Abstract
Background
Clinical outcomes of the Women's Health Initiative (WHI) calcium plus vitamin D supplementation trial have been reported during 7.0 years of active intervention. We now report outcomes 4.9 years after the intervention stopped and cumulative findings.
Methods
Postmenopausal women (N=36,282) were randomized; postintervention follow-up continued among 29,862 (86%) of surviving participants. Primary outcomes were hip fracture and colorectal cancer. Breast cancer, all cancers, cardiovascular disease (CVD), and total mortality were predetermined major study outcomes.
Results
Hip fracture incidence was comparable in the supplement and the placebo groups, postintervention hazard ratio (HR)=0.95, 95% confidence interval (95% CI: 0.78, 1.15) and overall HR=0.91 (95% CI: 0.79, 1.05). Overall, colorectal cancer incidence did not differ between randomization groups, HR=0.95 (95% CI: 0.80, 1.13). Throughout, there also was no difference in invasive breast cancer, CVD, and all-cause mortality between groups. In subgroup analyses, the invasive breast cancer effect varied by baseline vitamin D intake (p=0.03 for interaction). Women with vitamin D intakes >600 IU/d, had an increased risk of invasive breast cancer, HR=1.28 (95% CI; 1.03, 1.60). Over the entire study period, in post hoc analyses, the incidence of vertebral fractures, HR=0.87 (95% CI: 0.76, 0.98) and in situ breast cancers, HR=0.82 (95% CI: 0.68, 0.99) were lower among women randomized to supplementation.
Conclusion
After an average of 11 years, calcium and vitamin D supplementation did not decrease hip fracture or colorectal cancer incidence. Exploratory analyses found lower vertebral fracture and in situ breast cancer incidence in the supplement users. There was no effect on CVD or all-cause mortality.
Introduction
The Women's Health Initiative (WHI) calcium plus vitamin D trial assessed whether 1000 mg of elemental calcium as calcium carbonate1 with 400 IU of vitamin D3 reduced the risk of hip fracture and colorectal cancer in 36,282 postmenopausal women.2,3 During an intervention that averaged 7.0 years, hip fractures rates were similar between supplement use and placebo; among women adherent to study medication, there was a 29% decrease in hip fracture for supplement users compared to the placebo group.2 No significant differences were seen for colorectal cancer,3 in situ or invasive breast cancer,4 cardiovascular disease (CVD),5 overall cancer incidence,6 or all-cause mortality.7
Previous trial reports included only outcomes during the intervention phase. Herein, we report postintervention outcomes through an additional 4.9 years (mean) follow-up for a total follow-up of 11.1 years. These preplanned analyses assessed the long-term effects of calcium plus vitamin D supplementation on health outcomes using intent to treat analysis. We also examined whether effects changed postintervention or differed by subgroups defined by adherence, age, total baseline vitamin D intake, and use of calcium supplements at trial entry.
Methods
Intervention phase
The trial details have been reported.1–3 Briefly, postmenopausal women enrolled in the WHI dietary modification trial, hormone therapy trials, or both were invited to join the calcium plus vitamin supplementation D trial. Eligible women were 50 to 79 years old with anticipated 3-year survival. Personal supplemental calcium (up to 1000 mg/d) and vitamin D (up to 600 IU/d and subsequently raised to 1000 IU) were allowed.8,9 The study protocol was approved by institutional review boards at the participating institutions and all participants provided written informed consent.
A total of 36,282 participants were randomly assigned to daily calcium (1000 mg of calcium carbonate) plus vitamin D (400 IU D3) or matching placebo tablets (provided by GlaxoSmithKline). “Baseline” in this report refers to the baseline at the intervention phase. When the intervention phase ended as scheduled on March 30, 2005, vital status was known for 93% of women, of whom 4.6% were deceased. At that time, 76% were still taking study supplementation and 59% took 80% or more of it with little difference between groups.3 Women were told of their treatment assignment at study closeout. The postintervention phase began on the closeout date. The current report reflects findings through September 30, 2010. After intervention ended, subsequent follow-up required reconsent which was obtained for 86% of surviving participants with no differences in participation in the follow-up by randomized group. We collected information on calcium and vitamin D supplementation at the end of the extension period.
Clinical outcomes
Outcomes were ascertained semi-annually during the intervention and annually, postintervention. If a woman reported an event, we obtained permission from her to obtain her medical records. Medical records from each event were obtained to centrally adjudicate outcomes. Primary outcomes of the trial included hip fracture and colorectal cancer. Invasive breast cancer, all cancers, coronary heart disease (CHD) including myocardial infarction (MI), stroke, total CVD, and total mortality were prespecified as secondary outcomes. A global index of outcomes was created as a summary measure of the overall risks and benefits and included hip fracture, invasive breast and colorectal cancers and death. Vertebral fractures and in situ breast cancer were exploratory post hoc analyses. Nonhip fractures were adjudicated during the intervention phase and based on self-report thereafter. In WHI, 76% of self-reported fractures were confirmed by radiographic report.10
Other measurements
Demographic characteristics and medical history were collected using standardized questionnaires. Calcium intake before randomization was defined as the dietary calcium intake (assessed with a modified Block food-frequency questionnaire),11 the intake of calcium from supplements in the previous 2 weeks, and the intake of calcium from prescription medications (assessed though an interviewer-administered medication survey). Total vitamin D intake was similarly determined. Mammogram reports were obtained, reviewed, and coded for radiologist recommendation.
Statistical analyses
Baseline characteristics for women who reconsented were compared with women who did not reconsent and by randomization group using chi-square tests of association. Annualized rates of clinical events were estimated for the intervention phase, the postintervention phase, and overall by dividing the number of events by the corresponding person-time in each phase.
The primary analyses included all randomized participants using time to event methods based on the intention to treat principle.12 Hazard ratios (HRs) were estimated using Cox proportional hazards models13 stratified by age, prior disease (if appropriate), and randomization status in the WHI dietary modification14 and the hormone therapy trials.15,16 Models were constructed for each clinical outcome in which women contributed follow-up time until the end of the interval, the date of their first relevant clinical event, or the date of death or withdrawal from the study (whichever came first). Formal tests of differences between the HRs in the intervention and postintervention phase were calculated by inclusion of a binary term for trial phase as a time dependent variable as described.12 All statistical tests were two-sided.
Nominal p-values are reported without adjustment for multiple outcomes or sequential looks. Age, total baseline calcium and vitamin D intake, baseline calcium supplement use (yes/no), and hormone trial stratified analyses were tested for 14 outcomes. At the 0.05 level of significance, approximately four interaction p-values could be statistically significant based on chance alone. To determine whether reconsent influenced risk estimates, inverse-probability weighting analyses were conducted.17 Adherence sensitivity analyses were conducted by censoring follow-up 6 months after nonadherence (ingestion of <80% of study pills). For these analyses, participants who provided additional consent or were adherent were included in analyses that used the inverse of the participant's estimated reconsent or adherence probability as a weighting factor.
Based on differential supplement influence on invasive and in situ breast cancer, analyses examining mammogram diagnostic performance were conducted separately for invasive and in situ breast cancer. The sensitivity, specificity, and positive and negative predictive values for serial mammograms were evaluated as previously described.18 All statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, NC) and S-Plus software version 8.0 (TIBCO software, Inc, Sommerville, MA).
