Abstract
Aims
The rapidly increasing number of patients with implantable cardioverter-defibrillators (ICD) places a large burden on follow-up providers. This study investigated the possibility of longer in-office follow-up intervals in primary prevention ICD patients under remote monitoring with automatic daily data transmissions from the implant memory.
Methods and results
Conducted in 155 ICD recipients with MADIT II indications, the study compared the burden of scheduled and unscheduled ICD follow-up visits, quality of life (SF-36), and clinical outcomes in patients randomized to either 3- or 12-month follow-up intervals in the period between 3 and 27 months after implantation. Remote monitoring (Biotronik Home Monitoring) was used equally in all patients. In contrast to previous clinical studies, no calendar-based remote data checks were performed between scheduled in-office visits. Compared with the 3-month follow-up interval, the 12-month interval resulted in a minor increase in the number of unscheduled follow-ups (0.64 vs. 0.27 per patient-year; P = 0.03) and in a major reduction in the total number of in-office ICD follow-ups (1.60 vs. 3.85 per patient-year; P < 0.001). No significant difference was found in mortality, hospitalization rate, or hospitalization length during the 2-year observation period, but more patients were lost to follow-up in the 12-month group (10 vs. 3; P = 0.04). The SF-36 scores favoured the 12-month intervals in the domains ‘social functioning’ and ‘mental health’.
Conclusion
In prophylactic ICD recipients under automatic daily remote monitoring, the extension of the 3-month in-office follow-up interval to 12 months appeared to safely reduce the ICD follow-up burden during 27 months after implantation.
ClinicalTrials.gov Identifier
NCT00401466 (http://www.clinicaltrials.gov/ct2/show/NCT00401466).
Keywords: Implantable defibrillator, Office visits, Remote sensing technology, Patient schedule, Quality of life, Telemedicine
Introduction
Most patients receiving an implantable cardioverter-defibrillator (ICD) for a primary prevention indication have a low rate of events necessitating visits to the ICD clinic.1,2 However, regular follow-up is still considered important to monitor ICD function and the patient's condition. Expert consensus advocates clinical checks every 3–6 months, with an increased frequency in response to product advisories.3 Alternatively, ICD function and medical events recorded by the implant can be analysed using remote monitoring technology.3–6 Two large randomized clinical trials have shown that the replacement of regular clinic visits with remote data checks results in a faster reaction after events requiring intervention in parallel with a reduced number of in-hospital follow-up examinations.7,8 In both trials, longer in-hospital follow-up intervals combined with remote monitoring were compared with standard follow-up intervals without remote monitoring.7,8
Because remote monitoring is about to become the standard of care, it would be of interest to compare longer (e.g. yearly) and standard in-hospital follow-up intervals in patients undergoing the same kind of remote monitoring in both study arms in order to assess the isolated effect of less frequent in-hospital follow-up. Furthermore, the necessity of calendar-based remote follow-up sessions in the form used in the aforementioned trials7,8 can be questioned for remote systems capable of automatic physician alerting to relevant medical and technical events.
The REFORM trial randomized patients receiving prophylactic ICD treatment for MADIT II indications to either yearly or quarterly clinic visits. All patients were equally monitored using a remote monitoring system with fully automatic daily transmission and physician alerting after predefined events. The ICD follow-up burden (scheduled and unscheduled clinic visits), generic quality of life (QoL), and clinical outcomes were compared.
Methods
The Remote Follow-Up for ICD-Therapy in Patients Meeting MADIT II Criteria (REFORM) study was a randomized, non-blinded, parallel-design trial, in which three German and two Czech medical centres participated. The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Ethics Committees of the participating institutions. All patients gave their written informed consent to participate in the trial.
Patient selection
To be enrolled, patients had to meet the MADIT II trial enrolment criteria; that is, to be survivors of a myocardial infarction and to have a left-ventricular ejection fraction of <30%.9 The exclusion criteria were myocardial infarction within 30 days before enrolment, New York Heart Association functional class IV, a secondary prevention indication for ICD therapy, or living in an area lacking the GSM mobile phone coverage needed for remote monitoring transmission. Patients indicated for cardiac pacing were excluded for safety concerns because the ICDs used in this trial did not have the capability to automatically measure the pacing threshold. Furthermore, patients indicated for cardiac resynchronization therapy (CRT) according to clinically accepted criteria at the time of enrolment were not included because the required remote monitoring system was not available for devices providing CRT.
Remote monitoring
The ICDs used were equipped with the Home Monitoring (HM) capability (Biotronik, Berlin, Germany) described previously.5,6,10–15 Briefly, the implant transmits data once per day, comprising counts of arrhythmia episodes and therapies, rhythm information, and technical parameters. Transmission of intracardiac electrograms was available in only a few devices used in this study. The message sent by the implant is relayed by the patient device to the HM Service Center (HMSC) via the mobile phone network. The HMSC makes the data available to the treating physician on a secure internet site, and also sends alerts by e-mail or SMS if the data meet user-defined criteria. For long-term performance, HM was designed to require the patient only to switch on the patient device and keep it by the bedside. Transmission performance is calculated as the number of days with data transmission divided by the total number of days since the first message.
