Abstract
Differential sensing of dietary fat and fatty acids by the oral cavity is proposed to regulate the susceptibility to obesity. In the current experiments, animals that differ in their susceptibility to obesity were used to investigate the influence of the oral cavity on the preference for the polyunsaturated fatty acid, linoleic acid. In experiment 1, the preference for differing concentrations of linoleic acid was determined in obesity-prone Osborne-Mendel (OM) and obesity-resistant S5B/Pl (S5B) rats. The preference threshold for linoleic acid was lower in S5B rats, compared with OM rats. To determine whether differences in linoleic acid preference threshold were related to innate strain differences in the fatty acid receptors on the tongue, the expression of GPR120, GPR40, and CD36 on the circumvallate papillae were assessed in OM and S5B rats. Results indicated that the expression of CD36, GPR40, and GPR120 did not differ between these two strains. Numerous studies have examined the role of CD36 on fat intake; therefore, in experiment 3, RNA interference was used to decrease the expression of CD36 on the tongues of OM and S5B rats, and the effect of decreased CD36 expression on linoleic acid preference was determined. CD36 siRNA attenuated linoleic acid preference for the most preferred concentration in both OM and S5B rats. Overall, these data indicate that there are innate differences in the preference threshold for linoleic acid in obesity-prone and obesity-resistant rats. Experimentally reducing the expression of CD36 on the circumvallate papillae attenuated the preference for linoleic acid in both strains.
Keywords: preference threshold, obesity-prone, obesity-resistant, CD36, taste
consumption of an energy-dense, high-fat diet has been associated with an increased risk for developing obesity (23). The detection of dietary fat and subsequent fat intake are mediated by input from the oral cavity and by postingestive effects (12). The ability to sense dietary fat in the oral cavity has two main functions: 1) mediation of the cephalic phase response and 2) regulation of food intake (12, 31). Differences in fat/fatty acid sensors in the oral cavity have been proposed as potential mechanisms contributing to the susceptibility of becoming obese. This is characterized by a subset of individuals who become obese on a high-fat diet (HFD) and another subset of individuals that are resistant to becoming obese when consuming a HFD (15, 24, 31, 33). Understanding the detection and regulation of fat intake by fat/fatty acid sensors in the oral cavity may elucidate the mechanisms by which individuals develop preferences for high-fat foods, overconsume dietary fat, and subsequently become obese.
Demonstration of the presence of fatty acid-sensitive receptors and channels on the tongue has increased the significance of the oral cavity in the detection of fatty acids and dietary fat. These fatty acid-sensitive receptors and channels modulate the preference for and intake of fat/fatty acids and are regulated by the consumption of dietary fat (1, 4–9, 13, 14, 21, 25, 28, 29, 31, 32, 38, 41, 42, 46, 51). The detection of fatty acids by these mechanisms induces a signaling cascade, activating gustatory nerves, which transmit sensory information to the brain, particularly regions associated with the regulation of food intake and reward (12). Several types of long-chain polyunsaturated fatty acid (PUFA) G protein-coupled receptors are expressed in rat taste buds, including GPR40 [also known as Ffar1; (5)] and GPR120 [also known as O3FAR1; (5, 28–30)]. Although, both receptors are sensitive to PUFAs (i.e., linoleic acid), they are located on different types of taste cells (Type I and Type II, respectively) (5). Numerous studies have demonstrated the importance of delayed rectifying potassium (DRK) channels in detecting the presence of fatty acids, particularly PUFAs (14, 25). The most frequently studied fatty acid receptor thus far is CD36. A major function of CD36 is to facilitate the uptake of long-chain fatty acids. CD36 knockout mice exhibit a decreased preference for fatty acids and HFD, decreased cephalic response to fatty acids, decreased food intake, body weight, and body adiposity. These mice are protected from weight gain when eating a HFD (1, 10–12, 17, 21, 41). In the mouth, CD36 has been proposed as the taste receptor for dietary fat and/or fatty acids and is primarily expressed on Type II taste cells on the circumvallate papillae of the tongue (12, 21, 29, 42, 51).
Several studies have been conducted to elucidate differences in the detection of fatty acids in taste receptor cells in animal models that differ in their susceptibility to obesity (15, 16). These studies utilized obesity-prone Osborne-Mendel rats (OM) and obesity-resistant S5B/Pl rats (S5B). Using patch-clamp techniques, Gilbertson et al. (15, 16) demonstrated greater suppression on the potassium current of DRK channels by the PUFA, linoleic acid, in obesity-prone OM rats. S5B rats were more responsive to the presence of linoleic acid than OM rats, even though mRNA expression of one of nine DRK channels was higher in OM rats. The data from these studies indicated that the tongue plays an important role in the response to fatty acids in these obesity-prone and obesity-resistant strains and supports a role for the tongue in the susceptibility of obesity in these animals.
Obesity-prone OM and obesity-resistant S5B rats have been used for many years to study physiological, behavioral, and neurochemical mechanisms that contribute to the individual susceptibility to obesity (2, 27, 34–40, 44, 45, 48, 49). The OM rats consistently gain more weight and body fat when consuming a HFD and exhibit greater hyperphagia immediately following the introduction of the HFD, compared with the S5B rats (∼50 kcal vs. 15 kcal increase in 24 h) (37). The effects of various compounds on the intake and preference for a HFD have been examined in these strains, as well as the effects of HFD on alterations in hypothalamic neurochemistry, circulating hormone levels, and intestinal gene expression (2, 19, 22, 26, 37–40, 48, 49). Recently, Primeaux et al. (38) reported that the consumption of a HFD differentially altered expression of CD36 mRNA on the circumvallate papillae and in duodenal enterocytes of OM and S5B rats. In chow-fed OM and S5B rats, CD36 expression was similar; however, the consumption of a HFD for 3 days and 14 days significantly increased CD36 mRNA levels in obesity-prone OM rats, without altering CD36 mRNA in obesity-resistant S5B rats. These data indicated that CD36 on the tongue was regulated by fat intake in the obesity-prone rats and suggested that this receptor mediated the susceptibility to obesity in these rats by altering their ability to detect dietary fat in the oral cavity.
The majority of studies investigating OM and S5B rats have focused on the response to the adaptation to a HFD, which occurs following the consumption of HFD for at least 14 days (19, 26, 27, 37–39, 47–50). In the current series of experiments, OM and S5B rats were fed a standard chow diet, which allowed for the investigation of innate differences between these two strains on the preference for fatty acid and sucrose solutions, expression of fatty acid receptors on the tongue, and the ability of CD36 on the tongue to modulate the preference for fatty acids. Experiment 1 was conducted to determine whether there were strain differences in the preference threshold for the fatty acid, linoleic acid. On the basis of previous work (15), it was hypothesized that the obesity-prone OM rats would have a higher preference threshold than the S5B rats. No differences were expected between the two strains on sucrose preference. Our previous work failed to show differences in CD36 mRNA expression on the circumvallate papillae of chow-fed OM and S5B rats (38). Because our previous work did not investigate GPR40 or GPR120 mRNA or protein levels of these receptors, experiment 2 was designed to investigate the expression of CD36, GPR120, and GPR40 on the circumvallate papillae of OM and S5B rats. The most frequently studied fatty acid receptor is CD36. CD36 has been proposed as the fat taste receptor, and our previous study has reported a HFD-induced increase in CD36 mRNA on the circumvallate papillae of OM rats. Therefore, experiment 3 was conducted to 1) investigate whether RNA interference techniques would successfully reduce the expression of CD36 on the circumvallate papillae and 2) whether reducing CD36 on the circumvallate papillae would alter the detection of a preferred concentration of linoleic acid in OM and S5B rats. It was hypothesized that reducing CD36 expression on the circumvallate papillae would decrease the preference for linoleic acid in these strains.
