Abstract
The objective of this study was to investigate if a reduced milking frequency altered the effect of dietary energy restriction on the hepatic transcriptome of grazing dairy cows during early lactation. Multiparous Holstein-Friesian and Holstein-Friesian × Jersey cows (n = 120) were milked twice daily (2×) from calving until 34 ± 6 days in milk (mean ± SD). Cows were then allocated to one of four treatments in a 2 × 2 factorial arrangement. Treatments consisted of two milking frequencies [2× or once daily (1×)] and two feeding levels for 3 wk: adequately fed (AF) or underfed (UF, 60% of AF). Liver tissue was biopsied from 12 cows per treatment after 3 wk of treatment, and the hepatic transcriptome was profiled with an Agilent 4 × 44k bovine microarray. Over 2,900 genes were differentially expressed in response to the energy restriction; however, no effects resulted from changes to milking frequency. This may indicate that after 3 wk of 1× milking, any changes to the liver transcriptome that may have occurred earlier have returned to normal. After 3 wk of energy restriction, gene expression patterns indicate that glucose-sparing pathways were activated, and gluconeogenesis was increased in UF cows. Genes involved in hepatic stress were upregulated in response to the energy restriction indicative of the pressure energy restriction places on liver function. Other pathways upregulated included “cytoskeletal remodeling,” indicating that a 3 wk energy restriction resulted in molecular changes to assist tissue remodeling. Overall, 1× milking does not modify the hepatic transcriptome changes that occur in response to an energy restriction.
Keywords: once-daily milking, negative energy balance, homeorhesis
the ruminant liver has multiple roles in the lactating dairy cow (5), and multiple studies have investigated the hepatic transcriptome during lactation because of the importance of the liver in regulating nutrient supply (12). Altering the negative energy balance of an early-lactating cow, by changing milking frequency and nutrition, alters the hepatic transcript abundance of genes involved in cell signaling, metabolic diseases, and connective tissue development (31). Several of the pathways identified by these authors [peroxisome proliferator activated receptor (PPAR) signaling, bile secretion, biosynthesis of unsaturated fatty acids (FA), FA digestion and absorption] are involved in lipid metabolism (31), highlighting the importance of this process during early lactation. Additionally, ketosis induced by nutrient restriction in early-lactating cows was associated with the downregulation of genes involved in oxidative phosphorylation and the upregulation of genes involved in cytokine signaling, FA uptake by the liver, and FA oxidation (28). The physiological processes underlying negative energy balance during early lactation are also well researched, and the molecular changes in response to a short period of feed deprivation have been reported (10, 28, 31); however, there is limited literature regarding the effect of strategies used to alleviate the negative energy balance caused by a feed restriction.
Reducing milking frequency to once daily (1×) is a management strategy proposed to alleviate periods of negative energy balance, as the reduction in milking frequency reduces the energy demands of the mammary gland (42). Decreased milk production and, therefore, energy output through 1× milking result in greater plasma glucose concentrations and lower nonesterified fatty acid (NEFA) concentrations (32). Furthermore, in an experiment designed to investigate 1× milking during a feed shortage without the confounding effects of the lactation-induced negative energy balance, Kay et al. (27) reported greater plasma glucose, insulin, and insulin-like growth factor I, and lower plasma NEFA and β-hydroxybutyrate (BHBA) concentrations when cows were milked 1× during a feed restriction compared with milking cows twice daily (2×). These results reflect an improved metabolic status in cows milked 1× and led to the hypothesis that the improved energy status associated with 1× milking during an energy restriction would be reflected in changes in the hepatic transcriptome. Therefore, the objective of this experiment was to measure the hepatic transcriptome in cows milked 1× or 2×, to determine the effect of reduced MF during an energy deficit induced by feed restriction rather than the initiation of lactation.
MATERIALS AND METHODS
Experimental Design and Treatments
The study was conducted at the Westpac Trust Agriculture Research Station (Hawera, 39°35′ S 174°17′ E) from July 2009 to April 2010. All treatments and measurements were approved by the Ruakura Animal Ethics Committee, Hamilton, New Zealand.
The experimental design and grazing management were as described previously (27). In brief, multiparous Holstein-Friesian and Holstein-Friesian × Jersey dairy cows (n = 120, 4 ± 2 parity mean ± SD) were grazed on pasture as one herd [to target postgrazing residuals of >1,600 kg dry matter (DM)/ha] and milked twice daily (2×) for the first 34 ± 6 days in milk (mean ± SD) to avoid the confounding effects of the postpartum negative energy balance. Cows were then allocated to one of four treatments (n = 30 per treatment) in a 2 × 2 factorial arrangement. Treatments consisted of two milking frequencies (1× or 2×) and two feeding levels [adequately fed (AF) or underfed (UF)] and were imposed for 3 wk. Daily milking times were 0700 for 1× (24 h milking interval) and 0700 and 1500 for 2× (16/8 h milking interval). To reduce variability due to days in milk, cows were grouped into two experimental cohorts: cows that calved from 17 July to 7 August (n = 66) were included in cohort 1, and cows that calved from 8 August to 1 September (n = 54) were included in cohort 2. Both cohorts were managed identically. All cows grazed in the same paddock and were separated by double-stranded electric fences during the 3 wk treatment period to control pasture allowance and prevent back grazing. At the start of the treatment period, pasture allowances were incrementally increased or decreased over a 3-day period to target an average DM intake of 15 or 9 kg DM/cow per day in the AF and UF treatments, respectively (27). Following the treatment period, all animals were milked 2× and grazed together to target postgrazing residuals >1,600 kg DM/ha for 20 wk.
Animal Production Measures
The milk production, body weight (BW), body condition score (BCS, 10-point scale; Ref. 38), and plasma hormone and metabolite concentrations were measured as described previously (27). Individual milk yields were recorded at each milking (GEA, Oelde, Germany), and milk samples were collected weekly from the AM and PM milkings on the same day each week. Energy-corrected milk (ECM) was calculated as follows: ECM (kg/cow/day) = milk weight (kg/cow/day) × (383 × fat% + 242 × protein% + 783.2)/3,140 (41). Individual BW and BCS were determined weekly on the same day each week by the same person for 2 wk pretreatment until 10 wk posttreatment initiation and then fortnightly on the same day thereafter. Blood samples were collected weekly on the same day each week after the AM milking for 2 wk pretreatment until 10 wk posttreatment initiation, then fortnightly for 4 wk, and monthly thereafter. Samples were collected from the coccygeal vein of each cow into a 10 ml evacuated blood tube containing 100 IU of sodium heparin/ml, placed on ice, and centrifuged at 1,120 g for 12 min at 4°C. Plasma was harvested and stored at −20°C before being analyzed for glucose, insulin, BHBA, aspartate aminotransferase (AST), and NEFA. Insulin (22) was measured in duplicate by double-antibody radioimmunoassay, with inter- and intra-assay coefficient of variation (CV) <6%. Insulin antiserum (GP2, 21/7/80), donated by Peter Wynn (Commonwealth Scientific and Industrial Research Organisation, Division of Animal Production, Blacktown, New South Wales, Australia), was raised in guinea pigs with bovine insulin (BI 4499; Ely Lilly, West Ryde, Sydney, Australia). Validation for bovine plasma was as previously reported (8). The AST, NEFA, glucose, and BHBA analyses were performed at 37°C on a Modular P800 analyzer (Roche, Basel, Switzerland) by Gribbles Veterinary Pathology (Hamilton, New Zealand). The inter- and intra-assay CV were <2%.
Statistical analysis.
Pretreatment measurements of production data and hormone and metabolite data were used as a covariate, and means of daily data for week 3 were calculated for individual cows. Visual inspection of a residual plot was used to check for homogeneity of the variance and for the presence of outliers. Data were analyzed with mixed models fitted with REML in GenStat (version 14.1, VSN International, 2011), including cohort, feeding level, milking frequency, and the interaction of feeding level and milking frequency as fixed effects, with cow as a random effect. Differences were considered significant at P < 0.05.
