Abstract
Brainstem central chemoreceptors are critical to the hypercapnic ventilatory response, but their location and identity are poorly understood. When studied in vitro, serotonin-synthesizing (5-HT) neurons within the rat medullary raphé are intrinsically stimulated by CO2/acidosis. The contributions of these neurons to central chemosensitivity in vivo, however, are controversial. Lacking is documentation of CO2-sensitive 5-HT neurons in intact experimental preparations and understanding of their spatial and proportional distribution. Here we test the hypothesis that 5-HT neurons in the rat medullary raphé are sensitive to arterial hypercapnia. We use extracellular recording and hypercapnic challenge of spontaneously active medullary raphé neurons in the unanesthetized in situ perfused decerebrate brainstem preparation to assess chemosensitivity of individual cells. Juxtacellular labeling of a subset of recorded neurons and subsequent immunohistochemistry for the 5-HT-synthesizing enzyme tryptophan hydroxylase (TPH) identify or exclude this neurotransmitter phenotype in electrophysiologically characterized chemosensitive and insensitive cells. We show that the medullary raphé houses a heterogeneous population, including chemosensitive and insensitive 5-HT neurons. Of 124 recorded cells, 16 cells were juxtacellularly filled, visualized, and immunohistochemically identified as 5-HT synthesizing, based on TPH-immunoreactivity. Forty-four percent of 5-HT cells were CO2 stimulated (increased firing rate with hypercapnia), while 56% were unstimulated. Our results demonstrate that medullary raphé neurons are heterogeneous and clearly include a subset of 5-HT neurons that are excited by arterial hypercapnia. Together with data identifying intrinsically CO2-sensitive 5-HT neurons in vitro, these results support a role for such cells as central chemoreceptors in the intact system.
Keywords: serotonin, raphé, chemosensitivity, breathing
knowledge of the neurons and cellular basis with which the brain detects and responds to changes in CO2/pH (central chemosensitivity) is critical to understanding both homeostatic regulation of pH and the nature of diseases believed to result from failures in chemosensitivity, such as sleep apnea, congenital central hypoventilation syndrome (CCHS), sudden unexplained death in epilepsy (SUDEP), and sudden infant death syndrome (SIDS). In particular, vulnerability to SIDS is proposed to occur as a result of brainstem serotonergic dysfunction (Paterson et al. 2006; Kinney et al. 2009; Kinney and Thach 2009; Duncan et al. 2010). However, it is not known how this dysfunction contributes to these pathologies. Recent reviews of the cellular basis of central chemosensitivity suggest it is a complex system function involving multiple mechanisms and brainstem sites (Feldman et al. 2003; Putnam et al. 2004; Nattie and Li 2009; Darnall 2010; Guyenet et al. 2010, 2012). Single critical mechanisms have not been isolated and neither has a single locus been convincingly revealed as the seat of central chemosensitivity. Serotonin-synthesizing (5-HT) neurons in the brainstem raphé nuclei are proposed as one of a limited pool of potential chemosensitive cell types functioning as central chemoreceptors (Richerson 2004; Corcoran et al. 2009; Hodges and Richerson 2010; Johansen et al. 2012).
Serotonergic mechanisms are known to be involved in homeostatic responses to hypercapnia (Richerson 2004; Hodges et al. 2008). We have shown that neuroventilatory rhythmogenesis and burst pattern formation in the in situ perfused brainstem preparation do not depend on 5-HT neuron activity (Toppin et al. 2007) but that 5-HT activity is critical to central chemosensitivity (Corcoran et al. 2013). Rodents display perturbed ventilation when 5-HT function is altered genetically (Li and Nattie 2008; Hodges et al. 2008, 2009, 2011; Buchanan and Richerson 2010; Cummings et al. 2011b; Penatti et al. 2011a; Barrett et al. 2012) or disrupted by targeted pharmacalogical lesion (Nattie et al. 2004; Dias et al. 2007; Cummings et al. 2011a), by selective silencing (Ray et al. 2011), or by dietary tryptophan restriction (Penatti et al. 2011b). Intrinsically CO2-stimulated 5-HT neurons occur in the brainstem raphé nuclei in vitro, and 5-HT neurons occur in highly vascular regions connected to major homeostatic integration and respiratory control centers (Wang et al. 2001; Severson et al. 2003; Ptak et al. 2009). Hypercapnia induces immediate early gene (e.g., c-Fos) expression in raphé 5-HT neurons (Larnicol et al. 1994; Haxhiu et al. 2001; Johnson et al. 2005; Pete et al. 2002) and 5-HT release in respiratory motor nuclei in vivo (Harper et al. 2005). Extracellular single-unit raphé neuron recordings from freely moving cats have identified putative 5-HT neurons responding to hypercarbia with increased firing and sensitivities paralleling ventilatory sensitivity to CO2 (Veasey et al. 1995, 1997).
Collectively, these observations support raphé 5-HT neurons as important chemoreceptors. However, extrapolation of these findings to suggest that raphé 5-HT neurons serve as chemoreceptors in vivo has been criticized, based on the lack of documentation of chemosensitive 5-HT neurons within the raphé of intact preparations (Mulkey et al. 2004; Guyenet et al. 2005, 2008; DePuy et al. 2011; Takakura and Moreira 2013). These studies were performed on anesthetized rodents and/or targeted 5-HT neurons in regions outside the raphé. The potential for heterogeneity in chemosensitivity of raphé neurons has not been considered, and we have recently demonstrated that 5-HT neurons are inhibited by the anesthetic isoflurane in situ and in vitro (Johansen et al. 2012; Massey et al. 2012). Unknown are 1) if raphé 5-HT neurons are uniformly chemosensitive; 2) the neurotransmitters contained in and potentially distinguishing chemosensitive and insensitive raphé neurons; 3) which populations of 5-HT cells are responsive to CO2 in vivo; and 4) the mechanisms through which they function.
The present study documents the existence and locations of 5-HT neurons within the raphé that are chemosensitive or insensitive to moderate hypercapnia. We report functionally characterized individual neurons, conclusively identified as 5-HT by neurotransmitter phenotype, which may be chemoreceptive neurons in the intact animal. We tested the hypothesis that 5-HT neurons in the rat medullary raphé are sensitive to arterial hypercapnia. We demonstrate the presence of CO2-stimulated 5-HT medullary raphé neurons in situ, within a heterogeneous medullary raphé population of both chemosensitive and insensitive 5-HT neurons.
METHODS
Experimental animals and surgery.