MATERIALS AND METHODS
Animals.
The male obesity-prone Osborne-Mendel (OM) and obesity-resistant S5B/Pl (S5B) rats (8–9 wk old) used in these studies were bred in the American Association for Accreditation of Laboratory Animal Care-approved Pennington Biomedical Research Center vivarium. Male Sprague-Dawley rats (Harlan, Indianapolis, IN) (8–9 wk old) were used in experiment 3A. All rats were individually housed on a 12:12-h light-dark cycle (lights on at 0700) with food (standard chow) and water available ad libitum. All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
Experiment 1A: linoleic acid solution intake and preference in obesity-prone OM and obesity-resistant S5B rats.
Experimentally naive OM and S5B rats (9–10/strain) were given access to two water bottles. Procedures were based on Sclafani et al. (41). One bottle contained 0.3% Xanthan gum in deionized water, while the other bottle contained varying concentrations of linoleic acid (in 0.3% Xanthan gum and deionized water). The Xanthan gum solution was used to control for differences in texture between the solutions. A single concentration of linoleic acid was presented to each rat for 48 h. Rats were given increasing concentrations of linoleic acid [0.0025, 0.025, 0.25, 0.5, 1.0, 2.0% wt/wt; (41)]. Bottle position was reversed every 24 h to control for side preferences, and the solutions were replaced with fresh solutions. Consumption of the linoleic acid solution and the Xanthan gum solution was measured by weighing each bottle (g). Following 48 h access to the linoleic acid, all rats received deionized water in both bottles for a 48–72 h washout period. This pattern was repeated until rats had access to all concentrations of linoleic acid being tested. Total intake of the linoleic acid solution was determined, and the preference for each concentration of linoleic acid was calculated {[linoleic acid solution consumed (g)]/[linoleic acid solution consumed (g) + Xanthan gum solution consumed (g)]·100}. A percent intake of 50% is indicative of no preference between the solutions and suggests that the rats do not have a preference or an aversion toward the linoleic acid solution.
Experiment 1B: sucrose solution intake and preference in obesity-prone OM and obesity-resistant S5B rats.
Experimentally naïve OM and S5B rats (n = 9–10/strain) were used to assess sucrose preference threshold using a two-bottle paradigm, as described in experiment 1A. A single concentration of sucrose was presented to the rat for 48 h. Rats were given increasing concentrations of sucrose [0.5, 1.0, 2.0, 4.0, 8.0, 16.0% wt/wt (41)]. Total intake of the sucrose solution was determined and the preference for each concentration of sucrose were calculated {[sucrose solution consumed (g)/sucrose solution consumed (g) + deionized water consumed (g)]·100}. A preference of 50% would be indicative of no preference between the solutions and would suggest that the rats did not have a preference or an aversion for the sucrose solution.
Experiment 2: expression of fatty acid receptors on the circumvallate papillae of obesity-prone OM and obesity-resistant S5B rats.
Experimentally naive rats were killed between 0900 and 1100. The circumvallate papillae of the tongue was harvested immediately following death. For this procedure, the tongue was removed, cleaned and the epithelial layer of the tongue, encompassing the circumvallate papillae, was excised using a sterile scalpel blade. Samples were immediately frozen on dry ice and stored at −80°C until further processing.
RNA isolation and real-time PCR.
RNA was isolated from the circumvallate papillae (n = 8–10/strain) using Tri-Reagent (Molecular Research Center, Cincinnati, OH) and RNeasy Minikit procedures (Qiagen, Valencia, CA) based on a previous experiment (38). Briefly, the circumvallate papillae were homogenized in Tri-Reagent using a motorized tissue homogenizer, chloroform was added to the lysate, and the mixture was centrifuged (12,000 g) in phase lock tubes to separate RNA. Ethanol (70%) was added to the upper aqueous phase, applied to a filter column, and filtered by centrifugation (8000 g). Following multiple washes, the samples were subjected to an elution step using RNAase-free water. RT was conducted using the high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA). For RT, 1.0 μg of RNA from each sample was added to random primers (10×), dNTP (25×), MultiScribe RT (50 U/μl), and RT buffer (10×) and incubated in a thermal cycler (MyCycler thermal cycler, Bio-Rad, Hercules, CA) for 10 min at 27°C, and then for 120 min at 37°C. Primers were designed using Primer Express (Applied Biosystems). The following primers were used for GPR40 (free fatty acid receptor 1, Ffar1): 5′-CCCTGCCCGACTCAGTTTC-3′ and 5′-GGCAGCCCACATAGCAGAA-3′; GPR120 (omega-3 fatty acid receptor 1, O3FAR1): 5′-GACCAGGAAATTCCGATTTG-3′ and 5′-CTGGTGGCTCTCGGAGTATG-3′; and cyclophilin: 5′-CCCACCGTGTTCGACAT-3′ and 5′-CTGTCTTTGGAACTTTGTCTGC-3′. For real-time PCR, SYBR Green 2× Master Mix), forward and reverse primers (10 μM), and RT product (10 ng) were added to 96-well plates (CFX96 real-time system, Bio-Rad). The cycling parameters consisted of an initial 2-min incubation at 50°C, followed by 10 min at 95°C, then 15 s at 95°C, and a 1-min annealing/extension step at 60°C (40 cycles). The quantity of GPR40 and GPR120 mRNA levels were based on a standard curve and normalized to cyclophilin levels. The specific taste bud marker, α-gustducin, was systematically assessed in these studies to validate the purity of the papillae preparation (data not shown).
Protein isolation and Western blot analysis.
For protein isolation, samples (n = 5/strain) were incubated on ice in RIPA buffer (Sigma-Aldrich, St. Louis, MO) containing 1:100 protease inhibitor (Sigma-Aldrich), and 1:100 phosphatase inhibitor (Sigma-Aldrich). Samples were then homogenized for 30 s or until a smooth suspension resulted. Homogenized samples were centrifuged for 10 min at 14,000 rpm at 4°C. Supernatant was collected and measured for protein concentrations using a BCA protein assay kit (Pierce/Thermo Fisher Scientific, Rockford, IL). For Western blot analysis, equal amounts of protein (25 μg) were separated on a 10% Tris-HEPES-SDS premade gel (Pierce/Thermo Fisher Scientific) and were transferred to a PVDF membrane (Amersham, Little Chalfont, Buckinghamshire, UK), as indicated by the manufacturer. The membrane was then blocked in TBS (20 mM Tris-base, 150 mM NaCl, at pH 7.6) containing 5% nonfat dry milk overnight at 4°C. On day 2, the membrane was incubated for 1 h with primary antibody for GPR120 (1:500; Abcam, Cambridge, MA), GPR40 (1:500; Abcam), CD36 (1:500; Abcam), β-actin (1:1,000; Abcam), or α-gustducin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) against rabbit in TBS containing 5% nonfat dry milk. The membrane was washed with TBS containing 5% nonfat dry milk and 3 times with TBS containing 0.05% Tween-20 for 15 min. Following washing, the membrane was incubated for 45 min with horseradish peroxidase-conjugated anti-rabbit antiserum (1:10,000, Abcam). The membrane was washed as described above. Immunoreactivity was visualized using ECL Western blotting detection reagents (Amersham). Images were obtained using exposure to chemiluminescence film (Amersham). Bands were quantified using ImageJ densitometry. The specific taste bud marker α-gustducin was systematically assessed in these studies to validate the purity of the circumvallate papillae preparation (data not shown).
