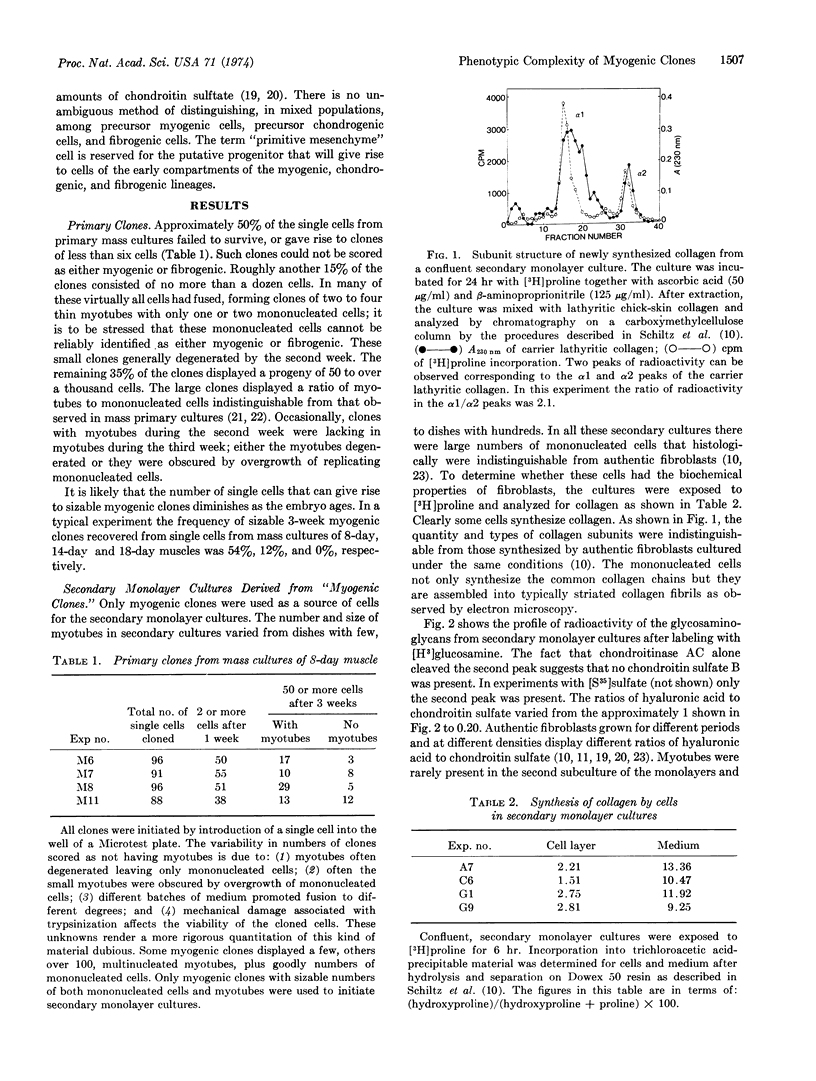
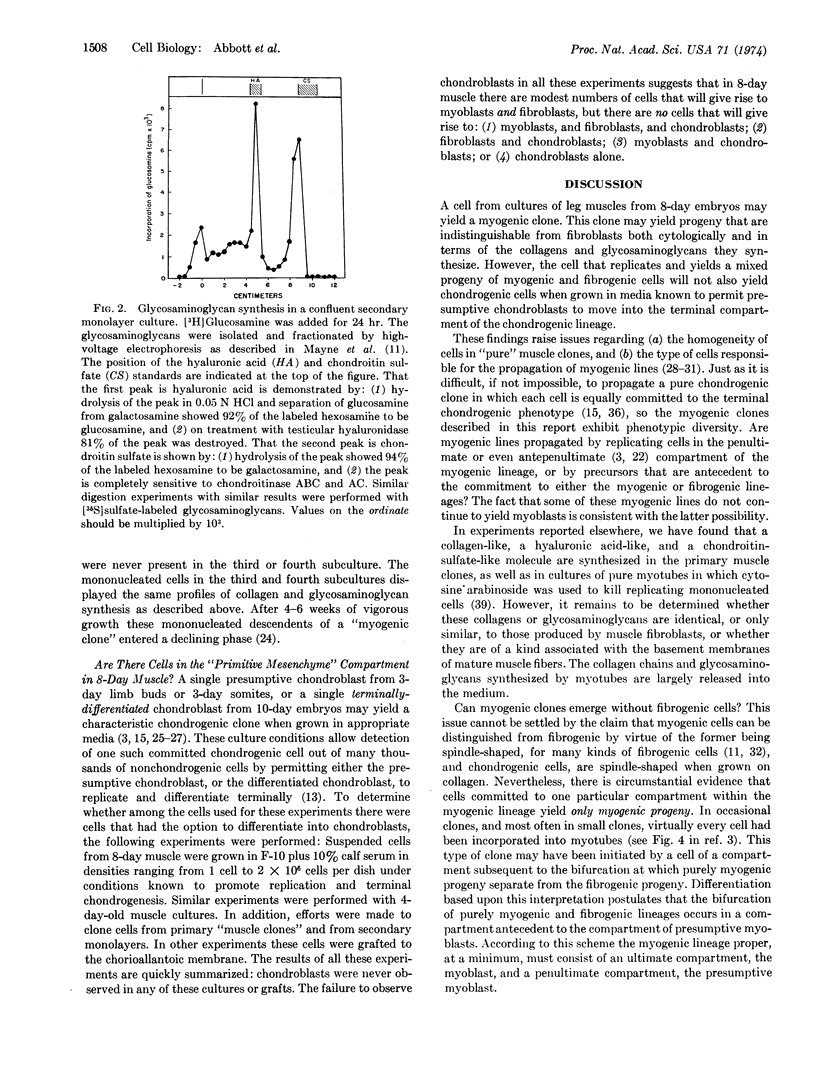
Abstract
A single cell isolated from cultured 8-day leg muscle may, when subcultured, yield a myogenic clone. A myogenic clone consists of myotubes and mononucleated cells. When such a myogenic clone is subcultured, large numbers of mononucleated cells are recovered. These mononucleated cells histologically and biochemically are indistinguishable from authentic fibroblasts cultured under the same conditions: they synthesize (α1)2α2 chains of collagen, large amounts of hyaluronic acid, and modest amounts of chondroitin sulfate. These mononucleated cells, however, will not chondrify when grown under culture conditions known to permit presumptive chondroblasts to differentiate terminally. These findings demonstrate that there is a population of single cells in 8-day muscle that is neither a myoblast nor a fibroblast, but is the common progenitor for cells in the myogenic and fibrogenic lineages: this progenitor, however, is beyond the point of readily yielding chondrogenic cells. These findings are discussed in terms of the limited number of phenotypic options open to differentiating cells in each of the successive compartments of their respective lineages.
Keywords: leg muscle, cell culture, fibroblasts
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells, V. The effect of 5-bromodeoxyuridine on cloned chondrocytes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1144–1151. doi: 10.1073/pnas.59.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott J., Mayne R., Holtzer H. Inhibition of cartilage development in organ cultures of chick somites by the thymidine analog, 5-bromo-2'-deoxyuridine. Dev Biol. 1972 Jun;28(2):430–442. doi: 10.1016/0012-1606(72)90024-3. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Mitosis and the processes of differentiation of myogenic cells in vitro. J Cell Biol. 1969 Apr;41(1):188–200. doi: 10.1083/jcb.41.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S., Abbott J., Holtzer S., Holtzer H. The loss of phenotypic traits by differentiated cells. VI. Behavior of the progeny of a single chondrocyte. J Exp Med. 1969 Aug 1;130(2):417–442. doi: 10.1084/jem.130.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski J. S., Przbylski R. J., Cox P. G. Ultrastructural studies of lizard (Anolis carolinensis) myogenesis in vitro. Dev Biol. 1973 Jul;33(1):80–99. doi: 10.1016/0012-1606(73)90166-8. [DOI] [PubMed] [Google Scholar]
