% NaCl reported by Chow et al. (126), who also demonstrated that this sex difference is an organizational effect of neonatal androgenization of the brain. Male-female sex differences have also been reported for some other fluids, including glucose-saccharine mixtures (750) and polysaccharides (650). As rats appear unusually reactive to these tastants, the translational relevance of these phenomena is uncertain.
Fig. 15.
Male-female and estrogen-controlled sex differences in hedonics in rats. A: intact male rats licked more 0.025 and 0.05 M sucrose than intact females, and estradiol-treated ovariectomized females licked more than control, oil-treated females in 10-s palatability tests; neither sex difference was detected in tests of higher sucrose concentrations. Solid squares denote intact male rats, while open circles denote oil-treated ovariectomized females (OVX-OIL), and gray circles denote estradiol-treated ovariectomized females (OVX-EB). aSignificantly different from OVX-OIL. bSignificantly different from OVX-EB. B: overnight intake of 0.025 M sucrose in the same rats, expressed as ml/100 g body wt; abbreviations are the same as above. That males drank more independent of their greater size suggests that the hedonic difference shown in A controlled intake. In contrast, the effect of estradiol on palatability did not influence overnight intake. *Significantly different from both groups of ovariectomized females. A and B: Reprinted from Physiology and Behavior, Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats, Kathleen S. Curtis, Linda M. Davis, Amy L. Johnson, Kelly L. Therrien, Robert J. Contreras, 80: 657–664, 2004; republished with permission from Elsevier; from Curtis et al. (152). C: intact rats tested during estrus (E/SAL, triangles) licked less 0.025 M sucrose solutions in 10-s palatability tests than rats tested during diestrus 2 (D2/SAL, circles). Intakes of 0.05–0.4 M sucrose solutions did not differ, suggesting that they were similarly palatable, and the effect obtained during the brief-access testing did not translate into an overnight effect (data not shown). aSignificantly different from diestrus, same sucrose concentration. Reprinted from Physiology and Behavior, vol. 86, Deann P. D. Atchley, Karen L. Weaver, and Lisa A. Eckel, Taste responses to dilute sucrose solutions are modulated by stage of the estrous cycle and fenfluramine treatment in female rats, 265–271, 2005, with permission from Elsevier; from Atchley et al. (23). D: estradiol failed to decrease 45-min sham intake of a 6.25% corn oil emulsion in ovariectomized rats, although estradiol significantly decreased real intake of the same solution; abbreviations are the same as in A. Rats received 2 μg estradiol benzoate or control injections once each 4th day and were tested on the day modeling estrus. *Significantly different from real intake in control rats. (Asarian L, Mangiaracina M, and Geary N, unpublished data).
The palatability of dilute sucrose solutions may vary during the ovarian cycle. Atchley et al. (23) reported that rats licked less 0.025 M sucrose during 10-s exposures during estrus than during diestrus 2, but no differences were obtained for more concentrated sucrose solutions (Fig. 15C). This cyclic change in palatability of 0.025 M sucrose did not appear to control amount eaten because there was no cyclic difference in overnight intake of 0.025 M sucrose. 5HT appeared to contribute to the palatability of 0.025 M sucrose during diestrus 2 because fenfluramine decreased 0.025 M brief-access sucrose licking during diestrus 2, but did not affect brief access licking during estrus or overnight (23).
If estrogen treatment decreases the palatability of sweet fluids in ovariectomized rats, the phenomenon must be subtle: 1) The change in brief-access licking of 0.025 M sucrose reported by Atchley et al. (23) did not occur in tests of 0.05–0.4 M sucrose. 2) Curtis et al. (152) reported that twice weekly treatment with 10 μg estradiol benzoate decreased 10-s intakes of 0.05 M sucrose, but not of higher or lower sucrose concentrations. 3) We found that an estradiol treatment regimen that did not affect 0.8 M sucrose intake in sham-eating tests decreased it during real-eating tests (260). Lick records revealed how rapidly postoropharyngeal effects of estradiol can appear: in real-eating tests, neither ovariectomy nor estradiol treatment affected lick rate during the first minute of sucrose access, but ovariectomy increased lick rate during minutes 2–4 compared with intact rats, and estradiol treatment decreased lick rate during minutes 2–4 compared with untreated ovariectomized rats (344).
Sex differences in the hedonics of fat flavor have not been extensively tested in rats. Stratford et al. (699) found that in male, but not female, rats, the addition of 88 μM linoleic acid increased the relative selection of a mixture of 40 mM monosodium glutamate and 5 μM ethanol in 10-min simultaneous-access tests. The addition of 88 μM linoleic acid, however, increased the relative selection of a 100 mM monosodium glutamate-5 μM ethanol solution in both sexes. They concluded that there is a sex difference in the ability of linoleic acid to increase the intensity and palatability of low concentrations of monosodium glutamate. Finally, as for sucrose, there appears to be no activational effect of estradiol on the palatability of fat flavor. We found that corn-oil emulsions elicited a concentration-dependent stimulation of sham eating in ovariectomized female rats, as reported earlier for male rats (485), but we failed to detect any effect of estradiol treatment on sham eating, despite that estradiol significantly decreased real eating of the same solutions (Asarian L, Mangiaracina M, and Geary N, unpublished data; Fig. 15D).
HUMANS: PSYCHOPHYSICS.
Determination of sex differences in flavor hedonics in humans requires comparisons of psychophysical ratings between different types of subjects, which is a classical problem in psychophysics. The general labeled-magnitude scale is the most valid tool available for this and is reshaping understanding of the relationships among hedonics, sex, and obesity (44, 45, 685). For example, Hayes and Duffy (316) reported a complex interaction between the density of fungiform papillae on the tongue and the pleasantness of sucrose-cream mixtures. As described above (please see Gustation), greater densities of fungiform papillae on the tongue are associated with increases in the perceived intensity of sweetness and creaminess of food, independent of the hedonic valence of the stimuli. In this study, Hayes and Duffy (316) found a sex difference in the relationship between fungiform papillae density and hedonic ratings. Women with fewer fungiform papillae and men with more fungiform papillae found most pleasant high-creamy, high-sweet flavors, whereas women with more fungiform papillae and men with fewer fungiform papillae found most pleasant medium-creamy, medium-sweet flavors. The effects were large: women and men who preferred most high-creamy, high-sweet flavors endorsed absolute intensities of liking that were approximately twofold larger than men and women who preferred most medium-creamy, medium-sweet flavors (Fig. 16). Given that absolute liking ratings of the most-preferred foods are positively correlated with BMI (45) and that obese women prefer high-fat, high-sugar foods (194, 451), these data suggest that a relative lack of fungiform papillae would increase a woman's risk for obesity. How the data relate to obesity risk in men is more difficult to predict, given that obese men prefer high-fat, high-protein foods to high-fat, high-sugar foods (194, 451). The complexity of interactions of sensory and hedonic flavor responses undoubtedly contributes to the variable findings on changes in food preferences over the menstrual cycle (e.g., 241, 359, 472) and in functional brain imaging studies (next section).
Fig. 16.
Sex and the density of fungiform papillae (FP) on the tongue interact to determine human hedonic responses to sweet-creamy flavor mixtures. Combinations of sugar solutions and cream were rated using the general labeled-magnitude-estimation scale, which enables valid comparisons of measurements of subjective experience between groups (e.g., between sexes). Men and women with low and high FP densities were analyzed separately. The x-axes are the sensory intensities (i.e., not hedonic intensities) of the sweetness of the stimuli, and the y-axes are the sensory intensities of their creaminess (0 = no sensation; 100 = the strongest imaginable sensation of any kind); note that the sensory intensities of the stimuli tested ranged from 0 to 90. The pleasantness of the stimuli (−100 = strongest imaginable disliking, 0 = neutral; 100 = strongest imaginable liking) are displayed as isohedonic contours; that is, each line indicates the various sweet-creamy combinations that were judged to have a particular pleasantness, the values of which are given on the contours. Insets on the graphs indicate the range of intensities of pleasantness observed. For example, men who had low FP density found the hedonic intensity of the stimuli to range from approximately −5 to ∼20, with maximum liking (smallest contour area) for stimuli with sensory intensity ∼50 sweet and ∼50 creamy. Note that men with low FP densities and women with high FP densities liked best intermediate sweet-creamy intensities best (maximum liking for men, ∼50 sweet, ∼50 creamy and for women, ∼30 sweet, and ∼40 creamy), but that their degrees of liking were moderate (<25 for men, <20 for women). In contrast, men with high FP densities and women with low FP densities liked higher sweet-creamy intensities best (90 sweet, 90 creamy were most liked by both sexes) and endorsed higher degrees of liking (>30 for men, >40 for women). Note also that flavor mixtures that women with low FP densities liked most (upper right part of graph) were disliked by women with high FP densities. Reprinted from Physiology and Behavior, Oral sensory phenotype identifies level of sugar and fat required for maximal liking, John E. Hayes and Valerie B. Duffy, 95: 77–87, 2008; republished with permission from Elsevier; from Hayes and Duffy (316).
HUMAN FUNCTIONAL BRAIN IMAGING: INTRODUCTION.
The neural bases of normal and disordered human eating, eating-related behavior, cognition, and affect can now be investigated with functional brain imaging, which indirectly measures levels of neuronal activity in circumscribed brain areas (111, 141, 173, 230, 410, 509, 753, 756). Progress in this exciting area is difficult for a number of reasons: 1) Images of brain responses related to cognition and affect are extraordinarily sensitive to within- and between-subjects variations in arousal, mood, habits, life experiences, food-related memories, social context, and other difficult-to-control factors. 2) Images of brain responses are only indirect measures of actual neural activity. They are based on indirect detection of cerebral glucose metabolism (positron-emission tomography) or, more frequently, indirect detection of cerebral blood flow (functional magnetic resonance imaging). Such measures cannot distinguish inhibition from excitation. Changes in blood flow or metabolism may arise from excitatory or inhibitory neural activation. In addition, differences in response intensity across regions might have more to do with differences in the neural architecture of microcircuits within the regions than with differential involvement in processing the stimuli tested. 3) Detection of brain activation depends both on the physical sensitivity of the imaging machinery and the information-processing algorithms used. For example, the present limit of spatial resolution of brief scans is not sufficient to differentiate activity in adjacent hypothalamic areas that have different functions. In addition, the temporal resolution is too poor to enable inferences about the sequence of information processing. 4) Images of larger telencephalic areas often differentiate activity within subareas for which no functional differences are known. 5) Psychological functions cannot be assigned unambiguously to brain areas. For example, the orbital frontal cortex (OFC), which many data suggest is the most important telencephalic node in the neural network mediating food hedonics, is clearly also involved in cognitive processes, such as decision making, risk evaluation, and expectation (66, 403, 409, 410, 530, 609). Furthermore, there are sex differences in some of these cognitive processes (403, 530).
Despite these difficulties, pictures of foods and tasting food reliably increase brain activation in a number of areas (111, 173, 410, 509, 753, 754, 756). Increased flavor intensity, independent of hedonic evaluation, usually increases activation in the middle insular cortex, cerebellum, and amygdala. Increased hedonic intensity usually increases activation in the OFC, anteroventral striatum, and nucleus accumbens (NAc), anterior insular cortex (AIC), and anterior cingulate cortex (ACC). The dorsolateral prefrontal (dlPFC) and parietal cortices are also often activated, which is usually interpreted to be associated with cognitive or “executive-control” aspects of the experience. These areas tend to be more activated in people with higher levels of dietary restraint (dietary restraint is discussed in Interaction with cognitive controls of eating). When the stimuli are pictures of foods, the fusiform gyrus, a higher-order visual processing area, is usually activated.
The involvement of the amygdala and NAc in human hedonics, as well as studies of the involvement of dopaminergic neurotransmission that we did not review (694, 695), parallel studies of flavor reward in rats (35, 515, 651). Similarly, studies of the responses of individual neurons in both subcortical and cortical brain areas in rhesus macaques to taste stimuli suggest that there is substantial overlap in the neural structures mediating flavor hedonics in monkeys and in humans (284, 606, 608). Thus, in addition to providing unique insights about human hedonics, neuroimaging data also support the use of animal models. Nevertheless, some species differences are to be expected. For example, neuroimaging and clinical data indicate that the AIC is necessary for normal affect related to bodily states, if not for all affect, and monkeys and rodents do not have a homologous structure (142, 143).
An important discovery is that food stimuli affect brain activation differently in normal-weight and obese persons (111, 173, 410, 509, 694, 753, 756). For example, obese persons typically display greater differential activation in response to pictures of high vs. low energy-density foods in the NAc, AIC, ACC, and OFC, suggesting that increased hedonic evaluation of food may contribute to the pathophysiology of obesity. A study by Stice et al. (696) supported this hypothesis. They found that palatable food and monetary rewards increased brain activation in several brain areas associated with hedonic evaluations in normal-weight children at high familial risk for obesity (by virtue of having two overweight or obese parents) compared with low-risk children (two normal-weight parents).
HUMAN FUNCTIONAL BRAIN IMAGING: SEX DIFFERENCES.
Male-female sex differences in brain activation in response to the flavors of normal foods (176, 675), to pure tastes (299), and to pictures of food (135, 231, 264, 378, 748) have been reported. In a recent review of this literature, Geliebter et al. (264) concluded that if brain areas are classed by their predominate functions, as described above, pictures of food stimuli elicit more activation in areas related to planning and executing behaviors in men and more activation in areas related to cognitive and affective processes in women. This conclusion, although certainly generally correct, belies the complexity of the data obtained in these studies.
The complex effects of sex on imaging responses is clearly reflected in Geliebter et al.'s (264) study. Subjects were weight-stable, healthy obese men and women. They ate a 1,000 kcal dinner, fasted 12 h, then ate either 750 ml liquid diet (∼740 kcal, fed state) or 750 ml water (fasted state), and were tested 95 min later. The difference in response between high and low energy-density foods was larger in men than women during the fed state in five brain areas and during the fasted state in five different brain areas. The high-low difference was larger in women than men in five brain areas during the fed state and during the fasted state in four different areas, as well as two of the same areas, the insula and the dlPFC. The insula responses occurred on the left side in both men and women, but not in similar subregions [Montreal Neurological Institute standard brain coordinates (right-left, anterior-posterior, superior-inferior), −32, −34, 20 and −28, 0, 30, respectively]. In the dlPFC, differences were detected in similar subregions, but occurred in the right hemisphere during the fed state and in the left hemisphere during the fasted state (coordinates 30, 40, 34, and −34, 38, and 32, respectively). Furthermore, in two other parts of the dlPFC, women showed smaller rather than larger responses than men in the fed state (coordinates 42, 34, 16, and 30, 22, 34). The only other area in which brain responses occurred in more than one condition was the right fusiform gyrus, where the high-low difference was larger in men than women in the fed state and larger in women than men in the fasted state. Aside from the lack of menstrual-cycle phase data, this is a well-planned and analyzed study. Nevertheless, current understanding of human brain function simply does not permit a coherent interpretation of most of the effects obtained.
It is possible that studies of brain responses to receipt of food might lead to simpler patterns of data. As far as we know, however, only two such studies have appeared. Haase at al. (299) studied brain responses to intraoral infusions of 0.3 ml 0.04 M caffeine (bitter), 0.01 M citric acid (sour), 0.64 M sucrose (sweet) or 0.16 M NaCl (salty) in normal-weight men and women during fed and fasted states [using a method similar to that of Geliebter et al. (264) described above]. Indeed, only two sex differences in brain responses to sucrose between the fed and fasted states were detected—the differential responses were larger in men than women in the insula and cerebellum (sex differences for other tastes occurred in several other areas). Smeets et al. (675) did a more naturalistic study. Brain responses to chocolate-flavored milk, administered in small amounts by an undescribed method, were tested in normal-weight men and women, first in a fasted state and then after eating solid bittersweet chocolate and water to satiety. Sex differences in the effect of satiation were detected only in three areas: men showed greater increases in activation in the ventral striatum and greater decreases in the medial PFC, while women showed greater decreases in the hypothalamus.
We are aware of two reports that women's brain activation in response to pictures of foods varies across the menstrual cycle. Frank et al. (232) reported that the differential effect of pictures of high energy-density foods vs. nonfoods was larger in the follicular phase than in the luteal phase in the NAc, amygdala, and hippocampus; the effect of low energy-density foods vs. nonfoods was significant only in the hippocampus. Alonso-Alonso et al. (4) tested the effects of pictures of food before and after a standard meal designed to contain 20% of their daily energy need; this was done once early (days 3–6 after the onset of menstruation) and once late (days 10–13) in the follicular phase of the menstrual cycle. The differential effect of fed vs. fasted states of activation in response to pictures of food was greater in the late vs. the early follicular phase in the inferior frontal gyrus and fusiform gyrus. The inferior frontal gyrus is considered to be an “executive-control” area and, as mentioned above, the fusiform gyrus is a higher-order visual-processing area; thus, neither is likely to be directly involved in the control of eating. Nevertheless, it is intriguing that, unique among cortical areas, the volume of the fusiform gyrus-parahippocampal gyrus area, as well as performance on hippocampal-dependent memory tasks, varied throughout the menstrual cycle (557, 572).
Physiological Sex Differences in Disordered Human Eating
Psychiatric eating disorders.
The current view is that the psychiatric eating disorders anorexia nervosa, bulimia nervosa, binge-eating disorder, and “eating disorders not otherwise specified” (which accounts for about half of eating-disorder patients) are caused by complex interactions among cultural, social, familial, and biological factors (5, 30, 365, 387, 460, 740, 777). Perhaps the strongest evidence that biological factors are important in psychiatric eating disorders is their high heritability (heritability is the fraction of phenotypic variance accounted for by genetic variation). Twin studies, for example, suggest that genetic variability accounts for ∼50–80% of the risks for anorexia nervosa and bulimia nervosa (365, 736, 740).
The various influences on psychiatric eating disorders are thought to interact dynamically, so that precipitating vulnerabilities spiral into vicious cycles involving increasing numbers of causal factors. Sex is clearly an important variable. The lifetime prevalence of psychiatric eating disorders (346, 351) and various symptoms of disordered eating (700) are about three-fold higher in women than in men in community samples, and these differences emerge around the onset of puberty (27, 631, 715). Subclinical symptoms of disordered eating are also more prevalent in females than males (329, 700) and were highly heritable in pubertal and postpubertal girls, but not in prepubertal girls (148). In addition, recovery from eating disorders is slower in women than in men, and rates of remission are higher (698).
That the pathogenesis of eating disorders involves biological factors naturally encourages studies of potential biological bases of these sex differences, but as our review reflects, few human studies have done so. Similarly, although the utility of animal models of disordered eating is increasingly emphasized (113, 138, 139, 362, 363, 665, 676), the contributions of physiological sex differences are not a major component of these research efforts.
Eating disorders affect gonadal-steroid hormone levels. Women who are acutely ill with anorexia nervosa have abnormally low gonadotropin, estrogen and progestin levels and usually amenorrhea, and women with bulimia nervosa frequently report menstrual dysfunction (30, 332, 334, 483, 566). In addition, testosterone levels are decreased in symptomatic women with anorexia nervosa and increased in symptomatic women with bulimia nervosa (30, 504, 709). Changes in gonadal steroid hormone levels, however, are consequences of weight loss and extreme dieting rather than being causes of disordered eating (7, 30, 277, 483, 586). Nevertheless, pharmacological treatments that affect gonadal hormone levels may be useful in the treatment of disordered eating, as least in selected patients (30, 63, 334, 366, 483, 504). For example, initial results suggest that antiandrogenic treatment may reduce binge eating in women with bulimia nervosa (504, 710).
ANOREXIA NERVOSA: HUMAN STUDIES.
Twin studies indicate that genetic factors account for up to 74 (395) to 88% (96) of the variance in anorexia nervosa. The genes accounting for this high heritability and their contribution to sex differences in anorexia nervosa are unknown. One potential clue comes from a family-based association study involving 321 French families (761) that suggested that polymorphisms in ESR1, the gene encoding ERα, may contribute. In this study, overtransmission of three ESR1 single-nucleotide polymorphisms (rs726281, rs3798577, and rs2295193), as well as a haplotype of ESR1 involving these and five additional single-nucleotide polymorphisms were associated with restricting-type anorexia nervosa (i.e., energy intake controlled by dieting rather than purging). This study (761) also failed to detect the previously reported (200, 616) association between the single nucleotide polymorphism rs1256049 in ESR2, which encodes ERβ, and anorexia nervosa. Further work is required to determine whether these effects can be replicated and extended to other populations. This has not yet succeeded. Rather, genome-wide association studies to date, although yielding some candidates for further research, have not identified genome-wide significance for variants in ESR1 or any other genes in anorexia nervosa (128, 354, 773, 781).
Klump and colleagues (146, 389) presented two lines of support for their hypothesis that disordered human eating is influenced by early organizational effects of gonadal steroid hormones similar to those discovered in animals (please see Development). 1) In normal-weight women with either male or female twins, higher ratios of the lengths of the second and fourth fingers, indicative of less prenatal androgen exposure, were significantly correlated with increased scores on a questionnaire measuring subclinical disordered-eating symptoms (389). 2) Disordered-eating scores were significantly higher in girls with female twins than girls with male twins, who presumably had greater prenatal androgen exposure (146). Furthermore, this difference in disordered eating scores emerged during mid-late puberty (147), suggesting that the developmental effect forms a substrate for a later activational effect of gonadal steroids (as described below). These associations, however, were detected in only one (577) of several (31, 95, 449, 571, 581) subsequent studies involving patients with eating disorders. Thus, Klump's organizational hypothesis requires further research.
