Abstract
Knowledge of the genetic relationships between β-D-N-acetylhexosaminidases A and B (EC 3.2.1.30) may help in understanding the hexosaminidase deficiency associated with GM2 gangliosidosis, a fatal lipid storage disease in man. Through the use of man-mouse somatic cell hybrids we have found that a gene involved in hexosaminidase A expression was linked to the genes coding for mannosephosphate isomerase and pyruvate kinase-3. The gene coding for hexosaminidase B was not linked to any of the genes coding for 25 enzyme markers tested. A combination of immunological and electrophoretic techniques was employed to identify human hexosaminidases A and B with certainty in cell hybrids. Discordant segregation of hexosaminidase A and hexosaminidase B in 60 clones indicated that the genes coding for their expression were not linked. However, hexosaminidase A was never expressed in cell hybrids in the absence of hexosaminidase B. This suggests that the gene responsible for the hexosaminidase A phenotype, linked to mannosephosphate isomerase and pyruvate kinase-3, requires the presence of the gene coding for hexosaminidase B for the expression of hexosaminidase A. These observations offer a genetic explanation for the biochemical and immunological relationships between hexosaminidases A and B and provide the framework for identifying the basic genetic defects responsible for GM2 gangliosidosis.
Keywords: GM2 gangliosidosis, lysosomal enzymes, anti-hexosaminidase sera, electrophoresis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew W. R., Rattazzi M. C. Immunochemical characterization of human beta-D-N-acetyl hexosaminidase from normal individuals and patients with Tay-Sachs disease. I. Antigenic differences between hexosaminidase A and hexosaminidase B. Int Arch Allergy Appl Immunol. 1974;46(4):512–524. doi: 10.1159/000231154. [DOI] [PubMed] [Google Scholar]
- Carroll M., Robinson D. Immunological properties of N-acetyl-beta-D-glucosaminidase of normal human liver and of GM2-gangliosidosis liver. Biochem J. 1973 Jan;131(1):91–96. doi: 10.1042/bj1310091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Malcolm L. A., Yoshida A., Giblett E. R. Phosphoglycerate kinase: an X-linked polymorphism in man. Am J Hum Genet. 1971 Jan;23(1):87–91. [PMC free article] [PubMed] [Google Scholar]
- Cohen M. M., Lin C. C., Sybert V., Orecchio E. J. Two human X-autosome translocations identified by autoradiography and fluorescence. Am J Hum Genet. 1972 Sep;24(5):583–597. [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Inherited variants of human nucleoside phosphorylase. Ann Hum Genet. 1971 May;34(4):395–408. doi: 10.1111/j.1469-1809.1971.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Egli F., Stalder G. Malformations of kidney and urinary tract in common chromosomal aberrations. I. Clinical studies. Humangenetik. 1973 Mar 23;18(1):1–15. doi: 10.1007/BF00279026. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Konecny P., Koenig H. Lysosomal hydrolases: Conversion of acidic to basic forms by neuraminidase. FEBS Lett. 1971 Feb 12;13(1):68–72. doi: 10.1016/0014-5793(71)80667-1. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle F. H. Controlled production of proliferating somatic cell hybrids. J Cell Biol. 1970 Apr;45(1):74–82. doi: 10.1083/jcb.45.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle R. H. Mapping of a human genetic regulator element by somatic cell genetic analysis. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1220–1227. doi: 10.1073/pnas.66.4.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEABACK D. H., WALKER P. G. Studies on glucosaminidase. 4. The fluorimetric assay of N-acetyl-beta-glucosaminidase. Biochem J. 1961 Jan;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
