Abstract
Feeding a diet high in fat and sucrose (HFS) during pregnancy and lactation is known to increase susceptibility to develop metabolic derangements later in life. A trait for increased behavioral activity may oppose these effects, since this would drain energy from milk produced to be made available to the offspring. To investigate these interactions, we assessed several components of behavioral energetics during lactation in control mice (C) and in mice of two lines selectively bred for high wheel-running activity (S1, S2) subjected to a HFS diet or a low-fat (LF) diet. Energy intake, litter growth, and milk energy output at peak lactation (MEO; assessed by subtracting maternal metabolic rate from energy intake) were elevated in HFS-feeding dams across all lines compared with the LF condition, an effect that was particularly evident in the S dams. This effect was not preceded by improved lactation behaviors assessed between postnatal days 1 and 7 (PND 1–7). In fact, S1 dams had less high-quality nursing, and S2 dams showed poorer pup retrieval than C dams during PND 1–7, and S dams had generally higher levels of physical activity at peak lactation. These data demonstrate that HFS feeding increases MEO underlying increased litter and pup growth, particularly in mice with a trait for increased behavioral physical activity.
Keywords: lactation, maternal behavior, maternal diet, metabolism, milk energy output, physical activity
physical activity levels can vary greatly between, as well as within animal species (11). Clearly, differences in physical activity can have major repercussions for energy balance regulation and fuel homeostasis. One approach to study this is to compare these regulations in control mice with those in mice from lines that have been selectively bred over multiple generations for increased running-wheel behavior. Activity-selected mice, and, in particular, females, have relatively low body masses, reduced body fat, increased mass-adjusted food consumption, increased daily energy expenditure, and a high maximal rate of oxygen consumption during forced treadmill exercise. These traits are expressed even if the mice are not allowed access to running wheels (12, 34, 35, 46). On a high-fat diet (with or without sucrose), the “physical activity” trait protects these mice against diet-induced obesity (DIO), although they are markedly hyperphagic compared with control mice that do become obese (46). The DIO resistance of highly active mice is, in part, attributable to a diet-induced augmentation of spontaneous locomotor activity and metabolic/endocrine changes that stimulate fuel metabolism (46). These effects are most pronounced in activity-selected females, pointing toward a sex-specific penetration of the physical hyperactivity trait (12).
While exercise training per se does not seem to negatively impact offspring growth and development (6, 21), a hyperactivity trait and associated increased metabolic rate (MR) when subjected to a high fat-sucrose (HFS) diet could be potential constraints to reproduction. Reproduction requires energy allocation to developing offspring, and this is particularly evident in lactating rodents (28). For this reason, we have studied behavioral energetics of lactation in control mice and in mice selectively bred for high running-wheel behavior (43) subjected to a standard low-fat (LF) control diet, or a HFS diet. Energetic costs of lactation in rodents can increase approximately four-fold above normal energy requirements (20). The maximum energy consumption and expenditure during this period—termed sustainable energy intake and sustainable metabolic rate, respectively—are probably limited intrinsically by aspects of physiology, and this concept has been outlined by Król and colleagues (26, 28, 42). When subjected to HFS feeding, mice during lactation may be channeling more energy in milk toward their offspring, thus leading to larger weight gain of pups compared with LF-feeding dams (47), although this has never been directly tested to our knowledge. We hypothesized that this effect would be limited in activity-selected mice, as HFS feeding in these mice may cause behavioral and metabolic effects that would drain energy away from offspring growth and development. This would be considered a “peripheral” limiting factor to offspring development (25).
MATERIALS AND METHODS
Animals and housing.
Mice from lines selectively bred for voluntary wheel-running behavior (the base population was the Hsd:ICR strain) were obtained from T. Garland Jr., Riverside, CA. Originally, eight mouse lines were created (four selected and four control) (43). Ten pairs from each of two selectively bred lines—here referred to as S1 and S2, but originally designation lines 7 and 8, respectively (17)—and from one randomly bred control line—here referred to as C, but originally designated line 2—were shipped to Groningen. These mice were in their 45th generation of selection upon arrival. We did not study selection lines 3 and 6, both of which exhibit the “minimuscle” phenotype, caused by a Mendelian recessive allele with many pleiotropic effects (10, 18). Some of the differences between S1 and S2 line were previously described by Hannon et al. (17). The lines were maintained at the animal facility of the Center for Behavior and Neurosciences in Groningen with further selection for wheel-running activity (until generation 52), with males separated from females after weaning and typically housed with three or four mice per cage (Macrolon Type II UNO Roestvaststaal BV, Zevenaar, NL) with ad libitum access to water and a fibered low-fat (LF) standard laboratory mouse chow (15.9 kJ/g; 62% carbohydrate, 13% fat, 24% protein; RMH-B 2181, HopeFarms BV, Woerden, NL) at 22 ± 1°C, and maintained on a 12:12-h light-dark cycle with lights on at 8 AM. Pine shavings and EnviroDry were used as bedding material. For the entire study, mice had no access to running wheels. All methods were approved by the Institutional Animal Use and Care Committee of the University of Groningen.
Virgin C, S1, and S2 female mice of 4.5 mo old weighing 35.2 ± 1.24 g, 27.1 ± 0.5 g, and 32.9 ± 0.9 g, respectively, were paired with males of corresponding lines. One week before pairing, half of each group was switched from the LF diet to a home-made HFS diet (19.4 kJ/g; 30% carbohydrate, 45% fat, and 24% protein). After 2 wk of pairing, males were removed, and females were left to deliver and raise their litters until PND 21. The presence of new-born litters was checked daily at onset and at the end of the light phase and designed as PND 0. Litters were not disturbed before PND 3 to allow formation of maternal olfactory bonding (45). From PND 3 onward, dams and litters were weighed, and litter sizes were counted.
Maternal behavior/pup-retrieving test.