The increased incidence of anorexia nervosa around puberty suggests that an activational effect of estrogens may cause anorexia nervosa, as originally hypothesized by Young (815, 816). Reports (389, 579) of significant correlations between salivary estradiol levels and subclinical eating-disorder symptoms across the menstrual cycle are consistent with this hypothesis. Whether any of these women progressed to anorexia nervosa, however, was not established. Misra et al. (486) performed a randomized, double-blind study in which female patients with anorexia nervosa (n = 110; mean age, 16 yr; mean BMI, 17.4 kg/m2; minimum duration of amenorrhea, 3 mo) received either placebo or a physiological regimen of estrogen treatment in addition to their ongoing treatment regimens. Estrogen treatment consisted of a fixed dose of estradiol in older patients and an escalating dose schedule that was designed to mimic peripubertal plasma estradiol levels in younger patients. After 18 mo, estradiol did not significantly affect body weight gain (4.6 ± 1.0 kg in estradiol-treated vs. 4.2 ± 1.0 kg in control patients) or BMI gain (1.6 ± 0.4 kg/m2 vs. 1.5 ± 0.4 kg/m2) but did significantly improve spine and hip bone mineral densities. These data suggest that estrogens did not exacerbate the symptoms of anorexia nervosa in these patients, but because eating was not measured and may have been too low in these patients to detect decreases, they are not conclusive.
ANOREXIA NERVOSA: ANIMAL MODELS.
The most popular animal model of anorexia nervosa is Routtenberg's (620, 621) activity-based anorexia model, which is self-starvation brought about in mice and rats by providing ad libitum access to an activity wheel and limiting food access to ∼1–2 h/day. Very few activity-based anorexia studies involve females. Doerries et al. (186) found that female Holtzman Sprague-Dawley rats ran more than weight-matched males, but ate more and lost weight slower. Female rats may not develop activity-based anorexia as reliably as males; in one study, females did not increase activity (79) and in another they increased activity but did not decrease eating (185).
Activity-based anorexia rapidly suppresses ovarian cycling in rats, paralleling the endocrine disturbances of anorexia nervosa (554) and other situations of insufficient energy intake (441, 442, 645, 770, 792). This activity-based-anorexia effect is due to an interaction of high activity and reduced food intake because neither alone was sufficient for it (184, 185, 784). A similar synergistic effect on ovarian cycling occurred in response to combinations of food restriction and psychosocial stress in cynomolgus monkeys (792). Increased activity and decreased eating combine to inhibit kisspeptin or GnRH neurons in the hypothalamus, which leads to reductions in LH and estrogens (175, 456, 666). Whether the reduced estrogen secretion is related to the weaker anorectic effect in female rats compared with male rats (185) is unknown.
There may also be a sex difference in the development of vulnerability to activity-based anorexia in rats. Hancock and Grant (307) reported that early, acute, maternal separation led to greater increases in activity and more profound anorexia in females than males when tested as adolescents, but more in males than females when tested as adults. These data suggest both 1) that early-life stress has complex effects on susceptibility to activity-based anorexia later in life and 2) that sex may affect the susceptibility to activity-based anorexia indirectly, via effects on stress reactivity, rather than by directly affecting physiological controls of eating.
Kas and colleagues (362, 363) suggest that the high heritability of anorexia nervosa can be fruitfully analyzed by studying genes implicated in the human syndrome that lead to phenotypic variations in animals. They point out that spontaneous activity is an excellent candidate phenotype because marked increases in physical activity frequently accompany anorexia nervosa and the propensity for spontaneous activity is highly heritable in mice. Given that estrogens control physical activity in female mice and rats and that female rodents normally display much higher levels of spontaneous activity than males (251, 433), this phenotype also seems to be a good choice for the investigation of sex differences. Such work, however, may have to be done outside the context of activity-based anorexia, however, because 1) activity-based-anorexia rats display high levels of activity in the absence of LH and estrogens and 2) estradiol treatment reduced activity in weight-reduced rats (656).
BINGE EATING.
Binge eating, defined as repeated episodes of eating abnormally large amounts of food together with the perception of loss of control over eating, is a core diagnostic criterion of bulimia nervosa and binge-eating disorder (5). The lifetime prevalence for bulimia nervosa is approximately three-fold higher in females than males, and that for binge eating disorder is about twice as high (346, 351). Binge eating also occurs outside these disorders, and a recent community sample estimated that 24% of women fall in this category (382). Importantly, the prevalence of binge eating considered as a separate symptom is also ∼2–6-fold higher in females than males (145, 196, 223, 320, 329, 584).
The heritability of bulimia nervosa is estimated in twin studies to account for 59 (772) to 95% (94) of the variance. The heritability of binge eating per se is nearly as high (35–85%) (93, 94, 394, 584, 708). This underscores the biological component of binge eating. As for anorexia nervosa, however, the genes underlying this high heritability remain unknown. Work by Davis et al. (164, 165) suggests that it may be related, in part, to gain-of-function polymorphisms in the dopamine D2-receptor and opioid μ-receptor genes. The authors hypothesized that these polymorphisms may predispose people to hedonically based or emotional eating and thus increase their risk for binge-eating disorder. It is important to replicate these findings and determine whether they can be extended to bulimia nervosa. Finally, further evidence that there is a biological component in the sex difference in binge-eating prevalence comes from fascinating recent rat studies indicating that female rats are more prone to display binge eating-like behavior than are male rats (29, 396) (Fig. 17).
Fig. 17.
Male-female sex difference in rats' propensities to binge eat. Thirty male and 30 female rats were offered standard chow ad libitum and a palatable commercial cake frosting 3 days/wk for 2 wk, a procedure that leads to increased palatable food intake in comparison to ad libitum access to the same food (138, 139). Four-hour palatable food intakes in each of the six tests were ranked across all rats, and individual differences in binge-eating propensity were scored. Data are percentages of male and female rats that were binge-eating prone (i.e., scored in the highest tertile of palatable food intake in 3 or more of the 6 tests and never scored in the lowest tertile) or binge-eating resistant (i.e., scored in the lowest tertile of palatable food intake in three or more of the six tests and never scored in the lowest tertile). *Significant sex difference, two-proportion z test, P < 0.001. Adapted from International Journal of Eating Disorders, Sex differences in binge eating patterns in male and female adult rats, Kelly L. Klump, Sarah Racine, Britny Hildebrandt, and Cheryl L. Sisk, 95: 77–87, 2008; republished with permission from John Wiley and Sons; from Klump et al. (396).
Klump et al. (388), as well as others (27, 631, 715) have also investigated the biological contributions to the sharp increases in the incidences of bulimia nervosa and binge-eating disorder that occur in girls at the onset of puberty (∼8–10 yr of age). In the Klump et al. study (388), they found 1) no detectable heritability of disordered eating in prepubertal girls; 2) heritability of ∼0.5 in girls during and after puberty; and 3) heritability of ∼0.5 in boys at all times. In another study (393), they detected significant heritability for disordered eating in 10–15-yr-old girls with higher plasma estradiol levels, but not in girls with lower estradiol levels. Pursuing these effects in a rat model, they found that a binge-eating trait emerged in vulnerable rats during puberty (398), but that ovariectomizing these rats increased binge size rather than eliminating binges (397). Taken together, these data suggest that 1) an organizational effect of estrogens at puberty acts on a genetically determined vulnerability to facilitate the development of bingeing in girls and 2) an activational effect of estrogens thereafter may limit binging (please see Ref. 149 for a review).
The estradiol metabolite 2-hydroxy-estradiol may mediate another activational effect of estrogens on bingeing. Catabolism of 2-hydroxy-estradiol, like catabolism of dopamine, is catalyzed by catechol-O-methyltransferase, and loss-of-function polymorphisms in the catechol-O-methyltransferase gene were linked to increased risk for anorexia nervosa and bulimia nervosa (481). Because increased plasma levels of estrogens, leading to increases in 2-hydroxy-estradiol, may competitively inhibit dopamine catabolism and increase synaptic dopamine and because altered dopaminergic mechanisms of food reward may contribute to binge eating (89, 780), catechol-O-methyltransferase could link estrogens to the propensity to binge. In support of this hypothesis, Babbs et al. (29) demonstrated that peripheral injections of 2-hydroxy-estradiol increased eating under binge conditions, but not under normal conditions, in a rat model.
There are several reports that binge frequency and disordered-eating symptoms are higher during the luteal and menstrual phases of the cycle than during the follicular phase (210, 272, 391, 426, 570, 578). Furthermore, within-subject analyses indicate that both binge frequency in patients (210, 391) and disordered-eating symptoms in community samples (390, 392, 578, 580) are positively correlated with preceding progesterone levels and negatively correlated with preceding estradiol levels, suggesting possibly causal relationships. Furthermore, the correlations between estradiol and progesterone levels and disordered eating were evident across a range of patient BMI, levels of dietary restraint, and levels of impulsivity (390, 580). Clearly, mechanistic work on the relationships between activational effects of estrogens and progestins and binge eating is warranted. Two issues that require further research in this context are whether binge size, in addition to frequency, varies across the cycle and whether cyclic effects on bingeing are related to the cyclic changes in normal eating (please see Ovarian cycle).
The hormonal control of binge size, but not of binge frequency, has been investigated in rat binge-eating models based on intermittent access to palatable food (138, 139). Binge size 1) did not vary across the ovarian cycle in intact rats (57), 2) was tonically, but not cyclically, decreased during a cyclic estradiol treatment regimen that both tonically and cyclically decreased normal eating in ovariectomized rats (817, 818), and 3) was not affected by cyclic treatment with a near-physiological progesterone dose, either alone or in combination with estradiol (817). Thus, how closely cyclic changes in binge frequency in women can be modeled in rats is not yet clear.
Finally, test-meal studies indicate that patients who binge eat display decreased perceived satiation together with decreased gastric emptying or increased gastric capacity (180, 263, 265), decreased postprandial plasma ghrelin drops (262), decreased postprandial plasma CCK increases (180, 269, 367, 555), and decreased postprandial plasma GLP-1 increases (506). Whether these are adaptations to binge eating or contribute causally to binge size is not certain. Nevertheless, in view of the sex differences in these mechanisms reviewed above, they merit testing as potential sources of sex differences in binge eating.
Obesity.
As reviewed above, the effects of loss of ovarian function and estrogen treatment on adiposity are clearly linked to changes in eating in mice and rats and may be so in women. Here, we discuss two further links among HPG function, eating, and obesity.
POLYCYSTIC OVARY SYNDROME.
Polycystic ovary syndrome is an endocrine disorder that includes abnormal ovarian morphology, hyperandrogenism (e.g., hirsutism) and oligomenorrhea or amenorrhea after puberty (211, 280, 334, 785). It affects ∼10% of reproductive-aged women. The majority of women with polycystic ovary syndrome are also obese, with atypical regional adipose-tissue distribution, i.e., predominately abdominal rather than gluteo-femoral adiposity. Because obesity, especially abdominal obesity, disrupts ovarian cycling and leads to signs of hyperandrogenism, obesity is presumed to cause or exacerbate polycystic ovary disorder (37, 211, 785). Progressive development of insulin resistance, hyperinsulinemia, and insulin-mediated inhibition of estrogen secretion may also release eating from estrogenic inhibition. In addition, some eating abnormalities that are associated with polycystic ovary syndrome are similar to those of bulimia nervosa, suggesting that increased androgen levels may underlie both (334, 335, 434, 505).
Meal-related gastrointestinal-hormone secretion is disordered in polycystic ovary syndrome: test meals decreased plasma ghrelin levels less (489) and increased plasma CCK level less (335) in patients with polycystic ovary syndrome than in weight-matched control women. Basal ghrelin secretion is also reduced in women with polycystic ovary syndrome, and increased during anti-androgen therapy in one small study (246). More work is required to determine the causes of these changes and whether they are related to disordered eating.
BARIATRIC SURGERY.
The most effective treatment for obesity is bariatric surgery. Because prevalence of morbid obesity in the USA is approximately twofold higher in women than men (226) and because women appear to suffer more from these disorders in terms of quality of life (24, 84, 273, 531, 762), >80% of bariatric surgery patients in the United States are women (568, 630). Both animal and human data suggest that physiological sex differences affect bariatric surgery outcome. 1) A retrospective analysis of >1,300 obese women who underwent either Roux-en-Y gastric bypass surgery or gastric banding indicated that presumptively premenopausal women (i.e., women age 20–45 yr) lost significantly more weight than presumptively postmenopausal women (i.e., age 55–65 yr of age) (522). This is consistent with the hypothesis that estrogens affect weight even in women in whom bariatric surgery has massively altered gastrointestinal food handling. 2) Estradiol treatment further decreased eating and body weight after Roux-en-Y gastric bypass surgery in ovariectomized rats (13) (Fig. 18). Thus, physiological sex differences in eating may be important issues in the surgical treatment of obesity.
Fig. 18.
Estradiol-treated ovariectomized rats (RYGB-E2) eat less (B) and gain less weight (A) after Roux-en-Y gastric-bypass surgery (RYGB) than untreated ovariectomized rats (RYGB-OIL), and these effects of estradiol were similar to those in rats with sham RYGB surgeries (SHAM-E2 and SHAM-OIL). Rats were fed solid, high-energy diet for 3 wk preoperatively (phase 1) and for 4 wk postoperatively (phase 2); they were then fed Ensure Plus liquid diet for 3 wk (phase 3). #SHAM-OIL significantly different from RYGB-OIL; *RYGB-OIL significantly different from RYGB-E2; +SHAM-OIL significantly different from RYGB-E2, all P < 0.05. Reprinted from Gastroenterology, Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats, Lori Asarian, Kathrin Abegg, Nori Geary, Marc Schiesser, Thomas A. Lutz, and Marco Bueter, 143: 325–327.e2, 2012; republished with permission from Elsevier; from Asarian et al. (13).
Conclusions
We reviewed the variety and complexity of physiological sex differences in eating in rats, mice and anthropoid primates, including phenomenology, peripheral and central neuroendocrine mechanisms, and pathophysiology. We discussed physiological sex differences in early development, puberty, and reproductive adulthood and senescence. The prominence and variety of physiological sex differences in rats, mice, and anthropoid primates with potential relevance for understanding normal and disordered human eating convince us of the importance of this area. For example 1) the physiological sex difference in intake of sweets that occurs in animals and women has clear potential implications for the development of countermeasures for worldwide epidemic of overeating and obesity, and 2) evidence for sex-linked physiological contributions to the vulnerability to psychiatric eating disorders may facilitate development of more efficacious treatment of these disorders. Furthermore, the numerous mechanistic approaches that already have been brought to bear on these and other sex differences in eating that we reviewed underscore their tractability to physiological and molecular analyses in humans, as well as in animals. Finally, powerful new methods are likely to accelerate progress in several aspects of physiological sex differences in eating. This is reflected, for example, in work under way on 1) the development of HPG-axis controls of eating, 2) links between gene variations and eating, and 3) functional brain imaging.
The greatest amount of data presently available concerns the activational effects of estrogens on eating in rats and mice. On the basis of these data, as well as the more limited results in anthropoid primates, we hypothesize that the population of ERα-expressing neurons in the cmNTS is an essential component of a local neuronal network that integrates a variety of peripheral afferent signals, as well as descending diencephalic and telencephalic influences to control meal size, food intake, and body weight in rats and mice, and probably in women. Fig. 19 diagrams this hypothesis and emphasizes its speculative nature. For example, we conclude that at present 1) CCK, ghrelin, and gustation are firmly established estrogen-sensitive peripheral controls, and, of these, only CCK satiation is certain to be influenced by cmNTS ERα, and 2) 5HT, AgRP, and orosensory hedonics are firmly established estrogen-sensitive central controls, and whether they are affected by cmNTS ERα or provide descending inputs to the local neuronal networks in which cmNTS ERα reside, is wholly speculative. As we also reviewed, however, these disparate data suggest several potential toeholds for efforts to test this and related hypotheses. For example, the apparent similarity of the roles of CCK, 5HT, and AgRP on the phasic estrogenic inhibition of eating during the ovarian cycle presents one such opportunity. Figure 19 also makes clear that there is an androgenic control of meal size, food intake, and body weight and that nothing is known about the central mechanisms mediating it. Finally, available data indicate that progestins do not contribute to the control of eating.
Fig. 19.
Schematic summary of the activational effects of gonadal steroid hormones on eating, emphasizing hypothesized neural mechanisms and their integration. The diagram is based on our review of rat, mouse, and anthropoid primate, including human, data. It is superimposed on a schematic midsagittal section of a rat brain, although most named structures are lateral to the midline. Red arrows and text boxes indicate estrogenic mechanisms; question marks and black font identify less well-established mechanisms. Estrogens acting on ERα on neurons in the cmNTS (filled oval) affect the neural processing of peripheral CCK signals (solid red arrow) so as to reduce meal size, food intake, and body weight; the same ERα neurons are hypothesized to be involved in the processing of a variety of other peripheral signals, especially ghrelin and gustatory signals, which are apparently affected by estrogens (dashed red arrows). ERα in the dorsal raphe and several hypothalamic areas are not strongly implicated in the control of eating (open red oval). A number of forebrain signaling molecules, especially 5HT and AgRP, as well as flavor hedonics contribute to the estrogenic control of eating, probably via hypothalamic and telencephalic mechanisms (dashed red arrows). Neural processing in these areas is presumably linked bidirectionally to the cmNTS and other brain stem areas (double-headed dashed red arrow), so that cmNTS ERα may also mediate these effects; non-ERα-expressing AgRP neurons in the Arc are the strongest candidates. Androgens acting on AR in unknown sites increase meal frequency, food intake, and body weight (green arrows and text boxes). In contrast, progestins appear not to have physiological effects on eating (blue, dashed text box). Challenges for future mechanistic studies of sex differences in eating include 1) establishing the physiological and pathophysiological roles of the estrogenic mechanisms shown and 2) identifying the androgenic mechanisms affecting eating.
We emphasize that the hypothesis that physiological sex differences in the controls of eating play causal roles in both normal and disordered human eating does not imply either 1) that physiological sex differences in eating alone are sufficient to cause disordered eating or 2) that physiological sex differences not directly involved in eating are not important in disordered eating, for example, variants in ESR1 that are related to personality differences in women (482). Rather, our view is that disordered eating results from dynamic interactions among numerous cultural, social, familial, and biological factors to which physiological sex differences, including sex differences in the physiology of eating, may contribute. Furthermore, we believe that physiological sex differences may be relevant to the treatment of disordered eating even if they are not causal. For example, a particular physiologically based therapy may be more or less efficacious in various states of HPG function, such as in premenopausal or postmenopausal women.
Despite the significant progress being made on the study of sex differences in the physiology of eating, many aspects of the problem remain nearly untouched. Examples of such specific issues include 1) how eating is released from estrogenic inhibition during the luteal phase in anthropoid primates, 2) how the activational effects of androgens on eating are mediated, 3) whether and how puberty and reproductive senescence affect eating, and 4) how basic physiological controls of eating contribute to disordered human eating.
In addition to these relatively specific issues, our review also indicates that more integrative approaches are called for. There are opportunities for integrative sex-difference studies of at least five kinds. 1) Almost no studies to date have addressed the neuroendocrine integration of peripheral and central eating-control mechanisms, such as the integration of brain stem and telencephalic neural mechanisms. This type of integration comprises one of the most exciting frontiers in the contemporary physiology of eating (291, 416, 494, 648, 800) and is an unfortunate lacuna in sex-difference research. 2) How hypothalamic and pituitary HPG-axis mechanisms, such as those mediated by kisspeptin or GnIH, are integrated with controls of eating mediated by gonadal steroids requires research. 3) Studies of physiological sex differences in eating must be better integrated with physiologies of growth, energy metabolism, and adiposity. Although there are certainly a number of efforts in this direction, particularly at the molecular genetic level, these should be better linked to the physiology of individual meals, where, in our opinion, sex difference studies are most advanced. 4) There are opportunities for more translational sex-difference studies integrating research advances in animal models and in humans. These include sex differences in peripheral and central controls of eating, in flavor hedonics, in binge eating, and in eating-disorder susceptibility genes, as well as many other phenomena that we reviewed. 5) Mood, anxiety, impulsivity, stress reactions, and other psychological processes often involve changes in eating and food-related cognitions and affect. These processes also display a variety of sex differences, and there are well-developed methods to assess them in animals and humans (6, 49, 50, 62, 183, 225, 238, 542, 624, 644, 653, 658, 703, 720). The study of interactions among such psychological processes and physiological sex differences in eating seem to us to be profitable avenues of investigation.
All of the problems outlined above have to be tackled to create an integrative regulatory physiology of HPG influences on eating. Viewed from the perspective of these challenges, what has been accomplished seems uneven and fragmentary. Sex is inappropriately neglected in the physiology of eating, as it is in most other branches of physiology and medicine (51, 64, 73, 109, 110, 466, 797, 826). Our review will have succeeded if it illuminates opportunities for meaningful research and attracts more attention to them.
We close by acknowledging several limitations of the review. 1) We reviewed only the “consummatory phase” of eating, i.e., eating per se, and not the “appetitive phase,” in which important sex differences are also to be expected. 2) As mentioned above, our focus on biological sex differences is not meant to suggest that there are sharp borders between purely biological and nonbiological causes of sex differences in behavior, and future work needs to address these gray zones. 3) Our emphasis on the role of eating in determining sex differences in adiposity should not be taken to discount the potential importance of sex differences in metabolism and energy expenditure. 4) Although rat and mouse models dominate the study of sex differences in eating and adiposity, it is not clear if these are always the most appropriate model species for humans. For example, as we discussed, these animals' ovarian cycles do not include a period comparable to the human luteal phase, and mice often fail to overeat after ovariectomy. Furthermore, the relative importance of ERα in the control of eating apparently differs among rodent species. Thus, future work should focus more on identifying which animal species provide the most appropriate models of particular human sex differences.
