Abstract
The fruit fly, Drosophila melanogaster, is a good experimental organism to study the underlying mechanism of heart rate (HR) regulation. It is already known that many neuromodulators (serotonin, dopamine, octopamine, acetylcholine) change the HR in Drosophila melanogaster larvae. In this study, we investigated the role of cAMP-PKA signaling pathway in HR regulation and 5-HT positive chronotropic action. In order to obtain insight into the 5-HT mechanism of action in larvae cardiomyocytes, genetic and pharmacological approaches were used. We used transgenic flies that expressed the hM4Di receptor [designer receptors exclusively activated by designer drugs (DREADDs)] as one tool. Our previous results showed that activation of hM4Di receptors (modified muscarinic acetylcholine receptors) decreases or arrests the heart from beating. In this study, it was hypothesized that the positive chronotropic effect of serotonin [5-hydroxytryptamine (5-HT)] are mediated by serotonin receptors coupled to the adenylyl cyclase pathway and downstream cAMP and PKA activity. Activation of hM4Di by clozapine-N-oxide (CNO) was predicted to block the effects of serotonin by inhibiting adenylyl cyclase activity through Gαi pathway activation. Interestingly, we found here that manipulation of adenylyl cyclase activity and cAMP levels had no significant effect on HR. The ability of hM4Di receptor activation to slow or stop the heart is therefore likely mediated by activation of GIRK channels to produce hyperpolarization of cardiomyocytes, and not through inhibition of adenylyl cyclase.
Keywords: 5-HT, M4D receptor, CNO, adenylyl cyclase, PKA
the heart in the fruit fly, drosophila melanogaster, is a model for investigating the underlying mechanism of cardiac diseases which are related to human cardiac disorders, such as congenital heart diseases (39) and dilated cardiomyopathy (25). Although, the Drosophila heart is simple and consists of a heart tube at the caudal region and anterior aortic region (9), it has been established as a model system to study the ionic basis of myocardiocytes (15). The myogenic heart of Drosophila larvae also is useful to examine modulators and their mode of actions, because modulators directly act on the myocardiocytes as there is no direct innervation on the pacemaker region. It has been previously shown that Ca2+ and K+ ions play a crucial role in generation of the cardiac action potential. Inhibition of L-type calcium channels or potassium channels by specific blockers significantly reduce the heart rate (HR) whereas the Na+ channel blocker, tetrodotoxin, does not have any effect on HR in Drosophila (18). To investigate the underlying mechanisms of modulation, ionic and second messenger cascades need to be determined.
Some neuromodulators and neurotransmitters, like serotonin, dopamine, octopamine, and acetylcholine are known to modulate heart performance in Drosophila melanogaster larvae (11, 20, 37). 5-Hydroxytryptamine (5-HT) is an essential neuromodulator that has many behavioral and physiological functions in the fly, such as learning and memory (22), courtship and mating (3), cardiac rate modulation (11, 41), and modulation of sensory-motor circuits (13). Moreover, 5-HT is assumed to be a modulator with an old evolutionary history because it is found in simple as well as in complex animals and even plants (2). There are four characterized 5-HT receptor genes in the Drosophila melanogaster genome, 5-HT1ADro, 5-HT1BDro, 5-HT2Dro, and 5-HT7Dro (3, 22, 24, 30). Recently, another 5-HT2Dro receptor subtype, 5-HT2BDro, has been identified in Drosophila (17). 5-HT1ADro, 5-HT1BDro inhibits adenylyl cyclase (AC) activity (36); 5-HT7Dro increases AC activity (40). Exogenous 5-HT application in semi-intact larvae increases the HR, but the underlying signaling mechanism has yet to be elucidated. However, we have shown that activation of 5-HT2Dro receptor mediates the positive chronotropic effect of 5-HT in Drosophila larval heart (Majeed ZR, Stacy A, Cooper RL, unpublished observations).
It is obvious that the Drosophila and vertebrates are different morphologically; notwithstanding, Drosophila and vertebrates use similar molecular pathways underlying cardiac development (7). Further, Drosophila and vertebrate hearts share crucial physiological and dynamic aspects, for example, cardiac output, and rate and duration of systole or diastole (10). The rationale for Drosophila larval heart study is to decode the undiscovered aspects of cardiac physiology and pathophysiology which in turn can be extended to the physiology of hearts in other animals, including humans.
In this study, we sought to elucidate the signaling pathway underlying the positive chronotropic effects of 5-HT in Drosophila melanogaster larvae by utilizing classical pharmacology with incorporation of a pharmacogenetic approach using designer receptors exclusively activated by designer drugs (DREADD) receptors (4). DREADDs are powerful new tools that allow a high degree of spatial and temporal control of neuronal and effector pathway activity. Significantly, DREADD control is reversible, and requires no specialized equipment. We used the UAS-Gal4 binary expression system (8) to express hM4Di receptors, which are positively coupled to Gαi in muscle fibers. The hM4Di is a modified human muscarinic acetylcholine M4 receptor, mutated such that it no longer has affinity for the native ligand, acetylcholine. Instead, this engineered receptor has high affinity for a chemical that is considered physiologically inert, clozapine-N-oxide (CNO) that has full agonist efficacy at DREADD receptors (1, 4, 29). With this approach one can rule out off-target effects of the natural ligand to specifically and remotely control effector pathway activity in defined target tissues that the DREADD receptor is expressed in.
In the pupal stage of Drosophila forskolin does not produce a change in the HR (21), indicating that cAMP levels are not important in the pupa; however, in the pupal stage significant alterations in endocrine function accompanied by significant morphological changes are occurring, and mechanisms of cardiac function may be different than in the larva. We previously demonstrated that 5-HT increases HR in larvae; therefore, we examine here the potential mechanisms of action of 5-HT in the larval stage heart. Our working model was that 5-HT activates Gαs, which activates adenylyl cyclase that in turn increases the level of cAMP. Increased cAMP leads to an increase in active PKA signaling and positive chronotropic modulation of the heart. In this scenario, blockade of adenylyl cyclase activity with pharmacological methods, or activation of Gαi through activation of hM4Di receptors expressed in the heart, would be predicted to decrease cAMP levels and PKA activation and block the positive chronotropic effects of 5-HT on the heart. Our data, however, indicate that cAMP levels do not contribute to either modulation of HR or the positive chronotropic effects of 5-HT in the Drosophila heart. It has been reported that forskolin does not noticeably change the HR in P1 pupal stage of Drosophila (21). This confirms that Drosophila HR in larvae also might not be markedly changed by activation of AC-PKA pathway; although, AC-PKA pathway might increase the contractility of heart.