Results
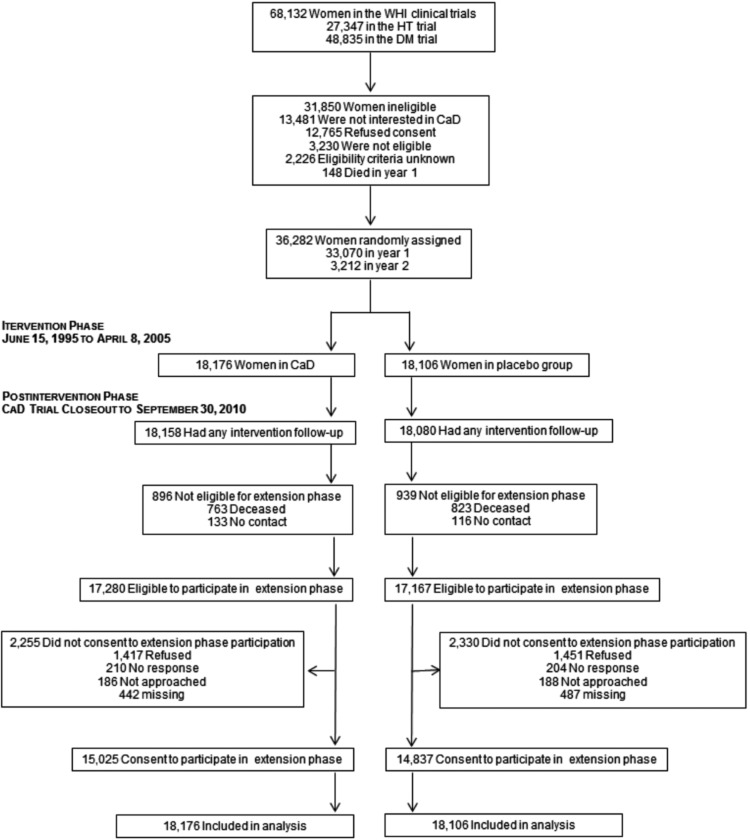
Participant movement through the study is outlined in Appendix Figure A1. The results include findings from all 36,282 randomized participants through the intervention phase and additional findings from the 29,862 women who reconsented. The latter were younger, more likely to be white, slightly less likely to have a history of stroke or MI or to be obese, reported higher education and higher use of calcium and/or vitamin D supplementation compared to women who did not re-consent (Appendix Table A1). There was no significant difference in the percentage of women who joined the extension study by randomized group. Of importance, the baseline characteristics of participants in the postintervention phase were similar by randomization assignment (Table 1).
Table 1.
Descriptive Characteristics of Women's Health Initiative Calcium Plus Vitamin D Trial Extension Study Participants at Baseline by Randomization Assignment
| |
CaD |
Placebo |
|
||
|---|---|---|---|---|---|
| |
(N=15,025) |
(N=14,837) |
|
||
| N | % | N | % | p-value | |
| Age group at screening, years | 0.80 | ||||
| 50–59 | 5729 | 38.1 | 5602 | 37.8 | |
| 60–69 | 6924 | 46.1 | 6883 | 46.4 | |
| 70–79 | 2372 | 15.8 | 2352 | 15.9 | |
| Race/ethnicity | 0.37 | ||||
| White | 12,694 | 84.5 | 12,648 | 85.2 | |
| Black | 1278 | 8.5 | 1222 | 8.2 | |
| Hispanic | 546 | 3.6 | 480 | 3.2 | |
| American Indian | 55 | 0.4 | 51 | 0.3 | |
| Asian/Pacific Islander | 295 | 2.0 | 274 | 1.8 | |
| Unknown | 157 | 1.0 | 162 | 1.1 | |
| Education | 0.53 | ||||
| None to some high school | 677 | 4.5 | 632 | 4.3 | |
| High school diploma or equivalent | 2715 | 18.2 | 2723 | 18.5 | |
| School after high school | 5901 | 39.5 | 5755 | 39.0 | |
| College degree or higher | 5640 | 37.8 | 5636 | 38.2 | |
| Gail 5-year risk of breast cancer | 0.84 | ||||
| <1.25 | 5176 | 34.4 | 5065 | 34.1 | |
| 1.25–1.74 | 4993 | 33.2 | 4965 | 33.5 | |
| ≥1.75 | 4856 | 32.3 | 4807 | 32.4 | |
| Medical history | |||||
| Kidney stones | 133 | 1.0 | 136 | 1.0 | 0.78 |
| Stroke | 111 | 0.7 | 128 | 0.9 | 0.23 |
| Myocardial infarction | 237 | 1.6 | 212 | 1.4 | 0.29 |
| Fracture | 5242 | 38.4 | 5120 | 38.1 | 0.56 |
| Prior hormone usea | 0.48 | ||||
| None | 4688 | 31.2 | 4589 | 30.9 | |
| Past | 2456 | 16.3 | 2346 | 15.8 | |
| Current estrogen-alone | 3768 | 25.1 | 3764 | 25.4 | |
| Current estrogen+progestin | 4113 | 27.4 | 4138 | 27.9 | |
| Body mass index, kg/m2 | 0.21 | ||||
| <25 | 3992 | 26.7 | 4038 | 27.4 | |
| 25–<30 | 5411 | 36.2 | 5385 | 36.5 | |
| ≥30 | 5545 | 37.1 | 5335 | 36.1 | |
| Physical activity, metabolic equivalents (MET), h/wk | 0.78 | ||||
| None | 2548 | 18.6 | 2491 | 18.4 | |
| 0.5–4.125 | 2848 | 20.7 | 2797 | 20.7 | |
| >4.125–9.5 | 2763 | 20.1 | 2709 | 20.0 | |
| >9.5–17.75 | 2823 | 20.6 | 2734 | 20.2 | |
| ≥17.75 | 2746 | 20.0 | 2788 | 20.6 | |
| Alcohol intake | 0.97 | ||||
| Nondrinker | 1502 | 10.1 | 1486 | 10.1 | |
| Past drinker | 2467 | 16.5 | 2486 | 16.9 | |
| <1 drink per month | 2086 | 14.0 | 2055 | 13.9 | |
| <1 drink per week | 3218 | 21.6 | 3143 | 21.3 | |
| 1–<7 drinks per week | 4016 | 26.9 | 3981 | 27.0 | |
| ≥7 drinks per week | 1625 | 10.9 | 1587 | 10.8 | |
| Smoking | 0.14 | ||||
| Never | 7774 | 52.2 | 7841 | 53.4 | |
| Past | 6045 | 40.6 | 5826 | 39.7 | |
| Current | 1060 | 7.1 | 1019 | 6.9 | |
| Total vitamin D (supplements+diet), IU/db | 0.12 | ||||
| <200 | 5538 | 37.5 | 5348 | 36.7 | |
| 200–<400 | 2765 | 18.7 | 2830 | 19.4 | |
| 400–<600 | 3498 | 23.7 | 3567 | 24.4 | |
| ≥600 | 2955 | 20.0 | 2845 | 19.5 | |
| Vitamin D supplement useb | 7238 | 48.2 | 7188 | 48.4 | 0.64 |
| Geographic region at randomization by latitude | 0.98 | ||||
| Southern, <35°N | 4187 | 27.9 | 4123 | 27.8 | |
| Middle, 35–40°N | 4266 | 28.4 | 4227 | 28.5 | |
| Northern, ≥40°N | 6572 | 43.7 | 6487 | 43.7 | |
| Total calcium intake (supplements+diet+medications), mg/db | 0.51 | ||||
| <800 | 4852 | 32.9 | 4728 | 32.4 | |
| 800–<1200 | 3904 | 26.5 | 3834 | 26.3 | |
| ≥1200 | 6000 | 40.7 | 6028 | 41.3 | |
| Calcium and/or vitamin D supplement useb | 8678 | 57.8 | 8662 | 58.4 | 0.27 |
| Hormone therapy trial participant | 6496 | 43.2 | 6420 | 43.3 | 0.95 |
| Diet modification trial participant | 10,543 | 70.2 | 10,467 | 70.5 | 0.48 |
Values incorporate hormone therapy use during year 1 of the clinical trial, including exposure to the hormone therapy trials.