GRANTS
The authors gratefully acknowledge research funding for our work from the U.S. National Institutes of Health Grants MH-51135 (to N. Geary), MH-65024 (to N. Geary), DK-54523 (to L. Asarian and N. Geary), and DK-092608 (to L. Asarian), as well as Grant 3100-122567 from the Swiss National Science Foundation (to N. Geary), from the Society for Women's Health Research (to N. Geary), and from the Novartis Foundation (to L. Asarian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.A. and N.G. conception and design of research; L.A. and N.G. interpreted results of experiments; L.A. and N.G. prepared figures; L.A. and N.G. edited and revised manuscript; L.A. and N.G. approved final version of manuscript; N.G. drafted manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the invaluable intellectual and technical contributions of the many colleagues who have assisted us in our research: colleagues whose helpful discussions improved our review, including Art Arnold (University of California, Los Angeles), Lisa Eckel (Florida State University), Richard Foltin (Columbia University Medical Center), Kelly Klump (Michigan State University), Lance Kriegsfeld (University of California, Berkeley), Timothy Moran (Johns Hopkins University School of Medicine), and Janet Polivy (Universtiy of Toronto). We also thank Barry E. Levin, Associate Editor, American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, for the invitation to prepare this review, as well as the three anonymous reviewers for their very helpful critiques of the initial submission.
REFERENCES
- 1.Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology 140: 4923–4931, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord 25: 1680–1688, 2001 [DOI] [PubMed] [Google Scholar]
- 3.al-Dahan MI, Thalmann RH. Progesterone regulates gamma-aminobutyric acid B (GABAB) receptors in the neocortex of female rats. Brain Res 727: 40–48, 1996 [PubMed] [Google Scholar]
- 4.Alonso-Alonso M, Ziemke F, Magkos F, Barrios FA, Brinkoetter M, Boyd I, Rifkin-Graboi A, Yannakoulia M, Rojas R, Pascual-Leone A, Mantzoros CS. Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: relation to fasting and feeding. Am J Clin Nutr 94: 377–384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders-5. Arlington, VA: American Psychiatric Association, 2013 [Google Scholar]
- 6.Anestis MD, Peterson CB, Bardone-Cone AM, Klein MH, Mitchell JE, Crosby RD, Wonderlich SA, Crow SJ, le Grange D, Joiner TE. Affective lability and impulsivity in a clinical sample of women with bulimia nervosa: the role of affect in severely dysregulated behavior. Int J Eat Disord 42: 259–266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arimura C, Nozaki T, Takakura S, Kawai K, Takii M, Sudo N, Kubo C. Predictors of menstrual resumption by patients with anorexia nervosa. Eat Weight Disord 15: e226–e233, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 55: 570–578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold AP, Chen X, Link JC, Itoh Y, Reue K. Cell-autonomous sex determination outside of the gonad. Dev Dyn 242: 371–379, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7: 413–442, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Asarian L. Loss of cholecystokinin and glucagon-like peptide-1-induced satiation in mice lacking serotonin 2C receptors. Am J Physiol Regul Integr Comp Physiol 296: R51–R56, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Asarian L. Membrane estrogen receptors and energy homeostasis. J Neurosci 26: 11255–11256, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, Bueter M. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology 143: 325–7.e2, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Asarian L, Boyle CN, Lutz TA. Estradiol increases the acute eating-inhibitory effect of amlyin in ovariectomized rats (Abstract). Appetite 57: S2, 2011 [Google Scholar]
- 15.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42: 461–471, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin's satiating action in ovariectomized rats. Peptides 20: 445–450, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148: 5656–5666, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361: 1251–1263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asarian L, Thammacharoen S, Geary N, Clegg DJ, Ogawa S, Lutz TA. Knock-down of estrogen receptor-α neurons in the nucleus tractus solitarii eliminates CCK-induced c-Fos expression in the paraventricular nucleus of the hypothalamus (Abstract). Appetite 54: 633, 2010 [Google Scholar]
- 20.Asarian L, Thammacharoen S, Lutz TA, Geary N. Selective RNAi knockdown of estrogen-receptor-α neurons in the nuceus tractus solitarii eliminates estradiol's inhibitory effects on food intake in ovareictomized rats (Abstract). Appetite 52: 817, 2009 [Google Scholar]
- 21.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374: 1606–1616, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature 488: 172–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atchley DP, Weaver KL, Eckel LA. Taste responses to dilute sucrose solutions are modulated by stage of the estrous cycle and fenfluramine treatment in female rats. Physiol Behav 86: 265–271, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Atlantis E, Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int J Obes (Lond) 32: 881–891, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Augustine RA, Grattan DR. Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology 149: 1049–1055, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Augustine RA, Ladyman SR, Grattan DR. From feeding one to feeding many: hormone-induced changes in bodyweight homeostasis during pregnancy. J Physiol 586: 387–397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin SB, Ziyadeh NJ, Forman S, Prokop LA, Keliher A, Jacobs D. Screening high school students for eating disorders: results of a national initiative. Prev Chronic Dis 5: A114, 2008 [PMC free article] [PubMed] [Google Scholar]
- 28.Aydin ZD. Determinants of age at natural menopause in the Isparta Menopause and Health Study: premenopausal body mass index gain rate and episodic weight loss. Menopause 17: 494–505, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Babbs RK, Wojnicki FH, Corwin RL. Effect of 2-hydroxyestradiol on binge intake in rats. Physiol Behav 103: 508–512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker JH, Girdler SS, Bulik CM. The role of reproductive hormones in the development and maintenance of eating disorders. Expert Rev Obstet Gynecol 7: 573–583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker JH, Maes HH, Lissner L, Aggen SH, Lichtenstein P, Kendler KS. Genetic risk factors for disordered eating in adolescent males and females. J Abnorm Psychol 118: 576–586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol 29: 1–16, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9: 220–226, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci 22: 9104–9112, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 191: 439–459, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 65: 137–145, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr 61: 39–43, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab 14: 289–299, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Bartness TJ, Keen-Rhinehart E, Dailey MJ, Teubner BJ. Neural and hormonal control of food hoarding. Am J Physiol Regul Integr Comp Physiol 301: R641–R655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartness TJ, Waldbillig RJ. Dietary self-selection in intact, ovariectomized, and estradiol-treated female rats. Behav Neurosci 98: 125–137, 1984 [DOI] [PubMed] [Google Scholar]
- 43.Bartoshuk LM. The psychophysics of taste. Am J Clin Nutr 31: 1068–1077, 1978 [DOI] [PubMed] [Google Scholar]
- 44.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav 82: 109–114, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci 361: 1137–1148, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav 56: 1165–1171, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Beal VA. Nutritional studies during pregnancy. I. Changes in intakes of calories, carbohydrates, fat, protein, and calcium. J Am Diet Assoc 58: 312–320, 1971 [PubMed] [Google Scholar]
- 48.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol 29: 36–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ 3: 14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35: 565–572, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beglinger C, Degen L. Fat in the intestine as a regulator of appetite—role of CCK. Physiol Behav 83: 617–621, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Beglinger C, Degen L. Gastrointestinal satiety signals in humans—physiological roles for GLP-1 and PYY? Physiol Behav 89: 460–464, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Begriche K, Sutton GM, Butler AA. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiol Behav 104: 546–554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, Nieschlag E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 15: 2478–2482, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Bell DD, Zucker I. Sex differences in body weight and eating: organization and activation by gonadal hormones in the rat. Physiol Behav 7: 27–34, 1971 [DOI] [PubMed] [Google Scholar]
- 57.Bello NT, Patinkin ZW, Moran TH. Opioidergic consequences of dietary-induced binge eating. Physiol Behav 104: 98–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belzer LM, Smulian JC, Lu SE, Tepper BJ. Food cravings and intake of sweet foods in healthy pregnancy and mild gestational diabetes mellitus. A prospective study. Appetite 55: 609–615, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 93: 1339–1344, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Bennink R, Peeters M, Van den Maegdenbergh V, Geypens B, Rutgeerts P, De Roo M, Mortelmans L. Comparison of total and compartmental gastric emptying and antral motility between healthy men and women. Eur J Nucl Med 25: 1293–1299, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Berbesque JC, Marlowe FW. Sex differences in food preferences of Hazda hunter-gatherers. Evol Psychol 7: 601–616, 2009 [Google Scholar]
- 62.Berg KC, Crosby RD, Cao L, Peterson CB, Engel SG, Mitchell JE, Wonderlich SA. Facets of negative affect prior to and following binge-only, purge-only, and binge/purge events in women with bulimia nervosa. J Abnorm Psychol 122: 111–118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergman L, Eriksson E. Marked symptom reduction in two women with bulimia nervosa treated with the testosterone receptor antagonist flutamide. Acta Psychiatr Scand 94: 137–139, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Berkley KJ. Vive la difference! Trends Neurosci 15: 331–332, 1992 [DOI] [PubMed] [Google Scholar]
- 65.Bernstein IL. Taste aversion learning: a contemporary perspective. Nutrition 15: 229–234, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 199: 457–480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 26: 393–428, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity Am J Physiol Regul Integr Comp Physiol 300: R1266–R1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berthoud HR, Munzberg H, Richards BK, Morrison CD. Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc 71: 390–400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bielert C, Busse C. Influences of ovarian hormones on the food intake and feeding of captive and wild female chacma baboons (Papio ursinus). Physiol Behav 30: 103–111, 1983 [DOI] [PubMed] [Google Scholar]
- 71.Black AE, Wiles SJ, Paul AA. The nutrient intakes of pregnant and lactating mothers of good socio-economic status in Cambridge, UK: some implications for recommended daily allowances of minor nutrients. Br J Nutr 56: 59–72, 1986 [DOI] [PubMed] [Google Scholar]
- 72.Blandau JR, Boling JL, Young WG. The length of heat in the albino rat as determined by the copulatory response. Anat Rec 79: 453–463, 1941 [Google Scholar]
- 73.Blaustein JD. Animals have a sex, and so should titles and methods sections of articles in endocrinology. Endocrinology 153: 2539–2540, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Blaustein JD. An estrogen by any other name. Endocrinology 149: 2697–2698, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Blaustein JD, Gentry RT, Roy EJ, Wade GN. Effects of ovariectomy and estradiol on body weight and food intake in gold thioglucose-treated mice. Physiol Behav 17: 1027–1030, 1976 [DOI] [PubMed] [Google Scholar]
- 76.Blaustein JD, Wade GN. Ovarian hormones and meal patterns in rats: effects of progesterone and role of gastrointestinal transit. Physiol Behav 19: 23–27, 1977 [DOI] [PubMed] [Google Scholar]
- 77.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav 17: 201–208, 1976 [DOI] [PubMed] [Google Scholar]
- 78.Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am J Clin Nutr 89: 372–381, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci 113: 1080–1089, 1999 [PubMed] [Google Scholar]
- 80.Bowen DJ. Possible explanations for excess weight gains in pregnancy: an animal model. Physiol Behav 46: 935–939, 1989 [DOI] [PubMed] [Google Scholar]
- 81.Bowen DJ. Taste and food preference changes across the course of pregnancy. Appetite 19: 233–242, 1992 [DOI] [PubMed] [Google Scholar]
- 82.Bowen DJ, Grunberg NE. Variations in food preference and consumption across the menstrual cycle. Physiol Behav 47: 287–291, 1990 [DOI] [PubMed] [Google Scholar]
- 83.Brawer JR, Beaudet A, Desjardins GC, Schipper HM. Pathologic effect of estradiol on the hypothalamus. Biol Reprod 49: 647–652, 1993 [DOI] [PubMed] [Google Scholar]
- 84.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 89: 2583–2589, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, Wishart JM, Jones KL, Horowitz M, Feinle-Bisset C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol 297: G602–G610, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Broberg DJ, Dorsa DM, Bernstein IL. Nausea in bulimic women in response to a palatable food. J Abnorm Psychol 99: 183–188, 1990 [DOI] [PubMed] [Google Scholar]
- 87.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA 95: 15043–15048, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci 31: 5574–5578, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Broft AI, Berner LA, Martinez D, Walsh BT. Bulimia nervosa and evidence for striatal dopamine dysregulation: a conceptual review. Physiol Behav 104: 122–127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown-Grant K, Exley D, Naftolin F. Peripheral plasma oestradiol and luteinizing hormone concentrations during the oestrous cycle of the rat. J Endocrinol 48: 295–296, 1970 [DOI] [PubMed] [Google Scholar]
- 91.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000 [DOI] [PubMed] [Google Scholar]
- 92.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav 58: 1067–1077, 1995 [DOI] [PubMed] [Google Scholar]
- 93.Bulik CM, Sullivan PF, Kendler KS. Genetic and environmental contributions to obesity and binge eating. Int J Eat Disord 33: 293–298, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biol Psychiatry 44: 1210–1218, 1998 [DOI] [PubMed] [Google Scholar]
- 95.Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry 63: 305–312, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: a review. Int J Eat Disord 27: 1–20, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Bulun SE, Adashi EY. The physiology and pathophysiology of the female reproductive tract. In: Williams Textbook of Endocrinology (10th ed.), edited by Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. Philadelphia, PA: Elsevier/Saunders, 2003, p. 587–664 [Google Scholar]
- 98.Burger H. The menopausal transition—endocrinology. J Sex Med 5: 2266–2273, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause 15: 603–612, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Burton-Freeman B, Davis PA, Schneeman BO. Interaction of fat availability and sex on postprandial satiety and cholecystokinin after mixed-food meals. Am J Clin Nutr 80: 1207–1214, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 94: 1704–1708, 1974 [DOI] [PubMed] [Google Scholar]
- 102.Butera PC. Estradiol and the control of food intake. Physiol Behav 99: 175–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butera PC, Beikirch RJ. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res 491: 266–273, 1989 [DOI] [PubMed] [Google Scholar]
- 104.Butera PC, Beikirch RJ, Willard DM. Changes in ingestive behaviors and body weight following intracranial application of 17α-estradiol. Physiol Behav 47: 1291–1293, 1990 [DOI] [PubMed] [Google Scholar]
- 105.Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav 53: 1235–1238, 1993 [DOI] [PubMed] [Google Scholar]
- 106.Butera PC, Willard DM, Raymond SA. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Res 576: 304–310, 1992 [DOI] [PubMed] [Google Scholar]
- 107.Butera PC, Wojcik DM, Clough SJ. Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physiol Behav 99: 142–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Butera PC, Xiong M, Davis RJ, Platania SP. Central implants of dilute estradiol enhance the satiety effect of CCK-8. Behav Neurosci 110: 823–830, 1996 [DOI] [PubMed] [Google Scholar]
- 109.Cahill L. A half-truth is a whole lie: on the necessity of investigating sex influences on the brain. Endocrinology 153: 2541–2543, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci 7: 477–484, 2006 [DOI] [PubMed] [Google Scholar]
- 111.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev 13: 43–56, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL. Influence of BMI and gender on postprandial hormone responses. Obesity (Silver Spring) 15: 2974–2983, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Casper RC, Sullivan EL, Tecott L. Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl) 199: 313–329, 2008 [DOI] [PubMed] [Google Scholar]
- 114.Cecchini DJ, Chattoraj SC, Fanous AS, Panda SK, Brennan TF, Edelin KC. Radioimmunoassay of 2-hydroxyestrone in plasma during the estrous cycle of the rat: interrelationships with estradiol, progesterone, and the gonadotropins. Endocrinology 112: 1122–1126, 1983 [DOI] [PubMed] [Google Scholar]
- 115.Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol Regul Integr Comp Physiol 276: R1366–R1373, 1999 [DOI] [PubMed] [Google Scholar]
- 116.Chambers KC. Hormonal influences on sexual dimorphism in rate of extinction of a conditioned taste aversion in rats. J Comp Physiol Psychol 90: 851–856, 1976 [DOI] [PubMed] [Google Scholar]
- 117.Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology 143: 1637–1642, 2002 [DOI] [PubMed] [Google Scholar]
- 118.Chen H, Zirkin BR. Long-term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc Natl Acad Sci USA 96: 14877–14881, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen P, Williams SM, Grove KL, Smith MS. Melanocortin 4 receptor-mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. J Neurosci 24: 5091–5100, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol Gastrointest Liver Physiol 268: G171–G176, 1995 [DOI] [PubMed] [Google Scholar]
- 121.Chen TS, Doong ML, Wang SW, Tsai SC, Lu CC, Shih HC, Chen YH, Chang FY, Lee SD, Wang PS. Gastric emptying and gastrointestinal transit during lactation in rats. Am J Physiol Gastrointest Liver Physiol 272: G626–G631, 1997 [DOI] [PubMed] [Google Scholar]
- 122.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet 8: e1002709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen X, McClusky R, Itoh Y, Reue K, Arnold AP. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 154: 1092–1104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol Endocrinol Metab 280: E315–E322, 2001 [DOI] [PubMed] [Google Scholar]
- 125.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol 213: 610–617, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN. Sex and sodium intake in the rat. Behav Neurosci 106: 172–180, 1992 [DOI] [PubMed] [Google Scholar]
- 127.Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty A, Ang BT, Chan L, Fraley GS. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 95: 305–316, 2012 [DOI] [PubMed] [Google Scholar]
- 128.Clarke TK, Weiss AR, Berrettini WH. The genetics of anorexia nervosa. Clin Pharmacol Ther 91: 181–188, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55: 978–987, 2006 [DOI] [PubMed] [Google Scholar]
- 130.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56: 1051–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 52: 682–687, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Cohen IT, Sherwin BB, Fleming AS. Food cravings, mood, and the menstrual cycle. Horm Behav 21: 457–470, 1987 [DOI] [PubMed] [Google Scholar]
- 133.Cohen LR, Woodside BC. Self-selection of protein during pregnancy and lactation in rats. Appetite 12: 119–136, 1989 [DOI] [PubMed] [Google Scholar]
- 134.Cone RD, Low MJ, Elmquist JE, Cameron JL. Neuroendocrinology. In: William's Textbook of Endocrinology (10th ed.), edited by Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. Philadephia, PA: Elsevier/Saunders, 2003, p. 81–176 [Google Scholar]
- 135.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav 99: 538–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corp ES, McQuade J, Moran TH, Smith GP. Characterization of type A and type B CCK receptor binding sites in rat vagus nerve. Brain Res 623: 161–166, 1993 [DOI] [PubMed] [Google Scholar]
- 137.Corsica JA, Spring BJ. Carbohydrate craving: a double-blind, placebo-controlled test of the self-medication hypothesis. Eat Behav 9: 447–454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav 104: 87–97, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav 82: 123–130, 2004 [DOI] [PubMed] [Google Scholar]
- 140.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20: 358–417, 1999 [DOI] [PubMed] [Google Scholar]
- 141.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70, 2009 [DOI] [PubMed] [Google Scholar]
- 142.Craig AD. A rat is not a monkey is not a human: comment on Mogil [Nature Rev. Neurosci 10, 283–294 (2009)] Nat Rev Neurosci 10: 466, 2009 [DOI] [PubMed] [Google Scholar]
- 143.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann NY Acad Sci 1225: 72–82, 2011 [DOI] [PubMed] [Google Scholar]
- 144.Crane JM, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J Obstet Gynaecol Can 31: 28–35, 2009 [DOI] [PubMed] [Google Scholar]
