Abstract
Exercise-induced changes in γ-aminobutyric acid (GABA) or nitric oxide signaling within the paraventricular nucleus (PVN) have not been studied in renovascular hypertension. We tested whether exercise training decreases mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) in two-kidney, one-clip (2K-1C) hypertensive rats due to enhanced nitric oxide or GABA signaling within PVN. Conscious, unrestrained male Sprague-Dawley rats with either sham (Sham) or right renal artery clipping (2K-1C) were assigned to sedentary (SED) or voluntary wheel running (ExT) for 6 or 12 wk. MAP and angiotensin II (ANG II) were elevated in 2K-1C SED rats. The 2K-1C ExT rats displayed lower MAP at 6 wk that did not decline further by 12 wk. Plasma ANG II was lower in 2K-1C ExT rats. Increases in MAP, heart rate, and RSNA to blockade of PVN nitric oxide in 2K-1C SED rats were attenuated compared with either Sham group. Exercise training restored the responses in 2K-1C ExT rats. The increase in MAP in response to bicuculline was inversely correlated with baseline MAP. The rise in MAP was lower in 2K-1C SED vs. either Sham group and was normalized in the 2K-1C ExT rats. Paradoxically, heart rate and RSNA responses were not diminished in 2K-1C SED rats but were significantly lower in the 2K-1C ExT rats. Thus the decrease in arterial pressure in 2K-1C hypertension associated with exercise training is likely due to diminished excitatory inputs to PVN because of lower ANG II and higher nitritergic tone rather than enhanced GABA inhibition of sympathetic output.
Keywords: Goldblatt hypertension, γ-aminobutyric acid, hemodynamics, nitric oxide, renal sympathetic nerve activity
numerous studies indicate that regular physical activity reduces cardiovascular risk not only in individuals with predisposing conditions (4, 19) but also in asymptomatic healthy subjects (42). In both humans (5, 30) and rats (49) with hypertension, moderate regular exercise decreases systemic arterial pressure. This depressor effect of exercise training is due, at least in part, to a decrease in efferent sympathetic tone (48) and improved autonomic function (13, 66).
Mounting evidence suggests that physical activity is associated with neuroplasticity in brain loci that regulate blood pressure (25, 44). In addition to brain stem cardiovascular regulatory centers such as the nucleus tractus solitarius and the rostral ventrolateral medulla, the paraventricular nucleus (PVN) of the hypothalamus is an important central site involved in sympathetic outflow and arterial pressure control that can be influenced by exercise (13, 25). The role of exercise training on γ-aminobutyric acid (GABA) and nitric oxide signaling mechanisms within the PVN and the associated improvement in sympathetic outflow have been extensively demonstrated in heart failure (66, 68). Studies exist indicating that exercise training also enhances PVN nitric oxide and GABA-mediated inhibition of sympathoexcitation in normotensive (27) and hypertensive rats (15). For example, exercise training normalizes the number of diaphorase positive, presumptively nitritergic neurons (15), and reduces proinflammatory cytokines (1) within the PVN of spontaneously hypertensive rats (SHR). In a rat model of hypertension induced by exogenously infused angiotensin II (ANG II), exercise training prevented arterial baroreflex dysfunction (50).
Renovascular disease resulting in reduced renal perfusion pressure is an important cause of secondary hypertension. Renal artery stenosis may result from fibromuscular dysplasia, but atherosclerotic renal artery disease is the most common with a prevalence as high as 20–54% in patients who have concurrent extrarenal atherosclerosis or heart failure (14). Moreover, atherosclerotic renal artery stenosis is an independent risk factor for adverse cardiovascular events (16). The hypertension that ensues is due to a complex interplay of the renin-angiotensin-aldosterone system (18), oxidative stress pathways (8), and the sympathetic nervous system (7). Most studies have focused on the role of the renin-angiotensin-aldosterone system, but it has long been known that sympathoexcitation is a critical factor in both experimental (29) and clinical (7) hypertension associated with unilateral renal artery stenosis, particularly in the later phase of hypertension (41).
Recent studies indicate that the PVN also plays an important role in renovascular hypertension (8, 9, 39). Studies on exercise training in two-kidney, one-clip (2K-1C) hypertension have largely focused on its effects on cardiac remodeling (57) rather than on its impact on sympathetic activity. Thus the present studies were designed to test the hypothesis that moderate exercise training by voluntary wheel running would decrease systemic blood pressure in the 2K-1C hypertensive rat. Furthermore, we hypothesize that the depressor effect of exercise will be associated with sympathoinhibition due to enhanced nitric oxide and/or GABA signaling within the PVN.
METHODS
Experiments were performed in male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) housed under controlled conditions (21–23°C; lights on, 0700-1900). They were permitted free access to water and standard rat chow. The rats were cared for in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Wayne State University Institutional Animal Care and Use Committee.
Renal artery clipping.
Five-week-old rats were anesthetized with combined ketamine (80 mg/kg) and xylazine (8 mg/kg ip). A right flank incision was made, and a silver clip (0.2 mm) was surgically placed around the right renal artery (2K-1C). Sham-clipped (Sham) rats underwent identical surgery but were not clipped. The flank incision was closed, and each rat was returned to its home cage and permitted to recover. At the end of surgery, each rat received butorphanol tartrate (0.2 mg/kg sc) for analgesia.
Exercise regimen.
Four to five days after sham or renal artery clipping, rats were randomly assigned to individual standard caging [sedentary (SED)] or to identical cages with access to running wheels [exercise training (ExT)] equipped with activity wheel monitors (Lafayette Instruments) by an investigator other than the person performing the clipping. Four groups of rats were studied after 6 wk of sedentary or voluntary wheel running: Sham SED, Sham ExT, 2K-1C SED, and 2K-1C ExT. A separate set of four groups were studied after 12 wk.
PVN cannula, vascular catheters, and renal nerve electrode placement.
At the end of the 6- or 12-wk regimen, each rat was anesthetized with ketamine and xylazine as before and then secured in a stereotaxic apparatus with the skull leveled between the bregma and lambda. A 26-gauge guide cannula (Plastics One) was inserted into the PVN such that the final injection after placement of the infusion cannula (see below) occurred at the following stereotaxic coordinates: ±0.6 lateral to the midline, −1.5 dorsal to bregma, and −8.6 ventral from the dorsal surface of the skull. The guide cannula was affixed with cranioplastic cement, and a dummy cannula was inserted to maintain patency. After recovery from the anesthetic at the end of surgery, each rat received butorphanol tartrate as above and was returned to its home cage and permitted to recover. At this point, access to the running wheels for the exercise-trained rats was closed to avoid injury to the rat and damage to the cannula should the cannula be caught within the wheel mechanism.
Four days later, each rat was anesthetized with pentobarbital sodium (50 mg/kg ip). If needed, supplemental doses of sodium pentobarbital were given intravenously after placement of the catheters to maintain an adequate plane of anesthesia. A midline ventral incision was made in the neck, and catheters were inserted into the right carotid artery and jugular vein. The catheters were filled with heparinized saline (100 U/ml), secured, tunneled subcutaneously, and exteriorized at the base of the neck. The incision was sutured, and the rat was then turned onto its right side and a left flank incision was made. The left renal nerve was isolated via a retroperitoneal approach. The nerve was carefully placed on electrodes constructed of Teflon-coated silver wire (0.0055-in. diameter; A-M Systems) with the exposed ends wound into single loops. The nerve and electrodes were covered with silicone gel (Kwik-Sil; World Precision Instruments), which was allowed to harden before closure. A ground wire was sewn into the surrounding tissue. The electrodes and ground wire were tunneled subcutaneously and also exteriorized at the base of the neck.
Each rat was returned to its home cage and permitted to recover. In all cases, the animals resumed normal grooming, eating, drinking, and cage activity. A minimum of 48 h was permitted after the renal nerve surgery for full recovery from anesthesia before performance of any test protocol (40).
Conditioning to the study chamber.
