Abstract
The dorsolateral prefrontal cortex matures late into adolescence or early adulthood. This pattern of maturation mirrors working memory abilities, which continue to improve into adulthood. However, the nature of the changes that prefrontal neuronal activity undergoes during this process is poorly understood. We investigated behavioral performance and neural activity in working memory tasks around the time of puberty, a developmental event associated with the release of sex hormones and significant neurological change. The developmental stages of male rhesus monkeys were evaluated with a series of morphometric, hormonal, and radiographic measures. Peripubertal monkeys were trained to perform an oculomotor delayed response task and a variation of this task involving a distractor stimulus. We found that the peripubertal monkeys tended to abort a relatively large fraction of trials, and these were associated with low levels of task-related neuronal activity. However, for completed trials, accuracy in the delayed saccade task was high and the appearance of a distractor stimulus did not impact performance significantly. In correct trials delay period activity was robust and was not eliminated by the presentation of a distracting stimulus, whereas in trials that resulted in errors the sustained cue-related activity was significantly weaker. Our results show that in peripubertal monkeys the prefrontal cortex is capable of generating robust persistent activity in the delay periods of working memory tasks, although in general it may be more prone to stochastic failure than in adults.
Keywords: development, eye movement, neurophysiology, persistent activity, principal sulcus
humans and nonhuman primates experience a protracted period of cognitive development, and the accrual of cognitive capacities during this period parallels the maturation of the prefrontal cortex (Casey et al. 2008; Chugani et al. 1987; Gogtay et al. 2004; Jernigan et al. 1991; Paus 2005; Pfefferbaum et al. 1994; Sowell et al. 2001; Spear 2000; Yakovlev and Lecours 1967). Working memory ability increases throughout childhood and adolescence, particularly when measured with working memory tasks that require retention of information in the face of distraction (Fry and Hale 2000; Gathercole et al. 2004). Performance in tasks that assess verbal and visuospatial working memory and executive control follows similar developmental trajectories (Gathercole et al. 2004). The age-related performance increases observed for cognitive tasks parallel long-term structural changes due to white and gray matter maturation within prefrontal cortex and other cortical regions (Bunge et al. 2002; Olesen et al. 2003). Human fMRI studies have also shown that the patterns of brain activation associated with working memory tasks, particularly those that require filtering of distraction, undergo distinct changes between childhood and adulthood (Burgund et al. 2006; Klingberg et al. 2002; Kwon et al. 2002; Luna et al. 2001; Olesen et al. 2007). Maturation of the connections of the prefrontal cortex with other areas is also predictive of working memory performance in children (Edin et al. 2007; Nagy et al. 2004; Olesen et al. 2003).
Experiments in animal models have provided neural correlates of working memory (Goldman-Rakic 1987; Miller 2000). In monkeys, individual prefrontal neurons exhibit sustained discharges that represent qualities of remembered stimuli (Constantinidis et al. 2001b; Funahashi et al. 1989; Fuster and Alexander 1971; Romo et al. 1999). Such correlates are well established in adult animals, but, like human cortex, monkey prefrontal cortex undergoes prolonged development, maturing throughout adolescence and into early adulthood (Bourgeois et al. 1994; Fuster 2002; Lewis 1997) and ultimately establishing a pattern of connectivity that is homologous to that in the human brain. Despite this evidence, it is still unclear whether or not prefrontal cortical neurons in peripubertal monkeys are immature with respect to their ability to implement cognitive capacities such as working memory.
We were therefore motivated to investigate the relationship between behavioral performance and neuronal activity in the oculomotor delayed response (ODR) task, which has been used extensively in the study of working memory, as well as in a similar task that involves a distractor stimulus. Our goal was to characterize the responses of prefrontal cortical neurons during task performance and establish whether they varied systematically between correct and error trials. Recordings were obtained at a time around the onset of puberty, a developmental landmark associated with the release of sex hormones and neurological changes in humans (Blakemore et al. 2010; Crone and Dahl 2012; Giedd et al. 2006). The male rhesus monkey (Macaca mulatta), the animal model we used in this study, enters puberty at ∼3.5 yr of age and reaches full sexual maturity at 5 yr, approximately equivalent to the human ages of 12 and 16 yr, respectively (Herman et al. 2006; Plant et al. 2005). Here we report that 1) cognitive performance in young animals is generally less reliable than in adults, 2) the levels of persistent activity observed in correct trials of working memory tasks are comparable in young and adult animals, and 3) prefrontal activity is significantly weaker for different types of error, suggesting that it contributes to multiple aspects of cognitive performance.
METHODS
Four male rhesus monkeys (M. mulatta) were used in this study. All surgical and animal use procedures were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee according to National Institutes of Health guidelines.
Developmental profiles.
The objective of this study was to assess working memory performance and the neural mechanisms that underlie it in nonhuman primates entering puberty, roughly equivalent to 12 yr of age for male humans (Crone and Dahl 2012). As in humans, the age of pubertal onset can vary considerably between individuals, making chronological age an imprecise index. We therefore tracked developmental measures on a quarterly basis over a period of time before, during, and after the behavioral training and neurophysiological recordings. Morphometric measures obtained included body weight, crown-to-rump length, chest circumference, and ulna and femur length. We ascertained testicular volume with an orchidometer (Prader Orchidometer, ESP, Rustington, UK). We checked for visible eruption of canines and determined bone maturation by X-rays of the upper and lower extremities. We additionally obtained blood samples and determined serum concentration of circulating hormones including testosterone and dihydrotestosterone (DHT) through extraction and enzyme immunoassay (performed at the Assay Services Unit of the Wisconsin National Primate Research Center). Developmental data were plotted relative to the onset of neurophysiological recordings. Considerable day-to-day variability is present in measures such as body weight and hormone concentrations, so we smoothed the corresponding data points with a three-point triangular filter.
Behavioral tasks.
Four monkeys were trained to perform an ODR task (Funahashi et al. 1989). This is a spatial working memory task that required them to remember the location of a cue stimulus flashed on a screen for 0.5 s. The cue was a 1° white square stimulus that could appear at one of eight locations arranged on a circle of 10° eccentricity. After a 1.5-s delay period, the fixation point was extinguished and the monkey was trained to make an eye movement to the remembered location of the cue within 0.6 s. The saccade needed to terminate on a 5–6° radius window centered on the stimulus (within 3–4° from the edge of the stimulus), and the monkey was required to hold fixation within this window for 0.1 s. Animals were rewarded with fruit juice for successful completion of a trial. Eye position was monitored with an infrared eye tracking system (ISCAN RK-716; ISCAN, Burlington, MA). Breaking fixation at any point before the offset of the fixation point aborted the trial and resulted in no reward. Stimulus presentation and online behavioral control were achieved by in-house software (Meyer and Constantinidis 2005). Two of the four monkeys were also trained to perform a variant of the task, the ODR with Distractor task (Powell and Goldberg 2000). This task involved a distractor presented after the cue, although the total duration of the trial was the same as in the standard ODR task. The location of the second stimulus, the distractor, was always diametric to the first, so in this sense it might have still conveyed information about the location of the rewarded location (as was the case in the Powell and Goldberg study). Nonetheless, the task allowed us to determine the ability of the monkeys to correctly choose the original stimulus and to quantify the neuronal activity associated with it. Monkeys were additionally trained to perform various variants of the antisaccade task and a delay-discounting task, after they had already mastered the ODR task. Data from these tasks are not analyzed here.
Surgery and neurophysiology.
Once the animals had reached asymptotic performance in the ODR tasks, a 20-mm-diameter recording cylinder was implanted over the prefrontal cortex of each animal. Localization of the recording cylinder and of electrode penetrations within the cylinder was based on MR imaging processed with the BrainSight system (Rogue Research, Montreal, QC, Canada). Recordings were collected with Epoxylite-coated tungsten electrodes with a diameter of 250 μm and an impedance of 4 MΩ at 1 kHz (FHC, Bowdoin, ME). Electrical signals recorded from the brain were amplified, band-pass filtered between 500 Hz and 8 kHz, and stored through a modular data acquisition system at 25-μs resolution (APM system, FHC). Recordings analyzed here were obtained from areas 8a and 46 of the dorsolateral prefrontal cortex.