All experiments were done in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the University of Alaska Fairbanks Institutional Animal Care and Use Committee. Experiments were conducted in preparations derived from juvenile (P20–P30; 60–150 g) male Simonsen albino rats (Sprague-Dawley derived; Simonsen Laboratories) in situ using the perfused decerebrate juvenile rat brainstem preparation, as per published methods (Toppin et al. 2007; Corcoran et al. 2013). Briefly, animals were heparinized (0.3 ml of 1,000 iu/ml ip; Baxter, Deerfield, IL) and then deeply anesthetized with isoflurane. Preparations were transected below the diaphragm, immersed in ice-chilled perfusate, and decerebrated rostral to the superior colliculi. Subsequent procedures were conducted in the absence of anesthesia as decerebration renders animals insensitive to pain. Preparations were placed prone in a stereotaxic head frame, and the descending aorta was cannulated retrogradely with a double-lumen catheter and perfused with solution at a temperature of 31°C. The perfusate contained the following (in mM): 1.0 MgSO4, 125 NaH2PO4, 4.0 KCl, 24 NaHCO3, 115 NaCl, 2.0 CaCl2, 10 d-glucose, and 0.18 Ficoll 70. Under baseline conditions, perfusing solutions were equilibrated with 95% O2-5% CO2 (Pco2: 33 mmHg, pH 7.4). The neuromuscular blocker gallamine triethidodide (60 mg/l) was added to the perfusate to eliminate movement. The pressure of aortic perfusion (measured with a blood pressure transducer attached to the second lumen) was increased gradually to 50–75 mmHg and then held constant. Perfusate was collected and recirculated. Neuronal recordings were always initiated under baseline conditions, followed by brief (5 min) hypercapnic challenges (91% O2-9% CO2; Pco2: 60 mmHg, pH 7.2, 5 min) before a return to baseline. Wilson et al. (2001) confirm that these procedures lead to brainstem tissue equilibration with the perfusate. The levels of O2 and CO2 in the perfusate were maintained by equilibrating a perfusate reservoir with gas mixtures produced with a precision gas mixer (GSM-2, CWE) and verified with a CO2 analyzer (CD-3A; Applied Electrochemistry). Lacking hemoglobin, solution hyperoxia (Po2 ≈600: mmHg) is necessary to maintain O2 content sufficient to meet tissue metabolic demands. This unavoidable hyperoxia was constant under all conditions. Baseline perfusate conditions approximated normocapnic plasma in vivo, and hypercapnic challenges produced conditions similar to those of plasma during a 4% elevation in inspired CO2.
Extracellular recording.
Extracellular recordings of medullary raphé neurons were made using pulled glass capillary electrodes (15–40 MΩ), filled with biotinamide hydrobromide (Life Technologies) dissolved at 5% in 0.5 M sodium acetate. We targeted regions of the medullary raphé (including the raphé obscurus, raphé magnus, and raphé pallidus) along the midline (0–0.1 mm lateral) between 0 and 3.25 mm caudal to interaural line, 10–12 mm below the dorsal surface. These are areas from which CO2-sensitive neurons have been identified in vitro. Electrodes were placed above raphé target areas and driven into the tissue using a fine stepping motor (2-μm steps; Burleigh Inchworm) held in a stereotaxic 5-axes micropositioner integrated with a digital brain atlas (Benchmark Angle Two; MyNeuroLab). Baseline firing was recorded for each unit in normocapnia, followed by a 5-min hypercapnic challenge and then a 5-min minimum normocapnic recovery period. Electrodes were connected to an Axon Multiclamp 700B intracellular amplifier (Molecular Devices) with high pass filter at 300 Hz and low pass filter at 1 kHz bessel via an Axon CV7B high impedance headstage (Molecular Devices). Signals were digitized using Spike 2 (CED) or LabChart (AD Instruments), sampled (>10 kHz), and stored as computer data files for subsequent analysis.
Biotinamide fills.
Extracellular recordings were made with an intracellular amplifier (Axon Multiclamp 700B) in current clamp mode, so that current could be injected through the electrode while action potentials (extracellular field potentials) were monitored. Neurons were indiscriminately assessed for response to hypercapnia. After the completion of the trial, if the neuron signal amplitude was sufficiently large, we attempted to individually fill the neuron with biotinamide using the juxtacellular labeling method (Pinault 1996; Winkler et al. 2006). Recorded neurons were individually filled with biotinamide by applying positive current pulses (400-ms duration, 50% duty cycle) of gradually increasing intensity (0–10 nA maximum in 0.2-nA steps) to each cell through the bridge circuit of the recording amplifier until entrainment of cell discharge to the current pulse was achieved. Cell entrainment was maintained for at least 30 s. These current pulses trigger the iontophoretic ejection of biotinamide, and entrainment facilitates uptake of this marker by the recorded and entrained cell. Entrainment was never initiated when multiple units were visible, and double neuron or ectopic labeling was not observed. Thirty minutes after termination of entrainment were allowed for biotinamide to disperse within the neuron before tissue fixation. The stereotaxic coordinates of the recording site were noted. During recording of single cells, fluctuations in spike amplitude were sometimes observed due to slight changes in relative extracellular position of the electrode and had no correlation with gas treatment or cell discharge. Spike height, width, and shape were monitored before, during, and after juxtacellular entrainment to ensure that only one cell was recorded and labeled (Pinault 1996; Winkler et al. 2006).
Neurons were selected for recording and hypercapnic challenges, and when we were confident that the electrode tip was located within the medullary raphé, the recorded unit was firing spontaneously, and the signal amplitude of the unit was sufficiently large to ensure a satisfactory recording. No discrimination was made based on unit firing characteristics or chemosensitivity. Chemosensitivity was assessed offline and was not always obvious during the hypercapnic trials. In this sense, cells were recorded and filled indiscriminately, based only on likelihood of being in the medullary raphé and exhibiting adequate spontaneous discharge. Therefore, visualized cells should comprise a population representative of the targeted medullary raphé regions.
Immunohistochemistry.
