Abstract
Nitric oxide (NO) is metabolized in plasma, in part by the ferroxidase ceruloplasmin (Cp), to form nitrite and nitrosothiols (SNOs), which are proposed to mediate protective responses to hypoxia and ischemia. We hypothesized that NO metabolism would be attenuated in fetal plasma due to low Cp activity. We measured Cp concentrations and activity in plasma samples collected from adults and fetuses of humans and sheep. We then added NO ([NO]: 1.5 or 100 μM) to plasma and aqueous buffer and measured rates of NO disappearance and the production of nitrite and SNO. Cp concentrations in fetal plasma were <15% of adult levels. In aqueous buffer, 1.5 μM NO disappeared with a half-life of 347 ± 64 s (means ± SE) but in plasma of humans the half-life was 19 ± 2 s (adult) and 11 ± 1 s (fetus, P = 0.004) and in sheep it was 31 ± 3 s (adult) and 43 ± 5 s (fetus, P = 0.04). Cp activity was not correlated with the overall elimination half-life of NO or with the amount of SNO ([NO]: 100 μM) or nitrite ([NO]: 1.5 or 100 μM) produced but correlated with SNO yields at 1.5 μM [NO] (r = 0.92, P = 0.04). Our data demonstrate that Cp is not essential to the increased rate of metabolism of NO in plasma relative to aqueous buffers and that it is not essential to the production of nitrite from NO. Cp may be involved in the conversion of NO to SNO in plasma under near-physiological concentrations of NO.
Keywords: ceruloplasmin, nitrite, nitrosothiol, fetal plasma
nitric oxide (no) is produced by the vascular endothelium and diffuses either abluminally into the adjacent vascular smooth muscle cells to cause vasodilation or luminally into the flowing blood (23). In the blood, NO is rapidly oxidized to nitrate by reaction with oxyhemoglobin (8) or binds with high affinity to the heme groups of deoxygenated hemoglobin to produce iron-nitrosyl hemoglobin (7). Both reactions proceed at nearly diffusion-limited rates and thus would severely limit the vasoactive effects of free NO. However, encapsulation of hemoglobin within the erythrocyte slows the diffusion of NO to the hemoglobin by a factor of ∼800 (16, 25, 48). This results in increased plasma NO concentrations, where it is metabolized to bioactive products such as nitrite (NO2−) and S-nitrosothiols (SNO; Refs. 38, 43, 49). Increasing evidence suggests that these NO metabolites play an important endocrine role in the regulation of blood flow and provide tissue protection during hypoxic and ischemic stress (28), yet questions remain about the specific mechanisms underlying their production from NO in plasma.
In oxygenated aqueous buffer, NO reacts with O2 to yield nitrite according to the following reaction:
| (reaction 1) |
This reaction, which is second order with respect to NO, has an overall rate constant of 2 to 4 × 106 M−2·s−1 (11, 24). However, at physiological concentrations of NO and O2, the reaction is too slow to account for the rate of NO disappearance from plasma (38, 43). One possibility is that plasma contains one or more metal-containing enzymes that would catalyze the oxidation of NO to nitrite or facilitate nitrosation chemistry resulting in the production of SNO. Ceruloplasmin is one enzyme proposed to play this role (19, 43).
Ceruloplasmin is a copper-containing multidomain protein best known as a plasma ferroxidase instrumental in the oxidation of Fe(II) to Fe(III) (34). An analogous one-electron oxidation of free radical NO by ceruloplasmin to form the nitrosonium ion (NO+) has been proposed to play an important role in the plasma metabolism of NO (19, 35, 43). The resulting NO+ may then nitrosate thiols in a first-order reaction with a rate constant of ∼1010 M−1·s−1 (32) to produce SNO:
| (reaction 2) |
However, reaction 2 is likely to only occur in hydrophobic phases, as NO+ also reacts with water at a nearly diffusion-limited rate to undergo a hydration reaction that results in nitrite (2).
| (reaction 3) |
| (reaction 4) |
There is some evidence that ceruloplasmin can contribute to the production of SNO as shown in reaction 2 (19, 35). However, other work indicates that, in plasma, ceruloplasmin predominantly catalyzes the conversion of NO to nitrite (reactions 3 and 4), with SNO constituting only a minor fraction of the products (43).
Plasma ceruloplasmin levels and activity vary significantly with developmental age in species including human (27, 36) and sheep (26). In humans, ceruloplasmin oxidase activity in adult plasma is >40-fold greater compared with newborns (36). The present study capitalizes on the difference between fetal and adult ceruloplasmin oxidase activities in humans and sheep to test the hypothesis that ceruloplasmin plays a predominant role in the overall metabolism of NO. Specifically, we compared rates of NO disappearance and the production of nitrite and SNO in plasma collected from adults or from cord blood of term newborns at the time of cesarean section for both humans and sheep.
MATERIALS AND METHODS
All procedures were conducted in accordance with animal protocols preapproved by the Loma Linda University Institutional Animal Care and Use Committee and human protocols preapproved by the Loma Linda University Institutional Review Board.
Chemicals and reagents.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. The NO donor PROLI 1-(hydroxy-NNO-azoxy)-l-proline disodium salt (NONOate) was obtained from Cayman Chemicals (Ann Arbor, MI).
Blood and tissue collection.
We conducted experiments using plasma and serum from four sources. Blood was collected from the external jugular vein of adult sheep. Anesthesia was then induced with an injection of pentobarbital sodium (10 mg/kg) followed by intubation and ventilation with 1–2% isoflurane balanced with 100% O2. Fetal sheep at 138–141 days of gestation (term = 145) were delivered through a midline incision in the maternal abdomen, and fetal blood was collected from the umbilical cord upon exposure. Liver samples were collected from nonpregnant ewes and fetal sheep for PCR analysis of ceruloplasmin.
Adult human blood was collected by forearm venipuncture of healthy volunteers after obtaining written informed consent. Following uncomplicated cesarean section, fetal human blood was collected from the umbilical vein of placentas.
Blood samples were collected into heparinized syringes and kept at room temperature for 30–40 min, during which time the native nitrite and SNO concentrations fell below the lower limit of detection (20 nM) (47). Plasma was then prepared by centrifugation of the blood at 2,500 rpm for 15 min at 4°C. To obtain serum, nonheparinized blood was first allowed to coagulate at room temperature for 30–40 min. Unless otherwise stated, all experiments were performed at room temperature with samples equilibrated to room air.
Ceruloplasmin oxidase activity measurement.
Ceruloplasmin oxidase activity was measured using methods described by Schosinsky et al. (42), as modified by Gorin and Gould (12). In this assay ceruloplasmin oxidizes the substrate o-dianisidine dihydrochloride to a colored substance that can be detected spectrophotometrically. Two tubes, each containing 750 μl of acetate buffer (pH 5.5) and 50 μl of serum were incubated at 30°C. Two hundred microliters of o-dianisidine reagent (250 mg/dl) were than added to each tube. By addition of 2 ml of sulfuric acid (9 M), the reaction in one tube was stopped after 5 min and in the other tube after 15 min. The absorbance of each solution was measured at 540 nm, and the oxidase activity of ceruloplasmin was calculated by the change of absorbance from 5 to 15 min. Results were expressed in units of ceruloplasmin activity per liter (U/l). Deionized water served as negative control.
Ceruloplasmin mRNA measurement (RT-qPCR).