MATERIALS AND METHODS
Transgenic fly strains.
In this study, two fly strains were used, UAS-hM4Di and 24B-GFP (a strain heterozygous for the 24B-GAL4 driver element that expresses in all larval muscle, and is also homozygous for the UAS-GFP element; from Dr. Charles Nichols, Louisiana State University Health Sciences Center). The UAS-hM4Di strain was crossed with 24B-GFP strain in order to express hM4Di (Gαi) coupled receptor in muscle fibers, including cardiomyocytes. In the F1 generation, half the flies contained the 24B-GAL4 expression element, detected by GFP expression, and the other half did not. The GFP-positive, hM4Di-expressing larvae were used as the experimental animals, and the non-GFP-positive, non-hM4Di-expressing (but UAS-hM4Di containing) ‘littermates’ were used as controls (background). The flies were reared at room temperature (22°C) in vials containing cornmeal-agar-dextrose-yeast medium.
Heart rate measurement.
Third instar larvae were dissected in the ventral side up position to expose the dorsal vessel (heart) in HL3 (saline solution) (NaCl 70 mM, KCl 5 mM, MgCl2.6H2O 20 mM, NaHCO3 10 mM, Trehalose 5 mM, sucrose 115 mM, BES 5 mM, and CaCl2.2H2O 1 mM with the pH adjusted to 7.1). Because heart performance is very sensitive to pH change, the pH was tightly regulated and adjusted as needed. Recently, the effects of various buffers have been probed to optimize the saline for monitoring heart rate (14). Drugs were applied at various concentrations as indicated in the Results. The preparation was left for 1 min in saline after dissection, and then heart beats were counted for the following minute. Exceptions are indicated on graphs. The difference in the HR before and after application of drugs was used to measure the effects of the various compounds.
Chemicals.
All the chemicals, serotonin hydrochloride [5-hydroxytryptamine (5-HT)], forskolin, SQ 22,536, N6, 2′-O-Dibutyryl adenosine 3′,5′ cyclic monophosphate sodium salt (dbcAMP), 2′,5′ dideoxyadenosine were purchased from Sigma-Aldrich, St. Louis, MO. Clozapine-N-oxide was kindly synthesized by Dr. David Nichols (Purdue University). 5-HT stock solution (1 mM) was prepared with HL3 saline and immediately before use, a 1 μM serotonin solution was made from the stock solution. Forskolin was dissolved in 1% dimethyl sulfoxide (DMSO) to obtain a 1 mM stock solution. The final concentration of DMSO was less than 0.1% (roughly about 0.03%) in 30 μM forskolin and 0.5% in 100 μM forskolin. 300 μM and 1 mM of dbcAMP were also prepared with HL3 saline. 200 μM of SQ 22,536 was made with saline from a 1 mM stock solution. A 1-mM dideoxyadenosine stock solution was made in DMSO, then 50 μM (0.04% final DMSO concentration) and 500 μM (0.2% final DMSO concentration) with dilutions in saline.
Statistical analysis.
All data are expressed as mean ± SEM. The paired t-test (before and after) was used to compare the difference of HR change after exchanging solution with saline containing chemicals. T-test was used to compare between background (UAS-hM4Di) and hM4Di expressing (UAS-hM4DiX24B-Gal4) larval HRs (SigmaPlot version 11.0). P ≤ 0.05 is considered as statistically significant. The number of asterisks are considered as P ≤ 0.05 (*), P ≤ 0.02 (**), and P ≤ 0.001 (***).
RESULTS
Effects of media change on heart rate.
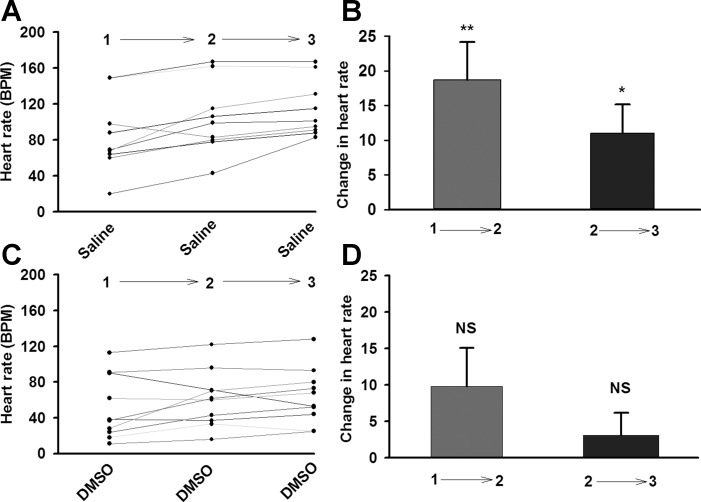
We previously found that the larval HR is sensitive to the physical exchange of the bathing media (9). In order to control for the exchange of the bathing media when exposing the heart to various compounds, a series of control experiments of saline bath exchanges was conducted. Also, because some of the drugs contained small concentrations of DMSO, the effect of low concentrations of DMSO alone and the effect of switching the DMSO bathing media were examined (Fig. 1). In addition, the HR was measured with an additional exchange of the bathing media twice for the same period as the first one, left for 1 min, and then examined for the following minute. Although the larvae were already exposed to saline during the dissection, there was still an average of 20 beats per minute (BPM) increase in HR with the first saline change. After another 2 min the heart was not as sensitive to the exchange of the saline, as only an average of 10 BPM was noted (Fig. 1B). Exchanging bathing media that contained DMSO did not produce as large of an increase in HR as saline. The second exchanges also resulted in a smaller alteration in HR (Fig. 1D).
Fig. 1.
A: The effect of changing saline with saline on larval heart rate in UAS-M4D X 24B-GFP flies, n = 9. B: Difference in heart rate before and after changing solution. C and D: The effect of changing saline containing DMSO with saline containing DMSO on larval heart rate in UAS-M4D X 24B-GFP flies; n = 10. P ≤ 0.05 (*), P ≤ 0.02 (**).
CNO concentration dependently inhibits heart rate in hM4Di expressing larvae.
