Abstract
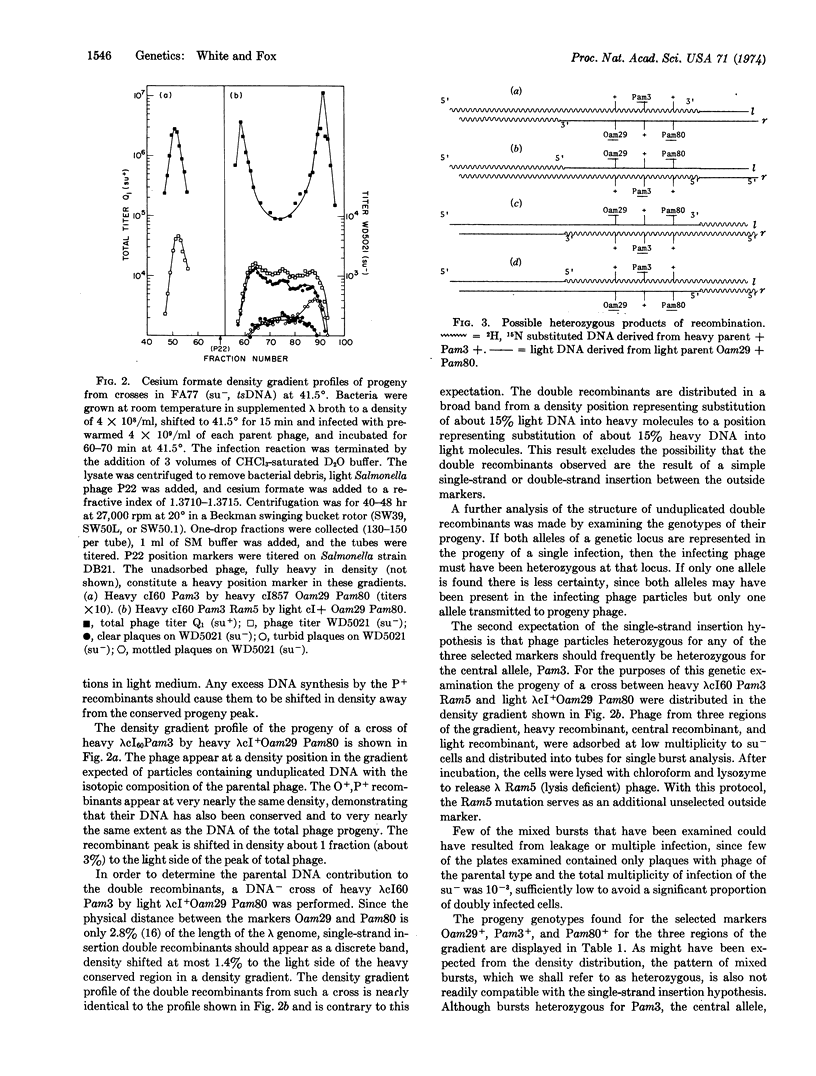
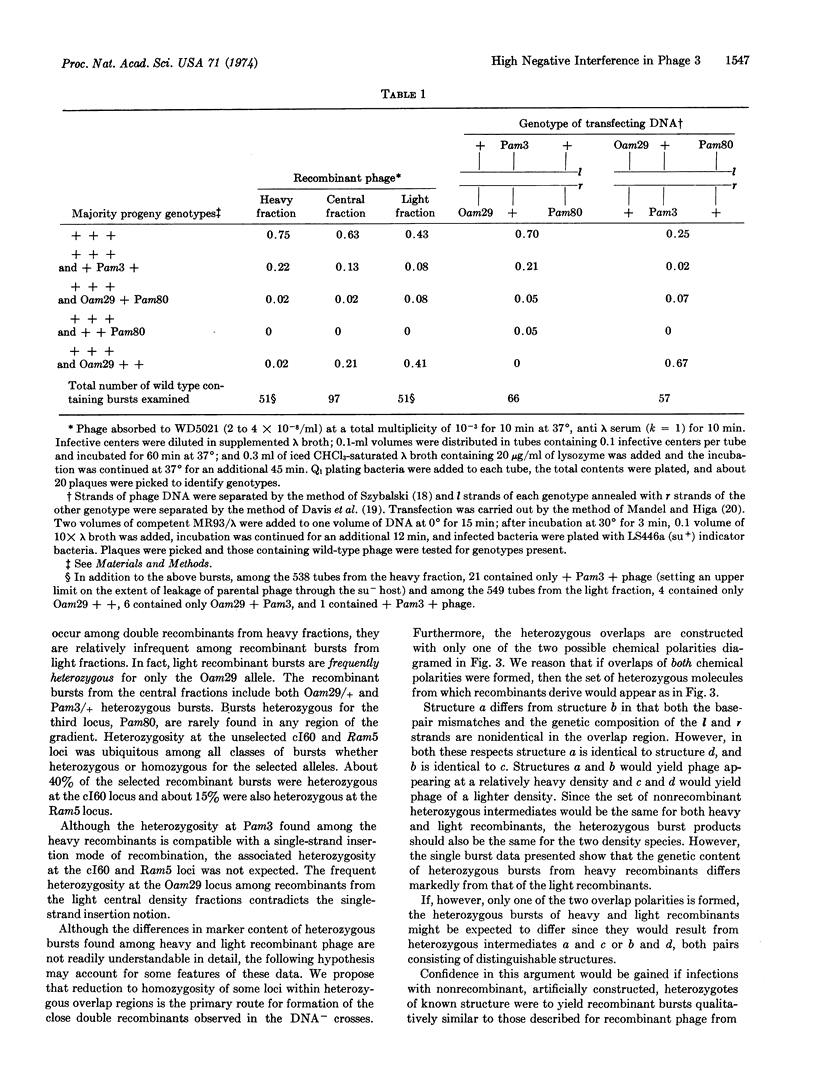
Two models designed to account for high negative interference phenomena have been examined. One proposal suggests that many recombination events are the result of insertion of a small single-stranded segment of DNA into a recipient molecule. An alternative explanation for the clustering of genetic exchanges is reduction to homozygosity of genetically heterozygous sites appearing within heteroduplex overlap regions. These proposals have been examined in phage λ by analyzing the structure of unduplicated recombinants arising from three-factor crosses with tightly linked markers. Double recombinants are found to have received substantial contributions of DNA from both parents. In addition, their patterns of genetic heterozygosity are not readily reconcilable with a single-strand insertion hypothesis. Reconstruction experiments with artifically constructed heteroduplex heterozygotes support the reduction to homozygosity hypothesis and specify the strand polarity of the heterozygous overlap region.
Keywords: bacteriophage λ, recombination, high negative interference, heteroduplex overlap
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P, Meselson M. Localized Negative Interference in Bacteriophage. Genetics. 1965 Mar;51(3):369–379. doi: 10.1093/genetics/51.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M, Doermann A H. High Negative Interference over Short Segments of the Genetic Structure of Bacteriophage T4. Genetics. 1958 May;43(3):332–353. doi: 10.1093/genetics/43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovnick A., Ballantyne G. H., Holm D. G. Studies on gene conversion and its relationship to linked exchange in Drosophila melanogaster. Genetics. 1971 Oct;69(2):179–209. doi: 10.1093/genetics/69.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J. B., Hogness D. S. The topography of lambda DNA: isolation of ordered fragments and the physical mapping of point mutations. J Mol Biol. 1972 Nov 14;71(2):363–381. doi: 10.1016/0022-2836(72)90357-9. [DOI] [PubMed] [Google Scholar]