Study protocol
Patients were evenly randomized to quarterly clinic visits (Q-group) or yearly clinic visits (Y-group). After implantation of a single- or dual-chamber ICD (Belos®, Lexos®, or Lumos®; Biotronik, Berlin, Germany), patients in both study arms received the HM patient device. They were under continuous, automatic remote monitoring during the entire study. The response to HM alerts was left to the investigators' discretion. No calendar-based remote data checks or remote follow-up sessions were scheduled. All treatments up to the 3-month follow-up were done according to institutional standards and were equal in both study arms (Figure 1).
Figure 1.
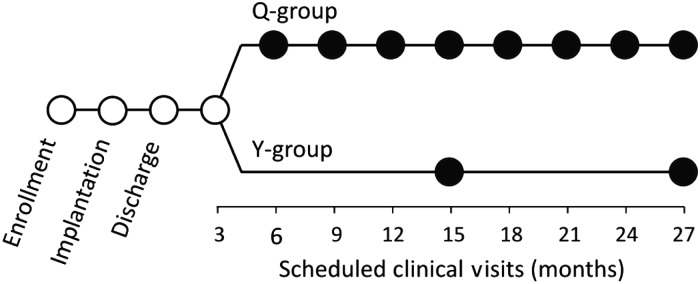
Clinical follow-up schedule. Full circles indicate the phase affected by patient randomization to quarterly (Q-group) or yearly (Y-group) routine in-office ICD follow-ups. For the whole duration of the study, all patients were under automatic, daily Home Monitoring surveillance, without calendar-based remote follow-up sessions. ICD, implantable cardioverter-defibrillator.
At the 3-month follow-up, all patients' ventricular function was re-evaluated. An improved left-ventricular ejection fraction (≥30%) or the development of a permanent pacing indication was ground for exclusion from the trial. The devices were programmed as follows: ventricular demand pacing with 40 beats per min (bpm); ventricular tachycardia zone from 180 bpm with anti-tachycardia pacing therapy; ventricular fibrillation zone from 200 bpm with shock therapy; stability and sudden onset criteria for arrhythmia discrimination ‘ON’. This programming was subject to adjustments after the occurrence of a first event.
Patients were followed for 2 years after the 3-month follow-up. They were instructed to refer to the investigational site for ICD-related issues and heart rhythm disorders. Care for the patients' concomitant diseases, most prominently heart failure, was provided by specialists as clinically indicated; however, they had no role in the study. Study data were collected by staff dedicated to the trial under the direct supervision of the principal investigators. Data management was provided by the sponsor. Data processing was supervised by the clinical team of the first author.
Scheduled follow-up visits
Patients assigned to the Q-group were scheduled to return for outpatient visits every 3 months, and those assigned to the Y-group had visits at 3, 15, and 27 months after discharge (Figure 1). A follow-up examination was classified as regular if occurring within a window of 4 weeks before or after an appropriate date relative to the discharge date. At each outpatient visit, the ICD was interrogated and tested and its memory was analysed in order to adapt patient management accordingly. The generic health-related QoL was assessed by the 36-item General Health Survey (SF-36) questionnaire at baseline, 15-month follow-up, and 27-month follow-up, in both study arms.
Unscheduled follow-up visits
Patients were seen outside of the scheduled visits for the following pre-specified HM alerts: (i) elective battery replacement indicator; (ii) any lead impedance out of range; (iii) delivery of a first shock after discharge of the patient from the hospital; (iv) delivery of ≥5 episodes of anti-tachycardia pacing; (v) delivery of ≥2 shocks per month for the first time, or occurrence of ≥2 supraventricular tachycardia or VT episodes per week. Patients could also be seen at unscheduled visits due to HM trend data or for any other reason, upon the patient's request or initiated by the investigator. Scheduled and unscheduled follow-up examinations were conducted similarly.
To get an estimate of additional patient contacts to medical professionals, the patients were instructed to notify such visits in a diary and were interviewed at each ICD follow-up.
Study hypothesis and endpoints
The primary study hypothesis was that the rate of unscheduled ICD follow-up visits in the Y-group would exceed the rate in the Q-group by <1.0 per patient-year, after the 3-month follow-up point. A confirmation of this non-inferiority hypothesis would mean that the total rate of scheduled and unscheduled ICD follow-up visits would be substantially lower in the Y-group than in the Q-group.
Secondary endpoints were the intra-individual difference in QoL scores between 27 months and baseline, total and cardiovascular mortality, and the rate and duration of all hospitalizations and cardiovascular hospitalizations. Furthermore, we made a post-hoc analysis of all available arrhythmia and therapy data (12 different items) extracted from the HMSC database. The data comprised, per study group, the numbers of patients fulfilling various criteria such as having episodes in different arrhythmia zones, started or delivered therapies, or unsuccessful maximum-energy shocks. The underlying arrhythmias in this analysis were not adjudicated by the investigator. Shock therapy was considered ineffective if the device delivered more than one shock during one arrhythmia episode, and this could include cases of repeated shocks in episodes of supraventricular tachycardia.