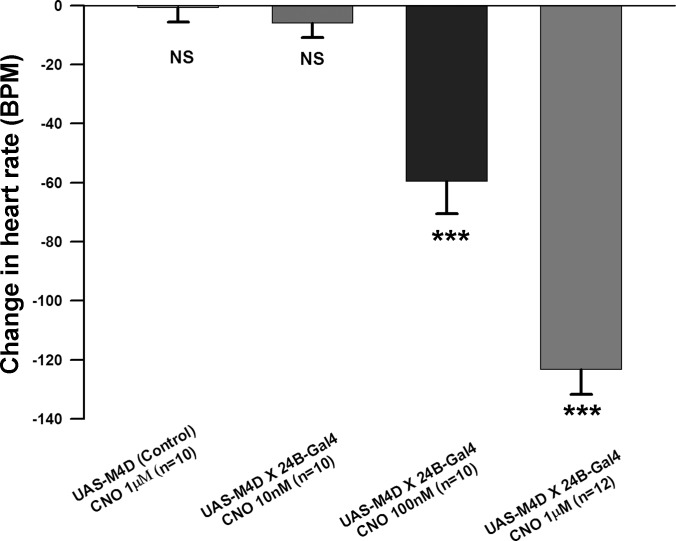
Increasing concentrations of CNO (10 nM, 100 nM, and 1 μM) were applied to the exposed heart of semi-intact larval preparations. Application of CNO decreases the HR in a concentration dependent manner in the UAS-M4D X 24B-GFP larvae (Fig. 2). CNO application at the highest concentration does not significantly change the HR in the nonexpressing UAS-M4D (control) larvae (Fig. 2). In this study, 500 nM CNO was chosen to use for the primary experiments because 1 μM stopped the heart from beating in all UAS-M4D expressing preparations. We observed that most of the hearts started to beat again when the saline + CNO was exchanged with saline only (not shown).
Fig. 2.
Concentration response curve for clozapine-N-oxide (CNO) at hM4Di receptors on the larval heart rate. The absolute change in rate [beats per minute (BPM)] is shown P ≤ 0.001 (***).
The presence of CNO to activate hM4Di in expressing larvae blocks the positive chronotropic effect of 5-HT.
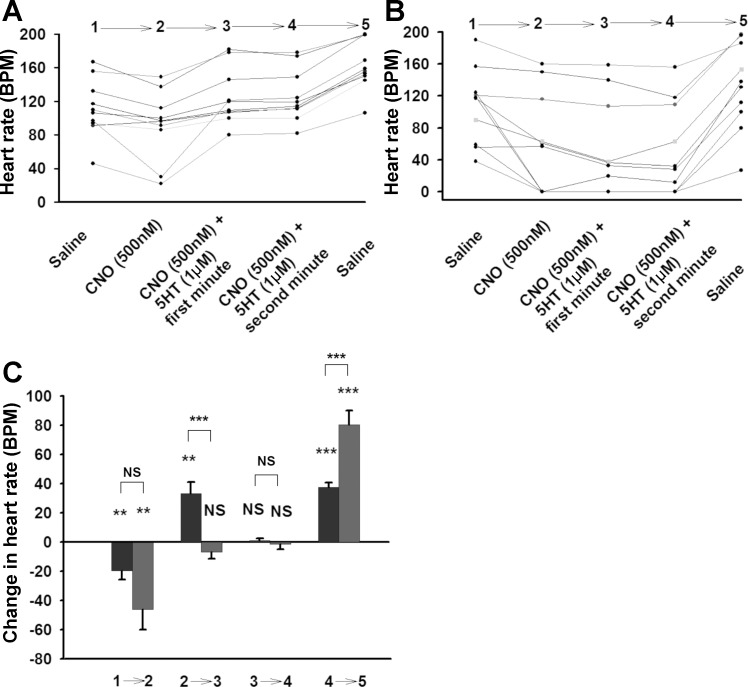
In flies that do not express hM4Di receptors (background), 5-HT markedly increases the HR, even in the presence of CNO (Fig. 3, A and C). These results confirm the positive chronotropic effects of 5-HT and that CNO does not block the plasma membrane receptor binding site for 5-HT. Only when hM4Di DREADD receptor expression was induced did CNO have an effect on the positive chronotropic effect of 5-HT (Fig. 3, B and C). We observed that CNO by itself slightly decreased HR in background larvae (Fig. 3, A and C). This may be due to leaky expression of the UAS-GAL4 element present in the background genotype rather than a direct effect of CNO on the heart. When the CNO + 5-HT containing saline was exchanged with saline (4→5), the HR significantly increased in both background and hM4Di-expressed larvae. This might be due to the 5-HT residual effect on the HR. The increase in HR on this final wash was 2-fold greater in the hM4Di expressing larvae, suggesting a rebound effect occurring from the removal of the repressing CNO mediated hM4Di signaling.
Fig. 3.
Effect of activation of hM4Di receptors on the action of 5-HT in larval heart. A: Background UAS-M4D, n = 10. B: UAS-M4D X 24B-GFP, n = 10. C: Comparison between background (dark grey) and M4D expressing group (light grey). The differences between genotypes are represented by NS (not significant) or asterisk(s) above the lines. The differences, which are due to the changing of one solution with another one in the same genotypes, are represented by NS or asterisk(s) above the bars. P ≤ 0.05 (*), P ≤ 0.02 (**), P ≤ 0.001 (***).
After 5-HT is applied to increase HR, the addition of CNO reverses the positive chronotropic effects of 5-HT.
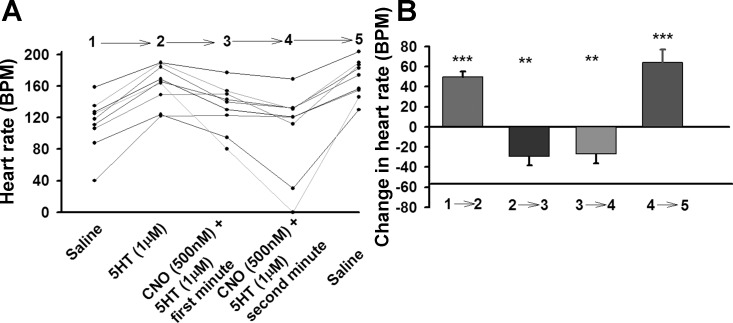
5-HT was applied prior to exposure of CNO in order to see if CNO can also reverse the effects of 5-HT in addition to block, as demonstrated above. 5-HT markedly increases the HR (Fig. 4). The addition of CNO reversed the effects of 5-HT (Fig. 4). When CNO + 5-HT saline was exchanged with saline alone (4→5), the HR increased again, as observed in the experiments shown in Fig. 3, indicating a possible rebound effect from removal of the repressing hM4Di signaling.