Experiment 3A: time course analysis of siRNA silencing of CD36 on the tongue.
RNA interference techniques were used to decrease the expression of the fatty acid receptor, CD36. CD36 siRNA was commercially designed and processed for in vivo use (Dharmacon/Thermo Fisher Scientific, Chicago, IL). Additionally, a nontargeting sequence (siGENOME nontargeting siRNA; Dharmacon) was used as a control. The siRNAs were prepared as suggested by the manufacturer prior to use.
Application of CD36 siRNA.
For application of siRNA, experimentally naive rats were anesthetized with isoflurane (1.5–3% in oxygen) between 0900 and 1000. CD36 expression is highest on the circumvallate papillae, and this region was targeted for siRNA application. The fungiform papillae and the foliate papillae exhibit minimal expression of CD36, and, therefore, were excluded from siRNA application. Once anesthetized, the rats were laid on their side, their mouths were opened, and large forceps were placed in their mouths, just behind their teeth to keep the mouth open during siRNA application. While the mouth was open, the head was tilted so that siRNA applied to the caudal one-quarter of the tongue would diffuse toward the back of the tongue and not toward the rostral three-quarters of the tongue. The tongue was gently extended forward until the circumvallate papillae were visualized. The caudal region of the tongue was cleaned and dried with sterile swabs. For application of siRNA, 5 μl of siRNA was slowly pipetted onto the most caudal one-quarter region of the tongue in a circular pattern. This ensured that siRNA was applied to the circumvallate papillae and the surrounding region. The mouth remained open for 3–5 min to allow for absorption of the siRNA. Following this procedure, rats were returned to their home cage. This process was repeated for 5 consecutive days at the same time of day. For the time course analysis of siRNA-induced decreases in CD36 expression on the circumvallate papillae, rats were euthanized prior to siRNA application (control condition), day 0 (1500 on last day of siRNA application), day 3, and day 7 following the last application of siRNA.
A separate group of experimentally naive rats was used to determine whether application of CD36 siRNA onto the circumvallate papillae would significantly alter CD36 siRNA expression in the duodenal enterocytes. Expression of CD36 in duodenal enterocytes is high, and alterations in CD36 in this region may affect food intake and fat preference. Rats were anesthetized as mentioned, and CD36 siRNA or a nontargeting control siRNA was applied to the caudal tongue/circumvallate papillae for 5 days. Rats were euthanized on day 0, and the circumvallate papillae were excised. Additionally, duodenal enterocytes were harvested as previously described (38).
RNA isolation and real-time PCR.
RNA was isolated from the excised circumvallate papillae (n = 5–8/time point) and duodenal enterocytes (n = 6–8) as described in experiment 2. Following RT, real-time PCR was performed as described in experiment 2. The following primers were used for CD36: 5′-GAGGTCCTTACACATACAGAGTTCGTT-3′ and 5′-ACAGACAGTGAAGGCTCAAAGATG-3′. The quantity of CD36 receptor mRNA levels were based on a standard curve and normalized to cyclophilin levels.
Protein isolation and Western blot.
Protein was isolated from the excised circumvallate papillae (n = 2–5/time point) as described in experiment 2. CD36 antibodies were used to detect changes in CD36 protein expression (1:500; Abcam) on the circumvallate papillae and were normalized to β-actin (1:1,000; Abcam). Because of the number of time points, two Western blots were run. Both blots contained a nontreated control group, and values from the time points were compared with their respective control group. The specific taste bud marker, α-gustducin (1:500; Santa Cruz Biotechnology) was systematically assessed in this study to validate the purity of the circumvallate papillae preparation (data not shown). Bands were quantified using ImageJ densitometry.
Experiment 3B: effects of decreased circumvallate papillae CD36 expression on linoleic acid and sucrose preference in obesity-prone OM and obesity-resistant S5B rat.
CD36 siRNA and nontargeting siRNA were applied to the tongues of experimentally naive OM and S5B rats (n = 8/group), as described in experiment 3A. Beginning 5 h following the last siRNA application, rats were given access to two water bottles. One bottle contained 0.3% Xanthan gum, and one bottle contained linoleic acid in 0.3% Xanthan gum, as described in experiment 1. The concentration of linoleic acid selected for each strain was based on the results from experiment 1, which indicated a difference in the preference threshold for linoleic acid between the two strains. OM and S5B rats were given access to their most preferred concentration of linoleic acid, 1.0% and 0.25%, respectively. A separate group of experimentally naive OM and S5B rats (n = 5/group) were given access to one bottle containing deionized water and one bottle containing 4.0% sucrose in deionized water as described in experiment 1. The sucrose preference test was used to assess the specificity of CD36 siRNA on fat preference. As described previously, rats were given continuous access of the two solutions for 24 h, at which time the solutions were weighed, replaced with fresh solutions, and bottle placement was reversed. A percent intake of 50% is indicative of no preference between the solutions. The circumvallate papillae was harvested following the sucrose preference test, and CD36 mRNA levels were assessed by real-time PCR, as described in experiment 3A.
Statistical analyses.
In experiment 1A and 1B, a repeated-measures ANOVA was used to determine whether linoleic acid and sucrose preference and intake differed across concentrations for each strain. Two-tailed between-subjects t-tests were used to determine the concentration of linoleic acid or sucrose, which differed from water intake. The Bonferroni correction factor was used to correct for multiple comparisons of preference (P < 0.0027). In experiment 2, two-tailed between-subjects t-tests were used to compare fatty acid receptor mRNA values and protein values on the circumvallate papillae between OM and S5B rats. In experiment 3A, a one-way ANOVA was used to determine whether CD36 siRNA produced a significant fold-change in CD36 mRNA and protein levels on the circumvallate papillae over time. For post hoc analyses, each time point was compared with the control condition. In the study to determine whether CD36 siRNA reduced CD36 mRNA expression in the duodenal enterocytes, a between-subjects t-test was used to assess differences between the CD36 siRNA condition and the nontargeting-control siRNA condition. In experiment 3B, a two-tailed between subjects t-test was used to determine whether CD36 siRNA altered the preference for and intake of linoleic acid or sucrose in OM and S5B rats. A significance level of P < 0.05 was used for all tests.
RESULTS
Experiment 1A: linoleic acid solution intake and preference in obesity-prone OM and obesity-resistant S5B rats.