Tissue Sampling
Liver tissue was biopsied at week 3 relative to treatment start from 12 cows randomly selected in each treatment and balanced for cohort. Controlled intravaginal drug-releasing devices containing progesterone (Zoetis, Auckland, New Zealand) were inserted 2 days prior to and removed on the day of biopsy to prevent estrous-associated behaviors that could have potentially damaged the healing biopsy sites. Percutaneous liver samples were collected as described previously (19, 30), immediately snap-frozen in liquid nitrogen, and stored at −80°C until RNA extraction.
Extraction of RNA
Total RNA was isolated from ∼30 mg of each liver biopsy as previously described (19) with a QIAGEN RNeasy kit (QIAGEN, Hilden, Germany). Extracted RNA was treated with DNase (Ambion DNA-free kit; Ambion, Austin, TX). RNA quantity, purity, and integrity were determined by using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE) and the Agilent 2100 Bioanalyzer with a RNA 6000 Nano LabChip kit (Agilent Technologies, Palo Alto, CA). Samples with an A260/A280 > 1.7 and an RNA integrity number > 7 (mean 8.7 ± 0.5 SD) were used for downstream analysis. All RNA samples were stored at −80°C. Of the 48 liver biopsies, RNA was successfully extracted from 45; in three samples the RNA was degraded. Final numbers were 2×AF = 12, 2×UF = 10, 1×AF = 12, 1×UF = 11.
Microarrays
cRNA synthesis, labeling, and purification.
Cy5-labeled experimental cRNA samples were generated from RNA isolated from liver tissue taken at week 3 using a Low Input Quick Amp (LIQA) kit (Agilent Technologies), following the manufacturer's instructions. For all samples, 150 ng of total RNA (n = 45) was used to generate first-strand cDNA. Agilent Spike-In B control RNA was included in each reaction. After the denaturation step (10 min at 65°C) and cRNA synthesis step (2 h at 40°C), the reactions were incubated at 70°C for 15 min to inactivate the AffinityScript enzyme. For labeling, cRNA samples were each mixed with 6 μl of Transcription Master Mix cocktail containing Cy5 dye and incubated at 40°C for 2 h. Purification was performed with RNeasy mini spin columns (QIAGEN), eluting in 30 μl of RNase-free water.
For the generation of the reference, equimolar amounts from all of the individuals at 3 wk were pooled to give 150 ng of total RNA used in eight first-strand reactions, which were then combined. Using a pooled reference from experimental samples reduces the number of extreme gene ratios that have large errors associated with them. It also ensures no loss of information when expression ratios are calculated (15, 44). Spike-In A control RNA was included in each reaction. After labeling with Cy3 and column purification as above, a common reference pool was created by combining all replicates.
Microarray hybridization and scanning.
Experimental samples of Cy5-labeled cRNA and the reference pool labeled with Cy3 were quantified on a NanoDrop ND-1000. All samples had sufficient dye incorporation with a mean (± SD) of 14.51 ± 2.25 pmol Cy5/μg cRNA as per manufacturer's recommendation (Agilent) and an appropriate RNA absorbance ratio with a mean of 2.27 ± 0.12. The Cy3 incorporation value for the reference pool was 12.61 pmol Cy3/μg cRNA and an RNA absorbance ratio of 2.1.
Frozen-labeled cRNA was transported to the Ramaciotti Centre for Gene Function Analysis (Sydney, Australia) where hybridization, scanning, and feature extraction were executed. Hybridization mixtures were loaded in random arrangements with respect to treatments (as each slide contained four arrays) onto 44K oligo bovine V1 microarrays (Agilent-015354) using Agilent SureHyb Hybridization Chambers and incubated for 17 h at 65°C. Slides were scanned after being washing on an Agilent SureScan Microarray Scanner at 5 μm resolution. The scanned images files were uploaded to the feature extraction software (Agilent feature extraction software version 10.7.3.1), where the features within each file were located, and data from each feature converted to a log2 ratio. Pixel outliers and background were removed, and any outlying features were flagged before a LOWESS dye normalization was used.
Data analysis and statistics.
We achieved data filtering through the use of GeneSpring GX 12.5 (Agilent), by importing the output from the feature extraction process. Data that were flagged as marginal or absent were excluded, leaving 15,789 from a possible 22,000 probes, as were probes that did not meet the raw intensity unit criteria (lower cut-off, 20th percentile; upper cut-off, 100th percentile), resulting in 14,360 probes. If probes met the raw intensity units in at least all of the samples in one group but not the other groups, they were not excluded so not to eliminate lowly expressed probes that may be reduced due to treatment effects. We identified transcripts where possible by querying microarray probe sequences against the bovine genome (Btau5.1) with BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi, Ref. 2). Data from probes annotated to the same gene were then combined to generate an average log2 value for each gene that was then exported for analysis. Using this gene-level approach for analyzing the data ensures only robust changes in gene expression were identified; however, this was at the expense of any variation in different transcript expression. The gene-level combination resulted in 13,882 genes of which 8,892 were fully annotated. The microarray data were uploaded to the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GSE47925).
Data were analyzed by mixed models fitted with REML in GenStat (version 14.1, VSN International, 2011), including cohort, feeding level, milking frequency, and the interaction of feeding level and milking frequency as fixed effects and cow as a random effect. Six Holstein-Friesian × Jersey cows and 39 Holstein-Friesian cows were used for the microarray; however, there were no breed effects or interactions with breed. Storey with Bootstrapping (43) was used to correct for multiple testing, with the definitions of false rejection rate and power as given in Genovese and Wasserman (18). This method was used as it takes into account the estimated proportion of nondifferentially expressed genes, thereby reducing false negatives. This method is increasingly being used to analyze microarray datasets from a variety of biological backgrounds (11, 25, 34). Differences were considered significant at a corrected P value of < 0.05, which corresponds to an uncorrected P value of 0.0294.
Pathway analysis.
A list of differentially expressed genes (corrected P < 0.05) due to the energy restriction and their corresponding fold-changes were used in functional pathway analysis. First, MetaCore from GeneGo (Thomson Reuters), an overrepresentation analysis, was used. No fold-change threshold was applied, and pathways or metabolic networks with an enrichment of P < 0.05 were classed as biologically relevant (i.e., significant). Second, the dynamic impact approach (DIA, Ref. 7) was utilized to determine the impact of treatments on pathways by calculating the overall impact and the direction of the impact (flux) due to expression changes in genes coding for proteins involved in those pathways. The impact and direction of the impact for KEGG pathways were calculated only for those pathways that were represented by at least 30% in the microarray compared with the entire bovine genome and with at least four annotated bovine genes within that pathway. Pathways with an impact >40 were considered relevant.
Quantitative Reverse Transcription-Quantitative PCR
cDNA synthesis.
For cDNA synthesis, 2 μg of each RNA sample and a final concentration of 27 μM of random pentadecamer primers (Sigma-Aldrich) were used. Total RNA was reverse transcribed with the Invitrogen Superscript III Supermix kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions (final volume = 40 μl). Reverse transcription controls were generated, whereby the above process was completed without the addition of superscript enzyme. The cDNA samples were stored at −20°C.
mRNA analyses.
The expression of 13 genes identified as significantly altered by the energy restriction was measured by real-time quantitative PCR (qPCR). The qPCR was performed using Roche real-time PCR master mix (LightCycler 480 Probes Master), and Roche Universal Probe Library assays were analyzed on the Roche LightCycler 480 (Roche Diagnostics, Mannheim, Germany). Assays were designed against publicly available bovine gene sequences (NCBI, http://www.ncbi.nlm.nih.gov/gene) using Roche Universal Probe Library design software. Assays were designed to span an intron-exon boundary of genes and specificity of assays tested by BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi, Ref. 2). The details of the assays are available in Table 1. Primers were manufactured by Invitrogen or Sigma at 50 or 25 nmol, respectively, and purified by desalting. The qPCR reaction volume was 10 μl consisting of 4 μl of cDNA and 6 μl of master mix (5 μl of Roche Probes Master, 0.4 μl of each 5 μM primer, 0.075 μl of probe, and 0.125 μl of H2O). Solutions were transferred by an epMotion 5075 robot (Eppendorf, NSW, Australia) before plates were transferred into the LightCycler 480. Standard cycling conditions were used [95°C for 10 min, (95°C for 10 s, 60°C for 30 s) × 45 cycles, 40°C for 40 s]. Each qPCR run included two negative controls (i.e., a no-template control with water added instead of cDNA and a reverse transcriptase negative control generated from a random sample) and three interrun calibrators. Interrun calibrators (24) were samples selected from various treatment groups. Triplicate measurements were performed for all samples, and standard curves with SD of <0.15 cycles were used for quantitation. We tested assays for specificity by running an agarose gel (3%, 130 V for 30 min) of the PCR products to ensure amplicon specificity and length. The efficiency of each assay was between 1.82 and 2.0, except for glutathione peroxidase 3 (GPX3, 1.65).
