Abstract
Erythrocytes participate in the matching of oxygen (O2) delivery with local need in skeletal muscle via the release of O2 and the vasodilator, ATP. It was reported that a concentration of insulin found in humans with insulin resistance inhibits low O2-induced ATP release. However, in vivo, insulin is coreleased with connecting peptide (C-peptide) at equimolar concentrations, but because of the shorter insulin half-life, the peptides circulate at ratios of C-peptide to insulin ranging from 1:1 to 6:1. Here, we investigate the hypothesis that C-peptide and insulin work synergistically to maintain low O2-induced ATP release from human erythrocytes. Using a thin-film tonometer to alter O2 tension, we determined that either C-peptide or insulin alone inhibits low O2-induced ATP release in a concentration-dependent manner; however, coadministration of the peptides at a 1:1 ratio does not (n = 5; P < 0.05). Because this ratio of C-peptide to insulin is not present in vivo for extended periods, we also investigated the effect of additional physiological ratios on ATP release. In the presence of insulin concentrations that would be found in fasting humans (0.05 nM), C-peptide to insulin ratios of 4:1 and 6:1 did not adversely affect low O2-induced ATP release. However, at a concentration of insulin found in the peripheral circulation of humans under postprandial conditions (0.5 nM), a ratio of C-peptide to insulin of 6:1 inhibited low O2-induced ATP release (n = 5). These findings demonstrate a heretofore unrecognized synergism between C-peptide and insulin that could have physiological importance in the regulation of perfusion distribution in skeletal muscle.
Keywords: phosphodiesterase 3, UT-15C, diabetes, microcirculation, adenosine triphosphate
the efficient matching of oxygen (O2) delivery to meet local O2 need in skeletal muscle requires a mechanism to direct blood flow (O2 delivery) to areas with reduced tissue oxygen tension (Po2). The cell responsible for the delivery of O2, the circulating erythrocyte, is in an ideal position to participate in such a regulatory mechanism. Exposure to reduced Po2 stimulates the release of O2, as well as the potent vasodilator ATP from erythrocytes. Importantly, erythrocyte-released ATP produces both local vasodilation and dilation that is conducted to upstream feed arterioles resulting in increases in O2 supply to local areas of O2 need (1, 4, 7, 39).
Low O2-induced ATP release from erythrocytes is the result of activation of a well-characterized signaling cascade that requires activation of the heterotrimeric G protein, Gi, as well as increases in cAMP that are regulated by the activity of phosphodiesterase 3 (PDE3) (14, 16, 36). Insulin is known to activate PDE3 in several tissues (18, 27, 46), and erythrocytes possess insulin receptors (12, 13, 33). Importantly, incubation of human erythrocytes with insulin was reported to inhibit low O2-induced ATP release via activation of PDE3 (15, 16). In support of the critical role for PDE3 in this signaling pathway, it was shown that the PDE3 inhibitor, cilostazol, attenuated insulin-induced inhibition of ATP release from healthy human erythrocytes in response to activation of Gi (16). Because low O2-induced ATP release from erythrocytes is involved in the optimal distribution of blood flow to skeletal muscle and circulating insulin is necessary for the control of blood glucose, some mechanism must exist, in vivo, to permit this important physiological response to low O2 to occur in the presence of insulin. One logical mechanism would be through the action of the connecting peptide that is coreleased with insulin (42).
Insulin is formed in β-cells of the pancreas as proinsulin, a single chain composed of an A-chain, a B-chain, and a 31-amino acid connecting peptide (C-peptide) (42). Upon enzymatic cleavage, mature insulin is separated from C-peptide, and both of these peptides are released together into the circulation. However, the half-life of C-peptide is approximately 30 min, while insulin has a half-life of only 3–5 min (5, 34, 42). Thus, circulating C-peptide levels equal or exceed those of insulin.
Although C-peptide was initially considered to be an inert by-product of insulin biosynthesis, recent studies have demonstrated several biological activities (10, 11, 20, 44), and the role of C-peptide in the microcirculation remains under investigation. It is of interest that, in humans with diabetes, C-peptide levels approaching physiological concentrations have been correlated with a reduced incidence of microvascular complications (2, 41). It has also been reported that C-peptide can oppose diabetes-associated renal and cerebral complications (45). Indeed, the value of therapeutic replacement of C-peptide in Type 1 diabetes has been borne out in clinical trials, with additional studies in progress at this time (3, 6). In contrast to the beneficial effects of physiological concentrations of C-peptide, the supraphysiological levels reached in humans with insulin resistance have been correlated with the development of atherosclerosis (32, 43). Thus, the role of C-peptide in the maintenance of normal vascular function remains controversial. For these reasons, it is critical to understand the effects of physiological concentrations and ratios of C-peptide to insulin on healthy human erythrocytes, cells that participate in the distribution of perfusion in skeletal muscle.
In spite of the fact that insulin and C-peptide are coreleased and circulate together, studies that examine the combined effects of the two peptides on the vasculature or on erythrocytes, in particular, are limited. Although it has been shown that C-peptide produces concentration-dependent vasodilation of skeletal muscle arterioles, only in the presence of a small, nonvasodilating concentration of insulin, these studies were performed in the absence of erythrocytes (24). It was also reported that C-peptide stimulates ATP release from erythrocytes; however, those studies used concentrations of C-peptide exceeding those found in vivo (25, 29, 30). In addition, those studies did not determine the effect of C-peptide on a physiological stimulus for ATP release from these cells and did not investigate any relationship between C-peptide and insulin (25, 29, 30).