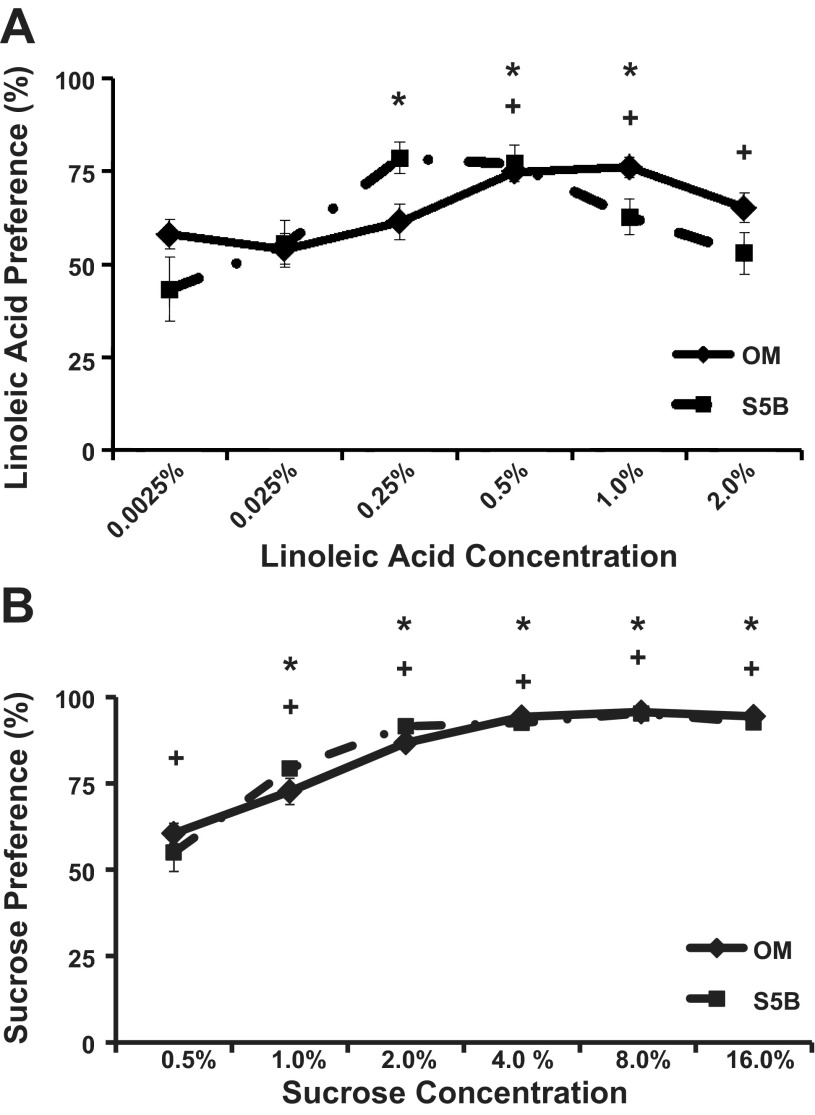
OM and S5B rats were given 48 h access to a two-bottle choice test with one bottle containing linoleic acid/0.3% Xanthan gum at increasing concentrations and one bottle containing 0.3% Xanthan gum (see Fig. 1A). Linoleic acid preference differed across concentrations [F(5,65) = 8.03, P < .001], but not across strains (P > 0.05). Linoleic acid preference was significantly higher than the preference for the Xanthan gum solution at 0.5%, 1.0%, and 2.0% linoleic acid in the OM rats. In S5B rats, an increase in the preference for linoleic acid was seen at 0.25%, 0.5%, and 1.0% linoleic acid. Linoleic acid intake (g) also differed across concentration [F(5,65) = 3.17, P < 0.02]. No significant differences between the strains were detected on linoleic acid intake (g). No interactions were detected for linoleic acid preference or linoleic acid intake.
Fig. 1.
Intake of and preference for increasing concentrations of linoleic acid and sucrose were assessed in Osborne-Mendel (OM) and obesity-resistant S5B/Pl (S5B) rats. A: obesity-resistant S5B rats exhibited a lower preference threshold for linoleic acid than obesity-prone OM rats (n = 10 rats/strain). B: OM rats exhibited a lower preference threshold for sucrose than S5B rats (n = 9–10 rats/strain). Data are shown as means ± SE. *P < 0.0027, water preference vs. linoleic acid preference in S5B; +P < 0.0027, water preference vs. linoleic acid preference in OM.
Experiment 1B: sucrose solution intake and preference in obesity-prone OM and obesity-resistant S5B rats.
OM and S5B rats were given 48-h access to a two-bottle choice test with one bottle containing sucrose at increasing concentrations and one bottle containing deionized water (see Fig. 1B). Sucrose preference differed across the concentration [F(5,85) = 32.66, P < 0.001] but did not differ across strains (P > 0.05). No interaction was detected. Sucrose preference was significantly higher than water preference at all concentrations in the OM rats. Sucrose preference was not higher than water preference at the lowest concentration (0.5%) in the S5B rats but was significantly higher at all other concentrations of sucrose. A strain difference in sucrose preference was not detected at any concentration. Sucrose intake (g) differed across concentrations [F(5,80) = 105.30, P < 0.001], but did not differ across strains (P > 0.05). No interaction was detected.
Experiment 2: expression of fatty acid receptors on the circumvallate papillae of obesity-prone OM and obesity-resistant S5B rats.
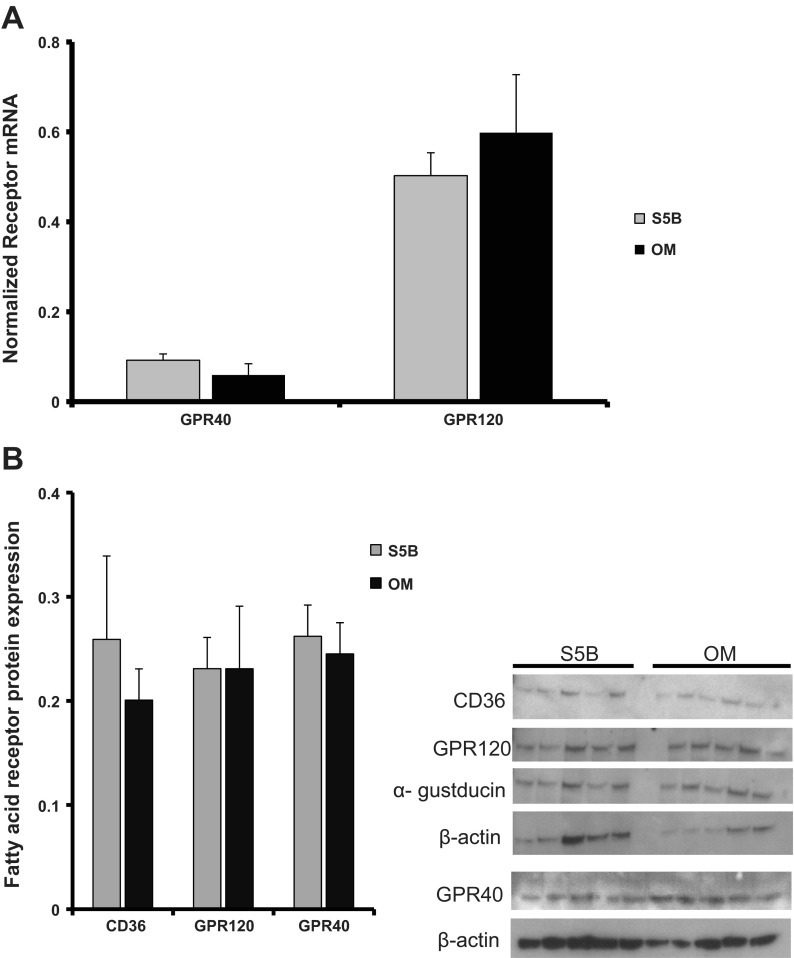
Expression of three fatty acid receptors on the circumvallate papillae of OM and S5B rats was assessed using real-time PCR and Western blot analysis. GPR40 mRNA expression and GPR120 mRNA expression on the circumvallate papillae were similar between OM and S5B rats fed a chow diet (see Fig. 2A). These data are consistent with previous data investigating differences in CD36 mRNA levels on the circumvallate papillae in these two strains (38). Protein expression of GPR40, GPR120, and CD36 on the circumvallate papillae did not differ between OM and S5B rats (see Fig. 2B).
Fig. 2.
Expression of three fatty acid receptors was assessed from the circumvallate papillae of OM and S5B rats. A: levels of GPR40 and GPR120 mRNA on the circumvallate papillae of OM and S5B rats did not differ (n = 8–10 rats/strain). B: protein expression of CD36, GPR120, and GPR40 on the circumvallate papillae of chow-fed OM and S5B rats did not differ (n = 5 rats/strain). Data are shown as means ± SE.