Table 1.
Characteristics of gene-specific qPCR assays
| Gene | GenBank Accession Number | Primer Direction | Primer Sequence (5′ to 3′) | UPL Probe Number and Sequence | Amplicon Size (bp) and Location | Dilution of cDNA |
|---|---|---|---|---|---|---|
| Endogenous control genes | ||||||
| COX4I1 | NM_001001439 | forward | CCAGAAGGCCTTGAAGGAG | 133 | 89 | 1/100 |
| reverse | CCTTGAACTTAAGGCGGTACA | GGAGAAGG | exon 3/4 | |||
| YWHAZ | BC102382 | forward | TGAAAATGAAAGGAGACTACTACCG | 30 | 84 | 1/100 |
| reverse | GCTGTGACTGGTCCACAATC | GGCTGAGG | exon 3/4 | |||
| genes of interest | ||||||
| ACSL1 | NM_001076085 | forward | ACTGCTCCACAAGGGCTTC | 50 | 93 | 1/100 |
| reverse | CATCCCTGTTCAATGATCACC | GCTCCAGA | exon 4/5 | |||
| ANGPTL4 | NM_001046043 | forward | CGCGGAGACAAGAACTGC | 92 | 76 | 1/10 |
| reverse | CGTTCAGGTTGGAGTGACTG | CAGGAGCC | exon 6/7 | |||
| AOX1 | NM_176668 | forward | CCTATTGGCCTTGGATCAGT | 37 | 73 | 1/100 |
| reverse | GCACAGACCCATCCAGATAAA | TGCCCTGG | exon 25/26 | |||
| APOA1 | NM_174242 | forward | GAAAGCCGTGGTGCTGAC | 76 | 99 | 1/100 |
| reverse | ACCCGATCCCAGGATGAC | TGGCTGTG | exon 2/3 | |||
| DECR1 | NM_001075423 | forward | GCTTCTTGGATTAATGGAGCTG | 55 | 91 | 1/100 |
| reverse | TGGTGACCTTCCTCAGACTGT | GGAGAGGA | exon 8/9 | |||
| FOXO1 | XM_583090 | forward | GATAAGGGCGACAGCAACAG | 11 | 65 | 1/10 |
| reverse | TGCAGGGACAGATTATGACG | GCTGGAAG | exon 2/3 | |||
| GATM | NM_001045878 | forward | TGCACTACATCGGGTCTCG | 69 | 114 | 1/100 |
| reverse | TGTCATCAGCTGCACAGGA | CTTCCTCC | exon 1/2 | |||
| GPX3 | NM_174077 | forward | CAGGGACAGGAGAAGTCGAA | 52 | 113 | 1/10 |
| reverse | GCGTACTGCTTAAAGGGGATG | GGGAGGAG | exon 1/2 | |||
| MT2A | NM_001075140 | forward | GGATCCCAACTGCTCCTG | 92 | 78 | 1/100 |
| reverse | GCGCACTTGCAATCTTTG | GGCTCCTG | exon 1/2 | |||
| PC | NM_177946 | forward | GAGGTGGTCCGCAAGATG | 39 | 107 | 1/100 |
| reverse | TCGTGCAGGGAAGTGATG | AGGTGGAG | exon 3/4 | |||
| SIRT5 | NM_001034295 | forward | ACTCGATGTACCTCTTGTGGAGT | 102 | 95 | 1/50 |
| reverse | TGAGTTTGGGGGTCTGGA | TGGCTGAG | exon 4/5 | |||
| THRSP | AY656814 | forward | ATGGGACAGGCCGTGTAG | 8 | 71 | 1/50 |
| reverse | TGCACCATGTCTTCATCACA | GAAGGCAG | exon 1/2 | |||
| TMED6 | NM_001075988 | forward | ACACGACTTTGCCATTGTGA | 79 | 109 | 1/10 |
| reverse | TCCTATTGTCCGCTGAACCT | CCAGGAGG | exon 1/2 | |||
qPCR, quantitative PCR; UPL, Universal Probe Library.
COX4I1, cytochrome c oxidase subunit IV isoform 1; YWAHZ, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta poly-peptide; ACSL1, acyl-CoA synthetase long-chain family member 1; ANGPTL4, angiopoietin-like 4; AOX1, aldehyde oxidase 1; APOA1, apolipoprotein A1; DECR1, 2,4-dienoyl CoA reductase 1, mitochondrial; FOXO1, forkhead box O1; GATM, glycine amidinotransferase; GPX3, glutathione peroxidase 3; MT2A, metallothionein 2A; PC, pyruvate carboxylase; SIRT5, sirtuin 5; THRSP, thyroid hormone responsive; TMED6, transmembrane emp24 protein transport domain containing 6.
The crossing point values for each amplification curve were calculated by the LightCycler 480 Abs Quant/2nd derivative max method (37) provided in the LightCycler 480 Relative Quantification software module (release 1.5.0.39). A five-point standard curve of threefold serial cDNA dilutions was used. Candidate endogenous control genes were selected based on high endogenous expression and stability across treatment groups. Cytochrome c oxidase subunit IV isoform 1 (COX4I1) family with sequence similarity 120A (FAM120A) and ribosomal protein, large, P0 (RPLP0) were selected from the microarray dataset, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein S15a (RPS15A), and tyrosine 3-mono-oxygenase/tryptophan 5-mono-oxygenase activation protein, zeta polypeptide (YWHAZ) were selected on the basis of previous studies. Endogenous control genes were tested across all samples, and their suitability determined with NormFinder and GeNorm (3, 45). The three most stable genes were COX4I1 (stability values, NormFinder = 0.044, GeNorm = 0.249), RPS15A (stability values, NormFinder = 0.054, GeNorm = 0.281), and YWHAZ (stability values, NormFinder = 0.071, GeNorm = 0.305) with a combined GeNorm V-value of 0.079; however, RPS15A had high intergroup variation so was excluded. We then used the Roche LightCycler 480 Software to perform advanced relative quantification analysis of gene expression using the normalization factor of the two endogenous control genes. The geometric mean of the interrun calibrators was calculated for each plate and applied to each sample as described in Ref. 24.
Calculations and statistical analysis for qPCR.
Data from each gene were log10-transformed prior to analysis, due to heterogeneity of variance. Data were analyzed by mixed models fitted with REML in GenStat (version 14.1, VSN International, 2011), including cohort, feeding level, milking frequency, and the interaction of feeding level and milking frequency as fixed effects, with cow as a random effect. Differences were considered significant at P < 0.05. Correlations between the expression ratios of the microarray and qPCR data were measured with the Pearson's correlation coefficient.
RESULTS
DM Intake
DM and energy intakes are described in Kay et al. (27). Briefly, average DM and metabolizable energy (ME) intake were 14.3 ± 3.6 kg DM/cow per day and 173 ± 44 MJ ME/cow per day, respectively, for AF treatments and 8.3 ± 2.9 kg dry matter/cow per day and 100 ± 35 MJ ME/cow per day, respectively, for the UF treatments.