Behavioral data analysis.
To analyze the patterns of eye movements in the ODR task we first determined the end point of saccades performed after the fixation point was turned off. During the 0.6-s interval following the offset of the fixation point, we determined the eye position corresponding to the time point of maximum eye velocity. The end point of the saccade was determined as the eye position at the last point of monotonic deviation away from the fixation point, following the point of maximum velocity. Reaction time was defined as the interval between the offset of the fixation point and the time that eye position exited the fixation window. Peak saccadic velocity was determined from the maximum eye position distance between successive samples during the saccade period, smoothed with an 8-ms triangular filter, and resampled at 4 ms.
Neural data analysis.
Recorded spike waveforms were sorted into separate units with an automated cluster analysis method based on the KlustaKwik algorithm (Harris et al. 2000). Firing rate of units was then determined by averaging spikes in each task epoch. We identified neurons that responded to the visual stimuli, evidenced by significantly elevated firing rate in the 0.5-s interval of stimulus presentation compared with the 1-s interval of fixation (paired t-test; P < 0.05). We similarly identified neurons that had significantly increased delay period activity following a stimulus by comparing the corresponding discharge rates with those during the baseline fixation interval (paired t-test; P < 0.05). Neurons with saccadic responses were defined by increased activity during the 0.5-s interval after the go signal compared with the baseline fixation interval (paired t-test; P < 0.05).
We characterized neuronal spatial selectivity by comparing the discharge rates during the presentation of single stimuli at each of the eight spatial locations. Spatial selectivity was determined separately for the cue, delay, and saccade periods. Population tuning curves were obtained by first rotating the firing rates of each neuron to the eight stimuli so that the peak rate would always be at the same location. We then averaged responses for each location relative to the peak location across all neurons and fitted them to a Gaussian curve of the form
where r represents average population firing rate at each location x, B the baseline, A the amplitude, μ the peak, and σ the standard deviation of the Gaussian.
Firing rates from multiple neurons were averaged to produce a population peristimulus time histogram (PSTH). We constructed PSTHs from correct trials as well as error trials. For the latter, we only included neurons from which at least two trials were available from the best or worst location of each neuron. We examined firing rate in error trials for which an error occurred after the end of the delay period or during the last 0.5 or 1.0 s before the end of the delay period. For this analysis, activity from multiple neurons was plotted for a common time interval prior to the error.
We estimated the Fano factor (variance divided by the mean) of each neuron's spike counts based on the method of Churchland and colleagues (Churchland et al. 2010). Data for each stimulus location were initially treated separately. This method proceeds in three steps. First, for each neuron spike counts are computed in a 100-ms sliding window moving in 20-ms steps; second, the variance and mean of the spike count across trials is calculated for each window and each neuron; and finally, again at each time window, a linear regression of the variance to the mean is performed, such that each neuron in the population contributes one point. The slope of this regression, which is a function of time, represents the Fano factor reported here.
RESULTS
We obtained behavioral data and neurophysiological recordings from areas 8 and 46 of the prefrontal cortex (Fig. 1A) from four male rhesus monkeys (M. mulatta) around the time of puberty but prior to reaching adulthood. To ascertain their developmental status we conducted development assays. We then evaluated their levels of performance in the behavioral tasks (Fig. 1, B and C) and the corresponding neural basis by recording single-unit activity in the prefrontal cortex.
Fig. 1.
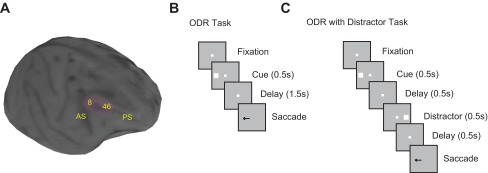
A: structural MRI of 1 adolescent monkey brain. The shaded areas indicate the recording sites in the dorsolateral prefrontal cortex (PFC). Black spots in the image are artifactual “shadows” created by ceramic screws in the skull. AS, arcuate sulcus; PS, principal sulcus. B: successive frames indicate the sequence of events in the oculomotor delayed response (ODR) task. Monkeys were required to remember the stimulus location and to saccade to it after a delay period. B: sequence of events in the ODR with Distractor task. The presentation of the stimulus is the same as in the ODR task, but 1 distractor is shown in the middle of the delay period.
Developmental profiles.
To determine the developmental stage of the animals, we conducted quarterly assays sampling a range of morphometric, hormonal, and radiographic measures. Representative data are shown in Fig. 2, including data for body mass, serum testosterone level, bone maturation, testicular size, and femur length for each monkey. In all graphs, data are aligned to the beginning of the acquisition of neural data at Q0. Data from the 3 quarters preceding and 3–5 quarters after recording onset are shown. The four animals had a median age of 4.3 yr at the last measurement prior to the onset of neurophysiological recordings (range: 4.0–5.2 yr). Neural data for monkeys 1708, 1709, and 1753 were collected across 2 quarters, whereas data for monkey 1752 were collected during a single 3-mo period. The median age at the first time point after the end of recordings was 4.7 yr. Testes had descended for all four monkeys prior to Q0; canines had erupted for only one monkey (subject 1752). Each of the measures examined were trending upward as expected for developing monkeys. Values in all cases are indicative of monkeys that are entering puberty but have not reached full maturity (Plant et al. 2005; Schlatt et al. 2008). It was also notable that a single measure could not be used to ascertain reliably the developmental stage of each animal; different individuals exhibited highest values for body mass, testis size, and femur length (Fig. 2).
Fig. 2.
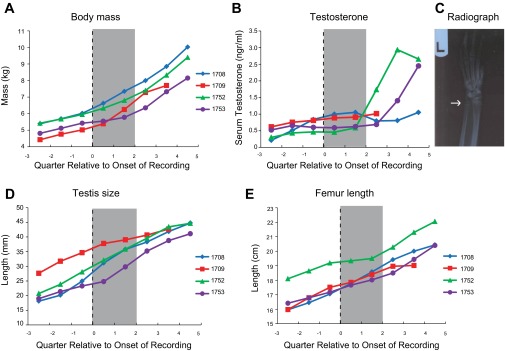
Developmental profile. A: body mass for each monkey as a function of time, evaluated in quarterly assays. Data are aligned to the onset of neurophysiological recordings (Q0). Shaded area covers the interval of recordings. B: serum testosterone concentration obtained from each monkey. C: sample radiography for monkey 1752; arrow indicates the epiphyseal plate, which has not fused yet. D: testis size as a function of time. E: femur length as a function of time.
Rhesus monkeys constitute the predominant animal model for childhood development, and there are ample data on the normative values for each of these measures at different developmental stages. For example, a body mass of 7–9 kg is typical for sexually mature adolescent male rhesus monkeys, whereas adults typically exceed 10 kg (Schlatt et al. 2008). Likewise, the onset of puberty in male rhesus monkeys is signaled by the pulsatile release of gonadotropin-releasing hormone, which is highly correlated with the circulating levels of testosterone (Plant 2001; Plant et al. 2005). Although it may vary as a function of breeding status and social dominance (Bercovitch 1993), typical levels of serum testosterone for adult males are 4 ng/ml (Mattison et al. 2011; Schlatt et al. 2008). The monkeys in this study were clearly below these levels (Fig. 2B), but each presented with descended testes that were increasing in size (Fig. 2C). The metrics plotted in Fig. 2, along with a host of other established developmental markers that we tracked (e.g., chest circumference, crown-to-rump length, canine eruption, epiphyseal closure), were all consistent with animals in the early adolescent stage.
Behavioral performance.
The four monkeys were initially trained in the ODR task, which has been extensively used to determine neural correlates of working memory in adult monkeys (Chafee and Goldman-Rakic 1998; Constantinidis et al. 2001a; Funahashi et al. 1989). The task required subjects to observe a visual cue presented on the screen, make an eye movement after a 1.5-s delay period to the location of the remembered stimulus, and stay within the target window for 100 ms (Fig. 1B). Once recordings were obtained, two animals (subjects 1709 and 1753) were trained to perform a variation of this task, in which a distractor stimulus appeared during the delay period at a location diametric to the original cue (Fig. 1C). This task is similar to tasks that have been used previously in adult animals (Powell and Goldberg 2000) and was used to test the effect of distracting stimuli during working memory, an ability that is presumed to be immature during adolescence. The animals were still required to make an eye movement to the location of the cue and ignore the distracting stimulus. The total trial duration was equal in the two tasks.