Experiment 3A: time course analysis of siRNA silencing of CD36 on the tongue.
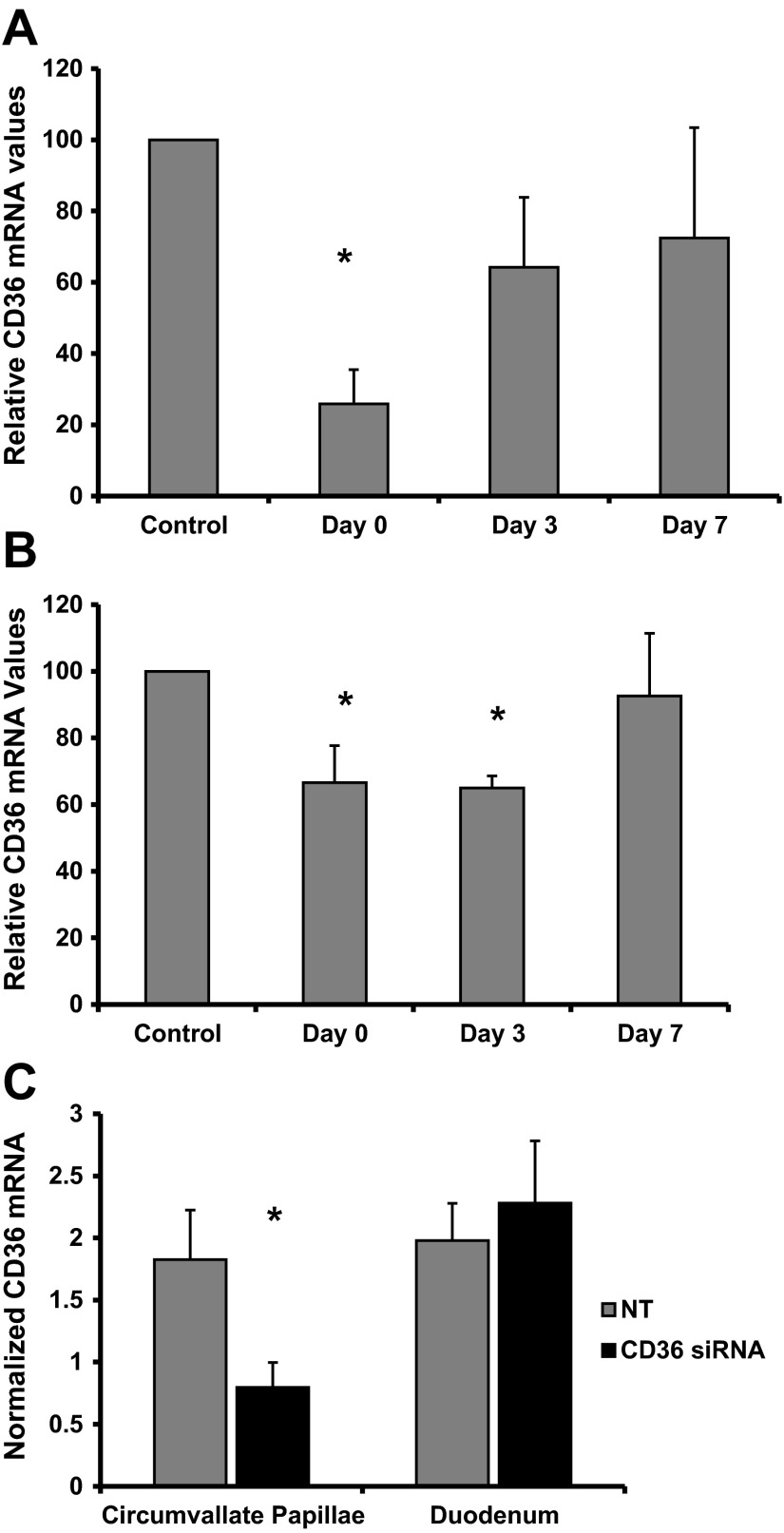
RNA interference techniques were used to decrease CD36 expression on the circumvallate papillae and a time course analysis of alterations in CD36 expression was conducted. CD36 levels are expressed as a percentage of control levels. Application of CD36 siRNA significantly altered CD36 mRNA levels [F(3,18) = 3.67, P < 0.05]. CD36 mRNA levels were reduced at the day 0 (0.15 ± 0.07) time point, which coincided with the last day of application of CD36 siRNA (P < 0.05; see Fig. 3A). CD36 mRNA was not decreased from control levels at the day 3 or day 7 time point (0.72 ± 0.16, 0.53 ± 0.10, and 0.50 ± 0.20, respectively). Application of CD36 siRNA significantly altered CD36 protein expression on the circumvallate papillae [F(3,10) = 6.42, P < 0.02]. CD36 levels were decreased at day 0 and day 3 (0.98 ± 0.16 and 0.96 ± 0.05, respectively) following siRNA application, compared with control levels (1.48 ± 0.02) (P < 0.05; see Fig. 3B). Protein expression of CD36 did not differ from control levels at day 7 (0.48 ± 0.04 vs. 0.44 ± 0.09, respectively). CD36 expression was normalized to β-actin (Western blot). α-Gustducin was used a marker of taste receptor specificity and did not differ between the time points (P > 0.05). A separate study was conducted to determine whether CD36 siRNA applied to the caudal tongue/circumvallate papillae significantly altered CD36 mRNA levels in duodenal enterocytes. CD36 mRNA expression was significantly decreased following CD36 siRNA application compared with nontargeting siRNA application on the circumvallate papillae [t(11) = 1.80, P < 0.02]. However, CD36 mRNA expression in the duodenal enterocytes was not significantly altered by the application of CD36 siRNA on the tongue (P > 0.05; see Fig. 3C).
Fig. 3.
A time course analysis of CD36 expression on the circumvallate papillae was conducted following 5-day application of CD36 siRNA. A: CD36 mRNA expression on the circumvallate papillae was decreased at day 0, which coincided with the last day of CD36 siRNA application (n = 5–8 rats/time point). B: CD36 protein expression on the circumvallate papillae was decreased at day 0 and day 3 following CD36 siRNA application (n = 2–5 rats/time point). C: CD36 mRNA expression on the circumvallate papillae was decreased at day 0 following CD36 siRNA application to the caudal tongue compared with application of nontargeting siRNA. CD36 siRNA application on the tongue did not alter CD36 mRNA expression in the duodenal enterocytes (n = 6–8/group) Data are shown as means ± SE; *P < 0.05.
Experiment 3B: effects of decreased circumvallate papillae CD36 expression on linoleic acid and sucrose preference in obesity-prone OM and obesity-resistant S5B rats.
