Abstract
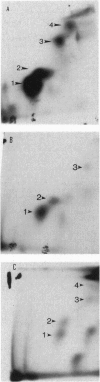
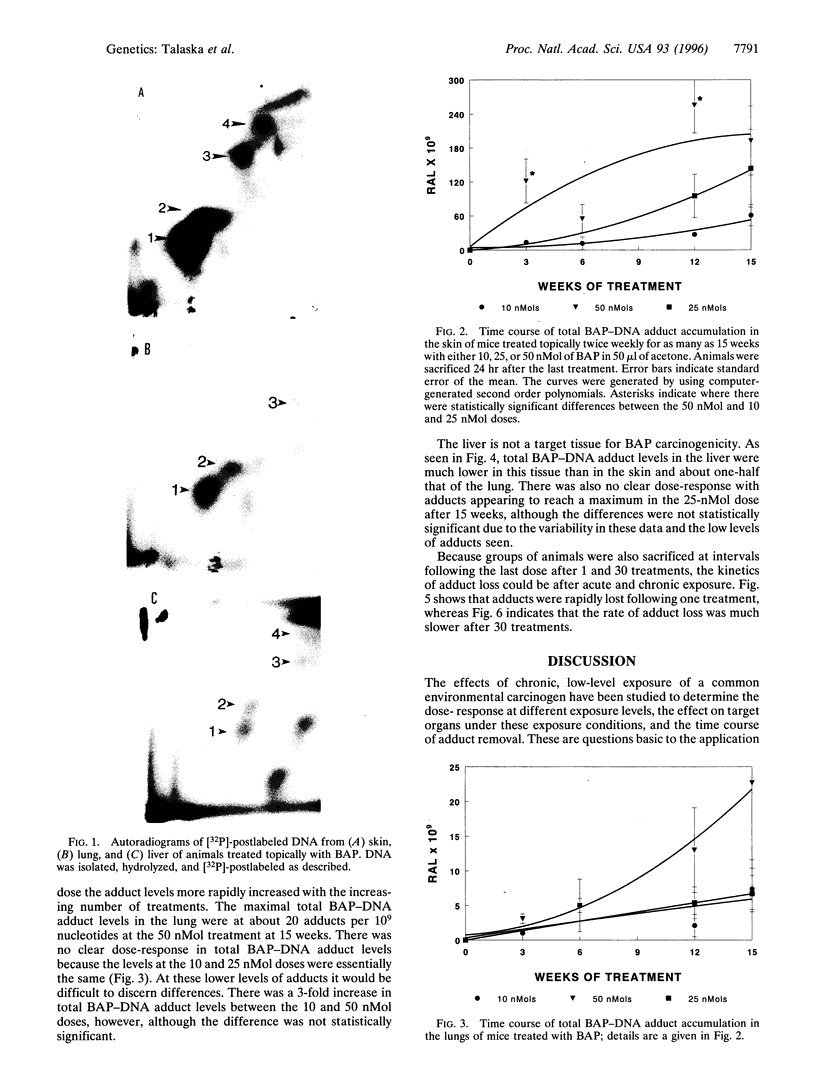
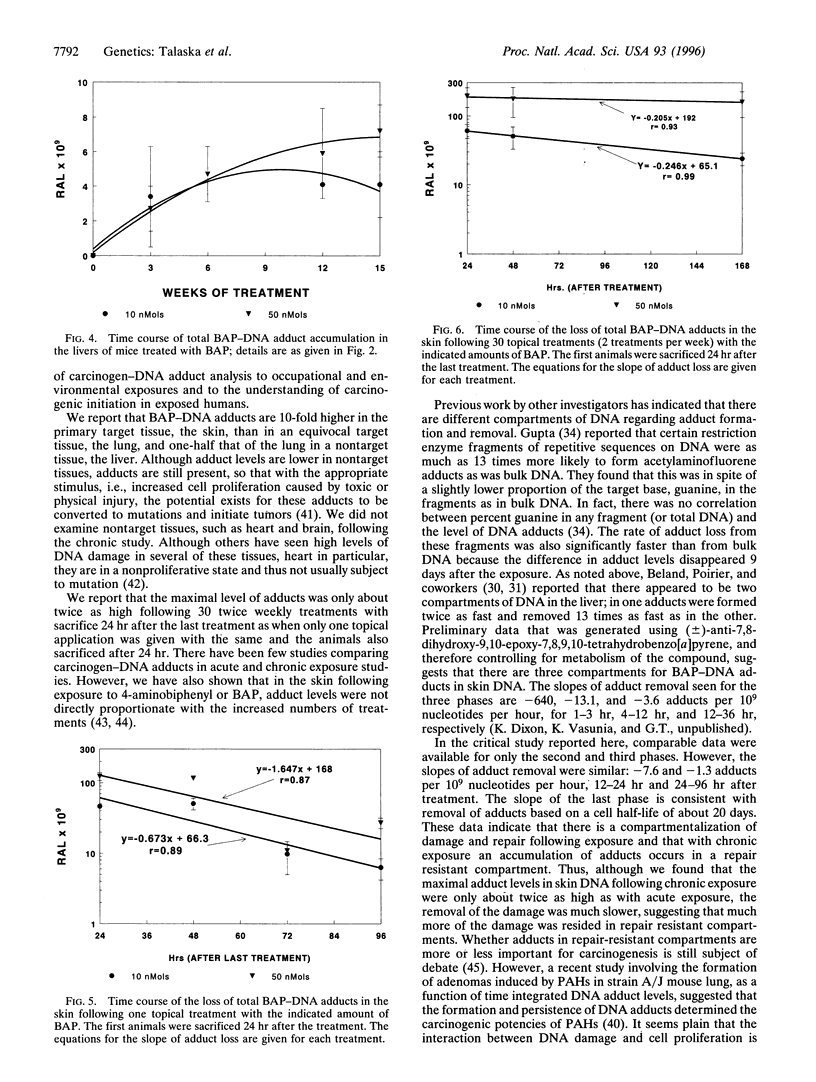
Carcinogen-DNA adduct measurements may become useful biomarkers of effective dose and/or early effect. However, validation of this biomarker is required at several levels to ensure that human exposure and response are accurately reflected. Important in this regard is an understanding of the relative biomarker levels in target and nontarget organs and the response of the biomarker under the chronic, low-dose conditions to which humans are exposed. We studied the differences between single and chronic topical application of benzo[a]pyrene (BAP) on the accumulation and removal of BAP-DNA adducts in skin, lung, and liver. Animals were treated with BAP at 10, 25, or 50 nMol topically once or twice per week for as long as 15 weeks. Animals were sacrificed either at 24, 48, or 72 hr after the last dose at 1 and 30 treatments, and after 24 hr for all other treatment groups. Adduct levels increased with increasing dose, but the slope of the dose-response was different in each organ. At low doses, accumulation was linear in skin and lung, but at high doses the adduct levels in the lung increased dramatically at the same time when the levels in the skin reached apparent steady state. In the liver adduct, levels were lower than in target tissues and apparent steady-state adduct levels were reached rapidly, the maxima being independent of dose, suggesting that activating metabolism was saturated in this organ. Removal of adducts from skin, the target organ, was more rapid following single treatment than with chronic exposure. This finding is consistent with earlier data, indicating that some areas of the genome are more resistant to repair. Thus, repeated exposure and repair cycles would be more likely to cause an increase in the proportion of carcinogen-DNA adducts in repair-resistant areas of the genome. These findings indicate that single-dose experiments may underestimate the potential for carcinogenicity for compounds that follow this pattern.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Miller M. L., Cody T. E., Talaska G., Underwood P., Andringa A. Epidermal cytokinetics, DNA adducts, and dermal inflammation in the mouse skin in response to repeated benzo[a]pyrene exposures. Toxicol Appl Pharmacol. 1996 Jan;136(1):67–74. doi: 10.1006/taap.1996.0007. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Huitfeldt H. S., Poirier M. C. DNA adduct formation and removal during chronic administration of a carcinogenic aromatic amine. Prog Exp Tumor Res. 1987;31:33–41. doi: 10.1159/000413901. [DOI] [PubMed] [Google Scholar]
- Boucheron J. A., Richardson F. C., Morgan P. H., Swenberg J. A. Molecular dosimetry of O4-ethyldeoxythymidine in rats continuously exposed to diethylnitrosamine. Cancer Res. 1987 Mar 15;47(6):1577–1581. [PubMed] [Google Scholar]