Vitamin D and calcium variables incorporate intake reported at baseline of the Calcium/D trial.
Comparison of intervention and postintervention findings
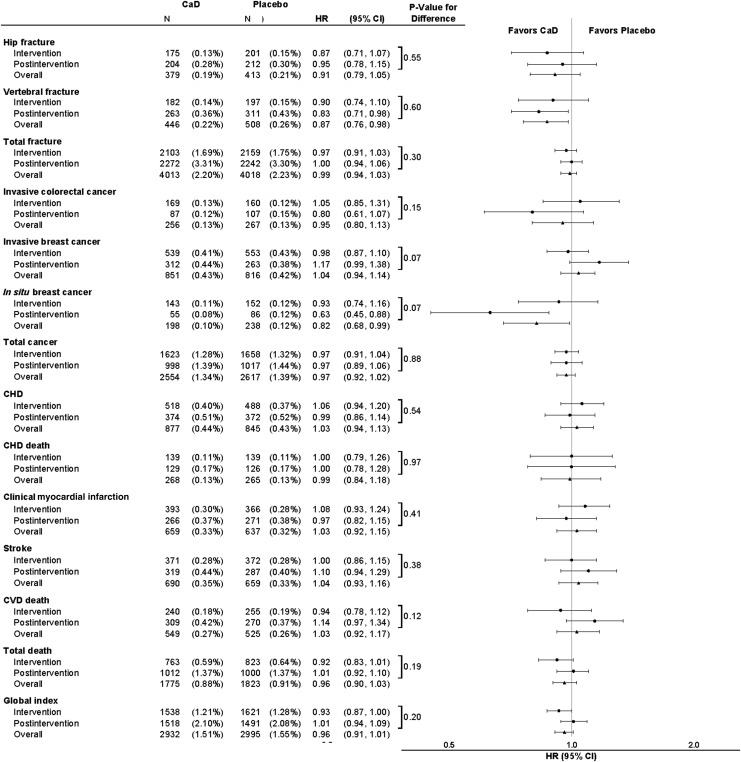
Incident clinical events by randomization assignment and corresponding HRs for the intervention, postintervention, and overall follow-up periods are summarized in Figures 1 and 2. Postintervention hip fractures were similar in the two randomized groups, HR=0.95 (95% CI: 0.78, 1.15). Vertebral fractures were 17% lower, HR=0.83 (95% CI: 0.71, 0.98) in the supplement group compared to placebo. Over the entire period, calcium and vitamin D supplementation significantly reduced clinical vertebral fractures but did not influence hip or other fractures.
FIG. 1.
Incident clinical events by randomization assignment and corresponding hazard ratios (HRs) for the intervention, postintervention, and overall follow-up period. The HR and 95% confidence interval (CI) for intervention period events are derived from a proportional hazards model stratified on age, prevalent condition (where appropriate), and randomization arm in the hormone therapy (HT) and diet modification (DM) trials, where time to event equals 0 on date of randomization. The HR and CI for postintervention period events are derived from a Cox proportional hazards model stratified on age, prevalent condition (where appropriate), and randomization arm in the HT and DM trials, where time to event equals 0 on calcium and vitamin D (CaD) trial close-out date. The HR and CI for the overall combined period events are derived from a proportional hazards model stratified by prevalent condition (where appropriate), age, HT and DM randomization arm, and trial phase (time-dependent), where time to event equals 0 on date of randomization. P-values for the difference between the intervention and postintervention periods are derived from a Cox proportional hazards models stratified by prevalent condition (where appropriate), age, HT and DM randomization arm, and trial phase (time-dependent), where time to event equals 0 on date of randomization. The p-value tests whether the HR for the intervention period equals the HR for the postintervention period. All nonhip fractures use adjudicated data during the clinical trial and self-reported data thereafter.
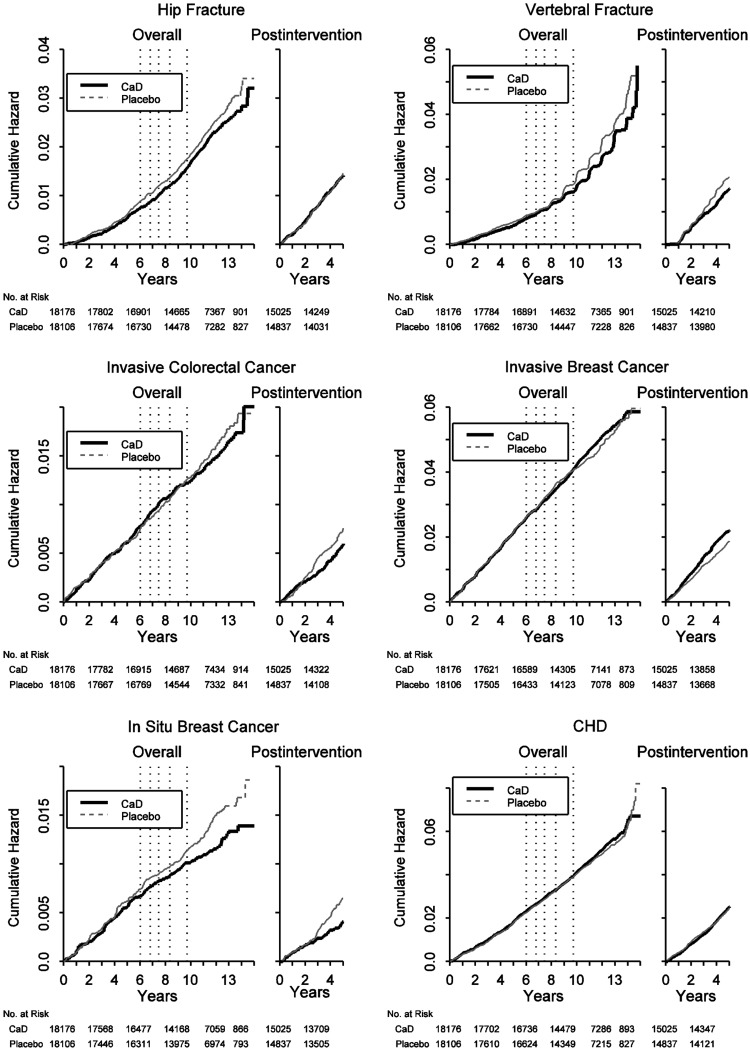
FIG. 2.
Risks and benefits by randomization assignment to calcium plus vitamin D supplementation or placebo before or after termination of the intervention. Kaplan Meier cumulative hazards for clinical outcomes, by time in the trial and time after termination of the intervention. Dotted vertical lines represent quintiles of duration of the intervention in the study population (elapsed time from randomization until the main study close-out). Overall curves include events from randomization to September 30, 2010. Postintervention curves include events from CaD study close-out to September 30, 2010. CHD, coronary heart disease.
The HR for colorectal cancer postintervention was HR=0.80 (95% CI: 0.61, 1.07), but the HR was close to unity overall indicating no overall difference by randomized group. Over the entire follow-up, the HR for invasive breast cancer was close to unity. Postintervention, there was some suggestion that invasive breast cancer incidence was increased in the supplement group HR=1.17 (95% CI: 0.99, 1.38), but results were not significant (p=0.07 comparing HR in the intervention and postintervention periods. In contrast, postintervention, in situ breast cancer incidence was significantly decreased in the supplement (0.08%) compared to the placebo group (0.12%; HR=0.63 [95% CI: 0.45, 0.88], p=0.07 for difference between phases). Overall, the risk of in situ breast cancer was 18% lower in the supplement group (HR=0.82; 95% CI: 0.68, 0.99).