The presence of ceruloplasmin mRNA in adult and fetal sheep liver was measured using reverse transcription quantitative real-time PCR (RT-qPCR). Total RNA was isolated from ∼30 mg samples of liver using the RNeasy Plus Mini Kit (Qiagen, Gaithersburg, MD) as per manufacturer's protocol. Reverse transcription of total RNA (1 μg) was performed using the QuantiTect Reverse Transcription Kit (Qiagen, Gaithersburg, MD). Ceruloplasmin primers were 5′-GGC TCC CAT GTG GCA CCC AA-3′ and 5′-CTC TGC CCG CAC TGG CTC AC-3′, and SYBR Green master mixes were prepared for PCR. Each qPCR reaction was performed in triplicate with primers for ceruloplasmin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The latter, a household gene, was used as an internal standard. Quantification of template replication was performed by the LightCycler 1.5 system and software (Roche, San Francisco, CA). Ceruloplasmin cycle threshold (Ct) results were normalized to GAPDH values as difference of cycle thresholds (dCt). The specificity of the PCR primer sets was verified using dissociation curve analysis, and no-template controls were performed.
Ceruloplasmin protein measurement (Western immunoblot).
Ceruloplasmin protein was assessed in plasma samples using Western immunoblot. Whole plasma protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA). Sixty-five micrograms of protein were separated using SDS-PAGE and then transferred to nitrocellulose membranes (Millipore, Temecula, CA). The membranes were blocked overnight in 5% milk, incubated with primary antibody against ceruloplasmin (ab 47785; Abcam, Cambridge, MA) for 1 h in 5% milk, washed, and incubated with secondary antibody, for 1 h in 5% milk. Following an additional wash, SuperSignal West Dura chemiluminescent substrate (Pierce, Rockford, IL) was used to visualize immuno-complexed bands (FluorChem SP Alpha Innotech, San Leandro, CA). After antibody visualization, each blot was stained by submersion in Amido Black (0.03% napthol blue black in 3% acetic acid) for 3 min to stain all proteins. Images were analyzed using ImageJ (National Institutes of Health, Bethesda, MD), which enabled determination of the ceruloplasmin quantity as a percentage of the total amount of proteins detected on the gel (for details see Ref. 1).
NO elimination in plasma.
For these experiments, NO as released from a donor substance was added to PBS or plasma. We opted for the rapid-release NO donor PROLI NONOate (PROLI; half time = 1.8 s for NO release at pH 7.4 and 37°C, with each molecule of PROLI releasing 2 molecules of NO). Previous reports have demonstrated that NO added to plasma disappears in a biphasic manner (39, 49), with the first ∼2 μM NO disappearing at a much more rapid rate (herein referred to as the “fast mode” of NO disappearance) than subsequent NO amounts (referred to as the “slow mode”). Therefore, to characterize NO metabolism in both the fast mode at low concentrations and the slow mode at concentrations >2 μM, these experiments were carried out at calculated initial NO concentrations of either 1.5 or 100 μM NO. This is referred to throughout the text as “addition” of 1.5 or 100 μM NO. Where indicated, samples were exposed to saturating concentrations of carbon monoxide gas (CO) to block ferrous hemes from reacting with NO. This is in contrast to “native” samples, which were studied without prior exposure to CO.
We measured the disappearance of free NO in plasma and in PBS as follows. NO was introduced into plasma or PBS at 37°C in a sealed reaction vessel (Harvard Apparatus, Holliston, MA) provided with a built-in propeller to ensure an instantaneous homogenous mixture. The vessel was filled to the brim to minimize any potential headspace gas and closed with a gas-tight seal. It held a highly selective amperometric NO probe with response time of <1 s and a detection limit of ∼5 nM (ISO-NOP; World Precision Instruments, Sarasota, FL). Responses were acquired at 1 Hz, and the probe was tested before each experiment over a 0.2 to 5 μM [NO] range. In each experiment, the time course of NO elimination was recorded following the addition of 1.5 μM NO. It is assumed that the disappearance of free NO in solution, as measured by the NO probe, reflects NO autoxidation in aqueous buffer and “metabolism” in the plasma samples.
Measurement of NO metabolites.
NO metabolites were measured by triiodide chemiluminescence (NOA 280; Sievers, Boulder, CO), which detects NO and other nitrogen oxide species (NOx) that include nitrite, SNO, iron-nitrosyls, and N-nitrosyls, as previously described (30), but not nitrate. To distinguish between these species, combined nitrite and free NO concentrations were determined by measurement of NOx concentrations before and after treatment of the samples with acidified sulfanilamide (0.5% for 3 min), which selectively removes free NO and nitrite. Similarly, SNO concentrations were measured by the difference of NOx concentrations in sulfanilamide-treated samples before and after treatment with 5 mM HgCl2 (3 min), which selectively removes SNO. The NOx signal remaining after sulfanilamide and HgCl2 treatment was considered to reflect combined iron-nitrosyl and N-nitrosyl concentrations. To measure only free and reversibly bound NO, samples were injected into PBS (pH 7.4) in a purge vessel that had never been exposed to triiodide, a precaution taken to eliminate any potential triiodide reagent contamination and false-positive measurement of free and reversibly bound NO.
The measurements of NO metabolites were performed at two different time points: at 1 min after addition of 1.5 μM NO and at 30 min after addition of 100 μM NO. As detailed above, these time points were taken to reflect a “fast” and a “slow” mode of NO metabolism, respectively.
The effects of free hemoglobin in the plasma on the metabolism of NO were evaluated by preequilibration of plasma samples with a many thousandfold molar excess of CO. This prevents the reaction of NO with iron heme because CO binds tightly to the heme center of hemoglobin.
Statistical analysis.
Results are presented as means ± SE. Differences in measured parameters were determined by one-way ANOVA or by Student's t-test. The disappearance of the first 90% of NO was used to calculate the apparent half-lives (t1/2, see below). Half-lives for the disappearance of NO were calculated by fitting a monoexponential equation (using Prism 5.0; Graphpad Software, La Jolla, CA) to the NO concentration vs. time curve measured following addition of PROLI. Half-life determinations in plasma were made from peaks observed following saturation of the fast mode of NO metabolism. Half-lives were determined for each plasma sample separately and then averaged by group. The Pearson correlation coefficient was determined for comparisons between ceruloplasmin activity and rates of NO metabolism or production of nitrite and SNO. Because ceruloplasmin activities were measured in different samples than those used for measurements of NO metabolism, the Pearson correlation was performed using mean values of these parameters for each of the four groups studied. Statistical analyses were carried out using Prism 5.0 with significance accepted at P ≤ 0.05.
RESULTS
Ceruloplasmin abundance, expression, and activity.
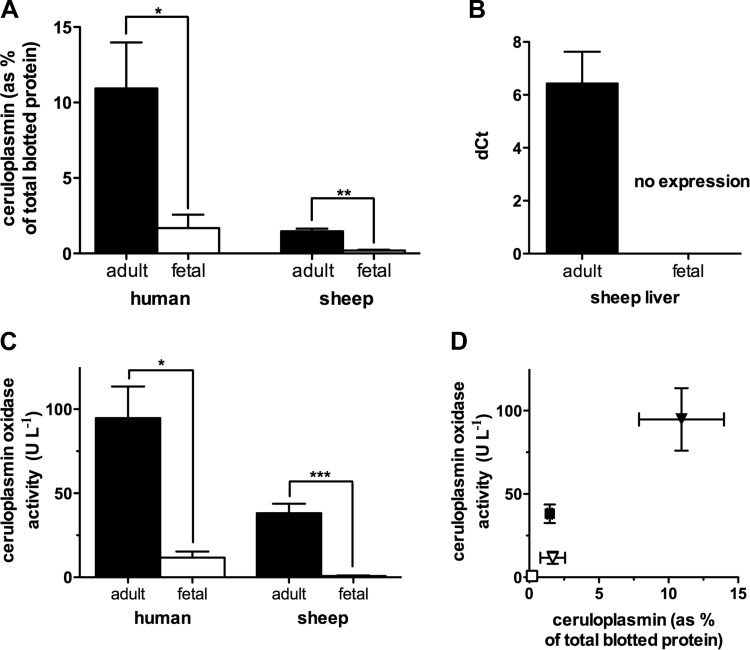
To quantify differences between adult and fetal ceruloplasmin, we measured abundance, expression, and oxidase activity. Total ceruloplasmin protein in plasma was measured by Western immunoblot using antibodies reactive to both mature, functional holoceruloplasmin and immature, nonfunctional apoceruloplasmin. Total ceruloplasmin levels in human fetal plasma averaged 15 ± 8% of adult levels (P = 0.04) and in fetal sheep plasma levels were 13 ± 2% of the adult (P = 0.003; Fig. 1A).