- Fedtke N., Boucheron J. A., Walker V. E., Swenberg J. A. Vinyl chloride-induced DNA adducts. II: Formation and persistence of 7-(2'-oxoethyl)guanine and N2,3-ethenoguanine in rat tissue DNA. Carcinogenesis. 1990 Aug;11(8):1287–1292. doi: 10.1093/carcin/11.8.1287. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gerde P., Cheng Y. S., Medinsky M. A. In vivo deposition of ultrafine aerosols in the nasal airway of the rat. Fundam Appl Toxicol. 1991 Feb;16(2):330–336. doi: 10.1016/0272-0590(91)90117-m. [DOI] [PubMed] [Google Scholar]
- Gerde P., Medinsky M. A., Bond J. A. Particle-associated polycyclic aromatic hydrocarbons--a reappraisal of their possible role in pulmonary carcinogenesis. Toxicol Appl Pharmacol. 1991 Mar 15;108(1):1–13. doi: 10.1016/0041-008x(91)90263-e. [DOI] [PubMed] [Google Scholar]
- Gerde P., Medinsky M. A., Bond J. A. The retention of polycyclic aromatic hydrocarbons in the bronchial airways and in the alveolar region--a theoretical comparison. Toxicol Appl Pharmacol. 1991 Feb;107(2):239–252. doi: 10.1016/0041-008x(91)90206-t. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Earley K., Sharma S. Use of human peripheral blood lymphocytes to measure DNA binding capacity of chemical carcinogens. Proc Natl Acad Sci U S A. 1988 May;85(10):3513–3517. doi: 10.1073/pnas.85.10.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res. 1991 Sep 15;51(18 Suppl):5023s–5044s. [PubMed] [Google Scholar]
- Harris C. C., Vahakangas K., Newman M. J., Trivers G. E., Shamsuddin A., Sinopoli N., Mann D. L., Wright W. E. Detection of benzo[a]pyrene diol epoxide-DNA adducts in peripheral blood lymphocytes and antibodies to the adducts in serum from coke oven workers. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6672–6676. doi: 10.1073/pnas.82.19.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen A., Becher G., Benestad C., Vahakangas K., Trivers G. E., Newman M. J., Harris C. C. Determination of polycyclic aromatic hydrocarbons in the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA, and antibodies to the adducts in sera from coke oven workers exposed to measured amounts of polycyclic aromatic hydrocarbons in the work atmosphere. Cancer Res. 1986 Aug;46(8):4178–4183. [PubMed] [Google Scholar]
- He S. L., Baker R. Micronuclei in mouse skin cells following in vivo exposure to benzo[a]pyrene, 7,12-dimethylbenz[a]anthracene, chrysene, pyrene and urethane. Environ Mol Mutagen. 1991;17(3):163–168. doi: 10.1002/em.2850170305. [DOI] [PubMed] [Google Scholar]
- Hemminki K. DNA adducts, mutations and cancer. Carcinogenesis. 1993 Oct;14(10):2007–2012. doi: 10.1093/carcin/14.10.2007. [DOI] [PubMed] [Google Scholar]