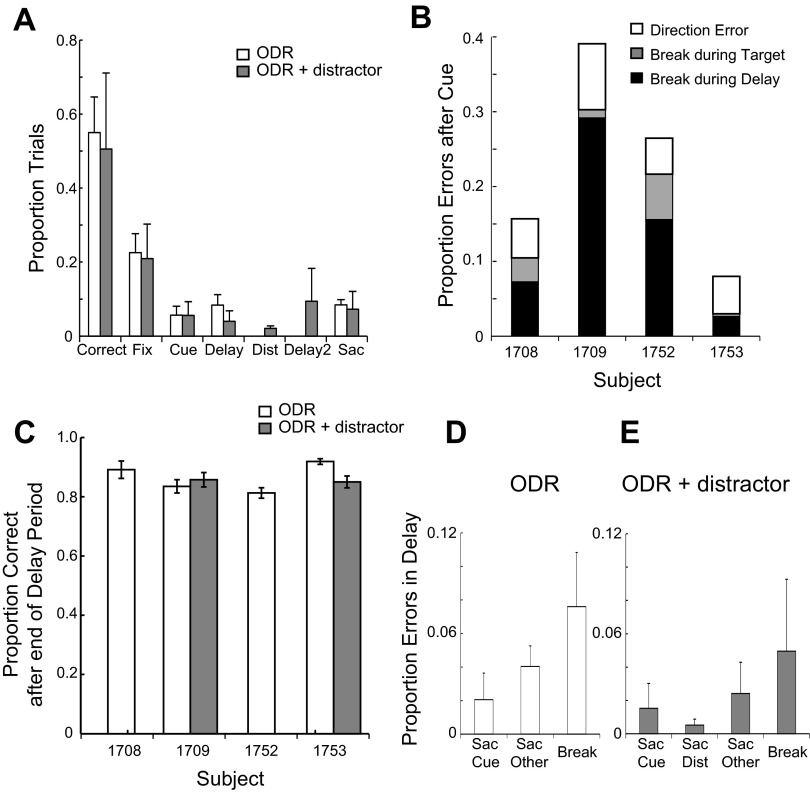
The monkeys exhibited several error types during execution of the ODR task, and errors occurred at all task intervals (Fig. 3A). Of those trials in which no error had occurred by the end of the cue presentation period, ∼13% ended because of breaks in fixation, including blinks (Fig. 3B). It should be noted that the duration of the delay period was only 1.5 s, compared with 3 s in most prior studies in adult animals, chosen precisely because of this propensity of peripubertal monkeys to break fixation. In an additional 3% of the trials that made it into the delay period, the monkeys correctly foveated the saccade target but did not remain within the target window for a sufficient time period to receive a reward (Fig. 3B). Excluding these nondirectional types of errors, all four monkeys made saccades toward the correct location of the target on >90% of the trials (average 93%). Directional errors, where the end point of the saccade was closer to a target other than the cued location, represented <10% of the completed trials (Fig. 3B). The percentage of correct trials among trials in which no break in fixation occurred prior to the end of the delay period was 94% (Fig. 3C). These included premature saccades to the cued location and other locations (Fig. 3D). Equivalent levels of performance in the ODR task have been reported in adult animals, albeit using a 3-s delay period. Correct performance in the range of 80–90% of trials (excluding breaks in fixation) was reported in prior studies (Chafee and Goldman-Rakic 1998; Funahashi et al. 1989). It should be noted, however, that the variance of the saccadic end point increases as a function of the delay period (Gnadt et al. 1991; White et al. 1994).
Fig. 3.
A: proportions of possible trial outcomes for the ODR and ODR with Distractor tasks. First 2 bars indicate correct trials in the 2 tasks. Subsequent bars indicate errors due to breaks in fixation during the initial fixation interval; errors during the cue presentation interval; errors during the delay interval; errors during the distractor presentation (in the ODR with Distractor task only); errors during the second delay period, following the distractor; and errors during the saccade interval. Mean values and SE are plotted across monkeys. B: proportions of errors after the cue presentation in the ODR task, separately for each of the monkeys. Types of errors plotted include directional saccade errors, where the monkey's eye movement was away from the cue location; target errors, where the monkey's eye movement was to the cue location but fixation was broken before the minimum time required to obtain a reward; and breaks in fixation during the delay period, prior to the offset of the fixation point, which instructed the animal to make a saccade. C: proportion of correct trials in the ODR and ODR with Distractor tasks, for trials that were completed up to the end of the delay period, shown separately for 4 monkeys. D: proportion of error trials during the delay period of the ODR task. Errors due to a saccade toward the cued location, errors due to a saccade to another location, and premature breaks in fixation at the target location are plotted. E: proportion of error trials during the second delay period (after the distractor) in the ODR with Distractor task. Errors due to a saccade toward the initial cue, errors due to a saccade to the distractor, errors due to a saccade to another location, and premature breaks in fixation at the target location are plotted.
Performance in the ODR task was stable over the time period that these results were collected; there was no significant effect of recording day on performance (regression analysis, P > 0.05 for each of the 4 monkeys). Means and standard deviations of reaction times were 277 ± 35, 209 ± 85, 258 ± 49, and 249 ± 35 ms, for subjects 1708, 1709, 1752, and 1753, respectively, although the duration of the delay period was fixed in our task and the offset of the fixation point may have been possible to anticipate. Peak velocities for correct trials were 488 ± 91, 449 ± 129, 587 ± 162, and 581 ± 120°/s, respectively. Mean and standard errors of amplitudes of these memory-guided saccades were 9.2 ± 1.6°, 8.7 ± 2.8°, 9.5 ± 2.0°, and 10.3 ± 2.3°, respectively. Saccadic precision (standard deviation around the saccadic end point) was 1.1°, 1.8°, 1.8°, and 1.3° for the four animals, respectively.
Performance in the ODR with Distractor task was qualitatively similar to performance in the ODR task. The average error rates in trials for which the monkeys successfully completed the delay period (including saccades away from the cue location and early breaks from the target) was 15% (Fig. 3C). A two-way ANOVA revealed no significant main effect of task (P > 0.2). When we compared the proportions of trials that resulted in a correct saccade toward the location of the target in the two tasks, there was an overall 4% decrease in the ODR with Distractor task (on average 88% compared with 92% in the ODR task, but only 1 of the 2 animals tested in both tasks showed a decrease; Fig. 3C). Errors in the second delay period included premature saccades to the cued location, the location of the distractor, and other locations (Fig. 3E). Saccadic precision was no worse than in the standard ODR task. Values of 1.7° and 1.2° were obtained for subjects 1709 and 1753, which were in fact slightly better than the equivalent precision values in the ODR task for the same animals (1.8° and 1.3°, respectively).
Neuronal responses in ODR task.
A total of 607 neurons were recorded in areas 8 and 46 of the dorsolateral prefrontal cortex of the four monkeys during execution of the ODR task (33, 133, 158, and 283 neurons from monkeys 1708, 1709, 1752, and 1753, respectively). During recordings, we sought neurons with any type of activity related to the task, although we relied on multiple electrode recordings and sometimes sampled neurons with no task-related response. A total of 532 neurons in our sample responded with elevated firing rate in at least one period of the task compared with the baseline during fixation. Examining separately the numbers of neurons that exhibited responses during the cue, delay, and saccade periods, we found 362 neurons responding to the cue, 263 neurons responding to the delay period, and 350 responding to the saccade.
Given the developmental stage of our monkeys, we were particularly interested in neurons that exhibited delay period activity. Expressed as a percentage of the neurons with any task-related responses, neurons that exhibited persistent activity during the delay period of the ODR task represented 49% of the total. This proportion was in the range of previous studies using the same task in adult monkeys: 51% was reported by Funahashi and colleagues (Funahashi et al. 1989) and 40% in another experiment (Chafee and Goldman-Rakic 1998); 57% of the neurons displayed delay period activity in the database of a third study (Constantinidis et al. 2001a). Individual examples of neurons with significantly elevated and spatially selective delay period activity are shown in Fig. 4 and Fig. 5. We observed delay period activity that remained fairly stable after the cue, as well as discharges that increased during the delay, similar to sustained and anticipatory responses described in adult monkeys in previous studies (Qi et al. 2010; Quintana and Fuster 1992; Romo et al. 1999).