Fig. 1.
Characterization of ceruloplasmin in adult and fetus. A: Western immunoblot data of plasma ceruloplasmin expressed as a percentage of total blotted protein (n = 3 and 4 for adult and fetal samples, respectively). Ceruloplasmin amounts were ∼7-fold lower in fetal plasma for both species, with P ≤ 0.05 for human and P ≤ 0.005 for sheep. B: quantitative real-time PCR (RT-qPCR) showed detectable adult sheep liver ceruloplasmin mRNA [difference of cycle thresholds (dCt) of 6.4 ± 2.7] while fetal samples did not show expression (n = 6). C: all serum samples except those from fetal sheep showed significant ceruloplasmin oxidase activity compared with that in de-ionized water (P ≤ 0.0001). Human adult serum (n = 6) had a significantly greater ceruloplasmin oxidase activity than human fetal serum (n = 4) with P ≤ 0.05. Sheep adult serum (n = 6) showed significant ceruloplasmin oxidase activity while sheep cord blood serum did not (n = 8, P ≤ 0.0001). D: ceruloplasmin oxidase activity correlates to the relative amount of ceruloplasmin in plasma (r = 0.95, P ≤ 0.05). Symbols indicate sheep: fetal (□) and adult (■); and human: fetal (▽) and adult (▼). *P ≤ 0.05, **P ≤ 0.005, and ***P ≤ 0.0001.
Ceruloplasmin is synthesized predominantly in the liver (10, 26, 41). Therefore, ceruloplasmin mRNA levels were measured by RT-qPCR in samples of adult and fetal sheep liver. Adult samples had a positive reaction indicative of the target nucleic acid (dCt = 6.4 ± 1.2), whereas fetal samples showed no detectable target (Fig. 1B).
Ceruloplasmin oxidase activity, measured in human serum, was eightfold greater in adult serum than in fetal serum (95 ± 19 vs. 11.7 ± 3.6 U/l; P = 0.008). The difference between adult and fetal serum was also observed in sheep, with adult values averaging 38.1 ± 5.6 U/l, whereas the oxidase activity measured in umbilical cord serum was not different from that in deionized water (serum: 0.8 ± 0.3 U/l; water: 0.4 ± 0.3 U/l; Fig. 1C). Interspecies differences were also significant with the human serum having more ceruloplasmin activity than the sheep (all differences P < 0.05; significances are not included on graph).
Ceruloplasmin oxidase activity relates directly to the amount of ceruloplasmin in plasma (r = 0.95; P < 0.05; Fig. 1D). While human fetal and sheep adult plasma have comparable amounts of ceruloplasmin protein, the lower ceruloplasmin oxidase activity of human fetal plasma is in line with previous observations that part of the fetal ceruloplasmin is nonfunctional apoceruloplasmin (27). These results are consistent in showing higher concentrations of ceruloplasmin in humans compared with sheep and the lack of this protein's presence in the near-term fetal sheep.
Rate of NO metabolism in plasma.
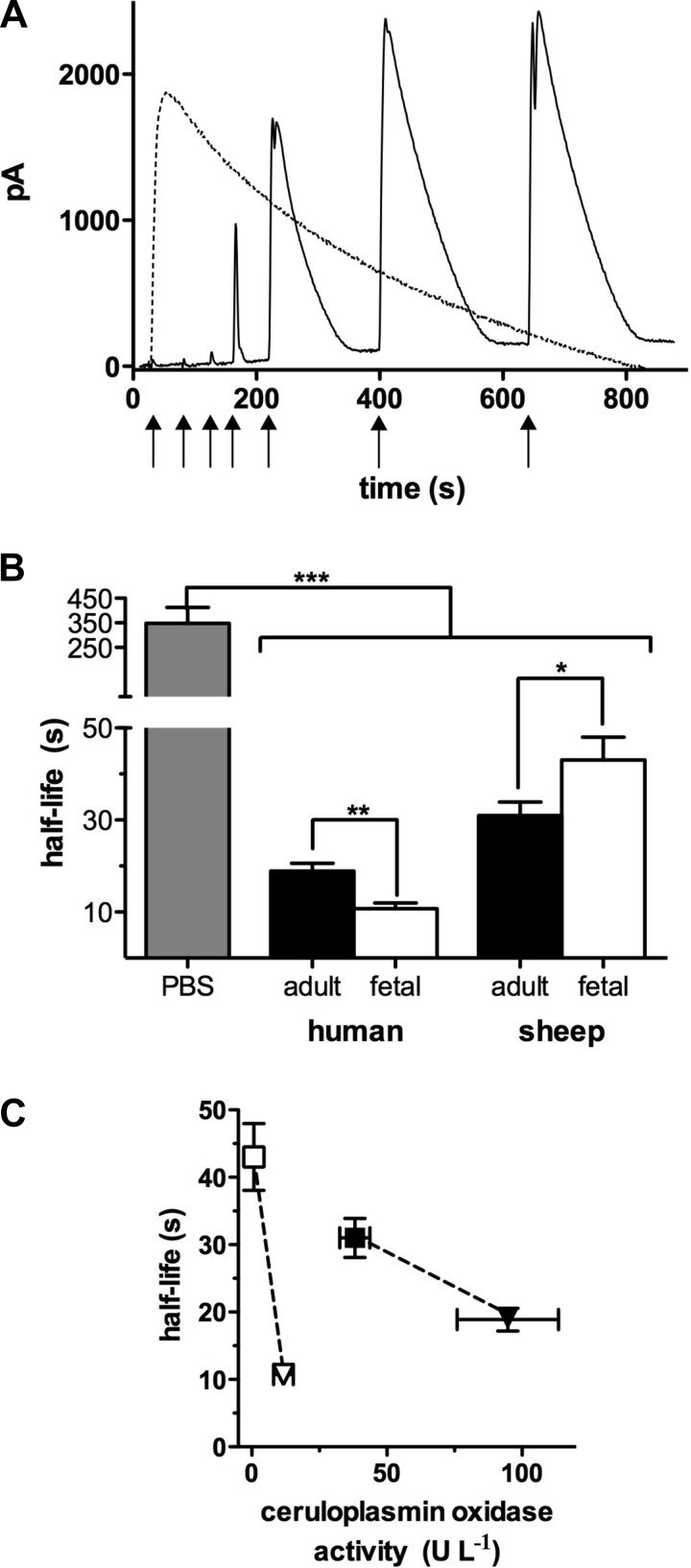
When 1.5 μM NO were added to aqueous buffer, NO was detected immediately by the NO probe and then disappeared with an apparent half-life of 347 ± 64 s (Fig. 2A, dashed black line). However, following a similar injection of 1.5 μM NO into either native or CO-treated plasma, free NO in solution was nearly undetectable. This indicates that NO was eliminated completely within the response time of the probe (1 s), consistent with the fast mode of NO metabolism. In this example of native plasma, NO became detectable after three more NO injections of the same amount each time (Fig. 2A, black line). On average, in adult sheep plasma pretreated with CO, the amount of NO that was injected before saturation of the fast mode of metabolism occurred was 2.3 ± 0.4 μM. When saturation of the fast mode reaction had occurred; further, 1.5-μM injections of donor resulted in reproducible NO peaks from which the half-life of the slow mode of NO elimination was calculated. Figure 2B shows that NO disappeared from human adult plasma with a half-life nearly twice that of fetal plasma (t1/2 = 19 ± 2 s vs. 11 ± 1 s; P = 0.004). In contrast, NO disappeared significantly faster from adult sheep plasma than fetal plasma (t1/2 = 31 ± 3 vs. 43 ± 5 s; P = 0.04). These values reflect the slow mode of NO metabolism. Interspecies differences were also significant with human plasma exhibiting a shorter half-life than sheep plasma for both adult and fetal groups (P = 0.01 and P = 0.0009, respectively, not on graph). As shown in Fig. 2C, there was an inverse relationship between ceruloplasmin oxidase activity and NO half-life for fetuses, and for adults.