On the second day after cannula placement and on each of the following days, the rats were conditioned to remain for 180 min within a custom-made Plexiglas study chamber (Braintree Scientific) that would be used during the experiment. The chamber allowed the rat to move forward and backward but not to turn around. They were returned to their home cage after each conditioning period.
Hemodynamic and renal sympathetic nerve activity measurements.
Arterial pressure was measured by connecting the arterial catheter to a pressure transducer (Gould P23 XL) that was coupled to an amplifier (Digi-Med BPA-200). Heart rate and mean arterial pressure (MAP) were derived by data-acquisition software (DasyLab; Biotech Products) using the arterial pressure pulse and averaged over 1-s intervals. Renal sympathetic nerve activity (RSNA) was amplified (5,000–20,000 times) and filtered (100–1,000 Hz) with a Grass P511 differential preamplifier and a high-impedance probe (HIP511GB). Both the probe and the rat were located within a shielded Faraday cage. The amplified and filtered neurogram signal was channeled to an oscilloscope and Grass AM8 audiomonitor for visual and auditory evaluation, respectively. The amplified nerve activity was digitized, rectified, integrated, and averaged over 1-s intervals. Background noise was determined at the end of experiment after administration of a bolus dose of the ganglionic blocker trimethaphan camsylate (20 mg/kg iv; Hoffman-La Roche). RSNA was defined as the amount of recorded nerve activity after subtraction of background noise.
Protocols.
All testing was performed in conscious rats. On the day of study, the rat was placed into the study chamber, the arterial catheter and nerve electrodes were connected to the data acquisition system, and the dummy cannula was replaced with an infusion cannula whose tip projected 1 mm below the guide cannula. Heart rate, arterial pressure, and RSNA were monitored continuously. After a minimum 30-min baseline period, the PVN was injected with the test agent: either 200 pmol bicuculline or 740 nmol Nω-nitro-l-arginine methyl ester (l-NAME) dissolved in 250 nl saline (54). Hemodynamic and RSNA parameters were monitored for 90 min after injection. Each rat was subjected only to one test agent on any given day in random order. A minimum of 24 h was permitted between test protocols. Some rats were studied only once if the quality of the renal nerve recording did not meet criteria set out by Guild et al. (21) before initiating the testing protocol.
On the day following all protocols, 10 2K-1C rats were subjected to phenylephrine (40 μg iv) to evaluate the ability to mount a pressor response. Baseline MAP and peak pressor responses were recorded.
After the last protocol was completed, rats were euthanized with sodium pentobarbital (100 mg/kg iv) and 250 nl 1,1-di-ictadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate were injected into the PVN to localize the injection. Then, the rat was perfused transcardially with 0.9% saline followed by 10% neutral buffered formalin. The brain was removed, dehydrated using an alcohol series, and embedded in paraffin. Coronal sections (60 μm) were examined by phase contrast microscopy, and the site and extent of injection were verified. Rats with injection sites outside the PVN were eliminated from analysis.
Radiotelemetry monitoring and plasma ANG II.
A separate set of sham-clipped or 2K-1C rats were assigned to radiotelemetry monitoring for subsequent plasma angiotensin II (ANG II measurement) after 12 wk of SED or ExT regimen. These rats had implantation of a radiotelemetry transducer (TA11PA-C40; Data Sciences) at the time of renal artery clipping or sham clipping. After the femoral artery was exposed and the proximal end was temporarily occluded, the gel-filled catheter attached to the transmitter device was inserted into the distal aorta via a 21-gauge needle. The catheter was advanced and secured with medical adhesive, and the transmitter placed subcutaneously and sutured to the underlying muscle. The skin was closed with surgical staples. The rat was given butorphanol tartrate for analgesia and was returned to its home cage with its individual receiver. Hemodynamic data was recorded for 1 day each week and then for the final week of the 12-wk protocol. At the end of the 12 wk, the rats were anesthetized with ketamine and xylazine. A midline abdominal incision was made, and the aorta was exposed and cannulated. Blood was collected into prechilled tubes containing a solution with inhibitors of proteolytic enzymes and angiotensin-converting enzyme: 5 mM EDTA, 10 μM pepstatin, 25 mM phenanthroline, and 20 μM enalaprilat to block degradation. The samples were immediately centrifuged at 4°C, and the plasma was removed and stored at −70°C until assay (39).
Plasma ANG II radioimmunoassay.