During PND 1–7, maternal care was assessed daily by two observers performing direct observations, as well as by observations from videotapes made from the dams and litters. The videotapes of all dams and litters were available and checked regularly to ensure consistency among observers. Maternal care was assessed at circadian time (CT) 3, CT9, and CT13, each period consisting of 100 min. Within each period, behaviors of dams were scored every 4 min, according to Champagne et al. (3), and adapted to our study. It discriminated the following categories: 1) Dam is not in contact with any pup. Contact with the tail alone is not considered to be “in contact”; 2) dam is in contact with three or more pups. She is with them but not in any defined nursing posture. If only the dam’s tail is touching the pups, then she is considered to be not in contact; 3) dam is retrieving one or more pups in her mouth and depositing it/them somewhere else; 4) dam is licking/grooming one or more pups; 5) one or more pups are away from the rest of the litter (split litter). The pups may or may not be clustered together in piles; 6) dam is licking/grooming one or two isolated pups; 7) dam is engaged in passive nursing (i.e., lying on her side or back) with at least 3 pups attached to nipples; and 8) dam is engaged in arched back nursing (ABN) 1–4: this is subdivided into four different positions with an increasing degree of active nursing behavior. This can range from ABN1 (the dam is lying flat on top of the pups) to ABN4 (back of the dam is arched maximally to give space allowing suckling).
Above-mentioned behavioral categories were also analyzed after merging them into “in contact” (total scores from 2, 4, 7, 8) and “not in contact” (total scores from 1, 3, 5, 6).
To test maternal behavioral responsiveness to standardized conditions, dams and litters were subjected to a pup-retrieving trial at PND 3 (4, 14). The test was performed in the middle of the light phase. Specifically, at the start of each trial, the dam was separated from her pups by placing her into another clean cage. The pups were then taken gently from the nest, while using odorless gloves and placed in the opposite corner of the cage. The distance between nest and new location of the pups was kept standard at 21 cm. After reintroducing the dam back to the empty nest, the latency to retrieve each pup was recorded, as well as the number of times the dam approached and then moved away from the displaced litter without retrieving a pup. A “move-away” was scored each time the dam was within 1 cm of a displaced pup and then moved away by a distance greater than her body length (excluding tail). The trial had a cut-off time of 10 min. It was noted when the dam started building a new nest at the location of the displaced pups. Pups not returned to the original nest at the end were returned by the observer.
Mother and offspring characteristics from parturition to weaning.
Maternal body mass was assessed on the day prior to breeding and weekly from pairing to parturition. Right after parturition [postnatal day 0 (PND 0)], litter size was determined by outside inspection (no handling) when the dam was not in the nest. Litter characteristics are presented in Fig. 1. Following parturition, litter size and the mass of the litters were assessed, and mean pup mass was calculated. Measurements were done at PND 3, 8, 13–15. Because maternal stress may play an important role in perinatal programming (33), particular care was taken to disturb the dams and litters as little as possible, and, when it was necessary, in a standardized manner.
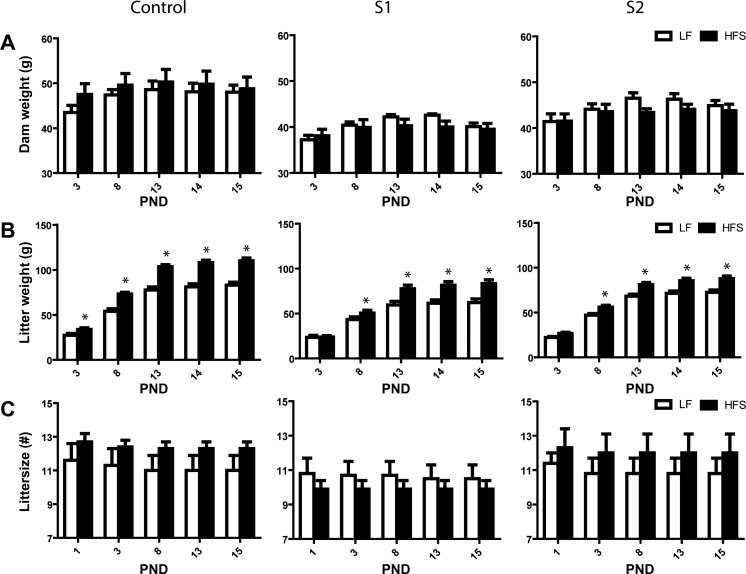
Fig. 1.
Dam weight (A), litter weight (B), and litter size (C) until end of peak lactation for control [C; low-fat (LF), n = 8; high-fat and high sucrose (HFS), n = 7] and highly active lines (S1: LF, n = 6; HFS, n = 7; S2, LF n = 8; HFS, n = 8) of mice. Values are expressed as means ± SE. Open bars represent LF, and closed bars represent HFS. *LF vs. HF, P < 0.05.
Food and water intake was assessed daily throughout lactation. Bedding was also checked for spilled or crumbled food (23). At peak lactation (between postnatal day 13 and 15), feces produced by the female and her offspring were collected. The energy content in dried, homogenized feces and food was determined using a bomb calorimeter CBB 330, standard benzoic acid 26.44 kJ/g, BCS-CRM No.90N (Boom BV, Meppel, The Netherlands).
Absorbed energy intake (AEI) of mothers at peak lactation was calculated from the difference between energy intake and fecal energy content. During PND 13–15, MR of dams in their home cages was estimated using the doubly labeled water (DLW) method (41). Milk energy output (MEO) was calculated by the difference between AEI and MR (27). 2H (>99.9%) water was provided by Sigma-Aldrich (Zwijndrecht, The Netherlands) and 18O (≈98%, specified to 0.1%) by ROTEM Industries (Rehovoth, Israel).
Mice were weighed to the nearest 0.1 g and injected intraperitoneally with a ∼0.12 g of enriched water (66.6% 18O, 33.3% 2H). The precise injectant was weighed to the nearest 0.0001 g before and after injection. After 1 h of equilibration, animals were bled at the tail tip, and two initial blood samples (20 μl) were collected in duplicate glass capillary tubes, which were immediately flame-sealed with an isobutane torch. A final blood sample was taken 48 h after the collection of the initial.