Fig. 4.
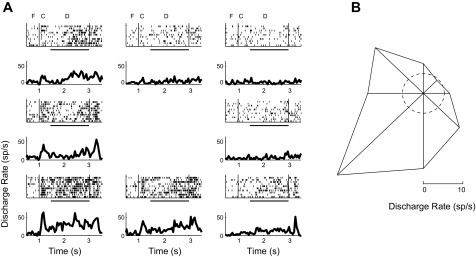
Persistent activity in a prefrontal neuron. A: rasters and peristimulus time histograms (PSTHs) of a single neuron during the ODR task. Spike rasters and histograms are arranged according to the spatial location of the cue (i.e., saccade target). Responses during the fixation interval (F), cue presentation (C), and delay period (D) are indicated. Horizontal bars represent the delay period. B: polar plot depicts the average firing rate during the delay period for each cue location; the dashed circle represents the average firing rate during the baseline, fixation period.
Fig. 5.
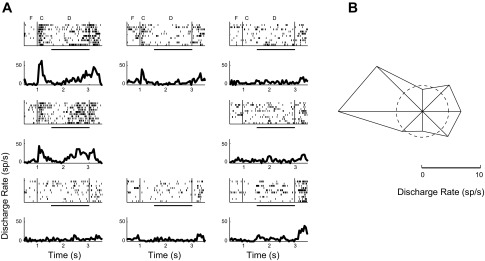
Rasters and PSTHs (A) and polar plot (B) of a second example neuron recorded during the ODR task. Conventions are the same as in Fig. 4.
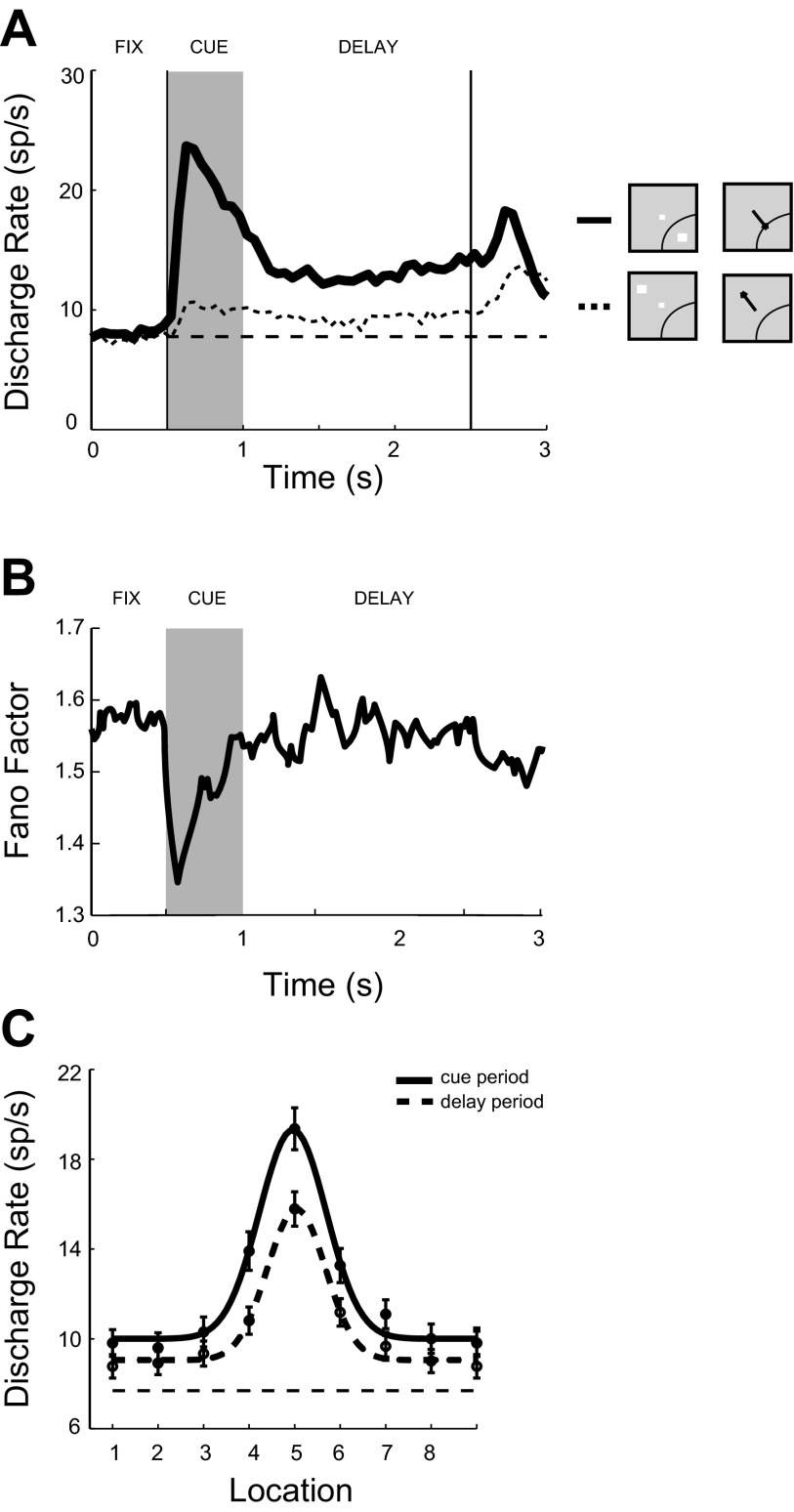
Across the sampled population, robust levels of delay period activity were present throughout the trial (Fig. 6A). Average firing rate during the best cue presentation (18.9 sp/s) was followed by delay period activity of 12.9 sp/s, which represented 68% of the cue response. These values were similar to those reported in adult animals (Funahashi et al. 1991). A reanalysis of a database of recordings from another study in adult animals (Constantinidis et al. 2001a) yielded average values of 15.8 sp/s for the best cue presentation and 11.6 sp/s for the delay period that followed it, which represented 73% of the cue response, very similar to the values recorded in the peripubertal animals—although again with the caveat that the delay period in the present study (1.5 s) was shorter than that used in previous reports (3 s).
Fig. 6.
Population responses of neurons with significantly elevated firing rate during the delay period of the ODR task (n = 263 neurons). A: average PSTH following the best cue location (solid line) or following the diametric location (dotted line). Horizontal line represents baseline fixation discharge rate. Labels indicate fixation (FIX), cue presentation (CUE), and delay (DELAY) periods. Insets: schematic illustrations of the position of the stimulus relative to the receptive field, which differed for each neuron. B: mean Fano factor values of neuronal discharges for the same population of neurons as in A. C: population tuning curves from the cue period and from the delay period.
We quantified the variability of neuronal discharges by computing the Fano factor of spike counts (variance divided by mean) in sliding 100-ms windows (Churchland et al. 2010). Data were pooled across stimulus conditions to obtain a single value for each task epoch (see methods). As previously reported for multiple brain areas of adult monkeys (Churchland et al. 2010), the Fano factor declined during the time of stimulus presentation for our data set (Fig. 6B). Averaged across the entire trial interval and all stimulus conditions, the mean and standard error values of the Fano factor were 1.54 ± 0.03 for neurons with delay period activity. This is somewhat higher than the values in the range of 1.3 obtained from an analysis of neuronal responses from the prefrontal cortex of adult monkeys using the ODR task (Compte et al. 2012) and other working memory tasks (Qi and Constantinidis 2012). It is therefore possible that elevated variability is characteristic of neuronal discharges in peripubertal animals. A caveat to this conclusion is that the variability we report here is still within the range of Fano factor values reported for the prefrontal cortex in the context of other tasks (Hussar and Pasternak 2010).