CVD event rates in a comparison of supplement and placebo groups were similar in the intervention and postintervention phases and close to unity overall. The suggestion of lower total mortality in the supplement group during the intervention was not maintained postintervention (HR=1.01; 95% CI: 0.92, 1.10). Overall, cancer deaths (data not shown) and deaths from all causes were similar in the two randomization groups.
Mammography use did not differ in the two randomized groups, and there was no consistent pattern of mammogram performance differences in sensitivity, specificity, or accuracy by randomized group for either invasive or in situ breast cancer (data not shown). The accuracy of the mammograms was >98% in both randomized groups.
Analyses stratified by age
Overall, the effect of supplementation on hip fracture did not differ by age (Table 2). Vertebral fracture risk was about 20% lower in the supplement group at least for women age >60. The risk of in situ breast cancer was consistently lower in women randomized to calcium plus vitamin D irrespective of age. For other outcomes there was little difference in the HR across age groups.
Table 2.
Cumulative Annualized Incidence Rates for Clinical Outcomes in the Women's Health Initiative Calcium and Vitamin D Supplementation Trial According to 10-Year Age Groups at Enrollment
| |
Overall combined phases |
||||||
|---|---|---|---|---|---|---|---|
| |
CaD |
Placebo |
|
|
|
||
| N | % | N | % | HR* | (95% CI)a | Interaction p-valuea | |
| Hip fracture | 0.18 | ||||||
| 50–59 | 55 | 0.07 | 36 | 0.05 | 1.47 | (0.97, 2.24) | |
| 60–69 | 141 | 0.16 | 175 | 0.19 | 0.80 | (0.64, 1.00) | |
| 70–79 | 183 | 0.57 | 202 | 0.64 | 0.90 | (0.73, 1.10) | |
| Vertebral fractureb | 0.33 | ||||||
| 50–59 | 97 | 0.12 | 85 | 0.11 | 1.12 | (0.84, 1.50) | |
| 60–69 | 210 | 0.23 | 249 | 0.28 | 0.83 | (0.69, 1.00) | |
| 70–79 | 139 | 0.43 | 174 | 0.55 | 0.79 | (0.63, 0.99) | |
| Total fractureb | 0.34 | ||||||
| 50–59 | 1284 | 1.79 | 1239 | 1.75 | 1.02 | (0.94, 1.10) | |
| 60–69 | 1841 | 2.24 | 1825 | 2.24 | 1.00 | (0.93, 1.06) | |
| 70–79 | 888 | 3.12 | 954 | 3.42 | 0.92 | (0.84, 1.01) | |
| Invasive colorectal cancer | 0.87 | ||||||
| 50–59 | 58 | 0.07 | 59 | 0.08 | 0.97 | (0.68, 1.40) | |
| 60–69 | 122 | 0.13 | 136 | 0.15 | 0.88 | (0.69, 1.13) | |
| 70–79 | 76 | 0.23 | 72 | 0.22 | 1.06 | (0.77, 1.46) | |
| Invasive breast cancer | 0.57 | ||||||
| 50–59 | 308 | 0.40 | 286 | 0.38 | 1.06 | (0.90, 1.25) | |
| 60–69 | 397 | 0.45 | 388 | 0.44 | 1.02 | (0.89, 1.18) | |
| 70–79 | 146 | 0.46 | 142 | 0.45 | 1.03 | (0.81, 1.29) | |
| In situ breast cancer | 0.82 | ||||||
| 50–59 | 74 | 0.10 | 89 | 0.12 | 0.82 | (0.60, 1.12) | |
| 60–69 | 93 | 0.11 | 110 | 0.13 | 0.85 | (0.64, 1.12) | |
| 70–79 | 31 | 0.10 | 39 | 0.12 | 0.75 | (0.46, 1.20) | |
| Total cancer | 0.13 | ||||||
| 50–59 | 816 | 1.10 | 781 | 1.07 | 1.03 | (0.93, 1.13) | |
| 60–69 | 1233 | 1.44 | 1268 | 1.49 | 0.97 | (0.89, 1.05) | |
| 70–79 | 505 | 1.65 | 568 | 1.88 | 0.88 | (0.78, 0.99) | |
| Coronary heart disease | 0.28 | ||||||
| 50–59 | 155 | 0.20 | 149 | 0.19 | 1.01 | (0.81, 1.27) | |
| 60–69 | 409 | 0.46 | 413 | 0.46 | 0.99 | (0.86, 1.13) | |
| 70–79 | 313 | 0.99 | 283 | 0.90 | 1.10 | (0.94, 1.30) | |
| Stroke | 0.98 | ||||||
| 50–59 | 117 | 0.15 | 106 | 0.14 | 1.08 | (0.83, 1.41) | |
| 60–69 | 318 | 0.35 | 307 | 0.34 | 1.03 | (0.88, 1.21) | |
| 70–79 | 255 | 0.80 | 246 | 0.78 | 1.02 | (0.86, 1.22) | |
| Total death | 0.71 | ||||||
| 50–59 | 302 | 0.39 | 307 | 0.40 | 0.97 | (0.82, 1.13) | |
| 60–69 | 822 | 0.90 | 831 | 0.92 | 0.99 | (0.90, 1.09) | |
| 70–79 | 651 | 1.99 | 685 | 2.12 | 0.93 | (0.84, 1.04) | |
| Global index | 0.48 | ||||||
| 50–59 | 667 | 0.88 | 644 | 0.86 | 0.94 | (0.84, 1.05) | |
| 60–69 | 1343 | 1.53 | 1385 | 1.59 | 0.97 | (0.90, 1.05) | |
| 70–79 | 922 | 2.98 | 966 | 3.16 | 0.95 | (0.87, 1.04) | |
From a proportional hazards model stratified by prevalent condition (where appropriate), age, hormone therapy and diet modification randomization arm, and trial phase (time-dependent). Time to event equals 0 on date of randomization.
All nonhip fractures use adjudicated data during the clinical trial and self-reported data thereafter.
Analyses stratified by vitamin D intake at baseline
In the combined trial phases, the incidence of hip fracture, vertebral fracture, all fractures, and colorectal cancer in women randomized to calcium plus vitamin D or placebo did not differ by total baseline vitamin D intake (Table 3). Results for invasive breast cancer showed a significant interaction between total vitamin D intake and calcium plus vitamin D, suggesting heterogeneity in the effects of calcium and vitamin D supplementation on invasive breast cancer by total vitamin D intake. A higher incidence in invasive breast cancer was observed in women with higher baseline vitamin D intakes (>600 IU/day; HR=1.28; 95% CI: 1.03, 1.60; p=0.03 for interaction). A similar but less pronounced pattern was seen for total cancer (p=0.07 for interaction), but not for in situ breast cancer. There was no significant effect of supplementation on CHD, stroke, total mortality, or the global index by baseline vitamin D intake.
Table 3.