- 145.Croll J, Neumark-Sztainer D, Story M, Ireland M. Prevalence and risk and protective factors related to disordered eating behaviors among adolescents: relationship to gender and ethnicity. J Adolesc Health 31: 166–175, 2002 [DOI] [PubMed] [Google Scholar]
- 146.Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry 65: 329–336, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Culbert KM, Breedlove SM, Sisk CL, Burt A, Klump KL. The emergence of sex differences in risk for disordered eating attitudes during puberty: A role for prenatal testosterone exposure. J Abnorm Psychol 122: 420–432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. J Abnorm Psychol 118: 788–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Culbert KM, Racine SE, Klump KL. The influence of gender and puberty on the heritability of disordered eating symptoms. Curr Top Behav Neurosci 6: 177–185, 2011 [DOI] [PubMed] [Google Scholar]
- 150.Currie PJ, Braver M, Mirza A, Sricharoon K. Sex differences in the reversal of fluoxetine-induced anorexia following raphe injections of 8-OH-DPAT. Psychopharmacology (Berl) 172: 359–364, 2004 [DOI] [PubMed] [Google Scholar]
- 151.Curtis KS. Estrogen and the central control of body fluid balance. Physiol Behav 97: 180–192, 2009 [DOI] [PubMed] [Google Scholar]
- 152.Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav 80: 657–664, 2004 [DOI] [PubMed] [Google Scholar]
- 153.Czaja JA. Food rejection by female rhesus monkeys during the menstrual cycle and early pregnancy. Physiol Behav 14: 579–587, 1975 [DOI] [PubMed] [Google Scholar]
- 154.Czaja JA. Ovarian influences on primate food intake: assessment of progesterone actions. Physiol Behav 21: 923–928, 1978 [DOI] [PubMed] [Google Scholar]
- 155.Czaja JA, Butera PC, McCaffrey TA. Independent effects of estradiol on water and food intake. Behav Neurosci 97: 210–220, 1983 [DOI] [PubMed] [Google Scholar]
- 156.Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav 6: 329–349, 1975 [DOI] [PubMed] [Google Scholar]
- 157.Dafopoulos K, Sourlas D, Kallitsaris A, Pournaras S, Messinis IE. Blood ghrelin, resistin, and adiponectin concentrations during the normal menstrual cycle. Fertil Steril 92: 1389–1394, 2009 [DOI] [PubMed] [Google Scholar]
- 158.Dagnault A, Richard D. Involvement of the medial preoptic area in the anorectic action of estrogens. Am J Physiol Regul Integr Comp Physiol 272: R311–R317, 1997 [DOI] [PubMed] [Google Scholar]
- 159.Dagnault A, Richard D. Lesions of hypothalamic paraventricular nuclei do not prevent the effect of estradiol on energy and fat balance. Am J Physiol Endocrinol Metab 267: E32–E38, 1994 [DOI] [PubMed] [Google Scholar]
- 160.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav 97: 229–238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dandona P, Dhindsa S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab 96: 2643–2651, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 141: 4295–4308, 2000 [DOI] [PubMed] [Google Scholar]
- 163.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med 28: 1204–1207, 1987 [PubMed] [Google Scholar]
- 164.Davis C, Levitan RD, Yilmaz Z, Kaplan AS, Carter JC, Kennedy JL. Binge eating disorder and the dopamine D2 receptor: genotypes and sub-phenotypes. Prog Neuropsychopharmacol Biol Psychiatry 38: 328–335, 2012 [DOI] [PubMed] [Google Scholar]
- 165.Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, King N, Curtis C, Kennedy JL. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 17: 1220–1225, 2009 [DOI] [PubMed] [Google Scholar]
- 166.Davis JD, Smith GP, Miesner J. Postpyloric stimuli are necessary for the normal control of meal size in real feeding and sham feeding rats. Am J Physiol Regul Integr Comp Physiol 265: R888–R895, 1993 [DOI] [PubMed] [Google Scholar]
- 167.Davis JD, Smith GP, Sayler JL. Reduction of intake in the rat due to gastric filling. Am J Physiol Regul Integr Comp Physiol 272: R1599–R1605, 1997 [DOI] [PubMed] [Google Scholar]
- 168.Davis PG, Krieger MS, Barfield RJ, McEwen BS, Pfaff DW. The site of action of intrahypothalamic estrogen implants in feminine sexual behavior: an autoradiographic analysis. Endocrinology 111: 1581–1586, 1982 [DOI] [PubMed] [Google Scholar]
- 169.Davis PG, McEwen BS, Pfaff DW. Localized behavioral effects of tritiated estradiol implants in the ventromedial hypothalamus of female rats. Endocrinology 104: 898–903, 1979 [DOI] [PubMed] [Google Scholar]
- 170.Davy BM, Van Walleghen EL, Orr JS. Sex differences in acute energy intake regulation. Appetite 49: 141–147, 2007 [DOI] [PubMed] [Google Scholar]
- 171.Davy SR, Benes BA, Driskell JA. Sex differences in dieting trends, eating habits, and nutrition beliefs of a group of midwestern college students. J Am Diet Assoc 106: 1673–1677, 2006 [DOI] [PubMed] [Google Scholar]
- 172.de Castro JM, Kreitzman SM. A microregulatory analysis of spontaneous human feeding patterns. Physiol Behav 35: 329–335, 1985 [DOI] [PubMed] [Google Scholar]
- 173.De Silva A, Salem V, Matthews PM, Dhillo WS. The use of functional MRI to study appetite control in the CNS. Exp Diabetes Res 2012: 764017, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22: 9005–9014, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dedes I. Kisspeptins and the control of gonadotrophin secretion. Syst Biol Reprod Med 58: 121–128, 2012 [DOI] [PubMed] [Google Scholar]
- 176.Del Parigi A, Chen K, Gautier JF, Salbe AD, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr 75: 1017–1022, 2002 [DOI] [PubMed] [Google Scholar]
- 177.Dempfle A, Hinney A, Heinzel-Gutenbrunner M, Raab M, Geller F, Gudermann T, Schafer H, Hebebrand J. Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J Med Genet 41: 795–800, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest 116: 561–570, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Desjardins GC, Brawer JR, Beaudet A. Estradiol is selectively neurotoxic to hypothalamic β-endorphin neurons. Endocrinology 132: 86–93, 1993 [DOI] [PubMed] [Google Scholar]
- 180.Devlin MJ, Walsh BT, Guss JL, Kissileff HR, Liddle RA, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr 65: 114–120, 1997 [DOI] [PubMed] [Google Scholar]
- 181.Di Lorenzo PM, Monroe S. Taste responses in the parabrachial pons of male, female and pregnant rats. Brain Res Bull 23: 219–227, 1989 [DOI] [PubMed] [Google Scholar]
- 182.Di Lorenzo PM, Monroe S. Taste responses in the parabrachial pons of ovariectomized rats. Brain Res Bull 25: 741–748, 1990 [DOI] [PubMed] [Google Scholar]
- 183.DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 15: 1330–1335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dimarco NM, Dart L, Sanborn CB. Modified activity-stress paradigm in an animal model of the female athlete triad. J Appl Physiol 103: 1469–1478, 2007 [DOI] [PubMed] [Google Scholar]
- 185.Dixon DP, Ackert AM, Eckel LA. Development of, and recovery from, activity-based anorexia in female rats. Physiol Behav 80: 273–279, 2003 [DOI] [PubMed] [Google Scholar]
- 186.Doerries LE, Stanley EZ, Aravich PF. Activity-based anorexia: relationship to gender and activity-stress ulcers. Physiol Behav 50: 945–949, 1991 [DOI] [PubMed] [Google Scholar]
- 187.Donato J, Jr, Cravo RM, Frazao R, Elias CF. Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendocrinology 93: 9–18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dovey TM, Staples PA, Gibson EL, Halford JC. Food neophobia and ‘picky/fussy’ eating in children: a review. Appetite 50: 181–193, 2008 [DOI] [PubMed] [Google Scholar]
- 189.Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol 299: 32–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Drewett RF. The meal patterns of the oestrous cycle and their motivational significance. Q J Exp Psychol 26: 489–494, 1974 [DOI] [PubMed] [Google Scholar]
- 191.Drewett RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav 21: 772–780, 1973 [DOI] [PubMed] [Google Scholar]
- 192.Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr 61: 1206–1212, 1995 [DOI] [PubMed] [Google Scholar]
- 193.Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav 51: 371–379, 1992 [DOI] [PubMed] [Google Scholar]
- 194.Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: carbohydrates versus fats. Appetite 18: 207–221, 1992 [DOI] [PubMed] [Google Scholar]
- 195.Duffey KJ, Popkin BM. Energy density, portion size, and eating occasions: contributions to increased energy intake in the United States, 1977–2006. PLoS Med 8: e1001050, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Duffy VB, Bartoshuk LM, Striegel-Moore R, Rodin J. Taste changes across pregnancy. Ann NY Acad Sci 855: 805–809, 1998 [DOI] [PubMed] [Google Scholar]
- 197.Dufour DL, Sauther ML. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. Am J Hum Biol 14: 584–602, 2002 [DOI] [PubMed] [Google Scholar]
- 198.Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod 12: 1142–1151, 1997 [DOI] [PubMed] [Google Scholar]
- 199.Earley CJ, Leonard BE. Androgens, estrogens and their anti-hormones: effects on body weight and food consumption. Pharmacol Biochem Behav 11: 211–214, 1979 [DOI] [PubMed] [Google Scholar]
- 200.Eastwood H, Brown KM, Markovic D, Pieri LF. Variation in the ESR1 and ESR2 genes and genetic susceptibility to anorexia nervosa. Mol Psychiatry 7: 86–89, 2002 [DOI] [PubMed] [Google Scholar]
- 201.Eck LH, Bennett AG, Egan BM, Ray JW, Mitchell CO, Smith MA, Klesges RC. Differences in macronutrient selections in users and nonusers of an oral contraceptive. Am J Clin Nutr 65: 419–424, 1997 [DOI] [PubMed] [Google Scholar]
- 202.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav 82: 35–41, 2004 [DOI] [PubMed] [Google Scholar]
- 203.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 104: 517–524, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Eckel LA, Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides 20: 451–456, 1999 [DOI] [PubMed] [Google Scholar]
- 205.Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 281: R738–R746, 2001 [DOI] [PubMed] [Google Scholar]
- 206.Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 283: R1378–R1385, 2002 [DOI] [PubMed] [Google Scholar]
- 207.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav 70: 397–405, 2000 [DOI] [PubMed] [Google Scholar]
- 208.Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol Regul Integr Comp Physiol 275: R186–R193, 1998 [DOI] [PubMed] [Google Scholar]
- 209.Eckel LA, Rivera HM, Atchley DP. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am J Physiol Regul Integr Comp Physiol 288: R1486–R1491, 2005 [DOI] [PubMed] [Google Scholar]
- 210.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med 37: 131–141, 2007 [DOI] [PubMed] [Google Scholar]
- 211.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 352: 1223–1236, 2005 [DOI] [PubMed] [Google Scholar]
- 212.Eng R, Gold RM, Wade GN. Ovariectomy-induced obesity is not prevented by subdiaphragmatic vagotomy in rats. Physiol Behav 22: 353–356, 1979 [DOI] [PubMed] [Google Scholar]
- 213.Essick GK, Chopra A, Guest S, McGlone F. Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol Behav 80: 289–302, 2003 [DOI] [PubMed] [Google Scholar]
- 214.Faas MM, Melgert BN, de Vos P. A brief review on how pregnancy and sex hormones interfere with taste and food intake. Chemosens Percept 3: 51–56, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fan W, Yanase T, Nishi Y, Chiba S, Okabe T, Nomura M, Yoshimatsu H, Kato S, Takayanagi R, Nawata H. Functional potentiation of leptin-signal transducer and activator of transcription 3 signaling by the androgen receptor. Endocrinology 149: 6028–6036, 2008 [DOI] [PubMed] [Google Scholar]
- 216.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 54: 1000–1008, 2005 [DOI] [PubMed] [Google Scholar]
- 217.Fantino M, Brinnel H. Body weight set-point changes during the ovarian cycle: experimental study of rats using hoarding behavior. Physiol Behav 36: 991–996, 1986 [DOI] [PubMed] [Google Scholar]
- 218.Farooqi IS, O'Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr 89: 980S–984S, 2009 [DOI] [PubMed] [Google Scholar]
- 219.Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106: 271–279, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87: 589–598, 2002 [DOI] [PubMed] [Google Scholar]
- 221.Ferin M, Tempone A, Zimmering PE, Van de Wiele RL. Effect of antibodies to 17β-estradiol and progesterone on the estrous cycle of the rat. Endocrinology 85: 1070–1078, 1969 [DOI] [PubMed] [Google Scholar]
- 222.Fessler DM. No time to eat: an adaptationist account of periovulatory behavioral changes. Q Rev Biol 78: 3–21, 2003 [DOI] [PubMed] [Google Scholar]
- 223.Field AE, Colditz GA, Peterson KE. Racial/ethnic and gender differences in concern with weight and in bulimic behaviors among adolescents. Obes Res 5: 447–454, 1997 [DOI] [PubMed] [Google Scholar]
- 224.Findlay AL, Fitzsimons JT, Kucharczyk J. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol 82: 215–225, 1979 [DOI] [PubMed] [Google Scholar]
- 225.Fischer S, Smith GT, Cyders MA. Another look at impulsivity: a meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clin Psychol Rev 28: 1413–1425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241, 2010 [DOI] [PubMed] [Google Scholar]
- 227.Fleming AS. Ovarian influences on food intake and body weight regulation in lactating rats. Physiol Behav 17: 969–978, 1976 [DOI] [PubMed] [Google Scholar]
- 228.Foltin RW, Woolverton WL, Schuster CR. Effects of psychomotor stimulants, alone and in pairs, on milk drinking in the rat after intraperitoneal and intragastric administration. J Pharmacol Exp Ther 226: 411–418, 1983 [PubMed] [Google Scholar]
- 229.Fong AK, Kretsch MJ. Changes in dietary intake, urinary nitrogen, and urinary volume across the menstrual cycle. Am J Clin Nutr 57: 43–46, 1993 [DOI] [PubMed] [Google Scholar]
- 230.Frank GK, Kaye WH. Current status of functional imaging in eating disorders. Int J Eat Disord 45: 723–736, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, Fritsche A, Preissl H. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res 1350: 159–166, 2010 [DOI] [PubMed] [Google Scholar]
- 232.Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 1363: 81–92, 2010 [DOI] [PubMed] [Google Scholar]
- 233.Frazao R, Cravo RM, Donato J, Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor alpha. J Neurosci 33: 2807–2820, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Freedman LS, Guenther PM, Krebs-Smith SM, Dodd KW, Midthune D. A population's distribution of Healthy Eating Index-2005 component scores can be estimated when more than one 24-hour recall is available. J Nutr 140: 1529–1534, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 97: 1673–1680, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Freeman ME. Neuroendocrine control of the ovarian cycle of the rat. In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JS. Amsterdam, The Netherlands: Elsevier/Academic, 2006, p. 2327–2388 [Google Scholar]
- 237.Fregly MJ. Effect of chronic treatment with estrogen on the dipsogenic response of rats to angiotensin. Pharmacol Biochem Behav 12: 131–136, 1980 [DOI] [PubMed] [Google Scholar]
- 238.French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 59: 541–549, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr 89: 973S–979S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Frye CA. Neurosteroids' effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology 34 Suppl 1: S143–S161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Frye CA, Crystal S, Ward KD, Kanarek RB. Menstrual cycle and dietary restraint influence taste preferences in young women. Physiol Behav 55: 561–567, 1994 [DOI] [PubMed] [Google Scholar]
- 242.Frye CA, Paris JJ. Progesterone turnover to its 5α-reduced metabolites in the ventral tegmental area of the midbrain is essential for initiating social and affective behavior and progesterone metabolism in female rats. J Endocrinol Invest 34: e188–e199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 30: 10205–10219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Furneaux EC, Langley-Evans AJ, Langley-Evans SC. Nausea and vomiting of pregnancy: endocrine basis and contribution to pregnancy outcome. Obstet Gynecol Surv 56: 775–782, 2001 [DOI] [PubMed] [Google Scholar]
- 245.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 143: 228–239, 1996 [DOI] [PubMed] [Google Scholar]
- 246.Gambineri A, Pagotto U, Tschop M, Vicennati V, Manicardi E, Carcello A, Cacciari M, De Iasio R, Pasquali R. Anti-androgen treatment increases circulating ghrelin levels in obese women with polycystic ovary syndrome. J Endocrinol Invest 26: 629–634, 2003 [DOI] [PubMed] [Google Scholar]
- 247.Gangestad SW, Thornhill R. Human oestrus. Proc Biol Sci 275: 991–1000, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol 292: G725–G733, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Garland HO, Atherton JC, Baylis C, Morgan MR, Milne CM. Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol 113: 435–444, 1987 [DOI] [PubMed] [Google Scholar]
- 250.Geary N. Counterpoint: physiologists should not distinguish “sex” and “gender”. Am J Physiol Regul Integr Comp Physiol 298: R1702–R1704, 2010 [DOI] [PubMed] [Google Scholar]
- 251.Geary N. Curt Richter and the female rat. Appetite 49: 376–387, 2007 [DOI] [PubMed] [Google Scholar]
- 252.Geary N. Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav 81: 719–733, 2004 [DOI] [PubMed] [Google Scholar]
- 253.Geary N. The estrogenic inhibition of eating. In: Handbook of Behavioral Neurobiology (2nd ed.), edited by Stricker EM, Woods SC. New York, NY: Kluwer Academic/Plenum Publishers, 2004, p. 307–345 [Google Scholar]
- 254.Geary N. Sex differences in disease anorexia. Nutrition 17: 499–507, 2001 [DOI] [PubMed] [Google Scholar]
- 255.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav 67: 141–147, 1999 [DOI] [PubMed] [Google Scholar]
- 256.Geary N, Asarian L. Estradiol increases glucagon's satiating potency in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 281: R1290–R1294, 2001 [DOI] [PubMed] [Google Scholar]
- 257.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142: 4751–4757, 2001 [DOI] [PubMed] [Google Scholar]
- 258.Geary N, Smith GP, Corp ES. The increased satiating potency of CCK-8 by estradiol is not mediated by upregulation of NTS CCK receptors. Brain Res 719: 179–186, 1996 [DOI] [PubMed] [Google Scholar]
- 259.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav 56: 281–289, 1994 [DOI] [PubMed] [Google Scholar]
- 260.Geary N, Trace D, Smith GP. Estradiol interacts with gastric or postgastric food stimuli to decrease sucrose ingestion in ovariectomized rats. Physiol Behav 57: 155–158, 1995 [DOI] [PubMed] [Google Scholar]
- 261.Geiselman PJ, Martin JR, Vanderweele DA, Novin D. Dietary self-selection in cycling and neonatally ovariectomized rats. Appetite 2: 87–101, 1981 [DOI] [PubMed] [Google Scholar]
- 262.Geliebter A, Hashim SA, Gluck ME. Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED). Physiol Behav 94: 696–699, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr 56: 656–661, 1992 [DOI] [PubMed] [Google Scholar]
- 264.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high- vs. low-energy food cues. Behav Brain Res 243: 91–96, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Geliebter A, Yahav EK, Gluck ME, Hashim SA. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav 81: 735–740, 2004 [DOI] [PubMed] [Google Scholar]
- 266.Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol 90: 18–25, 1976 [DOI] [PubMed] [Google Scholar]
- 267.Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J Comp Physiol Psychol 90: 747–754, 1976 [DOI] [PubMed] [Google Scholar]
- 268.George JT, Seminara SB. Kisspeptin and the hypothalamic control of reproduction: lessons from the human. Endocrinology 153: 5130–5136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Geracioti TD, Jr., Liddle RA. Impaired cholecystokinin secretion in bulimia nervosa. N Engl J Med 319: 683–688, 1988 [DOI] [PubMed] [Google Scholar]
- 270.Gill RC, Murphy PD, Hooper HR, Bowes KL, Kingma YJ. Effect of the menstrual cycle on gastric emptying. Digestion 36: 168–174, 1987 [DOI] [PubMed] [Google Scholar]
- 271.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62: 155–198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry 144: 1592–1595, 1987 [DOI] [PubMed] [Google Scholar]
- 273.Glass CM, Haas SA, Reither EN. The skinny on success: body mass, gender and occupational standing across the life course. Soc Forces 88: 1777–1806, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gloy V, Langhans W, Hillebrand JJ, Geary N, Asarian L. Ovariectomy and overeating palatable, energy-dense food increase subcutaneous adipose tissue more than intra-abdominal adipose tissue in rats. Biol Sex Differ 2: 6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gloy VL, Lutz TA, Langhans W, Geary N, Hillebrand JJ. Basal plasma levels of insulin, leptin, ghrelin, and amylin do not signal adiposity in rats recovering from forced overweight. Endocrinology 151: 4280–4288, 2010 [DOI] [PubMed] [Google Scholar]
- 276.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 153: 865–874, 2001 [DOI] [PubMed] [Google Scholar]
- 277.Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 151: 16–21, 1997 [DOI] [PubMed] [Google Scholar]
- 278.Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am J Clin Nutr 49: 252–258, 1989 [DOI] [PubMed] [Google Scholar]
- 279.Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of gonadotropin-releasing hormone 1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory luteinizing hormone surges. Endocrinology 149: 2333–2340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7: 219–231, 2011 [DOI] [PubMed] [Google Scholar]
- 281.Goodin SZ, Kiechler AR, Smith M, Wendt D, Strader AD. Effect of gonadectomy on AgRP-induced weight gain in rats. Am J Physiol Regul Integr Comp Physiol 295: R1747–R1753, 2008 [DOI] [PubMed] [Google Scholar]
- 282.Goodman RL. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology 102: 142–150, 1978 [DOI] [PubMed] [Google Scholar]
- 283.Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology 153: 5105–5118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Grabenhorst F, Rolls ET, Parris BA, d'Souza AA. How the brain represents the reward value of fat in the mouth. Cereb Cortex 20: 1082–1091, 2010 [DOI] [PubMed] [Google Scholar]
