Abstract
N-methyl-d-aspartate receptor (NMDAR)-mediated activity is required for whisker-related neural patterning in the rodent brain. Deletion of the essential NMDAR subunit NR1 gene in excitatory cortical neurons prevents whisker-specific barrel formation and impairs thalamocortical afferent patterning. We used electrophysiological and voltage-sensitive dye imaging methods to assess synaptic and sensory evoked cortical activity and immunohistochemistry to examine immediate early gene expression following whisker stimulation in cortex-specific NR1 knockout (CxNR1KO) mice. In mutant mice, layer IV neurons lacked NMDAR-mediated excitatory postsynaptic currents, and temporal summation of excitatory postsynaptic potentials (EPSPs) was impaired. Barrel neurons showed both phasic and tonic responses to whisker deflection. The averaged tonic response in CxNR1KO mice was significantly less than that in control mice due to impaired EPSP temporal summation. Electrophysiological estimation of the number of thalamic neurons innervating single barrel neurons indicated a significant increase in CxNR1KO mice. Similarly, voltage-sensitive dye optical signals in response to whisker stimulation were widespread. Immediate early gene expression following whisker stimulation also showed a diffuse expression pattern in the CxNR1KO cortex compared with whisker-specific expression patterns in controls. Thus, when NMDAR function is impaired, spatial discrimination of whisker inputs is severely compromised, and sensory stimulation evokes diffuse, topographically misaligned activity in the barrel cortex.
Keywords: barrel cortex, c-fos, immediate early gene expression, NMDA receptor, whisker
n-methyl-d-aspartate receptor (NMDAR)-mediated activity is critical for the development of whisker-specific neural patterns in the mouse brain. Genetic function blocking studies by targeted knockout of the NR1 or NR2B subunit genes led to failure in whisker-related pattern formation in the trigeminal brain-stem and downstream thalamic and cortical somatosensory centers (Iwasato et al. 1997; Kutsuwada et al. 1996; Li et al. 1994). Absence of NR1 subunit gene in excitatory cortical neurons (cortex-specific NR1 knockout, CxNR1KO mice) yielded a phenotype in which the thalamic and brain-stem whisker patterns were normal but in the neocortex layer IV stellate cells failed to form barrels (Iwasato et al. 2000). In wild-type mice, dendritic trees of layer IV stellate cells show a biased orientation toward patches of whisker-specific thalamocortical afferent (TCA) terminals (Datwani et al. 2002; Lee et al. 2005; Steffen and Van der Loos 1980). In CxNR1KO mice, this orientation bias was absent even though the TCAs formed rudimentary patterns related to the five rows of large whiskers (Datwani et al. 2002; Iwasato et al. 2000). Detailed analyses of single TCA arbors in these mice showed that there is extensive terminal branching, the area of which could occupy multiple barrels if they were present (Lee et al. 2005).
CxNR1KO mice were generated using Emx1cre and floxed NR1 mouse lines; therefore, all excitatory neurons in the neocortex, hippocampus and the olfactory bulb lack functional NMDARs (Iwasato et al. 2000). These mice are viable for up to a month and a half in age, are smaller in size, and display severe behavioral abnormalities. For the somatosensory system, not much is known at a functional level other than the detailed morphological analyses of the development of their barrel cortex (Datwani et al. 2002; Lee et al. 2005). In this study, we used thalamocortical slice preparations, in vivo electrophysiological recordings, voltage-sensitive dye imaging, and immunohistochemical analysis of immediate early gene, c-fos, expression following natural or artificial whisker stimulation to assess the consequences of impaired NMDAR function in the somatosensory cortex.
Our results first confirmed that layer IV excitatory neurons in CxNR1KO mice lack NMDAR-mediated postsynaptic responses. We discovered that in the absence of functional NMDARs, temporal summation of repetitive thalamic inputs is impaired, and the tonic response of cortical neurons to whisker deflection is reduced markedly. In line with morphological results, single layer IV excitatory neurons receive more convergent thalamic inputs in CxNR1KO mice than those in control mice. Activity-dependent voltage-sensitive dye imaging and immediate early gene, c-fos, expression in CxNR1KO mice also revealed activation of wider cortical areas and absence of whisker-barrel matching in the primary somatosensory (S1) cortex. These findings indicate that CxNR1KO mice have poor spatial discrimination of whisker-related inputs and impaired precision in topographic connectivity between the whisker barreloids in the thalamus and the barrels in the cortex.
MATERIALS AND METHODS
Generation of the CxNR1KO mouse line was described previously (Iwasato et al. 2000). Defects in thalamocortical axon patterning and cellular patterning in these mice were also reported in detail (Datwani et al. 2002; Lee et al. 2005). For the present study, we mated Emx1-Cre females and NR1-flox males to obtain CxNR1KO mice (Iwasato et al. 2000). We used Emx1Cre/+; NR1flox/− (CxNR1KO) and controls (Emx1Cre/+; NR1flox/+, NR1flox/− alone or NR1flox/+ mice) on 2- to 4-wk-old mice for in vitro thalamocortical slice physiology and voltage-sensitive dye imaging experiments. Juvenile (4–5 wk old) CxNR1KO and controls were used for in vivo electrophysiology and postnatal day (P) 15 mice for c-fos experiments. Genotypes were determined by PCR from tail lysate DNA samples as reported previously (Datwani et al. 2002; Iwasato et al. 2000; Lee et al. 2005). All animal handling was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) and a protocol approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee.
Whisker stimulation.
Previously, Staiger et al. (2000, 2002) showed that exploration of a novel environment induces expression of transcription factors and immediate early genes in a patterned fashion in the rat barrel cortex. We used a similar paradigm and obtained whisker row-specific immediate early gene, c-fos, expression in the barrel cortex and in the subcortical trigeminal centers of control mice. Adult CxNR1KO mice do not readily explore their environment and are smaller in size compared with controls. At younger ages, mutant mice are similar in their weight and behavior to their littermates. Thus we chose P15 for whisker stimulation in knockout and control animals. We first shaved all of the whiskers except C row whiskers. The following day, individual mice were placed in a plastic well with their heads poking out, and the intact row C whiskers were stimulated with a small horsehair paintbrush for 30 min. Forty-five minutes after the stimulation session, the mice were euthanized and perfused with 4% buffered paraformaldehyde (PFA). These experiments were carried out in four control and four 4-wk-old CxNR1KO mice.
Immunohistochemistry.
Cortices were dissected and flattened in PFA. The remainder of the brain was sectioned in the coronal plane at 50-μm thickness with a vibratome (Leica VT1000 S). After several rinses in phosphate buffer (PB), free-floating sections were incubated in antibody (Ab) solutions at 4°C for 48 h. We performed triple immunostaining using Abs against NeuN for neuronal labeling, vesicular glutamate transporters 1 or 2 for afferent terminals in the principal sensory trigeminal (PrV) nucleus (VGLUT1), ventroposteromedial (VPM) thalamic nucleus, and the barrel cortex (VGLUT2) and c-fos for activity-dependent immediate early gene expression in all three trigeminal stations. The primary Abs were rabbit polyclonal c-fos Ab (1:500; Santa Cruz Biotechnology), guinea pig polyclonal Ab VGLUT2 (1:500; Millipore Bioscience), guinea pig polyclonal Ab VGLUT1 (1:500; Millipore Bioscience), and mouse monoclonal Ab NeuN (1:500; Millipore Bioscience). After primary Abs were washed from the sections, fluorescent secondary Abs (FITC-conjugated donkey anti-guinea pig, 1:80; Cy3-conjugated donkey anti-rabbit, 1:125; and Alexa 647-conjugated donkey anti-mouse, 1:125; all from Jackson ImmunoResearch Laboratories) were applied for 1.5 h. Afterward, the sections were rinsed in PB several times, mounted onto glass slides, and coverslipped with fluorescence mounting medium.
The sections were examined under a Nikon Eclipse 90i fluorescent microscope, and regions of interest (ROI) were photographed using filters of different wavelengths appropriate for the fluorescent tag of each secondary Ab and using the NIS-Elements software.
Brain slice preparation for electrophysiology.
Mice (10 wild-type and 9 CxNR1KO) were anesthetized by intraperitoneal injection of pentobarbital (100 mg/kg) and decapitated. The brain was rapidly removed and immersed in cold (4°C) sucrose-based artificial cerebrospinal fluid (ACSF; in mM: 75 sucrose, 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 25 NaHCO3, 25 glucose, 0.5 CaCl2) bubbled with 95% O2-5% CO2 (pH 7.4). Thalamocortical slices were cut (400 μm) with a vibratome (Campden 7000smz) in sucrose-based ACSF at an angle of 55° from the midsagittal plane and 10° from the coronal plane (Agmon and Connors 1992; Lee et al. 2005). After 40-min incubation in sucrose-based ACSF at 35°C, the slices were transferred into normal ACSF (in mM: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 25 NaHCO3, 25 glucose, 2 CaCl2, pH 7.4) at room temperature for at least 1 h. The slice containing the thalamocortical pathway was transferred into a submerged-type recording chamber (RC-27L; Warner Instruments) and continuously perfused (>2 ml/min) with normal ACSF at room temperature. During electrophysiological recording, 50 μM picrotoxin was added into the ACSF to block GABAergic responses.