Fig. 4.
A: Effect of 5-HT application plus hM4Di activation on larval heart rate (UAS-M4D X 24B-GFP); n = 9. B: The difference in heart rate before and after change of solution. P ≤ 0.02 (**), P ≤ 0.001 (***).
CNO blocks the effects of forskolin.
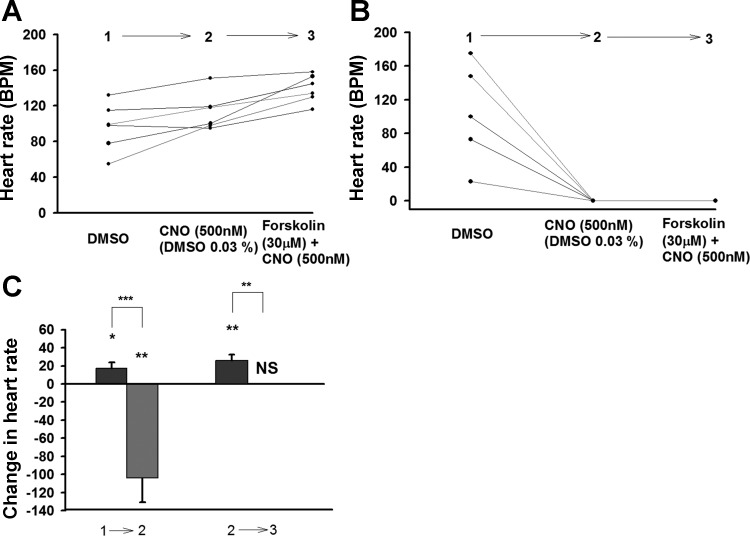
In order to know more about the effect of hM4Di expression on cardiac muscle fiber second messengers, forskolin, an AC activator was examined. In the presence of CNO forskolin increased the HR in background larvae (Fig. 5A). The presence of CNO blocked the positive chronotropic effect of forskolin in hM4Di-expressing larvae. Furthermore, not only was the HR completely arrested by CNO application, but forskolin was unable to overcome this blockade (Fig. 5, B and C).
Fig. 5.
Effect of hM4Di activation plus forskolin application on larval heart rate. A: Background strain UAS-M4D and heart rate with solution changing; n = 6. B: Change of heart rate in UAS-M4D X 24B-GFP, n = 5, with the same solutions as in A. C: Comparison between background (dark grey) and M4D expressing group on the effect of CNO and forskolin (light grey). The differences between genotypes are represented by NS (not significant) or asterisk(s) above the lines. The differences, which are due to the changing one solution with another one in the same genotypes, are represented by NS or asterisk(s) above the bars. P ≤ 0.05 (*), P ≤ 0.02 (**), P ≤ 0.001 (***).
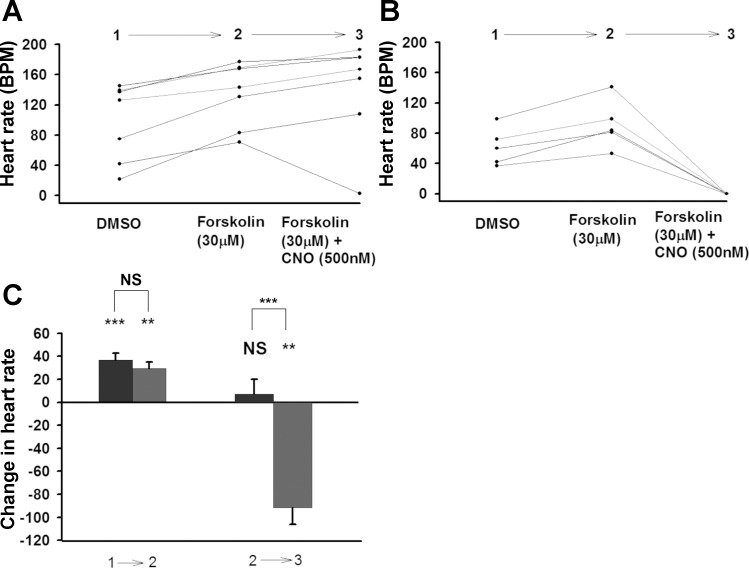
Forskolin (30 μM) alone was observed to increase the HR (Fig. 6). The subsequent addition of CNO had no inhibiting effect in the background larva but dramatically halted the HR in the hM4Di expressing larvae (Fig. 6). Since forskolin was not able to overcome the effects of hM4Di activation this may indicate that cAMP levels alone are not the primary mediator of HR. The effect of forskolin did not show a significant difference between two genotypes (Fig. 6C).
Fig. 6.
Effect of forskolin plus hM4Di activation on larval heart rate. A: Background, UAS-M4D, n = 7. B: UAS-M4D X 24B-GFP, n = 5. C: Comparison between background (dark grey) and M4D expressing group (light grey). The differences between genotypes are represented by the NS (not significant) or asterisk(s) above the lines. The differences, which are due to the changing one solution with another one in the same genotypes, are represented by NS or asterisk(s) above the bars. P ≤ 0.02 (**), P ≤ 0.001 (***).
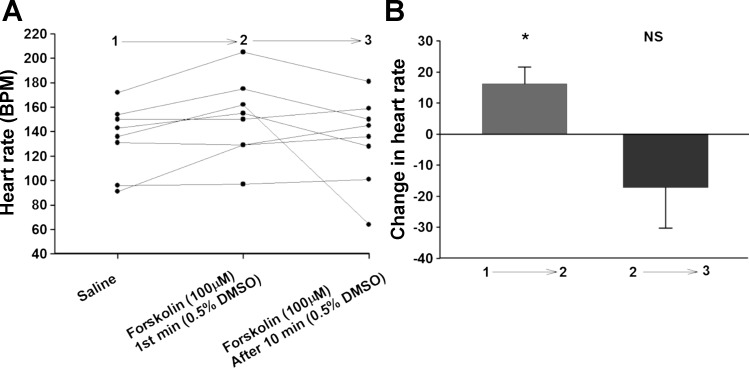
A higher concentration of forskolin (100 μM) was used to determine whether or not the 30 μM concentration was maximal or near maximal and thus producing a saturating effect. We also incubated with the higher concentration of forskolin for 10 min to observe if there was a time-dependent effect present on heart rate for forskolin exposure compared with an acute exposure. We observed that forskolin at 100 μM increased the HR at the beginning; however, it was not greater than that of 30 μM, and no further increase the HR occurred during the 10 min incubation time (Fig. 7, A and B), indicating that our original 30 μM concentration was likely saturating for forskolin.