A total of 139 of 263 neurons (53%) with significant delay period activity exhibited spatial tuning during the delay period, as evidenced by significant selectivity for location during the delay period (1-way ANOVA, P < 0.05). This percentage appeared to be within the range of tuned neurons with delay period activity reported in studies of adult animals, although different methods with quite disparate results have been employed previously: 79%, 43%, and 41% of neurons have been reported to exhibit significant tuning in three prior studies (Chafee and Goldman-Rakic 1998; Funahashi et al. 1989; Rao et al. 1999). Representative tuning functions are shown in the polar plots of Fig. 4 and Fig. 5; tuning obtained from the population average is shown in Fig. 6C. The standard deviation of the best Gaussian fit of population responses corresponded to 29° of angular distance between targets for the delay period. This was very similar to the standard deviations reported in the literature for adult animals (which were obtained by individual fits of Gaussian functions, averaged across neurons); a mean value of 27° was reported in two different studies (Chafee and Goldman-Rakic 1998; Funahashi et al. 1989).
Neuronal responses in ODR with Distractor task.
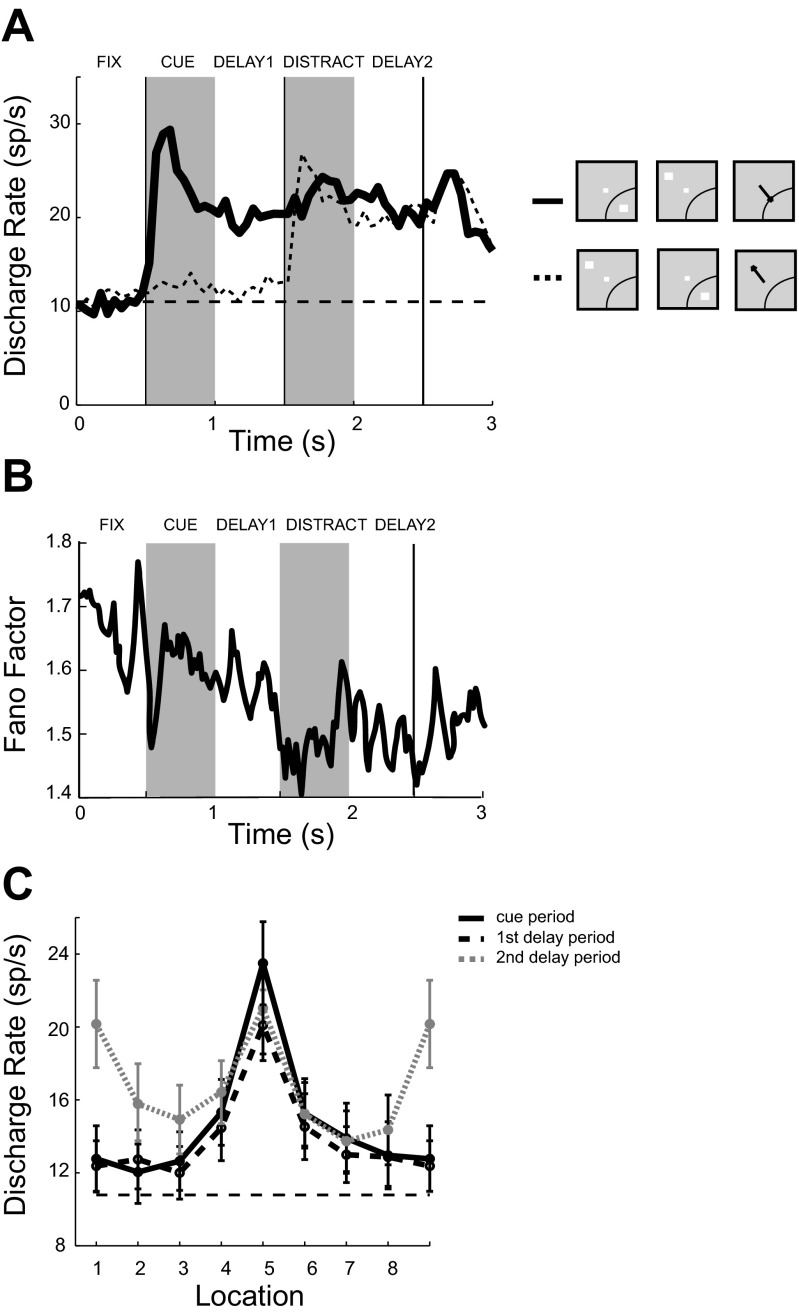
We additionally recorded 248 neurons from areas 8 and 46 of the dorsolateral prefrontal cortex of two monkeys (88 and 160 neurons from monkeys 1709 and 1753, respectively) while they performed the ODR with Distractor task (Fig. 1C). Of those, 211 neurons responded to the task, as evidenced by significantly elevated firing rate during at least one task epoch. A total of 117 neurons displayed significantly elevated firing during the cue presentation period, 81 during the first delay period, 113 during the distractor presentation, 106 during the second delay period, and 160 during the saccade period. The responses of a representative neuron are shown in Fig. 7. Increased activity following a stimulus at the preferred location persisted during the delay period and changed only slightly during the presentation of a distractor out of the receptive field (Fig. 7A, top left). The same neuron was also excited when the second, distracting stimulus appeared in the receptive field, and this distractor-related response was also sustained during the second delay period, albeit at a lower level (Fig. 7A, bottom right). This response pattern was representative of the population of prefrontal neurons (Fig. 8A). Overall, a robust response evoked by the cue appearing in the receptive field was followed by sustained activity in the first delay period, and the level of firing changed little during presentation of the distractor outside the receptive field during the second delay period (mean rate 21.3 sp/s). Slightly lower sustained activity in the second delay period (mean rate 20.3 sp/s) was seen when the distractor appeared in the neuron's receptive field. As a result, the spatial tuning of neurons during the second delay period appeared bimodal (Fig. 7B and Fig. 8C). Variability of discharges was similar to that obtained in the ODR task. The mean and standard error of the Fano factor across all neurons with delay period activity was 1.56 ± 0.09 (Fig. 8B).
Fig. 7.
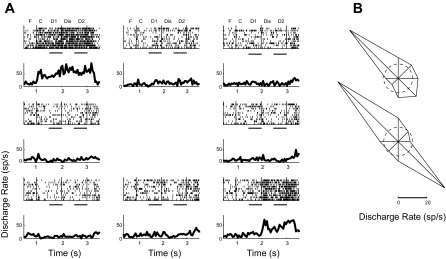
Activity in the ODR with Distractor task. A: rasters and PSTHs of a single neuron during the ODR with Distractor task. Responses during the fixation interval (F), cue presentation (C), first delay period (D1), distractor presentation (Dis), and second delay period (D2) are indicated. Horizontal bars represent the first and second delay periods. B: polar plots depict the average firing rate during the first (top) and second (bottom) delay periods for each cue location; the dashed circle represents the average firing rate during the baseline, fixation period.
Fig. 8.
Population responses of neurons with significant delay activity in the ODR with Distractor task. A: population PSTH (n = 88 neurons) following the best cue location and diametric distractor location (solid line) or following the worst cue location and best distractor location (dotted line). Horizontal line represents baseline fixation discharge rate. B: mean Fano factor values of neuronal discharges during the trial. C: population tuning curves from the cue period, from the first delay, and from the second delay (after distractor presentation). Labels in A and B indicate fixation (FIX), cue presentation (CUE), first delay (DELAY1), distractor presentation (DISTRACT), and second delay (DELAY2) periods. Horizontal dashed lines in A and C represent the baseline firing rate.
Analysis of error trials.