Electrophysiology.
Whole cell-patch micropipettes were pulled horizontally in four stages from borosilicate glass [K150F-4; World Precision Instruments (WPI)] with a P-87 puller (Sutter Instrument). The patch electrodes were backfilled with a cesium-based intracellular solution (in mM: 115 CsMeSO3, 10 NaCl, 1 KCl, 4 MgCl2, 1 MgCl2, 11 EGTA, 20 HEPES, 3 Na2-ATP, 0.5 Na2-GTP, 0.1 spermine, pH 7.25, >290 mosM) with a tip resistance of 5–9 MΩ. Layer IV excitatory neurons in the barrel cortex were visualized with infrared light/differential interference contrast optics of Olympus BX51WI upright microscope. After forming whole cell configuration, depolarizing current pulses were passed through the patch pipette to identify firing pattern of excitatory neurons (Agmon and Connors 1992; Beierlein et al. 2003) in current-clamp mode. A concentric stimulating electrode (TM33CCINS; WPI) was inserted into the VPM. Electrical pulses (0.3-ms duration, 0.2 Hz, 0–800 μA) were passed through the electrode to evoke excitatory postsynaptic responses in both current- and voltage-clamp mode. Paired pulses at an interval of 200 ms were delivered to test the paired-pulse ratio (PPR) of layer IV neurons. All biological data were acquired by an InstruTECH ITC-16 Data Acquisition Interface unit and stored on a Dell DM061 computer with PULSE (HEKA) software.
Multiple input index analysis.
Layer IV excitatory neurons were voltage-clamped at +60 mV to show excitatory postsynaptic currents (EPSCs) induced by stimulation of VPM at 0.2 Hz, and the stimulus intensity was gradually increased from 0 to 800 μA at steps of 10 μA as described previously (Lo et al. 2011). The peak amplitudes of EPSCs were measured and plotted against stimulus intensity. The amplitude of EPSCs enhanced in a stepwise manner following the increase in stimulus intensity. We first measured the baseline noise of recordings and calculated the standard deviation (SD) of the noise. The variation in amplitude of EPSCs was analyzed. If the amplitude of an EPSC was larger than the prior EPSC by >3 times of SD, a “jumping step” was defined, because the fluctuation of EPSCs induced by the same stimulus intensity was always <3 times of noise SD. The number of jumping steps (multiple input index, MII) provided an estimate of the lower limit number of VPM neurons that innervate the recorded cortical neuron.
In vivo electrophysiology.
Five-week-old control (n = 9) and knockout mice (n = 7) were anesthetized by intraperitoneal injection of urethane (1.5 g/kg body wt). Supplemental doses of urethane (0.5 g/kg) were given when needed to keep the animal without pain reflex. The head was fixed in a stereotaxic adaptor (515625M; Stoelting). A craniotomy was performed to expose the barrel cortex (1–3 mm posterior to bregma, 2–4 mm lateral from midline). Glass microelectrodes filled with 3 M NaCl (20–40 MΩ) were used for extracellular recordings and 3 M KAc (50–80 MΩ) for intracellular recordings from layer II to VI neurons in the barrel cortex. The whiskers on the contralateral side were stimulated by air puffs (20 psi, 300 ms at 0.33 Hz) generated by a pneumatic pressure pump (Picospritzer II; Parker Hannifin, Pine Brook, NJ) so that most of the whiskers were deflected rostrocaudally around 15° for 300 ms. The number of spikes during the 300-ms deflection was averaged for each cell.
Statistical analysis.
All data are expressed as means ± SE, and a Student's t-test was used.
Optical imaging.
Experiments were performed on six CxNR1KO and six control mice at 3 wk of age using MiCAM02 system (Brainvision, Tokyo, Japan). Animals were anesthetized with urethane, and their heads were shaved and placed into a stereotaxic frame. A 3- × 3-mm cranial opening was made over the left parietal cortex. The exposed dural surface was cleaned with hemostatic sponge dipped in ACSF. During the experiment, the body temperature of the animal was kept at 37°C by a temperature-controlled heating pad. The voltage-sensitive dye RH-1691 (Optical Imaging; 1.0 mg/ml in ACSF) was applied to the exposed dural surface for 45 min. After staining, the cortex was washed with dye-free ACSF for 15 min (Grinvald and Hildesheim 2004; Tsytsarev et al. 2009, 2010). The cortical surface was covered with high-density silicone oil and then sealed with a 0.1-mm-thick cover glass to suppress the brain pulsation originating from cardiovascular and respiratory movements.
MiCAM02 objective was positioned above the recording area with its optical axis perpendicular to the imaging area. The focusing plane was adjusted to the depth of 200 μm below the cortical surface (the border between layers II/III and IV in 3-wk-old mice). At the start of each optical recording, a grayscale image of the cortical surface was obtained and then moved down 200 μm to the ROI. Whereas the focal plane was 200 μm below the cortical surface, signals obtained are a combination of fluorescence coming from ∼150 μm below and above the focal plane as has been described in other, similar studies (Grinvald et al. 2001).
Each experiment consisted of 100 trials, 500 frames per trial, with the stimulus (whisker E2 deflection) presented at the 300th frame, 1 trial per stimulus. The intertrial interval was 8 s. Change in fluorescence was calculated as ΔF/F (in percentage; Tominaga et al. 2013) in the ROI using BrainVision Analyzer. Before whisker stimulation, all of the whiskers except row E whisker 2 (E2 whisker) were clipped close to the skin. A glass pipette (1.0 mm in diameter) fitted onto an XYZ manipulator was aimed at the E2 whisker. Air-puff stimulus of 25-ms duration was applied through a Picospritzer pressure valve connected to the glass pipette. The Picospritzer was coupled to the imaging system through the MiCAM02 computer so that the air could be puffed onto the whiskers and optical signals collected simultaneously. In a few exemplary cases, after the termination of the experiment, the animal was euthanized, and the brain was removed and processed for histology to assess the depth of layers II/III and IV.
For data analysis, we constructed a pseudocolor map using first-frame analysis and then averaging the data for each session (Ferezou et al. 2006; Orbach et al. 1985; Petersen et al. 2003; Shoham et al. 1999; Tsytsarev et al. 2009, 2010). Thus we obtained pseudocolor maps of the areas activated by whisker stimulus.
RESULTS
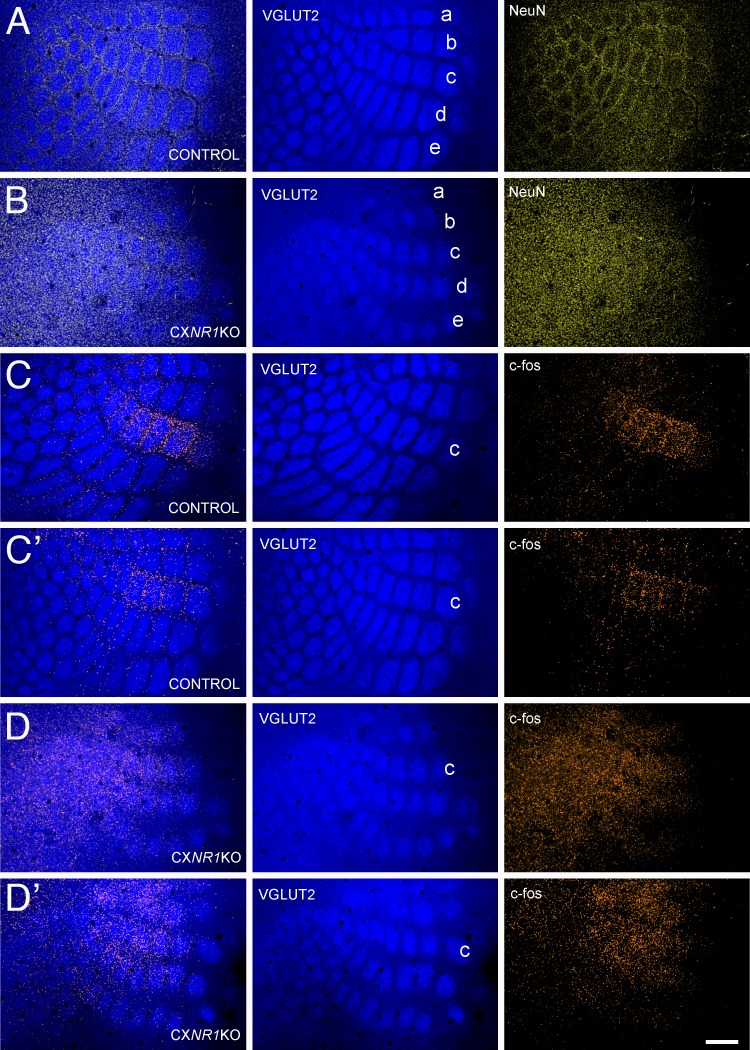
As previously reported, barrel cortex of CxNR1KO mice lack cellular patterns (barrels) even though TCAs form rudimentary patterns (Datwani et al. 2002; Iwasato et al. 2000). We first confirmed this phenotype by double-labeling the barrel cortex with VGLUT2 and NeuN Abs for TCA and cellular patterns, respectively. As illustrated in Fig. 1, A and B, distinct but less-developed and smaller TCA patterns can be detected in the mutant cortex, whereas cellular patterning is absent.