- Herbert R., Marcus M., Wolff M. S., Perera F. P., Andrews L., Godbold J. H., Rivera M., Stefanidis M., Lu X. Q., Landrigan P. J. A pilot study of detection of DNA adducts in white blood cells of roofers by 32P-postlabelling. IARC Sci Publ. 1990;(104):205–214. [PubMed] [Google Scholar]
- Herbert R., Marcus M., Wolff M. S., Perera F. P., Andrews L., Godbold J. H., Rivera M., Stefanidis M., Lu X. Q., Landrigan P. J. Detection of adducts of deoxyribonucleic acid in white blood cells of roofers by 32P-postlabeling. Relationship of adduct levels to measures of exposure to polycyclic aromatic hydrocarbons. Scand J Work Environ Health. 1990 Apr;16(2):135–143. doi: 10.5271/sjweh.1806. [DOI] [PubMed] [Google Scholar]
- Jahnke G. D., Thompson C. L., Walker M. P., Gallagher J. E., Lucier G. W., DiAugustine R. P. Multiple DNA adducts in lymphocytes of smokers and nonsmokers determined by 32P-postlabeling analysis. Carcinogenesis. 1990 Feb;11(2):205–211. doi: 10.1093/carcin/11.2.205. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Mass M. J., Jeffers A. J., Ross J. A., Nelson G., Galati A. J., Stoner G. D., Nesnow S. Ki-ras oncogene mutations in tumors and DNA adducts formed by benz[j]aceanthrylene and benzo[a]pyrene in the lungs of strain A/J mice. Mol Carcinog. 1993;8(3):186–192. doi: 10.1002/mc.2940080309. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Mumford J. L., Lee X., Lewtas J., Young T. L., Santella R. M. DNA adducts as biomarkers for assessing exposure to polycyclic aromatic hydrocarbons in tissues from Xuan Wei women with high exposure to coal combustion emissions and high lung cancer mortality. Environ Health Perspect. 1993 Mar;99:83–87. doi: 10.1289/ehp.939983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrebø S., Haugen A., Phillips D. H., Hewer A. Detection of polycyclic aromatic hydrocarbon-DNA adducts in white blood cells from coke oven workers: correlation with job categories. Cancer Res. 1992 Mar 15;52(6):1510–1514. [PubMed] [Google Scholar]
- Perera F. P., Santella R. M., Brenner D., Poirier M. C., Munshi A. A., Fischman H. K., Van Ryzin J. DNA adducts, protein adducts, and sister chromatid exchange in cigarette smokers and nonsmokers. J Natl Cancer Inst. 1987 Sep;79(3):449–456. [PubMed] [Google Scholar]
- Randerath E., Mittal D., Randerath K. Tissue distribution of covalent DNA damage in mice treated dermally with cigarette 'tar': preference for lung and heart DNA. Carcinogenesis. 1988 Jan;9(1):75–80. doi: 10.1093/carcin/9.1.75. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Rojas M., Alexandrov K., van Schooten F. J., Hillebrand M., Kriek E., Bartsch H. Validation of a new fluorometric assay for benzo[a]pyrene diolepoxide-DNA adducts in human white blood cells: comparisons with 32P-postlabeling and ELISA. Carcinogenesis. 1994 Mar;15(3):557–560. doi: 10.1093/carcin/15.3.557. [DOI] [PubMed] [Google Scholar]