- 285.Grattan DR, Ladyman SR, Augustine RA. Hormonal induction of leptin resistance during pregnancy. Physiol Behav 91: 366–374, 2007 [DOI] [PubMed] [Google Scholar]
- 286.Graves NS, Hayes H, Fan L, Curtis KS. Time course of behavioral, physiological, and morphological changes after estradiol treatment of ovariectomized rats. Physiol Behav 103: 261–267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gray JM, Greenwood MR. Time course of effects of ovarian hormones on food intake and metabolism. Am J Physiol Endocrinol Metab 243: E407–E412, 1982 [DOI] [PubMed] [Google Scholar]
- 288.Gray JM, Nunez AA, Siegel LI, Wade GN. Effects of testosterone on body weight and adipose tissue: role of aromatization. Physiol Behav 23: 465–469, 1979 [DOI] [PubMed] [Google Scholar]
- 289.Griffen JE, Ojeda SR. Textbook of Endocrine Physiology (6th ed.) New York, NY: Oxford Universtiy Press, 2012 [Google Scholar]
- 290.Griffin JE, Wilson JD. Disorders of the testes and the male reproductive tract In: Williams Textbook of Endocrinology (10th ed.), edited by Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. Philadelphia, PA: Elsevier/Saunders, 2003, p. 709–769 [Google Scholar]
- 291.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Grilo CM, Masheb RM, Brody M, Burke-Martindale CH, Rothschild BS. Binge eating and self-esteem predict body image dissatisfaction among obese men and women seeking bariatric surgery. Int J Eat Disord 37: 347–351, 2005 [DOI] [PubMed] [Google Scholar]
- 293.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8: 1289–1291, 2005 [DOI] [PubMed] [Google Scholar]
- 294.Grueso E, Rocha M, Puerta M. Plasma and cerebrospinal fluid leptin levels are maintained despite enhanced food intake in progesterone-treated rats. Eur J Endocrinol 144: 659–665, 2001 [DOI] [PubMed] [Google Scholar]
- 295.Gueguen N. The receptivity of women to courtship solicitation across the menstrual cycle: a field experiment. Biol Psychol 80: 321–324, 2009 [DOI] [PubMed] [Google Scholar]
- 296.Guertin TL. Eating behavior of bulimics, self-identified binge eaters, and non-eating-disordered individuals: what differentiates these populations? Clin Psychol Rev 19: 1–23, 1999 [DOI] [PubMed] [Google Scholar]
- 297.Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism 40: 1323–1326, 1991 [DOI] [PubMed] [Google Scholar]
- 298.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104: 288–291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite 57: 421–434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschop MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol 6: 689–697, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Halford JC, Harrold JA. 5-HT(2C) receptor agonists and the control of appetite. Handb Exp Pharmacol 209: 349–356, 2012 [DOI] [PubMed] [Google Scholar]
- 303.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med 25: 344–351, 2007 [DOI] [PubMed] [Google Scholar]
- 304.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 32: 275–282, 2008 [DOI] [PubMed] [Google Scholar]
- 305.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 53: 3024–3029, 2004 [DOI] [PubMed] [Google Scholar]
- 306.Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod 85: 431–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hancock SD, Grant VL. Sexually dimorphic effects of postnatal treatment on the development of activity-based anorexia in adolescent and adult rats. Dev Psychobiol 51: 679–695, 2009 [DOI] [PubMed] [Google Scholar]
- 308.Handelsman DJ. Aging in the hypothalamic-pituitary-testcular axis In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JD. St. Louis, MO: Elsevier, 2006, p. 2697–2728 [Google Scholar]
- 309.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology 143: 4172–4177, 2002 [DOI] [PubMed] [Google Scholar]
- 310.Harris RB, Bartness TJ, Grill HJ. Leptin responsiveness in chronically decerebrate rats. Endocrinology 148: 4623–4633, 2007 [DOI] [PubMed] [Google Scholar]
- 311.Harris RB, Kasser TR, Martin RJ. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. J Nutr 116: 2536–2546, 1986 [DOI] [PubMed] [Google Scholar]
- 312.Hartmann B, Kirchengast S, Albrecht A, Laml T, Bikas D, Huber J. Effects of hormone replacement therapy on growth hormone secretion patterns in correlation to somatometric parameters in healthy postmenopausal women. Maturitas 22: 239–246, 1995 [DOI] [PubMed] [Google Scholar]
- 313.Hashimoto I, Henricks DM, Anderson LL, Melampy RM. Progesterone and pregn-4-en-20 alpha-ol-3-one in ovarian venous blood during various reproductive states in the rat. Endocrinology 82: 333–341, 1968 [DOI] [PubMed] [Google Scholar]
- 314.Hashimoto I, Wiest WG. Correlation of the secretion of ovarian steroids with function of a single generation of corpora lutea in the immature rat. Endocrinology 84: 873–885, 1969 [DOI] [PubMed] [Google Scholar]
- 315.Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: a review. Neurosci Biobehav Rev 35: 1419–1449, 2011 [DOI] [PubMed] [Google Scholar]
- 316.Hayes JE, Duffy VB. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav 95: 77–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hayes JE, Duffy VB. Revisiting sugar-fat mixtures: sweetness and creaminess vary with phenotypic markers of oral sensation. Chem Senses 32: 225–236, 2007 [DOI] [PubMed] [Google Scholar]
- 318.Hayes MR. Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects. Physiol Behav 106: 413–416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Healy K, Conroy RM, Walsh N. The prevalence of binge-eating and bulimia in 1063 college students. J Psychiatr Res 19: 161–166, 1985 [DOI] [PubMed] [Google Scholar]
- 321.Heape W. The “sexual season” of mammals and the relation of the “pre-oestrum” to menstruation. Q J Microscopic Sci 44: 1–70, 1900 [Google Scholar]
- 322.Heatherton TF, Herman CP, Polivy J, King GA, McGree ST. The (mis)measurement of restraint: an analysis of conceptual and psychometric issues. J Abnorm Psychol 97: 19–28, 1988 [DOI] [PubMed] [Google Scholar]
- 323.Heil SH. Activational and organizational actions of gonadal hormones and the sex-specific effects of prolactin on food intake by rats. Dev Psychobiol 35: 61–67, 1999 [PubMed] [Google Scholar]
- 324.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Heisler LK, Kanarek RB, Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol Biochem Behav 63: 377–385, 1999 [DOI] [PubMed] [Google Scholar]
- 326.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007 [DOI] [PubMed] [Google Scholar]
- 327.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol 167: 1287–1294, 2008 [DOI] [PubMed] [Google Scholar]
- 328.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol 84: 66–72, 1975 [PubMed] [Google Scholar]
- 329.Hilbert A, de Zwaan M, Braehler E. How frequent are eating disturbances in the population? Norms of the eating disorder examination-questionnaire. PLoS One 7: e29125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294: E827–E832, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hillebrand JJ, Geary N. Do leptin and insulin signal adiposity? Forum Nutr 63: 111–122, 2010 [DOI] [PubMed] [Google Scholar]
- 332.Himmerich H, Schonknecht P, Heitmann S, Sheldrick AJ. Laboratory parameters and appetite regulators in patients with anorexia nervosa. J Psychiatr Pract 16: 82–92, 2010 [DOI] [PubMed] [Google Scholar]
- 333.Hintiryan H, Foster NN, Chambers KC. Dissociating the conditioning and the anorectic effects of estradiol in female rats. Behav Neurosci 123: 1226–1237, 2009 [DOI] [PubMed] [Google Scholar]
- 334.Hirschberg AL. Sex hormones, appetite and eating behaviour in women. Maturitas 71: 248–256, 2012 [DOI] [PubMed] [Google Scholar]
- 335.Hirschberg AL, Naessen S, Stridsberg M, Bystrom B, Holtet J. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol Endocrinol 19: 79–87, 2004 [DOI] [PubMed] [Google Scholar]
- 336.Ho SC, Wu S, Chan SG, Sham A. Menopausal transition and changes of body composition: a prospective study in Chinese perimenopausal women. Int J Obes (Lond) 34: 1265–1274, 2010 [DOI] [PubMed] [Google Scholar]
- 337.Hook EB. Dietary cravings and aversions during pregnancy. Am J Clin Nutr 31: 1355–1362, 1978 [DOI] [PubMed] [Google Scholar]
- 338.Hormes JM, Rozin P. Perimenstrual chocolate craving. What happens after menopause? Appetite 53: 256–259, 2009 [DOI] [PubMed] [Google Scholar]
- 339.Horner KM, Byrne NM, Cleghorn GJ, Naslund E, King NA. The effects of weight loss strategies on gastric emptying and appetite control. Obes Rev 12: 935–951, 2011 [DOI] [PubMed] [Google Scholar]
- 340.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Petrucco OM, Seamark R, Shearman DJ. The normal menstrual cycle has no effect on gastric emptying. Br J Obstet Gynaecol 92: 743–746, 1985 [DOI] [PubMed] [Google Scholar]
- 341.Houpt K. Domestic Animal Behavior for Veterinarians and Animal Scientists. Ames, IA: Blackwell, 2004 [Google Scholar]
- 342.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol 169: 105–112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hrupka BJ, Smith GP, Geary N. Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiol Behav 77: 233–241, 2002 [DOI] [PubMed] [Google Scholar]
- 344.Hrupka BJ, Smith GP, Geary N. Ovariectomy and estradiol affect postingestive controls of sucrose licking. Physiol Behav 61: 243–247, 1997 [DOI] [PubMed] [Google Scholar]
- 345.Huang YS, Doi R, Chowdhury P, Pasley JN, Nishikawa M, Huang TJ, Rayford PL. Effect of cholecystokinin on food intake at different stages of the estrous cycle in female rats. J Assoc Acad Minor Phys 4: 56–58, 1993 [PubMed] [Google Scholar]
- 346.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61: 348–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Hung AJ, Stanbury MG, Shanabrough M, Horvath TL, Garcia-Segura LM, Naftolin F. Estrogen, synaptic plasticity and hypothalamic reproductive aging. Exp Gerontol 38: 53–59, 2003 [DOI] [PubMed] [Google Scholar]
- 348.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997 [DOI] [PubMed] [Google Scholar]
- 349.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 96: 11–17, 1989 [DOI] [PubMed] [Google Scholar]
- 350.Isken F, Pfeiffer AF, Nogueiras R, Osterhoff MA, Ristow M, Thorens B, Tschop MH, Weickert MO. Deficiency of glucose-dependent insulinotropic polypeptide receptor prevents ovariectomy-induced obesity in mice. Am J Physiol Endocrinol Metab 295: E350–E355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Jacobi F, Wittchen HU, Holting C, Hofler M, Pfister H, Muller N, Lieb R. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS). Psychol Med 34: 597–611, 2004 [DOI] [PubMed] [Google Scholar]
- 352.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51: 171–180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH. Energy regulation over the menstrual cycle. Physiol Behav 56: 523–527, 1994 [DOI] [PubMed] [Google Scholar]
- 354.Jonassaint CR, Szatkiewicz JP, Bulik CM, Thornton LM, Bloss C, Berrettini WH, Kaye WH, Bergen AW, Magistretti P, Strober M, Keel PK, Brandt H, Crawford S, Crow S, Fichter MM, Goldman D, Halmi KA, Johnson C, Kaplan AS, Klump KL, La Via M, Mitchell JE, Rotondo A, Treasure J, Woodside DB. Absence of association between specific common variants of the obesity-related FTO gene and psychological and behavioral eating disorder phenotypes. Am J Med Genet B Neuropsychiatr Genet 156B: 454–461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Jones AB, Curtis KS. Differential effects of estradiol on drinking by ovariectomized rats in response to hypertonic NaCl or isoproterenol: Implications for hyper- vs. hypo-osmotic stimuli for water intake. Physiol Behav 98: 421–426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97: 12735–12740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol 203: 525–530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kanarek RB, Marks-Kaufman R, Lipeles BJ. Increased carbohydrate intake as a function of insulin administration in rats. Physiol Behav 25: 779–782, 1980 [DOI] [PubMed] [Google Scholar]
- 359.Kanarek RB, Ryu M, Przypek J. Preferences for foods with varying levels of salt and fat differ as a function of dietary restraint and exercise but not menstrual cycle. Physiol Behav 57: 821–826, 1995 [DOI] [PubMed] [Google Scholar]
- 360.Kaplan JM, Moran TH. Gastrointestinal signaling in the control of food intake. In: Handbook of Behavioral Neurobiology (2nd ed.), edited by Stricker E, Woods SC. New York: Kluwer Academic/Plenum Publishers, 2004, p. 275–306 [Google Scholar]
- 361.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ 3: 13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kas MJ, Adan RA. Animal models of eating disorder traits. Curr Top Behav Neurosci 6: 209–227, 2011 [DOI] [PubMed] [Google Scholar]
- 363.Kas MJ, Kaye WH, Foulds Mathes W, Bulik CM. Interspecies genetics of eating disorder traits. Am J Med Genet B Neuropsychiatr Genet 150B: 318–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Kauffman AS, Rissman EF. Neuroendocrine control of mating-induced ovulation. In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JD. St. Louis, MO: Elsevier/Academic, 2006, p. 2283–2326 [Google Scholar]
- 365.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav 94: 121–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Keating C, Tilbrook A, Kulkarni J. Oestrogen: an overlooked mediator in the neuropsychopharmacology of treatment response? Int J Neuropsychopharmacol 14: 553–566, 2011 [DOI] [PubMed] [Google Scholar]
- 367.Keel PK, Wolfe BE, Liddle RA, De Young KP, Jimerson DC. Clinical features and physiological response to a test meal in purging disorder and bulimia nervosa. Arch Gen Psychiatry 64: 1058–1066, 2007 [DOI] [PubMed] [Google Scholar]
- 368.Keen-Rhinehart E, Desai M, Ross MG. Central insulin sensitivity in male and female juvenile rats. Horm Behav 56: 275–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Kellokoski E, Poykko SM, Karjalainen AH, Ukkola O, Heikkinen J, Kesaniemi YA, Horkko S. Estrogen replacement therapy increases plasma ghrelin levels. J Clin Endocrinol Metab 90: 2954–2963, 2005 [DOI] [PubMed] [Google Scholar]
- 370.Kelly MJ, Moss RL, Dudley CA. The effects of microelectrophoretically applied estrogen, cortisol and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp Brain Res 30: 53–64, 1977 [DOI] [PubMed] [Google Scholar]
- 371.Kelly MJ, Moss RL, Dudley CA, Fawcett CP. The specificity of the response of preoptic-septal area neurons to estrogen: 17α-estradiol versus 17β-estradiol and the response of extrahypothalamic neurons. Exp Brain Res 30: 43–52, 1977 [DOI] [PubMed] [Google Scholar]
- 372.Kemnitz JW, Gibber JR, Lindsay KA, Eisele SG. Effects of ovarian hormones on eating behaviors, body weight, and glucoregulation in rhesus monkeys. Horm Behav 23: 235–250, 1989 [DOI] [PubMed] [Google Scholar]
- 373.Kemnitz JW, Gibber JR, Eisele SG, Lindsay KA. Relationship of reproductive condition to food intake and sucrose consumption of female rhesus monkeys. In: Current Perspectives in Primate Social Dynamics, edited by Taub DM, King FA. New York: Van Nostrand Reinhold, 1986, p. 274–286 [Google Scholar]
- 374.Kemnitz JW, Goy RW, Keesey RE. Effects of gonadectomy on hypothalamic obesity in male and female rats. Int J Obes (Lond) 1: 259–270, 1977 [PubMed] [Google Scholar]
- 375.Kemnitz JW, Sladky KK, Flitsch TJ, Pomerantz SM, Goy RW. Androgenic influences on body size and composition of adult rhesus monkeys. Am J Physiol Endocrinol Metab 255: E857–E864, 1988 [DOI] [PubMed] [Google Scholar]
- 376.Kenney NJ, Mook DG. Effects of ovariectomy on meal pattern in the albino rat. J Comp Physiol Psychol 87: 302–309, 1974 [DOI] [PubMed] [Google Scholar]
- 377.Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol 24: 131–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Killgore WD, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport 21: 354–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.King BM, Rollins BL, Grundmann SJ, Olivier LG. Excessive weight gains in female rats with transections of the stria terminalis. Physiol Behav 78: 563–568, 2003 [DOI] [PubMed] [Google Scholar]
- 380.King BM, Rollins BL, Stines SG, Cassis SA, McGuire HB, Lagarde ML. Sex differences in body weight gains following amygdaloid lesions in rats. Am J Physiol Regul Integr Comp Physiol 277: R975–R980, 1999 [DOI] [PubMed] [Google Scholar]
- 381.King JM. Effects of lesions of the amygdala, preoptic area, and hypothalamus on estradiol-induced activity in the female rat. J Comp Physiol Psychol 93: 360–367, 1979 [DOI] [PubMed] [Google Scholar]
- 382.Kinzl JF, Traweger C, Trefalt E, Mangweth B, Biebl W. Binge eating disorder in females: a population-based investigation. Int J Eat Disord 25: 287–292, 1999 [DOI] [PubMed] [Google Scholar]
- 383.Kirchner H, Heppner KM, Tschop MH. The role of ghrelin in the control of energy balance. Handb Exp Pharmacol: 161–184, 2012 [DOI] [PubMed] [Google Scholar]
- 384.Kisley LR, Sakai RR, Ma LY, Fluharty SJ. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am J Physiol Regul Integr Comp Physiol 276: R90–R96, 1999 [DOI] [PubMed] [Google Scholar]
- 385.Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol 285: R992–R998, 2003 [DOI] [PubMed] [Google Scholar]
- 386.Klingerman CM, Krishnamoorthy K, Patel K, Spiro AB, Struby C, Patel A, Schneider JE. Energetic challenges unmask the role of ovarian hormones in orchestrating ingestive and sex behaviors. Horm Behav 58: 563–574, 2010 [DOI] [PubMed] [Google Scholar]
- 387.Klump KL, Bulik CM, Kaye WH, Treasure J, Tyson E. Academy for eating disorders position paper: eating disorders are serious mental illnesses. Int J Eat Disord 42: 97–103, 2009 [DOI] [PubMed] [Google Scholar]
- 388.Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: evidence for a sex difference. Psychol Med 42: 627–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med 36: 539–546, 2006 [DOI] [PubMed] [Google Scholar]
- 390.Klump KL, Keel PK, Burt SA, Racine SE, Neale MC, Sisk CL, Boker S. Ovarian hormones and emotional eating associations across the menstrual cycle: an examination of the potential moderating effects of body mass index and dietary restraint. Int J Eat Disord 46: 256–263, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med 38: 1749–1757, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol 122: 131–137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Klump KL, Keel PK, Sisk C, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol Med 40: 1745–1753, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in preadolescent and adolescent female twins. J Abnorm Psychol 109: 239–251, 2000 [PubMed] [Google Scholar]
- 395.Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychol Med 31: 737–740, 2001 [DOI] [PubMed] [Google Scholar]
- 396.Klump KL, Racine S, Hildebrandt B, Sisk CL. Sex differences in binge eating patterns in male and female adult rats. Int J Eat Disord In press [DOI] [PubMed] [Google Scholar]
- 397.Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Horm Behav 59: 585–593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL. Binge eating proneness emerges during puberty in female rats: a longitudinal study. J Abnorm Psychol 120: 948–955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, Fisher RS. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol 92: 968–975, 1997 [PubMed] [Google Scholar]
- 400.Knight WD, Seth R, Boron J, Overton JM. Short-term physiological hyperleptinemia decreases arterial blood pressure. Regul Pept 154: 60–68, 2009 [DOI] [PubMed] [Google Scholar]
- 401.Knott CD. Changes in orangutan caloric intake, energy homeostasis and ketones in response to fluctuating fruit availability. Intl. J. Primatol. 19: 1061–1079, 1999 [Google Scholar]
- 402.Kochakian CD, Webster JA. Effect of testosterone propionate on the appetite, body weight and composition of the normal rat. Endocrinology 63: 737–742, 1958 [DOI] [PubMed] [Google Scholar]
- 403.Koscik T, Bechara A, Tranel D. Sex-related functional asymmetry in the limbic brain. Neuropsychopharmacology 35: 340–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior—facilitating genomic actions. Proc Natl Acad Sci USA 101: 12354–12357, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite 7: 1–17, 1986 [DOI] [PubMed] [Google Scholar]
- 406.Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav 79: 267–274, 2003 [DOI] [PubMed] [Google Scholar]
- 407.Kriegsfeld LJ, Gibson EM, Williams WP, 3rd, Zhao S, Mason AO, Bentley GE, Tsutsui K. The roles of RFamide-related peptide-3 in mammalian reproductive function and behaviour. J Neuroendocrinol 22: 692–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103: 2410–2415, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6: 691–702, 2005 [DOI] [PubMed] [Google Scholar]
- 410.Kringelbach ML, Stein A. Cortical mechanisms of human eating. Forum Nutr 63: 164–175, 2010 [DOI] [PubMed] [Google Scholar]
- 411.Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24: 152–182, 2003 [DOI] [PubMed] [Google Scholar]
- 412.Ladyman SR, Augustine RA, Grattan DR. Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol 22: 805–817, 2010 [DOI] [PubMed] [Google Scholar]
- 413.Ladyman SR, Tups A, Augustine RA, Swahn-Azavedo A, Kokay IC, Grattan DR. Loss of hypothalamic response to leptin during pregnancy associated with development of melanocortin resistance. J Neuroendocrinol 21: 449–456, 2009 [DOI] [PubMed] [Google Scholar]
- 414.Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav 97: 84–91, 2010 [DOI] [PubMed] [Google Scholar]
- 415.Landt M, Gingerich RL, Havel PJ, Mueller WM, Schoner B, Hale JE, Heiman ML. Radioimmunoassay of rat leptin: sexual dimorphism reversed from humans. Clin Chem 44: 565–570, 1998 [PubMed] [Google Scholar]
- 416.Langhans W, Geary N. Overview of the physiological control of eating. Forum Nutr 63: 9–53, 2010 [DOI] [PubMed] [Google Scholar]
- 417.Larner JM, Hochberg RB. The clearance and metabolism of estradiol and estradiol-17-esters in the rat. Endocrinology 117: 1209–1214, 1985 [DOI] [PubMed] [Google Scholar]
- 418.Larsson H, Elmstahl S, Berglund G, Ahren B. Evidence for leptin regulation of food intake in humans. J Clin Endocrinol Metab 83: 4382–4385, 1998 [DOI] [PubMed] [Google Scholar]