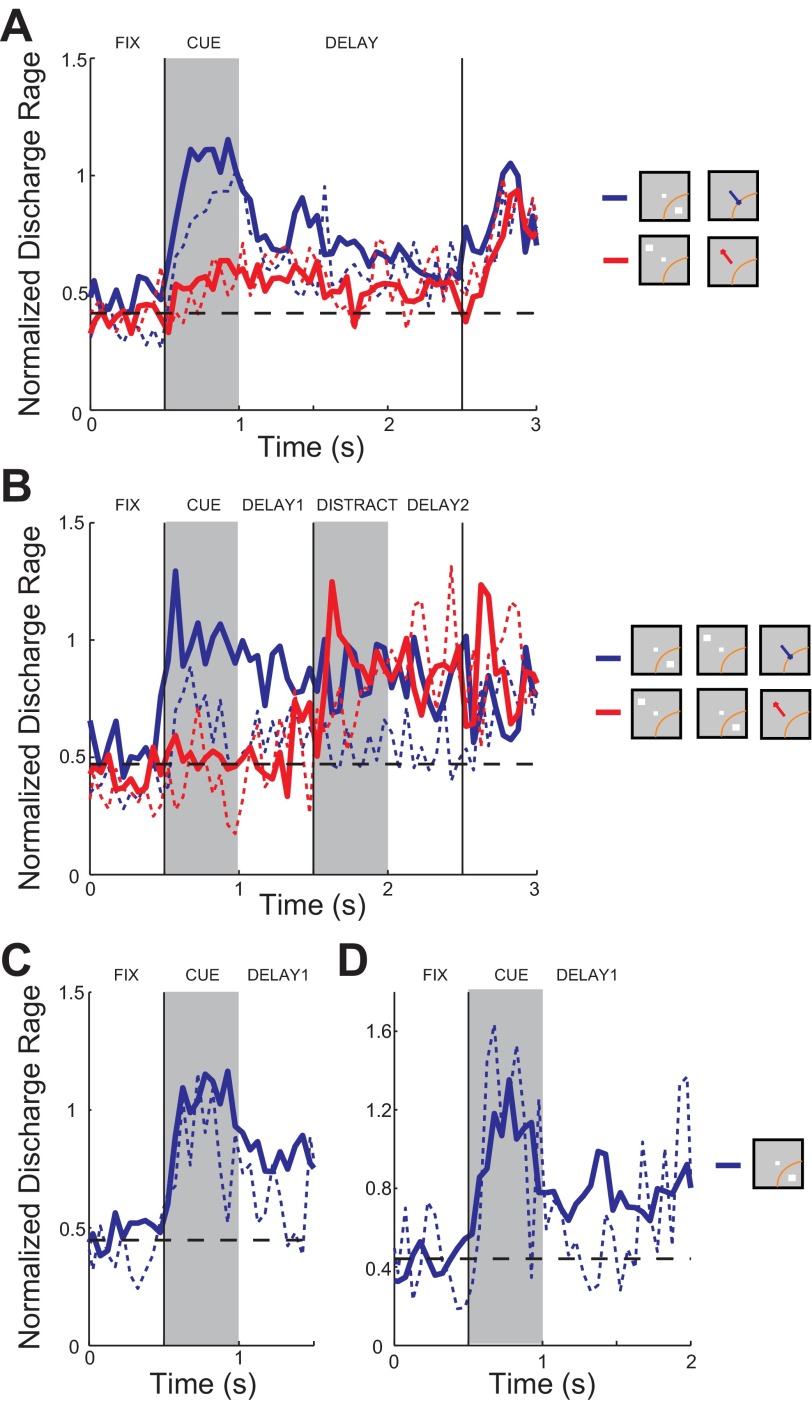
Considering that the animals made errors during all epochs of the task (Fig. 3A), we sought possible neural correlates of those behavioral outcomes by analyzing neural activity associated with specific types of error trials. For this purpose, we first analyzed trials in which an error was produced after the end of the delay period. These included errors due to saccades toward an incorrect direction, as well as premature breaks in fixation at the location of the remembered target (as shown in Fig. 3D). Neurons with at least two such error trials following the cue presentation at the best location were identified (n = 43; Fig. 9, blue traces). We also identified neurons with at least two error trials following the cue presentation at the diametric location (n = 40), which constituted a partially overlapping set with the previous group of neurons (Fig. 9A, red traces). Firing rates were normalized by dividing all rates of each neuron by the mean firing rate evoked by the best cue in correct trials. Normalized firing rates were then averaged across neurons. In the ODR task, firing rates were significantly lower in error trials compared with correct trials during both the cue presentation (paired t-test, P < 0.001) and the delay period (paired t-test, P < 0.05). This is similar to what has been described before in adult animals (Funahashi et al. 1989). No difference between correct and incorrect responses was found when the cue appeared at a location diametric to the best location in the receptive field (paired t-test, P > 0.05; Fig. 9A, compare solid and dashed red curves).
Fig. 9.
Neural activity in correct vs. error trials. A: population average firing rate from neurons with at least 2 error trials of any type in the ODR task. Normalized firing rate is shown in correct and error trials in which the cue appeared in the best location (blue traces, n = 43 neurons) and in the diametric location (red traces, n = 40 neurons). Solid and dashed lines indicate correct and error trials, respectively. B: population firing rate from the ODR with Distractor task. Averaged firing rate is shown in correct and error trials in which the cue appeared in the best location (blue traces, n = 10 neurons) and in the diametric location (red traces, n = 11 neurons). C: population firing rate in correct (solid) and error (dashed) trials aborted during the delay period. Error average is for all available trials in which a break in fixation occurred at least 0.5 s after the offset of the cue (n = 47 neurons). D: as in C but with error average for all trials in which an error occurred at least 1 s after the offset of the cue (n = 19 neurons).
Similar trends were present in the ODR with Distractor task (Fig. 9B), although smaller numbers of neurons were available with at least two error trials for the cue in the best location (n = 10; Fig. 9B, blue traces) and diametric location (n = 11; Fig. 9B, red traces). Importantly, in this task one target and one distractor are presented at diametric locations in every trial, so presumably the quantity that matters the most is the difference between the response to the target and the response to the distractor (note also, however, that the distractor is also informative of the location that leads to a reward, so its impact is not necessarily detrimental). When we analyzed the second delay period we found that activity was significantly higher (t-test, P < 0.05) in error trials in which the distractor appeared in the receptive field compared with error trials in which the cue appeared in the receptive field (red vs. blue dashed lines in Fig. 9B). In other words, trials characterized by either increased distractor-related activity or decreased cue-related activity during the second delay period were likely to end in errors.
Finally, we analyzed neuronal activity in trials that were aborted before the end of the delay period (because of blinks or premature saccades). We identified trials for which correct fixation lasted at least 0.5 s of the delay period (n = 47 neurons; Fig. 9C) and at least 1.0 s of the delay period (n = 19 neurons; Fig. 9D), the latter group being a subset of the first. Examining either group of neurons revealed that delay period activity was significantly reduced in cases that resulted in aborted trials (t-test, P < 0.05). Thus, as for spatial errors, premature response errors were also associated with decreased neuronal activity.
DISCUSSION
The present study investigated behavioral performance during working memory tasks and its neurophysiological correlates in peripubertal monkeys. Although it is widely thought that the prefrontal cortex and the cognitive functions it supports reach full maturity only in adulthood, the nature of activity in the developing prefrontal cortex has not been investigated. Our study revealed that peripubertal monkeys exhibit a propensity for spatially nonspecific errors (e.g., aborted trials) but are able to remember accurately the locations of visual targets and can do so even when challenged with a distractor stimulus at an alternate location. We also found that errors, both spatial and nonspatial, were characterized by diminished neuronal activation specifically during the delay period. On the other hand, during correct trials execution of the working memory task activated a substantial proportion of dorsolateral prefrontal neurons that generated robust levels of delay period activity, comparable to that reported in prior studies of adult animals.
Maturation of working memory in adolescence.
Data from human studies provide strong evidence that working memory capacity does not reach maturity in childhood but instead increases throughout adolescence; a virtually linear increase in performance with age has been observed in tasks of visuospatial working memory, executive control, and retention of information in the face of distraction (Fry and Hale 2000; Gathercole et al. 2004). These improvements in performance parallel long-term structural changes in the prefrontal cortex, such as myelination and increased axonal thickness in the underlying white matter as well as changes in gray matter within the prefrontal cortex and other brain regions connected with it (Bunge et al. 2002; Giedd et al. 2006; Nagy et al. 2004; Olesen et al. 2003). By some accounts, cortical thickness follows an inverted-U shape, peaking during the onset of puberty, the transition point between the juvenile and adolescent state (Blakemore et al. 2010; Crone and Dahl 2012; Giedd et al. 2006). Puberty also marks sex-specific differentiation in cortical structure and cognitive performance, implicating the release of sex hormones in shaping brain development (Blakemore et al. 2010; Nguyen et al. 2013).