- Coon H. G. Clonal stability and phenotypic expression of chick cartilage cells in vitro. Proc Natl Acad Sci U S A. 1966 Jan;55(1):66–73. doi: 10.1073/pnas.55.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Cellular interactions in mass cultures of human diploid fibroblasts. Nature. 1972 Mar 24;236(5343):152–155. doi: 10.1038/236152a0. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hauschka S. D., Konigsberg I. R. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966 Jan;55(1):119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaighn M. E., Ebert J. D., Stott P. M. The susceptibility of differentiating muscle clones to Rous sarcoma virus. Proc Natl Acad Sci U S A. 1966 Jul;56(1):133–140. doi: 10.1073/pnas.56.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Sanger J. W., Holtzer H. Inhibition of mucopolysaccharide synthesis by 5-bromodeoxyuridine in cultures of chick amnion cells. Dev Biol. 1971 Aug;25(4):547–567. doi: 10.1016/0012-1606(71)90005-4. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Shulman H. J., Meyer K. Cellular differentiation and the aging process in cartilaginous tissues. Mucopolysaccharide synthesis in cell cultures of chondrocytes. J Exp Med. 1968 Dec 1;128(6):1353–1362. doi: 10.1084/jem.128.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Kang A. H., Trelstad R. L., Gross J. Collagen heterogeneity within different growth regions of long bones of rachitic and non-rachitic chicks. Biochem J. 1972 May;127(4):715–720. doi: 10.1042/bj1270715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N. S., Trentin J. J. Differential proliferation of erythroid and granuloid spleen colonies following sublethal irradiation of the bone marrow donor. J Cell Physiol. 1970 Apr;75(2):225–229. doi: 10.1002/jcp.1040750211. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]