Fig. 2.
Nitric oxide (NO) disappearance curves. A: representative example of NO disappearance curves in PBS (dashed black line) and in native plasma (fetal sheep; black line). In PBS, injection of 1.5 μM NO (↑) immediately resulted in a full peak with a half-life of ∼350 s. In plasma, three injections of NO (each to achieve an initial NO concentration of 1.5 μM) were required before the probe current rose distinctly at the 4th injection. Starting with the 6th injection, probe responses were uniform, with an elimination half-life significantly shorter than in PBS. Plasma thus exhibits a distinct, fast and saturatable NO-metabolizing capacity (fast mode) not measurable with the amperometric probe. B: apparent t1/2 of NO in plasma from all groups, determined at full peak, was significantly shorter than in PBS (n = 6, P ≤ 0.0001), with an average rate of disappearance 17.5 ± 5.4 times faster in plasma than in PBS. The t1/2 of NO in human fetal plasma was significantly shorter than in human adult plasma (n = 5 and 6, respectively, P ≤ 0.005), whereas in sheep the t1/2 was significantly shorter in maternal plasma than in fetal plasma (n = 12 for both, P ≤ 0.05). C: there is no significant overall relationship between the plasma half-life of NO and ceruloplasmin oxidase activity (r = −0.36; P = 0.64) but within the group of fetuses (□, ▽) and adults (■, ▼), half-lives are higher at lower ceruloplasmin activities. Dashed lines highlight the comparison of the fetal and adult samples between species. *P ≤ 0.05, **P ≤ 0.005, and ***P ≤ 0.0001.
Conversion of NO to nitrite and SNO in plasma.
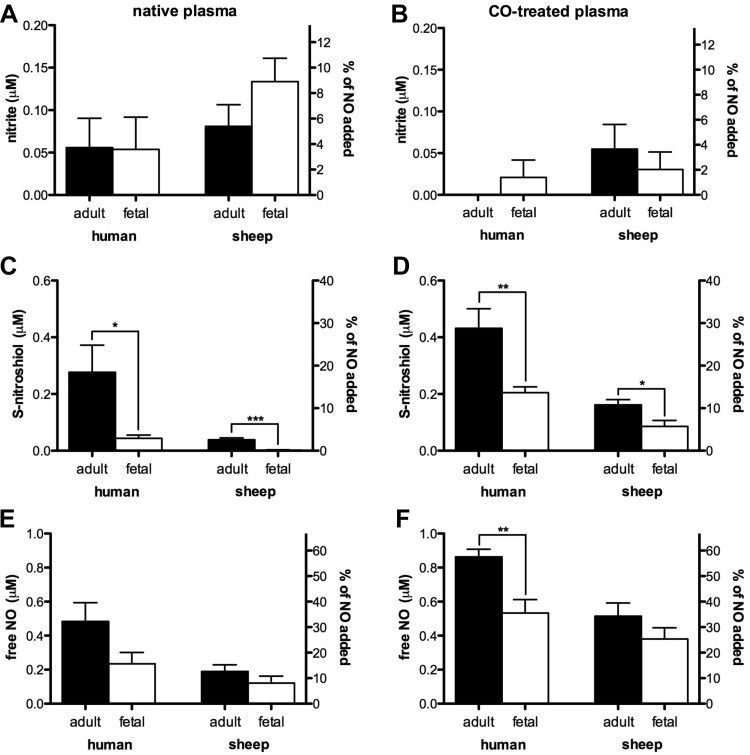
Initial endogenous NO, nitrite, and SNO concentrations were below the 20 nM lower limit of detection. Therefore, the products below reflect exclusively the metabolism of exogenous NO. To determine the products of the fast mode of NO metabolism, nitrite and SNO concentrations were measured 2 min after addition of 1.5 μM NO. At this concentration, a majority of the NO would be metabolized by the fast mode reaction. Also, we addressed the possibility that the rapid elimination of NO was due to hemoglobin present in the plasma as a result of hemolysis occurring during collection and handling of the samples. The experiments were performed with and without pretreatment of the plasma with CO, which renders the heme unavailable for reaction with NO. As shown in Fig. 3A, <5% of the 1.5 μM NO added to native human adult or fetal plasma was converted to nitrite, a yield not significantly greater than zero. Similar results were obtained following addition of NO to plasma pretreated with CO (Fig. 3B). Addition of 1.5 μM NO to native sheep adult or fetal plasma resulted in 0.085 ± 0.03 and 0.13 ± 0.03 μM nitrite, respectively, about 5–9% of the NO added. Pretreatment of the sheep plasmas with CO resulted in a reduction of nitrite yields to levels that were not significantly greater than zero. Except for the decrease in fetal sheep plasma nitrite (P = 0.02; not on graph), the decreases following CO treatment were not significant.
Fig. 3.
NO metabolites formed during the fast mode reaction, without (native plasma) and with (CO-treated plasma) equilibration with carbon monoxide. A: nitrite yield from plasma after 1-min incubation with 1.5 μM NO in native samples is not significantly different from zero, except for fetal sheep samples where a maximum of 9 ± 1.8% of NO injected was recovered as nitrite. ANOVA did not detect significant differences between groups. C: significantly more S-nitrosothiol (SNO) was measured in adult vs. fetal samples for both humans and sheep, with a maximum of 18.5 ± 6% of NO recovered as SNO (human adult). E: amount of free and reversibly bound NO detected in the samples ranged from 8 ± 3% (fetal sheep) to 32 ± 7% (human adult) but there were no significant differences between the four groups. When samples were pretreated with CO, there was a tendency of reduced nitrite production (B) and of increased yield of SNO (D) and free and reversibly bound NO (F), a trend that became significant in CO-treated human adult plasma (57.5 ± 3%, cf. E and F); in the latter 2 cases the difference between adult and fetal samples was maintained (sheep adult n = 11 and fetal n = 9; human adult n = 6 and fetal n = 7 for native plasma; and n = 6 except for sheep adult with n = 8 for CO-treated plasma). *P ≤ 0.05, **P ≤ 0.005, and ***P ≤ 0.0001.
Addition of 1.5 μM NO to native human adult and fetal plasma resulted in the production of 0.28 ± 0.1 and 0.044 ± 0.01 μM SNO, respectively (P = 0.02; Fig. 3C). In native sheep adult and fetal plasma, 1.5 μM NO produced 0.039 ± 0.006 and 0.002 ± 0.001 μM SNO, respectively (P < 0.0001). In addition, pretreatment of plasma with CO (Fig. 3D) resulted in significantly increased SNO yields in all four study groups (P < 0.001, statistics not shown on graphs), with the adult plasmas still producing more SNOs than the fetal plasmas.