Statistical analysis
The sample size was based on the primary study hypothesis and calculated on a Blackwelder-type test of non-inferiority. Unscheduled ICD follow-up visits were assumed to occur independently from one other, to have a daily probability (equivalent to 0.21 or 0.96 visits per patient-year in the Q-group and the Y-group, respectively), and, hence, to obey binomial distribution. Assuming a 2-year attrition rate of 28% in both groups, a sample of 150 patients needed to remain in the study after the 3-month follow-up (for 1-ß = 80%).
All patients with baseline QoL data were included in the intention-to-treat based QoL analysis. Missing 27-month QoL data were accounted for using the last-observation-carried-forward (LOCF) method. Due to slight post-implantation increase in patients' QoL and greater loss to follow-up in the Y-group, this approach can be considered conservative.
For normally distributed continuous data (verified using the Shapiro–Wilk test), mean values and standard deviations were calculated. For non-normally distributed data, median values, interquartile ranges (IQR), and mean values, if appropriate for comparison with reference data, are shown. For categorical data, the absolute and relative frequencies were calculated. Continuous data were compared using the t-test (if normally distributed) or the Wilcoxon–Mann–Whitney rank test (if non-normally distributed). Categorical data were compared with a χ² test according to Pearson's or Fisher's exact test as appropriate. One-sided statistical significance for the primary study hypothesis, or two-sided statistical significance for all other tests, was established as P < 0.05.
Results
Of 155 patients enrolled in the study, 78 were randomly assigned to the Q-group and 77 to the Y-group. The baseline characteristics of the two study groups were similar (Table 1). The patients were 63 ± 10 years old. The mean ejection fraction was 25 ± 5%. All patients had a history of myocardial infarction. As REFORM was planned before publication of major CRT trials, ventricular dyssynchrony and QRS duration were not systematically evaluated at enrolment.
Table 1.
Baseline patient characteristics
| Variable | All patients (n = 155) | Q-groupa (n = 78) | Y-groupb (n = 77) |
|---|---|---|---|
| Mean age ± SD, years | 63 ± 10 | 63 ± 10 | 63 ± 10 |
| Male gender, % | 85.8 | 88.5 | 83.1 |
| NYHA 0/I/II/III, % | 4/5/49/41 | 4/4/50/41 | 5/6/48/41 |
| Median time after MI, months | 19 | 19 | 19 |
| >6 months, % | 73.9 | 76.6 | 70.7 |
| >18 months, % | 51.1 | 51.1 | 51.2 |
| Revascularization before <3 monthsc | 29.9 | 24.4 | 33.8 |
| Mean LVEF ± SD, % | 25 ± 5 | 25 ± 6 | 25 ± 5 |
| Single-/dual-chamber ICD, % | 5.8/94.2 | 5.1/94.9 | 6.5/93.5 |
| Medication, % | |||
| Beta-blocker | 89.2 | 88.0 | 90.4 |
| ACE-inhibitor or ARB | 83.1 | 80.0 | 86.3 |
| Diuretic | 85.6 | 82.7 | 87.7 |
| Anti-arrhythmic | 10.8 | 10.7 | 11.0 |
No difference between groups was statistically significant.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; ICD, implantable cardioverter-defibrillator; LVEF, left-ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association.
aQuarterly vs. byearly scheduled in-office follow-up visits.
cRevascularization within 3 months before enrolment.
At the 3-month follow-up, nine patients (5.8%) were excluded due to either improved left-ventricular ejection fraction (≥30%) or development of a permanent pacing indication (Table 2). Additional 48 patients (31%) terminated the study prematurely due to death, heart transplantation, pacing system upgrade to biventricular ICD, or other reasons listed in Table 2, among which only loss to follow-up differed significantly between the two groups (three in the Q-group vs. 10 in the Y-group, P = 0.04). The remaining 98 patients (63.2%) completed the study as planned, at the 27-month follow-up. The median (IQR) follow-up duration in all 155 patients was 26.6 (14.6–27.5) months; in 57 patients with early study termination, 7.8 (3.5–15.1) months.
Table 2.
Reasons for early terminations and the length of follow-up
| All patients (n = 155) | Q-groupa (n = 78) | Y-groupb (n = 77) | |
|---|---|---|---|
| Exclusion at 3-month follow-up, n | 9 | 6 | 3 |
| Left-ventricular ejection fraction >30% | 8 | 5 | 3 |
| Pacing indication | 1 | 1 | 0 |
| Early termination for other reasons, n | 48 | 22 | 26 |
| Death | 13 | 7 | 6 |
| Heart transplantation | 2 | 1 | 1 |
| Pacing system upgrade to biventricular | 6 | 3 | 3 |
| Patient withdrawal | 7 | 4 | 3 |
| Moving away | 4 | 3 | 1 |
| Lost to follow-up | 13 | 3 | 10* |
| Other | 3 | 1 | 2 |
| Regular termination at 27-month follow-up, n | 98 | 50 | 48 |
| Median (IQR) follow-up duration, months | |||
| All patients (155) | 26.6 (14.6–27.5) | 26.8 (10.4–27.9) | 26.5 (15.6–27.0) |
| Patients terminating study regularly (98) | 27.1 (26.7–27.9) | 27.8 (27.0–28.0) | 26.8 (26.6–27.2)* |
| Patients terminating study early (57) | 7.8 (3.5–15.1) | 5.8 (3.1–10.9) | 14.6 (4.0–18.2)* |
aQuarterly vs. byearly scheduled in-office follow-up visits.