- 419.Lee CC, Kasa-Vubu JZ, Supiano MA. Differential effects of raloxifene and estrogen on insulin sensitivity in postmenopausal women. J Am Geriatr Soc 51: 683–688, 2003 [DOI] [PubMed] [Google Scholar]
- 420.Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E, Dowsett M, Grady D, Cummings SR. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab 91: 3791–3797, 2006 [DOI] [PubMed] [Google Scholar]
- 421.Lee TM, McClintock MK. Female rats in a laboratory display seasonal variation in fecundity. J Reprod Fertil 77: 51–59, 1986 [DOI] [PubMed] [Google Scholar]
- 422.Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 32 Suppl 7: S98–S108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Leibowitz SF, Akabayashi A, Alexander JT, Wang J. Gonadal steroids and hypothalamic galanin and neuropeptide Y: role in eating behavior and body weight control in female rats. Endocrinology 139: 1771–1780, 1998 [DOI] [PubMed] [Google Scholar]
- 424.Leidy LE. Timing of menopause in relation to body size and weight change. Hum Biol 68: 967–982, 1996 [PubMed] [Google Scholar]
- 425.Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci 6: 26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med 33: 51–60, 2003 [DOI] [PubMed] [Google Scholar]
- 427.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152: 2552–2557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Levin ER. Minireview: Extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol 25: 377–384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 55: 950–954, 1992 [DOI] [PubMed] [Google Scholar]
- 430.Li BY, Qiao GF, Feng B, Zhao RB, Lu YJ, Schild JH. Electrophysiological and neuroanatomical evidence of sexual dimorphism in aortic baroreceptor and vagal afferents in rat. Am J Physiol Regul Integr Comp Physiol 295: R1301–R1310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Li ET, Tsang LB, Lui SS. Menstrual cycle and voluntary food intake in young Chinese women. Appetite 33: 109–118, 1999 [DOI] [PubMed] [Google Scholar]
- 432.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor β is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord 26: 1103–1109, 2002 [DOI] [PubMed] [Google Scholar]
- 433.Lightfoot JT. Sex hormones' regulation of rodent physical activity: a review. Int J Biol Sci 4: 126–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Lim SS, Norman RJ, Clifton PM, Noakes M. Hyperandrogenemia, psychological distress, and food cravings in young women. Physiol Behav 98: 276–280, 2009 [DOI] [PubMed] [Google Scholar]
- 435.Linden A, Uvnas-Moberg K, Forsberg G, Bednar I, Sodersten P. Involvement of cholecystokinin in food intake: III. oestradiol potentiates the inhibitory effect of cholecystokinin octapeptide on food intake in ovariectomized rats. J Neuroendocrinol 2: 797–801, 1990 [DOI] [PubMed] [Google Scholar]
- 436.Lissner L, Stevens J, Levitsky DA, Rasmussen KM, Strupp BJ. Variation in energy intake during the menstrual cycle: implications for food-intake research. Am J Clin Nutr 48: 956–962, 1988 [DOI] [PubMed] [Google Scholar]
- 437.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 438.Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, Davidson WS, Liu M, Raybould HE, Woods SC, Tso P. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol 293: R1490–R1494, 2007 [DOI] [PubMed] [Google Scholar]
- 439.Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, Knoll JG, Wright H, Pfeifer GP, Ojeda SR. Epigenetic control of female puberty. Nat Neurosci 16: 281–289, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Long JA, Evans ME. The oestrous cycle of the rat and its associated phenomena. Mem Univ Calif 6: 1–103, 1922 [Google Scholar]
- 441.Loucks AB. The response of luteinizing hormone pulsatility to 5 days of low-energy availability disappears by 14 years of gynecological age. J Clin Endocrinol Metab 91: 3158–3164, 2006 [DOI] [PubMed] [Google Scholar]
- 442.Loucks AB, Redman LM. The effect of stress on menstrual function. Trends Endocrinol Metab 15: 466–471, 2004 [DOI] [PubMed] [Google Scholar]
- 443.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32: 949–958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Lu JK, LaPolt PS, Nass TE, Matt DW, Judd HL. Relation of circulating estradiol and progesterone to gonadotropin secretion and estrous cyclicity in aging female rats. Endocrinology 116: 1953–1959, 1985 [DOI] [PubMed] [Google Scholar]
- 445.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685, 2005 [DOI] [PubMed] [Google Scholar]
- 446.Lutz TA. Effects of amylin on eating and adiposity. Handb Exp Pharmacol: 231–250, 2012 [DOI] [PubMed] [Google Scholar]
- 447.Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol 298: R1475–R1484, 2010 [DOI] [PubMed] [Google Scholar]
- 448.Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Prog Brain Res 181: 177–192, 2010 [DOI] [PubMed] [Google Scholar]
- 449.Lydecker JA, Pisetsky EM, Mitchell KS, Thornton LM, Kendler KS, Reichborn-Kjennerud T, Lichtenstein P, Bulik CM, Mazzeo SE. Association between co-twin sex and eating disorders in opposite sex twin pairs: Evaluations in North American, Norwegian, and Swedish samples. J Psychosom Res 72: 73–77, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am J Clin Nutr 49: 1164–1168, 1989 [DOI] [PubMed] [Google Scholar]
- 451.Macdiarmid JI, Vail A, Cade JE, Blundell JE. The sugar-fat relationship revisited: differences in consumption between men and women of varying BMI. Int J Obes Relat Metab Disord 22: 1053–1061, 1998 [DOI] [PubMed] [Google Scholar]
- 452.Madrid JA, Lopez-Bote C, Martin E. Effect of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiol Behav 53: 329–335, 1993 [DOI] [PubMed] [Google Scholar]
- 453.Maletínská L, Matyšková R, Maixnerová J, Sykora D, Pychová M, Spolcová A, Blechová M, Drápalová J, Lacinová Z, Haluzik M, Zelezná B. The peptidic GHS-R antagonist [d-Lys(3)]GHRP-6 markedly improves adiposity and related metabolic abnormalities in a mouse model of postmenopausal obesity. Mol Cell Endocrinol 343: 55–62, 2011 [DOI] [PubMed] [Google Scholar]
- 454.Malpaux B. Seasonal reguation of reproduction in mammals. In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JD. St. Louis, MO: Elsevier/Academic, 2006, p. 2231–2281 [Google Scholar]
- 455.Mangiaracina M, Wolfe A, Azzara A, Schwartz GJ, Walsh BT, Geary N. The satiating potency of endogenous CCK increases after puberty in rats (Abstract). Appetite 42: 382, 2004 [Google Scholar]
- 456.Manning JM, Bronson FH. Suppression of puberty in rats by exercise: effects on hormone levels and reversal with GnRH infusion. Am J Physiol Regul Integr Comp Physiol 260: R717–R723, 1991 [DOI] [PubMed] [Google Scholar]
- 457.Marco A, Schroeder M, Weller A. Microstructural pattern of palatable food intake from weaning to adulthood in male and female OLETF rats. Behav Neurosci 123: 1251–1260, 2009 [DOI] [PubMed] [Google Scholar]
- 458.Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of the menstrual cycle on energy and nutrient intake. Am J Clin Nutr 60: 895–899, 1994 [DOI] [PubMed] [Google Scholar]
- 459.Matheny M, Zhang Y, Shapiro A, Tumer N, Scarpace PJ. Central overexpression of leptin antagonist reduces wheel running and underscores importance of endogenous leptin receptor activity in energy homeostasis. Am J Physiol Regul Integr Comp Physiol 297: R1254–R1261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite 52: 545–553, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides 25: 289–297, 2004 [DOI] [PubMed] [Google Scholar]
- 462.Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 22: 24–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107: 22693–22698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Mazzeo SE, Saunders R, Mitchell KS. Gender and binge eating among bariatric surgery candidates. Eat Behav 7: 47–52, 2006 [DOI] [PubMed] [Google Scholar]
- 466.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci 32: 2241–2247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.McCarthy MM, Caba M, Komisaruk BR, Beyer C. Modulation by estrogen and progesterone of the effect of muscimol on nociception in the spinal cord. Pharmacol Biochem Behav 37: 123–128, 1990 [DOI] [PubMed] [Google Scholar]
- 468.McCarthy MM, Coirini H, Schumacher M, Pfaff DW, McEwen BS, Schwartz-Giblin S. Ovarian steroid modulation of [3H]muscimol binding in the spinal cord of the rat. Brain Res 556: 321–323, 1991 [DOI] [PubMed] [Google Scholar]
- 469.McDermott LJ, Jorgensen DE, Byers DJ. Estradiol and progesterone suppress feeding induced by 2-deoxy-d-glucose. Physiol Behav 32: 731–736, 1984 [DOI] [PubMed] [Google Scholar]
- 470.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res 57: 357–384, 2002 [DOI] [PubMed] [Google Scholar]
- 471.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 20: 279–307, 1999 [DOI] [PubMed] [Google Scholar]
- 472.McVay MA, Copeland AL, Newman HS, Geiselman PJ. Food cravings and food cue responding across the menstrual cycle in a non-eating disordered sample. Appetite 59: 591–600, 2012 [DOI] [PubMed] [Google Scholar]
- 473.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am J Physiol Regul Integr Comp Physiol 299: R813–R822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, Di Carlo C, Nappi C, Di Carlo R. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology 145: 3115–3121, 2004 [DOI] [PubMed] [Google Scholar]
- 475.Mendelsohn ME, Karas RH. Rapid progress for non-nuclear estrogen receptor signaling. J Clin Invest 120: 2277–2279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Micevych P, Eckersell CB, Holland K, Smith A. Induction of CCK mRNA levels in the limbic-hypothalamic circuit: time course and site-specific effects of estrogen. J Neurobiol 30: 465–479, 1996 [DOI] [PubMed] [Google Scholar]
- 477.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol 290: 44–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol (Lausanne) 2: 90, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology 96: 103–110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Michener W, Rozin P, Freeman E, Gale L. The role of low progesterone and tension as triggers of perimenstrual chocolate and sweets craving: some negative experimental evidence. Physiol Behav 67: 417–420, 1999 [DOI] [PubMed] [Google Scholar]
- 481.Mikolajczyk E, Grzywacz A, Samochowiec J. The association of catechol-O-methyltransferase genotype with the phenotype of women with eating disorders. Brain Res 1307: 142–148, 2010 [DOI] [PubMed] [Google Scholar]
- 482.Miller A, Vo H, Huo L, Roca C, Schmidt PJ, Rubinow DR. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res 44: 788–794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Miller KK. Endocrine dysregulation in anorexia nervosa update. J Clin Endocrinol Metab 96: 2939–2949, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32: 81–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Mindell S, Smith GP, Greenberg D. Corn oil and mineral oil stimulate sham feeding in rats. Physiol Behav 48: 283–287, 1990 [DOI] [PubMed] [Google Scholar]
- 486.Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, Goldstein MA, Ebrahimi S, Clauss L, Weigel T, Mickley D, Schoenfeld DA, Herzog DB, Klibanski A. Physiological estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res 26: 2430–2438, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 153: 2800–2812, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Montague CT, Prins JB, Sanders L, Digby JE, O'Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 46: 342–347, 1997 [DOI] [PubMed] [Google Scholar]
- 489.Moran LJ, Noakes M, Clifton PM, Wittert GA, Tomlinson L, Galletly C, Luscombe ND, Norman RJ. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J Clin Endocrinol Metab 89: 3337–3344, 2004 [DOI] [PubMed] [Google Scholar]
- 490.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci 361: 1211–1218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 526: 95–102, 1990 [DOI] [PubMed] [Google Scholar]
- 492.Morris DH, Jones ME, Schoemaker MJ, McFadden E, Ashworth A, Swerdlow AJ. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the breakthrough generations study. Am J Epidemiol 175: 998–1005, 2012 [DOI] [PubMed] [Google Scholar]
- 493.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7: 1034–1039, 2004 [DOI] [PubMed] [Google Scholar]
- 494.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006 [DOI] [PubMed] [Google Scholar]
- 495.Moverare-Skrtic S, Venken K, Andersson N, Lindberg MK, Svensson J, Swanson C, Vanderschueren D, Oscarsson J, Gustafsson JA, Ohlsson C. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring) 14: 662–672, 2006 [DOI] [PubMed] [Google Scholar]
- 496.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 364: 2392–2404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Mulet T, Pico C, Oliver P, Palou A. Blood leptin homeostasis: sex-associated differences in circulating leptin levels in rats are independent of tissue leptin expression. Int J Biochem Cell Biol 35: 104–110, 2003 [DOI] [PubMed] [Google Scholar]
- 498.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 199: 105–112, 2008 [DOI] [PubMed] [Google Scholar]
- 499.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104: 2501–2506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556, 2008 [DOI] [PubMed] [Google Scholar]
- 501.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9: 117–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor α (ERα): a potential role for estrogen receptor beta (ERβ). Horm Metab Res 34: 758–763, 2002 [DOI] [PubMed] [Google Scholar]
- 503.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor β. Proc Natl Acad Sci USA 97: 11603–11608, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Naessen S, Carlstrom K, Bystrom B, Pierre Y, Hirschberg AL. Effects of an antiandrogenic oral contraceptive on appetite and eating behavior in bulimic women. Psychoneuroendocrinology 32: 548–554, 2007 [DOI] [PubMed] [Google Scholar]
- 505.Naessen S, Carlstrom K, Garoff L, Glant R, Hirschberg AL. Polycystic ovary syndrome in bulimic women—an evaluation based on the new diagnostic criteria. Gynecol Endocrinol 22: 388–394, 2006 [DOI] [PubMed] [Google Scholar]
- 506.Naessen S, Carlstrom K, Holst JJ, Hellstrom PM, Hirschberg AL. Women with bulimia nervosa exhibit attenuated secretion of glucagon-like peptide 1, pancreatic polypeptide, and insulin in response to a meal. Am J Clin Nutr 94: 967–972, 2011 [DOI] [PubMed] [Google Scholar]
- 507.Nance DM, Gorski RA. Neurohormonal determinants of sex differences in the hypothalamic regulation of feeding behavior and body weight in the rat. Pharmacol Biochem Behav 3: 155–162, 1975 [PubMed] [Google Scholar]
- 508.Neal-Perry G, Santoro NF. Aging in the hypothalamic-pituitary-ovarian axis In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JD. St. Louis, MO: Elsevier, 2006, p. 2729–2755 [Google Scholar]
- 509.Neary MT, Batterham RL. Gaining new insights into food reward with functional neuroimaging. Forum Nutr 63: 152–163, 2010 [DOI] [PubMed] [Google Scholar]
- 510.Neill JD, Freeman ME, Tillson SA. Control of the proestrus surge of prolactin and luteinizing hormone secretion by estrogens in the rat. Endocrinology 89: 1448–1453, 1971 [DOI] [PubMed] [Google Scholar]
- 511.Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod 20: 659–670, 1979 [DOI] [PubMed] [Google Scholar]
- 512.Nilsson S, Gustafsson JA. Estrogen receptors: therapies targeted to receptor subtypes. Clin Pharmacol Ther 89: 44–55, 2011 [DOI] [PubMed] [Google Scholar]
- 513.Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Munzberg H, Mauvais-Jarvis F. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology 152: 1661–1669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Nolan LJ, Guss JL, Liddle RA, Pi-Sunyer FX, Kissileff HR. Elevated plasma cholecystokinin and appetitive ratings after consumption of a liquid meal in humans. Nutrition 19: 553–557, 2003 [DOI] [PubMed] [Google Scholar]
- 515.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav 89: 531–535, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Nunez AA. Dose-dependent effects of testosterone on feeding and body weight in male rats. Behav Neural Biol 34: 445–449, 1982 [DOI] [PubMed] [Google Scholar]
- 517.Nunez AA, Gray JM, Wade GN. Food intake and adipose tissue lipoprotein lipase activity after hypothalamic estradiol benzoate implants in rats. Physiol Behav 25: 595–598, 1980 [DOI] [PubMed] [Google Scholar]
- 518.Nunez AA, Grundman M. Testosterone affects food intake and body weight of weanling male rats. Pharmacol Biochem Behav 16: 933–936, 1982 [DOI] [PubMed] [Google Scholar]
- 519.Nunez AA, Siegel LI, Wade GN. Central effects of testosterone on food intake in male rats. Physiol Behav 24: 469–471, 1980 [DOI] [PubMed] [Google Scholar]
- 520.O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JD. St. Louis, MO: Elsevier/Academic, 2006, p. 1017–1069 [Google Scholar]
- 521.O'Rahilly S, Farooqi IS. Genetics of obesity. Philos Trans R Soc Lond B Biol Sci 361: 1095–1105, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Ochner C, Teixeira J, Geary N, Asarian L. Greater short-term weight loss in women 20–45 versus 55–65 years of age following bariatric surgery. Obes Surg 23: 1650–1654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 278: 640–645, 2000 [DOI] [PubMed] [Google Scholar]
- 524.Oka K, Hirano T, Noguchi M. Changes in the concentration of testosterone in serum during the menstrual cycle, as determined by liquid chromatography. Clin Chem 34: 557–560, 1988 [PubMed] [Google Scholar]
- 525.Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord 27: 1020–1027, 2003 [DOI] [PubMed] [Google Scholar]
- 526.Olafsdottir AS, Skuladottir GV, Thorsdottir I, Hauksson A, Steingrimsdottir L. Maternal diet in early and late pregnancy in relation to weight gain. Int J Obes (Lond) 30: 492–499, 2006 [DOI] [PubMed] [Google Scholar]
- 527.Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci USA 106: 15932–15937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Olster DH, Blaustein JD. Biochemical and immunocytochemical assessment of neural progestin receptors following estradiol treatments that eliminate the sex difference in progesterone-facilitated lordosis in guinea pigs. J Neuroendocrinol 2: 79–86, 1990 [DOI] [PubMed] [Google Scholar]
- 529.Olster DH, Blaustein JD. Estradiol pulses induce progestin receptors selectively in substance P-immunoreactive neurons in the ventrolateral hypothalamus of female guinea pigs. J Neurobiol 23: 293–301, 1992 [DOI] [PubMed] [Google Scholar]
- 530.Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cogn 55: 134–147, 2004 [DOI] [PubMed] [Google Scholar]
- 531.Paeratakul S, White MA, Williamson DA, Ryan DH, Bray GA. Sex, race/ethnicity, socioeconomic status, and BMI in relation to self-perception of overweight. Obes Res 10: 345–350, 2002 [DOI] [PubMed] [Google Scholar]
- 532.Pagotto U, Gambineri A, Pelusi C, Genghini S, Cacciari M, Otto B, Castaneda T, Tschop M, Pasquali R. Testosterone replacement therapy restores normal ghrelin in hypogonadal men. J Clin Endocrinol Metab 88: 4139–4143, 2003 [DOI] [PubMed] [Google Scholar]
- 533.Palmer K, Gray Central vs JM. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav 37: 187–189, 1986 [DOI] [PubMed] [Google Scholar]
- 534.Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog Horm Res 53: 163–199, 1998 [PubMed] [Google Scholar]
- 535.Panotopoulos G, Ruiz JC, Raison J, Guy-Grand B, Basdevant A. Menopause, fat and lean distribution in obese women. Maturitas 25: 11–19, 1996 [DOI] [PubMed] [Google Scholar]
- 536.Paolisso G, Rizzo MR, Mazziotti G, Rotondi M, Tagliamonte MR, Varricchio G, Carella C, Varricchio M. Lack of association between changes in plasma leptin concentration and in food intake during the menstrual cycle. Eur J Clin Invest 29: 490–495, 1999 [DOI] [PubMed] [Google Scholar]
- 537.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese ERα-null mutant mice. J Clin Invest 121: 604–612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Parker GC, Bishop C, Coscina DV. Estrous cycle and food availability affect feeding induced by amygdala 5-HT receptor blockade. Pharmacol Biochem Behav 71: 701–707, 2002 [DOI] [PubMed] [Google Scholar]
- 539.Parnell W, Wilson N, Alexander D, Wohlers M, Williden M, Mann J, Gray A. Exploring the relationship between sugars and obesity. Public Health Nutr 11: 860–866, 2008 [DOI] [PubMed] [Google Scholar]
- 540.Patil CL, Abrams ET, Steinmetz AR, Young SL. Appetite sensations and nausea and vomiting in pregnancy: an overview of the explanations. Ecol Food Nutr 51: 394–417, 2012 [DOI] [PubMed] [Google Scholar]
- 541.Paulo RC, Brundage R, Cosma M, Mielke KL, Bowers CY, Veldhuis JD. Estrogen elevates the peak overnight production rate of acylated ghrelin. J Clin Endocrinol Metab 93: 4440–4447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Pearson CM, Combs JL, Zapolski TC, Smith GT. A longitudinal transactional risk model for early eating disorder onset. J Abnorm Psychol 121: 707–718, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Pelchat ML. Food cravings in young and elderly adults. Appetite 28: 103–113, 1997 [DOI] [PubMed] [Google Scholar]
- 544.Pelkman CL, Chow M, Heinbach RA, Rolls BJ. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am J Clin Nutr 73: 19–26, 2001 [DOI] [PubMed] [Google Scholar]
- 545.Pelleymounter MA, Baker MB, McCaleb M. Does estradiol mediate leptin's effects on adiposity and body weight? Am J Physiol Endocrinol Metab 276: E955–E963, 1999 [DOI] [PubMed] [Google Scholar]
- 546.Petersen S. Effects of testosterone upon feeding in male mice. Anim Behav 26: 945–952, 1978 [DOI] [PubMed] [Google Scholar]
- 547.Petersen S. The temporal pattern of feeding over the oestrous cycle of the mouse. Anim Behav 24: 939–955, 1976 [DOI] [PubMed] [Google Scholar]
- 548.Pfaff D, Waters E, Khan Q, Zhang X, Numan M. Minireview: estrogen receptor-initiated mechanisms causal to mammalian reproductive behaviors. Endocrinology 152: 1209–1217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Pfaff DW, Sakuma Y, Kow LM, Lee WL, Easton A. Hormonal, neural, and genomic mechanisms for female reproductive behaviors, motivation, and arousal. In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JD. Amsterdam, The Netherlands: Elsevier/Academic, 2006, p. 1825–1920 [Google Scholar]