Here, we investigated the effects of insulin and C-peptide alone and when combined at physiological ratios and concentrations (17, 22) on low O2-induced ATP release from human erythrocytes. The finding of a heretofore unrecognized positive synergistic effect of the peptides on this stimulus for ATP release in the microcirculation would have important implications for the prevention and treatment of peripheral vascular disease.
METHODS
Isolation of human erythrocytes.
Blood was obtained from healthy volunteers, 14 females and 9 males with an average age of 43 yr (range 20–65 yr), via venipuncture using a syringe containing heparin (500 units). Blood was centrifuged at 500 g at 4°C for 10 min, and the plasma, buffy coat, and uppermost layer of erythrocytes were removed by aspiration. The remaining erythrocytes were resuspended and washed three times in a physiological buffer containing (in mM) 21.0 Tris, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 5.5 glucose, and 0.5% BSA with the pH adjusted to 7.4. After the final wash, the hematocrit of the isolated erythrocytes was measured. Erythrocytes were prepared on the day of use. Informed consent was obtained from all subjects, and the protocol for blood removal was approved by the Institutional Review Board of Saint Louis University.
Measurement of ATP.
ATP was measured using the luciferin-luciferase assay. A 200-μl sample of the erythrocyte suspension (0.04% hematocrit) was injected into a cuvette containing 100 μl of firefly lantern extract (10 mg/ml distilled water, FLE 250; Sigma, St. Louis, MO) and 100 μl of d-luciferin solution (10 mg/20 ml distilled water; Research Products International, Mount Prospect, IL). The light emitted from the reaction with ATP was quantified using a luminometer (TD 20/20; Turner Designs). A standard curve was generated for each experiment. ATP values were normalized to the amount released from 4 × 108 cells.
Measurement of free hemoglobin.
To exclude the possibility that amounts of ATP measured were the result of erythrocyte lysis, samples used for measuring ATP were centrifuged at 800 g for 10 min at room temperature. The presence of hemoglobin in the supernatant was determined by measurement of light absorbance at 405 nm (21). Samples in which free hemoglobin increased were not included, resulting in the exclusion of only 12 of 88 experiments.
Determination of ATP release from erythrocytes in response to exposure to low O2 tension in the presence and absence of C-peptide and/or insulin.
Isolated erythrocytes were diluted to a 20% hematocrit in a second physiological buffer containing (in mM) 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 5.5 glucose, 24.8 NaHCO3, 5.5 dextrose, 0.5% BSA, pH 7.4 at 37°C. Erythrocyte suspensions were equilibrated for 30 min in a thin-film tonometer (model DEQ1; Cameron Instrument, Guelph, Ontario, Canada) with gas containing 15% O2, 6% CO2, and the balance N2 (normoxia, Po2 = 111 ± 6 mmHg) and ATP levels were determined. The erythrocytes were then exposed to reduced O2 (0% O2, 6% CO2, and the balance N2, Po2 = 16 ± 6 mmHg) for 10 min with ATP levels again determined. The effect of a 20-min pretreatment of erythrocytes with insulin (0.05, 0.1, or 0.5 nM) (Humalog U 100, Eli Lilly) or C-peptide (0.3 and 1 nM) (human, amino acid sequence 55–89, 97.4% pure by HPLC analysis; Sigma) on low O2-induced ATP release was determined. In separate studies, the effect of coadministration of C-peptide and insulin at various concentrations and ratios of C-peptide to insulin (1:1 to 6:1) on low O2-induced ATP release was also determined.
Determination of ATP release from erythrocytes in response to incubation with a prostacyclin analog in the presence and absence of insulin or C-peptide.
Isolated erythrocytes (20% hematocrit) were incubated with the prostacyclin analog, UT-15C (1 μM) (United Therapeutics), in the presence and absence of a 25-min pretreatment at room temperature with either 1 nM insulin or 1 nM C-peptide. UT-15C-induced ATP release was determined at 5, 10, and 15 min after the addition of the prostacyclin analog. The maximum response is reported.
Statistical analysis.
Statistical significance among experiments was determined using an ANOVA. In the event that the F-ratio indicated that a change had occurred, a Fisher's least significant difference test was performed to identify individual differences between groups. Results are reported as means ± SE. P values of 0.05 or less were considered to indicate a significant difference.
RESULTS
Effect of insulin on low O2-induced ATP release from erythrocytes.
