Abstract
Phosphoethanolamine methyltransferases (PMTs also known as PEAMTs) catalyze the three-step s-adenosylmethionione-dependent methylation of phosphoethanolamine to form phosphocholine. These enzymes play an important function in the synthesis of phosphatidylcholine, the major phospholipid in the membranes of lower and higher eukaryotes, as well as in the production of the compatible solute and osmoprotectant glycine betaine in plants. Genetic studies in plants, Caenhorhabditis elegans and Plasmodium falciparum have demonstrated that disruption of PMT activity results in severe defects in important cellular processes such as development, replication, survival and sexual maturation and differentiation. Here we report chemical shift assignments for PfPMT, the PMT from Plasmodium falciparum. X-ray crystal structures have been recently reported for complexes of PfPMT, but the structure of the apoenzyme remains unknown. The solution structure of the apoenzyme will help to elucidate important details of the mechanism of substrate binding by PfPMT, as residues comprising the substrate binding site are inaccessible to solvent in the conformation evident in the available crystal structures. In addition to enabling determination of the solution structure of the apoenzyme, the assignments will facilitate additional investigations into the interaction of PfPMT with its substrates and inhibitors.
Keywords: NMR, PfPMT, Malaria, Plasmodium falciparum, Phosphoethanolamine methyltransferase
Biological context
The PfPMT enzyme of Plasmodium falciparum, the agent of severe human malaria, is a defining member of a large family of phosphoethanolamine methyltransferases (PMTs) recently identified in plants, worms, frogs, and some proteobacteria (Bobenchik et al. 2011). This family is divided into four classes depending on the size of the enzyme members and whether they contain one or two catalytic domains (Bobenchik et al. 2011). Functional studies revealed that PfPMT plays a critical role in the synthesis of phosphatidylcholine (PtdCho) via a plant-like pathway involving serine decarboxylation and phosphoethanolamine methylation (Pessi et al. 2004). Genetic studies in P. falciparum have shown that this enzyme is critical for parasite development and survival within human erythrocytes (Witola et al. 2008). PtdCho constitutes half of the phospholipid content of the parasite membranes. Biochemical studies demonstrated that PtdCho synthesis occurs via two metabolic routes (Pessi et al. 2004; Bobenchik et al. 2011). The first route is the CDP-choline pathway, which uses host choline as a precursor. The second route is the serine decarboxylation-phosphoethanolamine methylation pathway, which uses serine, either transported from the host or generated by degradation of host proteins as a phospholipid precursor. The serine is first decarboxylated to produce ethanolamine, by an unknown serine decarboxylase. The ethanolamine is next phosphorylated by a parasite-specific ethanolamine kinase. A SAM-dependent triple methylation of the resulting phosphoethanolamine (P-Etn) by PfPMT results in the synthesis of phosphocholine (P-Cho). P-Cho then integrates into the CDP-choline pathway for the synthesis of PtdCho.
The 266 amino acid PfPMT enzyme is half the size that of plant PMT enzymes and contains a single SAM-dependent catalytic domain. Its specificity for its substrates and co-substrates was demonstrated using both biochemical and genetic studies (Reynolds et al. 2008). Interestingly, no PfPMT homologs are found in mammalian databases, suggesting that PfPMT could be an ideal target for development of novel inhibitors. Despite recent crystal structures solved for complexes of PfPMT, important questions about substrate recognition remain unresolved (Lee et al. 2012). Residues involved in substrate recognition by PfPMT are inaccessible to solvent in the crystal structures of the complexes, suggesting that conformation dynamics plays an important role in recognition. The detailed nature of the conformational changes remains the subject of speculation until the structure of the apoenzyme is determined.
Relatively little is known about the evolution of substrate specificity in PMTs. PfPMT has a single catalytic domain that catalyzes the three successive methyl transfer reactions in the formation of P-Cho from P-Etn (Pessi et al. 2004), in contrast to plant and worm PMTs that have catalytic domains with distinct specificities for different substrates (reviewed in Bobenchik et al. 2011). The solution structure of apo-PfPMT, which will be enabled by the chemical shift assignments, should yield significant new insights into the mechanism of substrate recognition, and as an exemplar of the simplest class of PMTs, further advance understanding of the evolution of specificity in substrate recognition by PMTs in other organisms.