Cumulative Annualized Incidence Rates for Clinical Outcomes in the Women's Health Initiative Calcium and Vitamin D Supplementation Trial According to Baseline Total Vitamin D Intake, IU/Day
| |
Overall combined phases |
||||||
|---|---|---|---|---|---|---|---|
| |
CaD |
Placebo |
|
|
|
||
| N | % | N | % | HRa | (95% CI)a | p-valuea | |
| Hip fracture | 0.97 | ||||||
| <200 | 138 | 0.18 | 140 | 0.19 | 0.93 | (0.74, 1.18) | |
| 200–<400 | 68 | 0.18 | 75 | 0.20 | 0.95 | (0.68, 1.33) | |
| 400–<600 | 80 | 0.17 | 112 | 0.24 | 0.74 | (0.55, 0.98) | |
| ≥600 | 87 | 0.23 | 77 | 0.21 | 1.07 | (0.79, 1.46) | |
| Vertebral fractureb | 0.70 | ||||||
| <200 | 143 | 0.19 | 156 | 0.21 | 0.88 | (0.70, 1.11) | |
| 200–<400 | 79 | 0.21 | 93 | 0.25 | 0.85 | (0.63, 1.15) | |
| 400–<600 | 100 | 0.22 | 127 | 0.27 | 0.78 | (0.60, 1.01) | |
| ≥600 | 116 | 0.31 | 127 | 0.35 | 0.84 | (0.65, 1.08) | |
| Total fractureb | 0.95 | ||||||
| <200 | 1456 | 2.11 | 1391 | 2.09 | 1.01 | (0.93, 1.08) | |
| 200–<400 | 708 | 2.07 | 771 | 2.25 | 0.93 | (0.84, 1.03) | |
| 400–<600 | 966 | 2.32 | 967 | 2.26 | 1.03 | (0.94, 1.13) | |
| ≥600 | 814 | 2.38 | 816 | 2.46 | 0.98 | (0.89, 1.08) | |
| Invasive colorectal cancer | 0.72 | ||||||
| <200 | 91 | 0.12 | 95 | 0.13 | 0.95 | (0.71, 1.27) | |
| 200–<400 | 46 | 0.12 | 60 | 0.16 | 0.79 | (0.54, 1.17) | |
| 400–<600 | 70 | 0.15 | 60 | 0.13 | 1.21 | (0.85, 1.71) | |
| ≥600 | 43 | 0.11 | 48 | 0.13 | 0.84 | (0.56, 1.28) | |
| Invasive breast cancer | 0.03 | ||||||
| <200 | 276 | 0.37 | 300 | 0.42 | 0.89 | (0.76, 1.05) | |
| 200–<400 | 178 | 0.49 | 168 | 0.45 | 1.10 | (0.89, 1.36) | |
| 400–<600 | 203 | 0.45 | 196 | 0.42 | 1.04 | (0.85, 1.27) | |
| ≥600 | 181 | 0.49 | 139 | 0.38 | 1.28 | (1.03, 1.60) | |
| In situ breast cancer | 0.31 | ||||||
| <200 | 73 | 0.10 | 81 | 0.11 | 0.87 | (0.64, 1.20) | |
| 200–<400 | 37 | 0.10 | 45 | 0.12 | 0.83 | (0.54, 1.28) | |
| 400–<600 | 44 | 0.10 | 66 | 0.14 | 0.66 | (0.45, 0.97) | |
| ≥600 | 39 | 0.11 | 40 | 0.11 | 0.91 | (0.58, 1.42) | |
| Total cancer | 0.07 | ||||||
| <200 | 888 | 1.23 | 942 | 1.36 | 0.91 | (0.83, 0.99) | |
| 200–<400 | 503 | 1.42 | 542 | 1.52 | 0.94 | (0.83, 1.06) | |
| 400–<600 | 588 | 1.34 | 611 | 1.36 | 0.98 | (0.88, 1.10) | |
| ≥600 | 529 | 1.49 | 472 | 1.34 | 1.11 | (0.98, 1.26) | |
| Coronary heart disease | 0.16 | ||||||
| <200 | 339 | 0.45 | 338 | 0.47 | 0.96 | (0.82, 1.11) | |
| 200–<400 | 142 | 0.38 | 166 | 0.44 | 0.86 | (0.69, 1.08) | |
| 400–<600 | 213 | 0.46 | 184 | 0.39 | 1.19 | (0.98, 1.46) | |
| ≥600 | 159 | 0.42 | 136 | 0.37 | 1.13 | (0.90, 1.43) | |
| Stroke | 0.68 | ||||||
| <200 | 242 | 0.32 | 227 | 0.31 | 1.03 | (0.86, 1.24) | |
| 200–<400 | 128 | 0.34 | 127 | 0.34 | 1.08 | (0.84, 1.38) | |
| 400–<600 | 174 | 0.38 | 153 | 0.32 | 1.18 | (0.95, 1.47) | |
| ≥600 | 130 | 0.34 | 132 | 0.36 | 0.97 | (0.76, 1.24) | |
| Total death | 0.19 | ||||||
| <200 | 632 | 0.83 | 655 | 0.89 | 0.93 | (0.83, 1.03) | |
| 200–<400 | 312 | 0.83 | 352 | 0.92 | 0.91 | (0.78, 1.06) | |
| 400–<600 | 410 | 0.88 | 429 | 0.90 | 0.98 | (0.85, 1.12) | |
| ≥600 | 376 | 0.99 | 341 | 0.92 | 1.07 | (0.92, 1.24) | |
| Global index | 0.13 | ||||||
| <200 | 1025 | 1.39 | 1073 | 1.51 | 0.92 | (0.84, 1.00) | |
| 200–<400 | 533 | 1.47 | 597 | 1.63 | 0.90 | (0.80, 1.01) | |
| 400–<600 | 691 | 1.54 | 709 | 1.55 | 1.01 | (0.91, 1.13) | |
| ≥600 | 617 | 1.69 | 550 | 1.53 | 1.04 | (0.93, 1.17) | |
From a proportional hazards model stratified by prevalent condition (where appropriate), age, hormone therapy and diet modification randomization arm, and trial phase (time-dependent). Time to event equals 0 on date of randomization.
All nonhip fractures use adjudicated data during the clinical trial and self-reported data thereafter.
Analyses stratified by calcium supplement use (with or without vitamin D)
There was little evidence of a differential effect of randomization assignment on most clinical outcomes when use of nonprotocol baseline calcium supplementation at baseline was considered (Table 4). However, women in the supplement group had approximately 9% lower relative risk of developing any cancer if they were not using calcium supplement at baseline (p=0.04 for interaction).
Table 4.