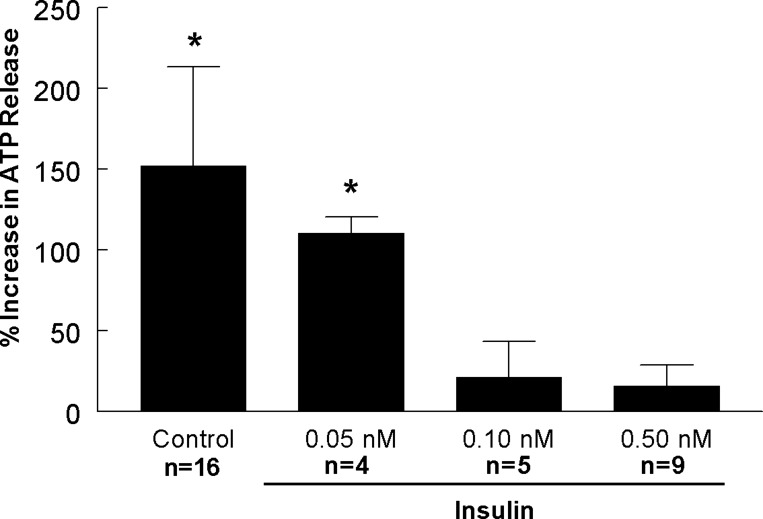
Erythrocytes release O2 and the vasodilator ATP in response to exposure to low O2 via a signaling pathway that requires increases in intracellular cAMP that are regulated by PDE3 (14, 16, 36). Insulin, at levels reported to be present in humans with insulin resistance, increases PDE3 activity, resulting in inhibition of ATP release (15, 16). Here, we determined the effect of insulin at concentrations that represent those observed in healthy humans on this physiological response (17, 22). Insulin at a concentration of 0.05 nM had no effect on low O2-induced ATP release. However, at concentrations of 0.1 nM and 0.5 nM, insulin inhibited the response to low O2 (Fig. 1). These data establish that insulin-induced inhibition of low O2-induced ATP release is concentration-dependent.
Fig. 1.
Effect of insulin on low O2-induced ATP release from erythrocytes. Isolated erythrocytes were incubated with vehicle (saline, control) or insulin at various concentrations for 20 min, while exposed to a gas mixture containing 15% O2, 6% CO2, and the balance N2 (normoxia) prior to measurement of ATP levels. This was followed by exposure to 0% O2, 6% CO2, and the balance N2 (low O2) for 10 min prior to a second measurement of ATP levels. Values are expressed as means ± SE. *Significant increase from normoxia (P < 0.05).
Effect of C-peptide on low O2-induced ATP release from erythrocytes.
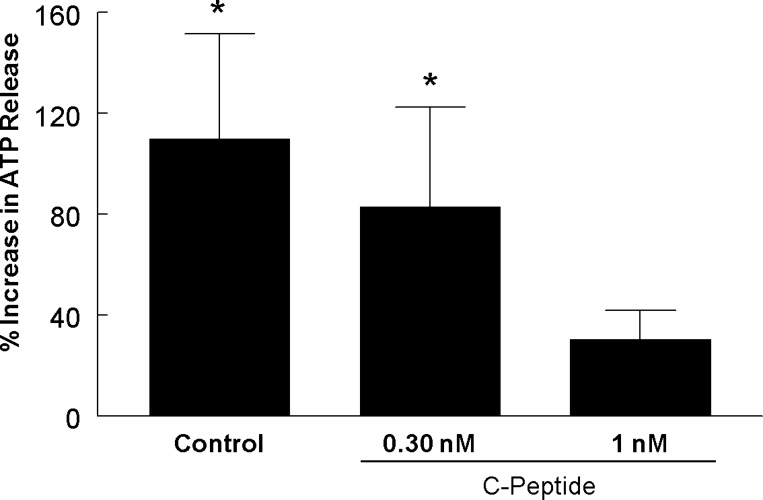
We next determined the effect of C-peptide in the absence of insulin on low O2-induced ATP release from human erythrocytes. At 1 nM, a concentration of C-peptide reached under postprandial conditions in healthy humans (17, 22), low O2-induced ATP release was inhibited (Fig. 2). However, when erythrocytes were incubated with 0.3 nM C-peptide, a concentration found during fasting, low O2-induced ATP release was not prevented (Fig. 2). Thus, similar to insulin, C-peptide-induced inhibition of low O2-induced ATP release is concentration-dependent.
Fig. 2.
Effect of C-peptide on low O2-induced ATP release from erythrocytes. Isolated erythrocytes were incubated with saline (control) or C-peptide at 0.3 nM (n = 4) or 1 nM (n = 6) for 20 min, while exposed to a gas mixture containing 15% O2, 6% CO2, and the balance N2 (normoxia) prior to measurement of ATP release. This was followed by exposure to 0% O2, 6% CO2, and the balance N2 (low O2) for 10 min prior to a second measurement of ATP release. Values are expressed as means ± SE. *Significant increase from normoxia (P < 0.05).
Effect of insulin or C-peptide on UT-15C-induced ATP release from erythrocytes.