Methods and experiments
Protein expression and purification
Full length PfPMT was expressed in the Escherichia coli BL21-CodonPlus strain (Stratagene) as a His-tag fusion protein. Isotope labeling was performed using M9 media, supplemented using a micronutrient mixture similar to one previously described (Weber et al. 1992), containing uniformly 15N-labeled ammonium chloride and uniformly 13C-labeled glucose. His-tagged PfPMT was purified using Ni–NTA Agarose (Qiagen) using a standard protocol of imidazole elution. The eluate was further purified by Superdex75 gel filtration chromatography using an AKTApurifier (GE) system. The final NMR sample contained 0.4 mM uniformly 13C/15N-labelled PfPMT in 50 mM HEPES, pH 6.9, 50 mM NaCl, 5 mM DTT, 1 mM EDTA and 10% (v/v) D2O.
NMR spectroscopy
Most NMR experiments were recorded at 25°C on Agilent VNMRS spectrometers operating at 500, 600, and 800 MHz, all equipped with cryogenically-cooled triple-resonance pulse field gradient probes. Supplemental data were collected using Bruker Avance spectrometers operating at 800 MHz (Landsman Research Facility, Brandeis) and 900 MHz (MIT), both equipped with TXI cryoprobes. The backbone and side-chain resonances were assigned using 15N-HSQC, HNCACB, CBCA(CO)NH, HBHA(CO)NH, 13C-HSQC, (H)CCH-TOCSY and H(C)CH-TOCSY experiments (Kay 1995). NMR data sets were processed using NMRPipe (Shen et al. 