Plasma samples for ANG II were processed by methods adapted from Navar et al. (47) for our laboratory (39). Briefly, 1 ml of plasma was extracted with 90% methanol in water. The eluates were taken to dryness under N2 and stored overnight at −20°C. The extracts were reconstituted in 0.5 ml assay buffer consisting of 50 mM sodium phosphate, 1 mM EDTA, 0.25 mM thimerosal, and 0.25% peptidase-free human serum albumin. All samples were assayed in duplicate using 125I-labeled ANG II (Perkin-Elmer, Billerica, MA) as the tracer and anti-ANG II antibody (Peninsula Laboratories, San Carlos, CA) at a final dilution of 1:660,000. Nonspecific binding was 2.1%, the lower limit of detection was 0.5 fmol/tube, and 50% binding was 15.2 fmol/tube.
Analyses and statistics.
All data are presented as the means ± SE. Two-way ANOVA for independent measures was used to evaluate the effect of exercise on the responses of MAP, heart rate, and RSNA to injections of bicuculline or l-NAME in Sham and 2K-1C groups. For comparisons of individual means among groups, one-way ANOVA was followed by Tukey Kramer post hoc analysis. A P value <0.05 was accepted as significant.
RESULTS
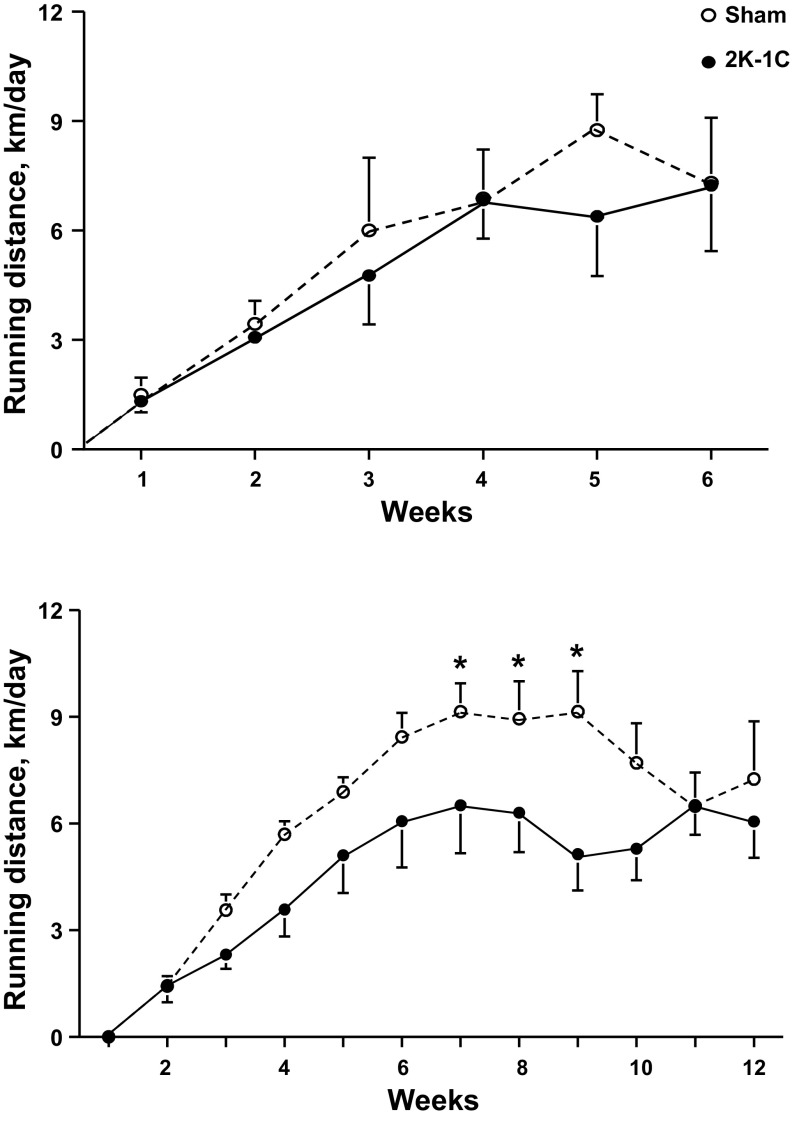
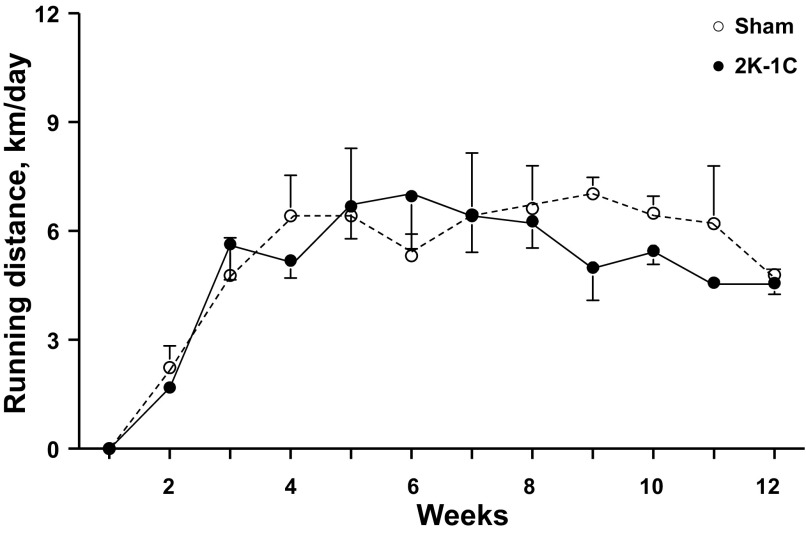
Table 1 shows that rat weights did not differ among the groups at the time of sham clipping or renal artery clipping. At 6 wk, the weights of the Sham ExT rats were significantly less than the those of the Sham SED rats, which did not differ from either of the 2K-1C groups. At 12 wk, both the Sham ExT and 2K-1C ExT rats had lower body weights than their sedentary counterparts even though overall the ExT rats ate more food (29 ± 2 g/day) than the SED rats (25 ± 1 g/day). The difference in food intake did not achieve significance (P = 0.078). Running distances were comparable between sham-clipped and 2K-1C rats studied at 6 wk (Fig. 1). In the rats studied at 12 wk, the 2K-1C rats tended to run less than the sham-clipped rats; the difference was significant during weeks 7–9.
Table 1.
Rat weights and running distances
| Initial |
6 wk |
12 wk |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Body wt, g | n | Body wt, g | Average Distance, km/day | n | Body wt, g | Average Distance, km/day | |
| Sham SED | 21 | 160 ± 4 | 11 | 357 ± 5 | 10 | 414 ± 11 | ||
| Sham ExT | 23 | 160 ± 4 | 9 | 324 ± 9* | 6.42 ± 0.25 | 14 | 373 ± 7* | 6.36 ± 0.76 |
| 2K-1C SED | 15 | 150 ± 2 | 7 | 340 ± 14 | 8 | 451 ± 14 | ||
| 2K-1C ExT | 18 | 162 ± 8 | 8 | 334 ± 13 | 5.93 ± 0.69 | 10 | 375 ± 10† | 5.64 ± 0.72 |
Values are means ± SE. Initial values are weights at time of clipping or sham clipping.
SED, sedentary; 2K-1C, two-kidney, one-clip; ExT, voluntary wheel running.
P < 0.05 vs. Sham SED;
P < 0.05 vs. 2K-1C SED.
Fig. 1.
Weekly running wheel distances for sham-clipped rats (n = 9) and two-kidney, one-clip (2K-1C) rats (n = 8) with voluntary exercise over 6 wk (top) or for sham-clipped rats (n = 14) and 2K-1C rats (n = 10) with voluntary exercise over 12 wk (bottom). *P < 0.05 vs. 2K-1C during the same week.
On the first day of the study protocol, baseline MAP was significantly higher in 2K-1C SED and 2K-1C ExT groups compared with the corresponding Sham groups in rats studied at 6 or at 12 wk (Table 2). MAP was similarly elevated in the 2K-1C SED and 2K-1C ExT rats before receiving bicuculline or l-NAME regardless of the day they were studied. MAP was significantly lower in the 2K-1C ExT rats after either 6 or 12 wk of the wheel running regimen compared with 2K-1C SED rats. Taken together, baseline heart rate was lower in the 2K-1C ExT rats vs. 2K-1C SED rats after 12 wk on the first study day. Heart rate was significantly lower in the 2K-1C ExT rats compared with 2K-1C SED after 6 or 12 wk of voluntary wheel running exercise only on the day these rats were tested with bicuculline.
Table 2.
Baseline hemodynamic parameters in all groups
| 6 wk |
12 wk |
|||||
|---|---|---|---|---|---|---|
| n | MAP, mmHg | HR, beats/min | n | MAP, mmHg | HR, beats/min | |
| All rats 1st study day | ||||||
| Sham SED | 14 | 132.7 ± 2.9 | 418 ± 9 | 11 | 132.0 ± 2.8 | 425 ± 11 |
| Sham ExT | 10 | 133.3 ± 3.1 | 409 ± 15 | 14 | 132.3 ± 2.7 | 399 ± 10 |
| 2K-1C SED | 9 | 188.4 ± 11.2* | 425 ± 9 | 8 | 186.5 ± 8.3* | 424 ± 11 |
| 2K-1C ExT | 11 | 160.0 ± 10.2*† | 402 ± 11 | 7 | 156.4 ± 2.7*† | 381 ± 6 |
| Before bicuculline | ||||||
| Sham SED | 12 | 131.8 ± 3.0 | 412 ± 9 | 9 | 132.9 ± 3.1 | 415 ± 12 |
| Sham ExT | 9 | 132.2 ± 3.3 | 400 ± 14 | 9 | 135.3 ± 3.8 | 379 ± 11 |
| 2K-1C SED | 8 | 193.8 ± 11.9* | 430 ± 8 | 6 | 187.2 ± 11.3* | 428 ± 15 |
| 2K-1C ExT | 8 | 155.5 ± 3.8*† | 395 ± 13 | 7 | 158.2 ± 3.0*† | 386 ± 6 |
| Before l-NAME | ||||||
| Sham SED | 9 | 131.8 ± 2.9 | 418 ± 10 | 10 | 131.1 ± 2.6 | 412 ± 11 |
| Sham ExT | 10 | 129.1 ± 4.0 | 402 ± 15 | 12 | 128.0 ± 2.0 | 397 ± 11 |
| 2K-1C SED | 7 | 181.8 ± 13.8* | 411 ± 13 | 7 | 192.3 ± 10.1* | 371 ± 22 |
| 2K-1C ExT | 9 | 161.8 ± 12.2* | 408 ± 12 | 7 | 162.8 ± 4.3*† | 380 ± 14 |
Values are means ± SE.
MAP, mean arterial pressure; l-NAME, Nω-nitro-l-arginine methyl ester.
P < 0.05 vs. corresponding Sham group the same day or treatment.
P < 0.05 vs. corresponding SED group same day or treatment.
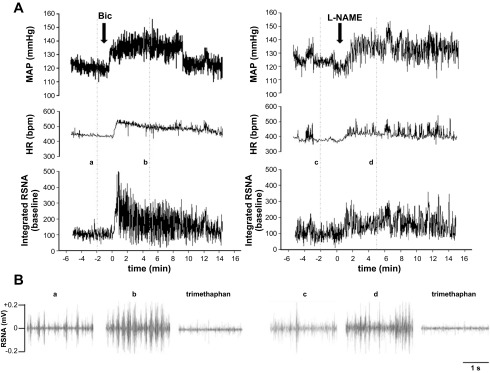
Typical increases in blood pressure, heart rate, and integrated RSNA responses to microinjection of either bicuculline or l-NAME into PVN in 12-wk Sham SED rats are shown in Fig. 2A. The pattern of RSNA activity is shown in Fig. 2B.