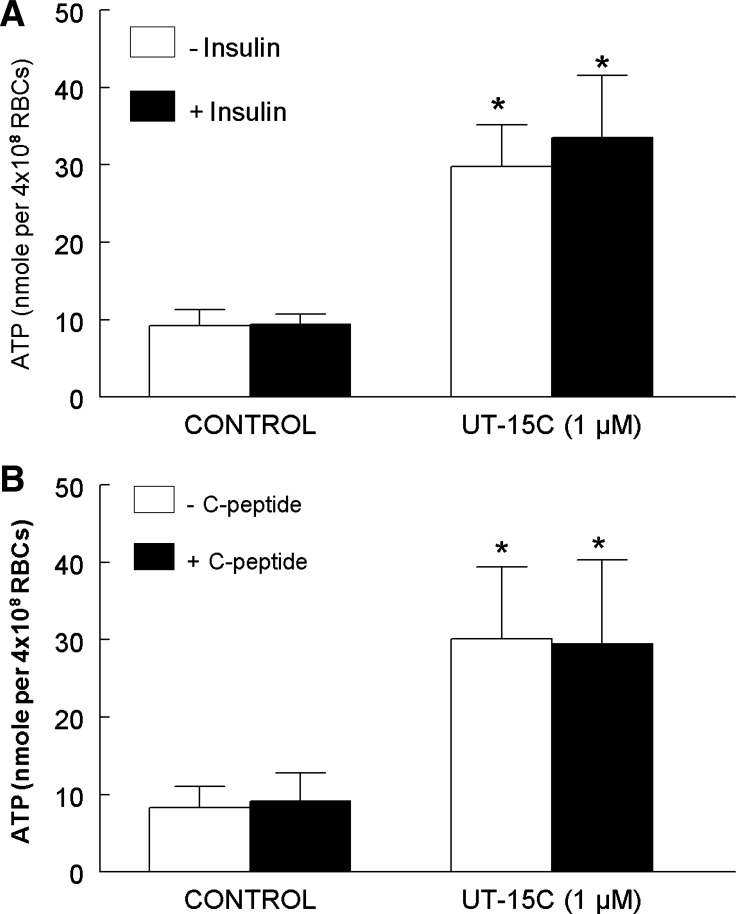
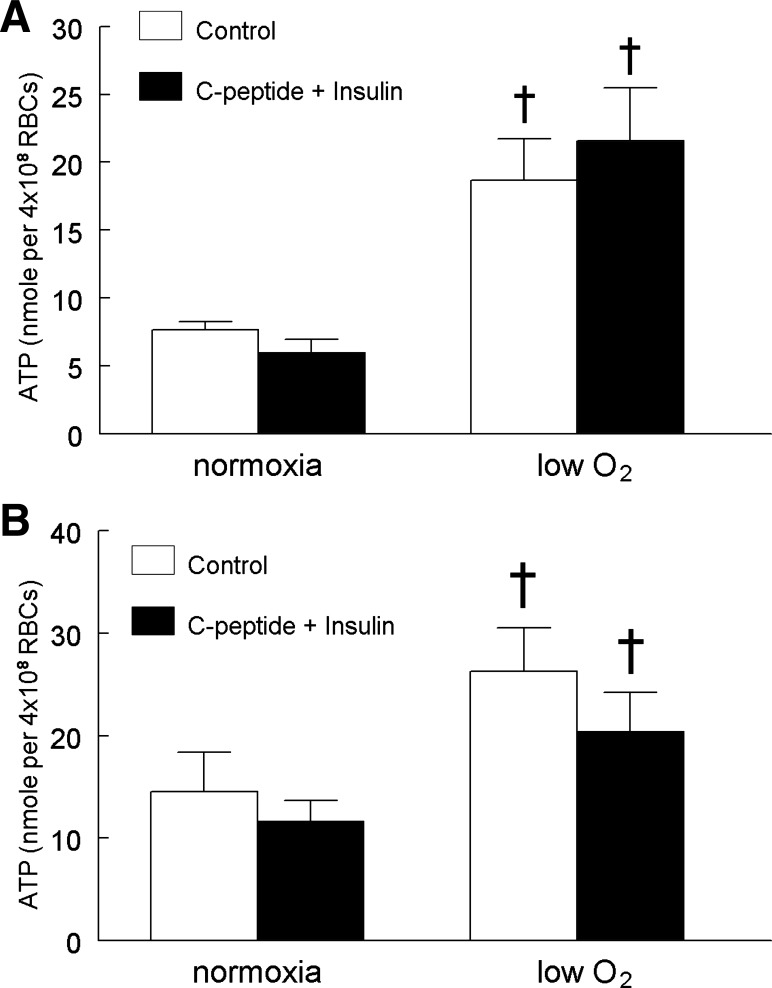
In addition to exposure to low O2, activation of erythrocyte prostacyclin receptors results in ATP release via a signaling pathway that differs from that initiated by exposure to low O2 (37, 40). We determined that pretreatment of erythrocytes with either 1 nM insulin or 1 nM C-peptide had no effect on ATP release stimulated by exposure of these cells to the prostacyclin analog, UT-15C (Fig. 3, A and B). Thus, the adverse effects of insulin and C-peptide on low O2-induced ATP release are not the result of a general inhibition of all signaling pathways for release of ATP from these cells.
Fig. 3.
Effect of insulin or C-peptide on UT-15C-induced ATP release from erythrocytes. Isolated erythrocytes were incubated with 1 μM UT-15C in the absence or presence of a 25-min preincubation with 1 nM insulin (A; n = 5) or 1 nM C-peptide (B; n = 5). The maximum ATP release stimulated by UT-15C treatment was reported. Values are expressed as means ± SE. *Significant increase from control (P < 0.05).
Effect of coadministration of C-peptide and insulin at various ratios and concentrations on low O2-induced ATP release from erythrocytes.