Fig. 1.
Altered cortical phenotype in CxNR1KO mice and immediate early gene, c-fos, expression in the barrel cortex following whisker stimulation. A and B: morphological appearance of the barrel cortex in control (A) and CxNR1KO (B) mice. Vesicular glutamate transporter 2 (VGLUT2) immunostaining reveals thalamocortical afferent (TCA) terminal patterns (blue) and NeuN immunostaining cellular, barrel patterns (yellow). The 1st micrograph in each horizontal series is an overlay of the 2 staining patterns. Note that in the CxNR1KO cortex TCA, patterns are smaller and less defined and there is no cellular patterning. C and C’: examples of barrel cortex from 2 different control cases immunostained for VGLUT-2 for TCA patterning (blue) and c-fos for immediate early gene expression (orange) following row C whisker stimulation. Whisker representations for rows A–E are indicated (also in Fig. 2). Note that c-fos immunoreactivity is mostly confined to C row barrels. D and D’: c-fos immunostaining in CxNR1KO cortex (orange) from 2 different cases shows diffuse staining without any patterning after row C whisker stimulation. Scale bar = 200 μm.
Immediate early gene c-fos expression following whisker stimulation.
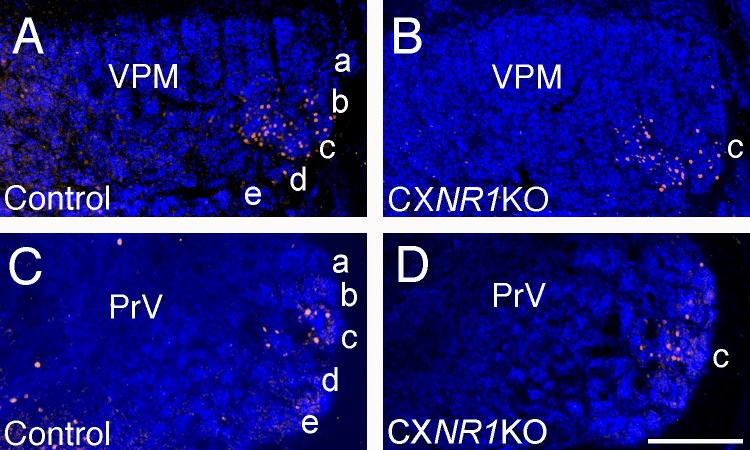
Unilateral clipping of all whiskers except row C and manual stimulation of the intact whiskers back and forth (anterior to posterior) with a brush yielded distinct and localized c-fos immunoreactivity in row C barrels (Fig. 1, C and C’). In striking contrast, similar experiments in CxNR1KO mice yielded c-fos immunolabeling diffusely distributed across the barrel field area without notable localization (Fig. 1, D and D’). Unlike the barrel cortex, C row whisker stimulation-induced c-fos immunoreactivity in subcortical trigeminal centers, the PrV and the VPM, were comparable and localized in both the control and mutant brains (Fig. 2). Thus the alterations in c-fos immunoreactivity patterns occurred only in the barrel cortex of CxNR1KO mice. Whisker stimulation-induced c-fos activity reveals a general picture of the functional state of the whisker-barrel pathway. We performed in vitro brain slice and in vivo electrophysiology to gain further insight into information processing along this pathway in the absence of functional NMDARs in the barrel cortex.
Fig. 2.
Whisker stimulation-induced c-fos expression in the ventroposteromedial thalamic nucleus (VPM) and the principal sensory trigeminal nucleus (PrV) is similar in control and CxNR1KO mice at postnatal day (P) 15. A and B: VGLUT2 (blue) and c-fos (orange) expression in the VPM following row C whisker stimulation in control (A) and CxNR1KO (B) mice. C and D: VGLUT1 (blue) and c-fos (orange) expression in the PrV following row C whisker stimulation in control (C) and CxNR1KO (D) mice. Scale bar = 200 μm for A and B and 80 μm for C and D.
Layer IV excitatory neurons of the barrel cortex lack NMDAR-mediated postsynaptic responses in CxNR1KO mice.
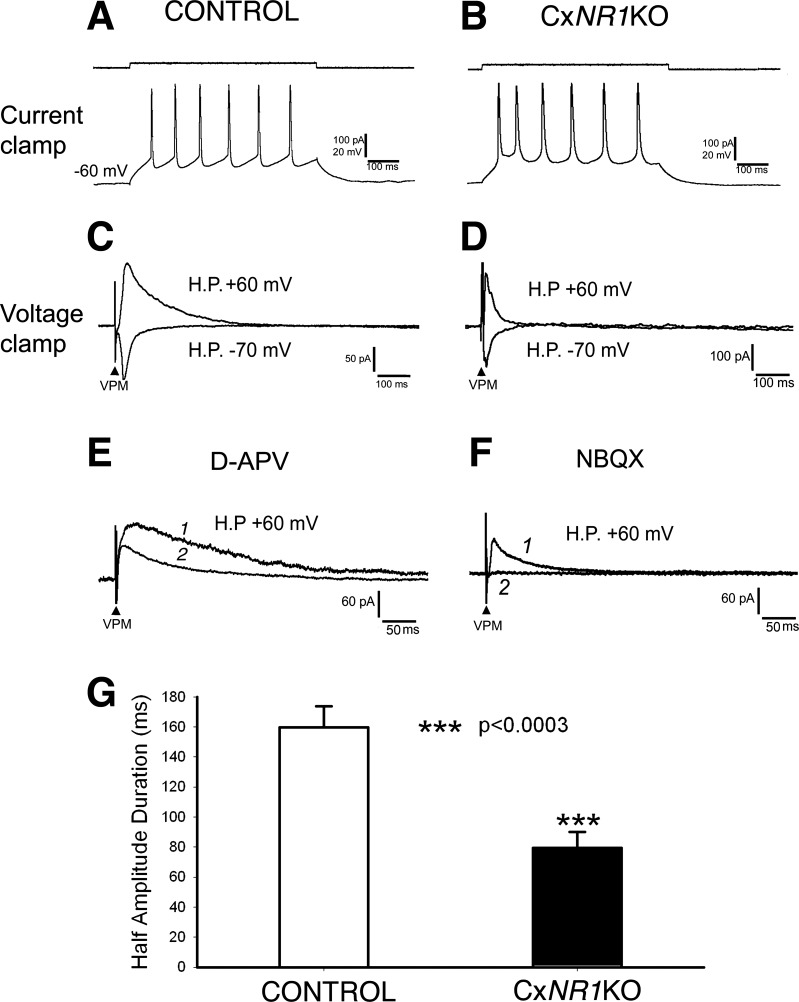
Because in the barrel cortex of CxNR1KO mice only excitatory neurons lack NR1 subunits (Iwasato et al. 2000), we analyzed electrophysiological data collected from layer IV excitatory neurons electrophysiologically defined in previous studies (Agmon and Connors 1992; Beierlein et al. 2003; Feldmeyer et al. 1999). In control mice, layer IV excitatory neurons responded to membrane depolarization with an adapting train of spikes (regular spike; Fig. 3A). Stimulation of the VPM nucleus induced an EPSC that had a longer duration at a holding potential of +60 mV and a short duration at −70 mV (Fig. 3C), suggesting that the EPSC was mediated by both AMPA receptors (AMPARs) and NMDARs. The EPSC at +60 mV was partially blocked by application of d-APV (100 μM), an NMDAR antagonist, and left the AMPAR-mediated EPSC intact (Fig. 3E, trace 1 vs. trace 2). To see the temporal difference between AMPAR-mediated EPSC and NMDAR-mediated (d-APV-sensitive) current, we show the record at a faster sweep speed. Note that at the peak of AMPAR-mediated EPSC (peak latency was ∼8 ms), the amplitude of NMDAR-mediated current is negligible, similar to our previous observation in the PrV (Lo and Zhao 2011). In CxNR1KO cortex, excitatory neurons also fired regular spikes (Fig. 3B). The EPSCs induced from stimulation of VPM had similar durations at different holding potentials (Fig. 3D). The EPSC at +60 mV was completely blocked by NBQX (10 μM), an AMPAR antagonist, indicating the absence of NMDAR-mediated postsynaptic response (Fig. 3F, trace 1 vs. trace 2, also at a faster sweep speed). Note that the amplitude of AMPAR-mediated EPSCs at +60 mV (Fig. 3, D–F) is considerably higher compared with that at −70 mV (Fig. 3, C and D), indicating that AMPARs of recorded neurons contain glutamate receptor subunit 2 (GluR2) without inward rectification as shown in GluR2-lacking AMPARs [for review, see Isaac et al. (2007)]. Absence of NMDAR-mediated currents in excitatory cortical neurons is predicted from in situ hybridization and Western blot data (Iwasato et al. 2000); the present result is the first physiological confirmation at a single-cell level.