After juxtacellular labeling, rats were perfused through the descending aorta with fixative, 4% paraformaldehyde in 0.1 M PBS. Brainstems were removed and stored overnight in the fixative, cryoprotected with 30% sucrose/PBS until infiltrated, and frozen in hexanes cooled with an ethanol/dry ice slurry. A series of coronal sections (30 μm) were cut through the medulla using a freezing microtome (cryostat), and mounted directly onto slides. Biotinamide introduced into single neurons by juxtacellular labeling was revealed with a streptavidin-Alexa 546 conjugate (no. S-11225; 4 μg/ml; Life Technologies). Sections were incubated in blocking buffer for 1 h (0.3% Triton X-100 and 5% normal goat serum in 0.1M PBS) and then overnight in antibody for the 5-HT-synthesizing enzyme tryptophan hydroxylase (TPH). We used mouse anti-TPH monocolonal primary antibody (no. T0768; 1:1000 dilution in blocking buffer; Sigma) followed by a 1-h incubation in a secondary Alexa 488-labeled goat anti-mouse antibody (no. A-11029; 1:500 dilution in 0.1 M PBS with 5% normal goat serum; Life Technologies). Immunohistochemical controls included incubation of medullary sections without primary antibody to rule out nonspecific binding of the fluorophores and incubation without fluorophores to rule out autofluorescence. The immunohistochemistry protocols were also performed on tissue known not to express target immmunoreactivity. 5-HT-related TPH-immunoreactivity (TPH-ir) was primarily localized in the raphé of medullary sections. Sections were air-dried, mounted with Vectashield (Vector Laboratories), and coverslipped. Low-magnification (×10) images were used to determine the location of biotinamide-labeled cells in relation to anatomical landmarks (ventral surface, pyramids, etc.), and cells were mapped onto the brain atlas at the appropriate location. Local biotinamide fluorescence and TPH-ir were visualized at ×40 to identify colocalization of TPH in biotinamide-labeled neurons. Sections were viewed to confirm sites of recording relative to major anatomical landmarks, and this placement was correlated with areas of the raphé and TPH phenotype of surrounding tissues. Fluorophores were individually excited, and emission spectra were collected separately to minimize interference using a Zeiss LSM510 confocal microscope: biotin-filled neuron, Alexa 546, 543-nm laser, filter BP 560–615; anti-TPH, Alexa 488, 488-nm laser, filter BP505–530. Images at one focal plane were collected with a ×10 objective, and z-stacks were collected with a ×40 objective. The ×40 images are presented as a collapsed projection of a z-stack.
Data analysis.
With the use of indirect immunofluorescence, 5-HT neurons were identified as those expressing TPH-ir. The number and location of cells containing both biotinamide and TPHir were noted. TPH-ir in tissues surrounding biotinamide-labeled neurons was also noted. We discriminated individual extracellular unit activity using computer spike sorting software (Spike 2, CED; Spike Histogram; AD Instruments). Stable 1- to 3-min periods of single unit firing were analyzed before, during, and after hypercapnic challenge (“baseline,” “hypercapnia,” and “recovery,” respectively) to provide a mean value for unit firing frequency (spikes/s), mean interspike interval (ms), standard deviation and standard error of interspike interval, and spike width. Subset analysis was used to classify neurons as unstimulated where relative frequencies failed to increase from baseline by ≥20%, or CO2 stimulated where relative frequencies increased from baseline by ≥20% and returned toward baseline upon return to normocapnia (Wang et al. 1998). Statistical differences were calculated using a one-way repeated-measures ANOVA or a two-tailed student's t-test as appropriate and Holm-Sidak pairwise multiple comparison procedures with an overall significance level set to P ≤ 0.05 (SigmaPlot 12). Values are expressed as means ± SE.
RESULTS
The medullary raphé contains CO2-stimulated and unstimulated neurons.
Single unit extracellular recordings from medullary raphé neurons illustrated that the medullary raphé contains CO2-stimulated and unstimulated neurons. Cell firing frequency and regularity were used to characterize putative 5-HT neurons (Mason 1997; Fornal et al. 1985; we followed this with conclusive identification based on juxtacellular labeling and TPH-ir). Using electrophysiological criteria, we found putative 5-HT neurons in the raphé that did exhibit a CO2-stimulated phenotype.
Electrophysiological characterization of neuronal chemosensitivity was obtained by extracellular recording through CO2 challenges for 124 neurons. Representative recordings are shown in Fig. 1. Sixteen of the one-hundred twenty-four electrophysiologically characterized cells were juxtacellularly filled, subsequently recovered, and successfully visualized to reveal positive TPH-ir, identifying them as 5-HT synthesizing. Representative CO2-stimulated and unstimulated neurons are illustrated in Fig. 2.
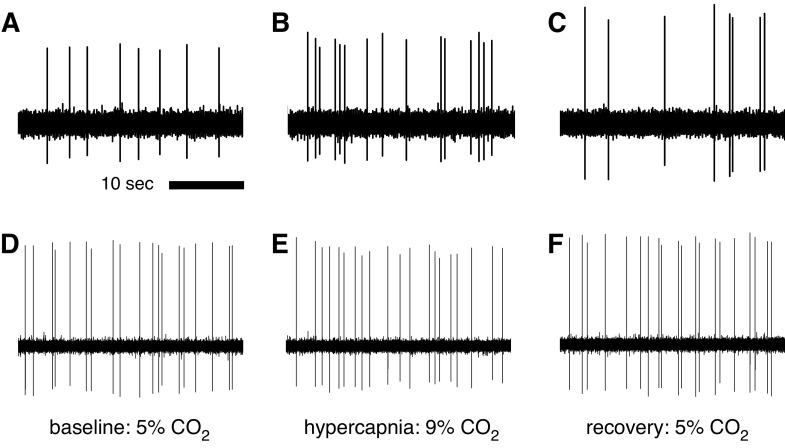
Fig. 1.
Single unit extracellular recordings of CO2-stimulated and unstimulated raphé serotonin-synthesizing (5-HT) neurons in situ. Recordings from a single CO2-stimulated raphé neuron show a firing frequency of 0.26 Hz during exposure to 5% CO2 (A), a 92% increase in firing frequency (to 0.51 Hz) with exposure to 9% CO2 (B), and recovery with return to baseline conditions (C). Recordings from a single unstimulated raphé neuron show firing frequencies of 0.70 Hz during exposure to 5% CO2 (D), 0.67 Hz during exposure to 9% CO2 (E), and 0.72 Hz during recovery normocapnia (F). Firing frequency and regularity analysis indicated these to be putative 5-HT neurons.