At PND 15, mice were subjected to indirect calorimetry over 3 h in the middle of the light phase to assess respiratory quotient (RQ); the latter was needed for the DLW analysis. Each animal was placed in a 2-liter Plexiglas metabolic chamber for 3 h. Bedding taken from their home cage was provided to reduce stress of novelty. During this period, females were separated from their litters, which remained in their home cage. Specifically, measurements included O2 consumption (V̇o2, ml/h) and CO2 production (V̇co2, ml/h) with an open-flow system (32) with inlet airflow set at 20 l/h (Brooks Type 5850 mass flow controller; Rijswijk, The Netherlands). We calculated RQ as V̇co2/V̇o2. During this time, voluntary activity of dams was assessed by a passive infrared (PIR) detection system (46). For clarity, the chronological order of the procedures mentioned above is presented in a time line shown in Fig. 2.
Fig. 2.
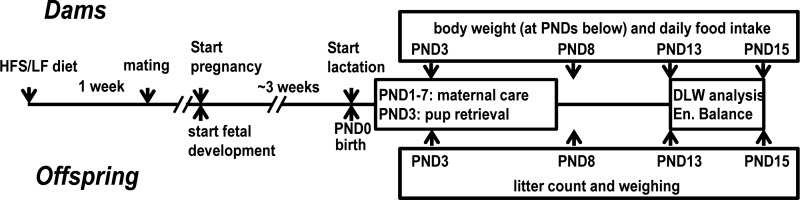
Timeline of procedures from start of the experiment until end of peak lactation. PND, postnatal day; DLW, doubly labeled water.
Analysis of DLW samples.
Determinations of 2H/1H and 18O/16O ratios in blood samples were performed at our Center for Isotope Research. A detailed description of the analytical procedures followed in our laboratory has been described previously (15). Briefly, samples were prepared by microdistillation (cryodistillation) at vacuum, first heating broken capillaries and cryogenically trapping the emerging water vapor with liquid nitrogen. Water samples were stored in glass vials and automatically injected into a high-temperature pyrolysis unit (Hekatech, Wegberg, Germany) (13), in which the injected water reacted with glassy carbon at 1420°C. The resultant H2 and CO gasses, emerging into a continuous helium flow through the system, were then led through a gas chromatography column to separate the two gases in time and finally fed into an isotope ratio mass spectrometer (GV Instruments IsoPrime, Manchester, UK) for analysis of δ18O and δ2H. Measurements were corrected for memory effects using an algorithm described by Guidotti et al. (15).
Body water pools were estimated by the intercept (ic) method (5) from the 18O (NO) dilution space:
where Eini and Ebkg are the enrichments at equilibrium and at background in the body, and tini-tinj is the time elapsed between injection of the DLW and sampling of the initial enrichment. Einj is the enrichment of the injected water. Eini-ic is the initial value extrapolated from the Eini back to the time of the injection (i.e., using the ic method) and ki is the 18O turnover rate given in h−1. E is expressed in parts per million. Final 2H and 18O dilution spaces were calculated from the final body mass, assuming the same percentage of body mass as measured for the initial dilution spaces, and ki is the 2H or 18O turnover rate is given in h−1.
Isotope turnover rates were calculated as follows:
where Efin represents the enrichment level of the final sample. Rate of CO2 production for each animal was calculated according to Speakman (41) using a single-pool model equation (Eq. 7.17). CO2 production was converted to energy expenditure using an equation derived from Weir et al. (48).
Statistical analysis.
Repeated-measures ANOVA (RM-ANOVA) was used to analyze differences in body mass and food intake of females throughout lactation. General linear model (GLM) univariate analysis was used to analyze energetics at peak lactation, maternal behavior, and litter characteristics followed by the Tukey post hoc test when appropriate. GLM analysis of covariance was used to assess contribution of maternal body mass (or fat-free mass) when appropriate. To assess litter size effects, we used this as a covariate, and we performed regression analysis with litter size, diet, and line as independent factors. A value of P < 0.05 (for two-tailed tests) was considered statistically significant for all tests.
RESULTS
Dams characteristics.
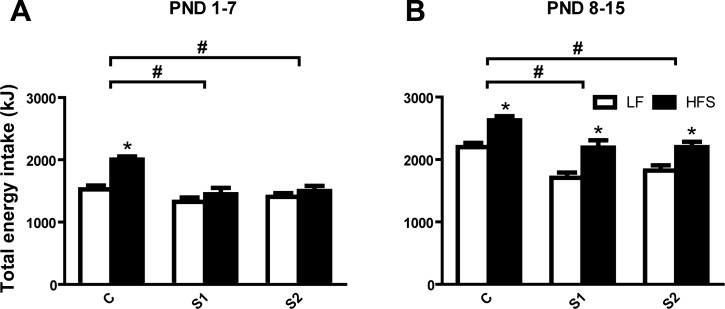
At PND 3, S1 dams weighed less than C dams (C vs. S1 P < 0.001) with S2 having an intermediate body weight (C vs. S2 P < 0.05). Over the period from PND 3 to PND 15, dams on the HFS diet gained less mass than the ones on the LF diet but started at PND 3 with a higher body mass [time×diet: F(5,190) = 2.81, P < 0.05], and S dams gained less weight than C dams, again with S2 being intermediate [line: F(2,38) = 12.88, P < 0.0001, see Fig. 1A]. From PND 1 to PND 15, daily food intake (daily data not shown) of lactating females was increasing over time [time: F(14,532) = 74.29, P < 0.0001] with S1 and S2 dams overall eating less food than C dams [line: F(2,38) = 23.65, P < 0.0001]. Assessment of cumulative energy intake over PND 1–7 (Fig. 3A) revealed that HFS feeding increased energy intake in the C line but not in the S1 and S2 lines during PND 1–7 [line×diet: F(2,38) = 4.33, P < 0.05]. During PND 8–15 (Fig. 3B), increased cumulative energy intake was observed in all lines when given HFS diet [F(1,38) = 37.74; P = 0.0001], with S lines having lower energy intake [line: F(2,38) = 17.47; P < 0.0001], but without an interaction between line and diet. Those effects were present even if dam body mass was included as a covariate.