Although NO was not detected by the amperometric probe following the initial addition of 1.5 μM PROLI to native plasma (see Fig. 2A), free and reversibly bound NO was detected by chemiluminescence in plasma samples injected into a purge vessel containing PBS. The amount of NO detected by this method, 1 min after addition of 1.5 μM NO tended to be greater in native adult human and sheep plasmas (0.48 ± 0.11 and 0.19 ± 0.04 μM, respectively) compared with fetal human and sheep plasmas (0.23 ± 0.07 and 0.12 ± 0.04 μM, respectively), although the differences were not statistically significant (Fig. 3E). Treatment of the plasma with CO resulted in an increase in free and reversibly bound NO concentrations in all four groups (P = 0.01 for human adult and fetal samples, P = 0.0008 for sheep adult samples, and P = 0.004 for fetal samples, not on graph). In CO-treated plasma, the amount of free and reversibly bound NO was significantly greater in human adult plasma compared with fetal (P < 0.005), but no difference was detected between sheep adult and fetal plasmas (Fig. 3F). No significant amounts of iron-nitrosyl or N-nitrosyl species were detected in any of the groups (data not shown). The portion of the 1.5 μM NO that was unaccounted for by nitrite, SNO, or free and reversibly bound NO was presumably converted to triiodide-stable nitrate. This portion measured 0.71 ± 0.2 μM and 1.17 ± 0.08 μM in human adult and fetal plasma (P = 0.03), respectively, and 1.19 ± 0.04 and 1.24 ± 0.04 μM in sheep adult and fetal plasma, respectively. After pretreatment of samples with CO, the amount of the 1.5 μM NO unaccounted for by nitrite, SNO, or free NO was 0.09 ± 0.05 and 0.6 ± 0.09 μM in human adult and fetal plasma (P < 0.001), respectively, and 0.65 ± 0.08 and 0.94 ± 0.06 μM in sheep adult and fetal plasma (P = 0.02), respectively (data not shown).
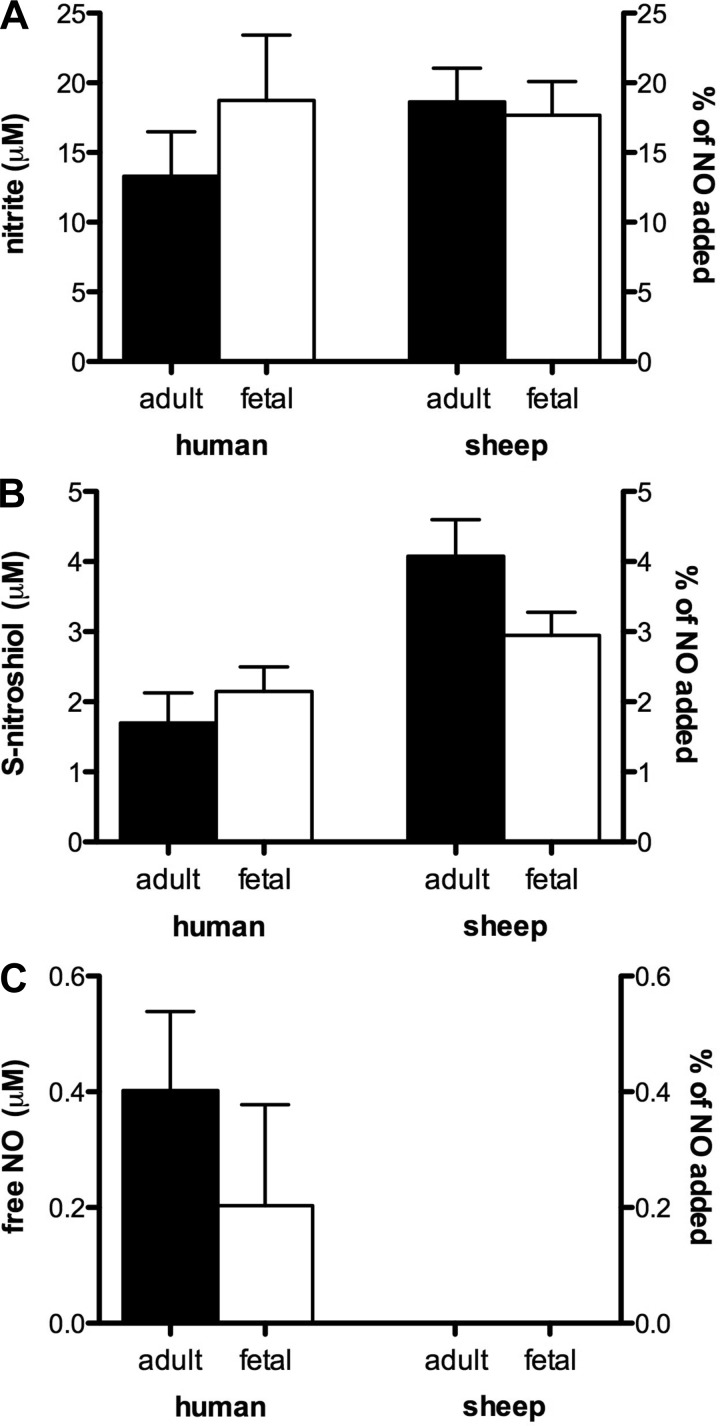
To assess the products of NO metabolism by the slow mode that predominates at cumulative NO concentrations above ∼2 μM, additional experiments were conducted in plasma using 100 μM NO (Fig. 4). After 30 min of incubation, there were no detectable differences between the four plasma groups with respect to nitrite or SNO production, with 15–20% of the added NO recovered as nitrite (Fig. 4A) and 2–4% recovered as SNO (Fig. 4B). No significant amounts of free and reversibly bound NO remained in any of the four study groups (Fig. 4C). As with the addition of 1.5 μM NO, there were no detectable levels of iron-nitrosyl or N-nitrosyl species in any samples. The portion of the added NO that was converted to triiodide-stable species (e.g., nitrate) was 84.5 ± 4 and 78.8 ± 5 μM in human adult and fetal plasma respectively (no significant difference) and 77.2 ± 3 and 79.4 ± 3 μM in sheep adult and fetal plasma respectively (no significant difference).
Fig. 4.
NO metabolites formed during the slow mode reaction in native plasma. A: 30-min incubation of 100 μM NO in native plasma produced nitrite concentrations in the four study groups ranging from 13 ± 3 μM (human adult) to 19 ± 5 μM (human fetus), with no significant differences between the groups. B: SNO production ranged from 2 ± 0.4 μM (human adult) to 4 ± 0.5 μM (sheep adult) without any significant differences between adult and fetal groups. C: direct measurement of free and reversibly bound NO yielded <0.4% of injected NO remaining in plasma, a value not significantly different from zero (n = 3 except for human adult with n = 4).