Production Measures
ECM production, BCS, BW, and plasma hormone and metabolite data from the whole lactation are presented in Kay et al. (27). Data from all cows involved in the trial and relevant to the present publication are presented in Table 2. In brief, at week 3, ECM was reduced with UF (P <0.001), such that UF2× cows produced 35% less ECM than AF2× cows, and UF1× cows produced 29% less ECM than AF1× cows. In contrast, AF1× and UF1× cows continued to produce 20 and 12% less ECM than AF2× and UF2× cows, respectively. Cows that were UF and milked 1× produced 43% less ECM compared with those that were AF and milked 2× (P <0.001).
Table 2.
Mean energy-corrected milk production and plasma AST, BHBA, glucose, insulin, and NEFA concentrations at 3 wk relative to treatment start
| Treatment |
SED |
P Value |
||||||
|---|---|---|---|---|---|---|---|---|
| AF2× | AF1× | UF2× | UF1× | MF | FL | MF×FL | ||
| Energy corrected milk, kg/day | 23.2 | 18.6 | 15.0 | 13.2 | 0.6 | <0.001 | <0.001 | <0.001 |
| AST, log10 IU/l | 1.89 (81) | 1.85 (73) | 1.93 (91) | 1.97 (107) | 0.03 | 0.91 | <0.001 | 0.09 |
| BHBA, log10 mmol/l | −0.32 (0.48) | −0.41 (0.39) | 0.07 (1.17) | −0.21 (0.62) | 0.04 | <0.001 | <0.001 | <0.01 |
| Glucose, log10 mmol/l | 0.62 (4.17) | 0.64 (4.37) | 0.53 (3.39) | 0.59 (3.89) | 0.01 | <0.001 | <0.001 | <0.05 |
| Insulin, log10 mmol/l | 0.48 (3.02) | 0.57 (3.72) | 0.25 (1.78) | 0.38 (2.40) | 0.05 | <0.01 | <0.001 | 0.59 |
| NEFA, log10 mmol/l | −0.36 (0.43) | −0.60 (0.25) | 0.05 (1.12) | −0.04 (0.92) | 0.04 | <0.001 | <0.001 | <0.01 |
Grazing cows (n = 120) were adequately fed (AF; 14.3 ± 3.6 kg dry matter/cow/day) or underfed (UF; 8.3 ± 2.9 kg dry matter/cow/day) and milked once (1×) or twice (2×) daily for 3 wk from treatment start. Plasma measures were log10 transformed and are displayed with back-transformed values in parentheses. Pretreatment measurements taken at −1 wk relative to the start of the treatment period were used as covariates.
AST, aspartate aminotransferase; BHBA, β-hydroxybutyrate; NEFA, nonesterified fatty acid; SED, standard error of the difference between the means; MF, milking frequency; FL, feeding level.
During week 3, plasma BHBA and NEFA content were greater (P <0.001) in UF cows and less (P <0.001) in 1× cows, compared with AF and 2× cows, respectively (Table 2). Plasma concentrations of BHBA and NEFA were 98 and 207% greater in UF cows, respectively. At week 3, plasma glucose and insulin concentrations were greater (P <0.01) in cows milked 1× compared with 2× and in AF compared with UF cows (Table 2). Plasma AST was also increased in UF cows compared with AF cows.
Microarray
There was no effect of 1× milking on hepatic gene transcription, and we detected no interactions between feeding level and milking frequency using a corrected P value of <0.05. However, there were 2,987 differentially expressed genes (DEG, corrected P <0.05) identified between AF and UF cows (Supplementary Table S1), of which 17 were upregulated >2.5-fold and eight were downregulated >2.5-fold, in UF relative to AF cows (Table 3).1
Table 3.
Differentially expressed genes upregulated or downregulated by at least 2.5-fold in cows UF for 3 wk compared with cows AF
| Gene Symbol | Accession | Fold Change | Gene Description |
|---|---|---|---|
| THRSP | NM_001040533 | −6.2 | thyroid hormone responsive |
| SCD | XM_002698490 | −4.3 | stearoyl-CoA desaturase (delta-9-desaturase) |
| TMED6 | NM_001075988 | −3.1 | transmembrane emp24 protein transport domain containing 6 |
| ANKRD37 | NM_001075392 | −3.1 | ankyrin repeat domain 37 |
| MBOAT2 | XM_001253490, XM_002691531 | −3.0 | PREDICTED: membrane bound O-acyltransferase domain containing 2 |
| MMP11 | XM_002694692 | −3.0 | PREDICTED: matrix metallopeptidase 11-like |
| FADS2 | NM_001083444 | −2.7 | fatty acid desaturase 2 |
| RASEF | NM_001105362 | −2.5 | RAS and EF-hand domain containing |
| HP | NM_001040470 | 2.5 | haptoglobin |
| LOC618787 | XM_871110 | 2.5 | PREDICTED: myosin-IXb-like |
| BRB | NM_173891 | 2.5 | brain ribonuclease |
| SLC13A5 | NM_001191446 | 2.6 | solute carrier family 13 (sodium-dependent citrate transporter), member 5 |
| APOA4 | NM_001037480 | 2.6 | apolipoprotein A-IV |
| INMT | NM_001192648 | 2.8 | indolethylamine N-methyltransferase |
| LOC100851509 | XM_003582052 | 3.0 | PREDICTED: collagen alpha-1 (XXVIII) chain-like |
| MT2A | NM_001075140 | 3.2 | metallothionein 2A |
| CGREF1 | NM_001045977 | 3.2 | cell growth regulator with EF-hand domain 1 |
| INHBA | NM_174363 | 3.6 | inhibin, beta A |
| SVOP | NM_001075441 | 3.7 | SV2-related protein homolog |
| G0S2 | NM_001192147 | 4.0 | G0/G1 switch 2 |
| RXRG | NM_001075408 | 4.1 | retinoid X receptor, gamma |
| ANGPTL4 | NM_001046043 | 4.3 | angiopoietin-like 4 |
| GPX3 | NM_174077 | 5.4 | glutathione peroxidase 3 (plasma) |
| FGF21 | XM_001789587 | 5.6 | PREDICTED: fibroblast growth factor 21 |
| MT1A | NM_001040492 | 9.2 | metallothionein-1A |
UF, 8.3 ± 2.9 kg dry matter/cow/day; AF, 14.3 ± 3.6 kg dry matter/cow/day. Genes with a negative fold change were downregulated in UF cows, whereas genes with a positive fold change were upregulated in UF cows.
qPCR
There were strong correlations between the expression ratios of the microarray and qPCR results (P <0.001, Table 4) across transcripts both up- or downregulated and with various degrees of fold-change.
Table 4.
Gene expression determined by qPCR in liver tissue from grazing cows AF or UF for 3 wk
| qPCR Data |
Expression Ratio UF vs. AF |
Correlation |
||||||
|---|---|---|---|---|---|---|---|---|
| Gene | AF | UF | SED | qPCR P Value | Array | qPCR | R | P |
| ACSL1 | 0.172 | 0.346 | 0.038 | <0.0001 | 1.60 | 2.01 | 0.89 | <0.0001 |
| ANGPTL4 | 0.360 | 1.023 | 0.079 | <0.0001 | 4.26 | 2.84 | 0.97 | <0.0001 |
| AOX1 | 0.499 | 0.407 | 0.029 | <0.001 | −1.59 | −1.23 | 0.65 | <0.0001 |
| APOA1 | 0.484 | 0.796 | 0.063 | <0.0001 | 2.50 | 1.64 | 0.96 | <0.0001 |
| DECR1 | 0.395 | 0.290 | 0.027 | <0.0001 | −1.34 | −1.36 | 0.75 | <0.0001 |
| FOXO1 | 0.456 | 0.547 | 0.037 | <0.05 | 1.16 | 1.20 | 0.71 | <0.0001 |
| GATM | 0.414 | 0.300 | 0.048 | <0.05 | −1.51 | −1.38 | 0.84 | <0.0001 |
| GPX3 | 0.798 | 1.784 | 0.096 | <0.0001 | 5.37 | 2.24 | 0.96 | <0.0001 |
| MT2A | 0.739 | 1.194 | 0.081 | <0.0001 | 3.15 | 1.62 | 0.96 | <0.0001 |
| PC | 0.313 | 0.475 | 0.049 | <0.01 | 1.47 | 1.52 | 0.51 | <0.001 |
| SIRT5 | 0.373 | 0.234 | 0.030 | <0.0001 | −1.24 | −1.59 | 0.72 | <0.0001 |
| THRSP | 1.807 | 0.737 | 0.105 | <0.0001 | −6.24 | −2.45 | 0.94 | <0.0001 |
| TMED6 | 0.871 | 0.430 | 0.053 | <0.0001 | −3.12 | −2.03 | 0.97 | <0.0001 |
Correlations between expression ratios were calculated with individual cow data and Pearson's coefficient. Abbreviations defined in Table 1 footnote.