- 550.Phillips GB, Jing T, Heymsfield SB. Does insulin resistance, visceral adiposity, or a sex hormone alteration underlie the metabolic syndrome? Studies in women. Metabolism 57: 838–844, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65: 369–382, 1959 [DOI] [PubMed] [Google Scholar]
- 552.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev 92: 1235–1316, 2012 [DOI] [PubMed] [Google Scholar]
- 553.Pinsky MR, Hellstrom WJ. Hypogonadism, ADAM, and hormone replacement. Ther Adv Urol 2: 99–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Pirke KM, Broocks A, Wilckens T, Marquard R, Schweiger U. Starvation-induced hyperactivity in the rat: the role of endocrine and neurotransmitter changes. Neurosci Biobehav Rev 17: 287–294, 1993 [DOI] [PubMed] [Google Scholar]
- 555.Pirke KM, Kellner MB, Friess E, Krieg JC, Fichter MM. Satiety and cholecystokinin. Int J Eat Disord 15: 63–69, 1994 [DOI] [PubMed] [Google Scholar]
- 556.Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol 33: 160–168, 2012 [DOI] [PubMed] [Google Scholar]
- 557.Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res 1348: 55–62, 2010 [DOI] [PubMed] [Google Scholar]
- 558.Pliner P, Fleming AS. Food intake, body weight, and sweetness preferences over the menstrual cycle in humans. Physiol Behav 30: 663–666, 1983 [DOI] [PubMed] [Google Scholar]
- 559.Poeschla B, Gibbs J, Simansky KJ, Greenberg D, Smith GP. Cholecystokinin-induced satiety depends on activation of 5-HT1C receptors. Am J Physiol Regul Integr Comp Physiol 264: R62–R64, 1993 [DOI] [PubMed] [Google Scholar]
- 560.Poeschla B, Gibbs J, Simansky KJ, Smith GP. The 5-HT1A agonist 8-OH-DPAT attenuates the satiating action of cholecystokinin. Pharmacol Biochem Behav 42: 541–543, 1992 [DOI] [PubMed] [Google Scholar]
- 561.Pohle-Krauza RJ, Carey KH, Pelkman CL. Dietary restraint and menstrual cycle phase modulated l-phenylalanine-induced satiety. Physiol Behav 93: 851–861, 2008 [DOI] [PubMed] [Google Scholar]
- 562.Polidori C, Geary N. Estradiol treatment fails to affect the feeding responses to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides 23: 1697–1700, 2002 [DOI] [PubMed] [Google Scholar]
- 563.Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol 70: 213–238, 2008 [DOI] [PubMed] [Google Scholar]
- 564.Pope JF, Skinner JD, Carruth BR. Cravings and aversions of pregnant adolescents. J Am Diet Assoc 92: 1479–1482, 1992 [PubMed] [Google Scholar]
- 565.Powell MR, Hendricks B. Body schema, gender, and other correlates in nonclinical populations. Genet Soc Gen Psychol Monogr 125: 333–412, 1999 [PubMed] [Google Scholar]
- 566.Poyastro Pinheiro A, Thornton LM, Plotonicov KH, Tozzi F, Klump KL, Berrettini WH, Brandt H, Crawford S, Crow S, Fichter MM, Goldman D, Halmi KA, Johnson C, Kaplan AS, Keel P, LaVia M, Mitchell J, Rotondo A, Strober M, Treasure J, Woodside DB, Von Holle A, Hamer R, Kaye WH, Bulik CM. Patterns of menstrual disturbance in eating disorders. Int J Eat Disord 40: 424–434, 2007 [DOI] [PubMed] [Google Scholar]
- 567.Pralong FP. Insulin and NPY pathways and the control of GnRH function and puberty onset. Mol Cell Endocrinol 324: 82–86, 2010 [DOI] [PubMed] [Google Scholar]
- 568.Pratt GM, Learn CA, Hughes GD, Clark BL, Warthen M, Pories W. Demographics and outcomes at American Society for Metabolic and Bariatric Surgery Centers of Excellence. Surg Endosc 23: 795–799, 2009 [DOI] [PubMed] [Google Scholar]
- 569.Prentice AM, Prentice A. Energy costs of lactation. Annu Rev Nutr 8: 63–79, 1988 [DOI] [PubMed] [Google Scholar]
- 570.Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics 28: 378–379, 1987 [DOI] [PubMed] [Google Scholar]
- 571.Procopio M, Marriott P. Intrauterine hormonal environment and risk of developing anorexia nervosa. Arch Gen Psychiatry 64: 1402–1407, 2007 [DOI] [PubMed] [Google Scholar]
- 572.Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus 18: 985–988, 2008 [DOI] [PubMed] [Google Scholar]
- 573.Prutkin J, Fisher EM, Etter L, Fast K, Gardner E, Lucchina LA, Snyder DJ, Tie K, Weiffenbach J, Bartoshuk LM. Genetic variation and inferences about perceived taste intensity in mice and men. Physiol Behav 69: 161–173, 2000 [DOI] [PubMed] [Google Scholar]
- 574.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol 22: 5027–5035, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Qiao GF, Li BY, Lu YJ, Fu YL, Schild JH. 17β-estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am J Physiol Cell Physiol 297: C654–C664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26: 5649–5655, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Quinton SJ, Smith AR, Joiner T. The 2 to 4 digit ratio (2D:4D) and eating disorder diagnosis in women. Pers Individ Dif 51: 402–405, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Racine SE, Culbert KM, Keel PK, Sisk CL, Alexandra Burt S, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int J Eat Disord 45: 333–344, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int J Eat Disord 45: 333–344, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Individual differences in the relationship between ovarian hormones and emotional eating across the menstrual cycle: a role for personality? Eat Behav 14: 161–166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Raevuori A, Kaprio J, Hoek HW, Sihvola E, Rissanen A, Keski-Rahkonen A. Anorexia and bulimia nervosa in same-sex and opposite-sex twins: lack of association with twin type in a nationwide study of Finnish twins. Am J Psychiatry 165: 1604–1610, 2008 [DOI] [PubMed] [Google Scholar]
- 582.Ramirez I. Estradiol-induced changes in lipoprotein lipase, eating, and body weight in rats. Am J Physiol Endocrinol Metab 240: E533–E538, 1981 [DOI] [PubMed] [Google Scholar]
- 583.Ramirez I. Relation between estrogen-induced hyperlipemia and food intake and body weight in rats. Physiol Behav 25: 511–518, 1980 [DOI] [PubMed] [Google Scholar]
- 584.Reichborn-Kjennerud T, Bulik CM, Kendler KS, Roysamb E, Maes H, Tambs K, Harris JR. Gender differences in binge-eating: a population-based twin study. Acta Psychiatr Scand 108: 196–202, 2003 [DOI] [PubMed] [Google Scholar]
- 585.Reimer RA, Debert CT, House JL, Poulin MJ. Dietary and metabolic differences in pre- versus postmenopausal women taking or not taking hormone replacement therapy. Physiol Behav 84: 303–312, 2005 [DOI] [PubMed] [Google Scholar]
- 586.Resch M, Szendei G, Haasz P. Bulimia from a gynecological view: hormonal changes. J Obstet Gynaecol 24: 907–910, 2004 [DOI] [PubMed] [Google Scholar]
- 587.Reubinoff BE, Wurtman J, Rojansky N, Adler D, Stein P, Schenker JG, Brzezinski A. Effects of hormone replacement therapy on weight, body composition, fat distribution, and food intake in early postmenopausal women: a prospective study. Fertil Steril 64: 963–968, 1995 [DOI] [PubMed] [Google Scholar]
- 588.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005 [DOI] [PubMed] [Google Scholar]
- 589.Richard D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol 250: R245–R249, 1986 [DOI] [PubMed] [Google Scholar]
- 590.Richter CP. Total self-regulatory functions in animals and human beings. Harvey Lecture Series 38: 63–103, 1942–1943 [Google Scholar]
- 591.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav 81: 249–273, 2004 [DOI] [PubMed] [Google Scholar]
- 592.Rivera HM, Eckel LA. Activation of central, but not peripheral, estrogen receptors is necessary for estradiol's anorexigenic effect in ovariectomized rats. Endocrinology 151: 5680–5688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav 86: 331–337, 2005 [DOI] [PubMed] [Google Scholar]
- 594.Rivera HM, Santollo J, Nikonova LV, Eckel LA. Estradiol increases the anorexia associated with increased 5-HT(2C) receptor activation in ovariectomized rats. Physiol Behav 105: 188–194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Rocha M, Bing C, Williams G, Puerta M. Physiologic estradiol levels enhance hypothalamic expression of the long form of the leptin receptor in intact rats. J Nutr Biochem 15: 328–334, 2004 [DOI] [PubMed] [Google Scholar]
- 596.Rock CL, Gorenflo DW, Drewnowski A, Demitrack MA. Nutritional characteristics, eating pathology, and hormonal status in young women. Am J Clin Nutr 64: 566–571, 1996 [DOI] [PubMed] [Google Scholar]
- 597.Rodin J, Mancuso J, Granger J, Nelbach E. Food cravings in relation to body mass index, restraint and estradiol levels: a repeated-measures study in healthy women. Appetite 17: 177–185, 1991 [DOI] [PubMed] [Google Scholar]
- 598.Roemmich JN, Clark PA, Berr SS, Mai V, Mantzoros CS, Flier JS, Weltman A, Rogol AD. Gender differences in leptin levels during puberty are related to the subcutaneous fat depot and sex steroids. Am J Physiol Endocrinol Metab 275: E543–E551, 1998 [DOI] [PubMed] [Google Scholar]
- 599.Roepke TA. Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol 21: 141–150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Ronnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 151: 4926–4937, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Roepke TA, Malyala A, Bosch MA, Kelly MJ, Ronnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 148: 4937–4951, 2007 [DOI] [PubMed] [Google Scholar]
- 602.Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci 16: 1560–1573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87: 39–44, 2006 [DOI] [PubMed] [Google Scholar]
- 604.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol 10: 133–142, 1991 [DOI] [PubMed] [Google Scholar]
- 606.Rolls ET. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci 361: 1123–1136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes (Lond) 35: 550–561, 2011 [DOI] [PubMed] [Google Scholar]
- 608.Rolls ET. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc Nutr Soc 71: 488–501, 2012 [DOI] [PubMed] [Google Scholar]
- 609.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol 86: 216–244, 2008 [DOI] [PubMed] [Google Scholar]
- 610.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92: 2744–2750, 2007 [DOI] [PubMed] [Google Scholar]
- 611.Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med 27: 207–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Rosenbaum M, Leibel RL. Clinical review 107: Role of gonadal steroids in the sexual dimorphisms in body composition and circulating concentrations of leptin. J Clin Endocrinol Metab 84: 1784–1789, 1999 [DOI] [PubMed] [Google Scholar]
- 613.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81: 3424–3427, 1996 [DOI] [PubMed] [Google Scholar]
- 614.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118: 2583–2591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Rosenblatt H, Dyrenfurth I, Ferin M, vande Wiele RL. Food intake and the menstrual cycle in rhesus monkeys. Physiol Behav 24: 447–449, 1980 [DOI] [PubMed] [Google Scholar]
- 616.Rosenkranz K, Hinney A, Ziegler A, Hermann H, Fichter M, Mayer H, Siegfried W, Young JK, Remschmidt H, Hebebrand J. Systematic mutation screening of the estrogen receptor β gene in probands of different weight extremes: identification of several genetic variants. J Clin Endocrinol Metab 83: 4524–4527, 1998 [DOI] [PubMed] [Google Scholar]
- 617.Ross GT, Cargille CM, Lipsett MB, Rayford PL, Marshall JR, Strott CA, Rodbard D. Pituitary and gonadal hormones in women during spontaneous and induced ovulatory cycles. Recent Prog Horm Res 26: 1–62, 1970 [DOI] [PubMed] [Google Scholar]
- 618.Roth JD, Erickson MR, Chen S, Parkes DG. GLP-1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. Br J Pharmacol 166: 121–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105: 7257–7262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Routtenberg A. “Self-starvation” of rats living in activity wheels: adaptation effects. J Comp Physiol Psychol 66: 234–238, 1968 [DOI] [PubMed] [Google Scholar]
- 621.Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol 64: 414–421, 1967 [DOI] [PubMed] [Google Scholar]
- 622.Rowland DL, Perrings TS, Thommes JA. Comparison of androgenic effects on food intake and body weight in adult rats. Physiol Behav 24: 205–209, 1980 [DOI] [PubMed] [Google Scholar]
- 623.Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite 17: 199–212, 1991 [DOI] [PubMed] [Google Scholar]
- 624.Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Front Neuroendocrinol 27: 210–216, 2006 [DOI] [PubMed] [Google Scholar]
- 625.Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev 8: 131–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Sakata I, Tanaka T, Yamazaki M, Tanizaki T, Zheng Z, Sakai T. Gastric estrogen directly induces ghrelin expression and production in the rat stomach. J Endocrinol 190: 749–757, 2006 [DOI] [PubMed] [Google Scholar]
- 627.Salamanca S, Uphouse L. Estradiol modulation of the hyperphagia induced by the 5-HT1A agonist, 8-OH-DPAT. Pharmacol Biochem Behav 43: 953–955, 1992 [DOI] [PubMed] [Google Scholar]
- 628.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 8: 538–554, 2006 [DOI] [PubMed] [Google Scholar]
- 629.Sammel MD, Freeman EW, Liu Z, Lin H, Guo W. Factors that influence entry into stages of the menopausal transition. Menopause 16: 1218–1227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg 192: 657–662, 2006 [DOI] [PubMed] [Google Scholar]
- 631.Sancho C, Arija MV, Asorey O, Canals J. Epidemiology of eating disorders: a two year follow up in an early adolescent school population. Eur Child Adolesc Psychiatry 16: 495–504, 2007 [DOI] [PubMed] [Google Scholar]
- 632.Sandoval DA, Ryan KK, de Kloet AD, Woods SC, Seeley RJ. Female rats are relatively more sensitive to reduced lipid versus reduced carbohydrate availability. Nutr Diabetes 2: e27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res 191: 173–177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Santollo J, Eckel LA. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol Behav 93: 842–850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERα is necessary for estradiol's anorexigenic effect in female rats. Horm Behav 58: 872–877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Santollo J, Torregrossa AM, Eckel LA. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm Behav 60: 86–93, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Santollo J, Wiley MD, Eckel LA. Acute activation of ERα decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293: R2194–R2201, 2007 [DOI] [PubMed] [Google Scholar]
- 638.Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril 71: 658–662, 1999 [DOI] [PubMed] [Google Scholar]
- 639.Savard R, Palmer JE, Greenwood MR. Effects of exercise training on regional adipose tissue metabolism in pregnant rats. Am J Physiol Regul Integr Comp Physiol 250: R837–R844, 1986 [DOI] [PubMed] [Google Scholar]
- 640.Sayegh R, Schiff I, Wurtman J, Spiers P, McDermott J, Wurtman R. The effect of a carbohydrate-rich beverage on mood, appetite, and cognitive function in women with premenstrual syndrome. Obstet Gynecol 86: 520–528, 1995 [DOI] [PubMed] [Google Scholar]
- 641.Scalera G. Effects of conditioned food aversions on nutritional behavior in humans. Nutr Neurosci 5: 159–188, 2002 [DOI] [PubMed] [Google Scholar]
- 642.Schieve LA, Cogswell ME, Scanlon KS. Trends in pregnancy weight gain within and outside ranges recommended by the Institute of Medicine in a WIC population. Matern Child Health J 2: 111–116, 1998 [DOI] [PubMed] [Google Scholar]
- 643.Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res 1123: 89–100, 2006 [DOI] [PubMed] [Google Scholar]
- 644.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann NY Acad Sci 1179: 70–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Schneider JE. Energy balance and reproduction. Physiol Behav 81: 289–317, 2004 [DOI] [PubMed] [Google Scholar]
- 646.Schneider JE, Klingerman CM, Abdulhay A. Sense and nonsense in metabolic control of reproduction. Front Endocrinol (Lausanne) 3: 26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Schumacher M, Coirini H, Pfaff DW, McEwen BS. Light-dark differences in behavioral sensitivity to oxytocin. Behav Neurosci 105: 487–492, 1991 [DOI] [PubMed] [Google Scholar]
- 648.Schwartz GJ. Integrative capacity of the caudal brainstem in the control of food intake. Philos Trans R Soc Lond B Biol Sci 361: 1275–1280, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Schwartz SM, Wade GN. Effects of estradiol and progesterone on food intake, body weight, and carcass adiposity in weanling rats. Am J Physiol Endocrinol Metab 240: E499–E503, 1981 [DOI] [PubMed] [Google Scholar]
- 650.Sclafani A, Hertwig H, Vigorito M, Feigin MB. Sex differences in polysaccharide and sugar preferences in rats. Neurosci Biobehav Rev 11: 241–251, 1987 [DOI] [PubMed] [Google Scholar]
- 651.Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav 104: 64–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30: 883–925, 2009 [DOI] [PubMed] [Google Scholar]
- 653.Scott KA, Melhorn SJ, Sakai RR. Effects of chronic social stress on obesity. Curr Obes Rep 1: 16–25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Sen S, Carpenter AH, Hochstadt J, Huddleston JY, Kustanovich V, Reynolds AA, Roberts S. Nutrition, weight gain and eating behavior in pregnancy: a review of experimental evidence for long-term effects on the risk of obesity in offspring. Physiol Behav 107: 138–145, 2012 [DOI] [PubMed] [Google Scholar]
- 655.Shaikh AA. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod 5: 297–307, 1971 [DOI] [PubMed] [Google Scholar]
- 656.Shelley DN, Dwyer E, Johnson C, Wittkowski KM, Pfaff DW. Interactions between estrogen effects and hunger effects in ovariectomized female mice. I. Measures of arousal. Horm Behav 52: 546–553, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Shen L, Wang DQ, Lo CM, Tso P, Davidson WS, Woods SC, Liu M. Estradiol increases the anorectic effect of central apolipoprotein A-IV. Endocrinology 151: 3163–3168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Shepard KN, Michopoulos V, Toufexis DJ, Wilson ME. Genetic, epigenetic and environmental impact on sex differences in social behavior. Physiol Behav 97: 157–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Shetty G, Wilson G, Hardy MP, Niu E, Huhtaniemi I, Meistrich ML. Inhibition of recovery of spermatogenesis in irradiated rats by different androgens. Endocrinology 143: 3385–3396, 2002 [DOI] [PubMed] [Google Scholar]
- 660.Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab 293: E316–E326, 2007 [DOI] [PubMed] [Google Scholar]
- 661.Shimizu H, Ohtani KI, Uehara Y, Abe Y, Takahashi H, Tsuchiya T, Sato N, Ibuki Y, Mori M. Orchiectomy and response to testosterone in the development of obesity in young Otsuka-Long-Evans-Tokushima Fatty (OLETF) rats. Int J Obes Relat Metab Disord 22: 318–324, 1998 [DOI] [PubMed] [Google Scholar]
- 662.Shimomura Y, Shimizu H, Takahashi M, Sato N, Uehara Y, Fukatsu A, Negishi M, Kobayashi I, Kobayashi S. The significance of decreased ambulatory activity during the generation by long-term observation of obesity in ovariectomized rats. Physiol Behav 47: 155–159, 1990 [DOI] [PubMed] [Google Scholar]
- 663.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388: 507–525, 1997 [DOI] [PubMed] [Google Scholar]
- 664.Siegel LI, Nunez AA, Wade GN. Effects of androgens on dietary self-selection and carcass composition in male rats. J Comp Physiol Psychol 95: 529–539, 1981 [DOI] [PubMed] [Google Scholar]
- 665.Siegfried Z, Berry EM, Hao S, Avraham Y. Animal models in the investigation of anorexia. Physiol Behav 79: 39–45, 2003 [DOI] [PubMed] [Google Scholar]
- 666.Silveira LF, Latronico AC. Approach to the patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab 98: 1781–1788, 2013 [DOI] [PubMed] [Google Scholar]
- 667.Simerly RB. Wired on hormones: endocrine regulation of hypothalamic development. Curr Opin Neurobiol 15: 81–85, 2005 [DOI] [PubMed] [Google Scholar]
- 668.Simonian SX, Herbison AE. Differential expression of estrogen receptor and neuropeptide Y by brainstem A1 and A2 noradrenaline neurons. Neuroscience 76: 517–529, 1997 [DOI] [PubMed] [Google Scholar]
- 669.Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen—the good, the bad, and the unexpected. Endocr Rev 26: 322–330, 2005 [DOI] [PubMed] [Google Scholar]
- 670.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci 7: 1040–1047, 2004 [DOI] [PubMed] [Google Scholar]
- 671.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26: 163–174, 2005 [DOI] [PubMed] [Google Scholar]
- 672.Sites CK, L'Hommedieu GD, Toth MJ, Brochu M, Cooper BC, Fairhurst PA. The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 90: 2701–2707, 2005 [DOI] [PubMed] [Google Scholar]
- 673.Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 150: 5351–5361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Slonaker JR. The effect of copulation, pregnancy, pseudopregnancy, and lactation on the voluntary activity and food consumption of the albino rat. Am J Physiol 71: 362–394, 1925 [Google Scholar]
- 675.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 83: 1297–1305, 2006 [DOI] [PubMed] [Google Scholar]
- 676.Smith GP. Animal models of human eating disorders. Ann NY Acad Sci 575: 63–72; discussion 72–64, 1989 [DOI] [PubMed] [Google Scholar]
- 677.Smith GP. Cholecystokinin and treatment of meal size: proof of principle. Obesity (Silver Spring) 14 Suppl 4: 168S–170S, 2006 [DOI] [PubMed] [Google Scholar]
- 678.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition 16: 814–820, 2000 [DOI] [PubMed] [Google Scholar]
- 679.Smith GP. Sham feeding in rats with chronic, reversible gastric fistulas. Curr Protoc Neurosci Chap. 8: Unit 8.6D, 2001 [DOI] [PubMed] [Google Scholar]
- 680.Smith JC. The history of the “Davis Rig”. Appetite 36: 93–98, 2001 [DOI] [PubMed] [Google Scholar]
- 681.Smith JT, Mark PJ, Waddell BJ. Developmental increases in plasma leptin binding activity and tissue Ob-Re mRNA expression in the rat. J Endocrinol 184: 535–541, 2005 [DOI] [PubMed] [Google Scholar]
- 682.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod 83: 568–577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Smith JT, Waddell BJ. Developmental changes in plasma leptin and hypothalamic leptin receptor expression in the rat: peripubertal changes and the emergence of sex differences. J Endocrinol 176: 313–319, 2003 [DOI] [PubMed] [Google Scholar]
- 684.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96: 219–226, 1975 [DOI] [PubMed] [Google Scholar]