Fig. 3.
Total energy intake (kJ) of LF (open bars) and HFS (solid bars) feeding lactating dams during the first week (PND 1–7; A) and second week (PND 8–15; B) after parturition in control (C) and selected (S1, S2) lines. Tukey post hoc test: *P < 0.05, diet; #P < 0.05, S1 or S2 vs. C. Values are expressed as means ± SE.
Litter characteristics.
Dams included in the study had litters varying between 6 and 15 pups (one S1 dam with a litter size of two was excluded to avoid stretching and skewing). Although litter size of S1 dams was slightly lower than in C and S2 dams, this effect did not reach significance [line: F(2,38) = 2.47, P = 0.098]. Over PND 1–15, pups were found missing in all lines [time: F(14,532) = 8.28, P < 0.0001], but S1 dams tended to lose fewer pups than C and S2 dams during the first days of lactation [time × line: F(28,532) = 1.42; P = 0.076] (Fig. 1C).
At PND 3, HFS-feeding dams had heavier litters than LF-feeding dams [diet: F(1,38) = 7.38, P < 0.01] and S1 and S2 dams had lighter litters than C dams [line: F(2,38) = 10.001, P < 0.001]; however, no interaction between line and diet was observed. Further testing showed that HFS feeding increased litter weight only in the C line (P < 0.05). During PND 3–15, LF litters grew less in S1 and S2 lines than in the C line (S2 being intermediate), an effect that was attenuated in the HFS condition, but particularly so in S lines [time×line×diet: F(8,152) = 2.62, P < 0.01] (Fig. 1B). Average pup mass per litter was only affected by a time×diet interaction [F(4,152) = 2.703, P < 0.0001] (data not shown).
Maternal behavior.
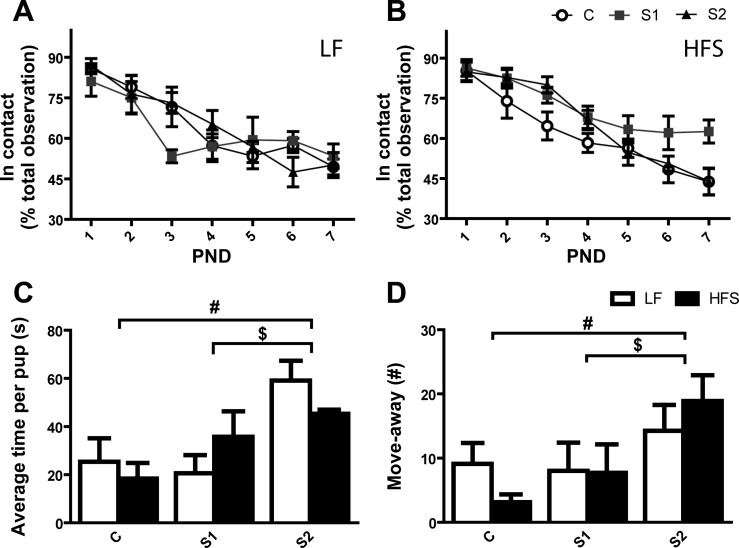
S1 dams showed a higher percentage of side nursing [F(2,38) = 3.29, P < 0.05] and ABN1 [F(2,38) = 4.80, P < 0.05] than S2 and C dams, and a lower percentage of ABN3 [F(2,38) = 10.42, P < 0.01] with S2 dams being intermediate (summary data can be found in Table 1). All lines declined “in contact with the litter” from PND 1 to PND 7 [F(6,228) = 57.95, P < 0.0001]. However, this decline in litter contact was less pronounced in S1 dams from PND 4 to PND 7 [time×line: F(12,228) = 2.29, P < 0.01], with the HFS feeding dams contributing most to this effect (Fig. 4, A and B).
Table 1.
Maternal behavior
| C |
S1 |
S2 |
||||
|---|---|---|---|---|---|---|
| LF | HFS | LF | HFS | LF | HFS | |
| In contact | 3.71 ± 0.38 | 4.02 ± 0.65 | 4.48 ± 0.46 | 4.88 ± 0.90 | 5.97 ± 0.68 | 5.93 ± 1.19 |
| Not in contact | 34.99 ± 2.83 | 38.09 ± 2.08 | 37.18 ± 1.16 | 28.86 ± 3.02 | 34.64 ± 2.50 | 33.51 ± 2.15 |
| Licking/grooming | 2.73 ± 0.54 | 2.35 ± 0.39 | 2.60 ± 0.49 | 4.36 ± 0.96 | 3.49 ± 0.70 | 3.97 ± 0.67 |
| Licking 1 pup | 0.07 ± 0.04 | 0.08 ± 0.08 | 0.00 ± 0.00 | 0.19 ± 0.10 | 0.00 ± 0.00 | 0.09 ± 0.09 |
| Split litter | 0.47 ± 0.21 | 0.89 ± 0.64 | 0.25 ± 0.15 | 0.09 ± 0.07 | 0.34 ± 0.22 | 1.03 ± 0.53 |
| Retrieving | 0.17 ± 0.07 | 0.09 ± 0.04 | 0.00 ± 0.00 | 0.15 ± 0.06 | 0.02 ± 0.02 | 0.09 ± 0.09 |
| Side Nursing | 2.24 ± 1.19 | 0.16 ± 0.11 | 5.84 ± 5.50 | 12.67 ± 5.90 | 4.60 ± 2.25 | 1.30 ± 1.17 |
| ABN1 | 1.41 ± 0.31 | 0.74 ± 0.18 | 2.79 ± 0.85 | 2.08 ± 0.49 | 1.47 ± 0.31 | 1.47 ± 0.38 |
| ABN2 | 24.63 ± 4.02 | 22.28 ± 1.78 | 30.84 ± 5.30 | 24.49 ± 5.73 | 24.15 ± 4.37 | 27.77 ± 3.90 |
| ABN3 | 22.75 ± 2.77 | 26.24 ± 1.72 | 13.55 ± 2.28 | 16.83 ± 1.56 | 18.97 ± 1.55 | 19.65 ± 1.90 |
| ABN4 | 6.83 ± 1.81 | 5.05 ± 1.21 | 2.48 ± 0.62 | 5.39 ± 1.82 | 6.34 ± 1.63 | 5.18 ± 1.32 |
Values are expressed as means ± SE. Average percentage of maternal behaviors in control mice (C) and selected lines (S1, S2) feeding low-fat (LF) or high-fat sucrose (HFS) diet during first week after parturition (PND 1–7). ABN1–4, arched back nursing, day 1–4. We assessed maternal care during three periods (each 100 min) of observations per day at CT 3, 9, 13. The total number of observation per day was 75 (every 4 min for 100 min, three times a day). Within each observation period, the behavior of each dam was scored every 4 min for a total of 25 observations per period and a total of 75 observations per dam per day.