Cumulative Analyzed Incidence Rates for Clinical Outcomes in the Women's Health Initiative Calcium and Vitamin D Supplementation Trial: Stratified by Personal Use of Calcium Supplements (With or Without Vitamin D) at Calcium and Vitamin D Randomization
| |
Overall combined phases |
||||||
|---|---|---|---|---|---|---|---|
| |
CaD |
Placebo |
|
|
|
||
| N | % | N | % | HR* | (95% CI)a | p-valuea | |
| Hip fracture | 0.28 | ||||||
| No | 153 | 0.16 | 180 | 0.20 | 0.84 | (0.67, 1.04) | |
| Yes | 226 | 0.21 | 233 | 0.22 | 0.96 | (0.80, 1.16) | |
| Vertebral fractureb | 0.76 | ||||||
| No | 172 | 0.18 | 192 | 0.21 | 0.89 | (0.72, 1.09) | |
| Yes | 274 | 0.25 | 316 | 0.29 | 0.84 | (0.71, 0.99) | |
| Total fractureb | 0.71 | ||||||
| No | 1765 | 2.08 | 1733 | 2.09 | 1.00 | (0.93, 1.06) | |
| Yes | 2248 | 2.31 | 2285 | 2.35 | 0.98 | (0.92, 1.04) | |
| Invasive colorectal cancer | 0.18 | ||||||
| No | 111 | 0.12 | 131 | 0.14 | 0.85 | (0.66, 1.10) | |
| Yes | 145 | 0.13 | 136 | 0.13 | 1.05 | (0.83, 1.32) | |
| Invasive breast cancer | 0.16 | ||||||
| No | 359 | 0.39 | 366 | 0.41 | 0.96 | (0.83, 1.11) | |
| Yes | 492 | 0.47 | 450 | 0.42 | 1.10 | (0.97, 1.25) | |
| In situ breast cancer | 0.17 | ||||||
| No | 76 | 0.08 | 106 | 0.12 | 0.69 | (0.52, 0.93) | |
| Yes | 122 | 0.12 | 132 | 0.13 | 0.91 | (0.71, 1.16) | |
| Total cancer | 0.04 | ||||||
| No | 1126 | 1.27 | 1204 | 1.40 | 0.91 | (0.84, 0.99) | |
| Yes | 1428 | 1.40 | 1413 | 1.38 | 1.02 | (0.94, 1.09) | |
| Coronary heart disease | 0.34 | ||||||
| No | 453 | 0.49 | 415 | 0.46 | 1.08 | (0.95, 1.23) | |
| Yes | 424 | 0.40 | 430 | 0.40 | 0.99 | (0.86, 1.13) | |
| Coronary heart disease deathc | 0.55 | ||||||
| No | 130 | 0.14 | 136 | 0.15 | 0.95 | (0.75, 1.22) | |
| Yes | 138 | 0.13 | 129 | 0.12 | 1.03 | (0.81, 1.32) | |
| Clinical myocardial infarction | 0.17 | ||||||
| No | 347 | 0.38 | 309 | 0.34 | 1.11 | (0.95, 1.29) | |
| Yes | 312 | 0.29 | 328 | 0.31 | 0.96 | (0.82, 1.12) | |
| Stroke | 0.30 | ||||||
| No | 340 | 0.37 | 305 | 0.34 | 1.11 | (0.95, 1.30) | |
| Yes | 350 | 0.33 | 354 | 0.33 | 0.99 | (0.85, 1.15) | |
| Cardiovascular disease death | 0.60 | ||||||
| No | 262 | 0.28 | 257 | 0.28 | 1.00 | (0.84, 1.19) | |
| Yes | 287 | 0.26 | 268 | 0.25 | 1.06 | (0.90, 1.25) | |
| Total death | 0.38 | ||||||
| No | 852 | 0.91 | 845 | 0.92 | 0.99 | (0.90, 1.09) | |
| Yes | 923 | 0.85 | 978 | 0.90 | 0.94 | (0.86, 1.02) | |
| Global index | 0.55 | ||||||
| No | 1325 | 1.46 | 1379 | 1.56 | 0.94 | (0.87, 1.02) | |
| Yes | 1607 | 1.54 | 1616 | 1.54 | 0.97 | (0.91, 1.04) | |
From a proportional hazards model stratified by prevalent condition (where appropriate), age, hormone therapy and diet modification randomization arm, and trial phase (time-dependent). Time to event equals 0 on date of randomization.
All nonhip fractures use adjudicated data during the clinical trial and self-reported data thereafter.
Coronary heart disease death includes definite and possible coronary heart disease death.
Sensitivity analyses
Among women who were adherent (took >80% of study pills), calcium plus vitamin D was associated with 29% lower hip fracture risk during the intervention phase. Overall, there was a 23% statistically significant reduction in hip fracture among adherent women (HR=0.77; 95% CI: 0.60, 0.99). Results for vertebral fracture were consistent with this observation but CI includes 1.0 (HR=0.81; 95% CI: 0.65, 1.02). Overall, there was little effect of calcium plus vitamin D supplementation among adherent women on colorectal or invasive breast cancer. The risk of in situ breast cancer was lower in adherent supplement group participants, but this association was not significant (HR=0.72; 95% CI: 0.51, 1.01). CVD and cancer mortality were similar in the two randomization groups in analyses limited to adherent women. However, all-cause mortality was lower in adherent women randomized to calcium plus vitamin D compared to placebo (HR=0.91; 95% CI: 0.82, 1.00), respectively. The summary index of overall benefits and risks suggested a greater benefit for supplementation among adherent women (HR=0.87; 95% CI, 0.80, 0.95).
Discussion
Over the entire intervention and postintervention period, there was no effect of calcium plus vitamin D supplementation on hip fractures or colorectal cancer, the primary outcomes of the trial. However, over the combined phases, women who were adherent to the supplement during the intervention had a significant 23% reduction in hip fracture risk and experienced greater benefits than risks. This observation is consistent with previous analyses of the calcium vitamin D trial, which demonstrated reduced hip fracture incidence with longer term use.19 Clinical vertebral fracture risk, a post hoc outcome was lower postintervention and during the cumulative follow-up period among women randomized to supplements: There would be four fewer vertebral fractures in 10,000 women taking supplements for 1 year. No overall influence of calcium plus vitamin D on invasive breast cancer was seen. Other major health risks and benefits of calcium plus vitamin D including CVD and death appeared balanced. Exploratory analysis revealed, a lower risk of in situ breast cancer postintervention among those randomized to supplements and was significant over the entire period: Absolute risk reduction in situ breast cancers would be two fewer women with in situ breast cancer in 10,000 women in 1 year.
While speculation has arisen regarding calcium supplementation and increased CVD risk,20,21 our current analyses after a total of 11 years provides no evidence of an effect on CHD events including CHD death, clinical MI, and stroke. This was true in both women who took a calcium supplement at study entry and those who were not taking a calcium supplement. A lower total mortality was suggested during the main trial with calcium plus vitamin D, but this was not maintained in the postintervention and overall there was no effect of calcium plus vitamin D on total mortality. In addition, the null effect of calcium plus vitamin D on colorectal cancer was consistent throughout the trial, the extension, and overall and in subgroups defined by age, total vitamin D intake, and use of calcium supplements at study baseline.
Cumulatively, calcium and vitamin D supplementation did not reduce invasive breast cancer, and thus the main findings do not support a causal association between supplement use and reduced breast cancer. However, in the postintervention period, a possible increased risk of invasive cancer was suggested. In addition, in subgroup analysis, the effect of calcium plus vitamin D differed by intake of total vitamin D. Overall, we found a significantly increased invasive breast cancer incidence in women with the highest baseline vitamin D intake randomized to the supplement group. This finding over the combined intervention and extension period is consistent with earlier analysis of the intervention.4 In general, epidemiologic studies on the association between vitamin D and calcium supplementation and breast cancer are mixed but most studies either supported an inverse association or a null association.22,23 The significant interaction between calcium plus vitamin D and higher total intake of vitamin D needs replication in future studies.
While a statistically significant overall reduction in situ breast cancer with no effect on invasive breast cancer incidence overall is puzzling, an influence of calcium plus vitamin D on breast cancer is biologically plausible. Vitamin D influences mammary gland development and function24,25 and some observational studies have associated lower 25-hydroxyvitamin D levels with lower invasive breast cancer risk. However, a recent meta-analysis of cohort studies found only a weak association.26
If women randomized to calcium plus vitamin D were less likely to obtain a routine mammogram then an ascertainment bias might explain the lower risk of in situ breast cancer in the supplement group. However, there was no difference in mammography use by randomized group. If vitamin D or calcium interfered with in situ breast cancer detection by mammography (by which diagnosis is commonly based on detection of calcifications) but not invasive breast cancer detection (by which diagnosis is commonly based on detection of a mass), a lower in situ breast cancer incidence could reflect diagnostic delay. However, mammogram performance was not compromised by supplement group randomization. Findings are mixed regarding the association of vitamin D use with mammographic breast density.26,27 A previous WHI report showed that calcium and vitamin D supplementation did not affect mammographic density.28
While some interventions, including neoadjuvant chemotherapy29,30 and raloxifene,31 have greater influence on invasive compared with in situ breast cancer, we could find no prior intervention that lowers in situ breast cancer incidence but doesn't influence invasive breast cancers. The annualized rates of invasive breast cancer were about 4 times higher than the rate of in situ cancer, and in situ cancers have better survival rates.32 Thus, the public health implication of this finding is uncertain. Additionally, in the current study, supplementation had no influence on benign proliferative breast disease.33 As a result, the lower in situ breast cancer incidence in the supplement group could represent a chance finding. However, emergence of lower in situ breast cancer incidence only occurred after a long period of supplement exposure and postexposure follow-up suggests a duration effect with perhaps an influence on subsequent invasive breast cancer to emerge after still longer follow-up. Cohorts with long-term supplement exposure information could address such a hypothesis.