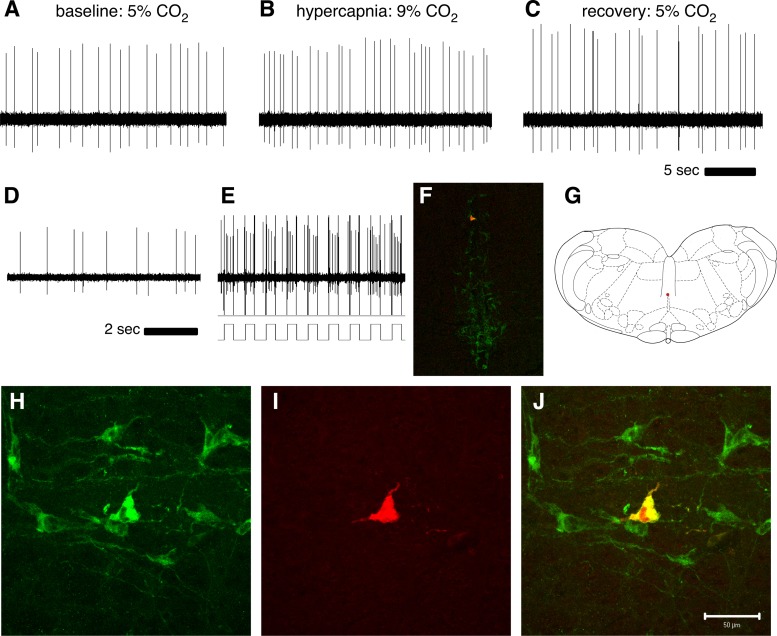
Fig. 2.
Extracellular recording of a CO2-stimulated 5-HT neuron. Recordings during normocapnic baseline (A; 088 Hz), hypercapnia (B; 124 Hz), and normocapnic recovery (C; 107 Hz) demonstrate a CO2-stimulated cell. Recording of this cell was maintained before (D) a juxtacellular entrainment attempt, during which the neuron was stimulated with 400-ms positive current pulses of gradually increasing amplitude Cell entrained with firing synchronous to 0.8 nAmp current pulses (E) and was filled with biotinamide as a result. Biotinamide fill (red) and tryptophan hydroxylase-immunoreactivity (TPH-ir; green) were visualized after histological processing, and a low magnification photomicrograph of a coronal section (F) shows the cell location within raphé magnus, near the dorsal border, on the midline (atlas representation in G; Paxinos and Watson 1998). Higher magnification images of staining in the same section reveal a population of TPH-ir cells (H), including the recorded cell with biotinamide fill (I), and its colocalization with TPH-ir (J), positively identifying the recorded CO2-stimulated cell as 5-HT synthesizing. Height and width analysis of individual spikes (not shown) confirmed recording of the same individual neuron throughout CO2 exposure and juxtacellular labeling.
CO2-stimulated and unstimulated 5-HT medullary raphé cells are distinct and have different baseline, hypercapnic, and recovery firing frequencies.
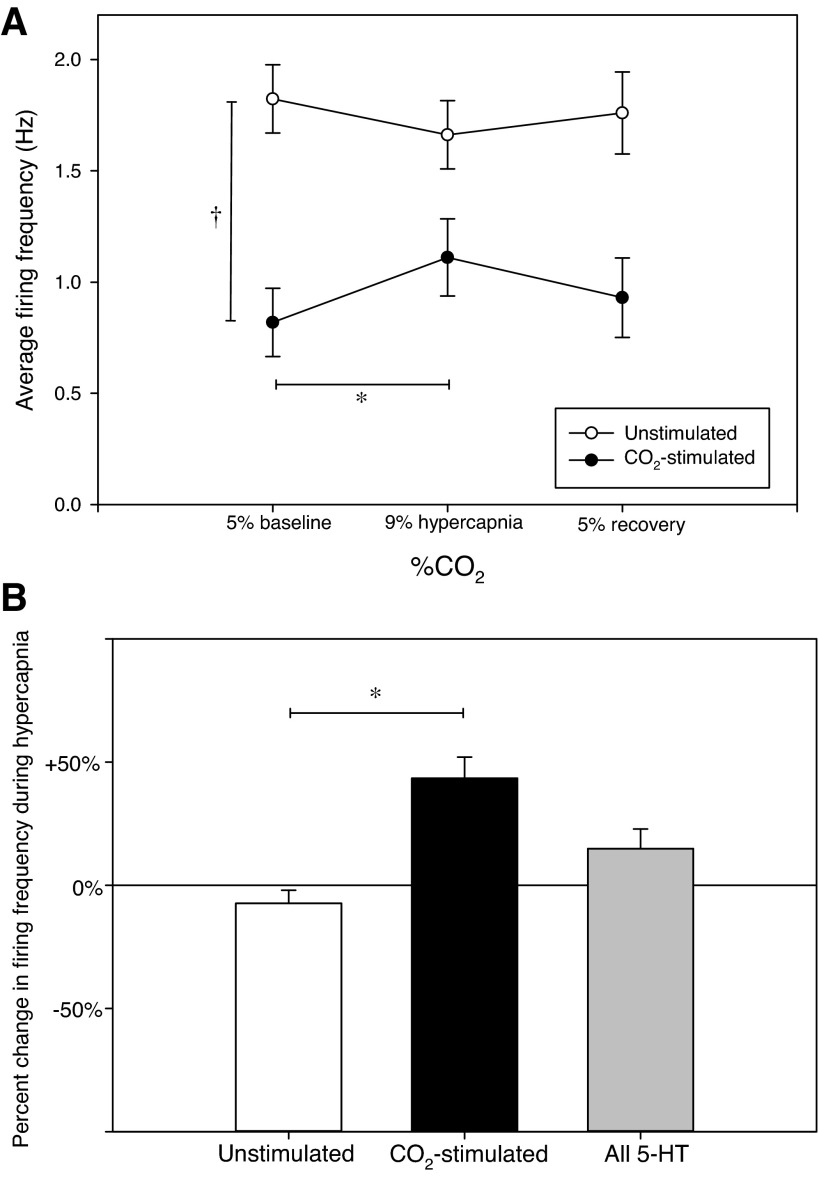
In the present study, we have based our interpretation of 5-HT neuron behavior solely on the definitively phenotyped 5-HT cells. Of the 16 juxtacellulary labeled 5-HT-synthesizing cells, seven (44%) were CO2 stimulated, and nine (56%) were unstimulated (Fig. 3A). Firing characteristics distinguished these as two homogeneous and distinct subsets that differ in mean spontaneous firing frequencies during all gas conditions. Mean baseline, hypercapnic, and recovery firing rates for the CO2-stimulated 5-HT cells were 0.82 ± 0.15, 1.11 ± 0.17, and 0.93 ± 0.18 Hz, respectively. As expected, ANOVA confirms that mean hypercapnic firing rates were increased from baseline in CO2-stimulated 5-HT cells (43%; P < 0.001). Mean baseline and recovery firing frequencies did not differ, confirming a return to baseline firing rate after 5 min of recovery normocapnia (P = 0.055). Mean baseline, hypercapnic, and recovery firing rates for the unstimulated 5-HT cells were 1.82 ± 0.15, 1.66 ± 0.15, and 1.76 ± 0.18 Hz, respectively. ANOVA confirms no hypercapnic response in the unstimulated 5-HT group (P = 0.436). Firing frequencies at baseline, hypercapnia, and recovery all differed between the CO2-stimulated and unstimulated 5-HT groups, with unstimulated cells exhibiting greater firing frequencies on average than CO2-stimulated cells (Fig. 3A; P < 0.05 for all gas conditions). Hypercapnic firing frequencies normalized to respective baseline firing frequencies confirm significant differences in hypercapnic responses between the CO2-stimulated and unstimulated 5-HT cells (Fig. 3B; t-test: P < 0.001). Hypercapnia produced a mean increase in firing frequency of 43% in CO2-stimulated 5-HT cells. Without consideration of heterogeneity between distinct CO2-stimulated and unstimulated 5-HT neuron subsets, 5-HT neurons collectively do not present consistent CO2 sensitivity (15% increase overall; ANOVA: F2,30 = 0.104, P = 0.902, n = 16).