Fig. 4.
Maternal behavior of dams of control (C) and selected (S1, S2) lines. Top: % of total observation time spent in contact with the litter when fed a LF (A) or HFS diet (B). Bottom: average time spent to retrieve a pup during the pup-retrieving test (C) and number of “move-aways” (D). A move-away was scored each time the dam was within 1 cm of a displaced pup and then moved away by a distance greater than her body length. LF (open bars) and HFS (black bars). Tukey post hoc test: #P < 0.05, S1 or S2 vs. C; $P < 0.05, S1 vs. S2. Values are expressed as means ± SE.
At PND 3, litters were subjected to a pup-retrieving test. S2 dams showed an impaired performance to retrieve the pups back to their nest with an overall higher average percentage of incomplete trials [line: F(2,38) = 7.59, P < 0.01] (Fig. 4C). Among eight S2 dams, only two completed the pup retrieval trial. The inability of S2 dams to successfully retrieve the litter was characterized by an increased number of “move-aways” [line: F(2,38) = 4.28, P < 0.05] (Fig. 4D). There were no differences between diet groups, and between lines S1 and C.
Peak lactation.
At peak lactation, S1 dams had overall lower body masses than C dams, with S2 dams being intermediate [line: F(2,38) = 10.73, P < 0.001]. Body composition of dams was assessed by isotope dilution and by assuming a constant hydration factor of fat-free mass of 0.73 (38). S dams showed a strong trend for a higher body fat content (fat%) and lower percentage fat-free mass (%FFM) than C dams (see Table 2).
Table 2.
Peak lactation energetics
| C |
S1 |
S2 |
||||
|---|---|---|---|---|---|---|
| LF | HFS | LF | HFS | LF | HFS | |
| Food intake, g/day | 20.7 ± 1 | 19 ± 0.8 | 14.7 ± 1.1# | 16.5 ± 1.4 | 16.1 ± 0.8# | 16.7 ± 1.2 |
| Energy intake, kJ/day | 329 ± 15 | 374 ± 16* | 233 ± 18# | 325 ± 27* | 256 ± 14# | 328 ± 24* |
| AEI, kJ/day | 301 ± 14 | 346 ± 15* | 213 ± 16# | 302 ± 25* | 229.8 ± 12# | 300 ± 22* |
| Body weight dam, g | 47.1 ± 1.5 | 49.6 ± 2.8 | 41.6 ± 0.4# | 39.9 ± 1.2 | 45.9 ± 1.1# | 44.1 ± 1.2 |
| MRa, kJ/day | 120.2 ± 6.1 | 120 ± 3.7 | 92.7 ± 8.6# | 106.3 ± 8.9 | 106.3 ± 3.4# | 99.7 ± 3.2 |
| RQ, V̇o2/CO2 | 1.04 ± 0.04 | 1.02 ± 0.05 | 1.1 ± 0.05 | 1 ± 0.04 | 1.01 ± 0.03 | 1.02 ± 0.04 |
| PIR | 5.46 ± 0.76 | 4.72 ± 0.26 | 9.94 ± 1.5# | 8.16 ± 0.92 | 6.74 ± 1.04 | 7.67 ± 0.73 |
| Fat% | 8.27 ± 0.59 | 7.3 ± 0.55 | 8.82 ± 1.2 | 9.77 ± 1.55 | 9.5 ± 1.22 | 10.79 ± 1.31 |
| Feces (n = 4) | ||||||
| Total dry weight | 5.852 ± 0.55 | 5.316 ± 0.3 | 3.618 ± 0.414 | 4.744 ± 1.43 | 4.98 ± 0.67242 | 4.869 ± 0.758 |
| Energy density, kJ/g | 15.96 ± 0.48 | 17.63 ± 0.77 | 16.12 ± 0.82 | 16.52 ± 0.73 | 16.9 ± 0.29#,$ | 19.1 ± 0.06* |
| Energy absorption, % | 91.69 ± 1.39 | 92.42 ± 0.5 | 91.27 ± 0.567 | 93.13 ± 1.54 | 89.95 ± 0.58139 | 91.59 ± 0.683 |
Values are expressed as means ± SE. Energetics during peak lactation (postnatal days 13–15) dams from the control line (C) and two selected lines (S1, S2) fed either an LF or an HFS diet. AEI, absorbed energy intake; MR, metabolic rate; RQ, respiratory quotient; PIR, passive infrared. Tukey post hoc test
P < 0.05, diet;
P < 0.05, S1 or S2 vs. C,
P < 0.05, S1 vs. S2.
Measured by doubly labeled water.
HFS-feeding dams had overall heavier litters [F(1,38) = 60.50, P < 0.0001], and S dams raised lighter litters at peak lactation than C dams, with S2 dams having litters of intermediate mass [F(2,38) = 27.00, P < 0.0001]. Average pup mass was increased in litters of HFS-feeding dams [F(1,38) = 19.01, P < 0.01]. Average pup mass was not affected by line or line×diet interaction.
Fecal mass, fecal energy content, and absorption efficiency were assessed during peak lactation in a subcohort of dam/litter (n = 4) groups. Fecal mass, energy absorption (in kJ), and % absorbed energy were not statistically different in C and S lines. Fecal energy content of S2 animals was higher than of C and S1 animals irrespective of diet [F(2,18) = 4.02, P < 0.05] and HFS-feeding mice had a higher fecal energy content than LF-feeding mice [F(1,18) = 8.32, P < 0.01], irrespective of line.