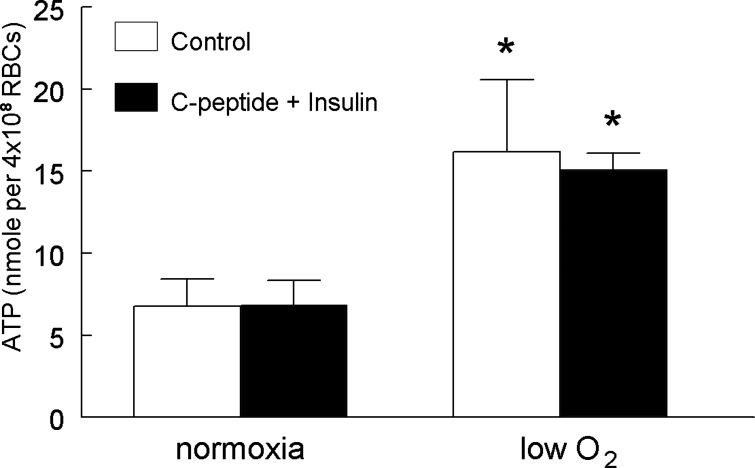
Although administration of either C-peptide or insulin alone at postprandial concentrations had inhibitory effects on low O2-induced ATP release, this is not representative of the in vivo relationship between these two peptides. These peptides are coreleased from the pancreas in equimolar amounts, but because of differing degradation rates, a range of C-peptide to insulin ratios is present physiologically with C-peptide levels equaling or exceeding those of insulin (17, 22). We examined the effect of coadministration of C-peptide and insulin at a 1:1 ratio using concentrations (1 nM each) that inhibited low O2-induced ATP release when the peptides were administered alone (Figs. 1 and 2). Importantly, coadministration of the peptides at this concentration did not inhibit low O2-induced ATP release (Fig. 4).
Fig. 4.
Effect of a 1:1 ratio of C-peptide to insulin on low O2-induced ATP release from erythrocytes. Isolated erythrocytes were incubated with saline (control) or 1 nM C-peptide and 1 nM insulin (n = 5) for 20 min while exposed to a gas mixture containing 15% O2, 6% CO2, and the balance N2 (normoxia). ATP release was determined during normoxia and after a 10-min exposure to 0% O2, 6% CO2, and the balance N2 (low O2). Values are expressed as means ± SE. *Significant increase from normoxia (P < 0.05).
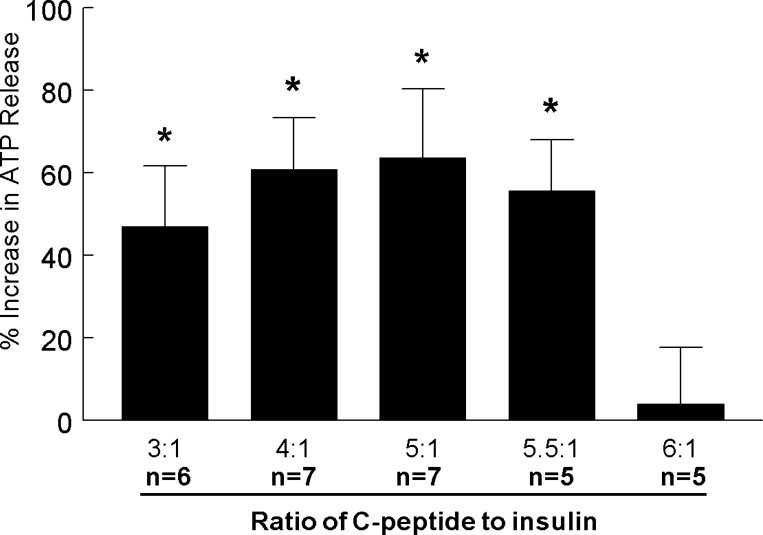
We next examined the effects of a range of C-peptide to insulin ratios on low O2-induced ATP release from human erythrocytes with the concentration of insulin kept constant at 0.5 nM. Under these conditions, C-peptide-to-insulin ratios of 3:1, 4:1, 5:1, and 5.5:1 did not inhibit low O2-induced ATP release (Fig. 5). However, at a ratio of 6:1 (3 nM C-peptide and 0.5 nM insulin), low O2-induced ATP release was inhibited similarly to that seen when C-peptide was administered alone (Figs. 3 and 6).
Fig. 5.
Effect of physiological ratios of C-peptide to insulin on low O2-induced ATP release from erythrocytes. Isolated erythrocytes were incubated with various ratios of C-peptide (1.5, 2.0, 2.5, 2.75, or 3.0 nM) to insulin (0.5 nM) for 20 min, while exposed to a gas mixture containing 15% O2, 6% CO2, and the balance N2 (normoxia). ATP release was determined during normoxia and after a 10-min exposure to 0% O2, 6% CO2, and the balance N2 (low O2). Values are expressed as means ± SE. *Significant increase from normoxia (P < 0.05).
Fig. 6.
Effect of physiological ratios of C-peptide to insulin at lower concentrations on low O2-induced ATP release from erythrocytes. Isolated erythrocytes were incubated with saline (control), 0.20 nM C-peptide and 0.05 nM insulin (4:1 ratio, n = 4; A), or 0.30 nM C-peptide and 0.05 nM insulin (6:1 ratio, n = 4; B) for 20 min while exposed to a gas mixture containing 15% O2, 6% CO2, and the balance N2 (normoxia). ATP release was determined during normoxia and after a 10-min exposure to 0% O2, 6% CO2, and the balance N2 (low O2). Values are expressed as means ± SE. †Significant increase from normoxia (P < 0.01).