*P < 0.05. No other difference between groups was significant.
One patient assigned to the Y-group preferred to see the physician more often than once a year. According to the intention-to-treat principle, he was evaluated within the Y-group, with all clinic visits other than those at 3, 15, and 27 months classified as unscheduled patient-initiated follow-ups.
The first HM transmission occurred at 5 ± 18 days (median, 1 day) after patient discharge. Subsequently, HM data were received on 87.1% (83 648 out of 96 066) of all days, including all enrolled patients. The transmission performance did not differ significantly between the Q-group (85.0%) and Y-group (89.0%).
Primary study hypothesis
Of 145 patients (72 Q-group, 73 Y-group) remaining in the study after the 3-month follow-up, 50 patients (17Q/33Y) had 94 unscheduled in-office ICD follow-ups (24Q, 70Y) until 27 months. The mean (median; IQR) number of unscheduled follow-ups per patient-year was 0.27 (0.00; 0.00–0.35) in the Q-group and 0.64 (0.00; 0.00–1.00) in the Y-group (P = 0.03). As the increase in the Y-group did not exceed one additional visit per patient-year (P < 0.001), the primary study hypothesis was met.
The reasons for 94 unscheduled follow-ups are listed in Table 3. Nearly two-thirds of unscheduled follow-ups were initiated by physicians (n = 61; 64.9%) and one-third was initiated by patients (n = 33; 35.1%). The most frequently claimed reason was ‘arrhythmia and/or ICD therapy’ (five in the Q-group, 21 in the Y-group). This marked difference between the two groups can be attributed to a less need to see the affected patient outside regular follow-up schedule in the Q-group, because of the relative proximity of the next regular visit. Similarly, the number of unscheduled visits caused by ‘patient malaise or need for reassurance’ in susceptible patients increased directly proportionally to the prolongation of the regular follow-up interval, with nine such visits in the Y-group and one in the Q-group. Furthermore, during ‘hospital stay or visits unrelated to ICD treatment,’ unscheduled follow-ups were undertaken more frequently in the Y-group (n = 10) than in the Q-group (n = 5), reflecting a greater likelihood for the physician to check the ICD function opportunely in a patient who was not supposed to be seen soon (or was not seen recently) on a regular basis.
Table 3.
Reasons for unscheduled implantable cardioverter-defibrillator follow-up visits after the 3-month follow-up
| All patients | Q-groupa | Y-groupb | |
|---|---|---|---|
| Reason, total number (physician-/patient- initiated) | |||
| Arrhythmia or ICD therapy | 26 (11/15) | 5 (1/4) | 21 (10/11) |
| Malaise or need for reassurance | 10 (0/10) | 1 (0/1) | 9 (0/9) |
| Hospital stay or visits unrelated to ICD treatment | 15 (14/1) | 5 (5/0) | 10 (9/1) |
| Scheduling problem | 10 (8/2) | 3 (2/1) | 7 (6/1) |
| Implanted device test | 2 (0/2) | 0 (0/0) | 2 (0/2) |
| Reason not clearly indicated | 31 (28/3) | 10 (9/1) | 21 (19/2) |
| Total | 94 (61/33) | 24 (17/7) | 70 (44/26) |
ICD, implantable cardioverter-defibrillator.
aQuarterly vs. byearly scheduled in-office follow-up visits.
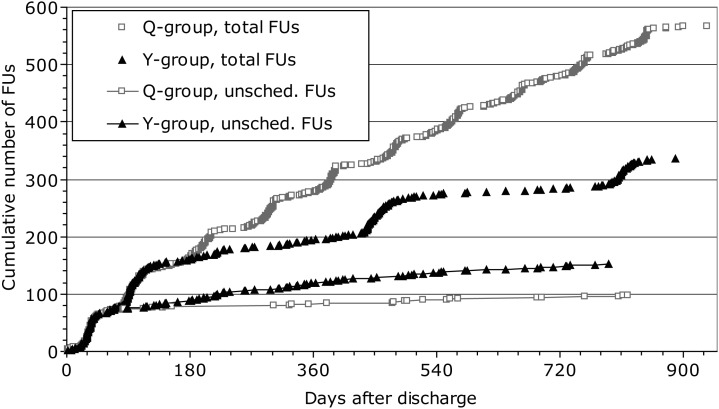
The total number of scheduled and unscheduled follow-up visits after the 3-month follow-up was 421 (Q-group) and 184 (Y-group). The corresponding mean (median; IQR) values per patient-year were 3.85 (3.90; 3.42–4.15) in the Q-group and 1.60 (1.10; 1.00–2.01) in the Y-group (P < 0.001) (Figure 2). Of note, the total number of follow-up visits up to and including the mandatory 3-month follow-up was 144 (Q-group) vs. 146 (Y-group). The cumulative number of unscheduled and total follow-up visits after device implantation in each group is illustrated in Figure 3.
Figure 2.