Fig. 7.
Effect of 100 μM forskolin on larval heart rate. A: Background, UAS-M4D, n = 8, heart rate of individual preparations. B: Background, UAS-M4D, n = 8, shows the change in heart rate after application of forskolin. P ≤ 0.02 (**), P ≤ 0.001 (***).
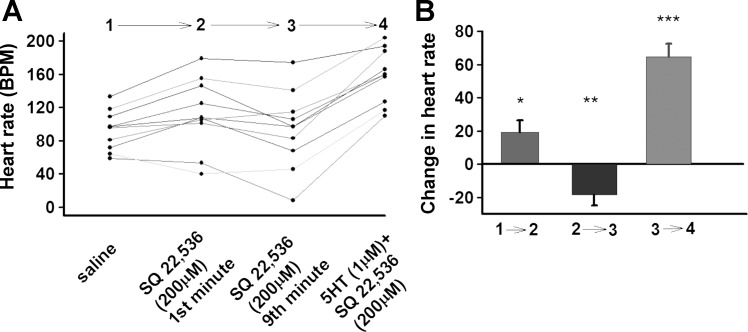
To further probe the role of cAMP, we used the AC inhibitor SQ 22,536 (SQ). Application of SQ had no effect in the first minute (Fig. 8). Because no inhibitory effect of SQ on the HR was observed within the first few minutes, we prolonged the interval to 9 min. At the ninth minute of incubation the HR decreased significantly (P < 0.02), but it did not stop (Fig. 8). This decrement in HR might be due to the lengthy incubation period, rather than the drug. However, it was shown if the semi-intact heart preparation (Canton-S flies) that were incubated inside the saline (pH 7.00) for 10 min the HR do not show a marked change (data not shown). Taken together, these results show that SQ has a slightly inhibitory effect on the HR. When the SQ solution was exchanged with SQ + 5-HT solution, HR significantly increased (Fig. 8). These results indicate that SQ cannot override the positive chronotropic effect of 5-HT. Either the SQ is not effective in blocking Drosophila AC as it is for mammalian AC, or perhaps manipulating cAMP levels alone is not sufficient to mediate an increase in HR, in agreement with our results using forskolin.
Fig. 8.
A and B: Effect of SQ 22,536 and SQ + 5-HT combination on larval heart rate (UAS-M4D X 24B-GFP); n = 10. P ≤ 0.05 (*), P ≤ 0.02 (**), P ≤ 0.001 (***).
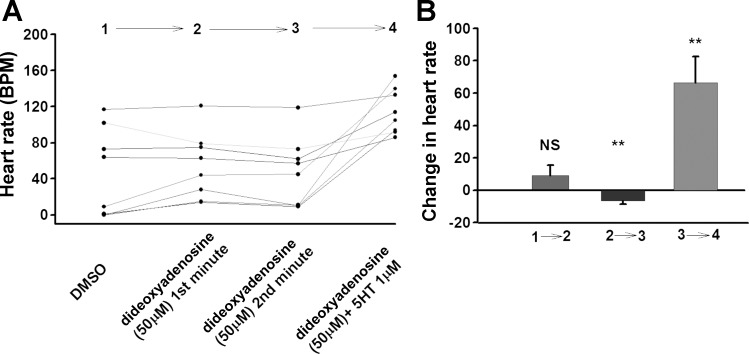
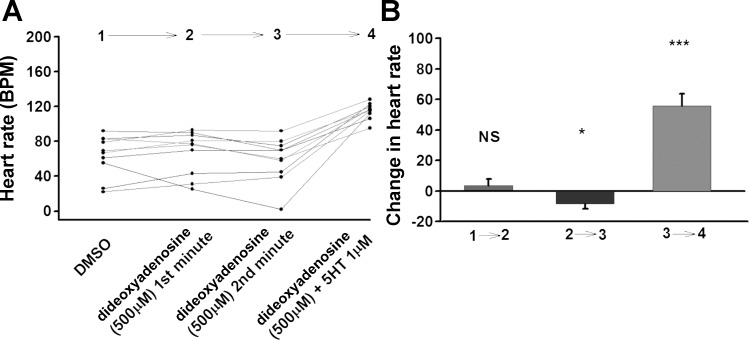
We next tried another AC inhibitor, dideoxyadenosine. Application of dideoxyadenosine (50 and 500 μM) did not produce a noticeable change in HR in hM4Di expressing larvae (Fig. 9 and 10). By the second minute HR showed a very slight decrease in hM4Di expressing larvae. However, when dideoxyadenosine (50 or 500 μM) solution was exchanged with dideoxyadenosine + 5-HT, a marked increase in the HR occurred (Fig. 9 and 10).
Fig. 9.
A: Effect of dideoxyadenosine and dideoxyadenosine + 5-HT combination on larval heart rate (UAS-M4D X 24B-GFP), n = 8. B: The difference in heart rate before and after change of solution. P ≤ 0.02 (**), P ≤ 0.001 (***).
Fig. 10.
A: Effect of a high concentration of dideoxyadenosine and dideoxyadenosine + 5-HT combination on larval heart rate (UAS-M4D X 24B-GFP), n = 10. B: The difference in heart rate before and after change of solution. P ≤ 0.05 (*), P ≤ 0.001 (***).