Fig. 3.
Layer IV excitatory neurons of the barrel cortex lack N-methyl-d-aspartate receptor (NMDAR)-mediated postsynaptic responses in CxNR1KO mice. A: in control mice, layer IV excitatory neurons respond to membrane depolarization with an adapting train of spikes (regular spike). C: at a holding potential (H.P.) of +60-mV, stimulation of the VPM induces excitatory postsynaptic current (EPSC) with a longer duration, whereas at −70 mV it has a short duration, indicating that the EPSC is mediated by AMPA receptors (AMPARs) and NMDARs. E: at +60 mV, the EPSC is partially blocked by application of an NMDAR antagonist, d-APV (100 μM; trace 1, before; trace 2, after d-APV). To see the temporal difference between AMPAR-mediated EPSC and NMDAR-mediated (d-APV-sensitive) current, we show the record at a faster sweep speed. B: in CxNR1KO mice, layer IV excitatory neurons also fire regular spikes. D: the EPSCs induced from stimulation of VPM have similar durations at different holding potentials. F: the EPSC at +60 mV is completely blocked by AMPAR antagonist NBQX (10 μM, trace 1, before; trace 2, after NBQX, also at a faster sweep speed). G: in control mice, the averaged half-amplitude duration of EPSCs at +60 mV is 159.7 ± 14.0 ms (n = 15), whereas that in CxNR1KO mice is 79.6 ± 10.4 ms, which is significantly shorter (P < 0.0003) than control mice.
We measured the half-amplitude duration of EPSCs. In control mice, the averaged half-amplitude duration of EPSCs at +60 mV was 159.7 ± 14.0 ms (n = 15), whereas that in CxNR1KO mice was 79.6 ± 10.4 ms (n = 11), ∼50% shorter (P < 0.0003) than control mice (Fig. 3G). Next, we tested the effect of shortened postsynaptic responses on synaptic transmission of thalamic inputs.
Temporal summation of repetitive thalamic afferents is impaired in CxNR1KO barrel cortex.
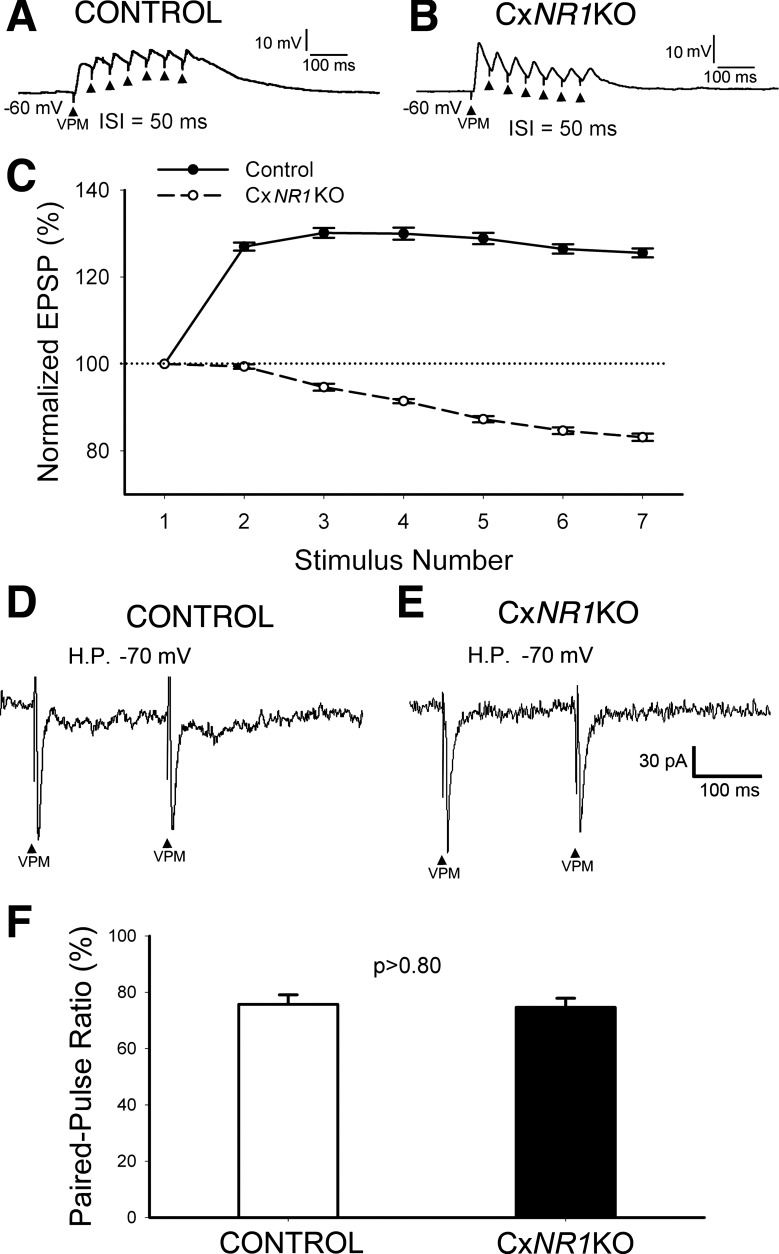
As reported earlier, 37% VPM neurons display slowly adapting responses with tonic discharges of 23.74 ± 18.86 spikes per second (Simons and Carvell 1989). We stimulated VPM with seven pulses at 20 Hz (interstimulus interval = 50 ms) to test postsynaptic responses. In control mice, all excitatory postsynaptic potentials (EPSPs) overlapped and built up a membrane depolarization (Fig. 4A). The curve of averaged temporal summation of EPSPs (Fig. 4C; n = 16, from 5 neurons) indicated that the peaks of subsequent EPSPs (measured from peak to −60 mV) were higher than the peak of the 1st EPSP. Specifically, when stimulus intensity increases, each EPSP can trigger a postsynaptic spike, suggesting that the thalamic afferent signals can be transmitted to the barrel cortex faithfully. However, in CxNR1KO mice, the EPSPs could not build up a membrane depolarization (Fig. 4B). The averaged peaks of subsequent EPSPs were gradually lower than the 1st (Fig. 4C; n = 20, from 6 neurons). Thus the temporal summation of EPSPs at 20 Hz of layer IV excitatory neurons was impaired in CxNR1KO mice. That means only early EPSPs can trigger spikes so that the transmission of tonic thalamic signals to barrel cortex is impaired in CxNR1KO mice. Therefore, the best way to test temporal summation is to count the number of spikes (spiking probability) induced by the same stimulus. The outcome of temporal summation of EPSPs depends on two factors: duration of EPSP and presynaptic release probability. We used a paired-pulse protocol with 200-ms interstimulus interval (Lo and Erzurumlu 2011) to test presynaptic release probability. For these experiments, the PPR was measured from AMPAR-mediated EPSCs at a holding potential of −70 mV.
Fig. 4.
Temporal summation of repetitive thalamic inputs is impaired in CxNR1KO mice. A: all excitatory postsynaptic potentials (EPSPs) induced by stimulation of VPM with 7 pulses at 20 Hz are overlapped to build up a membrane depolarization in control mice. C: plot averaged peak amplitude shows temporal summation curve of EPSPs (solid line, n = 16). B: in CxNR1KO mice, EPSPs cannot build up a membrane depolarization. The averaged peaks of subsequent EPSPs are gradually lower than the 1st (Fig. 2C, dash line; n = 20). D–F: in both control and CxNR1KO mice, layer IV excitatory cells show paired-pulse depression (PPD), and there is no significant difference in the paired-pulse ratio.
An example record from a control mouse is presented in Fig. 4D. Averaged PPR was 75.7 ± 3% (Fig. 4F; n = 18). An example record from a CxNR1KO mouse is given in Fig. 4E. Averaged PPR in CxNR1KO mice was 74.7 ± 3.2% (Fig. 4F; n = 12), which is about the same as that of control mice (P > 0.80). Thus the impaired temporal summation of subsequent EPSPs in CxNR1KO mice is caused by the short duration of EPSPs. Next, we performed in vivo experiments to test the responses to whisker deflection of cortical neurons in control and CxNR1KO mice.