Energetic traits during peak lactation (PND 13–16) are shown in Table 2. Total food intake (in grams) was lower in S1 and S2 dams compared with C dams [F(2,38) = 10.47, P < 0.001] irrespective of diet. Energy intake (in kilojoules) was higher in the HFS-feeding dams [diet: F(1,38) = 30.73, P < 0.0001] than LF-feeding dams, and S1 and S2 dams consumed overall less energy than C dams [line: F(2,38) = 7.67, P < 0.01]. MR, measured by the DLW method, was lower in S dams than in C dams [line: F(2,38) = 5.09, P < 0.05], irrespective of dietary condition. This line difference was not observed when body mass or FFM was used as a covariate. With respect to home cage activity, a line, but not a diet effect on PIR activity over the 3-h indirect calorimetry measurements [F(2,38) = 9.08, P < 0.001] was found. Post hoc analysis revealed that S1 offspring had higher PIR activity than C, with S2 having an intermediate phenotype (Table 2).
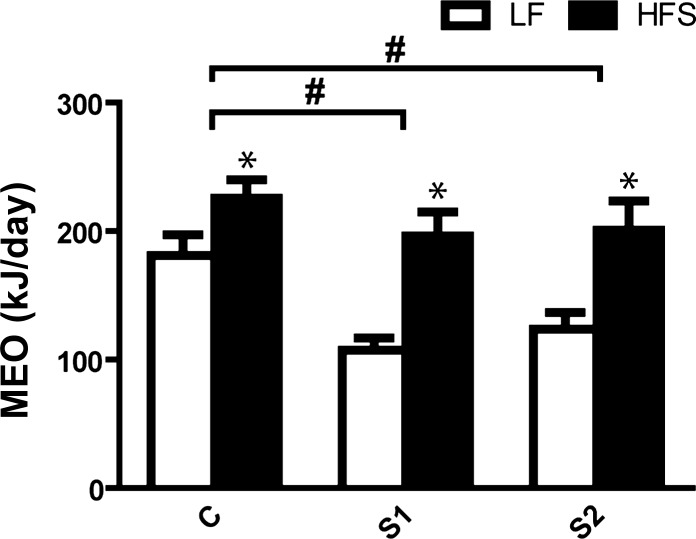
With LF feeding, MEO was markedly lower in S1 and S2 dams than in C dams [F(2,38) = 5.18, P < 0.01, Fig. 5]. An overall increased MEO by HFS-feeding [F(1,38) = 25.52, P < 0.0001] was observed above the ones observed in the LF, which was particularly evident in the S lines (Fig. 5). These stronger increases in the S lines (S1: 83%, S2: 62%) than in the C line (24%) caused MEO to be indifferent between the lines in the HFS condition. When corrected for body mass, this percent difference in MEO in S1 (90%) and S2 (70%), but not in C dams (18%), became even more pronounced. Statistical outcomes were not affected when using litter size as a covariate. Neither did litter size remain a significant factor in a backward regression analysis, with line and diet as other independent factors. Finally, by post hoc removing cases with relatively high (>14) litters size (2 in C-HFS group; 2 in S2-HFS group) and with relatively low (<8) litter sizes (2 in S1-HFS group), average (10.5–11.7), and median (11–12) litter sizes were exactly similar in all groups, and this did not yield a different pattern of MEO outcomes and neither did it lead to changes in statistical outcomes. This is a strong indication that subtle differences in litter size were not a relevant factor in differently limiting MEO.
Fig. 5.
Milk energy output (MEO) quantified by DLW analysis in control (C) and selected (S1, S2) lines feeding LF (open bars) or HFS (black bars) during peak lactation. Tukey post hoc test: *P < 0.05, diet; #P < 0.05, S1 or S2. Values are expressed as means ± SE.
DISCUSSION
Previous rodent studies have shown that HFS feeding during pregnancy and lactation causes advanced pup growth and development relative to LF feeding (19, 40, 44). This can be associated with increased body length (8); however, increases in lean mass are less obvious (24). The HFS-induced pup growth and development may be dampened by intense behavioral and metabolic activities (37). To study the energetic bases of these effects, we 1) quantified MEO from dams to pups under HFS and LF diet conditions and 2) assessed whether a trait of increased physical activity would impact negatively on MEO.
In the assessment of potential differences in MEO during lactation, it is important to consider nursing behavior of lactating dams. This is particularly important during the first week after parturition, because early maternal behavior is crucial for mother-pup bonding, pup development, and thrift (45). In our analysis, merging several maternal behavioral categories into percentage contact time showed very high (i.e., ∼80% of time scored) maternal care during the early postnatal days, and then slowly decreased to levels around 40–50% being “in contact” on postnatal day 7. This decline of dams being “in contact” with their litter has been observed before (2, 39) and probably reflects a reciprocal daily increase in feeding behavior to increase MEO (39). To our surprise, we did not observe differences between HFS-feeding and LF-feeding dams on nursing or related maternal behaviors. Although increased nursing behavior in HFS-feeding dams was anticipated on the basis of data from Purcell et al. (33) in lactating rats, this was not observed in our mice. We did observe, however, that S1 dams performed more low-care nursing behavior than C dams. The reduced level of high-quality nursing appeared to be compensated with overall more time spent with the litter, a situation that was particularly increased when S1 dams were subjected to the HFS diet. Contrary to S1 dams, S2 dams showed similar maternal care as C dams, but performed less well in the pup retrieval test relative to C and S1 dams. Girard et al. (14) did not observe differences in maternal behavior on PND 9 and PND 16 between four C and four S lines, which may indicate that maternal care differences between lines are lost at a later stage of lactation. In conclusion, S1 dams showed subtle impairments in maternal care over the first postpartum week relative to control dams, and S2 dams showed poorer pup retrieval. These effects in the S lines could have been relevant for energy availability to the offspring, pup growth and development, and pup survival at any point of the preweaning period.