Fig. 2.
A: representative tracings of mean arterial pressure (MAP), heart rate (HR), and integrated renal sympathetic nerve activity (RSNA) in sedentary, sham-clipped rats after either 200 pmol bicuculline (left) or 740 nmol Nω-nitro-l-arginine methyl ester (l-NAME; right). B: examples of the pattern of RSNA at the specified time points (a–d) indicated in A and after trimethaphan camsylate.
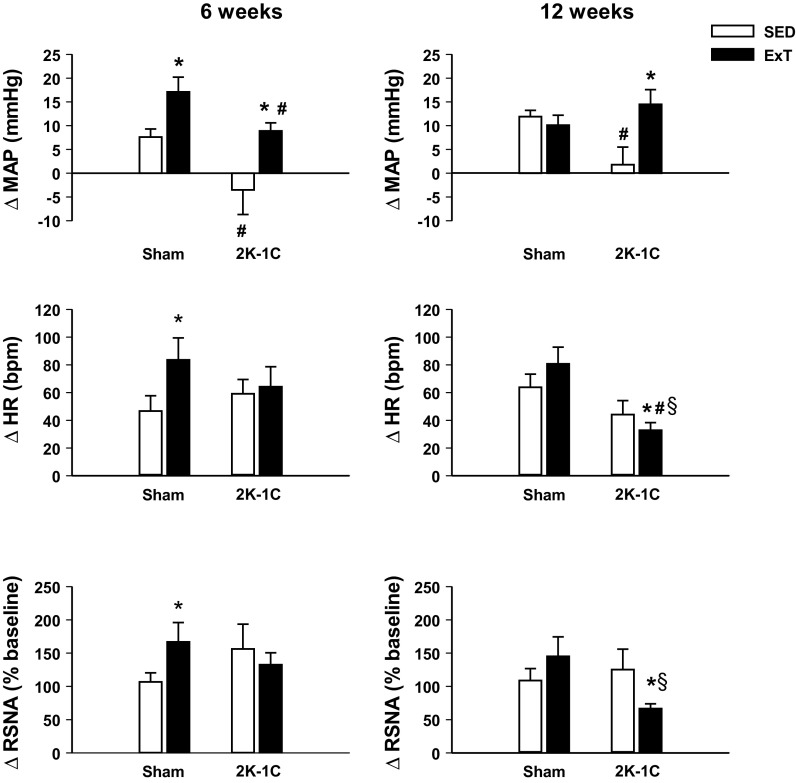
At 6 wk, both the Sham ExT and 2K-1C ExT groups displayed a significantly greater rise in MAP in response to bicuculline than the Sham SED and 2K-1C SED groups, respectively (Fig. 3, left). The heart rate and RSNA responses were significantly greater in the Sham ExT group than the Sham SED, whereas these responses were similar in the 2K-1C SED and ExT rats.
Fig. 3.
Changes in MAP, HR, and RSNA 5 min after injection of 200 pmol bicuculline into paraventricular nucleus of conscious sham-clipped (sham) and 2K-1C rats after 6 wk (left) or 12 wk (right) of sedentary housing (SED, clear bars) or voluntary wheel running exercise training (ExT, black bars) regimen. Different animals studied at 6 and 12 wk; number of observations in each group is given in Table 1. Values are means ± SE. *P < 0.05 vs. SED; #P < 0.05 vs. sham, same time and regimen; §P < 0.05 vs. 6 wk, corresponding group.
In the 12-wk groups, the increase in MAP by the 2K-1C SED rats with bicuculline was smaller than that of the Sham SED rats. The increase in MAP by the 2K-1C ExT group was significantly greater than in the 2K-1C SED rats and no different from that in the Sham groups. The heart rate and RSNA response of the 12-wk 2K-1C ExT rats were significantly lower compared with that of the 2K-1C SED rats as well as both Sham groups (Fig. 3, right).
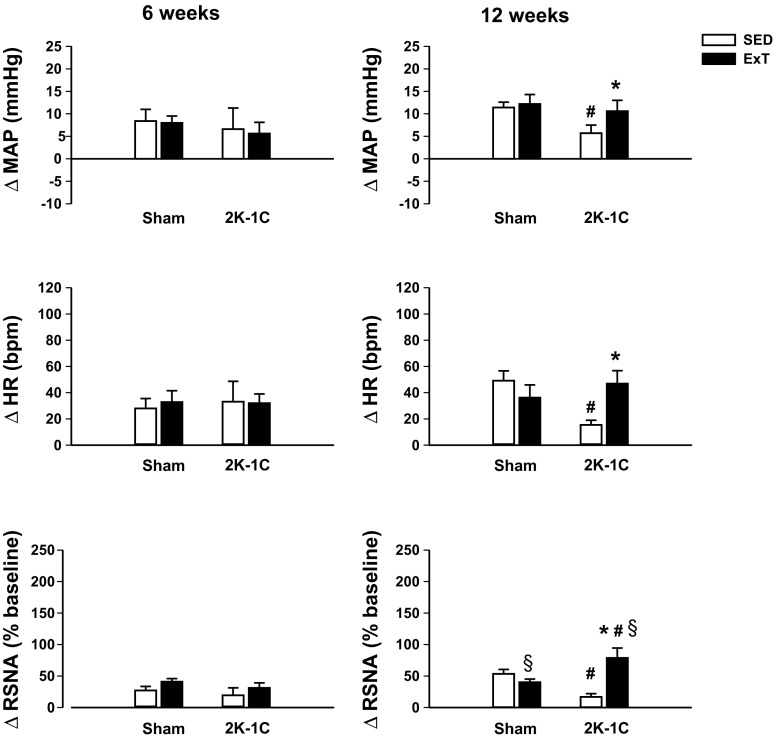
At 6 wk, the hemodynamic and RSNA responses to PVN injection of l-NAME were similar in Sham and 2K-1C groups regardless of the sedentary or exercise regimen (Fig. 4, left).
Fig. 4.
Changes in MAP, HR, and renal RSNA 5 min after injection of 740 nmol l-NAME into paraventricular nucleus of conscious sham-clipped (sham) and 2K-1C rats after 6 wk (left) or 12 wk (right) of sedentary housing (SED, clear bars) or voluntary wheel running exercise training (ExT, black bars) regimen. Different animals studied at 6 and 12 wk; number of observations in each group is given in Table 1. Values are means ± SE. *P < 0.05 vs. SED; #P < 0.05 vs. sham, same time, and regimen; §P < 0.05 vs. 6 wk, corresponding group.
At 12 wk, the increase in MAP with l-NAME in the 2K-1C SED group was significantly lower than that seen in the Sham groups (Fig. 4, right). The rise in MAP in the 2K-1C ExT group was similar to that in the Sham rats, and the heart rate and RSNA responses followed the same pattern as MAP. The increase in RSNA was also greater than that observed in 2K-1C ExT rats studied after only 6 wk of exercise training.