Reduction of tonic responses to whisker deflection of barrel cortical neurons in CxNR1KO mice.
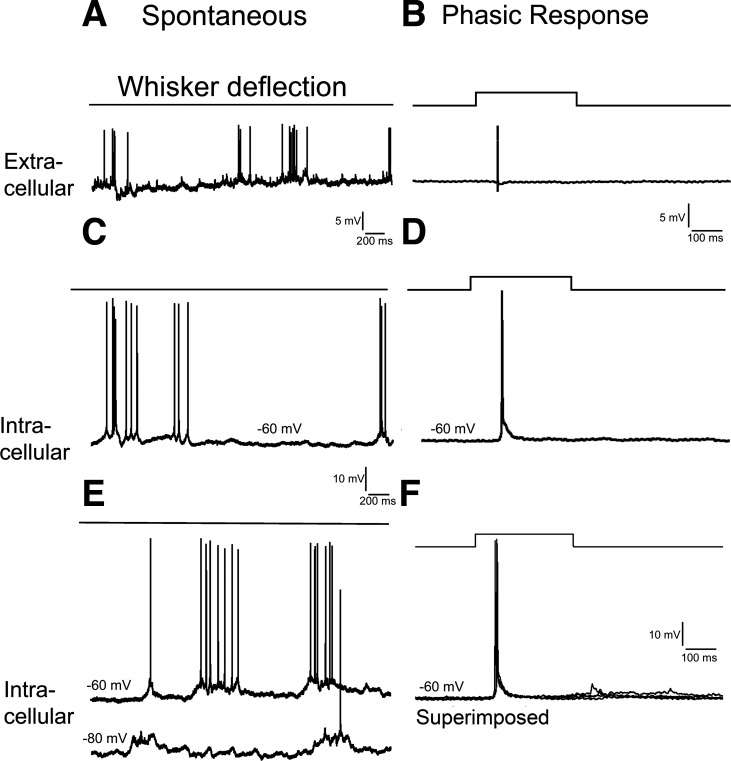
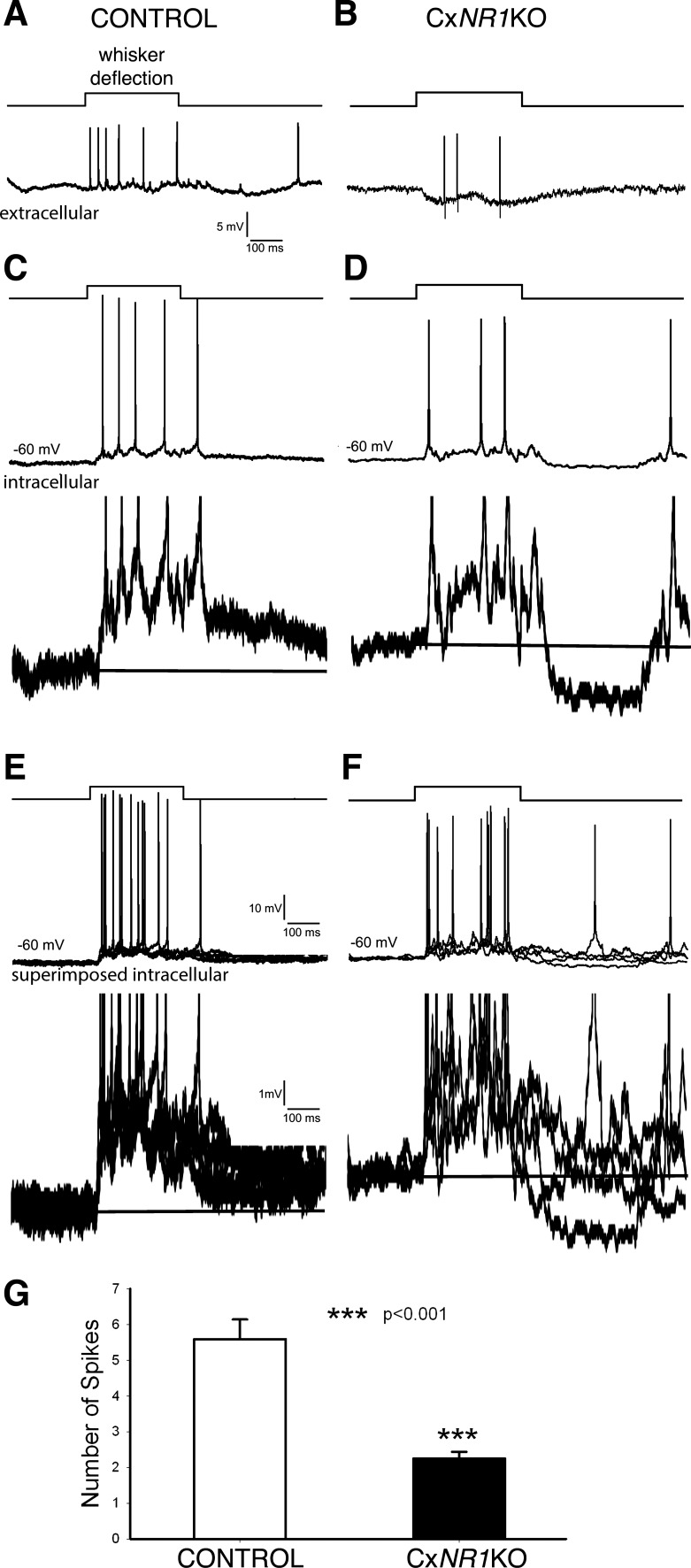
To investigate further temporal summation in the absence of functional NMDARs in excitatory barrel neurons, we performed in vivo experiments and recorded cortical responses to whisker deflection. Both extracellular (Fig. 5A) and intracellular recordings (Fig. 5, C and E) with sharp microelectrodes from barrel cortex showed regular spontaneous activities that are induced by up and down of the membrane potential (Fig. 5E, bottom trace; Petersen et al. 2003). In both control and CxNR1KO mice, there were 2 types of responses to whisker deflection. Phasic (rapidly adapting) response contained 1–2 spikes during whisker deflection with an average of 1.5 ± 0.1 spikes (n = 12, from 4 mice). Example records of phasic responses are given in Fig. 5, B, D, and F. In control mice, tonic (slowly adapting) response contained a train of spikes with an average of 5.6 ± 0.6 spikes (n = 16) that was different from phasic response (P < 0.0001). Example records of extracellular and intracellular tonic responses from control mice are presented in Fig. 6, A and C. Note that superimposed 5 traces of intracellular records (Fig. 6E, bottom trace) revealed a sustained depolarization that gave rise to repetitive spikes. This depolarization was composed of temporal summation of EPSPs, counteracted by feedforward IPSPs from the primary whisker, lateral IPSPs from surrounding whiskers, and spike afterhyperpolarizations. Example records of tonic responses from CxNR1KO mice are shown in Fig. 6, B and D, with much fewer spikes during whisker deflection. Superimposed five traces of intracellular recordings (Fig. 6F, bottom trace) reveal an unstable depolarization with fewer spikes riding on it (Fig. 6F, top trace). We divided the recorded neurons into three groups according to their depth in the neocortex (Quairiaux et al. 2007): layer II-III (150–350 μm), layer IV (350–480 μm), and layer V-VI (480–1,000 μM). In both control and CxNR1KO mice, the spike number of tonic responses showed no layer specificity (P > 0.44), thus we pooled neurons from all layers. An average of spike number of tonic responses in control mice was 5.6 ± 0.6 (n = 16, from 6 wild-type mice; Fig. 6G) and that for CxNR1KO mice was 2.3 ± 0.2 (n = 9, from 4 CxNR1KO mice; Fig. 6G). Thus the tonic responses in CxNR1KO mice reduced by ∼55% (P > 0.001).
Fig. 5.
Spontaneous activities and phasic responses to whisker deflection in barrel cortex of anesthetized control mice. A and C: extracellular and sharp electrode intracellular recordings show repetitive spike trains without whisker deflection. The spike train results from a sustained membrane depolarization (E, bottom trace). B and D: extracellular and intracellular recordings show phasic responses to whisker deflection. Note that the spike is induced by a short EPSP (D), and superimposed 5 traces (F) indicate the EPSPs are of similar latencies.
Fig. 6.
Comparison of tonic responses to whisker deflection between anesthetized control and CxNR1KO mice. A and B: extracellular recordings. C and D: sharp electrode intracellular recordings show different spike numbers (top traces) and difference in membrane depolarization (10 times enlarged bottom traces) between control and CxNR1KO mice. E and F: superimposed 5 traces show that in control mice the depolarization is sustained, whereas in CxNR1KO mice the depolarization is not stable (bottom traces). G: averaged spike numbers during whisker deflection are significantly deferent between control and CxNR1KO mice.