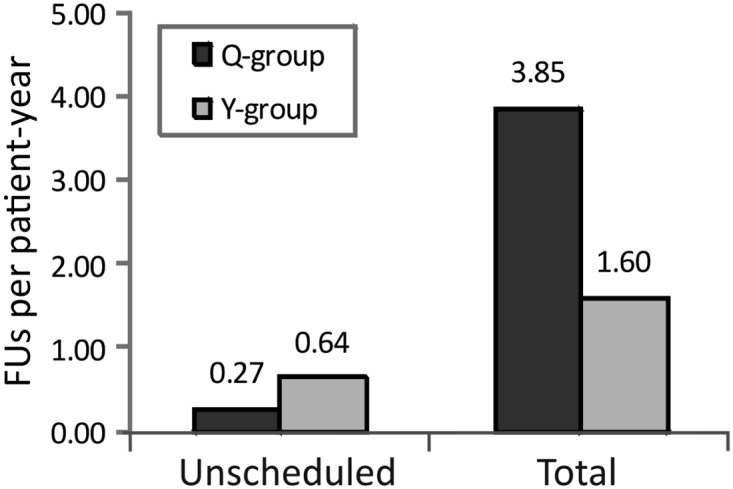
The mean numbers of FUs per patient-year after the 3-month follow-up. ICD, implantable cardioverter-defibrillator; FU, in-office ICD follow-up; Q-group, quarterly routine FU; Y-group, yearly routine FU. All patients were under close Home Monitoring surveillance.
Figure 3.
Cumulative number of unscheduled and total follow-up visits from post-implant discharge up to and including 27-month follow-up. Even if not required by study protocol, 1-month follow-up was performed in most patents and is categorized as unscheduled follow-up; it had no influence on study endpoints, for it occurred before the active study period. Unsched., unscheduled; other abbreviations as in Figure 2.
Secondary study endpoints and further analyses
Thirteen patients (8.4%) died during the study (Table 2), all except two for cardiovascular reasons. Of the 145 patients remaining in the study after the 3-month follow-up, 70 patients experienced 170 hospitalizations (0.74 per patient-year), lasting for a median (IQR) of 5 (2–10) days. Cardiovascular reasons caused 68% of the hospitalizations, lasting for 4 (2–9) days. There were no significant differences between the study groups in these aspects. Likewise, the 12 items extracted from the HMSC database did not differ between the two study arms (all P ≥ 0.4). As shown in Table 4, 50 patients received ICD therapy, 45 had at least one shock charging, and six had an unsuccessful maximum energy shock.
Table 4.
Hospitalizations and implantable cardioverter-defibrillator therapy delivery after the 3-month follow-up
| All patients (n = 155) | Q-groupa (n = 78) | Y-groupb (n = 77) | |
|---|---|---|---|
| Patients remaining after the 3-month FU, n | 145 | 72 | 73 |
| Cumulative FU duration after the 3-month FU, years | 229.5 | 111.6 | 117.9 |
| Hospitalizations for all causes, n | 170 | 80 | 90 |
| Patients with hospitalization | 70 | 32 | 38 |
| Hospitalizations per patient-year | 0.74 | 0.72 | 0.76 |
| Median (IQR) length of hospital stay, days | 5 (2–10) | 5 (2–10) | 4 (2–9) |
| Hospitalizations for adverse CV events, n | 116 | 54 | 62 |
| Patients with CV hospitalization | 55 | 26 | 29 |
| CV hospitalizations per patient-year | 0.51 | 0.48 | 0.53 |
| Median (IQR) length of hospital stay, days | 4 (2–9) | 3 (2–8) | 3.5 (2–8) |
| Patients with ICD therapy delivery, n | |||
| Patients receiving any ICD therapy | 50 | 24 | 26 |
| Patients with charged shock(s) | 45 | 21 | 24 |
| Patients with unsuccessful maximum energy shocks | 6 | 2 | 4 |
No difference between groups was statistically significant.
CV, cardiovascular; FU, follow-up; ICD, implantable cardioverter-defibrillator; IQR, interquartile range.
aQuarterly vs. byearly scheduled in-office follow-up visits.
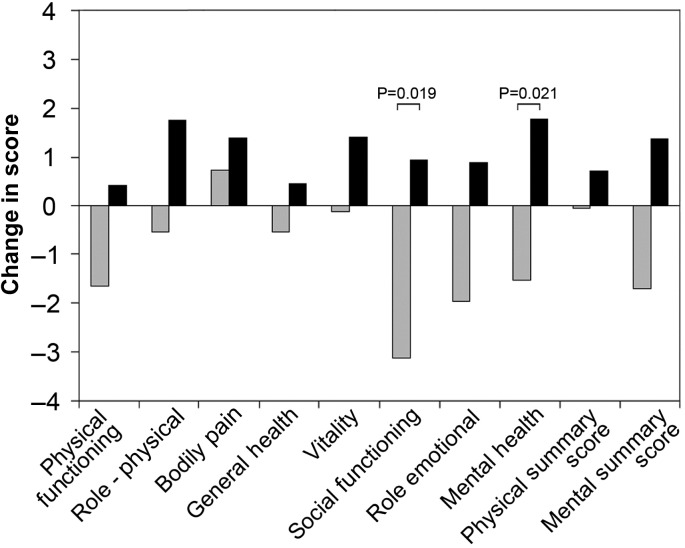
The SF-36 questionnaire was filled out by 112 patients at baseline, of which 54 answered the questionnaire also at the 27-month follow-up, and 23 answered it at the 15-month but not the 27-month follow-up. Evaluated according to the LOCF method, the mean intra-individual changes in different SF-36 domains from baseline to 27 months are shown in Figure 4. A significant difference was found in the domains ‘social functioning’ and ‘mental health,’ in favour of the Y-group.