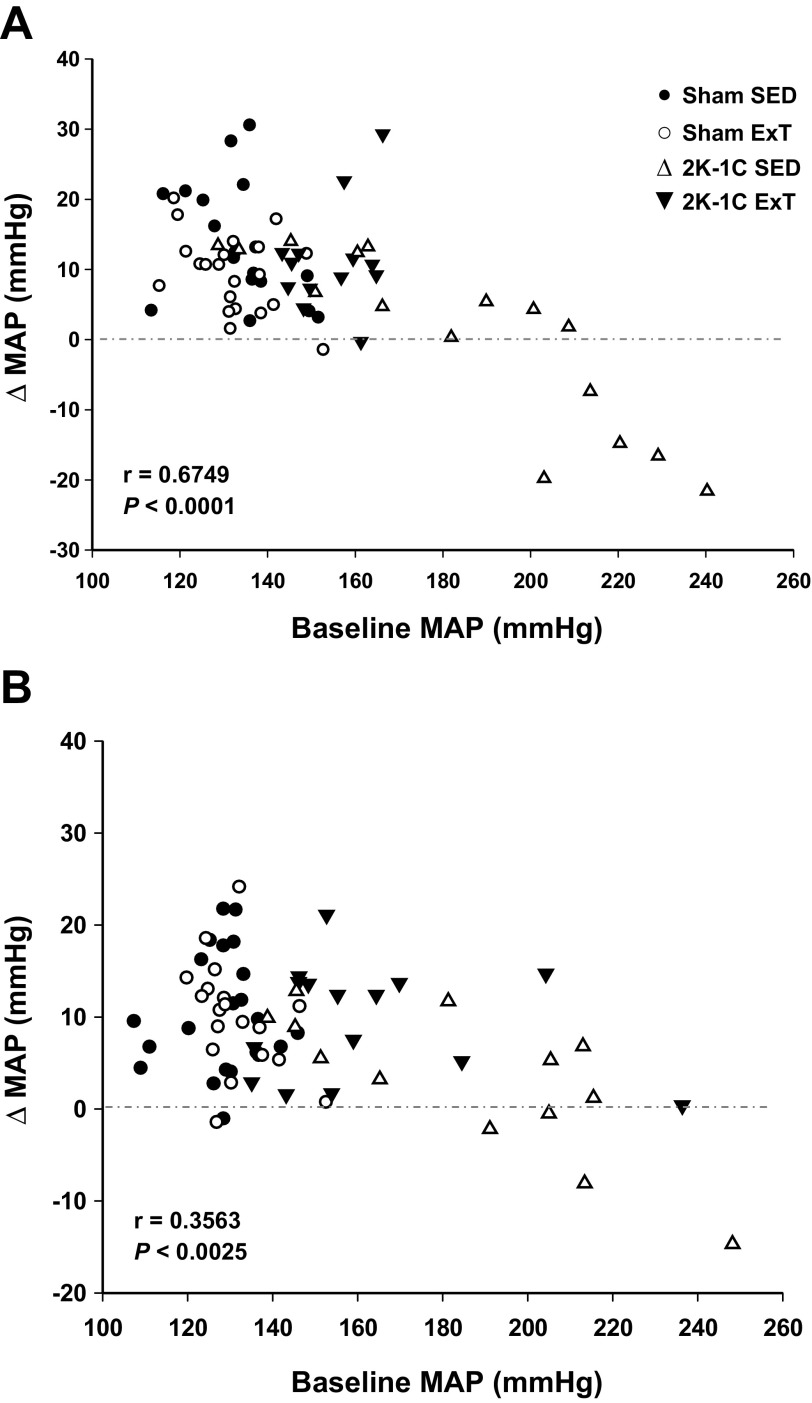
Since it appeared that the change in MAP in response to bicuculline was related to baseline MAP, we performed a post hoc analysis. When the change in MAP by all rats was plotted against baseline MAP, a significant inverse linear relationship was observed (Fig. 5A). Rats with the highest baseline MAP were all 2K-1C SED rats and displayed the smallest increase to bicuculline with some rats responding with a decrease in MAP. Thus we analyzed data from 2K-1C SED and 2K-1C ExT rats excluding those with MAP >200 mmHg. In this subgroup, baseline MAP was similar in 2K-1C SED (160.3 ± 2.5 mmHg) and 2K-1C ExT rats (162.7 ± 1.3 mmHg). The 2K-1C ExT rats still displayed a greater rise in MAP after bicuculline, 15.1 ± 1.0 mmHg, compared with 2K-1C SED rats, 7.5 ± 1.2 mmHg (P < 0.05). Similar to the analysis of the entire cohort (Fig. 3), the increase in heart rate was significantly smaller in the 2K-1C ExT vs. 2K-1C SED rats, 44 ± 5 vs. 81 ± 8.3 beats/min (P < 0.01) as was the rise in RSNA, 77.8 ± 5.3 vs. 192 ± 24.9% baseline (P < 0.01).
Fig. 5.
Change in MAP after injection of bicuculline (A) or l-NAME (B) into paraventricular nucleus (PVN) as a function of resting MAP for each observation.
Although the correlation was less strong, the relationship between the change in MAP in response to l-NAME vs. baseline MAP was also significant (Fig. 5B). As with bicuculline, subanalysis did not alter the findings with all rats shown in Fig. 4.
Importantly, the increase in MAP after 40 μg intravenous phenylephrine did not differ regardless of baseline MAP in the 2K-1C rats. MAP rose 39.1 ± 3.5 mmHg in 2K-1C rats with baseline MAP between 140 and 200 mmHg (n = 16) and 33.9 ± 6.1 mmHg in 2K-1C rats with baseline >200 mmHg (n = 7; P > 0.05).
The overall average running distances for the set of rats monitored by telemetry (Table 3) are shown in Fig. 6 and were not significantly different from those for 12-wk ExT rats in Table 1. The pattern of running was similar for both sets of 2K-1C ExT rats (compare Figs. 1 and 6). Running distances for the Sham ExT rats with telemetry transmitters were no different from the 2K-1C ExT rats in this set but tended to run less than the Sham ExT rats in the first set of experiments during weeks 7–9 (compare Figs. 1 and 6). Baseline MAP and heart rates for these groups are shown in Table 3 and were similar to those obtained after instrumentation and conditioning but before PVN injection in the first set of experiments (Table 2). As before, 2K-1C ExT rats had significantly lower MAP than 2K-1C SED rats (Table 3). The plasma ANG II level was 2.5-fold higher in the 2K-1C SED group compared with Sham SED rats. 2K-1C ExT rats had significantly lower plasma ANG II levels than the 2K-1C SED rats.
Table 3.
Plasma angiotensin II levels at 12 wk
| n | MAP, mmHg | Heart Rate, beats/min | Plasma ANG II, fmol/ml | Average Distance, km/day | |
|---|---|---|---|---|---|
| Sham SED | 4 | 133.8 ± 3.8 | 415 ± 12 | 92.8 ± 28.8 | — |
| Sham ExT | 4 | 132.0 ± 3.4 | 379 ± 11* | 34.4 ± 16.4 | 5.8 ± 0.2 |
| 2K-1C SED | 4 | 170.0 ± 4.1* | 397 ± 13 | 232.3 ± 35.8* | — |
| 2K-1C ExT | 4 | 143.8 ± 7.9*† | 392 ± 5 | 109.4 ± 27.7† | 5.3 ± 0.1 |
Values are means ± SE.
P < 0.05 vs. Sham SED;
P < 0.05 vs. 2K-1C SED.
Fig. 6.
Weekly voluntary running wheel distances for 12-wk exercise-trained sham-clipped rats (n = 4) and 2K-1C rats (n = 4) with hemodynamic parameters monitored by telemetry depicted in Table 3. There were no statistical differences between the groups at any time point.