Finally, we determined whether the concentration of the two peptides, as well as their ratio, was critical for maintenance of low O2-induced ATP release. In these studies, the concentration of insulin was reduced 10-fold (0.05 nM) to values that represent those found during fasting. At both the higher (Fig. 5) and lower (Fig. 6A) concentrations of insulin, a 4:1 ratio of the peptides did not inhibit low O2-induced ATP release. However, although a 6:1 ratio of the two peptides inhibited ATP release at the higher concentrations (0.5 nM insulin, Fig. 5), at the lower concentrations, that same ratio did not (0.05 nM, Fig. 6B). These results provide support for the hypothesis that both the ratios and concentrations of C-peptide and insulin are critical in modulating the effect of these peptides on low O2-induced ATP release from human erythrocytes.
DISCUSSION
It is well recognized that erythrocytes are far more than simple O2 carriers, but are, in fact, complex cells that contribute actively to the control of local vascular caliber (1, 4, 7, 9, 39). We and others have shown that erythrocytes of healthy humans stimulate vasodilation in the skeletal muscle microcirculation via their ability to release ATP in response to exposure to reduced O2 tension (1, 4, 8, 35, 38). This erythrocyte-released ATP then binds to purinergic receptors on the endothelium (23, 26, 31), inducing both local vasodilation, as well as dilation, that is conducted to feed arterioles resulting in increased blood flow and O2 delivery (1, 4, 7, 39). Thus, in skeletal muscle, the ability of erythrocytes to release both O2 and ATP in response to exposure to low O2 permits these circulating cells to participate in the regulation of blood flow to provide optimal delivery of O2 and nutrients.
The release of ATP from erythrocytes exposed to low O2 requires activation of a signaling pathway that is inhibited in the presence of insulin at a concentration found in humans with insulin resistance (15). Here, we report that, in the absence of C-peptide, concentrations of insulin that are present in humans in the postprandial period (17, 22) also inhibit low O2-induced ATP release from erythrocytes (Fig. 1). Studies in which insulin alone is administered ignore a potential synergistic role for C-peptide in the microcirculation.
Although C-peptide was previously considered to be an inactive by-product of insulin synthesis and release, more recently, this peptide has been reported to have biological activity (10, 11, 20, 44). The potential importance of physiological levels of C-peptide in circulatory control is suggested by reports that in Type 1 and Type 2 diabetes, higher residual endogenous C-peptide levels correlate negatively with the incidence of neuropathy, nephropathy, and retinopathy (2, 41). In contrast, C-peptide may not be beneficial at supraphysiological concentrations, such as those observed in prediabetes (32, 43). In insulin-resistant individuals, elevated C-peptide has been associated with increased levels of the proinflammatory cytokine, IL-6 (19), and C-peptide has been found in arteriosclerotic lesions in humans with diabetes (28). However, these studies do not address the relationship between C-peptide and insulin in circulatory control.
Previous studies investigating the effects of C-peptide on erythrocytes reported that the peptide directly promoted ATP release (25, 29, 30). However, those studies used either supraphysiological concentrations of C-peptide (17) and/or did not address either a physiological stimulus for ATP release or a time course for ATP release that is relevant for the moment-to-moment regulation of blood flow and oxygen delivery in vivo (25, 29, 30). We report here that C-peptide alone, at a concentration reported to be present in humans during the postprandial period (1 nM) (17), inhibits low O2-induced ATP release from human erythrocytes (Fig. 2). Although studies in which C-peptide is administered in the absence of insulin are interesting, they do not replicate the physiological relationship between the two peptides. In contrast, insulin is administered to humans with diabetes in the absence of C-peptide. Therefore, we investigated the effect of several concentrations of insulin alone on low O2-induced ATP release (Fig. 1). We determined that both C-peptide and insulin, given alone, inhibit low O2-induced ATP release in a concentration-dependent manner (Figs. 1 and 2). It is important to note that this inhibition of low O2-induced ATP release is not due to a general failure to release ATP from erythrocytes since the peptides do not inhibit ATP release in response to prostacyclin receptor activation, which initiates a distinct signaling pathway for ATP release from erythrocytes (Fig. 3) (37, 40).
Although incubation with either C-peptide or insulin alone can inhibit low O2-induced ATP release from erythrocytes, it is important to recognize that these peptides are coreleased and circulate together. Therefore, it is critical to determine whether C-peptide and insulin act synergistically with respect to this important physiological response. In support of such a relationship, it has been reported that C-peptide stimulates concentration-dependent vasodilation of isolated arterioles only when a small, nonvasodilating concentration of insulin is present (24). In that study, coadministration of insulin with increasing concentrations of C-peptide resulted in greater and more uniform increases in vasodilation than was seen with C-peptide alone. Although this result demonstrates a synergistic effect of C-peptide and insulin directly on arterioles, no previous studies have addressed the effect of physiological concentrations and ratios of C-peptide to insulin on ATP release from erythrocytes. Here, we report that at a ratio of C-peptide to insulin of 1:1 and at a concentration of each peptide of 1 nM, a concentration that has been measured in the portal vein (22), these peptides do not inhibit low O2-induced ATP release from human erythrocytes (Fig. 4). However, although insulin is coreleased with C-peptide in vivo, due to differing clearance rates, circulating C-peptide levels exceed those of insulin (17, 22, 42).