Figure 4.
Mean intra-individual changes in quality-of-life (SF-36) scores from baseline to 27 months are shown, generated according to the last-observation-carried-forward method. Grey columns denote the Q-group (57 patients); black columns, the Y-group (55 patients). A positive column height indicates improved quality of life at 27 months and a negative height indicates a deterioration. A statistically significant difference in the heights of the paired white and black columns is indicated by the P-value seen above the columns.
Patients from the Q-group reported 1005 additional visits to medical professionals after the 3-month follow-up, and patients from the Y-group 942 visits. Neither the percentage of patients reporting such visits (75% in the Q-group vs. 65% in the Y-group, including those who terminated the study prematurely) nor the number of visits per patient (median 7.7 vs. 7.9, respectively) differed statistically significantly between the two groups.
Loss to follow-up
Of the 10 patients lost to follow-up in the Y-group, four had HM data transmissions up to 27 months after implantation (they did not die), one patient was lost at day 5, before any methodological difference took place between the two groups (Figure 1), and five patients were lost at ∼3, 7, 8, 10, and 24 months after implantation. In these five patients, and in the three patients in the Q-group who were lost to follow-up at ∼3, 16, and 17 months, death cannot be excluded. Even if all these uncertain patients had died, the mortality in both groups would have remained similar (10-Q vs. 11-Y; P = 0.80).
Discussion
Main findings
In remotely monitored patients with primary prevention ICDs and no cardiac pacing indications, scheduling yearly instead of quarterly follow-up visits resulted in 0.37 more unscheduled follow-up visits per patient-year. However, the total rate of follow-up visits was reduced by 58% from 3.85 to 1.60 per patient-year. A favourable impact of longer follow-up interval on the patients' QoL was observed, while no impact on patient mortality, hospitalization rate, or other clinical outcomes was evident after the 2-year observation period.
Reduction of follow-up burden
Since the completion of the MADIT II trial, primary prevention of sudden cardiac death has become the major indication for ICD implantation. The resulting proliferation of ICDs and the parallel introduction of CRT have created a new challenge for individual caregivers and the healthcare system. The simplest solution, in-office follow-up interval lengthening, may delay the detection of potentially important medical events and device failures,16–20 and is therefore not acceptable without a remote access to the information stored in implanted devices' memories.5–8,21,22
Previously, the TRUST7 and CONNECT8 trials investigated remote follow-up in ICD recipients. TRUST enrolled a typical contemporary ICD population and used the same remote monitoring system as REFORM. Replacing follow-up visits at 6, 9, and 12 months by scheduled checks of remote data in the HM study arm reduced the number of total in-hospital follow-ups by 45%.7 Even higher reduction in REFORM (58%) can be attributed to starting the observation period after the mandatory 3-month follow-up. The numbers of unscheduled visits per patient-year were similar in TRUST (0.78, HM group) and REFORM (0.64, Y-group). In a mixed population of ICD and CRT patients, the CONNECT trial replaced visits at 3, 6, 9, and 12 months with remote follow-ups performed using a substantially different technology (Medtronic CareLink).8 The increase in the number of unscheduled clinic visits in CONNECT (0.29 per patient-year)8 was, however, similar to that in TRUST (0.28) and REFORM (0.37).
Quality of life
The term ‘follow-up burden’ is mainly understood as the hospital's burden, taxed by limited resources and budgets. The patient, however, may also perceive follow-up visits as a burden.23 In REFORM, mental health and social functioning were improved in the Y-group at 27 months compared to baseline, while they deteriorated in the Q-group. The recent literature mostly relates QoL in ICD recipients to the number of ICD shocks. However, the necessity of frequent follow-up examinations may also require consideration, since patients undergoing less frequent clinical examinations might feel less ill and less distracted from their normal lives. REFORM offers a first indication that most patients have positive attitude to longer follow-up intervals, although the cohort studied was too small for final answer. As some patients may not agree with the mainstream opinion (one patient randomized to the Y-group wanted to see his physician more often), physicians should carefully discuss the pros and cons of remote monitoring concepts with patients.24
Safety aspects
The main concern about lengthening follow-up visit intervals is that patient condition deterioration may remain undetected for a longer time. Additional physiological sensors are therefore desirable to mirror patient health status.25 However, in REFORM, no significant difference was found between the Y-group and the Q-group regarding mortality, hospitalization rate, or hospitalization length, neither for all causes nor for cardiovascular causes. Likewise, the incidence of arrhythmias and therapies, as indicated by a variety of device counters, did not differ significantly between the two groups. These findings are in agreement with TRUST where mortality and adverse event rate were equal in both groups. Albeit, patient exclusion criteria were more stringent in REFORM in that patients with any cardiac pacing indication were excluded; in TRUST, only pacemaker dependent patients were left out. Also CONNECT identified no safety issues, but unlike REFORM and TRUST patients, CONNECT patients were contacted by telephone for remote follow-ups and had thereby the opportunity to report health-related issues. Recently, the EVOLVO study showed that remote monitoring of heart failure patients with ICDs or an ICD for CRT reduces emergency department and urgent in-office visits and, in general, total healthcare use.26
The REFORM study adds three safety relevant aspects to the growing evidence favouring longer follow-up intervals in remotely monitored patients. First, the follow-up period was 27 months, or 1 year longer than that in TRUST and CONNECT. Secondly, we did not schedule remote data checks in 3-month intervals, but relied entirely on automatic HM alerts. Our results support the assumption that regular remote data checks are not needed in a sufficiently reliable and frequent automatic alerting system. Thirdly, both our study groups were under the equal remote surveillance. One might argue that TRUST and CONNECT only show that the safety of longer follow-up intervals with remote monitoring is not inferior to standard follow-up intervals without remote monitoring. The best possible care in theory, however, should combine standard follow-up intervals with remote monitoring. Yet, REFORM findings do not support the superiority of this option over 12-month follow-up intervals.