In the postintervention period, a significant 17% reduction in clinical vertebral fractures emerged with no difference in the effect of calcium plus vitamin D supplementation on vertebral fractures between the intervention and postintervention period. Overall, calcium plus vitamin D reduced clinical vertebral fractures. Although these results are from a post hoc analysis, these results are important because vertebral fractures are the most common osteoporotic fracture, occurring in an estimated 30%–50% of individuals age 50 and older.34 Vertebral fractures are major risk factors for future fractures including hip fractures.35,36 They also are associated with significant morbidity and mortality.37–39 Thus, our results suggest that calcium plus vitamin D supplementation could reduce the burden of vertebral fractures over the long term, although more research is needed before this conclusion can be applied to clinical practice. Failure to see an effect on hip fractures may reflect low statistical power because we under-enrolled women age 70–79 in the trial, which thus influenced the observed number of hip fractures. Failure to see an effect on all fractures may reflect the heterogeneous etiology of different fracture sites.
Study strengths include the randomized double blind study design, large sample size, long follow-up, and high percentage of women who agreed to the extended follow-up. The women who reconsented to the extension differed by select characteristics, but of importance, there were no differences in characteristics by randomized group. Limitations include less than optimal adherence and the inability to separate the effects of calcium and vitamin D. Some have considered the vitamin D3 dose of 400 IU daily to be a study limitation. However, the dose followed the Institute of Medicine (IOM) recommendations available during the trial.8 These IOM recommendations were recently increased for vitamin D to 600 IU daily for those <70 years old and 800 IU daily for those 71 years.40 Since about half of the current study participants were taking nonprotocol vitamin D, the mean vitamin D intake in the supplement groups was 773 IU daily (mean), meeting current recommendations. For the time period of the extension, we have no information on dietary intake of calcium and vitamin D. However, 44% of those in the intervention and 42% of those in the placebo reported taking calcium and vitamin D supplements at the end of the extension. Only hip fractures were adjudicated during the extension and the validity of self-reported clinical vertebral fractures was low.10 Finally, during the intervention phase a 17% higher risk of kidney stones was found among women in the supplement group.2 Postintervention, these data were not collected.
In summary, after an average of 11 years, calcium and vitamin D supplementation did not decrease hip fracture or colorectal cancer, coprimary outcomes. Throughout, there was no difference in invasive breast cancer, CVD, or all-cause mortality between groups. Exploratory analyses found lower vertebral fracture and in situ breast cancers in supplement users, although absolute risk reduction was small. The incidence of invasive breast cancer differed across baseline intake of vitamin D with a significantly higher incidence for those with higher intakes. Overall, a decreased risk of hip fracture was observed only among women adherent during the intervention. Future research should explore the relationships with vertebral fractures and in situ and invasive breast cancers including potential mechanistic studies.
Appendix
Appendix Figure A1.
Women's Health Initiative calcium and vitamin D (CaD) trial through extended follow-up.
Appendix Table A1.
Baseline Characteristics of Calcium and Vitamin D Trial Participants by Extension Study Participation Status
| |
Nonextension participant |
Extension participant |
|
||
|---|---|---|---|---|---|
| |
(N=6420) |
(N=29,862) |
|
||
| N | % | N | % | p-value | |
| Age group at screening, years | <0.0001 | ||||
| 50–59 | 2091 | 32.6 | 11,331 | 37.9 | |
| 60–69 | 2712 | 42.2 | 13,807 | 46.2 | |
| 70–79 | 1617 | 25.2 | 4724 | 15.8 | |
| Race/ethnicity | <0.0001 | ||||
| White | 4813 | 75.0 | 25,342 | 84.9 | |
| Black | 815 | 12.7 | 2500 | 8.4 | |
| Hispanic | 476 | 7.4 | 1026 | 3.4 | |
| American Indian | 43 | 0.7 | 106 | 0.4 | |
| Asian/Pacific Islander | 152 | 2.4 | 569 | 1.9 | |
| Unknown | 121 | 1.9 | 319 | 1.1 | |
| Education | <0.0001 | ||||
| None to some high school | 594 | 9.3 | 1309 | 4.4 | |
| High school diploma or equivalent | 1235 | 19.4 | 5438 | 18.3 | |
| School after high school | 2717 | 42.6 | 11,656 | 39.3 | |
| College degree or higher | 1826 | 28.7 | 11,276 | 38.0 | |
| Gail 5-year risk of breast cancer | <0.0001 | ||||
| <1.25 | 2417 | 37.6 | 10,241 | 34.3 | |
| 1.25–1.74 | 1912 | 29.8 | 9958 | 33.3 | |
| ≥1.75 | 2091 | 32.6 | 9663 | 32.4 | |
| Medical history | |||||
| Kidney stones | 74 | 1.3 | 269 | 1.0 | 0.04 |
| Stroke | 109 | 1.7 | 239 | 0.8 | <0.0001 |
| Myocardial infarction | 205 | 3.2 | 449 | 1.5 | <0.0001 |
| Fracture | 2177 | 38.3 | 10,362 | 38.2 | 0.99 |
| Colorectal cancer | 12 | 0.2 | 39 | 0.1 | 0.27 |
| Breast cancer | 13 | 0.2 | 44 | 0.1 | <0.0001 |
| Prior hormone usea | <0.0001 | ||||
| None | 2219 | 34.6 | 9277 | 31.1 | |
| Past | 1141 | 17.8 | 4802 | 16.1 | |
| Current estrogen-alone | 1592 | 24.8 | 7532 | 25.2 | |
| Current estrogen+progestin | 1468 | 22.9 | 8251 | 27.6 | |
| Body mass index, kg/m2 | <0.0001 | ||||
| <25 | 1549 | 24.2 | 8030 | 27.0 | |
| 25–<30 | 2167 | 33.9 | 10,796 | 36.3 | |
| ≥30 | 2682 | 41.9 | 10,880 | 36.6 | |
| Physical activity, MET h/wk | <0.0001 | ||||
| None | 1285 | 22.4 | 5039 | 18.5 | |
| 0.5–4.125 | 1261 | 21.9 | 5645 | 20.7 | |
| >4.125–9.5 | 1113 | 19.4 | 5472 | 20.1 | |
| >9.5–17.75 | 1028 | 17.9 | 5557 | 20.4 | |
| ≥17.75 | 1060 | 18.4 | 5534 | 20.3 | |
| Alcohol intake | <0.0001 | ||||
| Nondrinker | 766 | 12.1 | 2988 | 10.1 | |
| Past drinker | 1448 | 22.8 | 4953 | 16.7 | |
| <1 drink per month | 907 | 14.3 | 4141 | 14.0 | |
| <1 drink per week | 1253 | 19.7 | 6361 | 21.5 | |
| 1–<7 drinks per week | 1383 | 21.8 | 7997 | 27.0 | |
| ≥7 drinks per week | 596 | 9.4 | 3212 | 10.8 | |
| Smoking | <0.0001 | ||||
| Never | 3138 | 49.5 | 15,615 | 52.8 | |
| Past | 2517 | 39.7 | 11,871 | 40.2 | |
| Current | 682 | 10.8 | 2079 | 7.0 | |
| Total vitamin D (supplements+diet), IU/db | <0.0001 | ||||
| <200 | 2612 | 41.9 | 10,886 | 37.1 | |
| 200–<400 | 1207 | 19.4 | 5595 | 19.1 | |
| 400–<600 | 1418 | 22.8 | 7065 | 24.1 | |
| ≥600 | 991 | 15.9 | 5800 | 19.8 | |
| Vitamin D supplement useb | 2741 | 42.7 | 14,426 | 48.3 | <0.0001 |
| Geographic region at randomization by latitude | <0.0001 | ||||
| Southern, <35°N | 2563 | 39.9 | 8310 | 27.8 | |
| Middle, 35–40°N | 1550 | 24.1 | 8493 | 28.4 | |
| Northern, ≥40°N | 2307 | 35.9 | 13,059 | 43.7 | |
| Total calcium intake (supplements+diet+medications), mg/db | <0.0001 | ||||
| <800 | 2527 | 40.6 | 9580 | 32.6 | |
| 800–<1200 | 1632 | 26.2 | 7738 | 26.4 | |
| ≥1200 | 2069 | 33.2 | 12,028 | 41.0 | |
| Calcium and/or vitamin D supplement useb | 3275 | 51.0 | 17,340 | 58.1 | <0.0001 |
| Hormone therapy trial participant | 3173 | 49.4 | 12,916 | 43.3 | <0.0001 |
| Diet modification trial participant | 4200 | 65.4 | 21,010 | 70.4 | <0.0001 |
| CaD trial randomization assignment | |||||
| Intervention | 3151 | 49.1 | 15,025 | 50.3 | |
| Control | 3269 | 50.9 | 14,837 | 49.7 | |
Values incorporate hormone therapy use during year 1 of the clinical trial, including exposure to the Hormone Therapy trials.