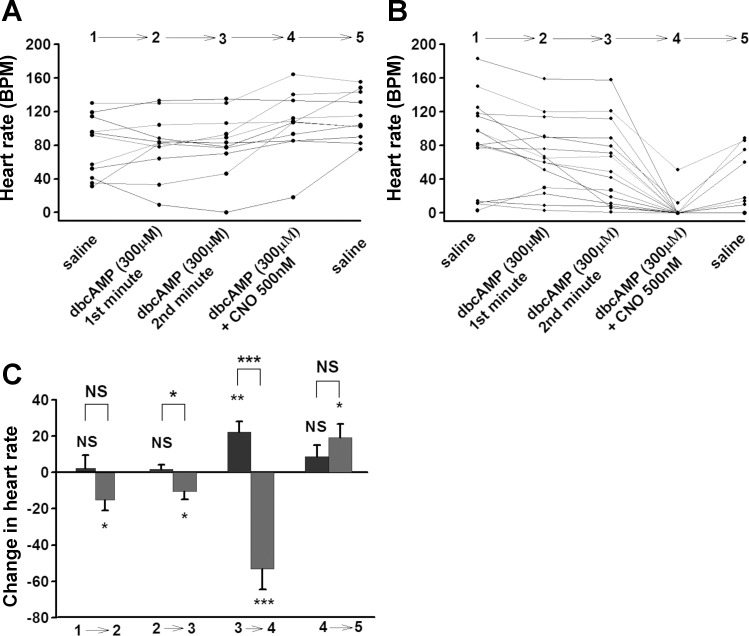
In a different approach, we attempted to manipulate cAMP levels by using the cAMP analog dbcAMP, which both inhibits phosphodiesterase and mimics the effects of cAMP on downstream effectors like PKA. dbcAMP is resistant to cleaving by phosphodiesterase (19). Application of dbcAMP (300 μM) does not change the HR in background larvae (Fig. 11, A and C). Because no effect was observed with the initial exposure of dbcAMP on the HR, the HR was measured for the following second minute. Again, there was no significant change in HR with a longer exposure to dbcAMP (Fig. 11, A and C). When the dbcAMP solution was exchanged with dbcAMP + CNO solution, HR slightly increased in background larvae (Fig. 11, A and C). Application of dbcAMP slightly decreased HR in hM4Di expressing larvae (Fig. 11, B and C). When the dbcAMP solution was exchanged with the dbcAMP + CNO solution, the HR dramatically decreased in the hM4Di expressing larvae (Fig. 11, B and C). dbcAMP did not have a significant difference between two genotypes.
Fig. 11.
Effect of dbcAMP and dbcAMp+CNO combination on larval heart rate. A: Background UAS-M4D, n = 11. B: UAS-M4D X 24B-GFP, n = 15. C: Comparison between background (dark grey) and M4D expressing group (light grey). The differences between genotypes are represented by NS (not significant) or asterisk(s) above the line. The differences, which are due to changing one solution with another one in the same genotypes, are represented by NS or asterisk(s) above the bars. P ≤ 0.05 (*), P ≤ 0.02 (**), P ≤ 0.001 (***).
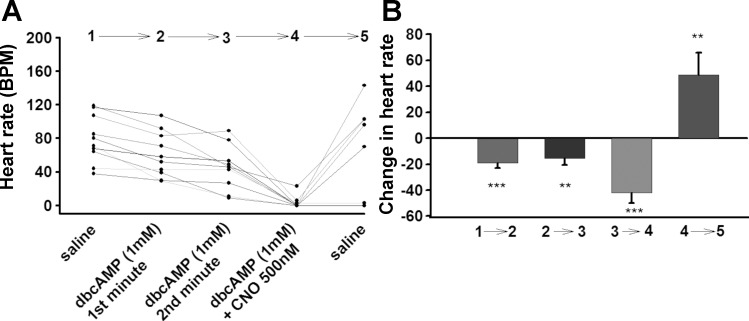
Because there was no significant change after application of 300 μM dbcAMP, a higher concentration of dbcAMP (1 mM) was used. Application of dbcAMP at this higher concentration only slightly decreased the HR in the first and second minute (Fig. 12) to the same degree as with the lower concentration (Fig. 11). However, when the dbcAMP solution was exchanged with dbcAMP + CNO, the HR markedly decreased (Fig. 12). Changing dbcAMP + CNO solution with saline alone showed a marked increase in HR (Fig. 12).
Fig. 12.
Effect of dbcAMP (1 mM) and dbcAMP + CNO combination on larval heart rate. UAS-M4D X 24B-GFP (n = 10). B: The difference in heart rate before and after change of solution.P ≤ 0.05 (*), P ≤ 0.02 (**), P ≤ 0.001 (***).
DISCUSSION
Drosophila has been established to be a good model for the study of cardiac physiology in part because of malleable genetics and conserved development with mammalian heart (33). Moreover, fly and mammalian hearts use many of the similar signaling pathways during development (39). In mammals, 5-HT receptor dysfunction or overactivation leads to abnormal cardiac development and physiological disturbance (23, 28). Clearly, 5-HT and proper activation of relevant 5-HT receptors is critical for normal cardiac development and function in mammals (5). 5-HT receptors are all G-protein coupled receptors (GPCRs), with the exception of the 5-HT3 receptor that is a ligand gated ion channel (31). Modified DREADD GPCR receptors have been shown to be excellent tools to further understand the function of specific GPCRs, like serotonin receptors, and their signaling pathways in normal physiological processes in both fly and mammals (4, 29). For example, the hM4D1 DRAEDD receptor we have used here has also been used to understand the role of serotonin in respiratory control (34) and to determine key structural components of the native muscarinic M4 acetylcholine receptor itself (27). These pharamcogenetic approaches have opened up new avenues to further understand the roles of GPCR receptors in physiology and are promising tools to obtain additional insight into underlying molecular mechanisms of human diseases.
Here, we initiated studies to elucidate effector pathways underlying HR regulation in the Drosophila heart and to further explore the positive chronotropic role of serotonin on the Drosophila larva heart. Our original hypothesis was that Drosophila larva HR, like mammalian HR, was governed by adenylyl cyclase activity and its downstream effectors and second messengers. Further, that the positive chronotropic effect of 5-HT was due to positive modulation of adenylyl cyclase activity. To address this hypothesis, we used both pharmacological and pharmocogenetic approaches using a combination of drugs and the modified mammalian G-protein coupled receptor (hM4Di, coupled to Gαi) to provide an insight into potential signaling pathways underlying HR and the effects of 5-HT in Drosophila larval heart. We initially observed that activation of hM4Di can slow or stop the heart. Moreover, that hM4Di activation can override the positive chronotropic effect of 5-HT. These observations were consistent with our hypothesis, with the prediction that hM4Di activation would activate Gαi, reduce adenylyl cyclase activity, and slow the heart.
It has been shown, however, that forskolin does not noticeably change the HR in P1 pupal stage of Drosophila (21). We extended the investigation in larvae and showed, in general, manipulation of adenylyl cyclase activity and cAMP levels with the dbcAMP, SQ 22,536 and dideoxyadenosine did not have a noticeable impact on the HR. In rodents adenosine analogs can retard the positive chronotropic effect of norepinephrine (35). There was a positive effect of forskolin; however, the effect was only slight. Also, dbcAMP slightly decreased the HR. This may be due to activation of targets by cAMP that could bring about ion channel activation, such as K+ channels. This might result in depolarization of the pacemaker cell membrane potential. Further, adding dbcAMP is not the same as stimulating AC as there are various isoforms of AC that might be differentially activated by forskolin whereas addition of dbcAMP maintains a high level of cAMP, bypassing activation of AC.