The development of the prefrontal cortex is associated not only with structural changes but also with changing patterns of activation. Human fMRI studies indicate a progressive increase of prefrontal activation between childhood and adulthood in tasks that require working memory, inhibition of prepotent responses, and filtering of distractors (Burgund et al. 2006; Klingberg et al. 2002; Kwon et al. 2002; Luna et al. 2001; Olesen et al. 2007). These changes included increased activation specifically during the delay period of visual spatial tasks analogous to the tasks that we used in our study (Kwon et al. 2002; Olesen et al. 2007). The level of activation in visual spatial working memory tasks in childhood is also predictive of a broader range of cognitive abilities in later life, such as arithmetic performance (Dumontheil and Klingberg 2012). Modeling studies further suggest that the pattern of changes in the BOLD signal observed between childhood and adulthood can be best explained by strengthening of connections between the prefrontal and posterior parietal cortex (Edin et al. 2007).
Considering the strong evidence for increasing prefrontal activation from childhood into adulthood, single-neuron activity in a working memory task such as the ODR task might be expected to be weaker in immature subjects. When considering the large number of errors observed in the task, which were associated with decreased activation in the delay period (Fig. 9), neuronal responses in the adolescent prefrontal cortex may indeed be viewed as weaker than those of adult animals. However, we also found that the peripubertal prefrontal cortex was capable of generating robust delay period activity in correct ODR trials. It is conceivable that lower overall activation during adolescence may be most evident for more complex or challenging tasks and that prefrontal activity may mature at different rates in association with the acquisition of different cognitive abilities. Finally, it should also be noted that BOLD activation is driven by both excitatory and inhibitory activity in the cortex, and in any case it is only an indirect measure of single-neuron spiking (Logothetis et al. 2001).
Monkey cognitive maturation.
The male rhesus monkey enters puberty at ∼3.5 yr of age (Herman et al. 2006; Plant et al. 2005), equivalent to ∼12 yr in humans (Crone and Dahl 2012). Monkeys have a median life span of 25 yr and can live up to 40 yr in captivity, suggesting that their rate of aging is roughly three times that of humans (Roth et al. 2004). The monkey prefrontal cortex continues to undergo anatomical maturation during adolescence and early adulthood, similar to the human pattern of development (Bourgeois et al. 1994; Fuster 2002), though some developmental studies suggest the prepubertal 2- to 3-yr-old macaque already begins to undergo some of the biochemical and anatomical changes that characterize the human adolescent prefrontal cortex (Hoftman and Lewis 2011; Lewis 1997).
Working memory abilities in monkeys begin to develop in infancy. Monkeys as young as 4 mo old are able to perform delayed response tasks with 2- to 5-s delay periods at >90% accuracy, similar to 12-mo-old human infants (Diamond 1990). Similarly, 9-mo-old monkeys were able to perform delayed alternation tasks at high levels of performance (Alexander and Goldman 1978). Monkeys of ∼3 yr of age can learn more complex working memory tasks such as the delayed match to sample task, although performance levels may not exceed 60% correct with a 1-s period and decline with increasing delay periods (Verrico et al. 2011).
Cortical inactivation and lesion studies paint a complex picture of prefrontal cortical involvement in cognitive functions in early development. Cooling of the prefrontal cortex at ages of 19–31 mo has negligible effects (<10% decline) on performance of the delayed response task, which monkeys of this age are already able to perform essentially error free (Alexander and Goldman 1978). In contrast, prefrontal cooling in monkeys older than 33 mo of age has substantial effects (>20% decline). The effects of cooling on delayed alternation are similarly greatest after the age of 33 mo (Alexander 1982; Alexander and Goldman 1978). Modest effects on delayed response performance are also observed after complete bilateral lesions of the prefrontal cortex in infant 4-mo-old monkeys (Diamond and Goldman-Rakic 1989). On the basis of these results, it was concluded that the prefrontal cortex is not fully developed before 2.5–3 yr of age, that execution of working memory tasks at younger ages may depend on other brain areas, and that working memory functions become increasingly dependent on prefrontal activation after the age of 2.5–3 yr (Alexander and Goldman 1978). Our results are consistent with such an interpretation, as they show robust activity in the prefrontal cortex at ∼4 yr of age. These results still leave open the possibility that more complex working memory tasks, or tasks requiring other types of executive function, are associated with prefrontal activity that emerges later in life.
Behavioral performance in peripubertal monkeys.
The peripubertal monkeys in the present study committed several types of errors when performing working memory tasks. The most common were breaks in fixation during the (relatively short) delay period or failure to fixate for the requisite duration at the saccade target location. Such nonspatial errors may be associated with impulsivity and/or an inability to maintain focus on the task, which itself may be the result of loss in working memory in various components of the current task. Consistent with this view, when monkeys did make an eye movement at the conclusion of the delay period as required, they attained a level of performance >90% correct, similar to that reported in the ODR task for adult animals (Chafee and Goldman-Rakic 1998; Funahashi et al. 1991). Furthermore, the addition of a distracting stimulus had no significant impact on performance, also consistent with prior findings in adult animals (Powell and Goldberg 2000). These results suggest that the ability to perform at least a simple spatial working memory task while resisting the effect of a distracting stimulus is already present in peripubertal animals.
On the other hand, breaks in fixation during the delay period of the task may reflect the stochastic failure of the prefrontal network to maintain task-relevant information—not necessarily spatial only—over the delay, leading the subject to abort the trial prematurely. Supporting this view, decreased activity during the delay period was associated with trials that resulted in both directional and nondirectional errors. Analogous patterns of nonspecific errors in the delay period have not been reported in the literature for adult monkeys. Such errors may be a unique characteristic of adolescent monkeys; however, it will be essential for future investigation to confirm that peripubertal fixation performance is qualitatively different from that of the adult. Our findings do not rule out the possibility that more subtle measures of performance, such as the precision of the saccadic end point, also improve further after adolescence. Saccadic variability in the ODR task increases as a function of delay period duration (White et al. 1994), and the difficulty of these peripubertal monkeys to perform the ODR task with a 3-s delay period limits our ability to compare saccadic variability with those in adult monkeys. At the same time, the ability to direct attention and to maintain focus on the task at hand appears to be limited in these animals.
Neural activity in peripubertal monkeys.
Delay period activity was observed during ODR task performance in peripubertal monkeys, and its incidence, magnitude, and characteristics in correct trials were consistent with those previously reported for adult animals. The percentage of neurons that displayed delay period activity was within the range reported in previous studies in adult monkeys performing the ODR task (Chafee and Goldman-Rakic 1998; Constantinidis et al. 2001a; Funahashi et al. 1989). Neurons with rising or slightly decreasing firing rate throughout the course of the delay period were also observed, as in adult monkeys (Qi et al. 2010; Quintana and Fuster 1992). Error trials were characterized by decreased firing rate, similar to studies in adult animals (Funahashi et al. 1989), and this was true both for directional errors and nonspecific errors, such as blinks, during the delay period. The level of activity during the delay period relative to that evoked during the stimulus presentation period was also comparable to that described in previous studies (Constantinidis et al. 2001a; Funahashi et al. 1991), with possibly higher variability than that of adult animals (Compte et al. 2012). It has recently been proposed that the average rates of delay period responses (but not stimulus-elicited responses) decline monotonically with age (Wang et al. 2011); our results show that high levels of delay period activity are achieved early in life, prior to adulthood. The spatial tuning of the delay period activity was also within the range of tuning values obtained in adult monkeys (Chafee and Goldman-Rakic 1998; Funahashi et al. 1989; Rao et al. 1999).
Results from the distractor task demonstrated that prefrontal activity in peripubertal monkeys survives the presentation of a distracting stimulus, a property that appears to be unique for the prefrontal cortex (Qi et al. 2010; Suzuki and Gottlieb 2013). However, the response to the distractor stimulus itself was very robust and elicited delay period activity only slightly less vigorous than that of the remembered stimulus (Fig. 8A). A possible result of prefrontal maturation may be the more effective filtering of distractor responses, at least in the context of some tasks (Suzuki and Gottlieb 2013). We should point out that although the second stimulus presentation was irrelevant for the eventual eye movement, our task was structured such that in a block of trials the two stimuli always appeared at diametric locations. In this sense, the second stimulus may not have been truly a distractor, as it was also predictive of the correct eye movement location via an additional spatial transformation.