Because MEO is maximized under conditions of peak lactation (27) and dams were weight-stable during this period, this allowed us to calculate MEO by subtracting MR from AEI (27). We were able to confirm that MEO at peak lactation was greater in HFS-feeding mice than in LF-feeding mice. To our knowledge, this is the first demonstration using the DLW method to show that HFS-feeding causes an elevation of milk energy availability at peak lactation, which then increases litter mass (1, 9, 16). At least three mechanisms could underlie this effect. As opposed to LF feeding, HFS feeding increases fat and “fast carbohydrate” absorption, which can immediately be converted to milk ingredients without first having to be processed by energy-demanding mechanisms, such as de novo lipogenesis (31, 36). Second, the HFS diet is more energetically dense and, thus, allows dams to derive more energy from it. Third, because dietary fat has a lower postingestive thermic effect than complex carbohydrates (7) and because energy intake may be constrained by heat loss under conditions of peak lactation (28), sustainable energy intake could have been elevated more on the HFS diet than on the LF diet without risk of overheating. The explanation that subtle differences in litter size played a role in affecting MEO is not likely because inclusion of litter size as a cofactor did not contribute to differences in MEO.
When viewing the mouse lines separately, S1 and S2 dams had markedly lower levels of MEO than C dams under LF feeding conditions. Contrary to our expectation, however, this was not caused by a higher MR in S1 and S2 dams, analogous to what we previously observed under nonlactating conditions in S females (46), but rather by a lower level of food intake by S dams compared with C dams. This effect appeared to be body (lean) mass-related. As a result of the lower MEO, litter masses of S dams were lower than those of C dams (Fig. 1B). However, MEO in S dams showed an increase upon HFS-feeding relative to LF-feeding (S1 +83%, S2 +62%), which was much stronger than in C dams subjected to HFS diet (+24%). When expressed per unit body mass, this effect was further amplified in S relative to C dams. A HFS-induced increase in food intake that was stronger in S dams than in C dams combined with an unresponsively low MR contributed to this effect. A potential mechanism may be related to thermoregulatory effects mentioned above. Thus, although we did not directly assess thermoregulation in the present study, it may be speculated that S dams were able to transmit more heat to the environment than C dams in the HFS condition, potentially because of a higher surface-to-volume ratio (22). In turn, this would cause sustainable energy intake to be limited at a higher level in the S dams (26–28). Increased food intake in HFS-feeding S dams, however, was only observed during PND 8–PND 15, while HFS-induced increases in food intake in C dams were observed during PND 1–PND 7, as well as PND 8–PND 15. Thus, although MEO in C and S dams were indistinguishable under the HFS feeding condition at peak lactation (PND 13–PND 15), body masses of S litters did not reach those of C litters, presumably because of the hyperphagia unresponsiveness of the S dams to the HFS diet during PND 1–7. Indeed, the HFS diet increased litter weight in the C line from PND 3 onward, whereas this effect could be observed in the S lines only from postnatal day PND 8 onward. The mechanism underlying this unresponsiveness is not known, but it seems to fit the overall lower level of quality nursing and reduced pup retrieval by S1 and S2 dams, respectively, as shown in our behavioral analysis over PND 1–7.
In conclusion, HFS feeding during lactation clearly causes an increase in MEO from dams to the offspring. Combined with findings of Wahlig et al. (47), showing that milk of HFS-feeding mice is richer in fat than that of LF-feeding dams, this could collectively underlie increased litter growth. The increased MEO in such a critical window not only allows increased growth and development in the offspring, but, at the same time, could increase the susceptibility in the offspring to develop metabolic derangement later in life, such as increased adiposity and insulin resistance (1, 9, 16, 29, 47). In fact, in a follow-up study, we observed that the preweaning HFS diet increased the adult proneness to diet-induced metabolic derangements irrespective of line (Guidotti S, Meyer N, Scheurink A, Garland Jr T, and van Dijk G, unpublished data).
Mice selectively bred for increased running-wheel behavior showed impaired MEO under LF condition, but this was surprisingly increased to the same level as controls when subjected to HFS feeding. This contrasts sharply with their behavioral energetics under nonreproductive conditions, during which HFS feeding augments wheel-running activity (30) and MR (46) even further. Whereas nonreproducing S females are diet-induced obesity-resistant (i.e., as opposed to C females), there was even a strong trend toward an increase in body fat content in lactating S dams compared with lactating C dams feeding HFS diet. Although the S dams were still more physically active (assessed by PIR activity) than the C dams, the data suggest that the hypermetabolic state of the S dams is offset by lactation, and apparently does not prevent HFS feeding-induced weight gain in the offspring. While we are currently investigating the underlying neurobiological causes and consequences of these prioritizing mechanisms, such an effect may be relevant for obesogenic programming in human offspring too (29), irrespective of the physical activity phenotype of the mother.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.G. and G.v.D. conception and design of research; S.G. performed experiments; S.G. and G.v.D. analyzed data; S.G., I.J., A.J.S., and G.v.D. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; S.G., H.A.M., and G.v.D. approved final version of manuscript; I.J., K.A.S., T.G.J., H.A.M., A.J.S., and G.v.D. edited and revised manuscript.
ACKNOWLEDGMENTS
Jan Bruggink, Gerard Overkamp, Berthe Verstappen-Dumoulin are thanked for technical assistance. Tobias-Navarro Schroeder and Jantien Zandvoort are thanked for their help in performing the experiment. TG is supported by NSF grant IOS-1121273, and GvD is supported by the Dutch Diabetes Research Foundation.