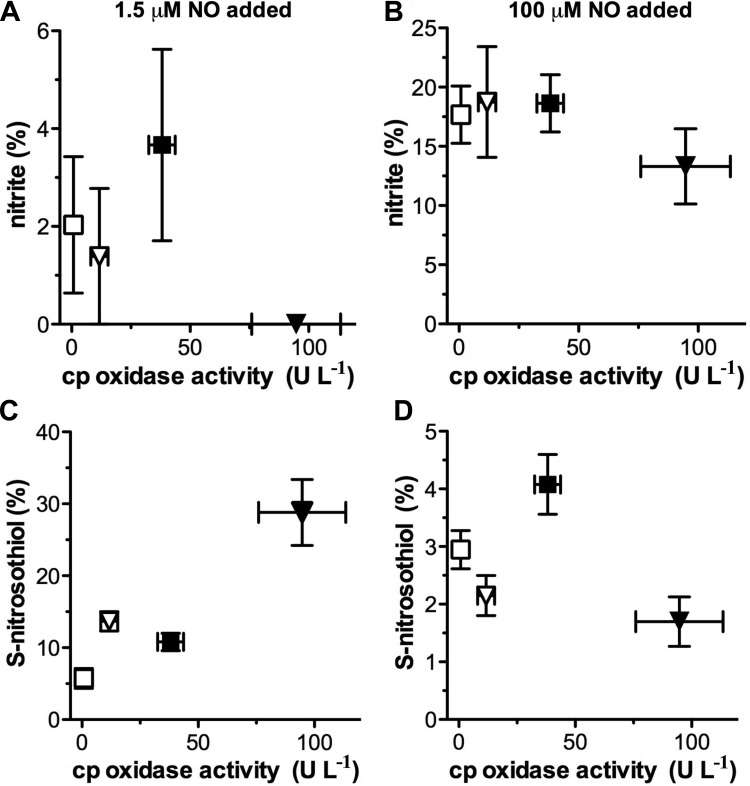
Correlation of ceruloplasmin oxidase activity with nitrite and SNO formation.
To summarize the comparison between ceruloplasmin oxidase activity and production of either nitrite or SNO, we plotted the means ± SE values measured for ceruloplasmin oxidase activity (U/l) in serum (Fig. 1C) against the means ± SE of nitrite or SNO measured after addition of 1.5 μM to plasma pretreated with CO (Fig. 3, B and D) or after 100 μM NO in native plasma (Fig. 4, A and B) for each of the four groups (Fig. 5). Ceruloplasmin oxidase activity of serum preequilibrated with CO did not differ from that of native serum (result not shown).
Fig. 5.
Relationship between ceruloplasmin (cp) oxidase activity and nitrite and SNO formation during the fast (A and C) and slow (B and D) NO reaction mode. Ceruloplasmin oxidase activity (Fig. 1C) is plotted against yields of nitrite and SNO (Fig. 3, B and D). There was no significant correlation between nitrite production and ceruloplasmin activity following addition of either 1.5 μM (A) or 100 μM NO (B). There was also no significant correlation between ceruloplasmin activity and SNO production following addition of 100 μM NO (D). However, SNO production was significantly correlated with ceruloplasmin activity following addition of 1.5 μM NO (C; r = 0.92, P = 0.04). Notably, this is associated with an absent nitrite production (cf. Figs. 3B and 5A) at high oxidase activity values. Symbols indicate sheep: fetal (□) and adult (■); and human: fetal (▽) and adult (▼).
Although ceruloplasmin oxidase activity varied considerably, there was no correlation with nitrite production either during the fast (1.5 μM NO) or slow NO reaction (100 μM NO) as shown in Fig. 5, A and B. Likewise, SNO production following addition of 100 μM NO did not vary significantly between adult and fetal plasmas (Fig. 5D). However, SNO concentrations during the fast mode reaction period increased significantly in both human and sheep adult plasma compared with their fetal counterparts, resulting in a positive correlation between ceruloplasmin oxidase activity and SNO production from NO (Fig. 5C; r = 0.918; P = 0.04). This correlation with SNO production is possible despite the lack of correlation with the total metabolism rate since only a relatively small fraction of NO is metabolized to SNO.
DISCUSSION
Ceruloplasmin is proposed to play a key role in the conversion of NO to nitrite and SNO in the plasma. Because fetal ceruloplasmin levels are markedly lower than those of adults, the current experiments test the hypothesis that the production of nitrite and SNO from NO and the overall metabolism of NO will be different in fetal and adult plasma. The principle findings are 1) while the study groups have a wide variation in ceruloplasmin levels and activity, there are no differences in the amounts of nitrite produced from NO; 2) similar to ceruloplasmin oxidase activity, production of SNO from near-physiological concentrations of NO is higher in adults of both species; and 3) within adults or fetuses, overall metabolism of NO is lower at low ceruloplasmin oxidase activity levels. This suggests NO metabolism is multimodal and is not mediated by a single function like that of ceruloplasmin within each of the four experimental groups.
Ceruloplasmin concentrations and oxidase activity.
We found no evidence of ceruloplasmin transcription in the fetal sheep liver. This result confirms the finding by Lockhart and Mercer (26), who by RNA hybridization did not identify hepatic expression of ceruloplasmin RNA in fetal sheep. Although Western immunoblot detected a small amount of ceruloplasmin protein in fetal plasma of both humans and sheep, at that stage of development it is likely to be apoceruloplasmin from extrahepatic sources, which is known to be void of oxidase activity (50). Accordingly, we were not able to detect any ceruloplasmin oxidase activity in fetal sheep plasma, and activity in human fetal plasma was ∼12% of that in the adult. This is consistent with radial immunodiffusion results of Louro et al. (27) who measured ceruloplasmin oxidase activity in 50 full-term newborns to be ∼16% of adult levels.
Overall rate of NO metabolism.
Consistent with previous reports (38, 49), the current study demonstrates that, even after saturation of the initial fast mode of clearance of NO from plasma, NO disappears from fetal and adult plasma by a reaction at a rate that is nearly 10–30 times faster than reaction 1 in PBS. Although ceruloplasmin has been demonstrated previously to catalyze the production of both nitrite and SNO, our study suggests that it does not play an exclusive role in the overall rate of NO clearance. Specifically in sheep fetus, with no plasma ceruloplasmin oxidase activity, NO clearance is still distinctly faster than in PBS, and in the human fetus, which has some ceruloplasmin activity, the NO metabolic rate is higher than its respective adult, which has a high ceruloplasmin activity. This indicates that factors besides ceruloplasmin level, which differ between the fetus and adult, affect NO metabolism. For example, the heme of hemoglobin has been proposed to catalyze SNO formation (6), and the adult and fetus have distinct hemoglobin species raising the possibility that fetal hemoglobin in the plasma promotes the formation of SNO more efficiently than adult hemoglobin. However, the fact that the greater SNO forming capacity of adult plasma compared with fetal plasma holds true in samples equilibrated with excess CO makes the involvment of hemoglobin or any other heme-containing proteins less likely and other factors yet unknown may be involved.
Production of nitrite by ceruloplasmin.
Plasma nitrite concentrations are in the low- to mid-nanomolar range (approximately 100–600 nM/l) in most mammalian species studied to date (20). Up to 80% of plasma nitrite is derived from NO generated by endothelial NO synthase (20, 40), suggesting that the oxidation of NO to nitrite in plasma is a key contributor to plasma nitrite concentrations. Nitrite concentrations in placental cord blood plasma are the same as those of the mother in both sheep (4) and humans (unpublished data from our laboratory) at the time of birth. Although nitrite can cross the placenta (45), the extent to which fetal plasma nitrite concentrations are derived from the transfer of nitrite from maternal blood via the placenta is unknown. Plasma concentrations in the human newborn fall by ∼30% within 12 h after birth (4), but again the role that plasma NO oxidation to nitrite plays is unclear as little is known about how overall production of NO by edothelial NO synthase varies during the first few hours of life.
Work by Shiva et al. (43) has implicated plasma ceruloplasmin as a catalyst for the production of nitrite from NO. Their studies demonstrated that removal of ceruloplasmin from human plasma by immunodepletion decreased the yield of nitrite from 100 μM NO at 30 min to levels comparable to those in saline buffer and that addition of ceruloplasmin to PBS or immunodepleted plasma (both containing erythrocytes at 50% hematocrit) increased nitrite yields. Similarly, endogenous plasma nitrite concentrations are significantly lower in both ceruloplasmin knockout mice and in humans with a genetic mutation resulting in aceruloplasminemia (43). Our current results indicate that ceruloplasmin is not a major catalyst for oxidation of NO to nitrite in fetal plasma because fetal levels were undetectable or nearly 10-fold less than the adult, and yet we measured similar nitrite yields following addition of NO to fetal and adult plasmas. It seems likely that reaction 1, which is indepedent of ceruloplasmin and too slow to account for the overall NO metabolic rate, is still sufficient to generate the small amounts of nitrite (<9%) from NO observed in our study.