To evaluate further the synergistic relationship between C-peptide and insulin on low O2-induced ATP release from healthy human erythrocytes, we measured ATP release in the absence and presence of varying ratios of the two peptides. In these studies, the concentration of insulin was held constant at 0.5 nM, a concentration that can be achieved in healthy humans under postprandial conditions (17, 22). When C-peptide and insulin were coadministered at ratios of 1:1 to 5.5:1 (C-peptide to insulin) low O2-induced ATP release from healthy human erythrocytes was maintained (Fig. 5). However, when the ratio of C-peptide to 0.5 nM insulin was increased to 6:1, low O2-induced ATP release is compromised (Fig. 5). A ratio of C-peptide to insulin of 6:1 with an insulin concentration of 0.5 nM could be anticipated to be present in humans under postprandial conditions (17, 22). In contrast, low O2-induced ATP release was not inhibited at the same ratio of the two peptides (6:1) when the concentration of insulin was reduced to represent values that are present in the fasting state (0.05 nM) as shown in Fig. 6B (17, 22). Thus, both the ratio and concentration of C-peptide and insulin are critical factors that determine the effect of these peptides on low O2-induced ATP release from human erythrocytes.
Perspectives and Significance
Our data are consistent with the hypothesis that corelease of C-peptide and insulin in vivo prevents adverse effects of insulin alone on low O2-induced ATP release from erythrocytes permitting these cells to participate fully in the optimal matching of O2 delivery with need in skeletal muscle. These findings also suggest that administration of the combination of C-peptide with insulin, at appropriate physiological ratios, could aid in the prevention and treatment of the peripheral vascular disease associated with diabetes. In light of the use of C-peptide in clinical trials for the treatment of humans with diabetes, it becomes increasingly important to understand the relationship between C-peptide and insulin in erythrocyte physiology.
GRANTS
This work was supported by grants from the American Diabetes Association (BS-150), the National Institutes of Health (HL-089094). The authors thank United Therapeutics for the gift of UT-15C.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.P.R., A.H.S., M.L.E., and R.S.S. conception and design of research; J.P.R. and R.S.S. performed experiments; J.P.R. and R.S.S. analyzed data; J.P.R. and R.S.S. interpreted results of experiments; J.P.R. prepared figures; J.P.R. and R.S.S. drafted manuscript; J.P.R., A.H.S., M.L.E., and R.S.S. edited and revised manuscript; J.P.R., A.H.S., M.L.E., and R.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank J. L. Sprague for inspiration. The authors also wish to thank E. A. Bowles for technical support and assistance in preparing this manuscript.
REFERENCES
- 1.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Bo S, Gentile L, Castiglione A, Prandi V, Canil S, Ghigo E, Ciccone G. C-peptide and the risk for incident complications and mortality in type 2 diabetic patients: a retrospective cohort study after a 14-year follow-up. Eur J Endocrinol 167: 173–180, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Cebix Incorporated A Phase 2b, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of CBX129801 (Ersatta™), Long-Acting Synthetic C-Peptide, in Type 1 Diabetes Mellitus Subjects With Mild to Moderate Diabetic Peripheral Neuropathy. Bethesda, MD: National Library of Medicine (US) 2000. (accessed July 8, 2013) Available from http://clinicaltrials.gov/show/NCT01681290 NLM Identifier: NCT01681290 [Google Scholar]
- 4.Dietrich HH. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278: H1294–H1298, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 19: 608–624, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Ekberg K, Brismar T, Johansson BL, Lindstrom P, Juntti-Berggren L, Norrby A, Berne C, Arnqvist HJ, Bolinder J, Wahren J. C-Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care 30: 71–76, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ellsworth ML. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 24: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellsworth ML, Sprague RS. Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP. J Physiol 590: 4985–4991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forst T, De La Tour DD, Kunt T, Pfutzner A, Goitom K, Pohlmann T, Schneider S, Johansson BL, Wahren J, Lobig M, Engelbach M, Beyer J, Vague P. Effects of proinsulin C-peptide on nitric oxide, microvascular blood flow and erythrocyte Na+,K+-ATPase activity in diabetes mellitus type I. Clin Sci (Lond) 98: 283–290, 2000 [PubMed] [Google Scholar]
- 11.Forst T, Kunt T. Effects of C-peptide on microvascular blood flow and blood hemorheology. Exp Diabesity Res 5: 51–64, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambhir KK, Agarwal VR. Red blood cell insulin receptors in health and disease. Biochem Med Metab Biol 45: 133–153, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Gambhir KK, Archer JA, Bradley CJ. Characteristics of human erythrocyte insulin receptors. Diabetes 27: 701–708, 1978 [DOI] [PubMed] [Google Scholar]
- 14.Goldman D, Fraser GM, Ellis CG, Sprague RS, Ellsworth ML, Stephenson AH. Toward a multiscale description of microvascular flow regulation: O2-dependent release of ATP from human erythrocytes and the distribution of ATP in capillary networks. Front Physiol 3: 246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson MS, Ellsworth ML, Achilleus D, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Insulin inhibits low oxygen-induced ATP release from human erythrocytes: implication for vascular control. Microcirculation 16: 424–433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson MS, Stephenson AH, Bowles EA, Sprague RS. Insulin inhibits human erythrocyte cAMP accumulation and ATP release: role of phosphodiesterase 3 and phosphoinositide 3-kinase. Exp Biol Med (Maywood) 235: 256–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heding LG, Rasmussen SM. Human C-peptide in normal and diabetic subjects. Diabetologia 11: 201–206, 1975 [DOI] [PubMed] [Google Scholar]