Increase in thalamic inputs to layer IV excitatory neurons in CxNR1KO mice.
Morphological studies showed dendritic trees of layer IV spiny stellate cells fail to orient and single TCA terminals form widespread arbors in CxNR1KO mice (Datwani et al. 2002; Lee et al. 2005). c-fos Immunolabeling and voltage-sensitive dye imaging following whisker stimulation also corroborated morphological results at a functional level. However, we do not know whether the overlapping widespread thalamic afferent arbors converge on single cortical neurons. We employed an additional electrophysiological approach, MII, that has been effectively used in several other systems to assess functional convergence (Arsenault and Zhang 2006; Crepel et al. 1976; Lo et al. 2002, 2011; Lu and Constantine-Paton 2004; Mariani and Changeux 1981; Stevens et al. 2007). We used this approach to estimate the number of VPM neurons converging on single layer IV neurons in the barrel cortex in control and CxNR1KO mice. During these recordings, we paid attention to monosynaptic responses. Occasionally, we recorded polysynaptic EPSCs or EPSPs from layer IV excitatory cells, but the peak latency of the polysynaptic responses is longer than the monosynaptic response. As shown in Fig. 7, A and B, the latencies of the EPSCs are almost fixed, and we measured the amplitude of the first peaks. Thus our MII analysis is derived from monosynaptic EPSCs.
Fig. 7.
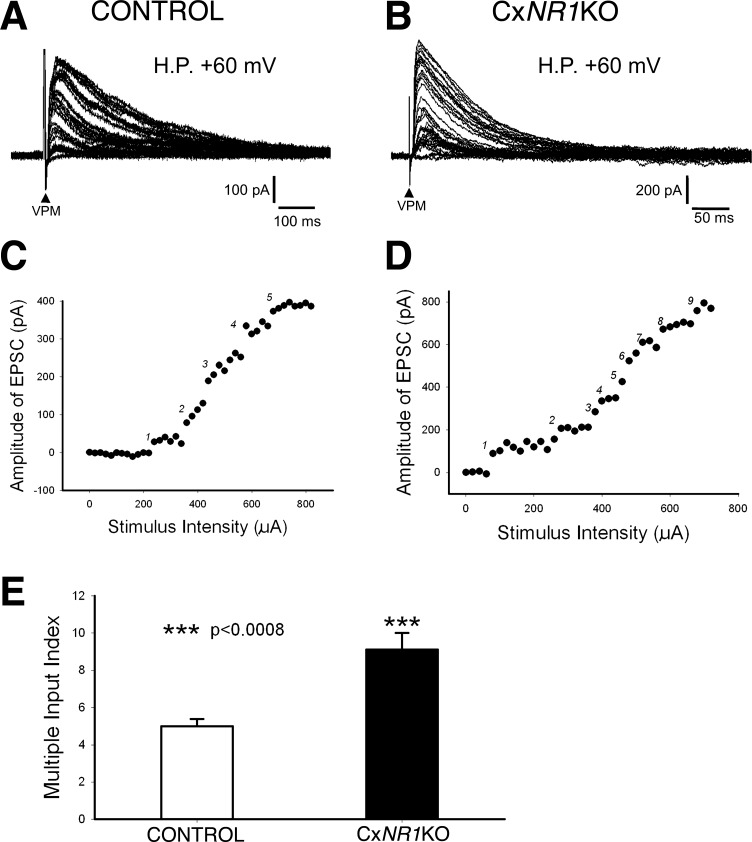
Layer IV excitatory neurons receive more thalamic afferents in CxNR1KO mice than in controls. A: example records from a layer IV excitatory neuron of a control mouse showing EPSCs induced by increasing stimulus intensity form 5 groups separated by big gaps. C: plot peak amplitude of EPSCs in A against stimulus intensity reveals 5 jumping steps [multiple input index (MII) = 5]. B: example records from a layer IV excitatory neuron of a CxNR1KO mouse showing more gaps separating the EPSCs induced by increasing stimulus intensity. D: plot peak amplitude of EPSCs in C against stimulus intensity reveals 9 jumping steps (MII = 9). E: averaged MII in CxNR1KO mice (black bar) is significantly higher (P < 0.0008) than that of control mice (white bar).
At a holding potential of +60 mV, EPSCs induced by incremental increases in stimulus intensity can be gathered into five groups separated by big gaps (Fig. 7A). Plot of peak amplitude of EPSCs against stimulus intensity revealed five jumping steps (Fig. 7C). Therefore, the recorded neuron receives inputs from at least five VPM neurons. Averaged MII in control mice was 5.0 ± 0.4 (n = 15; Fig. 4E). In CxNR1KO mice, the number of jumping steps of EPSCs was much higher. Example records from a layer IV neuron in a CxNR1KO mouse showed nine jumping steps in EPSC amplitude (Fig. 7, B and D). Averaged MII in CxNR1KO mice was 9.1 ± 0.9 (n = 11), suggesting that single layer IV excitatory neurons in CxNR1KO mice receive thalamic inputs from about two times more VPM neurons compared with control mice (P < 0.0008).
The above-described findings should be interpreted with the caveat that the thalamocortical slice cannot contain the entire VPM to SI cortex connections. Thus the MII analysis in thalamocortical slices does not reveal the exact number of VPM cells that converge on a single layer IV cell. Nonetheless, this approach gives a reliable estimate of the lowest number of VPM neurons innervating single cortical cells (Lo et al. 2011).
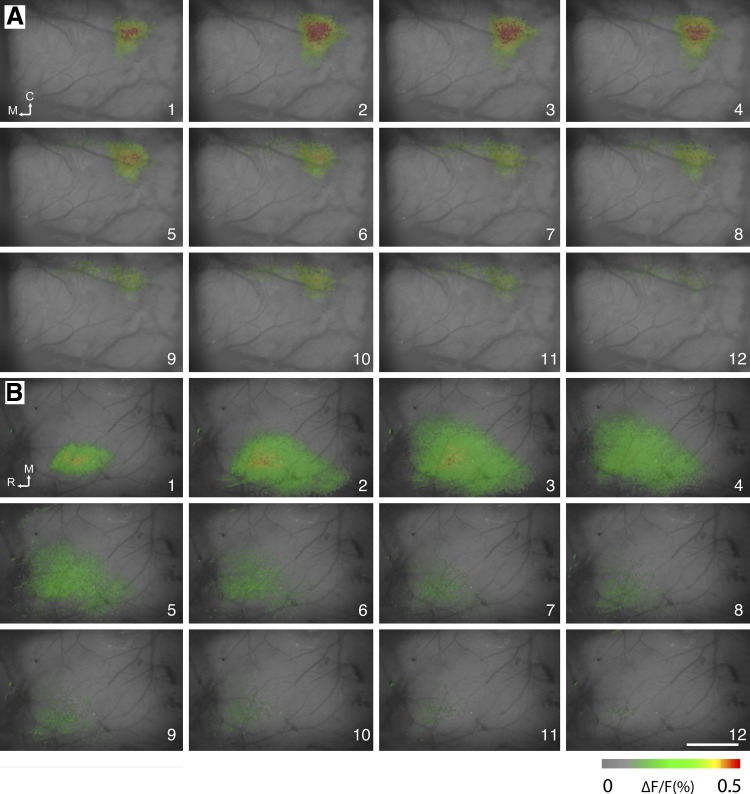
A far more widespread cortical activation area was seen in optical signals following E2 whisker stimulation in CxNR1KO mice. For all examined animals, whisker stimulation elicited optical signals in the barrel field of the contralateral hemisphere. Voltage-sensitive dye signal usually appeared just after the frame when the stimulus was presented and reached its peak 10–20 ms after the stimulus onset and then decreased and returned to baseline within 20–30 ms. There was a striking difference in spatial distribution of the optical signals between the control and mutant barrel cortex (Fig. 8). In control mice, optical signals were located in a discrete spot corresponding to the E2 barrel region, as it has been reported in previous, similar imaging studies in mice (Ferezou et al. 2006). In contrast, in CxNR1KO mice, signals were weak and spread across a conspicuously wide area (1–2 mm2).
Fig. 8.
Voltage-sensitive dye optical images from layer III-IV border (200-μm depth from the surface) of the barrel cortex following E2 whisker stimulation. Fluorescence changes (ΔF/F) are shown in pseudocolors in series of frames from control (A) and in CxNR1KO (B) mice. The stimulus onset was at the beginning of frame 1. Frame number is indicated at the bottom right corner of each image. Duration of each frame is 5 ms. Calibration bar is 1 mm. C, caudal; M, medial; R, rostral.