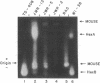
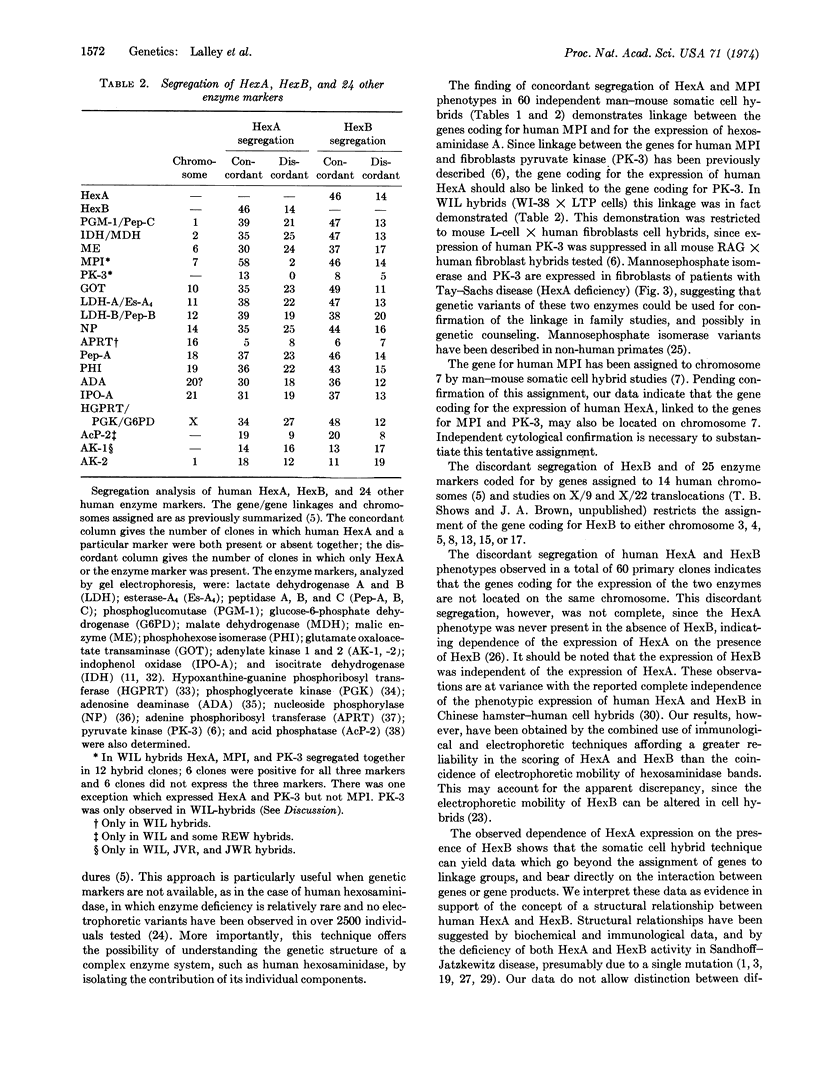
- McMorris F. A., Chen T. R., Ricciuti F., Tischfield J., Creagan R., Ruddle F. Chromosome assignments in man of the genes for two hexosephosphate isomerases. Science. 1973 Mar 16;179(4078):1129–1131. doi: 10.1126/science.179.4078.1129. [DOI] [PubMed] [Google Scholar]
- Mowbray S., Watson B., Harris H. A search for electrophoretic variants of human adenine phosphoribosyl transferase. Ann Hum Genet. 1972 Nov;36(2):153–162. doi: 10.1111/j.1469-1809.1972.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Chapman V. M., Ruddle F. H. Polymorphism and linkage for mannosephosphate isomerase in Mus musculus. Biochem Genet. 1973 Jan;8(1):47–53. doi: 10.1007/BF00485556. [DOI] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter H., Schmitt J. Genetic polymorphism of mannosephosphate isomerase in Primates. Humangenetik. 1973 Sep 20;19(3):325–326. doi: 10.1007/BF00278411. [DOI] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle F. H. Linkage analysis in man by somatic cell genetics. Nature. 1973 Mar 16;242(5394):165–169. doi: 10.1038/242165a0. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Shin S., Meera Khan P., Cook P. R. Characterization of hypoxanthine-guanine phosphoribosyl transferase in man--mouse somatic cell hybrids by an improved electrophoretic method. Biochem Genet. 1971 Feb;5(1):91–99. doi: 10.1007/BF00485734. [DOI] [PubMed] [Google Scholar]
- Shows T. B. Genetics of human-mouse somatic cell hybrids: linkage of human genes for isocitrate dehydrogenase and malate dehydrogenase. Biochem Genet. 1972 Dec;7(3):193–204. doi: 10.1007/BF00484817. [DOI] [PubMed] [Google Scholar]
- Shows T. B. Genetics of human-mouse somatic cell hybrids: linkage of human genes for lactate dehydrogenase-A and esterase-A 4 . Proc Natl Acad Sci U S A. 1972 Feb;69(2):348–352. doi: 10.1073/pnas.69.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Ruddle F. H. Function of the lactate dehydrogenase B gene in mouse erythrocytes: evidence for control by a regulatory gene. Proc Natl Acad Sci U S A. 1968 Oct;61(2):574–581. doi: 10.1073/pnas.61.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Hexosaminidase-A and hexosaminidase-B: studies in Tay-Sachs' and Sandhoff's disease. Nature. 1973 Feb 16;241(5390):463–463. doi: 10.1038/241463a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki K. Partial deficiency of hexosaminidase component a in juvenile gm2-gangliosidosis. Neurology. 1970 Sep;20(9):848–851. doi: 10.1212/wnl.20.9.848. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Stokes D. C., Corney G., Harris H. Differences between the N-acetyl hexosaminidase isozymes in serum and tissues. Ann Hum Genet. 1974 Jan;37(3):287–302. doi: 10.1111/j.1469-1809.1974.tb01836.x. [DOI] [PubMed] [Google Scholar]