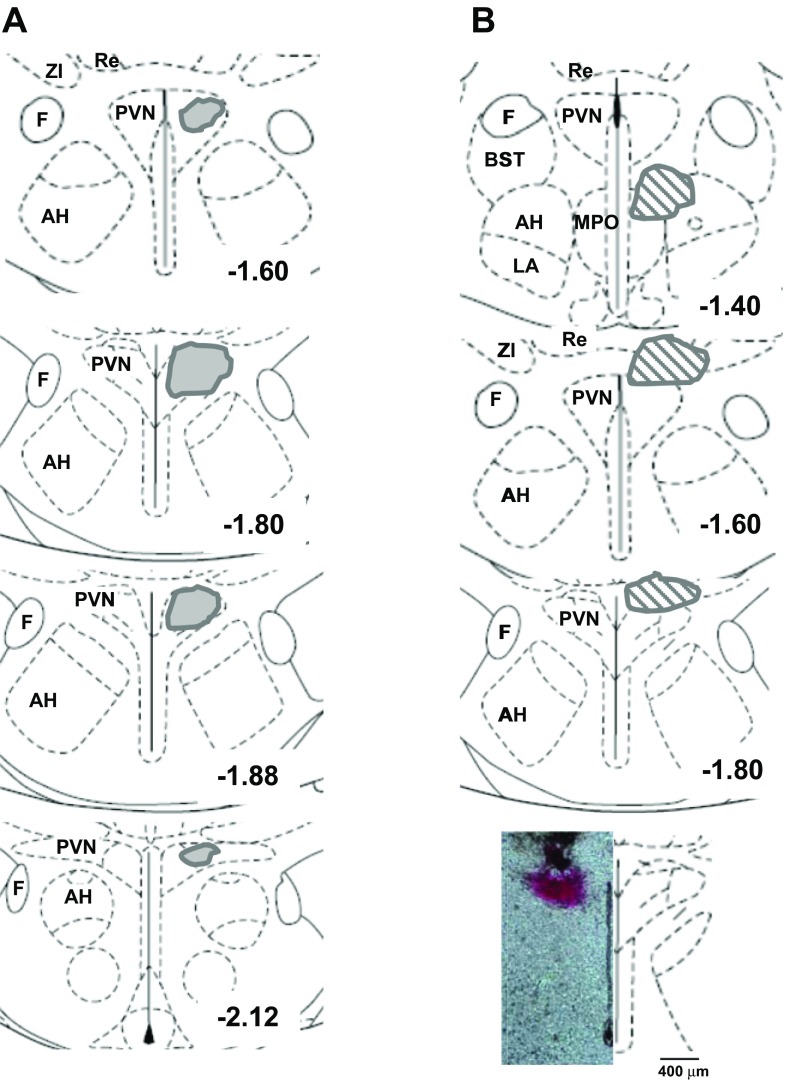
Figure 7A shows the areas identified within the PVN over which the injections spread for the rats that were used in the analysis. Injections outside the PVN are depicted in Fig. 7B. Two rats with injections in the area of the thalamus and zona incerta displayed seizure-like stereotypic behavior; these rats were euthanized and not studied further. Injection of bicuculline into the region between the median preoptic nucleus and lateral hypothalamic area resulted in profound increases in MAP, heart rate, and RSNA in one animal (36.3 mmHg, 144 beats/min and 288.2% baseline). In a different rat injected with l-NAME into this area, increases were also observed in MAP (32.8 mmHg), heart rate (48 beats/min), and RSNA (70.4% baseline). Saline injection into the PVN resulted in no change in any of the parameters from baseline values (not shown).
Fig. 7.
Schematic diagrams of the coronal sections through the PVN showing the area of spread encompassed by the microinjections: injections included in analysis (A) and injections outside the PVN (B) not included in the analysis. Bottom: actual injection. AH, anterior hypothalamic area; F, fornix; LA, lateroanterior hypothalamic nucleus; MPO, median preoptic nucleus; Re, reuniens thalamic nucleus; ZI, zona incerta. Numbers indicate distance posterior to bregma.
DISCUSSION
The present studies support our hypothesis that voluntary wheel running exercise decreases systemic arterial pressure in conscious 2K-1C rats. The data highlight five major findings. First, the pressure-lowering effect is clearly evident after 6 wk with no further decline after 12 wk of exercise. Second, the distances run by all the groups were comparable to those reported for Sprague-Dawley rats (15, 31, 43, 58). Nonetheless, the pressure-lowering effects of exercise training are observed only in the hypertensive 2K-1C rats despite similar or even greater running distances by the sham-clipped rats. Third, plasma ANG II is elevated at 12 wk in the 2K-1C rats and declines with exercise training to levels no different from that of normotensive sham-clipped rats. Fourth, the smaller changes in arterial pressure, heart rate, and RSNA after blockade of nitric oxide in sedentary 2K-1C rats suggest a lower nitritergic tone within the PVN that is restored by exercise to a level comparable to that of sham-clipped animals. Finally, although GABA inhibition evokes a greater rise in arterial pressure in exercised 2K-1C rats, the changes in heart rate and RSNA are paradoxically lower, which suggests that exercise exerts a differential influence on nitric oxide and GABAergic mechanisms within the PVN in this model.
Current evidence supports the concept that PVN nitric oxide plays a pivotal role in modulating arterial pressure in several models of hypertension including the SHR (53), the Dahl salt-sensitive strain (17), and neurogenic hypertension (63). Blockade of endogenous nitric oxide generation within the PVN results in higher arterial pressure and sympathoexcitatory responses (64, 65), and administration of exogenous nitric oxide donors typically exerts the opposite effects (26). The same has been reported in hypertension models that involve an activated renin angiotensin system, such as the mRen2 (27) transgenic rat (37) and the 2K-1C rat (12, 35, 54). However, the attenuated hemodynamic and RSNA responses by the 12-wk sedentary 2K-1C rats indicate that PVN nitritergic signaling is diminished compared with sham-clipped sedentary rats. These findings are consistent with data in renal wrap hypertension (23) and heart failure (51), both of which exhibit an activated renin-angiotensin system.
Regular exercise exerts a more profound impact on PVN nitric oxide signaling in 2K-1C hypertensive rats than in sham-clipped rats. Exercise enhances the heart rate and RSNA responses by 2K-1C rats to inhibition of nitric oxide generation such that the responses are at least as robust as those of sham-clipped animals, consistent with moderate regular exercise increasing nitric oxide signaling within the PVN of 2K-1C rats. The increase in nitric oxide may, at least in part, be due to the lower ANG II levels in the exercising 2K-1C rats since blockade of ANG II formation or receptor activation in SHR has been shown to increase hypothalamic nitric oxide synthase activity (53). Other potential mechanisms include exercise-training augmentation of cardiac vagal afferent inputs to PVN neurons (22, 32, 38), increases in the number of nitric oxide synthase-positive PVN neurons (15, 66), or an increase in the sensitivity of neurons to nitric oxide itself (66).
Since baseline arterial pressure is already lower in the 6-wk exercised 2K-1C rats, the lack of observed differences with PVN nitric oxide blockade at this time point suggests that a longer period of regular exercise is required for changes in modulation of heart rate and RSNA and that exercise may exert its effects earlier at other central cardioregulatory sites and/or via other sympathetic nerves (e.g., splanchnic or adrenal nerves).
Existing evidence supports the concept that the sympathoinhibitory actions of nitric oxide are mediated by GABA, which exerts a tonic inhibitory action within the PVN (27, 33, 65). Compared with sham-clipped rats, the increase in arterial pressure with GABA inhibition in conscious sedentary 2K-1C rats is reduced at both 6 and 12 wk, suggesting a decrease in GABAergic tone in the hypertensive rats. However, the heart rate and RSNA responses are not concomitantly blunted as would have been expected if GABA were mediating the effects of nitric oxide. The greater rise in arterial pressure by exercised 2K-1C rats is consistent with exercise enhancing PVN GABAergic tone within the PVN, but heart rate and RSNA responses rather than mirroring the augmented responses with l-NAME are attenuated compared with those of sedentary 2K-1C rats. While combined blockade may have provided additional insights, these disparities suggest that, at least in the 2K-1C model, the relationship between PVN nitric oxide and GABA may be more complex.
One or more possible mechanisms may account for these findings. A substantial component of sympathoexcitation in 2K-1C hypertension is due to activation of neurons at the subfornical organ by a combination of afferent nerve inputs from the clipped kidney and high plasma ANG II activation of AT1 receptors (6, 11). The subfornical organ, in turn, sends glutamatergic projections to the PVN (36) where glutamate receptors are upregulated in 2K-1C hypertension (2). Nitric oxide is known to decrease expression of glutamate receptors (67), thereby directly decreasing excitatory neurotransmission independent of its effects on GABA inhibition (20, 60). Furthermore, although AT1 receptor expression is increased within the PVN of 2K-1C rats (9), whether exercise alters this expression has not yet been studied. Nonetheless, diminished activation of AT1 receptors due to the lower levels of plasma ANG II in the exercised rats may contribute to the attenuated response to GABA inhibition (10). It has been proposed that the balance between excitatory and inhibitory inputs in PVN of 2K-1C rats favors excitatory inputs over time (3, 59). Importantly, nitric oxide can attenuate glutamatergic signaling within the PVN (20). If so, then exercise may decrease arterial pressure in 2K-1C hypertension primarily by diminishing underlying ANG II and glutamatergic excitatory inputs to PVN presympathetic neurons that drive cardiac and renal sympathetic nerves to a greater extent than it enhances GABA inhibition of sympathetic output.