Fig. 3.
CO2-stimulated and unstimulated 5-HT medullary raphé cells have different baseline firing frequencies and hypercapnic sensitivities. Of 16 juxtacellulary labeled 5-HT cells, 7 (44%) were CO2 stimulated and 9 (56%) were unstimulated (A). Hypercapnia caused a mean 43% increase (*F2,12 = 16.014, P = 0.001, n = 7) in firing rate of CO2-stimulated 5-HT cells (●) and normocapnic recovery returned firing frequencies to baseline levels. Hypercapnia and recovery normocapnia did not affect firing rate of unstimulated 5-HT cells (○; *F2,16 = 0.874, P = 0.436, n = 9). Mean baseline, hypercapnic, and recovery firing frequencies differed between CO2-stimulated and unstimulated 5-HT neurons during all gas conditions (†baseline t14 = 4.545, P < 0.001; hypercapnia t14 = 2.377, P = 0.003; recovery t14 = 3.164, P = 0.007). B: hypercapnic firing frequencies normalized to respective baseline firing frequencies confirm differences in hypercapnic responses between the CO2-stimulated and unstimulated 5-HT cells (*t14 = −5.234, P < 0.001). 5-HT neurons collectively do not present consistent chemosensitivity (grey bar).
CO2-stimulated 5-HT cells are clustered in the rostral medullary raphé.
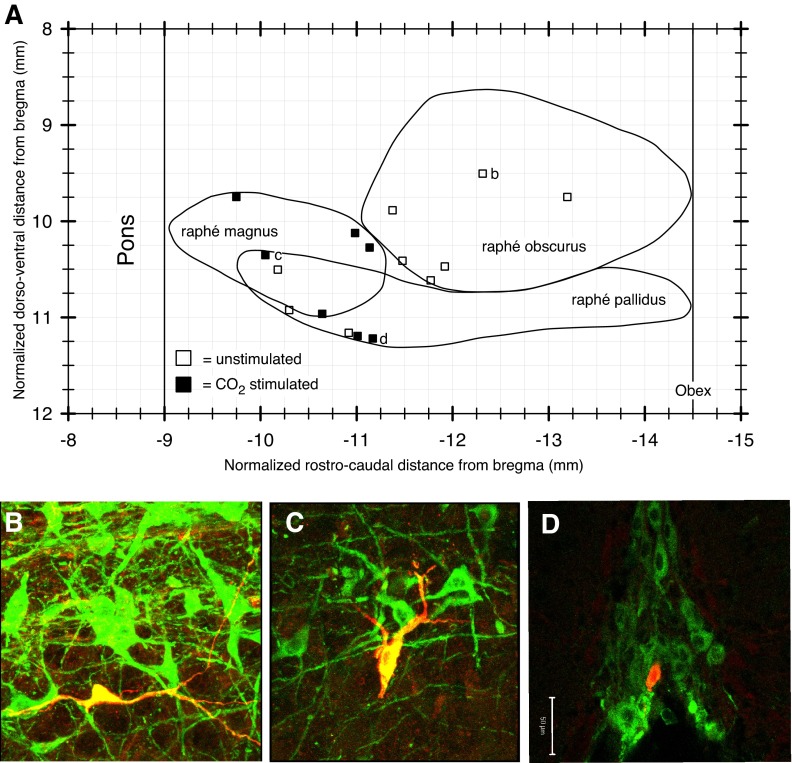
We determined the relative anatomical locations, based on stereotaxic coordinates, and histological examination, of biotin-filled 5-HT neurons. All cells were found within the medullary raphé. A plot (Fig. 4A) of the location of the cells on a sagittal representation of the medullary raphé nuclei indicates that the average location of the CO2-stimulated 5-HT neurons is somewhat more rostral than that of the unstimulated 5-HT cells. All CO2-stimulated 5-HT cells were located in either the rostral half of raphé pallidus or in raphé magnus, which is the most rostral nucleus of the medullary raphé. The unstimulated 5-HT cells were found throughout the medullary raphé, with the majority (6 out of 9) in the raphé obscurus. The TPH-ir of an unstimulated 5-HT cell in the raphé obscurus is shown in Fig. 4B. Figure 4C shows the location and TPH-ir of an individual CO2-stimulated 5-HT cell in raphé magnus. Figure 4D shows a CO2-stimulated 5-HT cell in raphé pallidus, on the ventral surface of the brainstem, apposed to the basilar artery cavity. All cells were proximal to the midline (within 0.1 mm). The CO2-stimulated 5-HT cells described here are clustered in the rostral medullary raphé midline (raphé magnus and raphé pallidus), likely within the anatomical region thought to arise from the r5 rhombomeric 5-HT sublineage (Jensen et al. 2008; Brust et al. 2010). We observed no correlation with 5-HT neuron chemosensitivity and any aspect of morphologhy (e.g., size, shape, and arborization pattern) or spike shape.
Fig. 4.
CO2-stimulated 5-HT cells are clustered in the rostral medullary raphé midline (raphé magnus and raphé pallidus). A: position of juxtacellularly labeled 5-HT neurons plotted onto a sagittal outline of the medullary raphé nuclei. Representative ×40 photomicrographs show an unstimulated raphé obscurus cell (B), a CO2-stimulated raphé magnus cell (C), and a CO2-stimulated raphé pallidus cell (D). Biotinamide fills are red, and TPH-ir is green (B—D).