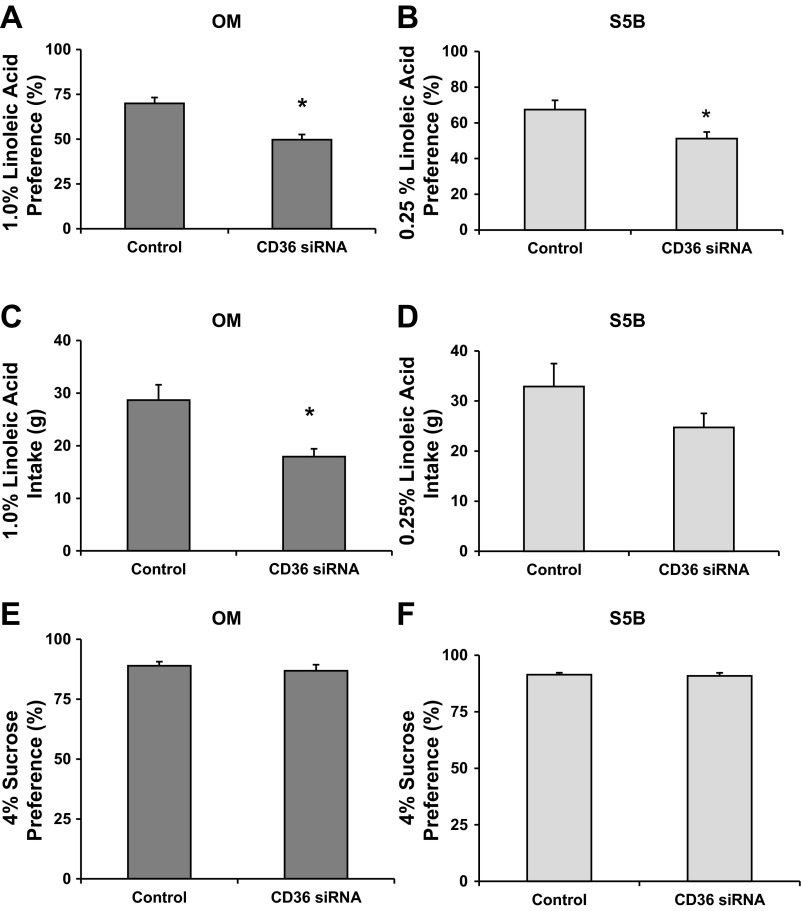
The application of CD36 siRNA significantly decreased the preference for a 1.0% linoleic acid solution in obesity-prone OM rats [t(14)=4.62, P < .001; see Fig. 4A]. The application of CD36 siRNA to the circumvallate papillae attenuated the preference for 0.25% linoleic acid solution in obesity-resistant S5B rats [t(13) = 2.51, P < 0.05; see Fig. 4B]. The amount of 1.0% linoleic acid solution consumed by the OM rats was significantly reduced by application of CD36 siRNA [t(14) = 3.28, P < 0.01; see Fig. 4C]; however, CD36 siRNA application did not significantly reduce the consumption of the 0.25% linoleic acid solution by the S5B rats (see Fig. 4D). Sucrose preference was not affected by application of CD36 siRNA in OM rats (P > 0.05; see Fig. 4E) or in S5B rats (P > 0.05; see Fig. 4F). Sucrose intake was not affected by the application of CD36 siRNA on the circumvallate papillae in either strain (P > 0.05, data not shown). CD36 mRNA levels on the circumvallate papillae were assessed following the sucrose preference test. CD36 mRNA levels did not differ significantly between CD36 siRNA and control group values; however, there was a 25–30% decrease in CD36 mRNA levels in the CD36 siRNA-treated group (data not shown). These values coincide with CD36 mRNA expression in the time course experiment (day 3).
Fig. 4.
Forty-eight-hour preference for and intake of the most preferred concentration of linoleic acid and sucrose following CD36 siRNA application to the circumvallate papillae was assessed in OM and S5B rats. A: application of CD36 siRNA attenuated the preference for 1.0% linoleic acid solution in OM rats (n = 8 rats/group). B: application of CD36 siRNA attenuated the preference for the 0.25% linoleic acid solution in S5B rats (n = 8 rats/group). C: application of CD36 siRNA to the circumvallate papillae decreased the intake of the 1.0% linoleic acid solution in OM rats. D: application of CD36 siRNA to the circumvallate papillae did not significantly decrease intake of the 0.25% linoleic acid solution in S5B rats. E: application of CD36 siRNA did not alter 4.0% sucrose preference in OM rats (n = 5 rats/group). F: application of CD36 siRNA did not alter 4.0% sucrose preference in S5B rats (n = 5 rats/group). Data are shown as means ± SE; *P < 0.05.
DISCUSSION
The consumption of foods high in dietary fat has been associated with increased rates of obesity seen throughout the world. The orosensory detection of dietary fat mediates the cephalic phase response to dietary fat and contributes to the intake of fat. Individual differences in oral fat perception and fat preference thresholds in humans are hypothesized to influence their propensity for developing diet-induced obesity. It may be hypothesized that those people who exhibit a reduced oral fat perception may consume high-fat foods to compensate for a weak signal from the oral cavity, which would lead to the overconsumption of the fatty food, and eventually, obesity (18, 20, 43). An alternate hypothesis would suggest that increased sensing of dietary fat via the oral cavity would lead to an increase in fat intake, and subsequent obesity. In the current series of experiments, animal models that differed in their susceptibility to obesity were used to examine the influence of the oral cavity on the preference for the PUFA, linoleic acid. Obesity-prone OM and obesity-resistant S5B rats were fed a standard chow diet, instead of a HFD, to investigate innate differences between these two strains on the preference threshold for linoleic acid and sucrose solutions, expression of fatty acid receptors on the tongue, and the ability of CD36 on the tongue to modulate the preference of linoleic acid and sucrose. On the basis of previous work by Gilbertson et al. (15), we hypothesized that the obesity-prone OM rats would have a higher preference threshold for linoleic acid, as reflected by the necessity for a higher concentration of linoleic acid to produce a preference. We also predicted that the preference for linoleic acid, but not sucrose, in these strains would be regulated by CD36 on circumvallate papillae of the tongue.
In experiment 1A, OM and S5B rats were given 48-h access to increasing concentrations of the PUFA, linoleic acid (0.0025–2.0%), and the control solution (0.3% Xanthan gum). As hypothesized, the obesity-resistant S5B rats had a lower preference threshold for linoleic acid (0.25%) than the obesity-prone OM rats (see Fig. 1A). The OM rats reached their linoleic acid preference threshold at the 0.5% concentration and continued to prefer the linoleic acid solution for the higher concentrations. The S5B rats did not exhibit a preference for linoleic acid at the highest concentration tested (2.0%). Linoleic acid preference data indicated that the S5B rats reach their peak preference at a lower concentration of linoleic acid (78.7% preference, 0.25%), compared with OM rats (76.2% preference, 1.0%). These data suggest that the obesity-prone rats exhibit a different preference threshold for fatty acids, which may indicate a deficit in the oral sensing of fatty acid. However, since linoleic acid was available for 48 h, it is possible that the higher linoleic acid preference threshold in OM rats is due to postingestive effects. Our previous data indicate that CD36 mRNA expression is lower in the duodenal enterocytes of OM rats (38), which may modulate differences in fat preference in these strains. Experiment 1B assessed differences in sucrose preference and consumption between the OM and S5B rats. OM rats exhibited a lower preference threshold for sucrose than S5B rats and preferred the sucrose solution over the water at the 0.5% concentration (see Fig. 1B). At the highest concentrations of sucrose presented (4.0%, 8.0%, 16.0%), preferences exceeded 90% for both strains. Although these results are of interest as a possible strain difference affecting the susceptibility to obesity, the current studies focused on fatty acid/fat preference, not sucrose preference. Therefore, future studies should investigate potential differences in sucrose intake and preference in OM and S5B rats and factors that mediate these differences. As predicted, the obesity-prone rats have an innate difference in their preference for linoleic acid. Our data suggest that the obesity-prone OM rats prefer a higher concentration of fat than obesity-resistant rats and that at the highest concentration of linoleic acid tested, OM rats maintained their preference, while the S5B rats no longer preferred the linoleic acid. This is in congruence with studies suggesting that people that have a higher threshold for detecting fats may be more likely to overconsume high-fat foods, which increases their risk for developing obesity (18, 20, 43).