Our results are in agreement with reports of delay period activity present in the delayed response task in 3-yr-old monkeys, albeit without control of fixation (Alexander 1982). A few prior physiological studies have also reported delay period activity in young monkeys, inferred based on either the actual age reported (3–4 yr old) or weight (3–5 kg) of the subjects (Hasegawa et al. 1998; Takeda and Funahashi 2002). Our study extends these findings by determining developmental measures indicating the onset of puberty and by characterizing the cue- and distractor-related responses, as well as the nature of errors in the task. Overall, the present findings indicate that the peripubertal prefrontal cortex is capable of generating robust delay period activity. However, such activity does not always result in successful trials with activation tending to wane in trials that ultimately resulted in errors. Future studies will be needed to determine whether differences in the prefrontal activity of adult and adolescent monkeys emerge in the context of tasks that impose different and/or greater cognitive demands.
GRANTS
Research reported in this article was supported by the National Institute of Mental Health under award number R33 MH-86946, by the Tab Williams Family Endowment Fund, and in part by Grant P51 RR-000167 from the National Center for Research Resources (NCRR) of the National Institutes of Health (NIH) to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program Grants RR-15459-01 and RR-020141-01. This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.Z., D.Z., X.-L.Q., C.J.L., and A.J.B. performed experiments; X.Z., D.Z., X.-L.Q., C.J.L., A.J.B., and C.C. analyzed data; X.Z., D.Z., C.J.L., A.J.B., E.S., T.R.S., and C.C. interpreted results of experiments; X.Z. and C.C. prepared figures; X.Z., D.Z., X.-L.Q., C.J.L., E.S., T.R.S., and C.C. edited and revised manuscript; X.Z., D.Z., X.-L.Q., C.J.L., A.J.B., E.S., T.R.S., and C.C. approved final version of manuscript; E.S., T.R.S., and C.C. conception and design of research; E.S., T.R.S., and C.C. drafted manuscript.
ACKNOWLEDGMENTS
We thank Kathini Palaninathan for technical assistance.
REFERENCES
- Alexander GE. Functional development of frontal association cortex in monkeys: behavioural and electrophysiological studies. Neurosci Res Prog Bull 20: 471–479, 1982 [PubMed] [Google Scholar]
- Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res 143: 233–249, 1978 [DOI] [PubMed] [Google Scholar]
- Bercovitch FB. Dominance rank and reproductive maturation in male rhesus macaques (Macaca mulatta). J Reprod Fertil 99: 113–120, 1993 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp 31: 926–933, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 4: 78–96, 1994 [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron 33: 301–311, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Lugar HM, Miezin FM, Schlaggar BL, Petersen SE. The development of sustained and transient neural activity. Neuroimage 29: 812–821, 2006 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann NY Acad Sci 1124: 111–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940, 1998 [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol 22: 487–497, 1987 [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, Bradley DC, Smith MA, Kohn A, Movshon JA, Armstrong KM, Moore T, Chang SW, Snyder LH, Lisberger SG, Priebe NJ, Finn IM, Ferster D, Ryu SI, Santhanam G, Sahani M, Shenoy KV. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci 13: 369–378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Nykamp DQ, Constantinidis C. Bump attractor dynamics in prefrontal cortex underlie behavioral precision in spatial working memory (Abstract). Soc Neurosci Abstr 2012: 597–520, 2012 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. Coding specificity in cortical microcircuits: a multiple electrode analysis of primate prefrontal cortex. J Neurosci 21: 3646–3655, 2001a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat Neurosci 4: 311–316, 2001b [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci 13: 636–650, 2012 [DOI] [PubMed] [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann NY Acad Sci 608: 637–669, 1990 [DOI] [PubMed] [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget's AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res 74: 24–40, 1989 [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb Cortex 22: 1078–1085, 2012 [DOI] [PubMed] [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegner J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. J Cogn Neurosci 19: 750–760, 2007 [DOI] [PubMed] [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol 54: 1–34, 2000 [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989 [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 65: 1464–1483, 1991 [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. J Neurocytol 31: 373–385, 2002 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971 [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol 40: 177–190, 2004 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Mol Cell Endocrinol 254–255: 154–162, 2006 [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Bracewell RM, Andersen RA. Sensorimotor transformation during eye movements to remembered visual targets. Vision Res 31: 693–715, 1991 [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Handbook of Physiology. The Nervous System. Higher Functions of the Brain. Bethesda, MD: Am Physiol Soc, 1987, sect. 1, vol. V, part 1, chapt. 9, p. 373–417 [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000 [DOI] [PubMed] [Google Scholar]
- Hasegawa R, Sawaguchi T, Kubota K. Monkey prefrontal neuronal activity coding the forthcoming saccade in an oculomotor delayed matching-to-sample task. J Neurophysiol 79: 322–333, 1998 [DOI] [PubMed] [Google Scholar]
- Herman RA, Zehr JL, Wallen K. Prenatal androgen blockade accelerates pubertal development in male rhesus monkeys. Psychoneuroendocrinology 31: 118–130, 2006 [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull 37: 493–503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussar C, Pasternak T. Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task. Proc Natl Acad Sci USA 107: 21842–21847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI. I. Localization of age-related changes. Biol Psychiatry 29: 55–67, 1991 [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol 24: 781–791, 2002 [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci USA 99: 13336–13341, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology 16: 385–398, 1997 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001 [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13: 786–793, 2001 [DOI] [PubMed] [Google Scholar]
- Mattison DR, Plant TM, Lin HM, Chen HC, Chen JJ, Twaddle NC, Doerge D, Slikker W, Jr, Patton RE, Hotchkiss CE, Callicott RJ, Schrader SM, Turner TW, Kesner JS, Vitiello B, Petibone DM, Morris SM. Pubertal delay in male nonhuman primates (Macaca mulatta) treated with methylphenidate. Proc Natl Acad Sci USA 108: 16301–16306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Constantinidis C. A software solution for the control of visual behavioral experimentation. J Neurosci Methods 142: 27–34, 2005 [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci 1: 59–65, 2000 [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 16: 1227–1233, 2004 [DOI] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama SBrain Development Cooperative Group Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex 23: 1424–1432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegner J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb Cortex 17: 1047–1054, 2007 [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res 18: 48–57, 2003 [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 9: 60–68, 2005 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 51: 874–887, 1994 [DOI] [PubMed] [Google Scholar]
- Plant TM. Neurobiological bases underlying the control of the onset of puberty in the rhesus monkey: a representative higher primate. Front Neuroendocrinol 22: 107–139, 2001 [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, Simorangkir D, Marshall GR. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann NY Acad Sci 1061: 149–162, 2005 [DOI] [PubMed] [Google Scholar]
- Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J Neurophysiol 84: 301–310, 2000 [DOI] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C. Variability of prefrontal neuronal discharges before and after training in a working memory task. PLoS One 7: e41053, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Katsuki F, Meyer T, Rawley JB, Zhou X, Douglas KL, Constantinidis C. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci 4: 12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana J, Fuster JM. Mnemonic and predictive functions of cortical neurons in a memory task. Neuroreport 3: 721–724, 1992 [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol 81: 1903–1916, 1999 [DOI] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399: 470–473, 1999 [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science 305: 1423–1426, 2004 [DOI] [PubMed] [Google Scholar]
- Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta). Biol Reprod 79: 93–99, 2008 [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc 7: 312–322, 2001 [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463, 2000 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci 16: 98–104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Prefrontal task-related activity representing visual cue location or saccade direction in spatial working memory tasks. J Neurophysiol 87: 567–588, 2002 [DOI] [PubMed] [Google Scholar]
- Verrico CD, Liu S, Asafu-Adjei JK, Sampson AR, Bradberry CW, Lewis DA. Acquisition and baseline performance of working memory tasks by adolescent rhesus monkeys. Brain Res 1378: 91–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature 476: 210–213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Sparks DL, Stanford TR. Saccades to remembered target locations: an analysis of systematic and variable errors. Vision Res 34: 79–92, 1994 [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. (editors). The Myelogenetic Cycles of Regional Maturation of the Brain. Oxford, UK: Blackwell, 1967, p. 3–70 [Google Scholar]