DISCUSSION
The fact that CO2-sensitive 5-HT neurons have not been conclusively identified in intact experimental preparations limits the extrapolation of in vitro characterizations of intrinsically CO2-sensitive raphé 5-HT neurons and the resulting conclusion that these cells contribute to central chemosensitivity in vivo. Here we provide the first application of the juxtacellular labeling technique, in conjunction with electrophysiological characterization, to conclusively identify the neurotransmitter phenotype of specific chemosensitive and insensitive raphé neurons in an unanesthetized rodent preparation with an intact brainstem respiratory network. We have confirmed the presence of CO2-stimulated and unstimulated neurons in the intact raphé (Veasey et al. 1995) and for the first time shown that a subset of neurons identified to be TPH-ir are stimulated by moderate levels of hypercapnia. Furthermore, we have shown the distribution of CO2-stimulated 5-HT neurons across the raphé. These are the first characterizations of the heterogeneity of raphé chemosensitivity in situ. The findings demonstrate the utility of combining these methods to identify functionally characterized individual neurons whose activity may make a contribution to central chemosensitivity in the intact animal. Our recordings illustrate that CO2-sensitive neurons are common within the raphé. These recordings, when combined with juxtacellular labeling and immunohistochemical neurotransmitter phenotyping, confirm the presence of CO2-sensitive raphé 5-HT neurons. As we conclusively identify a subset of 5-HT neurons that are not chemosensitive to the applied stimuli, our data also caution against the supposition of raphé and 5-HT neuron homogeneity.
As with previous studies, we classified neurons as “CO2 stimulated” if they presented a minimum 20% increase in firing frequency with exposure to arterial hypercapnia or “unstimulated” if they did not (Wang et al. 1998). The current study was designed to test the hypothesis that CO2-stimulated raphé neurons conclusively identified as 5-HT occur under a physiologically relevant level of chemostimulation and in a relatively intact preparation lacking the influences of anesthesia. As we show that a proportion of 5-HT neurons are CO2 stimulated, our data support this hypothesis. Results indicate heterogeneity with respect to general CO2sensitivity of neurons within the raphé; CO2 sensitivity of specifically identified raphé 5-HT neurons; and distribution of CO2-sensitive 5-HT neurons across raphé subregions. Without consideration of this heterogeneity, 5-HT neurons collectively would not present consistent chemosensitivity (Fig. 3B) (Mulkey et al. 2004; DePuy et al. 2011; Takakura and Moreira 2013). We are mindful that unstimulated cells may have been generally insensitive or simply insensitive with respect to firing frequency within the range of chemostimulation used and under the conditions of study. We cannot exclude the possibility that such cells would present chemosensitivity under different conditions.
Of the labeled 5-HT cells included in this study, the CO2-stimulated cells have a lower baseline firing rate than the unstimulated 5-HT cells (Fig. 3A). Baseline firing rate itself may determine whether or not a 5-HT cell will respond to hypercapnia. The maximum baseline firing frequency for any of the 5-HT cells was 2.3 Hz. The maximum observed firing frequency of any of the 5-HT cells during hypercapnic exposure was 2.5 Hz. It may be that 2.5 Hz approximates a “ceiling” for 5-HT neuron firing in situ, and that CO2-insensitive fast-firing 5-HT cells may already be at their physiological maximum firing frequency. Alternatively, chemosensitive and insensitive 5-HT neurons may simply represent different classes of neurons with different properties. Characteristics that distinguish CO2-sensitive 5-HT cells from insensitive 5-HT cells in situ are unknown.
The relative anatomical distribution of CO2-sensitive and insensitive 5-HT neurons suggest that different classes of 5-HT cells are clustered in particular subregions (Fig. 4). Results agree with other demonstrations of 5-HT neuron groups defined by anatomy (Dahlström and Fuxe 1964), developmental origin (Wallace and Lauder 1983; Jensen et al. 2008; Wylie et al. 2010), and chemoresponsiveness or other physiological function (Jacobs et al. 2002; Madden and Morrison 2006; Brust et al. 2010; Ray et al. 2011). Central 5-HT cells are not homogeneous (Jacobs and Azmitia 1992; Kiyasova et al. 2011) but rather show variety in function, morphology, connectivity, receptor subtypes, and electrophysiological characteristics (Dahlström and Fuxe 1964; Allers and Sharp 2003; Kirby et al. 2003; Kocsis et al. 2006; Hajós et al. 2007; Schweimer and Ungless 2010; Bang et al. 2011; Calizo et al. 2011). The varying functions and widespread projections of the 5-HT system support a need for distinct subpopulations of 5-HT cells. 5-HT neuron subtypes with specific molecular developmental histories and requirements have been defined, based on genetic lineage (Jensen et al. 2008; Kiyasova and Gaspar 2011). Furthermore, these subtypes have functional and pathophysiological heterogeneity (Kinney et al. 2011). The 5-HT neurons identified here as chemosensitive likely constitute at least one of these neuron categories, distinct in anatomical architecture, function, and developmental lineage (Brust et al. 2010; Ray et al. 2011). Many studies have demonstrated that focal cell-specific lesions and genetic or pharmocalogical disruption of serotonergic processes affect ventilatory chemosensitivity in vivo (Hodges et al. 2004, 2008, 2009, 2011; Nattie et al. 2004; Taylor et al. 2005; Dias et al. 2007; Erickson et al. 2007; Li and Nattie 2008; Cummings et al. 2011a, 2011b; Penatti et al. 2011a; Ray et al. 2011; Barrett et al. 2012). The rostral portion of the medullary raphé appears to be especially important in these disruptions.
We observe that 44% of recorded medullary raphé 5-HT cells were CO2 stimulated and that these show a 43% mean increase in firing with hypercapnia. These findings are similar to those of Veasey et al. (1995, 1997) who report 22% of putative 5-HT neurons in the medulla and 25% of those in dorsal raphé nucleus show a similar increase in firing rate during CO2 breathing in awake, freely moving cats. Collectively, in situ and in vitro data indicate that raphé 5-HT neurons are heterogeneous and include a subset of CO2-sensitive neurons. Because of the sample size of the recorded neurons in the present study (n = 124, 16 of which were identified as TPH-ir), the characterization of chemosensitivity (or lack thereof) of recorded neurons should accurately reflect overall characteristics of the medullary raphé region in situ. We have indiscriminately selected a random sample representative of the medullary raphé as a whole and shown that spontaneously active CO2-sensitive 5-HT cells are located in raphé magnus and raphé pallidus. No CO2-stimulated 5-HT cells were conclusively identified in the raphé obscurus. Although several CO2-stimulated neurons (n = 10) were recorded in this region, these cells were not immunohistochemically phenotyped or anatomically visualized.