Pathway Analysis
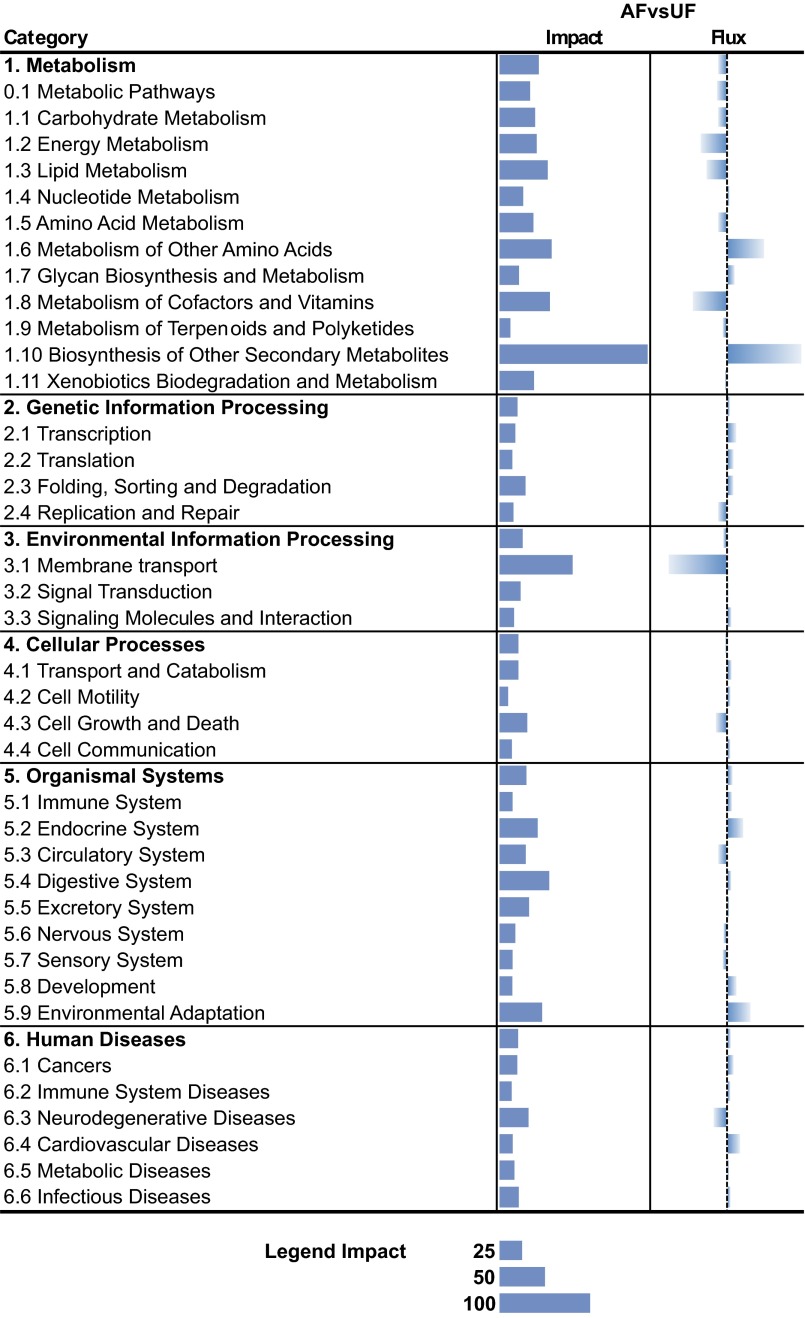
The overall effect of DEG due to the energy restriction on overall categories of KEGG pathways is presented in Fig. 1. The most affected pathways (impact >40, n = 31) and the direction of the impact within these categories as determined by the DIA are presented in Fig. 2. The data indicate that pathways related to metabolism were the most affected overall by energy restriction, with a larger inhibition of main metabolic pathways, such as energy and lipid metabolism, and the metabolism of vitamins. Within metabolism, the data indicate that feed-restricted cows had an overall inhibition of hepatic lipid synthesis, as indicated by the lower expression of genes in several pathways, such as of “biosynthesis of unsaturated fatty acids,” “fatty acid,” and “steroid biosynthesis” (Fig. 2). Energy-restricted cows had a lower production of energy through the mitochondria compared with AF cows, as indicated by the inhibition of “oxidative phosphorylation” (Fig. 2). The UF cows had a larger induction of secondary metabolism (Fig. 1) compared with AF cows, which was the most affected category of pathways. The “metabolism of cofactors and vitamins” was inhibited in UF compared with AF cows (Fig. 1) with the strongest inhibition observed for “vitamin B6 metabolism” (Fig. 2). The data also indicate a slightly higher induction of pathways related to transcription, translation, and cell motility in energy-restricted cows. Among those, the “PPAR signaling pathway” was most affected (Fig. 2). Finally, cell growth and death-related pathways were overall inhibited by energy restriction (Fig. 1), with “P53 signaling pathway” being the most affected pathway (Fig. 2).
Fig. 1.
Impact of differentially expressed genes (DEG) between adequately fed (AF) and underfed (UF) cows on categories and subcategories of pathways as calculated by the Dynamic Impact Approach. Solid filled bars denote the “impact” (i.e., overall effect of the DEG on the category of pathways) and the gradient-filled bars denote the “flux” (or direction of the impact due to the effect of the DEG on categories of pathways, i.e., reduced in UF vs. AF center-to-left bars or induced in UF vs. AF center-to-right bars).
Fig. 2.
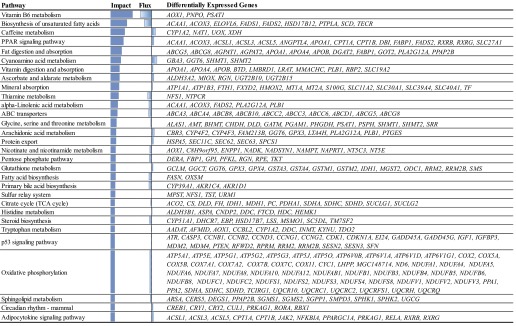
Pathways identified by the Dynamic Impact Approach as having a high impact (>40), in grazing cows UF (8.3 ± 2.9 kg dry matter/cow/day) compared with AF (14.3 ± 3.6 kg dry matter/cow/day) cows. KEGG pathways are ranked on their impact with their flux indicated as inhibited (to the left) or activated (to the right).
Networks and metabolic pathways identified by GeneGo are described in Tables 5 and 6. Lipid and carbohydrate metabolism were strongly represented as important metabolic networks, while oxidative phosphorylation, cytoskeleton remodeling, and cell signaling pathways were identified as key pathways altered by the energy restriction.
Table 5.