2009). Nonuniformly sampled data were processed with the Rowland NMR Toolkit (http://rnmrtk.uchc.edu). Spectra were analyzed using Sparky (Goddard, T. D. and Kneller, D. G., SPARKY-NMR, 2003, University of California San Francisco). The backbone Φ and ψ torsion angles were derived from chemical shifts of backbone atoms using TALOS+ (Shen et al. 2009).
Assignments and data deposition
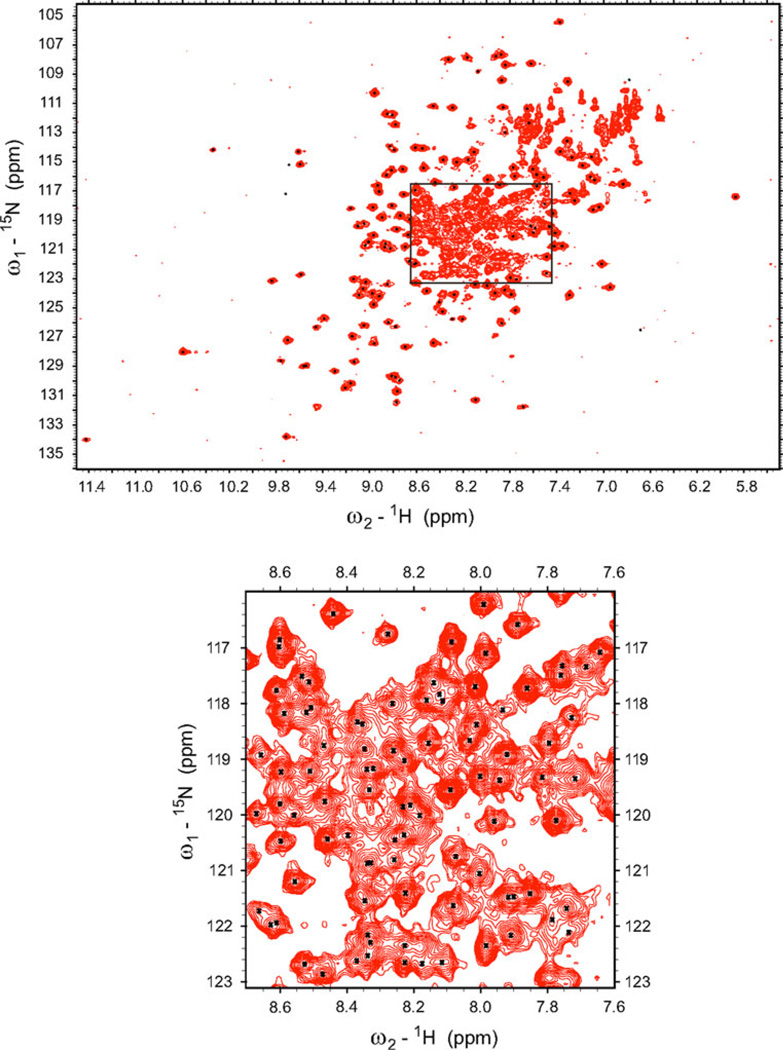
The 1H-15N HSQC spectrum of the full length, 266 amino acid residue long, PfPMT is shown in Figure 1a. The assigned backbone N and HN resonances are annotated in the supplemental material, Fig. 1S. The resonance assignments were made for 95% of backbone residues and 75% of side chain residues. The assignment of remaining resonances was not possible due to spectral overlap and ambiguity. The regular secondary structure elements of the PfPMT were predicted based on 1Hα, 13Cα, 13Cβ and 13C′ secondary chemical shifts (Wishart and Sykes 1994) and are shown in Fig. 1b.
Fig. 1.
15N-1H HSQC spectrum of uniformly 15N-labeled PfPMT. Assigned backbone resonances are indicated by black dots in the center of a peak. The annotated spectrum is available in “Supplementary materials”
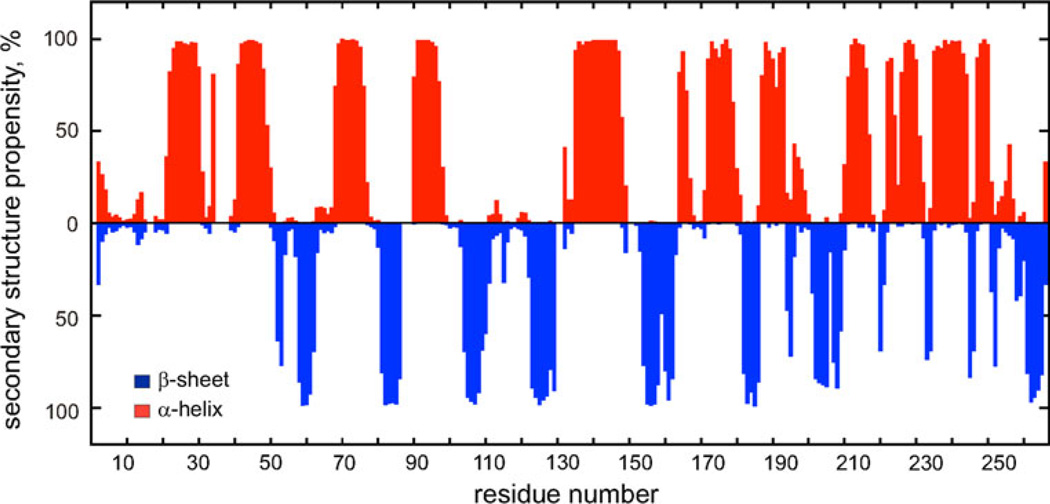
The 1H, 13C and 15N chemical shifts of PfPMT have been deposited into the BioMagResBank (http://www.bmrb.wisc.edu; accession no. 18303) (Fig. 2).
Fig. 2.