To determine whether there were inherent differences in fatty acid receptor expression on the circumvallate papillae that may account for the differences in linoleic acid preference thresholds between the strains, experiment 2 quantified the expression of three fatty acid receptors in OM and S5B rats fed a chow diet. Previous data demonstrated that CD36 mRNA levels did not differ on the circumvallate papillae of OM and S5B rats (38). In the current experiment, GPR40 mRNA and GPR120 mRNA on the circumvallate papillae were not significantly different between OM and S5B rats (see Fig. 2A). Using Western blot analysis, we assessed CD36, GPR40, and GPR120 protein expression in chow-fed OM and S5B rats (see Fig. 2B). As with mRNA expression, significant strain differences between CD36, GPR40, and GPR120 on the circumvallate papillae were not detected. On the basis of these data and previous data (38), we concluded that there were no innate differences in the mRNA or protein expression of these three fatty acid receptors on the circumvallate papillae of OM and S5B rats. These results were somewhat unexpected since previous studies have reported that the preference for linoleic acid is decreased in CD36 knockout mice, GPR40 knockout mice, and GPR120 knockout mice (5, 21, 29, 41), although these models were whole-organism knockouts. On the basis of the results from experiment 1, we expected the mRNA or protein expression on the circumvallate papillae of at least one of these fatty acid receptors to be reduced in OM rats, which may have accounted for the higher linoleic acid preference threshold. Because these fatty acid receptors are also expressed in other regions, including the duodenum, postingestive effects are likely to play a principal role in the preference for linoleic acid in these strains and in the knockout models.
Since differential expression of CD36, GPR40, or GPR120 was not detected when these strains were fed a standard chow diet, but there was a difference in their preference threshold for linoleic acid, the DRK channels are a another possible mechanism by which sensitivity and preference to linoleic acid are altered (15, 35). The DRK channels on the tongue have been studied previously in OM and S5B rats, and these studies report that the expression of these channels is differentially regulated in OM and S5B rats. One of nine DRK channels was more highly expressed on the tongues of OM rats, although the S5B rats are more sensitive to the presence of linoleic acid using patch-clamp techniques. Gilbertson et al. (15) hypothesized that the ratio of fatty acid-sensitive to fatty-acid insensitive DRK channels in the OM and S5B rats differed. The OM rats had more fatty-acid insensitive channels compared with fatty-acid-sensitive channels. Other studies have suggested that the different fatty acid receptors play a role in different aspects of the consumption of HFD. Martin et al. (29) reported that both CD36 and GPR120 mRNA expression on the circumvallate papillae display a diurnal rhythm. CD36 mRNA levels are higher prior to the dark cycle, while GPR120 mRNA expression is higher during the dark cycle. Additionally, the consumption of a meal leads to a decrease in the expression of CD36 mRNA levels, and fasting leads to an increase in CD36 mRNA levels (29). The current study did not assess the effects of meals or diurnal rhythms on the expression of fatty acid receptors. Also, as mentioned earlier, because of the long exposure to the linoleic acid solution (48 h), it is possible that postingestive effects play a large role in the differential preference threshold between the strains. More studies are needed in OM and S5B rats to elucidate the role of fatty acid receptors in the intestine and their role on fatty acid sensing.
CD36 is the most frequently studied fatty acid receptor and has been declared a potential fat taste receptor. In experiment 3, RNA interference was used to decrease the expression of CD36 on the tongue and to examine the functional role of CD36 on the tongues of OM and S5B on linoleic acid preference and intake. To our knowledge, this is the first time this technique has been used to successfully decrease both mRNA and protein expression of a gene on the tongue. In experiment 3A, a time course analysis was conducted to determine decreases in CD36 mRNA and CD36 protein expression following CD36 siRNA application. The results indicate that a 5-day application of CD36 siRNA to the caudal region of the tongue decreased CD36 mRNA levels by ∼65% on day 0, which corresponded to the last day of CD36 siRNA application (see Fig. 3A). Additionally, CD36 protein expression was decreased by ∼35% at day 0 and day 3 (see Fig. 3B), suggesting a more prolonged effect on translation of the receptor. These data provide support for the use of RNA interference techniques for the short-term partial silencing of genes on the tongue of rats. The rate of turnover for taste buds is ∼10 days (3); therefore, any technique used to experimentally alter expression of genes on the tongue will be transient. A separate study determined that during the peak decrease in siRNA-induced CD36 mRNA expression on the circumvallate papillae (day 0), CD36 mRNA levels in the duodenal enterocytes was not altered. These data suggest CD36 siRNA application to the caudal tongue/circumvallate papillae is region-specific and does not directly affect intestinal expression of CD36.
In experiment 3B, CD36 siRNA or a nontargeting siRNA were applied to the caudal region of the tongue for 5 days in OM and S5B rats. Following the final application of siRNA, rats were given a choice between the concentration of linoleic acid that had the highest preference for each strain (based on experiment 1A) and the control solution. In both OM and S5B rats, CD36 siRNA treatment was able to attenuate the preference for the linoleic acid concentration previously preferred, suggesting that these rats no longer preferred the previously preferred concentration of linoleic acid. (see Fig. 4, A and B). Additionally, CD36 siRNA application decreased the total amount of linoleic acid solution (g) consumed by the obesity-prone OM rats (see Fig. 4C). The functional specificity of CD36 siRNA was evaluated using a sucrose preference test. CD36 siRNA application did not alter sucrose preference in either strain. Assessment of CD36 mRNA levels on the circumvallate papillae revealed that following the sucrose preference test, there was a 25–30% decrease in CD36 mRNA expression in the group receiving CD36 siRNA. These data are consistent with the time course study. As mentioned, CD36 siRNA application is transient and, therefore, the behavioral effects are expected to be transient, which is a limitation to the current approach. Overall, these data indicate that experimentally reducing CD36 on the caudal region of the tongue significantly decreases the preference for the PUFA, linoleic acid, without altering sucrose preference or altering duodenal expression of CD36, thereby supporting CD36 as an oral fatty acid sensor. In this study, as in experiment 1, rats were given access to the linoleic acid solution for 48 h. CD36 siRNA was able to attenuate the preference for linoleic acid during this long exposure, further highlighting the role of the oral cavity in fat sensing.
Perspectives and Significance
The purpose of the current series of experiments was to examine the influence of the oral cavity on the preference for the PUFA, linoleic acid, in rats, which differed in their susceptibility to obesity. Obesity-prone OM and obesity-resistant S5B rats exhibited a differential preference threshold for linoleic acid. As described in some obese people, a higher concentration of linoleic acid was required to reach the peak preference in obesity-prone rats. The investigation of fatty acid receptor expression on the circumvallate papillae revealed that there were no innate differences in the expression of CD36, GPR40, or GPR120 between these two strains of rats, suggesting that differential expression of DRK channels regulates linoleic acid preference, an unknown/uninvestigated receptor/channel in the oral cavity, or differential postingestive factors regulates innate linoleic acid preference. The functional role of CD36 on the circumvallate papillae was measured in these models since CD36 has been associated with fat taste. CD36 siRNA application to the circumvallate papillae was able to attenuate the 48-h preference for linoleic acid in both strains. The current data and previous studies indicate that the regulation of fat intake is a complex phenomenon that is influenced by multiple receptors and channels on the tongue. The expression profile of various fatty acid receptors and channels is likely to differ and be responsible for various aspects of fat consumption.