Pathways identified by GeneGo as significantly (corrected P value <0.05) altered in grazing cows UF compared with AF cows
| Pathway | P Value | % DEG | DEG |
|---|---|---|---|
| Oxidative phosphorylation | <0.0001 | 17.1 | NDUFA1, NDUFB3, NDUFS2, NDUFS3, NDUFS4, ATP5J, COX2, COX5A, COX7C, NDUFA4, NDUFA8, NDUFB6, NDUFC1, NDUFV3UQCR10, UQCRC2, UQCRH, UQCRQ |
| Immune response: histamine H1 receptor signaling | <0.001 | 20.8 | GNA11, NFATC1, JUN, PLCB3, PPP3CA, F3, GNB2/GNG12G, ICAM1, MAPK13, RELA |
| Transcription: transcription regulation of aminoacid metabolism | <0.001 | 28.0 | FECH, GCLM, HMBS, JUND/FOSL2, KEAP1, ODC1, PRKCE/PRKD2 |
| Cytoskeleton remodeling: TGF, WNT and cytoskeletal remodeling | <0.001 | 14.4 | ACTA2/ACTG1/ACTG2, ACTG1, CAV1, DVL2, FRAT1, JUN, KDR, MDM2, MKNK1, MYL12B, MYL4, COL4A5, MAPK13, NLK, RPS6KA5, TCF7 |
| Cytoskeleton remodeling: Cytoskeleton remodeling | <0.001 | 14.7 | ACTG1, CAV1, JUN, KDR, MKNK1, MYL12B, MYL4, COL4A5, EIF4A1, EIF4G1, EIF4G3, MAPK13, PTEN, RPS6KA5, TCF7 |
| Cell cycle: role of SCF complex in cell cycle regulation | <0.001 | 24.1 | CUL1, NEDD8, PLK1, CDT1, RBX1, SKP2 |
| Signal transduction: cAMP signaling | <0.001 | 21.1 | ADCY5, PHKG2, PPP3CA/B, PRKAR2A, PRKD2, RAP2A, ADCY6, GNB2/GNG12, |
| Protein folding and maturation: bradykinin/kallidin maturation | <0.01 | 21.9 | CPN2, THOP1, KNG1 |
| Development: beta-adrenergic receptors signaling via cAMP | <0.01 | 17.3 | ATP2A2, CAMK2D, PPARGC1A, PPP2CB, PPP3CB, PRKAR2A, ADCY6, MAPK13, PPKAR1B, |
| Development: notch signaling pathway | <0.01 | 18.6 | CUL1, HES1, JAG1, KAT2B, TLE3, GCN5, NFKBIA, NOTCH1, RBBP7, SAP30, SNW1 |
Boldfaced genes are upregulated, lightfaced genes are downregulated by energy restriction.
DEG, differentially expressed genes.
Table 6.
Metabolic networks identified by GeneGo as significantly (corrected P value <0.05) altered in grazing cows UF compared with AF cows
| Metabolic Network | P Value | % DEG | DEG |
|---|---|---|---|
| (L)-valine pathways and transport | <0.001 | 10.9 | ACSL1, JUN/JUND/FOSL2, PLCB3, SLC43A1, VARS, ACSL5, ICAM1, LHPP, LTA4H, PPA1 |
| GalNAcbeta1-3Gal pathway | <0.01 | 10.6 | CXCR4, EDNRA, EDNRB, F2RL2, GNAT1, PROC, AARS, GNA14, GNB2/GNG12, KHK, LARS2, LTA4H |
| Carbohydrate metabolism: TCA and tricarboxylic acids transport | <0.01 | 11.7 | DLD, IDH1, PC SLC25A21, SLC33A1, MDH1, SDHA, SDHC, SDHD, SLC13A2, SLC13A5, SUCLG2 |
| O-hexadecanoyl-(L)-carnitine pathway | <0.01 | 10.8 | ADCY5, CPT1A, CPT1B, GNAT1, PLCB3, ADCY6, GNB2/GNG12 |
| Lipid metabolism: fatty acid beta-oxidation | <0.01 | 8.9 | ACADVL, ACSL1, CPT1A, CPT1B, EHHADH, HSD17B4, HSD17B10, ACSL5, HMGCL |
| Phosphatidylinositol-4,5-diphosphate pathway | <0.01 | 9.4 | ACTN3, INPP5B, PLCB3, SPTBN2, ICAM1, INPP5F, OSBPL5, OSBPL9, OSBPL10, PLCD4, PTEN |
| Carbohydrate metabolism: Glycolysis, gluconeogenesis and glucose transport | <0.01 | 8.0 | ENO3, FBP1, FBP2, LDHB, SLC2A8, ALDOB, GPI, KHK, PGAM1, PGAM4, |
| Decanoylcarnitine pathway | <0.01 | 9.3 | CPT1A, CPT1B, CROT, FOXA2, SLC22A5, TLE3, GLI1, RORA, |
| Lauroylcarnitine pathway | <0.05 | 8.7 | CPT1A, CPT1B, CROT, FOXA2, SLC22A5, TLE3, GLI1, RORA, |
| 1-Akyl-glycerol_3-phosphoethanolamine pathway | <0.05 | 14.3 | GNA11, JUN, ICAM1, PAFAH1B2 |
| Sphingomyelin pathway | <0.05 | 7.8 | MED1, PLCB3, SORT1, SPHK1, CERS5, FABP1, RORA, SIRPA |
Boldfaced genes are upregulated, lightfaced genes are downregulated by energy restriction.
DISCUSSION
In the present study, restricting the energy intake of grazing dairy cows for 3 wk during early lactation induced significant changes in hepatic gene expression. The effects of 3 wk of nutrient restriction on the hepatic transcriptome in grazing dairy cows, such as the coordinated response to maintain glucose production, are similar to previous reports on the short-term effects of energy restriction in housed cows fed a more energy-dense diet (1, 10, 28, 31). This supports the premise that similar mechanisms are enacted in response to energy deficits in different dairy systems.
On examination of metabolite concentrations, it appeared that a lower milking frequency reduced the degree of negative energy balance caused by energy restriction. UF cows milked 1× had reduced plasma concentrations of NEFA and BHBA and greater glucose concentrations than UF cows milked 2×. However, there was no effect on the hepatic transcriptome as there were no interactions between milking frequency and the energy restriction, indicating that by 3 wk of an energy restriction, the liver has adapted to any differences in output between cows milked 1× and those milked 2×. It is possible, that had the liver been biopsied during the first week of the energy restriction and the hepatic transcriptome measured when the liver was still adapting, transcriptional differences may have been measured between cows milked 1× and those milked 2×.
Carbohydrate Metabolism
Underfeeding cows altered pathways involved in carbohydrate metabolism, probably reflecting the need to maintain plasma glucose concentrations during the period of the energy restriction (33). Pathways that utilize glucose and important glucose precursors, such as “tricarboxylic acid (TCA) cycle” and “pentose phosphate” were inhibited by underfeeding. “Thiamine metabolism” and “vitamin B6 metabolism” were also inhibited as thiamine (vitamin B1) is an important cofactor for reactions in the TCA cycle and the pentose phosphate pathway (17), and vitamin B6 is an important cofactor in glycogen breakdown (glycogenolysis). Even though glycogenolysis is crucial during the first few hours of starvation to provide gluconeogenic precursors, this process is switched off during the later stages of starvation (33). Therefore, genes involved in processes that utilize gluconeogenic precursors [succinate dehydrogenase complex, subunit A (SDHA)] and the metabolism of cofactors that support these processes [pyridoxamine 5′-phosphate oxidase (PNPO), nitrogen fixation 1 homolog (NFS1)] were downregulated (−1.24-, −1.28-, and −1.26-fold respectively), thereby supporting increased glucose production.
The activated pathways in UF cows compared with AF cows associated with carbohydrate metabolism, such as “ascorbate and aldarate metabolism” and the GeneGo pathway “glycolysis, gluconeogenesis, and glucose transport,” and the increased expression of genes involved in these processes [fructose-1,6-bisphosphatase 1 (FBP1), 1.30-fold; pyruvate carboxylase (PC), 1.47-fold] reflect an increase in the synthesis of glucose precursors and an increase in hepatic gluconeogenesis. Increased production of pyruvate from acetyl-coA can occur through greater expression of forkhead box O1 (FOXO1) and pyruvate dehydrogenase kinase, isozyme 4 (PDK4), which were both upregulated by underfeeding (1.16- and 2.25-fold, respectively). FOXO1 activity is reportedly enhanced during periods of low insulin concentrations (46), as was the case in UF animals. FOXO1 activates transcription of PDK4 (21), subsequently inhibiting the pyruvate dehydrogenase complex. This limits conversion of pyruvate to acetyl-CoA and enhances the use of pyruvate for gluconeogenesis (36). The increased glucose production may have also come from greater lactate utilization; lactate dehydrogenase B (LDHB) was upregulated 1.22-fold in UF cows, likely increasing the production of pyruvate from lactate (33). This upregulation of LDHB is crucial during an energy restriction, as glucose production from ruminally derived propionate is limited by the feed intake. However, lactate is continuously produced by red blood cells and other anaerobic tissues, and gluconeogenesis from lactate is constant during several weeks of energy restriction (33). Increased expression of LDHB and PC in energy-restricted cows has been reported previously (10, 31), indicating that increased gluconeogenesis from lactate is important during periods of nutritional deficit.