- FOX M. S. Biological effects of the decay of incorporated radioactive phosphorus in transforming deoxyribonucleate. J Mol Biol. 1963 Jan;6:85–94. doi: 10.1016/s0022-2836(63)80083-2. [DOI] [PubMed] [Google Scholar]
- Fox M. S. On the mechanism of integration of transforming deoxyribonucleate. J Gen Physiol. 1966 Jul;49(6):183–196. doi: 10.1085/jgp.49.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTCHKISS R. D. Size limitations governing the incorporation of genetic material in the bacterial transformations and other non-reciprocal recombinations. Symp Soc Exp Biol. 1958;12:49–59. [PubMed] [Google Scholar]
- KELLENBERGER G., ZICHICHI M. L., EPSTEIN H. T. Heterozygosis and recombination of bacteriophage. Virology. 1962 May;17:44–55. doi: 10.1016/0042-6822(62)90080-6. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. Replication of bacteriophage DNA. I. Replication of DNA of lambda phage defective in early functions. J Mol Biol. 1968 Dec 14;38(2):217–225. doi: 10.1016/0022-2836(68)90407-5. [DOI] [PubMed] [Google Scholar]
- Russo V. E. On the physical structure of lambda recombinant DNA. Mol Gen Genet. 1973 May 28;122(4):353–366. doi: 10.1007/BF00269436. [DOI] [PubMed] [Google Scholar]
- Salamini F., Lorenzoni C. Genetical analysis of glossy mutants of maize. 3. Intracistron recombination and high negative interference at the gl-1 locus. Mol Gen Genet. 1970;108(3):225–232. doi: 10.1007/BF00283352. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethals: a new class of nonsense suppressors in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jun;63(2):392–399. doi: 10.1073/pnas.63.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., McMilin K. D., Stahl M. M., Malone R. E., Nozu Y., Russo V. E. A role for recombination in the production of "free-loader" lambda bacteriophage particles. J Mol Biol. 1972 Jul 14;68(1):57–67. doi: 10.1016/0022-2836(72)90262-8. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE H. L., HASTINGS P. J. THE ANALYSIS OF GENETIC RECOMBINATION ON THE POLARON HYBRID DNA MODEL. Genet Res. 1965 Feb;6:27–92. doi: 10.1017/s0016672300003955. [DOI] [PubMed] [Google Scholar]