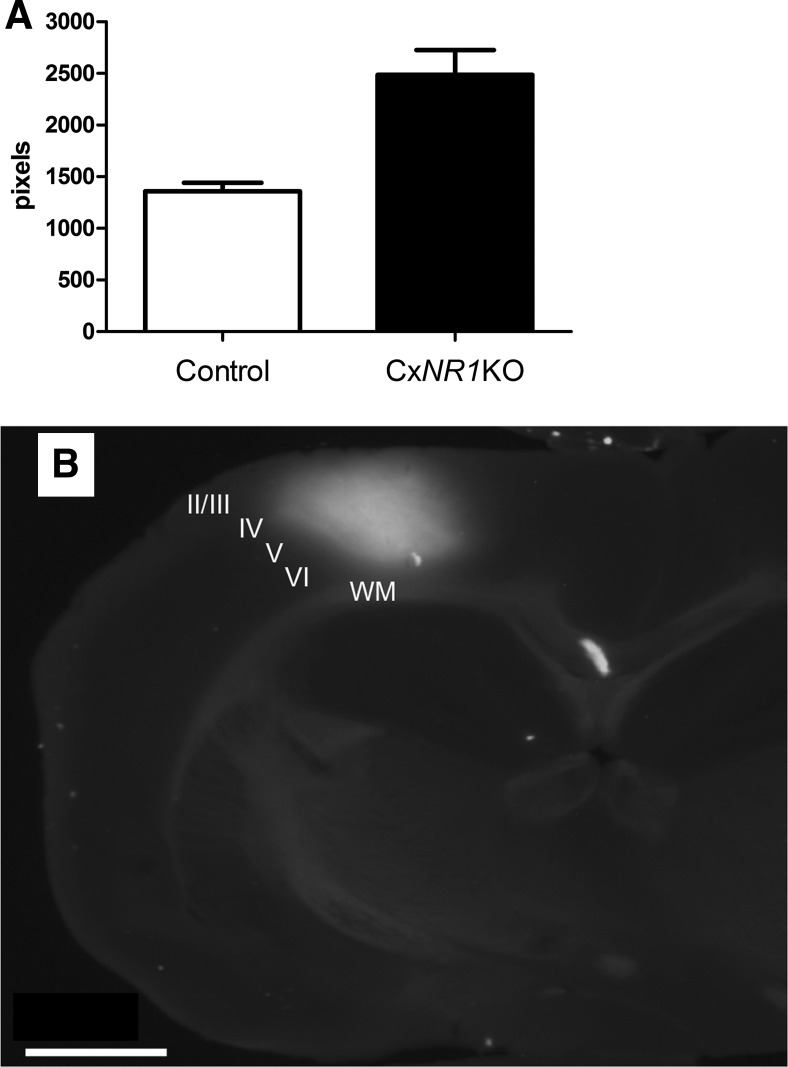
We performed comparative analysis of the areas activated by whisker stimulation. The sizes of the activated areas were calculated and averaged after setting the threshold at half of the maximum signal strength (Tsytsarev et al. 2009, 2010). At maximal size, the activity field covered an area of 2,972 pixels (1.86 mm2) in CxNR1KO and 1,137 pixels (0.71 mm2) in control animals, and the confidences were 591 (0.37 mm2) and 202 (0.13 mm2), respectively (Fig. 9A).
Fig. 9.
Quantitative comparison of areas activated by whisker stimulation in CxNR1KO and control animals and an exemplary dye spread in the cortex. A: activated area sizes are averaged after setting the threshold at half of the maximum signal strength. At its maximum, the activated area corresponded to 2,972 pixels in CxNR1KO cortex, significantly higher (t-test, 2-tailed, 2-sampled equal variants, P = 0.001301) than control cortices (1,137 pixels); the confidences are 591 and 202, respectively. B: example of RH-1691 dye spread in a coronal slice from the neocortex of a P22 mouse. The location of the fluorescence is over the D and E row of barrels, and the dye spans all cortical layers but is most prominent in layers II–IV. Cortical layers are indicated by Roman numerals. WM, white matter. Scale bar = 1 mm.
The difference between sizes of the activated areas of the CxNR1KO and control animals is statistically significant (t-test, 2-tailed, 2-sampled equal variants, P = 0.001301). Figure 9B also illustrates the dye spread along the mediolateral and dorsoventral axes of the neocortex. We could see that the voltage-sensitive dye RH-1691 (Optical Imaging) loading extended across all cortical layers. It is important to note that in the imaging experiments we do not have real depth resolution. The fluorescence signal in each pixel consists of the signal from whole “column” of tissue below this pixel. We set the plane of focus 200 μm below the surface, but this can only partially cut off the signal from above and below the focal plane. Different technical approaches showed that RH-1691 is preferentially located in the layers II–III (Ferezou et al. 2006; Lippert et al. 2007). Layers I–III contain ∼70% of the dye and most likely the fluorescent signal (Lippert et al. 2007). Illumination intensity decreases with depth (because of dye concentration), whereas light scattering increases; activity in layers V–VI has smaller contribution to the signal. Therefore, the voltage-sensitive dye signal in our experiments is most likely a combination of spikes and synaptic activity in layers II–IV.
DISCUSSION
Postsynaptic NMDAR-mediated activity is blocked in the barrel cortex following selective deletion of the NR1 gene in CxNR1KO mice (Iwasato et al. 2000). In these mutants, the whiskers on the snout and subcortical whisker-related patterns appear normal, whereas the structural features of the barrel cortex are not. Patterned aggregation (barrels) of layer IV spiny stellate neurons fail to form, normally skewed dendritic field orientation is absent, and the TCAs form exuberant terminal arbors within which there is rudimentary clustering (Datwani et al. 2002; Iwasato et al. 2000; Lee et al. 2005). Similar barrel cortex defects have also been noted in mice with genetic defects in other components of the glutamatergic pathway such as metabotropic GluR5 (mGluR5) and PLC-β (Ballester-Rosado et al. 2010; Hannan et al. 1998, 2001). Although increasing numbers of genetically modified mouse models with barrel cortex defects are emerging [see Erzurumlu and Gaspar (2012) and Wu et al. (2011) for recent reviews], few studies have investigated the functional and behavioral consequences of altered barrel cortex phenotypes.
The behavioral tests specific for the whisker-barrel pathway function are sparse. Overall, diminished mobility in CxNR1KO mice and lack of exploratory behavior also make it very difficult to assess the behavioral consequences of cortical NMDAR disruption. In most instances, these mutants appear almost catatonic as they grow up. However, it is possible to compare the activity-dependent and electrophysiological characteristics of the thalamocortical pathway by other means as we have done in this study.
CxNR1KO mice do not readily explore novel environments, but it is possible to stimulate individual whiskers and elicit immediate early gene expression patterns in subcortical trigeminal centers similar to control mice. Previous voltage-sensitive dye imaging studies also indicated that the synaptic communication from the whiskers all the way to the neocortex is not blocked; it becomes defective at the cortical level (Iwasato et al. 2000). Our whisker stimulation and c-fos immunostaining experiments also show that there is a patterned, stimulus-induced c-fos expression in a specific manner within the PrV and in the VPM, but in the barrel cortex this expression becomes diffuse and not whisker-specific. This is what one would expect from previously reported exuberant branching of TCAs in the barrel cortex (Lee et al. 2005).
Initial voltage-sensitive dye imaging of the CxNR1KO barrel cortex confirmed lack of NMDAR-mediated excitation (Iwasato et al. 2000). The present study further confirms this observation at a cellular level showing layer IV excitatory neurons in CxNR1KO mice do not have NMDAR-mediated EPSC. Thus the half-amplitude duration of EPSC in CxNR1KO mice is ∼50% shorter than that of control mice at ages of P8–P28. This may be true also for adult mice because the switch from NR2B to NR2A subunits of NMDAR is largely completed (Lu et al. 2001) and the kinetics of NMDAR-mediated EPSP is stabilized after P7.
In juvenile CxNR1KO mice, the tonic response to whisker deflection is reduced by ∼55% due to the shortening of EPSPs. In the trigeminal ganglion cells, tonic responses represent amplitude of whisker deflection (Shoykhet et al. 2000; Stuttgen et al. 2006). The impaired tonic responses in barrel cortex of the CxNR1KO mice should lead to a failure in differentiating whisker deflection parameters.