Production of SNO by ceruloplasmin.
The reaction of nitrogen oxides with thiols to form SNO has significant ramifications on the bioactivity of NO in blood because many of these SNOs preserve the bioactivity of NO while protecting it from scavenging reactions such as those with oxyhemoglobin. Since the first measurement of SNO in plasma nearly two decades ago (46), much research has focused on the vasoactive role of circulating species ranging from small molecular weight nitrosoglutathione (GSNO) and nitrosocysteine to large proteins such as SNO-albumin and SNO-hemoglobin (15). However, despite growing interest in the physiological roles of SNO, the mechanism(s) by which they are formed is not fully understood.
Free radical NO is not effective as a thiol nitrosating agent without first being oxidized either by reaction with O2 to form higher nitrogen oxides such as NO2, N2O3, and N2O4 (5) or by reaction with transition metals to form nitrosonium (NO+). Inoue et al. (19) reported evidence that ceruloplasmin catalyzes the production of glutathione-SNO in the presence of glutathione and free radical NO by transfer of the unpaired NO electron to the type I copper of ceruloplasmin. The resulting NO+ is proposed to nitrosate nearby thiols. However, at physiological pH NO+ cannot exist as a free species in aqueous solutions as NO+ reacts with water at a nearly diffusion-limited rate to form nitrite (2, 17). Hence, its reaction with a sulfhydryl does not appear to be kinetically feasible in aqueous solutions without a hypothetical concerted coordination of the thiol at the site of NO+ formation. Despite this mechanistic constraint, we found a positive correlation between ceruloplasmin oxidase activity and SNO formation 1 min after addition of 1.5 μM NO (Fig. 5C). The fetal sheep plasma had no measurable ceruloplasmin oxidase activity and no measurable SNO formation. Similarly, human fetal samples had 12.3% of the ceruloplasmin oxidase activity of their adult counterparts and correspondingly produced 15.8% as much SNO. The SNO concentrations recovered after a 1-min incubation of 1.5 μM NO (i.e., resulting from fast mode metabolism) are consistent with previously published results (38). These data are also in agreement with previous evidence that ceruloplasmin purified from numerous species and cell lines catalyzes SNO formation from free radical NO (19, 35). The SNO concentrations we measured after a 30-min incubation of 100 μM NO (i.e., resulting from slow mode metabolism) are in line with previous reports (38, 43).
In contrast to our experiments with 1.5 μM NO, no correlation between ceruloplasmin oxidase activity and SNO production was observed at 100 μM of NO, consistent with previous studies in which 100 μM NO was added to plasma that was either immunodepleted of or supplemented with ceruloplasmin (43). The difference between the results observed at near-physiological 1.5 μM vs. supraphysiological 100 μM NO concentrations may be due to a shift in the prevailing pathway of NO metabolism to reaction 1 instead of SNO-producing to reactions, as the rate constant of reaction 1 would be increased by nearly 100-fold due to the second-order dependence on NO concentrations. Additionally, 100 μM NO may be above the Km of ceruloplasmin for NO metabolism, although the Michaelis-Menten kinetics for this function of ceruloplasmin have not been characterized.
It should be noted that the observed correlations between SNO production and ceruloplasmin concentrations in fetal and adult plasma are merely associative and may be coincidental with some other differences that result in greater SNO formation in adult plasma. One possibility is that plasma thiol concentrations are greater in adult plasma compared with the fetus, resulting in more substrate for SNO production from NO. Alternatively, SNO production can be catalyzed effectively by free copper (19), raising the possibility that the difference between fetal and adult plasma is due to lower free copper in the fetal plasma. However, both of these possibilities are not supported by reports that the plasma thiol load and free copper concentrations are similar in both the fetus and adult (22, 37, 44). Another alternative pathway for SNO production is suggested in recent work by Kolesnik et al. (21), who provide evidence that SNOs can form by direct reaction of free radical NO with thiyl radicals (R-S) and that this reaction is of significance at physiological NO concentrations. This possibility cannot be excluded without further study.
The fast mode reaction and the effect of carbon monoxide.
It took multiple injections of 1.5 μM NO into plasma before free NO in solution became detectable with the amperometric probe (Fig. 2A). The fast disappearance of NO may be caused by an unspecified “fast mode” of reactions or may reflect a NO “sink” by compounds that are capable of tightly binding or sequestering NO quickly. The reactions of NO with the heme centers of oxy- and deoxyhemoglobin represent one possibility. Free hemoglobin is a normal constituent of plasma in the range of 0.5 μM (9), a level that may be higher in our plasma samples following their handling during preparation. NO will immediately bind to deoxyhemoglobin or will react with oxyhemoglobin to form nitrate, and thus an amperometric probe will not detect unbound NO until the available deoxy- and oxyhemoglobin are exhausted. It is also possible that a portion of the added free NO disappears due to reversibly binding to plasma constituents (such as binding to the heme of deoxyhemoglobin) or by reversible reactions such as the formation of labile nitrosothiols. In these cases an equilibrium will exist between free NO and the labile NO species, with the amperometric probe only measuring the portion of NO that is free in solution. In contrast, injection of the sample into the purge vessel will result in removal of the free NO, thus shifting the equilibrium towards liberation of the labile NO species. This possibility is supported by our finding that although the amperometric probe detected no free NO following injection of up to 1.5 μM NO (Fig. 2A), injection of the samples into a purge vessel resulted in the release of ∼0.2 to 0.5 μM NO as detected by chemiluminescence (Fig. 3E).
Because gases are distinctly more soluble in lipids than in water, it is also possible that the concentration of free NO in aqueous phases is reduced by the presence of lipid particles in plasma. The effect depends on the water:oil partition coefficient for NO and the type and concentration of lipids in plasma. The effect of plasma lipids was not quantified in the present experiments, but its role for NO clearance could be significant. This includes the possibility that the rate of the NO reaction with O2 may be accelerated in lipid micelles within the plasma, since both NO and O2 partition preferentially into hydrophobic lipid compartments, effectively increasing their concentrations. This “lens effect” has previously been demonstrated to increase the rate of NO autoxidation by up to 30-fold, leading to the production of intermediates such as NO2 and N2O3, both of which can react further in aqueous solutions to form nitrite (11, 31) or, in the case of N2O3, nitrosate thiol groups. Lipid concentrations in cord blood plasma are reportedly ∼50% lower than those of the adult (33), suggesting that the faster rate of NO disappearance in the human cord plasma in our study compared with adults is not related to lipid concentrations. Lipid concentrations were not measured in our study, and the impact of the lens effect on the ratio of nitrite and SNO production from NO in plasma is not known, making this a topic for further study.