In addition, GABAergic tone may decrease with the duration and/or magnitude of hypertension in 2K-1C rats. In 13-wk-old hypertensive SHR, bicuculline failed to increase and even decreased PVN neuronal firing rates compared with younger normotensive SHR (34). Such a mechanism would be consistent with the apparent paradoxical decrease in arterial pressure to bicuculline in sedentary 2K-1C rats with MAP >200 mmHg. This was not due to an inability to mount a pressor reaction since the rise in arterial pressure with phenylephrine was the same. Notably, the post hoc analysis indicates that the attenuated heart rate and RSNA responses by 2K-1C rats are dependent on exercise and independent of baseline arterial pressure. A role for the duration of hypertension cannot be excluded especially since the increases in heart rate and RSNA at 12 wk were less than at 6 wk. Furthermore, it is possible that the affinity or number of GABA receptors may be altered; however, this was not evident in renal wrap hypertension (23). Finally, differential control by GABA within cardiovascular regulatory nuclei to which PVN neurons project such as the nucleus tractus solitarius (55) or rostral ventrolateral medulla cannot be excluded (45).
Activation of efferent renal sympathetic nerves is a powerful stimulus for renin secretion (46) and thereby increasing plasma ANG II. The lower plasma ANG II levels in the 2K-1C rats are consistent with the sympathoinhibitory influence of exercise. Thus it is possible that a component of the decrease in systemic arterial pressure in exercised 2K-1C rats may also be due to attenuation of the direct vasoconstrictive action of ANG II on resistance vascular beds.
Technical considerations and limitations.
Harlan Sprague-Dawley rats have been observed by other laboratories to have higher baseline arterial pressures (20, 52), but this has not been universally observed (24, 62). The protocols in the present studies required multiple survival surgeries. Although the renal artery clipping (or sham clipping) is remote from the time the animals undergo the study protocols, the PVN cannula and catheter insertion surgery and renal electrode placement surgery are more proximate to the studies and may have contributed to stress that resulted in higher baseline arterial pressures. We timed the PVN cannula placement to permit recovery from brain edema. The 4-day period was based on earlier studies with subfornical organ cannulas where recovery can be monitored by recovery of water intake behavior (39). It is unfortunate that to have a reliable good quality sympathetic nerve activity signal in a conscious rat, the electrodes could not be placed during the PVN cannula surgery thereby permitting a longer recovery period. With the advent of nerve telemetry becoming available, this limitation may be circumvented in the future. The potential effect of detraining during the 4–6 days after cannula insertion before testing can also not be totally eliminated and may have exerted a greater impact on the 6-wk compared with the 12-wk exercise regimen. Nonetheless, these studies have attempted to balance the advantages and disadvantages of studying rats in the conscious state vs. those that occur with anesthetized animals.
In summary, voluntary wheel exercise training by 2K-1C rats results in lower arterial pressure, heart rate, and plasma ANG II levels. Sedentary 2K-1C rats exhibit attenuated hemodynamic and RSNA responses to blockade of nitric oxide generation within the PVN. This impaired nitritergic signaling is reversed by 12 wk of moderate exercise. Likewise, the blunted increase in arterial pressure with GABA antagonism in sedentary 2K-1C rats was improved by exercise training at 6 wk and restored to that seen with normotensive sham-clipped animals at 12 wk. Paradoxically, heart rate and RSNA responses to GABA inhibition were not diminished in the sedentary 2K-1C rats and exercise training decreased rather than enhanced both responses. Although the changes in arterial pressure were highly and inversely correlated with baseline systemic pressure, the changes in heart rate and RSNA with GABA blockade were dependent on exercise or sedentary status rather than baseline arterial pressure.
Perspectives and Significance
The present findings have important implications for exploring the benefit and risks of exercise in humans with renovascular disease since this entity is gaining greater prevalence in the population (28). In contrast to heart failure where the increase in arterial pressure and RSNA responses to PVN nitric oxide and GABA inhibition after regular exercise are both enhanced (64, 65, 68), PVN mechanisms in 2K-1C hypertension display some distinguishing characteristics. Whereas the arterial pressure response is enhanced by both nitric oxide and GABA blockade, the renal sympathetic response to GABA antagonism is actually diminished. This indicates that the neural plasticity conferred by exercise training in 2K-1C hypertension may influence excitatory pathways initiated by ANG II to a greater extent than inhibitory pathways. Thus caution should be used in generalizing mechanisms among different diseases and perhaps even among different types of hypertension. Furthermore, the paradoxical response to GABA antagonism in very hypertensive 2K-1C rats shows that underlying mechanisms may differ depending on the level of resting arterial pressure. This may, in part, explain the disparate results that have been observed with exercise training in humans (61) and strongly advocates for close monitoring of exercise regimens in hypertensive individuals with higher resting blood pressures (56).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-079102 and a grant from Veterans Administration Research and Rehabilitation (to N. F. Rossi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.F.R. and M.M.-S. conception and design of research; N.F.R., H.C., and M.M.-S. performed experiments; N.F.R. and M.M.-S. analyzed data; N.F.R. and M.M.-S. interpreted results of experiments; N.F.R. and M.M.-S. prepared figures; N.F.R. drafted manuscript; N.F.R. edited and revised manuscript; N.F.R., H.C., and M.M.-S. approved final version of manuscript.