In CxNR1KO mice, the dendritic field of spiny stellate (layer IV excitatory) cells covers a larger territory and exhibits profuse branching with increased spine density compared with control mice (Datwani et al. 2002). In normal mice, TCA arbors are largely confined to a single barrel and are discontinuous in their distribution (Lee et al. 2005; Petersen 2007). The arbors in CxNR1KO mice expand laterally to cover a larger area spanning multiple presumptive barrel territories (Lee et al. 2005). Thus exuberant TCA arborization suggests that thalamic afferents from different whisker barreloids have overlapping synaptic territories. This overlap may or may not result in convergence onto the same postsynaptic partners. Our MII analyses revealed that different thalamic afferents indeed converge onto single layer IV excitatory neurons. The MII analysis has been used to estimate the number of innervating fibers or neurons to single target in the olivocerebellar (Crepel et al. 1976; Mariani and Changeux 1981), retinogeniculate (Lo et al. 2002; Stevens et al. 2007), retinocollicular (Lu and Constantine-Paton 2004), and medial lemniscal pathways (Arsenault and Zhang 2006) and the first relay station of the trigeminal pathway, PrV (Lo et al. 2011). The criterion for multiple innervation is the abrupt jump in the amplitude of postsynaptic responses on progressively increasing stimulus intensity. A jumping step represents additional activation of a group of fibers or neurons by a given stimulus intensity. Thus the number of jumping steps provides an estimation of the lower limit of inputs to a neuron. Whereas previous studies judged the steps entirely by eye, we have been defining the number of steps with statistical methods (Lo et al. 2011; see also materials and methods). In the present study, we found an increase in MII in CxNR1KO mice indicating that layer IV neurons in CxNR1KO mice receive thalamic inputs from more VPM neurons than control mice. We predict that the overlapped thalamic afferents lead to a defect in spatial discrimination of the whisker-induced sensory information.
Voltage-sensitive dye imaging results also support our conclusions derived from immunocytochemical detection of immediate early gene expression experiments. As in previous studies in both rats and mice (Ferezou et al. 2006; Petersen et al. 2003; Tsytsarev et al. 2010), single brief whisker deflections evoked highly focalized optical responses in normal mouse cortex, but, in contrast, the response was widespread over the barrel cortex of CxNR1KO mice. These activity-dependent indices further confirm our morphological observations that CxNR1KO mice have exuberant thalamocortical projections (Lee et al. 2005).
In conclusion, the results of the present study show that targeted deletion of the gene encoding for the essential subunit of the NMDARs in cortical excitatory neurons in the developing mouse brain leads to significant functional impairment in somatosensory information processing later in life. In CxNR1KO mice, typical activation of the barrel cortex in a one-whisker, one-barrel fashion is compromised; spatial discrimination and tonic response-encoded information from the whiskers are weakened.
GRANTS
National Institute of Neurological Disorders and Stroke Grant NS-039050 (R. S. Erzurumlu) supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.-S.L. and R.S.E. conception and design of research; F.-S.L., F.A., and V.T. performed experiments; F.-S.L., F.A., and V.T. analyzed data; F.-S.L., V.T., and R.S.E. interpreted results of experiments; F.-S.L. and F.A. prepared figures; F.-S.L. and R.S.E. drafted manuscript; R.S.E. edited and revised manuscript; R.S.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. T. Iwasato, S. Itohara, and S. Tonegawa for providing the founder mouse lines, C. Bernardelli for mouse breeding and genotyping, and S. Zhao for help with immunohistochemistry. We also thank B. Okura, K. Tsubokura, and T. Sakuraba from Brainvision for their help with MiCAM02 installation and data analysis.
REFERENCES
- Agmon A, Connors BW. Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. J Neurosci 12: 319–329, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault D, Zhang ZW. Developmental remodelling of the lemniscal synapse in the ventral basal thalamus of the mouse. J Physiol 573: 121–132, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester-Rosado CJ, Albright MJ, Wu CS, Liao CC, Zhu J, Xu J, Lee LJ, Lu HC. mGluR5 in cortical excitatory neurons exerts both cell-autonomous and -nonautonomous influences on cortical somatosensory circuit formation. J Neurosci 30: 16896–16909, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003 [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol 7: 567–578, 1976 [DOI] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci 21: 477–492, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci 35: 1540–1553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J Physiol 521: 169–190, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron 50: 617–629, 2006 [DOI] [PubMed] [Google Scholar]
- Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci 5: 874–885, 2004 [DOI] [PubMed] [Google Scholar]
- Grinvald A, Shoham D, Shmuel A, Glaser D, Vanzetta I, Shtoyerman E, Slovin H, Sterkin A, Wijnbergen C, Hildesheim R, Arieli A. In-vivo optical imaging of cortical architecture and dynamics. In: Modern Techniques in Neuroscience Research, edited by Windhorst U, Johansson H. New York: Springer-Verlag, 2001 [Google Scholar]
- Hannan AJ, Blakemore C, Katsnelson A, Vitalis T, Huber KM, Bear M, Roder J, Kim D, Shin HS, Kind PC. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosci 4: 282–288, 2001 [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Kind PC, Blakemore C. Phospholipase C-beta1 expression correlates with neuronal differentiation and synaptic plasticity in rat somatosensory cortex. Neuropharmacology 37: 593–605, 1998 [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54: 859–871, 2007 [DOI] [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron 19: 1201–1210, 1997 [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature 406: 726–731, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron 16: 333–344, 1996 [DOI] [PubMed] [Google Scholar]
- Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol 485: 280–292, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell 76: 427–437, 1994 [DOI] [PubMed] [Google Scholar]
- Lippert MT, Takagaki K, Xu W, Huang X, Wu JY. Methods for voltage-sensitive dye imaging of rat cortical activity with high signal-to-noise ratio. J Neurophysiol 98: 502–512, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. Peripheral nerve damage does not alter release properties of developing central trigeminal afferents. J Neurophysiol 105: 1681–1688, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Zhao S. N-methyl-d-aspartate receptor subunit composition in the rat trigeminal principal nucleus remains constant during postnatal development and following neonatal denervation. Neuroscience 178: 240–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Zhao S, Erzurumlu RS. Astrocytes promote peripheral nerve injury-induced reactive synaptogenesis in the neonatal CNS. J Neurophysiol 106: 2876–2887, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Ziburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca2+-mediated plateau potential in developing relay cells of the LGN. J Neurophysiol 87: 1175–1185, 2002 [DOI] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron 32: 619–634, 2001 [DOI] [PubMed] [Google Scholar]
- Lu W, Constantine-Paton M. Eye opening rapidly induces synaptic potentiation and refinement. Neuron 43: 237–249, 2004 [DOI] [PubMed] [Google Scholar]
- Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci 1: 696–702, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach HS, Cohen LB, Grinvald A. Optical mapping of electrical activity in rat somatosensory and visual cortex. J Neurosci 5: 1886–1895, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron 56: 339–355, 2007 [DOI] [PubMed] [Google Scholar]
- Petersen CC, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 23: 1298–1309, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quairiaux C, Armstrong-James M, Welker E. Modified sensory processing in the barrel cortex of the adult mouse after chronic whisker stimulation. J Neurophysiol 97: 2130–2147, 2007 [DOI] [PubMed] [Google Scholar]
- Shoham D, Glaser DE, Arieli A, Kenet T, Wijnbergen C, Toledo Y, Hildesheim R, Grinvald A. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron 24: 791–802, 1999 [DOI] [PubMed] [Google Scholar]
- Shoykhet M, Doherty D, Simons DJ. Coding of deflection velocity and amplitude by whisker primary afferent neurons: implications for higher level processing. Somatosens Mot Res 17: 171–180, 2000 [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol 61: 311–330, 1989 [DOI] [PubMed] [Google Scholar]
- Staiger JF, Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K. Exploration of a novel environment leads to the expression of inducible transcription factors in barrel-related columns. Neuroscience 99: 7–16, 2000 [DOI] [PubMed] [Google Scholar]
- Staiger JF, Masanneck C, Bisler S, Schleicher A, Zuschratter W, Zilles K. Excitatory and inhibitory neurons express c-Fos in barrel-related columns after exploration of a novel environment. Neuroscience 109: 687–699, 2002 [DOI] [PubMed] [Google Scholar]
- Steffen H, Van der Loos H. Early lesions of mouse vibrissal follicles: their influence on dendrite orientation in the cortical barrelfield. Exp Brain Res 40: 419–431, 1980 [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell 131: 1164–1178, 2007 [DOI] [PubMed] [Google Scholar]
- Stuttgen MC, Ruter J, Schwarz C. Two psychophysical channels of whisker deflection in rats align with two neuronal classes of primary afferents. J Neurosci 26: 7933–7941, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga TK, Riichi K, Tominaga Y. VSD imaging method of ex vivo brain preparation. J Neurosci Neuroeng 2: 211–219, 2013 [Google Scholar]
- Tsytsarev V, Fukuyama H, Pope D, Pumbo E, Kimura M. Optical imaging of interaural time difference representation in rat auditory cortex. Front Neuroeng 2: 2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsytsarev V, Pope D, Pumbo E, Yablonskii A, Hofmann M. Study of the cortical representation of whisker directional deflection using voltage-sensitive dye optical imaging. Neuroimage 53: 233–238, 2010 [DOI] [PubMed] [Google Scholar]
- Wu CS, Ballester Rosado CJ, Lu HC. What can we get from ‘barrels’: the rodent barrel cortex as a model for studying the establishment of neural circuits. Eur J Neurosci 34: 1663–1676, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]