Our observation of CO2-sensitive 5-HT neurons in the rostral medullary raphé may reflect higher concentrations of such cells in that area, although this may simply reflect a coincidental bias due to sample size. However, a scarcity of CO2-sensitive 5-HT cells in the caudal medullary raphé is reported by others (Mulkey et al. 2004; DePuy et al. 2011; Veasey et al. 1995). It should not be presumed that 5-HT cells located in different regions are homogeneous and consistently chemosensitive (Mulkey et al. 2004; DePuy et al 2011; Takakura and Moreira 2013). Rather, as we show, medullary raphé 5-HT neurons are heterogenous and certainly include populations that are CO2 stimulated.
Respiratory pathologies occur in SIDS, CCHS, SUDEP, Prader-Willi Syndrome, obstructive sleep apnea, panic disorder, and neurodegenerative diseases. Medullary 5-HT neuron dysfunction is thought to play a role in all of these disorders (Hilaire et al. 2010; Kinney 2009; Richerson et al. 2001; Sowers et al. 2013). In infants that have died from SIDS, brain tissue has been found to have lower levels of 5-HT, TPH, and/or decrease in 5-HT1A receptor binding (Paterson et al. 2006; Kinney et al. 2009; Kinney and Thach 2009; Duncan et al. 2010). SIDS is thought result from the brain's inability to restore homeostasis following a life-threatening challenge (a failure of state-dependent respiratory and autonomic control; Kinney et al. 2009). During development, even transient disturbances of 5-HT can permanently change the structure of cell networks and receptor expression (Nakamura and Hasegawa 2007). The ubiquitous projections of serotonin neurons (the 5-HT system) and the wealth of evidence for 5-HT modulation of not only breathing and chemosensitivity but also anxiety, cerebrovascular control, arousal, pain, and thermoregulation (Richerson 2010) support an overall function of the 5-HT system in global control of neural tissue homeostasis or motor control (Jacobs and Fornal 1997; Azmitia 2007); the 5-HT system is thought to play a general gain-setting role in sensory and motor systems (Mason 2011).
Control of breathing and central chemoreception are complex functions that involve many brainstem regions, messengers, and cellular mechanisms. The relationship between structure and function and the physiology underlying both of these functions, and the role of the 5-HT system in these functions have been only somewhat elucidated. 5-HT could effect neuronal development, chemosensitivity, or excitatory modulation of chemoreceptor and respiratory neurons. Substance P, 5-HT, and thyrotropin-releasing hormone are all released by raphé 5-HT neurons in response to stimulation. These transmitters each stimulate ventilatory motor output, both in vivo and in vitro by increasing excitability of rhythm-generating, premotor, and motor neurons, thus increasing ventilation for the ultimate effect of restoring homeostatic levels of blood CO2 (Dekin et al. 1985; Ptak et al. 2009; Doi and Ramirez 2008; Corcoran et al. 2009). In addition to direct chemosensation, 5-HT neurons have been shown to play a modulatory role in chemoreception, by enhancing the hypercapnic response of the rest of the respiratory network (Hodges et al. 2008). This evidence collectively forms the basis for the serotonin hypothesis of central chemosensitivity, which contends that serotonergic brainstem neurons are respiratory chemosensors. Since 5-HT neurons are intrinsically chemosensitive in vitro, they are stimulated by hypercapnia in vivo, they stimulate respiratory output in vivo, and the hypercapnic ventilatory response is attenuated when 5-HT neurons are disrupted, 5-HT neurons are proposed to function as chemoreceptors. This hypothesis does not contend that 5-HT neurons are the exclusive sensory transducers of hypercapnia in the brain. Rather, 5-HT neurons are one component of a network of chemosensitive brain sites (Richerson 2004).
Since raphé cells contribute to several different crucial homeostatic processes, there is no surprise in our observations that not all 5-HT raphé cells are chemosensitive and that chemosensitive neurons occur as a substantial subset within a heterogeneous population in the medullary raphé. Although we have demonstrated that CO2-stimulated raphé 5-HT neurons occur in situ, these data do not show that raphé 5-HT neurons are chemoreceptors per se. However, a great deal of in vitro evidence suggests that raphé neurons are intrinsically chemosensitive, project to and stimulate respiratory neurons, and thus have the capacity of central chemoreceptors. Lacking has been documentation in intact unanesthetized preparations that raphé 5-HT neurons increase firing rate in response to hypercapnia. The present study provides critical evidence in support of the overall conclusion that raphé 5-HT neurons are chemoreceptors contributing to central chemosensitivity.
GRANTS
Research supported by National Institutes of Health Grants 2U54-NS-041069-06A1 (to M. B. Harris and G. B. Richerson), P20-GM-103395 (to M. B. Harris), P20-NS-076916 (to G. B. Richerson), and R01-HD-052772 (to G. B. Richerson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.E.I. performed experiments; K E.I. analyzed data; K.E.I. and M.B.H. interpreted results of experiments; K.E.I. prepared figures; K.E.I., G.B.R., and M.B.H. edited and revised manuscript; K.E.I., G.B.R., and M.B.H. approved final version of manuscript; K. E. I., G.B.R., and M.B.H. conception and design of research; K.E.I. drafted manuscript.
ACKNOWLEDGMENTS
We thank Shaun Morrison for generous and critical technical assistance.