The collective results reflect changes to hepatic metabolism that spare gluconeogenic precursors from oxidation and increase gluconeogenesis in an attempt to maintain steady glucose concentrations during the energy restriction.
Lipid Metabolism
Lipid-related pathways in liver were inhibited by the 3 wk energy restriction, including the biosynthesis of FA, fat digestion and absorption, bile acid biosynthesis, and the sphingomyelin pathway, all indicative of reduced synthesis and hepatic output of lipids, including cholesterols (10, 28), as indicated by the inhibition of “ABC transporters” pathway. In contrast, FA oxidation was slightly activated. The energy restriction increased lipid mobilization from adipose tissue (20) and increased NEFA concentrations in the plasma of UF cows. Hepatic absorption of NEFA appears to be constant at ∼25% rate (13), and it is strongly linked to FA oxidation (4, 26). This pathway contains acyl-CoA synthetase long-chain family member 1 (ACSL1) and carnitine palmitoyltransferase 1A (CPT1A); both transcripts were upregulated (1.6- and 1.2-fold, respectively) by energy restriction. Additionally, fibroblast growth factor 21 (FGF21), which plays a role in regulating hepatic lipid metabolism in conditions of negative energy balance (41), was upregulated 5.63-fold in UF cows. Increased FGF21 transcript abundance has been reported previously in mice that either ate a ketogenic diet or were fasted (35) and has also been reported to be increased in cows in negative energy balance (41). Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PPARGC1A) expression is regulated by FGF21, which in turn increases FA oxidation (35). Consistent with this result, the expression of PPARGC1A was also upregulated 1.35-fold in the liver of UF cows in the present experiment.
FA oxidation is crucial during periods of energy restriction (33); however, previous reports on the effects of negative energy balance on transcription of FA oxidation genes are conflicting (10, 28, 31). It has been suggested that increased FA oxidation is mediated by the expression of a few “key” genes in this pathway (e.g., CPT1A) and driven by abundance of substrate rather than increase in abundance of enzymes involved in the pathway (6). Therefore, the increase in NEFA plasma concentrations in UF cows with a slight induction of FA oxidation is supportive of an overall increase in FA oxidation in UF compared with AF cows. However, the length or severity of the negative energy balance may affect the expression of genes involved in this process (10, 28, 31).
The upregulation of FA oxidation and inhibition of the TCA cycle during an energy restriction may result in ketone body synthesis; ketone bodies can be used for energy to spare glucose (33). Plasma concentrations of BHBA were greater in UF cows; however, transcript abundance of genes involved in ketone body synthesis did not change [3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2), 3-hydroxybutyrate dehydrogenase, type 2 (BDH2)]. Increased ketogenesis is in agreement with the increased FA oxidation and NEFA absorption in the liver and, as previously discussed, may be driven by substrate concentrations (6) or posttranscriptional regulation of HMGSC2 (23). Increased FA oxidation and BHBA synthesis indicate that the liver has adapted to the increased requirement for ketogenesis in UF cows by 3 wk of nutrient restriction.
Hepatic Stress
The increased expression of genes encoding the acute-phase proteins haptoglobin (HP, 2.53-fold) and heme oxygenase (decycling) 2 (HMOX2, 1.22-fold) in response to the energy restriction is indicative of an increased hepatic stress. Additionally, angiopoietin-like 4 (ANGPTL4) was upregulated 4.26-fold in UF cows and has recently been implicated in the acute-phase response (29). Hepatic upregulation of this gene in response to ketosis and during early lactation has been reported previously (28, 31). Plasma concentrations of AST were also increased in UF cows compared with AF cows, indicating liver damage or stress. The activation of the pathway “arachidonic acid metabolism” and in particular the increased expression of both glutathione peroxidase 3 (GPX3, 5.37-fold) and gamma-glutamyltransferase 6 (GGT6, 1.65-fold) indicate disturbances in glutathione homeostasis, crucial in the response to oxidative stress (47). This combination of both the gene expression results and the blood concentrations of AST indicates that the liver is under stress during the response to an energy restriction. Further work is required to determine if there is damage that limits the ability of the liver to respond adequately to the energy restriction.
Cytoskeleton Remodeling
Two pathways (“TGF, WNT, and cytoskeletal remodeling” and “cytoskeleton remodeling”) were identified by GeneGo as altered, with several genes upregulated in response to the energy restriction. Additionally, “TGF-beta signaling pathway,” “Notch signaling pathway,” and “P53 signaling” were all downregulated in UF compared with AF cows. These results indicate a decrease in cell cycling, differentiation, and remodeling of the liver cytoskeleton. Studies investigating the effects of feed restriction in beef cattle report decreases in liver size (39) and increases in mRNA expression of multiple myosin and actin genes (9), in agreement with the cytoskeletal remodeling identified in the current paper. The decrease in liver size was suggested to have a nutrient sparing effect as the size of the liver greatly affects energy expenditure (16). Therefore a potential reduction in liver size or activity in energy-restricted cows may be a survival mechanism to spare energy.
Cellular Energy Status
Inhibition of pathways such as “oxidative phosphorylation” and “nicotinate and nicotinamide metabolism” indicates that mitochondrial respiration was reduced in UF compared with AF cows. Nicotinate and nicotinamide are precursors of nicotinamide-adenine dinucleotide, which is crucial for oxidative phosphorylation and many other redox reactions (40). Nicotinamide-adenine dinucleotide can also be synthesized from tryptophan (40), the metabolism of which was also reduced by UF in the current study. Reduced oxidative phosphorylation due to feed restriction in dairy cows has been reported previously (1, 28) with the authors suggesting that this was because of reduced metabolic activity of the liver. This premise is supported by food restriction experiments in mice, whereby liver mass and mitochondrial respiration rates were reduced in mice fed 50% less for 3 days (14). These results indicate that during an energy restriction in dairy cows, genes involved in oxidative phosphorylation are downregulated, indicating liver mitochondria are less metabolically active during an energy restriction.
Conclusion
In summary, a 3 wk feed restriction in early lactation induced many gene transcription changes in the liver, resulting in >2,900 DEG. Pathways identified from this list of genes were involved in the metabolism of glucose and fatty acids and acted in a coordinated response to spare gluconeogenic precursors and increase gluconeogenesis. There was increased use of FA as fuel and increased ketogenesis. Upregulation of the expression of genes involved in acute-phase response and higher blood concentration of liver tissue damage markers indicate that the hepatic changes in response to the energy restriction do negatively affect liver health. No genes were differentially expressed due to milking frequency. It is possible, that for a short-period of restriction, 1× may have assisted in promoting a more positive energy balance (within 1 wk) as indicated by plasma measures; however, by 3 wk, homeorhetic mechanisms, such as the reduction in milk production, may have reduced the requirement for altered hepatic gene transcription.