Elements of PfPMT secondary structure. Secondary structure propensities calculated based on 1Hα, 13Cα, 13Cβ and C′ secondary chemical shifts (Wishart and Sykes 1994) are shown as red and blue bars for α-helix and β-sheet, respectively
Supplementary Material
Acknowledgments
Support from the US National Institutes of Health (grants GM47467 to J.C.H., AI51507 to C.B.M, GM092369 to S.K. Weller), the US Department of Defense (grant PR033005 to C.B.M.), the United States Department of Agriculture (grant NIFA-2010-38505-21257 to J.C.H.) and the Burroughs Wellcome Fund (grant 1006267 to C.B.M) is gratefully acknowledged. The 800 MHz NMR spectrometer at UCHC was purchased with support from NIH grant RR023041. The 900 MHz NMR spectrometer at the MIT/Harvard Center for Magnetic Resonance is supported by NIH grant EB002026. We thank Ms. Li Luo for technical assistance.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12104-012-9372-3) contains supplementary material, which is available to authorized users.
Contributor Information
Irina Bezsonova, Department of Molecular, Microbial and Structural Biology, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 6030-3305, USA.
Iulian Rujan, Department of Molecular, Microbial and Structural Biology, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 6030-3305, USA.
April M. Bobenchik, Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT 06520, USA
Vitaliy Gorbatyuk, Department of Molecular, Microbial and Structural Biology, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 6030-3305, USA.
Mark W. Maciejewski, Department of Molecular, Microbial and Structural Biology, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 6030-3305, USA
Oksana Gorbatyuk, Department of Molecular, Microbial and Structural Biology, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 6030-3305, USA.
Bing Hao, Department of Molecular, Microbial and Structural Biology, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 6030-3305, USA.
Haribabu Arthanari, Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, 240 Longwood Ave., Boston, MA 02115, USA.
Choukri Ben Mamoun, Email: choukri.benmamoun@yale.edu, Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT 06520, USA.
Jeffrey C. Hoch, Email: hoch@uchc.edu, Department of Molecular, Microbial and Structural Biology, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 6030-3305, USA.
References
- Bobenchik AM, Augagneur Y, Hao B, Hoch JC, Ben Mamoun C. Phosphoethanolamine methyltransferases in phosphocholine biosynthesis: functions and potential for antiparasite therapy. FEMS Microbiol Rev. 2011;35(4):609–619. doi: 10.1111/j.1574-6976.2011.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LE. Pulsed field gradient multi-dimensional NMR methods for the study of protein structure and dynamics in solution. Prog Biophys Mol Biol. 1995;63(3):277–299. doi: 10.1016/0079-6107(95)00007-0. [DOI] [PubMed] [Google Scholar]
- Lee SG, Kim Y, Alpert TD, Nagata A, Jez JM. Structure and reaction mechanism of phosphoethanolamine methyltransferase from the malaria parasite Plasmodium falciparum: an antiparasitic drug target. J Biol Chem. 2012;287(2):1426–1434. doi: 10.1074/jbc.M111.315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessi G, Kociubinski G, Mamoun CB. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc Natl Acad Sci USA. 2004;101:6206–6211. doi: 10.1073/pnas.0307742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JM, Takebe S, Choi JY, El Bissati K, Witola WH, Bobenchik AM, Hoch JC, Voelker DR, Mamoun CB. Biochemical and genetic analysis of the phosphoethanolamine methyltransferase of the human malaria parasite Plasmodium falciparum. J Biol Chem. 2008;283(12):7894–7900. doi: 10.1074/jbc.M709869200. [DOI] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44(4):213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DJ, Gittis AG, Mullen GP, Abeygunawardana C, Lattman EE, Mildvan AS. NMR docking of a substrate into the X-ray structure of staphylococcal nuclease. Proteins. 1992;13(4):275–287. doi: 10.1002/prot.340130402. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR. 1994;4(2):171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- Witola WH, El Bissati K, Pessi G, Xie C, Roepe PD, Mamoun CB. Disruption of the Plasmodium falciparum PfPMT gene results in a complete loss of phosphatidylcholine biosynthesis via the serine-decarboxylase-phosphoethanolamine-methyltransferase pathway and severe growth and survival defects. J Biol Chem. 2008;283(41):27636–27643. doi: 10.1074/jbc.M804360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.