Our findings indicate that cAMP signaling does not contribute to regulation of HR in Drosophila larvae as it does in mammals, and that cAMP is not a component of the positive chronotropic effects of 5-HT in the fly heart. Supporting this conclusion is that 5-HT is still able to produce a large increase in HR in the presence of AC inhibitors. As an additional tool to probe mechanisms underlying HR, we employed the designer receptor hM4Di, which is positively coupled to Gαi signaling in both mammals and the fly, as well as the G-coupled inwardly rectifying potassium channel (GIRK) to produce silencing. For example, CNO activation of hM4Di in mammalian systems results in the hyperpolarization and electrical silencing of hippocampal neurons in culture (1). We previously observed that CNO significantly slowed or stopped the heart when applied to Drosophila hearts expressing hM4Di receptors (4) and observe the same effect here. Significantly, pharmacological manipulation of AC or cAMP levels in the presence of activated hM4Di had no effect on the negative chronotropic effects of activated hM4Di. Forskolin was unable to override the repressing effects of CNO. Together, the responses likely indicate that the main effects of hM4Di activation on HR are probably mediated through Gβγ activation of GIRK channels with subsequent hyperpolarization and silencing of cardiomyocytes rather than through negative modulation of cAMP levels through Gαi signaling.
When we applied 5-HT to the larvae preparations, we observed that 5-HT markedly increased the HR, in agreement with our earlier studies. Our original hypothesis was that 5-HT activates AC, which in turn lead to the activation of PKA and phosphorylation of Ca2+ channels that lead to inward Ca2+ currents and contraction. If this were correct, then the positive chronotropic effects of 5-HT action could be blocked by decreasing AC activity or levels of cAMP. Since our multiple attempts at manipulation of AC and cAMP levels had no or little effect on HR indicate that HR in the fly is in fact not substantially mediated by cAMP. The inhibitory effect of activation of hM4Di we observed is therefore likely mediated through Gβγ activation of GIRK channels and silencing of cardiomyocytes, rather than inhibition of AC through Gαi to decrease cAMP levels and PKA activity.
Toward understanding the mechanism of action of serotonin on the heart, it needs to be determined if there may be different 5-HT receptors on heart muscle fibers acting through multiple signaling mechanisms. The known fly 5-HT receptors are all G-protein coupled receptors: 5-HT1ADro and 1BDro inhibit adenylyl cyclase through activation of Gαi; 5-HT7Dro activates adenylyl cyclase through Gαs activation; 5-HT2Dro signaling pathway has not been identified yet (6, 32, 36). A remaining possibility for the positive chronotropic effect of 5-HT could be that 5-HT2Dro receptors are mediating HR. 5-HT2 receptors couple with Gαq and activation of phospholipase C (PLC). PLC cleaves PIP2 to IP3 and diacylglycerol (DAG). IP3 leads to increases in intracellular Ca2+ (16), which could lead to contraction. However, we previously demonstrated that mutation or misexpression of the 5-HT2Dro receptor does not have a dramatic effect on Drosophila larval HR (12). A second 5-HT2Dro receptor has recently been reported (17), and it may be this receptor mediating the effects if Gαq pathways are involved. Recently, a pharmacological study has shown that activation of 5-HT2 receptor by 5-HT2 agonist increases HR and ketanserin, which is 5-HT2 antagonist, markedly decreases the action of 5-HT on HR in Drosophila larvae (Majeed ZR, Stacy A, Cooper RL, unpublished observations). Therefore, we speculate that a PLC pathway might mediate the positive chronotropic effect of 5-HT. However, to confirm this suspicion, various pharmacological agents should be used to manipulate the PLC pathway to observe how it effects 5-HT action related to HR. Another possibility is that 5-HT1ADro, 5-HT1BDro, and 5-HT7Dro receptors are mediating HR through coupling to other effector pathways than adenylyl cyclase. GPCRs couple to a wide range of effectors, and certain drugs and natural ligands can activate these pathways differentially through functional selectivity (38). In this scenario the 5-HT1ADro or 5-HT7Dro receptors could mediate HR through noncanonical pathways yet to be determined. In summary, we present evidence here that the positive chronotropic effect of 5-HT in the Drosophila larva HR is not governed by the adenylyl cyclase signaling pathway, and that the negative chronotropic effects of hM4Di activation are likely due to electrical silencing rather than negative modulation of AC.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.R.M. and R.L.C. performed experiments; Z.R.M. analyzed data; Z.R.M., C.D.N., and R.L.C. interpreted results of experiments; Z.R.M. prepared figures; Z.R.M., C.D.N., and R.L.C. drafted manuscript; Z.R.M., C.D.N., and R.L.C. edited and revised manuscript; Z.R.M., C.D.N., and R.L.C. approved final version of manuscript; C.D.N. and R.L.C. conception and design of research.
GRANTS
This work was funded by National Institute of Mental Health Grant R01MH083689 (C.D.N), Higher Committee for Education Development (HCED) in Iraq (Z.R.M), and personal funds (R.L.C).