GRANTS
This work was supported, in part, by P20-RR-021945 from the National Center for Research Resources and National Institutes of Health Center Grant 1P30 DK-072476 to Pennington Biomedical Research Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.S.-Y.C., E.M.B., T.D.A., A.L.S., K.P.A., and S.D.P. performed experiments; C.S.-Y.C., E.M.B., and S.D.P. analyzed data; C.S.-Y.C. and S.D.P. interpreted results of experiments; C.S.-Y.C. and S.D.P. prepared figures; C.S.-Y.C. and S.D.P. drafted manuscript; C.S.-Y.C., T.D.A., and S.D.P. edited and revised manuscript; C.S.-Y.C., E.M.B., T.D.A., A.L.S., K.P.A., and S.D.P. approved final version of manuscript; S.D.P. conception and design of research.
ACKNOWLEDGMENTS
This research was supported by Louisiana Health Science Center-New Orleans to S. D. Primeaux.
REFERENCES
- 1.Abumrad NA. CD36 may determine our desire for dietary fats. J Clin Invest 115: 2965–2967, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA. Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides 27: 3292–3298, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Beidler LM, Smallman RL. Renewal of cells within the taste buds. J Cell Biol 27: 263–272, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder PC, Deckelbaum RJ. CD36:taste the difference? Curr Opin Clin Nutr Metab Care 9: 77–78, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30: 8376–8382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chale-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses 32: 423–431, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Degrace-Passilly P, Besnard P. CD36 and taste of fat. Curr Opin Clin Nutr Metab Care 15: 107–111, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Dramane G, Abdoul-Azize S, Hichami A, Vogtle T, Akpona S, Chouabe C, Sadou H, Nieswandt B, Besnard P, Khan NA. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J Clin Invest 122: 2267–2282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem 283: 12949–12959, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an importnt role in fatty acid and lipoprotein metabolism. J Biol Chem 274: 19055–19062, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Febbraio M, Guy E, Coburn C, Knapp FF, Beets AL, Abumrad NA, Silverstein RL. The impact of overexpression and deficiency of fatty acid traslocase (FAT)/CD36. Mol Cell Biochem 239: 193–197, 2002 [PubMed] [Google Scholar]
- 12.Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J 22: 1458–1468, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gaillard D, Passilly-Degrace P, Besnard P. Molecular mechanisms of fat preference and overeating. Ann NY Acad Sci 1141: 163–175, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Gilbertson TA, Fontenot TD, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol Cell Physiol 272: C1203–C1210, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Gilbertson TA, Liu L, Kim I, Burks KA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav 86: 681–690, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann NY Acad Sci 855: 165–168, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56: 1872–1880, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hayes JE, Duffy VB. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav 95: 77–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishihara Y, White CL, Kageyama H, Kageyama A, York DA, Bray GA. Effects of diet and time of the day on serum and CSF leptin levels in Osborne-Mendel and S5B/Pl rats. Obes Res 12: 1067–1076, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Keller KL. Genetic influences on oral fat perception and preference. J Food Sci 77: S143–S147, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115: 3177–3184, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, York DA. Comparisons of the effects of enterostatin on food intake and gastric emptying in rats. Brain Res 745: 205–209, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr 49: 79–90, 1995 [PubMed] [Google Scholar]
- 24.Little TJ, Feinle-Bisset C. Oral and gastrointestinal sensing of dietary fat and appetite regulation in humans: modification by diet and obesity. Front Neurosci 4: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Hansen DR, Kim I, Gilbertson TA. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol 289: C868–C880, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Liu X, York DA, Bray GA. Regulation of ghrelin gene expression in stomach and feeding response to a ghrelin analogue in two strains of rats. Peptides 25: 2171–2177, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Madiehe AM, Schaffhauser AO, Braymer DH, Bray GA, York DA. Differential expression of leptin receptor in high- and low-fat-fed Osborne-Mendel and S5B/Pl rats. Obes Res 8: 467–474, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Martin C, Passilly-Degrace P, Chevrot M, Ancel D, Sparks SM, Drucker DJ, Besnard P. Lipid-mediated release of GLP-1 by mosue taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res 53: 2256–2265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin C, Passilly-Degrace P, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 6: e24014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura S, Mizushige T, Yoneda T, Iwanaga T, Tsuzuki S, Inoue K, Fushiki T. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res (Tokyo) 28: 49–55, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Mattes RD. Fat taste and lipid metabolism in humans. Physiol Behav 86: 691–697, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Mizushige T, Inoue K, Fushiki T. Why is fat so tasty? Chemical reception of fatty acid on tongue. J Nutr Sci Vitaminol (Tokyo) 53: 1–4, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res 53: 561–566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrescu O, Cheema AF, Fan X, Bradbury MW, Berk PD. Differences in adiposyte long chain fatty acid uptake in Osborne-Mendel and S5B/Pl rats in response to high-fat diets. Int J Obes 32: 853–862, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Pittman D, Smith KR, Crawley ME, Corbin CH, Hansen DR, Watson KJ, Gilbertson TA. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: strain and sex differences. Chem Sens 33: 449–460, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Primeaux SD, Barnes MJ, Bray GA. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone, but not obesity-resistant rats. Behav Brain Res 180: 190–196, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of Exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes 34: 1427–1433, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Primeaux SD, Braymer DH, Bray GA. CD36 mRNA in the gastrointestinal tract is differntially regulated by dietary fat intake in obesity-prone and obesity-resistant rats. Dig Dis Sci 58: 72–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Primeaux SD, Braymer DH, Bray GA. High fat diet differentially regulates the expression of oflactory receptors in the duodenum of obesity-prone and obesity-resistant rats. Dig Dis Sci 58: 363–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res 10: 1188–1196, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol 293: R1823–R1832, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and procine taste buds from circumvallate and foliate papillae. Acta Histochem 113: 839–843, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr 104: 145–152, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Thanos PK, Cho J, Kim R, Michaelides M, Primeaux S, Bray G, Wang GJ, Volkow ND. Bromocriptine increased operant responding for high fat food but decreased chow intake in both obesity-prone and resistant rats. Behav Brain Res 217: 165–170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thanos PK, Kim R, Cho J, Michaelides M, Anderson BJ, Primeaux SD, Bray GA, Wang GJ, Robinson JK, Volkow ND. Obesity-resistant S5B rats showed greater cocaine conditioned place preference than the obesity-prone OM rat. Physiol Behav 101: 713–718, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker RM, Mattes RD. Are free fatty acids effective taste stimuli in humans? J Food Sci 77: S148–S150, 2012 [DOI] [PubMed] [Google Scholar]
- 47.White CL, Braymer HD, York DA, Bray GA. Effect of a high or low ambient perinatal temperature on adult obesity in Osborne-Mendel and S5B/Pl rats. Am J Physiol Regul Integr Comp Physiol 288: R1376–R1384, 2005 [DOI] [PubMed] [Google Scholar]
- 48.White CL, Ishihara Y, York DA, Bray GA. Effect of meta-chlorophenylpiperazine and cholecystokinin on food intake of Osborne-Mendel and S5B/P1 rats. Obesity 15: 624–631, 2007 [DOI] [PubMed] [Google Scholar]
- 49.White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, York DA. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides 26: 2331–2338, 2005 [DOI] [PubMed] [Google Scholar]
- 50.White CL, Kashima K, Bray GA, York DA. Effect of serotonin 1-A agonist on food intake of Osborne-Mendel and S5B/P1 rats. Physiol Behav 68: 715–722, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Zhang XJ, Zhou LH, Ban X, Liu DX, Jiang W, Liu XM. Decreased expression of CD36 in circumvallate taste buds of high-fat induced obese rats. Acta Histochem 113: 663–667, 2011 [DOI] [PubMed] [Google Scholar]