The only concerning result is that more patients were lost to follow-up in the Y-group (10 vs. 3; P = 0.04). The administration staff in follow-up facilities may therefore need to adapt their efforts in tracking patients with longer follow-up intervals. Based on the information provided in the last section of Results, however, it is unlikely that the larger loss to follow-up in the Y-group may have hidden a significant increase in mortality. Moreover, as hospitalizations were calculated per patient-year, the greater patient attrition in the Y-group should not have influenced group comparisons importantly (Table 4). Of note, cumulative follow-up duration was numerically even longer in the Y-group (117.9 months) than in the Q-group (111.6 months) (Table 4).
Both TRUST and CONNECT investigated the median time from clinical event to clinical decision and found remarkable improvements in the remote monitoring arms (<2 days to clinical decision in TRUST and 4.6 days in CONNECT).7,8 REFORM did not investigate this issue because remote monitoring was equally used in both study arms, but it corroborated the other two trials in that follow-up interval lengthening is feasible and that physician can rely on remote monitoring information to decide on the necessity of an unscheduled in-office ICD follow-up. As the reimbursement for, and legal aspects of, remote monitoring are unsolved in many countries, a broader clinical implementation of the concepts proposed in these trials may, however, be delayed.27
Study limitations
The investigators were aware of the patients' group assignment when deciding on the need for unscheduled ICD follow-ups. This could have introduced a bias such may be inclination not to initiate unscheduled ICD follow-ups for borderline reasons in the Y-group. A blinded approach, however, was not possible because all patient's data were needed to judge on the necessity of an in-office follow-up. Likewise, the QoL results could have been affected by the patient's knowledge of group assignment.
Our analysis was restricted to ICD-related visits because the follow-up burden at ICD and CRT follow-up clinics has been increasing exponentially. Although we also assessed the number of patient contacts to other physicians (for concomitant diseases) based on patient diary and on interviews at each ICD follow-up, these data might have been biased towards less contacts reported in the Y-group because patients may have more difficulty in remembering all contacts since 12 months than since 3 months ago. The total economic effect of our follow-up concept will need to be investigated.
More than one-third of the enrolled patients did not reach the regular end of the study. The drop-out rate was only slightly higher than expected and was reasonably balanced between study groups, eventually not affecting the evaluation of primary study hypothesis that reached statistical significance. On the other hand, a larger study cohort would have enhanced the accuracy of secondary endpoints evaluation. In addition, only half of the enrolled patients contributed to the QoL analysis; the rest of patients did not fill out the QoL questionnaire either at baseline or at a later time-point.
REFORM results are not transferrable to patients receiving ICDs for secondary prevention or an ICD for CRT, who potentially have different needs for remote monitoring and for in-office follow-up visits. The follow-up duration of 27 months was insufficient to evaluate the results towards the end of the device's life cycle, when the risk of failures increases.
Conclusion
In prophylactic ICD recipients without cardiac pacing indications and under automatic daily remote monitoring, the extension of the 3-month follow-up interval to 12 months appeared to safely reduce the ICD follow-up burden during 27 months after implantation. In contrast to the previous trials, we did not schedule regular remote data checks but fully relied on automatic HM alerts. The SF-36 scores favoured the 12-month interval in the domains ‘social functioning’ and ‘mental health’.
Funding
The study was supported by Biotronik SE & Co. KG, Berlin, Germany
Conflict of interest: G.H. received research grants, travel grants and honoraria for lectures from Biotronik.
Acknowledgements
The authors acknowledge the contribution of Heinrich Beilmann, PhD, to study management, Jürgen Schrader, PhD, to data analysis and manuscript conception, Jochen Proff to publication process coordination and scientific discussion, and Dejan Danilovic, PhD, to critical reading and editorial assistance.