- 685.Snyder DJ, Bartoshuk LM. Epidemiological studies of taste function: discussion and perspectives. Ann NY Acad Sci 1170: 574–580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Sorensen MB, Rasmussen V, Jensen G, Ottesen B. Temporal changes in clinic and ambulatory blood pressure during cyclic post-menopausal hormone replacement therapy. J Hypertens 18: 1387–1391, 2000 [DOI] [PubMed] [Google Scholar]
- 687.Souquet AM, Rowland NE. Dexfenfluramine: action with estradiol on food intake and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 258: R211–R215, 1990 [DOI] [PubMed] [Google Scholar]
- 688.Spary EJ, Maqbool A, Batten TF. Changes in oestrogen receptor alpha expression in the nucleus of the solitary tract of the rat over the oestrous cycle and following ovariectomy. J Neuroendocrinol 22: 492–502, 2010 [DOI] [PubMed] [Google Scholar]
- 689.Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat 38: 185–196, 2009 [DOI] [PubMed] [Google Scholar]
- 690.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A. Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology 91: 142–154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Steingrimsdottir L, Greenwood MR, Brasel JA. Effect of pregnancy, lactation and a high-fat diet on adipose tissue in Osborne-Mendel rats. J Nutr 110: 600–609, 1980 [DOI] [PubMed] [Google Scholar]
- 692.Stengel A, Wang L, Goebel-Stengel M, Tache Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 22: 253–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77: 810–824, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Stice E, Dagher A. Genetic variation in dopaminergic reward in humans. Forum Nutr 63: 176–185, 2010 [DOI] [PubMed] [Google Scholar]
- 695.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322: 449–452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 31: 4360–4366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Stotsenburg JM. The effect of spaying and semi-spaying young albino rats (Mus norvegicus albinus) on the growth in body weight and body length. Anat Rec 7: 183–194, 1913 [Google Scholar]
- 698.Stoving RK, Andries A, Brixen K, Bilenberg N, Horder K. Gender differences in outcome of eating disorders: a retrospective cohort study. Psychiatry Res 186: 362–366, 2011 [DOI] [PubMed] [Google Scholar]
- 699.Stratford JM, Curtis KS, Contreras RJ. Linoleic acid increases chorda tympani nerve responses to and behavioral preferences for monosodium glutamate by male and female rats. Am J Physiol Regul Integr Comp Physiol 295: R764–R772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Striegel-Moore RH, Rosselli F, Perrin N, DeBar L, Wilson GT, May A, Kraemer HC. Gender difference in the prevalence of eating disorder symptoms. Int J Eat Disord 42: 471–474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Strohmayer AJ, Smith GP. The meal pattern of genetically obese (ob/ob) mice. Appetite 8: 111–123, 1987 [DOI] [PubMed] [Google Scholar]
- 702.Strubbe JH, Gorissen J. Meal patterning in the lactating rat. Physiol Behav 25: 775–777, 1980 [DOI] [PubMed] [Google Scholar]
- 703.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29: 71–83, 1985 [DOI] [PubMed] [Google Scholar]
- 704.Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O'Rahilly S, Farooqi IS, Froguel P, Meyre D. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 57: 2511–2518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res 13: 2072–2080, 2005 [DOI] [PubMed] [Google Scholar]
- 706.Sullivan EL, Koegler FH, Cameron JL. Individual differences in physical activity are closely associated with changes in body weight in adult female rhesus monkeys (Macaca mulatta). Am J Physiol Regul Integr Comp Physiol 291: R633–R642, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Sullivan EL, Shearin J, Koegler FH, Cameron JL. Selective estrogen receptor modulator promotes weight loss in ovariectomized female rhesus monkeys (Macaca mulatta) by decreasing food intake and increasing activity. Am J Physiol Endocrinol Metab 302: E759–E767, 2012 [DOI] [PubMed] [Google Scholar]
- 708.Sullivan PF, Bulik CM, Kendler KS. Genetic epidemiology of binging and vomiting. Br J Psychiatry 173: 75–79, 1998 [DOI] [PubMed] [Google Scholar]
- 709.Sundblad C, Bergman L, Eriksson E. High levels of free testosterone in women with bulimia nervosa. Acta Psychiatr Scand 90: 397–398, 1994 [DOI] [PubMed] [Google Scholar]
- 710.Sundblad C, Landen M, Eriksson T, Bergman L, Eriksson E. Effects of the androgen antagonist flutamide and the serotonin reuptake inhibitor citalopram in bulimia nervosa: a placebo-controlled pilot study. J Clin Psychopharmacol 25: 85–88, 2005 [DOI] [PubMed] [Google Scholar]
- 711.Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol 2012: 625434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 147: 2183–2196, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Suzuki Y, Kurose Y, Takahashi H, Asakuma S, Azuma Y, Kobayashi S. The differences in feeding-inhibitory responses to peripheral and central leptin between non-lactating and lactating rats. J Endocrinol 207: 105–111, 2010 [DOI] [PubMed] [Google Scholar]
- 714.Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism 44: 369–373, 1995 [DOI] [PubMed] [Google Scholar]
- 715.Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry 68: 714–723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr 90: 1453–1456, 2009 [DOI] [PubMed] [Google Scholar]
- 717.Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav 54: 471–477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Swithers SE, McCurley M, Scheibler A, Doerflinger A. Differential effects of lipoprivation and food deprivation on chow and milk intake in 25- and 30-day-old rats. Appetite 45: 86–93, 2005 [DOI] [PubMed] [Google Scholar]
- 719.Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene 197: 169–175, 1997 [DOI] [PubMed] [Google Scholar]
- 720.Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress 14: 468–474, 2011 [DOI] [PubMed] [Google Scholar]
- 721.Tanentsapf I, Heitmann BL, Adegboye AR. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth 11: 81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Tao YX. The melanocortin-4 receptor: pharmacology, pathophysiology, and physiology Endocr Rev 31: 506–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Tao YX. Mutations in melanocortin-4 receptor and human obesity. Prog Mol Biol Transl Sci 88: 173–204, 2009 [DOI] [PubMed] [Google Scholar]
- 724.Tapper CM, Brown-Grant K. The secretion and metabolic clearance rates of oestradiol in the rat. J Endocrinol 64: 215–227, 1975 [DOI] [PubMed] [Google Scholar]
- 725.Tarasuk V, Beaton GH. Menstrual-cycle patterns in energy and macronutrient intake. Am J Clin Nutr 53: 442–447, 1991 [DOI] [PubMed] [Google Scholar]
- 726.Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinol (Copenh) 72: 551–568, 1973 [DOI] [PubMed] [Google Scholar]
- 727.Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol Behav 7: 847–852, 1971 [DOI] [PubMed] [Google Scholar]
- 728.Taya K, Komura H, Watanabe G, Sasamoto S. Peripheral blood levels of immunoreactive inhibin during pseudopregnancy, pregnancy and lactation in the rat. J Endocrinol 121: 545–552, 1989 [DOI] [PubMed] [Google Scholar]
- 729.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 93: 359–404, 2013 [DOI] [PubMed] [Google Scholar]
- 730.Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-Mullerian hormone. J Clin Endocrinol Metab 98: 729–735, 2013 [DOI] [PubMed] [Google Scholar]
- 731.Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-alpha agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res 1268: 88–96, 2009 [DOI] [PubMed] [Google Scholar]
- 732.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 149: 1609–1617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Thompson FE, McNeel TS, Dowling EC, Midthune D, Morrissette M, Zeruto CA. Interrelationships of added sugars intake, socioeconomic status, and race/ethnicity in adults in the United States: National Health Interview Survey, 2005. J Am Diet Assoc 109: 1376–1383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Thompson ME, Cox VC. The effects of estradiol on body weight and food intake in normal weight VMH-lesioned rats. Physiol Behav 22: 627–629, 1979 [DOI] [PubMed] [Google Scholar]
- 735.Thorneycroft IH, Mishell DR, Jr, Stone SC, Kharma KM, Nakamura RM. The relation of serum 17-hydroxyprogesterone and estradiol-17β levels during the human menstrual cycle. Am J Obstet Gynecol 111: 947–951, 1971 [DOI] [PubMed] [Google Scholar]
- 736.Thornton LM, Mazzeo SE, Bulik CM. The heritability of eating disorders: methods and current findings. Curr Top Behav Neurosci 6: 141–156, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Tobias JH, Steer CD, Vilarino-Guell C, Brown MA. Effect of an estrogen receptor-alpha intron 4 polymorphism on fat mass in 11-year-old children. J Clin Endocrinol Metab 92: 2286–2291, 2007 [DOI] [PubMed] [Google Scholar]
- 738.Tomelleri R, Grunewald KK. Menstrual cycle and food cravings in young college women. J Am Diet Assoc 87: 311–315, 1987 [PubMed] [Google Scholar]
- 739.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord 24: 226–231, 2000 [DOI] [PubMed] [Google Scholar]
- 740.Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet 375: 583–593, 2010 [DOI] [PubMed] [Google Scholar]
- 741.Tremollieres FA, Pouilles JM, Ribot CA. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am J Obstet Gynecol 175: 1594–1600, 1996 [DOI] [PubMed] [Google Scholar]
- 742.Trevaskis JL, Turek VF, Wittmer C, Griffin PS, Wilson JK, Reynolds JM, Zhao Y, Mack CM, Parkes DG, Roth JD. Enhanced amylin-mediated body weight loss in estradiol-deficient diet-induced obese rats. Endocrinology 151: 5657–5668, 2010 [DOI] [PubMed] [Google Scholar]
- 743.Trujillo ML, Spuch C, Carro E, Senaris R. Hyperphagia and central mechanisms for leptin resistance during pregnancy. Endocrinology 152: 1355–1365, 2011 [DOI] [PubMed] [Google Scholar]
- 744.Tso P, Liu Apolipoprotein AIV M, food intake, obesity. Physiol Behav 83: 631–643, 2004 [DOI] [PubMed] [Google Scholar]
- 745.Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. Gen Comp Endocrinol 177: 305–314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol 517: 841–855, 2009 [DOI] [PubMed] [Google Scholar]
- 747.Ubuka T, Son YL, Tobari Y, Tsutsui K. Gonadotropin-inhibitory hormone action in the brain and pituitary. Front Endocrinol (Lausanne) 3: 148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 169: 111–119, 2006 [DOI] [PubMed] [Google Scholar]
- 749.Uphouse L, Salamanca S,., Caldarola-Pastuszka M. Gender and estrous cycle differences in the response to the 5-HT1A agonist 8-OH-DPAT. Pharmacol Biochem Behav 40: 901–906, 1991 [DOI] [PubMed] [Google Scholar]
- 750.Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science 156: 942–943, 1967 [DOI] [PubMed] [Google Scholar]
- 751.Valle A, Catala-Niell A, Colom B, Garcia-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab 289: E15–E22, 2005 [DOI] [PubMed] [Google Scholar]
- 752.Van den Eynde F, Koskina A, Syrad H, Guillaume S, Broadbent H, Campbell IC, Schmidt U. State and trait food craving in people with bulimic eating disorders. Eat Behav 13: 414–417, 2012 [DOI] [PubMed] [Google Scholar]
- 753.Van den Eynde F, Treasure J. Neuroimaging in eating disorders and obesity: implications for research. Child Adolesc Psychiatr Clin N Am 18: 95–115, 2009 [DOI] [PubMed] [Google Scholar]
- 754.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 55: 296–303, 2011 [DOI] [PubMed] [Google Scholar]
- 755.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Van Vugt DA. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update 16: 276–292, 2010 [DOI] [PubMed] [Google Scholar]
- 757.Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol 488: 152–179, 2005 [DOI] [PubMed] [Google Scholar]
- 758.Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, Blaha V. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol Behav 68: 99–107, 1999 [DOI] [PubMed] [Google Scholar]
- 759.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev 28: 1–19, 2007 [DOI] [PubMed] [Google Scholar]
- 760.Verhagen JV, Giza BK, Scott TR. Effect of amiloride on gustatory responses in the ventroposteromedial nucleus of the thalamus in rats. J Neurophysiol 93: 157–166, 2005 [DOI] [PubMed] [Google Scholar]
- 761.Versini A, Ramoz N, Le Strat Y, Scherag S, Ehrlich S, Boni C, Hinney A, Hebebrand J, Romo L, Guelfi JD, Gorwood P. Estrogen receptor 1 gene (ESR1) is associated with restrictive anorexia nervosa. Neuropsychopharmacology 35: 1818–1825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Viner RM, Cole TJ. Adult socioeconomic, educational, social, and psychological outcomes of childhood obesity: a national birth cohort study. BMJ 330: 1354, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Voorhoeve PG, van Mechelen W, Uitterlinden AG, Delemarre-van de Waal HA, Lamberts SW. Estrogen receptor-alpha gene polymorphisms and body composition in children and adolescents. Horm Res Paediatr 76: 86–92, 2011 [DOI] [PubMed] [Google Scholar]
- 764.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav 8: 523–534, 1972 [DOI] [PubMed] [Google Scholar]
- 765.Wade GN. Interaction between estradiol-17 beta and growth hormone in control of food intake in weanling rats. J Comp Physiol Psychol 86: 359–362, 1974 [DOI] [PubMed] [Google Scholar]
- 766.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol 88: 183–193, 1975 [DOI] [PubMed] [Google Scholar]
- 767.Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav 22: 583–593, 1979 [DOI] [PubMed] [Google Scholar]
- 768.Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes (Lond) 9 Suppl 1: 83–92, 1985 [PubMed] [Google Scholar]
- 769.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol 287: R1277–R1296, 2004 [DOI] [PubMed] [Google Scholar]
- 770.Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev 16: 235–272, 1992 [DOI] [PubMed] [Google Scholar]
- 771.Wade GN, Zucker I. Development of hormonal control over food intake and body weight in female rats. J Comp Physiol Psychol 70: 213–220, 1970 [DOI] [PubMed] [Google Scholar]
- 772.Wade T, Martin NG, Neale MC, Tiggemann M, Treloar SA, Bucholz KK, Madden PA, Heath AC. The structure of genetic and environmental risk factors for three measures of disordered eating. Psychol Med 29: 925–934, 1999 [DOI] [PubMed] [Google Scholar]
- 773.Wade TD, Gordon S, Medland S, Bulik CM, Heath AC, Montgomery GW, Martin NG. Genetic variants associated with disordered eating. Int J Eat Disord, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Wager-Srdar SA, Gannon M, Levine AS. The effect of cholecystokinin on food intake in gonadectomized and intact rats: the influence of sex hormones. Physiol Behav 40: 25–28, 1987 [DOI] [PubMed] [Google Scholar]
- 775.Wager-Srdar SA, Levine AS. The effect of cholecystokinin-octapeptide on food intake and consummatory behavior in lactating rats. Physiol Behav 50: 331–336, 1991 [DOI] [PubMed] [Google Scholar]
- 776.Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr 131: 2351–2357, 2001 [DOI] [PubMed] [Google Scholar]
- 777.Walsh BT, Devlin MJ. Eating disorders: progress and problems. Science 280: 1387–1390, 1998 [DOI] [PubMed] [Google Scholar]
- 778.Wang GH. Age and sex differences in the amount of daily food-intake of the albino rat. Am J Physiol 71: 729–773, 1925 [Google Scholar]
- 779.Wang GH. The changes in the amount of daily food-intake in the albino rat during pergnancy and lactation. Am J Physiol 71: 736–741, 1925 [Google Scholar]
- 780.Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 19: 1601–1608, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Wang K, Zhang H, Bloss CS, Duvvuri V, Kaye W, Schork NJ, Berrettini W, Hakonarson H. A genome-wide association study on common SNPs and rare CNVs in anorexia nervosa. Mol Psychiatry 16: 949–959, 2011 [DOI] [PubMed] [Google Scholar]
- 782.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 121: e1604–1614, 2008 [DOI] [PubMed] [Google Scholar]
- 783.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med 27: 107–116, 2004 [DOI] [PubMed] [Google Scholar]
- 784.Watanabe K, Hara C, Ogawa N. Feeding conditions and estrous cycle of female rats under the activity-stress procedure from aspects of anorexia nervosa. Physiol Behav 51: 827–832, 1992 [DOI] [PubMed] [Google Scholar]
- 785.Weaver JU. Classical endocrine diseases causing obesity. Front Horm Res 36: 212–228, 2008 [DOI] [PubMed] [Google Scholar]
- 786.Weingarten HP, Elston D. The phenomenology of food cravings. Appetite 15: 231–246, 1990 [DOI] [PubMed] [Google Scholar]
- 787.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351: 987–997, 2004 [DOI] [PubMed] [Google Scholar]
- 788.Westenhoefer J. Age and gender dependent profile of food choice. Forum Nutr: 44–51, 2005 [DOI] [PubMed] [Google Scholar]
- 789.Westerterp KR, Goris AH. Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care 5: 489–493, 2002 [DOI] [PubMed] [Google Scholar]
- 790.Whitaker KW, Totoki K, Reyes TM. Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutr Metab Cardiovasc Dis, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Williams DL. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology 150: 2997–3001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab 293: E270–E276, 2007 [DOI] [PubMed] [Google Scholar]
- 793.Williams WP, 3rd, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne) 3: 60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Williamson DA, Martin CK, York-Crowe E, Anton SD, Redman LM, Han H, Ravussin E. Measurement of dietary restraint: validity tests of four questionnaires. Appetite 48: 183–192, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab 296: E490–E496, 2009 [DOI] [PubMed] [Google Scholar]
- 796.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol 166: 520–528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Wizemann TM, Pardue ML. Exploring the biological contributions to human health: Does sex matter? Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 798.Wolnerhanssen B, Beglinger C. Therapeutic potential of gut peptides. Forum Nutr 63: 54–63, 2010 [DOI] [PubMed] [Google Scholar]
- 799.Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab 9: 489–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab 93: S37–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos Trans R Soc Lond B Biol Sci 361: 1219–1235, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Woodside B. Prolactin and the hyperphagia of lactation. Physiol Behav 91: 375–382, 2007 [DOI] [PubMed] [Google Scholar]
- 803.Woodward CJ, Emery PW. Energy balance in rats given chronic hormone treatment. 2. Effects of corticosterone. Br J Nutr 61: 445–452, 1989 [DOI] [PubMed] [Google Scholar]
- 804.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336: 293–306, 1993 [DOI] [PubMed] [Google Scholar]
- 805.Wright CL, Schwarz JS, Dean SL, McCarthy MM. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol Metab 21: 553–561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by estradiol in ovariectomized rats. Scand J Gastroenterol 37: 1133–1139, 2002 [DOI] [PubMed] [Google Scholar]
- 807.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139: 61–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Wurtman JJ, Baum MJ. Estrogen reduces total food and carbohydrate intake, but not protein intake, in female rats. Physiol Behav 24: 823–827, 1980 [DOI] [PubMed] [Google Scholar]
- 809.Wurtman JJ, Brzezinski A, Wurtman RJ, Laferrere B. Effect of nutrient intake on premenstrual depression. Am J Obstet Gynecol 161: 1228–1234, 1989 [DOI] [PubMed] [Google Scholar]
- 810.Xiao E, Kim AJ, Dutia R, Conwell I, Ferin M, Wardlaw SL. Effects of estradiol on cerebrospinal fluid levels of agouti-related protein in ovariectomized rhesus monkeys. Endocrinology 151: 1002–1009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Xu J, Kirigiti MA, Grove KL, Smith MS. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology 150: 4231–4240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14: 453–465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Yanovski S. Sugar and fat: cravings and aversions. J Nutr 133: 835S–837S, 2003 [DOI] [PubMed] [Google Scholar]
- 814.Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, Miller DD, Dalton JT, Narayanan R. Estrogen receptor-β-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem 285: 31292–31303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Young JK. Anorexia nervosa and estrogen: current status of the hypothesis. Neurosci Biobehav Rev 34: 1195–1200, 2010 [DOI] [PubMed] [Google Scholar]
- 816.Young JK. Estrogen and the etiology of anorexia nervosa. Neurosci Biobehav Rev 15: 327–331, 1991 [DOI] [PubMed] [Google Scholar]
- 817.Yu Z, Geary N, Corwin RL. Individual effects of estradiol and progesterone on food intake and body weight in ovariectomized binge rats. Physiol Behav 104: 687–693, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiol Behav 95: 501–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Yuan DL, Chambers KC. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Horm Behav 36: 1–16, 1999 [DOI] [PubMed] [Google Scholar]
- 820.Zandian M, Ioakimidis I, Bergh C, Leon M, Sodersten P. A sex difference in the response to fasting. Physiol Behav 103: 530–534, 2011 [DOI] [PubMed] [Google Scholar]
- 821.Zeleznik AJ, Pohl CR. Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Knobil and Neill's Physiology of Reproduction (3rd ed.), edited by Neill JD. Amsterdam, The Netherlands: Elsevier/Academic, 2006, p. 2449–2510 [Google Scholar]
- 822.Zellner DA, Garriga-Trillo A, Centeno S, Wadsworth E. Chocolate craving and the menstrual cycle. Appetite 42: 119–121, 2004 [DOI] [PubMed] [Google Scholar]
- 823.Zellner DA, Garriga-Trillo A, Rohm E, Centeno S, Parker S. Food liking and craving: A cross-cultural approach. Appetite 33: 61–70, 1999 [DOI] [PubMed] [Google Scholar]
- 824.Zhang J, Matheny MK, Tumer N, Mitchell MK, Scarpace PJ. Leptin antagonist reveals that the normalization of caloric intake and the thermic effect of food after high-fat feeding are leptin dependent. Am J Physiol Regul Integr Comp Physiol 292: R868–R874, 2007 [DOI] [PubMed] [Google Scholar]
- 825.Zucker I. Progesterone in the experimental control of the behavioural sex cycle in the female rat. J Endocrinol 38: 269–277, 1967 [DOI] [PubMed] [Google Scholar]
- 826.Zucker I, Beery AK. Males still dominate animal studies. Nature 465: 690, 2010 [DOI] [PubMed] [Google Scholar]