Perspectives and Significance
The fetal cardiovasculature is in a high-flow, low-resistance state relative to that of the newborn and adult. It has been known for many years that endothelial-derived NO plays an essential role in maintaining low systemic vascular resistance in the fetus (3, 13, 14, 18). Increasing evidence suggests that the effects of NO are distributed throughout the body by its more stable metabolites such as nitrite and SNO. Furthermore, the bioactivity of these metabolites is heightened under hypoxic conditions (15, 29), lending to the possibility that they play a critical role in maintaining homeostasis in the relatively hypoxemic in utero environment of the fetus. To what extent these metabolites are derived from the maternal circulation or produced in the fetus itself, and how these metabolites are affected by removal of the umbilical circulation and the sudden increased oxygenation that occurs at parturition, remain to be determined. This study's finding that ceruloplasmin is not essential to the overall increased rate of NO disappearance in fetal plasma relative to aqueous buffers, and that it is not essential to the production of nitrite from NO, also renews the question of what major factors account for NO metabolism in plasma. One such factor, the impact of the presence of a hydrophobic lipid phase within the blood plasma, is under active investigation by our laboratory.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-095973 (to A. B. Blood).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.V., H.J.S., L.D.L., G.G.P., and A.B.B. conception and design of research; K.V. performed experiments; K.V. analyzed data; K.V., H.J.S., G.G.P., and A.B.B. interpreted results of experiments; K.V. and A.B.B. prepared figures; K.V. drafted manuscript; K.V., H.J.S., L.D.L., G.G.P., and A.B.B. edited and revised manuscript; K.V. and A.B.B. approved final version of manuscript.
REFERENCES
- 1.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172: 250–254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss NS, Watts DW. The spectra and equilibria of nitrosonium ion, nitro-acidium ion, and nitrous acid in solutions of sulphuric, hydrochloric, and phosphoric acids. Aust J Chem 9: 319–332, 1956 [Google Scholar]
- 3.Blood AB, Terry MH, Merritt TA, Papamatheakis DG, Blood Q, Ross JM, Power GG, Longo LD, Wilson SM. Effect of chronic perinatal hypoxia on the role of Rho-kinase in pulmonary artery contraction in newborn lambs. Am J Physiol Regul Integr Comp Physiol 304: R136–R146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG. Increased nitrite reductase activity of fetal versus adult ovine hemoglobin. Am J Physiol Heart Circ Physiol 296: H237–H246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broniowska KA, Hogg N. The chemical biology of S-nitrosothiols. Antioxid Redox Signal 17: 969–980, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carver J, Doctor A, Zaman K, Gaston B. S-nitrosothiol formation. Methods Enzymol 396: 95–105, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cassoly R, Gibson Q. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol 91: 301–313, 1975 [DOI] [PubMed] [Google Scholar]
- 8.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35: 6976–6983, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Fairbanks VF, Ziesmer SC, O'Brien PC. Methods for measuring plasma hemoglobin in micromolar concentration compared. Clin Chem 38: 132–140, 1992 [PubMed] [Google Scholar]
- 10.Fleming RE, Gitlin JD. Primary structure of rat ceruloplasmin and analysis of tissue-specific gene expression during development. J Biol Chem 265: 7701–7707, 1990 [PubMed] [Google Scholar]
- 11.Ford PC, Wink DA, Stanbury DM. Autoxidation kinetics of aqueous nitric oxide. FEBS Lett 326: 1–3, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Gorin AB, Gould J. Immunoglobulin synthesis in the lungs and caudal mediastinal lymph node of sheep. J Immunol 123: 1339–1342, 1979 [PubMed] [Google Scholar]
- 13.Green LR, Bennet L, Hanson MA. The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol 497: 271–277, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris AP, Helou S, Gleason CA, Traystman RJ, Koehler RC. Fetal cerebral and peripheral circulatory responses to hypoxia after nitric oxide synthase inhibition. Am J Physiol Regul Integr Comp Physiol 281: R381–R390, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang C, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci USA 98: 11771–11776, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes MN. Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim Biophys Acta 1411: 263–272, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Hunter CJ, Blood AB, White CR, Pearce WJ, Power GG. Role of nitric oxide in hypoxic cerebral vasodilatation in the ovine fetus. J Physiol 549: 625–633, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J Biol Chem 274: 27069–27075, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35: 790–796, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kolesnik B, Palten K, Schrammel A, Stessel H, Schmidt K, Mayer B, Gorren AC. Efficient nitrosation of glutathione by nitric oxide. Free Radic Biol Med 63: 51–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuster A, Tea I, Ferchaud-Roucher V, Le Borgne S, Plouzennec C, Winer N, Roze JC, Robins RJ, Darmaun D. Cord blood glutathione depletion in preterm infants: correlation with maternal cysteine depletion. PLoS One 6: e27626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide 1: 18–30, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Lewis RS, Deen WM. Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions. Chem Res Toxicol 7: 568–574, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem 273: 18709–18713, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lockhart PJ, Mercer JF. Cloning and expression analysis of the sheep ceruloplasmin cDNA. Gene 236: 251–257, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Louro MO, Cocho JA, Tutor JC. Specific oxidase activity of cord serum ceruloplasmin in the newborn. Clin Chem Lab Med 38: 1289–1292, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun 396: 39–45, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008 [DOI] [PubMed] [Google Scholar]
- 30.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci 851: 93–105, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Moller MN, Li Q, Vitturi DA, Robinson JM, Lancaster JR, Jr, Denicola A. Membrane “lens” effect: focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem Res Toxicol 20: 709–714, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Morakinyo MK, Strongin RM, Simoyi RH. Modulation of homocysteine toxicity by S-nitrosothiol formation: a mechanistic approach. J Phys Chem B 114: 9894–9904, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Nakai T, Tamai T, Yamada S, Kobayashi T, Hayashi T, Kutsumi Y, Oida K, Takeda R. Plasma lipids and lipoproteins of Japanese adults and umbilical cord blood. Artery 9: 132–150, 1981 [PubMed] [Google Scholar]
- 34.Osaki S, Walaas O. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase. 2. Rate constants at various steps and formation of a possible enzyme-substrate complex. J Biol Chem 242: 2653–2657, 1967 [PubMed] [Google Scholar]
- 35.Paradis M, Gagne J, Mateescu MA, Paquin J. The effects of nitric oxide-oxidase and putative glutathione-peroxidase activities of ceruloplasmin on the viability of cardiomyocytes exposed to hydrogen peroxide. Free Radic Biol Med 49: 2019–2027, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Perveen S, Altaf W, Vohra N, Bautista ML, Harper RG, Wapnir RA. Effect of gestational age on cord blood plasma copper, zinc, magnesium and albumin. Early Hum Dev 69: 15–23, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Raijmakers MT, Roes EM, Steegers EA, van Der Wildt B, Peters WH. Umbilical cord and maternal plasma thiol concentrations in normal pregnancy. Clin Chem 47: 749–751, 2001 [PubMed] [Google Scholar]
- 38.Rassaf T, Kleinbongard P, Preik M, Dejam A, Gharini P, Lauer T, Erckenbrecht J, Duschin A, Schulz R, Heusch G, Feelisch M, Kelm M. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO–experimental and clinical study on the fate of NO in human blood. Circ Res 91: 470–477, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Rhodes PM, Leone AM, Francis PL, Struthers AD, Moncada S. The l-arginine-nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem Biophys Res Commun 209: 590–596, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology 47: 2089–2111, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem 20: 1556–1563, 1974 [PubMed] [Google Scholar]
- 43.Shiva S, Wang X, Ringwood LA, Xu XY, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol 2: 486–493, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Shokeir MH. Investigations on the nature of ceruloplasmin deficiency in the newborn. Clin Genet 2: 223–227, 1971 [DOI] [PubMed] [Google Scholar]
- 45.Shuval HI, Gruener N. Epidemiological and toxicological aspects of nitrates and nitrites in the environment. Am J Public Health 62: 1045–1052, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA 89: 444–448, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res 39: 797–815, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol Heart Circ Physiol 274: H1705–H1714, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci USA 101: 11477–11482, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziak M, Meier M, Novak-Hofer I, Roth J. Ceruloplasmin carries the anionic glycan oligo/poly alpha2,8 deaminoneuraminic acid. Biochem Biophys Res Commun 295: 597–602, 2002 [DOI] [PubMed] [Google Scholar]