References
- 1.Klein RC, Raitt MH, Wilkoff BL, Beckman KJ, Coromilas J, Wyse DG, Friedman PL, Martins JB, Epstein AE, Hallstrom AP, Ledingham RB, Belco KM, Greene HL. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. J Cardiovasc Electrophysiol. 2003;14:940–948. doi: 10.1046/j.1540-8167.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 3.Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE. HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Europace. 2008;10:707–725. doi: 10.1093/europace/eun122. [DOI] [PubMed] [Google Scholar]
- 4.Heidbuchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, Ector H. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. 2008;10:351–357. doi: 10.1093/europace/eun010. [DOI] [PubMed] [Google Scholar]
- 5.Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace. 2009;11:701–709. doi: 10.1093/europace/eup110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung W, Rillig A, Birkemeyer R, Miljak T, Meyerfeldt U. Advances in remote monitoring of implantable pacemakers, cardioverter defibrillators and cardiac resynchronization therapy systems. J Interv Card Electrophysiol. 2008;23:73–85. doi: 10.1007/s10840-008-9311-5. [DOI] [PubMed] [Google Scholar]
- 7.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 8.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30(Suppl. 1):S2–S12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen JC, Kottkamp H, Zabel M, Aliot E, Kreutzer U, Bauer A, Schuchert A, Neuser H, Schumacher B, Schmidinger H, Stix G, Clementy J, Danilovic D, Hindricks G. Automatic home monitoring of implantable cardioverter defibrillators. Europace. 2008;10:729–735. doi: 10.1093/europace/eun099. [DOI] [PubMed] [Google Scholar]
- 12.Zartner P, Handke R, Photiadis J, Brecher AM, Schneider MB. Performance of an autonomous telemonitoring system in children and young adults with congenital heart diseases. Pacing Clin Electrophysiol. 2008;31:1291–1299. doi: 10.1111/j.1540-8159.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- 13.Theuns DA, Rivero-Ayerza M, Knops P, Res JC, Jordaens L. Analysis of 57,148 transmissions by remote monitoring of implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2009;32(Suppl. 1):S63–S65. doi: 10.1111/j.1540-8159.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- 14.Spencker S, Coban N, Koch L, Schirdewan A, Muller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11:483–488. doi: 10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 15.Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace. 2008;10:164–170. doi: 10.1093/europace/eum289. [DOI] [PubMed] [Google Scholar]
- 16.Gould PA, Krahn AD. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295:1907–1911. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- 17.Ruffy R. The device recalls nightmare: help might be on the way. Pacing Clin Electrophysiol. 2007;30(Suppl. 1):S1. doi: 10.1111/j.1540-8159.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 18.Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm. 2009;6:605–610. doi: 10.1016/j.hrthm.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Guedon-Moreau L, Chevalier P, Marquie C, Kouakam C, Klug D, Lacroix D, Brigadeau F, Kacet S. Contributions of remote monitoring to the follow-up of implantable cardioverter-defibrillator leads under advisory. Eur Heart J. 2010;31:2246–2252. doi: 10.1093/eurheartj/ehq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser RG, Abdelhadi R, McGriff D, Retel LK. Deaths caused by the failure of Riata and Riata ST implantable cardioverter-defibrillator leads. Heart Rhythm. 2012;9:227–35. doi: 10.1016/j.hrthm.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 21.Raatikainen MJ, Uusimaa P, van Ginneken MM, Janssen JP, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace. 2008;10:1145–1151. doi: 10.1093/europace/eun203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusumoto F, Goldschlager N. Subject of the year: remote monitoring. J Interv Card Electrophysiol. 2009;25:89–90. doi: 10.1007/s10840-009-9401-z. [DOI] [PubMed] [Google Scholar]
- 23.Gramegna L, Tomasi C, Gasparini G, Scaboro G, Zanon F, Boaretto G, Tomei R, Tomasi L. In-hospital follow-up of implantable cardioverter defibrillator and pacemaker carriers: patients' inconvenience and points of view. A four-hospital Italian survey. Europace. 2012;14:345–350. doi: 10.1093/europace/eur334. [DOI] [PubMed] [Google Scholar]
- 24.Matlock DD. Big Brother is watching you: what do patients think about ICD home monitoring? Circulation. 2010;122:319–321. doi: 10.1161/CIRCULATIONAHA.110.966515. [DOI] [PubMed] [Google Scholar]
- 25.Sack S, Wende CM, Nagele H, Katz A, Bauer WR, Barr CS, Malinowski K, Schwacke H, Leyva F, Proff J, Berdyshev S, Paul V. Potential value of automated daily screening of cardiac resynchronization therapy defibrillator diagnostics for prediction of major cardiovascular events: results from Home-CARE (Home Monitoring in Cardiac Resynchronization Therapy) study. Eur J Heart Fail. 2011;13:1019–1027. doi: 10.1093/eurjhf/hfr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, Parati G, Borghi G, Zanaboni P, Valsecchi S, Marzegalli M. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation. 2012;125:2985–2992. doi: 10.1161/CIRCULATIONAHA.111.088971. [DOI] [PubMed] [Google Scholar]
- 27.Vinck I, De Laet C, Stroobandt S, Van Brabandt H. Legal and organizational aspects of remote cardiac monitoring: the example of implantable cardioverter defibrillators. Europace. 2012;14:1230–1235. doi: 10.1093/europace/eus004. [DOI] [PubMed] [Google Scholar]