GRANTS
This research was funded by New Zealand dairy farmers through DairyNZ (AN802) and by the New Zealand Ministry of Business, Innovation, and Employment (DRCXO801).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.M.G., J.K.K., C.V.P., R.G.S., and J.R.R. conception and design of research; T.M.G. performed experiments; T.M.G., M.B., and C.G.W. analyzed data; T.M.G., J.K.K., C.V.P., M.B., A.G.R., and J.R.R. interpreted results of experiments; T.M.G. drafted manuscript; T.M.G., J.K.K., C.V.P., M.B., C.G.W., A.G.R., R.G.S., and J.R.R. approved final version of manuscript; J.K.K., C.V.P., M.B., C.G.W., A.G.R., R.G.S., and J.R.R. edited and revised manuscript; M.B. prepared figures.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge DairyNZ farm and technical staff, in particular, Stuart Morgan for implementation of this experiment; Klaus Lehnert for microarray design; and Barbara Dow for statistical analysis.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Akbar H, Bionaz M, Carlson DB, Rodriguez-Zas SL, Everts RE, Lewin HA, Drackley JK, Loor JJ. Feed restriction, but not l-carnitine infusion, alters the liver transcriptome by inhibiting sterol synthesis and mitochondrial oxidative phosphorylation and increasing gluconeogenesis in mid-lactation dairy cows. J Dairy Sci 96: 2201–2213, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 215: 403–410, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data:” a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bauchart D, Gruffat D, Durand D. Lipid absorption and hepatic metabolism in ruminants. Proc Nutr Soc 55: 39–47, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bauman DE, Currie BW. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J Dairy Sci 63: 1514–1529, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Bionaz M, Loor JJ. Ruminant metabolic systems biology: reconstruction and integration of transcriptome dynamics underlying functional responses of tissues to nutrition and physiological state. Gene Reg Syst Biol 6: 109–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bionaz M, Periasamy K, Rodriguez-Zas SL, Hurley WL, Loor JJ. A novel dynamic impact approach (DIA) for functional analysis of time-course omics studies: validation using the bovine mammary transcriptome. PLoS One 7: e32455, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chagas LM, Gore PJS, Meier S, Macdonald KA, Verkerk GA. Effect of monopropylene glycol on luteinizing hormone, metabolites, and postpartum anovulatory intervals in primiparous dairy cows. J Dairy Sci 90: 1168–1175, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Connor EE, Kahl S, Elsasser TH, Parker JS, Li RW, Van Tassell CP, Baldwin RL, Barao SM. Enhanced mitochondrial complex gene function and reduced liver size may mediate improved feed efficiency of beef cattle during compensatory growth. Funct Integr Genomics 10: 39–51, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Doelman J, Cao H, Purdie NG, Kim JJM, Swanson KC, Osborne VR, Tey J, Ali A, Feng Z, Karrow NA, Cant JP. Transcript profiling of the ruminant liver indicates a unique program of transcriptional regulation of ketogenic enzymes during food restriction. Comp Biochem Physiol D 7: 303–310, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Dolezal AL, Obrian GR, Nielsen DM, Woloshuk CP, Boston RS, Payne GA. Localization, morphology and transcriptional profile of Aspergillus flavus during seed colonization. Mol Plant Pathol [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drackley JK, Overton TR, Douglas GN. Adaptations of glucose and long-chain fatty acid metabolism in liver of dairy cows during the periparturient period. J Dairy Sci 84: E100–E112, 2001 [Google Scholar]
- 13.Drackley JK. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci 82: 2259–2273, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Dumas J. Food restriction affects energy metabolism in rat liver mitochondria. Biochim Biophys Acta 1670: 126–131, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Eisen MB, Brown PO. DNA arrays for analysis of gene expression. Meth Enzymol 303: 179–205, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Ferrell C. Contribution of visceral organs to animal energy expenditures. J Anim Sci 6, Suppl 3: 23–34, 1988 [Google Scholar]
- 17.Frank RAW, Leeper FJ, Luisi BF. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell Mol Life Sci 64: 892–905, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovese C, Wasserman L. Operating characteristics and extensions of the false discovery rate procedure. J R Stat Soc Series B 64: 499–518, 2002 [Google Scholar]
- 19.Grala TM, Lucy MC, Phyn CVC, Sheahan AJ, Lee JM, Roche JR. Somatotropic axis and concentrate supplementation in grazing dairy cows of genetically diverse origin. J Dairy Sci 94: 303–315, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Grala TM, Roche JR, Phyn CVC, Rius AG, Boyle RH, Snell RG, Kay JK. Expression of key lipid metabolism genes in adipose tissue is not altered by once-daily milking during a feed restriction of grazing dairy cows. J Dairy Sci [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Gross DN, van den Heuvel APJ, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene 27: 2320–2336, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hales CN, Randle PJ. Immunoassay of insulin with insulin-antibody precipitate. Biochem J 88: 137–146, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegardt F. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J 582: 569–582, 1999 [PMC free article] [PubMed] [Google Scholar]
- 24.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho NT, Furge K, Fu W, Busik J, Khoo SK, Lu Q, Lenski M, Wirth J, Hurvitz E, Dodge N, Resau J, Paneth N. Gene expression in archived newborn blood spots distinguishes infants who will later develop cerebral palsy from matched controls. Pediatr Res 73: 450–456, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, Cameron R, Abumrad NA. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem 274: 26761–26766, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Kay JK, Phyn CVC, Rius AG, Morgan SR, Grala TM, Roche JR. Once-daily milking during a feed deficit decreases milk production but improves energy status in early lactating grazing dairy cows. J Dairy Sci 96: 6274–6284, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Loor JJ, Everts RE, Bionaz M, Dann HM, Morin DE, Oliveira R, Rodriguez-Zas SL, Drackley JK, Lewin HA. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol Genomics 32: 105–116, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lu B, Moser A, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun 391: 1737–1741, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Lucy MC, Verkerk GA, Whyte BE, Macdonald KA, Burton L, Cursons RT, Roche JR, Holmes CW. Somatotropic axis components and nutrient partitioning in genetically diverse dairy cows managed under different feed allowances in a pasture system. J Dairy Sci 92: 526–539, 2009 [DOI] [PubMed] [Google Scholar]
- 31.McCarthy SD, Waters SM, Kenny D, Diskin MG, Fitzpatrick R, Patton J, Wathes DC, Morris DG. Negative energy balance and hepatic gene expression patterns in high-yielding dairy cows during the early postpartum period: a global approach. Physiol Genomics 42A: 188–199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNamara S, Murphy J, Omara F, Rath M, Mee J. Effect of milking frequency in early lactation on energy metabolism, milk production and reproductive performance of dairy cows. Livest Sci 117: 70–78, 2008 [Google Scholar]
- 33.Newsholme E, Leech A. Biochemistry for the Medical Sciences. Chichester: John Wiley and Sons, 1988 [Google Scholar]
- 34.Peterson MP, Rosvall KA, Choi JH, Ziegenfus C, Tang H, Colbourne JK, Ketterson ED. Testosterone affects neural gene expression differently in male and female juncos: a role for hormones in mediating sexual dimorphism and conflict. PLoS One 8: e61784, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewera SA, Burgess SC. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106: 10853–10858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randle PJ, Garland PB, Hales CN, Newsholme E. The glucose fatty-acid cycle. Lancet 1: 785–789, 1963 [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen R. Quantification on the LightCycler. In: Rapid Cycle Real-Time PCR: Methods and Application, edited by Meuer S, Wittwer C, Nakagawara K. Berlin: Springer, 2001, p. 21–34 [Google Scholar]
- 38.Roche JR, Dillon PG, Stockdale CR, Baumgard LH, VanBaale JM. Relationships among international body condition scoring systems. J Dairy Sci 87: 3076–3079, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Sainz R, Bentley B. Visceral organ mass and cellularity in growth-restricted and refed beef steers. J Anim Sci 75: 1229–1236, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther 324: 883–893, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Schoenberg KM, Giesy SL, Harvatine KJ, Waldron MR, Cheng C, Kharitonenkov A, Boisclair YR. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 152: 4652–4661, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Stelwagen K. Effect of milking frequency on mammary functioning and shape of the lactation curve. J Dairy Sci 84: E204–E211, 2001 [Google Scholar]
- 43.Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B 64: 479–498, 2002 [Google Scholar]
- 44.van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AAM, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415: 530–536, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White MF. Insulin signaling in health and disease. Science 302: 1710, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med 30: 29–41, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.