REFERENCES
- 1.Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol 106: 1069–1085, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergamaschi C, Campos RR, Schor N, Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension 26: 1117–1120, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol 518: 567–585, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 170: 126–135, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boman K, Gerdts E, Wachtell K, Dahlof B, Nieminen MS, Olofsson M, Papademetriou V, Devereux RB. Exercise and cardiovascular outcomes in hypertensive patients in relation to structure and function of left ventricular hypertrophy: the LIFE study. Eur J Cardiovasc Prev Rehabil 16: 242–248, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst 3: 311–320, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Campese VM, Ku E, Park J. Sympathetic renal innervation and resistant hypertension. Int J Hypertens 2011: 814354, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos RR, Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT. The role of oxidative stress in renovascular hypertension. Clin Exp Pharmacol Physiol 38: 144–152, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Chen AD, Zhang SJ, Yuan N, Xu Y, De W, Gao XY, Zhu GQ. Angiotensin AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol 96: 94–103, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Ciriello J. Afferent renal inputs onto subfornical organ neurons responsive to angiotensin II. Am J Physiol Regul Integr Comp Physiol 272: R1684–R1689, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Dawson CA, Jhamandas JH, Krukoff TL. Activation by systemic angiotensin II of neurochemically identified neurons in rat hypothalamic paraventricular nucleus. J Neuroendocrinol 10: 453–459, 1998. [DOI] [PubMed] [Google Scholar]
- 13.de Abreu SB, Lenhard A, Mehanna A, de Souza HC, Correa FM, Hasser EM, Martins-Pinge MC. Role of paraventricular nucleus in exercise training-induced autonomic modulation in conscious rats. Auton Neurosci 148: 28–35, 2009. [DOI] [PubMed] [Google Scholar]
- 14.de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens 27: 1333–1340, 2009. [DOI] [PubMed] [Google Scholar]
- 15.DiCarlo SE, Zheng H, Collins HL, Rodenbaugh DW, Patel KP. Daily exercise normalizes the number of diaphorase (NOS) positive neurons in the hypothalamus of hypertensive rats. Brain Res 955: 153–160, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med 165: 207–213, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gabor A, Leenen FH. Mechanisms mediating sodium-induced pressor responses in the PVN of Dahl rats. Am J Physiol Regul Integr Comp Physiol 301: R1338–R1349, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Garovic V, Textor SC. Renovascular hypertension: current concepts. Semin Nephrol 25: 261–271, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, Thomas SB, Brown J, McTigue K, Hames KC, Lang W, Jakicic JM. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 304: 1795–1802, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin K, Polichnowski A, Licea-Vargas H, Picken M, Long J, Williamson G, Bidani A. Large BP-dependent and -independent differences in susceptibility to nephropathy after nitric oxide inhibition in Sprague-Dawley rats from two major suppliers. Am J Physiol Renal Physiol 302: F173–F182, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guild SJ, Barrett CJ, McBryde FD, Van Vliet BN, Head GA, Burke SL, Malpas SC. Quantifying sympathetic nerve activity: problems, pitfalls and the need for standardization. Exp Physiol 95: 41–50, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Guo ZL, Moazzami AR. Involvement of nuclei in the hypothalamus in cardiac sympathoexcitatory reflexes in cats. Brain Res 1006: 36–48, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Haywood JR, Mifflin SW, Craig T, Calderon A, Hensler JG, Hinojosa-Laborde C. Gamma-Aminobutyric acid (GABA)–A function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension. Hypertension 37: 614–618, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Henze M, Tiniakov R, Samarel A, Holmes E, Scrogin K. Chronic fluoxetine reduces autonomic control of cardiac rhythms in rats with congestive heart failure. Am J Physiol Heart Circ Physiol 304: H444–H454, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Higa-Taniguchi KT, Silva FC, Silva HM, Michelini LC, Stern JE. Exercise training-induced remodeling of paraventricular nucleus (nor)adrenergic innervation in normotensive and hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292: R1717–R1727, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol Regul Integr Comp Physiol 266: R306–R313, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Hsu YC, Chen HI, Kuo YM, Yu L, Huang TY, Chen SJ, Chuang JI, Wu FS, Jen CJ. Chronic treadmill running in normotensive rats resets the resting blood pressure to lower levels by upregulating the hypothalamic GABAergic system. J Hypertens 29: 2339–2348, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Kalra PA, Guo H, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in the United States. Kidney Int 77: 37–43, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension 4: 166–174, 1982. [PubMed] [Google Scholar]
- 30.Kokkinos P, Pittaras A, Manolis A, Panagiotakos D, Narayan P, Manjoros D, Amdur RL, Singh S. Exercise capacity and 24-h blood pressure in prehypertensive men and women. Am J Hypertens 19: 251–258, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Kramer JM, Beatty JA, Little HR, Plowey ED, Waldrop TG. Chronic exercise alters caudal hypothalamic regulation of the cardiovascular system in hypertensive rats. Am J Physiol Regul Integr Comp Physiol 280: R389–R397, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Lebrun CJ, Blume A, Herdegen T, Seifert K, Bravo R, Unger T. Angiotensin II induces a complex activation of transcription factors in the rat brain: expression of Fos, Jun and Krox proteins. Neuroscience 65: 93–99, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol 88: 2664–2674, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol 290: H1110–H1119, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Ferguson AV. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol 265: R302–R309, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 302: R424–R432, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lon S, Szczepanska-Sadowska E, Paczwa P, Ganten D. Enhanced blood pressure buffering role of the brain nitrergic system in renin transgenic rats. Brain Res 842: 384–391, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res 454: 123–130, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Maliszewska-Scislo M, Chen H, Augustyniak RA, Seth D, Rossi NF. Subfornical organ differentially modulates baroreflex function in normotensive and two-kidney, one-clip hypertensive rats. Am J Physiol Regul Integr Comp Physiol 295: R741–R750, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maliszewska-Scislo M, Scislo TJ, Rossi NF. Effect of blockade of endogenous angiotensin II on baroreflex function in conscious diabetic rats. Am J Physiol Heart Circ Physiol 284: H1601–H1611, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension 17: 707–719, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116: 2110–2118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller PJ. Influence of sedentary versus physically active conditions on regulation of plasma renin activity and vasopressin. Am J Physiol Regul Integr Comp Physiol 295: R727–R732, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller PJ. Physical (in)activity-dependent alterations at the rostral ventrolateral medulla: influence on sympathetic nervous system regulation. Am J Physiol Regul Integr Comp Physiol 298: R1468–R1474, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller PJ, Mischel NA, Scislo TJ. Differential activation of adrenal, renal, and lumbar sympathetic nerves following stimulation of the rostral ventrolateral medulla of the rat. Am J Physiol Regul Integr Comp Physiol 300: R1230–R1240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura A, Johns EJ. Effect of renal nerves on expression of renin and angiotensinogen genes in rat kidneys. Am J Physiol Endocrinol Metab 266: E230–E241, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. J Renin Angiotensin Aldosterone Syst 2: S176–S184, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Negrao CE, Irigoyen MC, Moreira ED, Brum PC, Freire PM, Krieger EM. Effect of exercise training on RSNA, baroreflex control, and blood pressure responsiveness. Am J Physiol Regul Integr Comp Physiol 265: R365–R370, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Overton JM, Tipton CM, Matthes RD, Leininger JR. Voluntary exercise and its effects on young SHR and stroke-prone hypertensive rats. J Appl Physiol 61: 318–324, 1986. [DOI] [PubMed] [Google Scholar]
- 50.Pan YX, Gao L, Wang WZ, Zheng H, Liu D, Patel KP, Zucker IH, Wang W. Exercise training prevents arterial baroreflex dysfunction in rats treated with central angiotensin II. Hypertension 49: 519–527, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol 302: H527–H537, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol Regul Integr Comp Physiol 275: R1719–R1723, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Qadri F, Arens T, Schwarz EC, Hauser W, Dendorfer A, Dominiak P. Brain nitric oxide synthase activity in spontaneously hypertensive rats during the development of hypertension. J Hypertens 21: 1687–1694, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Rossi NF, Maliszewska-Scislo M, Chen H, Black SM, Sharma S, Ravikov R, Augustyniak RA. Neuronal nitric oxide synthase within paraventricular nucleus: blood pressure and baroreflex in two-kidney, one-clip hypertensive rats. Exp Physiol 95: 845–857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scislo TJ, Tan N, O'Leary DS. Differential role of nitric oxide in regional sympathetic responses to stimulation of NTS A2a adenosine receptors. Am J Physiol Heart Circ Physiol 288: H638–H649, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Sharman JE, Stowasser M. Australian association for exercise and sports science position statement on exercise and hypertension. J Sci Med Sport 12: 252–257, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Soares ER, Lima WG, Machado RP, Carneiro CM, Silva ME, Rodrigues MC, De Castro UG, Santos RA, Campagnole-Santos MJ, Alzamora AC. Cardiac and renal effects induced by different exercise workloads in renovascular hypertensive rats. Braz J Med Biol Res 44: 573–582, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Tanabe K, Masuda K, Hirayama A, Nagase S, Kono I, Kuno S. Effect of spontaneous exercise on antioxidant capacity in rat muscles determined by electron spin resonance. Acta Physiol (Oxf) 186: 119–125, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Golledge J. Neuronal nitric oxide synthase and sympathetic nerve activity in neurovascular and metabolic systems. Curr Neurovasc Res 10: 81–89, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Watkins ND, Cork SC, Pyner S. An immunohistochemical investigation of the relationship between neuronal nitric oxide synthase, GABA and presympathetic paraventricular neurons in the hypothalamus. Neuroscience 159: 1079–1088, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation 121: 2109–2116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension 60: 1023–1030, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye S, Zhong H, Duong VN, Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension 39: 1101–1106, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 273: R864–R872, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332–H2341, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension 58: 966–973, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann NY Acad Sci 940: 431–443, 2001. [PubMed] [Google Scholar]