- Ross J. A., Nelson G. B., Wilson K. H., Rabinowitz J. R., Galati A., Stoner G. D., Nesnow S., Mass M. J. Adenomas induced by polycyclic aromatic hydrocarbons in strain A/J mouse lung correlate with time-integrated DNA adduct levels. Cancer Res. 1995 Mar 1;55(5):1039–1044. [PubMed] [Google Scholar]
- Schurdak M. E., Randerath K. Effects of route of administration on tissue distribution of DNA adducts in mice: comparison of 7H-dibenzo(c,g)carbazole, benzo(a)pyrene, and 2-acetylaminofluorene. Cancer Res. 1989 May 15;49(10):2633–2638. [PubMed] [Google Scholar]
- Talaska G., Roh J. H., Getek T. 32P-postlabelling and mass spectrometric methods for analysis of bulky, polyaromatic carcinogen-DNA adducts in humans. J Chromatogr. 1992 Sep 16;580(1-2):293–323. doi: 10.1016/0378-4347(92)80540-7. [DOI] [PubMed] [Google Scholar]
- Talaska G., al-Juburi A. Z., Kadlubar F. F. Smoking related carcinogen-DNA adducts in biopsy samples of human urinary bladder: identification of N-(deoxyguanosin-8-yl)-4-aminobiphenyl as a major adduct. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5350–5354. doi: 10.1073/pnas.88.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. L., McCoy Z., Lambert J. M., Andries M. J., Lucier G. W. Relationships among benzo(a)pyrene metabolism, benzo(a)pyrene-diol-epoxide:DNA adduct formation, and sister chromatid exchanges in human lymphocytes from smokers and nonsmokers. Cancer Res. 1989 Dec 1;49(23):6503–6511. [PubMed] [Google Scholar]
- Warshawsky D., Barkley W., Miller M. L., LaDow K., Andringa A. Carcinogenicity of 7H-dibenzo[c,g]carbazole, dibenz[a,j]acridine and benzo[a]pyrene in mouse skin and liver following topical application. Toxicology. 1994 Nov 11;93(2-3):135–149. doi: 10.1016/0300-483x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Warshawsky D., Livingston G. K., Fonouni-Fard M., LaDow K. Induction of micronuclei and sister chromatid exchanges by polycyclic and N-heterocyclic aromatic hydrocarbons in cultured human lymphocytes. Environ Mol Mutagen. 1995;26(2):109–118. doi: 10.1002/em.2850260204. [DOI] [PubMed] [Google Scholar]
- Wei S. J., Chang R. L., Hennig E., Cui X. X., Merkler K. A., Wong C. Q., Yagi H., Jerina D. M., Conney A. H. Mutagenic selectivity at the HPRT locus in V-79 cells: comparison of mutations caused by bay-region benzo[a]pyrene 7,8-diol-9,-10-epoxide enantiomers with high and low carcinogenic activity. Carcinogenesis. 1994 Aug;15(8):1729–1735. doi: 10.1093/carcin/15.8.1729. [DOI] [PubMed] [Google Scholar]
- Wiencke J. K., McDowell M. L., Bodell W. J. Molecular dosimetry of DNA adducts and sister chromatid exchanges in human lymphocytes treated with benzo[a]pyrene. Carcinogenesis. 1990 Sep;11(9):1497–1502. doi: 10.1093/carcin/11.9.1497. [DOI] [PubMed] [Google Scholar]