- 18.Heimann E, Jones HA, Resjo S, Manganiello VC, Stenson L, Degerman E. Expression and regulation of cyclic nucleotide phosphodiesterases in human and rat pancreatic islets. PLoS One 5: e14191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heliovaara MK, Teppo AM, Karonen SL, Tuominen JA, Ebeling P. Plasma IL-6 concentration is inversely related to insulin sensitivity, and acute-phase proteins associate with glucose and lipid metabolism in healthy subjects. Diabetes Obes Metab 7: 729–736, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hills CE, Brunskill NJ. Cellular and physiological effects of C-peptide. Clin Sci (Lond) 116: 565–574, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Holownia P, Bishop E, Newman DJ, John WG, Price CP. Adaptation of latex-enhanced assay for percent glycohemoglobin to a Dade Dimension analyzer. Clin Chem 43: 76–84, 1997 [PubMed] [Google Scholar]
- 22.Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest 55: 1278–1283, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houston DA, Burnstock G, Vanhoutte PM. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther 241: 501–506, 1987 [PubMed] [Google Scholar]
- 24.Jensen ME, Messina EJ. C-peptide induces a concentration-dependent dilation of skeletal muscle arterioles only in presence of insulin. Am J Physiol Heart Circ Physiol 276: H1223–H1228, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Keltner Z, Meyer JA, Johnson EM, Palumbo AM, Spence DM, Reid GE. Mass spectrometric characterization and activity of zinc-activated proinsulin C-peptide and C-peptide mutants. Analyst 135: 278–288, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Kennedy C, Delbro D, Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol 107: 161–168, 1985 [DOI] [PubMed] [Google Scholar]
- 27.Lindh R, Ahmad F, Resjo S, James P, Yang JS, Fales HM, Manganiello V, Degerman E. Multisite phosphorylation of adipocyte and hepatocyte phosphodiesterase 3B. Biochim Biophys Acta 1773: 584–592, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Marx N, Walcher D, Raichle C, Aleksic M, Bach H, Grub M, Hombach V, Libby P, Zieske A, Homma S, Strong J. C-peptide colocalizes with macrophages in early arteriosclerotic lesions of diabetic subjects and induces monocyte chemotaxis in vitro. Arterioscler Thromb Vasc Biol 24: 540–545, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Medawala W, McCahill P, Giebink A, Meyer J, Ku CJ, Spence DM. A molecular level understanding of zinc activation of C-peptide and its effects on cellular communication in the bloodstream. Rev Diabet Stud 6: 148–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer JA, Froelich JM, Reid GE, Karunarathne WK, Spence DM. Metal-activated C-peptide facilitates glucose clearance and the release of a nitric oxide stimulus via the GLUT1 transporter. Diabetologia 51: 175–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motte S, Pirotton S, Boeynaems JM. Heterogeneity of ATP receptors in aortic endothelial cells. Involvement of P2y and P2u receptors in inositol phosphate response. Circ Res 72: 504–510, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Nordquist L, Johansson M. Proinsulin C-peptide: friend or foe in the development of diabetes-associated complications? Vasc Health Risk Manag 4: 1283–1288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada Y. Characteristics of human erythrocyte insulin binding sites. Acta Med Okayama 35: 125–135, 1981 [DOI] [PubMed] [Google Scholar]
- 34.Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest 77: 98–105, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprague RS, Bowles EA, Achilleus D, Ellsworth ML. Erythrocytes as controllers of perfusion distribution in the microvasculature of skeletal muscle. Acta Physiol (Oxf) 202: 285–292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprague RS, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML. A selective phosphodiesterase 3 inhibitor rescues low Po2-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control. Am J Physiol Heart Circ Physiol 301: H2466–H2472, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation 15: 461–471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep 61: 183–190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol 285: H693–H700, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Sridharan M, Bowles EA, Richards JP, Krantic M, Davis KL, Dietrich KA, Stephenson AH, Ellsworth ML, Sprague RS. Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel. Am J Physiol Heart Circ Physiol 302: H553–H559, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 26: 832–836, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Steiner DF. The proinsulin C-peptide—a multirole model. Exp Diabesity Res 5: 7–14, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasic D, Walcher D. C-peptide: a new mediator of atherosclerosis in diabetes. Mediators Inflamm 2012: 858692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahren J, Ekberg K, Johansson J, Henriksson M, Pramanik A, Johansson BL, Rigler R, Jornvall H. Role of C-peptide in human physiology. Am J Physiol Endocrinol Metab 278: E759–E768, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Wahren J, Kallas A, Sima AA. The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes 61: 761–772, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wijkander J, Landstrom TR, Manganiello V, Belfrage P, Degerman E. Insulin-induced phosphorylation and activation of phosphodiesterase 3B in rat adipocytes: possible role for protein kinase B but not mitogen-activated protein kinase or p70 S6 kinase. Endocrinology 139: 219–227, 1998 [DOI] [PubMed] [Google Scholar]