REFERENCES
- 1.Armburster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104: 5163–5168, 2007. 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azmitia EC. Serotonin and brain: Evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol 77: 31–56, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Becnel J, Johnson O, Luo J, Nässel DR, Nichols CD. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS One 6(6): e20800, 2011. 10.1371/journal.pone.0020800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becnel J, Johnson O, Majeed ZR, Tran V, Yu B, Roth BL, Cooper RL, Nichols CD. A pharmocogenetic approach for controlling behavior, neuronal signaling, and physiology in Drosophila. Cell Reports 4(5): 1049–1059, 2013. 10.1016/j.celrep.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 60: 355–366, 2009. 10.1146/annurev.med.60.042307.110802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: Lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol 48(1): 13–38, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bodmer R, Venkatesh TV. Heart development in Drosophila and vertebrates: Conservation of molecular mechanisms. Dev Genet 22(3): 181–186, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118(2): 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Cammarato A, Ahrens CH, Alayari NN, Qeli E, Rucker J, Reedy MC, Zmasek CM, Gucek M, Cole RN, Van Eyk JE, Bodmer R, O'Rourke B, Bernstein SI, Foster DB. A mighty small heart: The cardiac proteome of adult Drosophila melanogaster. PLoS One 25, 6(4): e18497, 2011. 10.1371/journal.pone.0018497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choma MA, Suter MJ, Vakoc BJ, Bouma BE, Tearney GJ. Physiological homology between Drosophila melanogaster and vertebrate cardiovascular systems. Dis Model Mech 4(3): 411–420, 2011. 10.1242/dmm.005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasari S, Cooper RL. Direct influence of serotonin on the larval heart of Drosophila melanogaster. J Comp Physiol B 176(4): 349–57, 2006. 10.1007/s00360-005-0058-3 [DOI] [PubMed] [Google Scholar]
- 12.Dasari S, Wang L, Harrison DA, Cooper RL. Reduced and misexpression of 5-HT2 receptors alters development, behavior and CNS activity in Drosophila melanogaster. Int J Zool Res 5(3): 101–114, 2009. 10.3923/ijzr.2009.101.114 [DOI] [Google Scholar]
- 13.Dasari S, Cooper RL. Modulation of sensory-CNS-motor circuits by serotonin, octopamine, and dopamine in semi-intact Drosophila larva. Neurosci Res 48(2): 221–227, 2004. 10.1016/j.neures.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 14.deCastro C, Titlow J, Majeed ZR, Cooper RL. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J Comp Physiol, in press [DOI] [PubMed] [Google Scholar]
- 15.Desai-Shah M, Papoy AR, Ward M, Cooper RL. Roles of the Sarcoplasmic/Endoplasmic reticulum Ca2+-ATPase, plasma membrane Ca2+-ATPase and Na+/Ca2+ exchanger in regulation of heart rate in larval Drosophila. Open Physiol J 3: 16–36, 2010 [Google Scholar]
- 16.Fukami K, Inanobe S, Kanemaru K, Nakamura Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res 49(4): 429–437, 2010. 10.1016/j.plipres.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 17.Gasque G, Conway S, Huang J, Rao Y, Vosshall LB. Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci Rep 3: srep02120, 2013. 10.1038/srep02120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu GG, Singh S. Pharmacological analysis of heartbeat in Drosophila. J Neurobiol 28(3): 269–280, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Henion WF, Sutherland EW, Posternak T. Effects of derivatives of adenosine 3′,5′-phosphate on liver slices and intact animals. Biochim Biophys Acta 148(1): 106–113, 1967 [DOI] [PubMed] [Google Scholar]
- 20.Johnson E, Ringo J, Dowse H. Modulation of Drosophila heartbeat by neurotransmitters. J Comp Physiol B 167: 89–97, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Johnson E, Sherry T, Ringo J, Dowse H. Modulation of the cardiac pacemaker of Drosophila: cellular mechanisms. J Comp Physiol B 172(3): 227–36, 2002. 10.1007/s00360-001-0246-8 [DOI] [PubMed] [Google Scholar]
- 22.Johnson O, Becnel J, Nichols CD. Serotonin 5-HT1A-like, 5-HT2, and 5-HT7 receptors modulate learning and memory in Drosophila. FASEB J) 586.11, 2009 [Google Scholar]
- 23.Lairez O, Cognet T, Schaak S, Calise D, Guilbeau-Frugier C, Parini A, Mialet-Perez J. Role of serotonin 5-HT2A receptors in the development of cardiac hypertrophy in response to aortic constriction in mice. J Neural Transm 120(6): 927–935, 2013. 10.1007/s00702-013-1011-3 [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Becnel J, Nichols CD, Nässel DR. Insulin-producing cells in the brain of adult Drosophila are regulated by the serotonin 5-HT1A receptor. Cell Mol Life Sci 69(3): 471–484, 2012. 10.1007/s00018-011-0789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Bradu A, Podoleanu AG, Bloor JW. Arrhythmia caused by a Drosophila tropomyosin mutation is revealed using a novel optical coherence tomography instrument. PLoS One 5(12): e14348, 2010. 10.1371/journal.pone.0014348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nawaratne V, Leach K, Suratman N, Loiacono RE, Felder CC, Armbruster BN, Roth BL, Sexton PM, Christopoulos A. New insights into the function of M4 muscarinic acetylcholine receptors gained using a novel allosteric modulator and a DREADD (designer receptor exclusively activated by a designer drug). Mol Pharmacol 74(4): 1119–1131, 2008. 10.1124/mol.108.049353 [DOI] [PubMed] [Google Scholar]
- 28.Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci U S A 97(17): 9508–9513, 2000. 10.1073/pnas.97.17.9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols CD, Roth BL. Engineered G-protein coupled receptors are powerful tools to investigate biological processes and behaviors. Front Mol Neurosci 2: 16, 2009. 10.3389/neuro.02.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther 112(3): 677–700, 2006. 10.1016/j.pharmthera.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 31.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev 108(5): 1614–1641, 2008. 10.1021/cr078224o [DOI] [PubMed] [Google Scholar]
- 32.Obosi LA, Schuette DG, Europe-Finner GN, Beadle DJ, Hen R, King LA, Bermudez I. Functional characterisation of the Drosophila 5-HTdro1 and 5-HTdro2B serotonin receptors in insect cells: Activation of a G(alpha) s-like protein by 5-HTdro1 but lack of coupling to inhibitory G-proteins by 5-HTdro2B. FEBS Lett 381(3): 233–236, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev 63(2): 411–436 , 2011. 10.1124/pr.110.003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333(6042): 637–642, 2011. 10.1126/science.1205295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samet MK, Rutledge CO. Antagonism of the positive chronotropic effect of norepinephrine by purine nucleosides in rat atria. J Pharmacol Exp Ther 232(1): 106–110, 1985 [PubMed] [Google Scholar]
- 36.Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signaling properties and expression patterns. EMBO J 11(1): 7–17, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Titlow JS, Rufer JM, King KE, Cooper RL. Pharmacological analysis of dopamine modulation in the Drosophila melanogaster larval heart. Physiol Rep 1(2): 1–9, 2013. 10.1002/phy2.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320(1): 1–13, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Vogler G, Bodmer R, Akasaka T. Drosophila model of congenital heart diseases, congenital heart disease-selected aspects, P. Syamasundar Rao (Ed.). Available from: http://www.intechopen.com/books/congenital-heart-disease-selected-aspects/drosophila-model-of-congenital-heart-diseases, 2012. 10.5772/26739 [DOI] [Google Scholar]
- 40.Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, Hen R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci U S A 87(22): 8940–8944, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zornick E, Paisley K, Nichols R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptides 20(1): 45–51, 1999. 10.1016/S0196-9781(98)00151-X [DOI] [PubMed] [Google Scholar]