REFERENCES
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphé nucleus using juxtacellular labelling methods in vivo. Neuroscience 122: 193–204, 2003 [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin and brain: evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol 77: 31–56, 2007 [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci 35: 85–96, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KT, Kinney HC, Li A, Daubenspeck JA, Leiter JC, Nattie EE. Subtle alterations in breathing and heart rate control in the 5-HT1A receptor knockout mouse in early postnatal development. J Appl Physiol 113: 1585–1593, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust RD, Richerson GB, Wu Y, Dymecki SM. Mapping chemosensitivity onto genetically-defined serotonergic neuron subtypes (Online). Program no. 188.16. 2010 Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, 2010 [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA 107: 16354–16359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, Pan YZ, Lemos JC, Craige C, Heemstra LA, Beck SG. Raphé serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61: 524–543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Richerson GB, Harris MB. Serotonergic mechanisms are necessary for central respiratory chemoresponsiveness in situ. Respir Physiol Neurobiol 186: 214–220, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol 589: 5247–5256, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Commons KG, Hewitt JC, Daubenspeck JA, Li A, Kinney HC, Nattie EE. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: Implications for SIDS. J Appl Physiol 111: 825–833, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brainstem neurons. Acta Physiol Scan Suppl 232: 1–55, 1964 [PubMed] [Google Scholar]
- Darnall RA. The role of CO2 and central chemoreception in the control of breathing in the fetus and the neonate. Respir Physiol Neurobiol 173: 201–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science 229: 67–69, 1985 [DOI] [PubMed] [Google Scholar]
- DePuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphé obscurus serotonergic neurons in mice. J Neurosci 31: 1981–1990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG. Raphé magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol 103: 1780–1788, 2007 [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA 303: 430–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol 159: 85–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. 2003 Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Exp Neurol 88: 590–608, 1985 [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol 90: 247–253; discussion 253–257; 2005 [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol 518: 3883–3906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Abbott SB, Stornetta RL. The respiratory chemoreception conundrum: light at the end of the tunnel? Brain Res 1511: 126–137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Allers KA, Jennings K, Sharp T, Charette G, Sk A, Kocsis B. Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphé nucleus using juxtacellular labelling methods. Eur J Neurosci 25: 119–126, 2007 [DOI] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR. Hypercapnic exposure in congenital central hypoventilation syndrome reveals CNS respiratory control mechanisms. J Neurophysiol 93: 1647–1658, 2005 [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Tolentino-Silva F, Pete G, Kc P, Mack SO. Monoaminergic neurons, chemosensation and arousal. Respir Physiol 129: 191–209, 2001 [DOI] [PubMed] [Google Scholar]
- Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol 174: 76–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol 173: 256–263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol 96: 1815–1824, 2004 [DOI] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol 177: 133–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 72: 165–229, 1992 [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol 7: 820–825, 1997 [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martn-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40: 45–52, 2002 [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci 11: 417–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen SL, Iceman KE, Richerson GB, Harris MB. Influence of isoflurane on CO2 sensitive and insensitive raphé neurons (Online). Program no. 897.08. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012 [Google Scholar]
- Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol 19: 327–341, 2005 [DOI] [PubMed] [Google Scholar]
- Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol 51: 223–233, 2009 [DOI] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med 361: 795–805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol 4: 517–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Broadbelt KG, Haynes RL, Rognum IJ, Paterson DS. The serotonergic anatomy of the developing human medulla oblongata: Implications for pediatric disorders of homeostasis. J Chem Neuroanat 41: 182–199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphé nucleus: electrophysiological and immunohistochemical studies. Neuroscience 116: 669–683, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Gaspar P. Development of raphé serotonin neurons from specification to guidance. Eur J Neurosci 34: 1553–1562, 2011 [DOI] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar AP. Genetically defined morphologically and functionally unique subset of 5-ht neurons in the mouse raphé nuclei. J Neurosci 31: 2756–2768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: identification of raphé neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci USA 103: 1059–1064, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larnicol N, Wallois F, Berquin P, Gros F, Rose D. C-fos-like immunoreactivity in the cat's neuraxis following moderate hypoxia or hypercapnia. J Physiol (Paris) 88: 81–88, 1994 [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia. J Physiol 586: 2321–2329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol 577: 525–537, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol 77: 1087–1098, 1997, [DOI] [PubMed] [Google Scholar]
- Mason P. 2011 From descending pain modulation to obesity via the medullary raphé. Pain 152: S20–4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey CA, Richerson GB, Wu Y. Isoflurane eliminates serotonin (5-HT) neuron chemosensitivity in vitro and markedly depresses the hypercapnic ventilatory response in vivo (Online). Program no. 897.04. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012 [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hasegawa H. Developmental role of tryptophan hydroxylase in the nervous system. Mol Neurobiol 35: 45–54, 2007 [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106: 1464–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson GB, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556: 235–253, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson AC. Stereotaxic Atlas of the Rat Brain. New York: Academic, 1998 [Google Scholar]
- Penatti E, Barina A, Schram K, Li A, Nattie E. Serotonin transporter null male mouse pups have lower ventilation in air and 5% CO2 at postnatal ages P15 and P25. Respir Physiol Neurobiol 177: 6165, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti EM, Barina AE, Raju SC, Li A, Kinney HC, Commons KG, Nattie EE. Maternal dietary tryptophan deficiency alters cardiorespiratory control in rat pups. J Appl Physiol 110: 318–328, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pete G, Mack SO, Haxhiu MA, Walbaum S, Gauda EB. CO2-induced c-Fos expression in brainstem preprotachykinin mRNA containing neurons. Respir Physiol Neurobiol 130: 265–274, 2002 [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods 65: 113–136, 1996 [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raph neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333: 637–642, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004 [DOI] [PubMed] [Google Scholar]
- Richerson GB. Respiratory plasticity in sleep apnoea: should it be harnessed or restrained? J Physiol 588: 3–4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129: 175–189, 2001 [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–266; discussion 266–269; 2005 [DOI] [PubMed] [Google Scholar]
- Schweimer JV, Ungless MA. Phasic responses in dorsal raphé serotonin neurons to noxious stimuli. Neuroscience 171: 1209–1215, 2010 [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci 6: 1139–1140, 2003 [DOI] [PubMed] [Google Scholar]
- Sowers LP, Massey CA, Gehlbach BK, Granner MA, Richerson GB. Sudden unexpected death in epilepsy: Fatal post-ictal respiratory and arousal mechanisms. Respir Physiol Neurobiol pii: S1569–9048, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS. Arterial chemoreceptor activation reduces the activity of parapyramidal serotonergic neurons in rats. Neuroscience 237C: 199–207, 2013 [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566: 543–557, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppin VAL, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol 103: 220–227, 2007 [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphé neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphé neurons to specific motor challenges in freely moving cats. Neuroscience 79: 161–169, 1997 [DOI] [PubMed] [Google Scholar]
- Wallace JA, Lauder JM. Development of the serotonergic system in the rat embryo: an immunocytochemical study. Brain Res Bull 10: 459–479, 1983 [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphé neurones in primary tissue culture. J Physiol 51: 433–450, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis stimulated neurons of the medullary raphé are serotonergic. J Neurophysiol 85: 2224–2235, 2001 [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Remmers JE, Paton JF. Brain stem Po2 and pH of the working heart-brain stem preparation during vascular perfusion with aqueous medium. Am J Physiol Regul Integr Comp Physiol 281: R528–R538, 2001 [DOI] [PubMed] [Google Scholar]
- Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol 96: 3465–3473, 2006 [DOI] [PubMed] [Google Scholar]
- Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, Fox S, Maeno H, Deneris ES. Distinct transcriptomes define rostral and caudal serotonin neurons. J Neurosci 30: 670–684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]