REFERENCES
- 1.Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a “junk food” diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol 586: 3219–3230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champagne FA, Curley JP, Keverne EB, Bateson PPG. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav 91: 325–334, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79: 359–371, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Salmon C. Differences in patterns of pup care in Mus musculus domesticus. VIII Effects of previous experience and parity in XLII inbred mice. Physiol Behav 40: 177–180, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Coward WA, Prentice AM. Isotope method for the measurement of carbon dioxide production rate in man. Am J Clin Nutr 41: 659–663, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Dewey KG, McCrory MA. Effects of dieting and physical activity on pregnancy and lactation. Am J Clin Nutr 59: 446S–452S, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Donato KA. Efficiency and utilization of various energy sources for growth. Am J Clin Nutr 45: 164–167, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150: 4999–5009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr 102: 514–519, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Garland T, Jr, Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA. Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evolution 56: 1267–1275, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 214: 206–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garland T, Jr, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, Middleton KM. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc Biol Sci 278: 574–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehre M, Geilmann H, Richter J, Werner RA, Brand WA. Continuous flow 2H/1H and 18O/16O analysis of water samples with dual inlet precision. Rapid Commun Mass Spectrom 18: 2650–2660, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Girard I, Swallow JG, Carter PA, Koteja P, Rhodes JS, Garland T. Maternal-care behavior and life-history traits in house mice (Mus domesticus) artificially selected for high voluntary wheel-running activity. Behav Processes 57: 37–50, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Guidotti S, Jansen HG, Aerts-Bijma AT, Verstappen-Dumoulin BMAA, van Dijk G, Meijer HAJ. Doubly labelled water analysis: Preparation, memory correction, calibration and quality assurance for δ2H and δ18O measurements over four orders of magnitudes. Rapid Commun Mass Spectrom 27: 1055–1066, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav 57: 681–686, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Hannon RM, Meek TH, Acosta W, Maciel RC, Schutz H, TG Sex-specific heterosis in line crosses of mice selectively bred for high locomotor activity. Behav Genet 41: 615–624, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann J, Garland T, Jr, Hannon RM, Kelly SA, Muñoz G, Pomp D. Fine mapping of “mini-muscle,” a recessive mutation causing reduced hindlimb muscle mass in mice. J Hered 99: 679–687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol 587: 905–915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson MS, Thomson SC, Speakman JR. Limits to sustained energy intake. I. Lactation in the laboratory mouse Mus musculus. J Exp Biol 204: 1925–1935, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Karasawa K, Suwa J, Kimura S. Voluntary exercise during pregnancy and lactation and its effect on lactational performance in mice. J Nutr Sci Vitaminol (Tokyo) 27: 333–339, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Kleiber M. Body size and metabolic rate. Physiol Rev 27: 511–541, 1947 [DOI] [PubMed] [Google Scholar]
- 23.Koteja P, Carter PA, Swallow JG, GarlandJr T. Food wasting by house mice: variation among individuals, families, and genetic lines. Physiol Behav 80: 375–383, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Krasnow SM, Nguyen MLT, Marks DL. Increased maternal fat consumption during pregnancy alters body composition in neonatal mice. Am J Physiol Endocrinol Metab 301: E1243–E1253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Król E, Johnson MS, Speakman JR. Limits to sustained energy intake. VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J Exp Biol 206: 4283–4291, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Król E, Murphy M, Speakman JR. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J Exp Biol 210: 4233–4243, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Król E, Speakman JR. Limits to sustained energy intake. VII. Milk energy output in laboratory mice at thermoneutrality. J Exp Biol 206: 4267–4281, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Król E, Speakman JR. Limits to sustained energy intake. VI. Energetics of lactation in laboratory mice at thermoneutrality. J Exp Biol 206: 4255–4266, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Lucas A. Programming by early nutrition in man. Ciba Found Symp 156: 38–50; discussion 50–55, 1991 [PubMed] [Google Scholar]
- 30.Meek TH, Eisenmann JC, Garland T. Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int J Obes 34: 960–969, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr 17: 159–183, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Oklejewicz M, Hut RA, Daan S, Loudon AS, Stirland AJ. Metabolic rate changes proportionally to circadian frequency in tau mutant Syrian hamsters. J Biol Rhythms 12: 413–422, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KLK. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav 104: 474–479, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezende EL, Chappell MA, Gomes FR, Malisch JL, Garland T. Maximal metabolic rates during voluntary exercise, forced exercise, and cold exposure in house mice selectively bred for high wheel-running. J Exp Biol 208: 2447–2458, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Rezende EL, Kelly SA, Gomes FR, Chappell MA, Garland T., Jr Effects of size, sex, and voluntary running speeds on costs of locomotion in lines of laboratory mice selectively bred for high wheel-running activity. Physiol Biochem Zool 79: 83–99, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Rudolph MC, Neville MC, Anderson SM. Lipid synthesis in lactation: diet and the fatty acid switch. J Mammary Gland Biol Neoplasia 12: 269–281, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Schubert KA, de Vries G, Vaanholt LM, Meijer HAJ, Daan S, Verhulst S. Maternal energy allocation to offspring increases with environmental quality in house mice. Am Nat 173: 831–840, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Sheng HP, Huggins RA. A review of body composition studies with emphasis on total body water and fat. Am J Clin Nutr 32: 630–647, 1979 [DOI] [PubMed] [Google Scholar]
- 39.Shoji H, Kato K. Maternal behavior of primiparous females in inbred strains of mice: A detailed descriptive analysis. Physiol Behav 89: 320–328, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLos One 4: 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speakman JR. Doubly Labelled Water: Theory and Practice. London: Chapman and Hall, 1997, p. 420 [Google Scholar]
- 42.Speakman JR, Król E. Limits to sustained energy intake. IX. a review of hypotheses. J Comp Physiol B 175: 375–394, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet 28: 227–237, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol 92: 287–298, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Teicher MH, Blass EM. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science 198: 635–636, 1977 [DOI] [PubMed] [Google Scholar]
- 46.Vaanholt LM, Jonas I, Doornbos M, Schubert KA, Nyakas C, Garland T, Visser GH, van Dijk G. Metabolic and behavioral responses to high-fat feeding in mice selectively bred for high wheel-running activity. Int J Obes (Lond) 32: 1566–1575, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Wahlig JL, Bales ES, Jackman MR, Johnson GC, McManaman JL, MacLean PS. Impact of high-fat diet and obesity on energy balance and fuel utilization during the metabolic challenge of lactation. Obesity 20: 65–75, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition 6: 213–221, 1990 [PubMed] [Google Scholar]