Vitamin D and calcium variables incorporate intake reported at year 1 of the clinical trial.
MET, metabolic equivalents; CaD, calcium and vitamin D.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute and the General Clinical Research Center program of the National Center for Research Resources, Department of Health and Human Services (N01WH32112). The active study drug and placebo were supplied by Glaxo SmithKline Consumer Healthcare (Pittsburgh).
Author Disclosure Statement
No competing financial interest exists.
References
- 1.Jackson RD. LaCroix AZ. Cauley JA, et al. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 Suppl):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 2.Jackson RD. LaCroix AZ. Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 3.Wactawski-Wende J. Kotchen JM. Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT. Johnson KC. Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsia J. Heiss G. Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 6.Brunner RL. Wactawski-Wende J. Caan BJ, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women's Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr Cancer. 2011;63:827–841. doi: 10.1080/01635581.2011.594208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaCroix AZ. Kotchen J. Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women's Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yates AA. Schlicker SA. Suitor CW. Dietary reference intakes: the new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J Am Diet Assoc. 1998;98:699–706. doi: 10.1016/S0002-8223(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 10.Chen Z. Kooperberg C. Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women's Health Initiative observational study and clinical trials. Menopause. 2004;11:264–274. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 11.Patterson RE. Kristal AR. Tinker LF, et al. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 12.Heiss G. Wallace R. Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 13.Cox DR. Regression analysis and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 14.Prentice RL. Thomson CA. Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534–1543. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossouw JE. Anderson GL. Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GL. Limacher M. Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 17.Robins JM. Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 18.Chlebowski RT. Anderson G. Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168:370–377. doi: 10.1001/archinternmed.2007.123. quiz 345. [DOI] [PubMed] [Google Scholar]
- 19.Prentice RL. Pettinger MB. Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567–580. doi: 10.1007/s00198-012-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolland MJ. Bacon CJ. Horne AM, et al. Vitamin D insufficiency and health outcomes over 5 y in older women. Am J Clin Nutr. 2010;91:82–89. doi: 10.3945/ajcn.2009.28424. [DOI] [PubMed] [Google Scholar]
- 21.Li K. Kaaks R. Linseisen J. Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg) Heart. 2012;98:920–925. doi: 10.1136/heartjnl-2011-301345. [DOI] [PubMed] [Google Scholar]
- 22.McCullough ML. Rodriguez C. Diver WR, et al. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2898–2904. doi: 10.1158/1055-9965.EPI-05-0611. [DOI] [PubMed] [Google Scholar]
- 23.Gissel T. Rejnmark L. Mosekilde L. Vestergaard P. Intake of vitamin D and risk of breast cancer—a meta-analysis. J Steroid Biochem Mol Biol. 2008;111:195–199. doi: 10.1016/j.jsbmb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Lopes N. Paredes J. Costa JL. Ylstra B. Schmitt F. Vitamin D and the mammary gland: a review on its role in normal development and breast cancer. Breast Cancer Res. 2012;14:211. doi: 10.1186/bcr3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinser G. Packman K. Welsh J. Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development. 2002;129:3067–3076. doi: 10.1242/dev.129.13.3067. [DOI] [PubMed] [Google Scholar]
- 26.Green AK. Hankinson SE. Bertone-Johnson ER. Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127:667–674. doi: 10.1002/ijc.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight JA. Vachon CM. Vierkant RA, et al. No association between 25-hydroxyvitamin D and mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15:1988–1992. doi: 10.1158/1055-9965.EPI-06-0241. [DOI] [PubMed] [Google Scholar]
- 28.Bertone-Johnson ER. McTiernan A. Thomson CA, et al. Vitamin D and calcium supplementation and one-year change in mammographic density in the women's health initiative calcium and vitamin D trial. Cancer Epidemiol Biomarkers Prev. 2012;21:462–473. doi: 10.1158/1055-9965.EPI-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RL. Lakhani SR. Ring AE, et al. Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer. 2006;94:358–362. doi: 10.1038/sj.bjc.6602950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazouni C. Peintinger F. Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25:2650–2655. doi: 10.1200/JCO.2006.08.2271. [DOI] [PubMed] [Google Scholar]
- 31.Vogel VG. Costantino JP. Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:139–141. doi: 10.1093/jncimonographs/lgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohan TE. Negassa A. Chlebowski RT, et al. A randomized controlled trial of calcium plus vitamin D supplementation and risk of benign proliferative breast disease. Breast Cancer Res Treat. 2009;116:339–350. doi: 10.1007/s10549-008-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christiansen BA. Bouxsein ML. Biomechanics of vertebral fractures and the vertebral fracture cascade. Curr Osteoporos Rep. 2010;8:198–204. doi: 10.1007/s11914-010-0031-2. [DOI] [PubMed] [Google Scholar]
- 35.Klotzbuecher CM. Ross PD. Landsman PB. Abbott TA., 3rd Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 36.Black DM. Arden NK. Palermo L. Pearson J. Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821–828. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- 37.Ettinger B. Black DM. Nevitt MC, et al. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1992;7:449–456. doi: 10.1002/jbmr.5650070413. [DOI] [PubMed] [Google Scholar]
- 38.Kado DM. Duong T. Stone KL, et al. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int. 2003;14:589–594. doi: 10.1007/s00198-003-1412-5. [DOI] [PubMed] [Google Scholar]
- 39.Cauley JA. Thompson DE. Ensrud KC. Scott JC. Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for calcium and vitamin D consensus report. Washington DC: National Academy Press; 2010. [Google Scholar]