Abstract
Polarized cells such as epithelial cells and neurons exhibit different plasma membrane domains with distinct protein compositions. Recent studies have shown that sorting of transmembrane proteins to the basolateral domain of epithelial cells and the somatodendritic domain of neurons is mediated by recognition of signals in the cytosolic domains of the proteins by adaptors. These adaptors are components of protein coats associated with the trans-Golgi network and/or recycling endosomes. The clathrin-associated adaptor protein 1 (AP-1) complex plays a preeminent role in this process, although other adaptors and coat proteins, such as AP-4, ARH, Numb, exomer, and retromer, have also been implicated.
Cell polarity
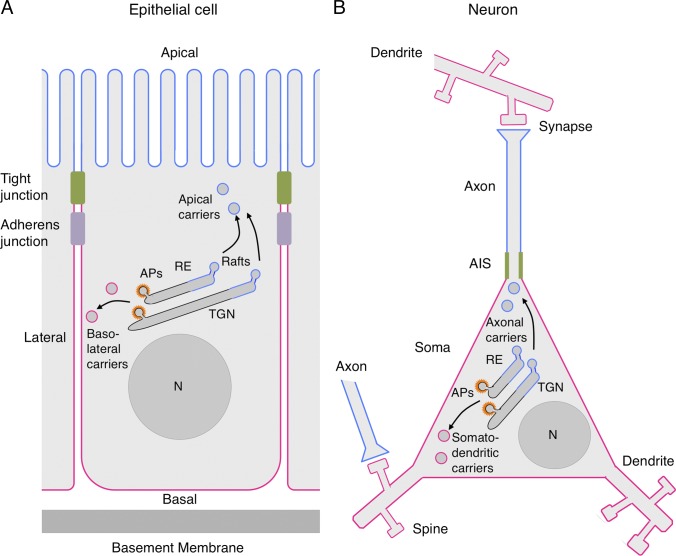
Virtually all cells exhibit an asymmetric arrangement of cytoplasmic organelles and the cytoskeleton that is referred to as “cell polarity”. This asymmetry extends to the plasma membrane, which is differentiated into two or more domains with distinct protein and lipid compositions. Cell polarity is particularly critical for multicellular organisms, in which each cell must be perfectly integrated into a complex body plan. Polarity is manifested in many forms, depending on the cell type. For example, the plasma membrane of epithelial cells is differentiated into an apical domain that faces the exterior or lumen of organs, a lateral domain that contacts neighboring cells, and a basal domain that sits on a basement membrane (Fig. 1 A; Bryant and Mostov, 2008; Mellman and Nelson, 2008; Apodaca et al., 2012). The lateral and basal domains share many properties, for which they are often referred to as the “basolateral” domain. Neurons are another prominent type of polarized cell, in which the cytoplasm is organized into dendrites, soma, and axon (Fig. 1 B; Arimura and Kaibuchi, 2007; Lasiecka et al., 2009). Neuronal asymmetry can be extreme, as best exemplified by the axons of spinal motor neurons, which in humans can reach distances of up to one meter from the soma. In some respects, the dendrites and soma are also considered parts of a common “somatodendritic” domain. The establishment and maintenance of polarity in various cell types depend on many factors, including extracellular diffusible molecules and their surface receptors, interactions with the extracellular matrix and with other cells, intracellular landmark complexes, signal-transduction pathways, cytoskeletal structures, and protein traffic. These different aspects of cell polarity have been extensively reviewed in the literature (Arimura and Kaibuchi, 2007; Bryant and Mostov, 2008; Mellman and Nelson, 2008; Lasiecka et al., 2009; Apodaca et al., 2012). In this article I will focus on a specific question: how are transmembrane proteins sorted to different plasma membrane domains in various polarized cell types? The discussion will particularly center on the role of adaptor proteins (APs) in cargo recognition for polarized sorting.
Figure 1.
Schematic representation of a polarized epithelial cell and a neuron. (A) The scheme depicts a polarized epithelial cell flanked by two neighboring cells. Tight junctions block the passage of substances through the intercellular space and also serve as a boundary between the apical (blue) and basolateral (red) domains of the plasma membrane. Adherens junctions hold cells together and regulate their proliferation. Vesicular carriers that transport proteins to the apical and basolateral plasma membrane domains are shown to emanate from both the trans-Golgi network (TGN) and recycling endosomes (RE), reflecting polarized sorting in both the biosynthetic and recycling pathways, respectively. Apical carriers are shown to arise from cholesterol- and glycosphingolipid-enriched “raft” domains, whereas basolateral carriers arise from adaptor protein (AP)–enriched “coat” domains. (B) Depiction of a neuron making synapses with two other neurons. Synapses are shown as contacts between an axon terminal and a dendritic spine, but post-synaptic sites also occur on the dendritic shaft or soma. The boundary between the axonal (blue) and somatodendritic (red) domains of the plasma membrane is at the axon initial segment (AIS, green). Axonal and somatodendritic carriers are shown emanating from the TGN and RE, as in epithelial cells. Although both schemes depict polarized sorting occurring by segregation into two types of transport carriers at the TGN/RE, selective retention at or removal from a particular domain of the plasma membrane, as well as transcytosis, also determine polarized distribution of some proteins in both epithelial cells and neurons (Garrido et al., 2001; Gan et al., 2002; Sampo et al., 2003 Anderson et al., 2005; Yap et al., 2008). A trans-endosomal route involving transport from the TGN to RE in the biosynthetic pathway has also been demonstrated (Ang et al., 2004). N, nucleus.
Signals for basolateral sorting in epithelial cells
The mechanisms of polarized sorting have been studied in greater detail in epithelial cells (Fig. 1 A). Sorting of transmembrane proteins to the apical and basolateral surfaces is thought to occur by segregation into two types of vesicular transport carriers emanating from the trans-Golgi network (TGN) and/or recycling endosomes (RE; Fig. 1 A). While the TGN participates in the sorting of newly synthesized proteins, RE are involved in returning internalized proteins to the cell surface. Biosynthetic transport from the TGN to the cell surface via RE has also been demonstrated (Ang et al., 2004). Polarized sorting depends on recognition of specific determinants on the cargo proteins. Apical sorting determinants are highly diverse and vaguely defined (Weisz and Rodriguez-Boulan, 2009; Cao et al., 2012). For transmembrane proteins, they have been variously mapped to the extracellular, transmembrane, and cytosolic domains, but their exact nature and mode of action remain poorly understood. A leading theory is that apical determinants promote direct or indirect partitioning into glycosphingolipid- and cholesterol-rich membrane microdomains (i.e., lipid rafts) at the TGN and/or RE, from where apically bound carriers arise (Fig. 1 A). On the other hand, basolateral sorting determinants are often discrete and more precisely defined, earning them the more stringent designation of “signals”. Basolateral signals are generally located in the cytosolic domains of the proteins and in some cases consist of amino acid arrays that fit canonical motifs such as YXXØ and [DE]XXXL[LI] (amino acids denoted in single letter code; X is any amino acid and Ø is a bulky hydrophobic amino acid; Fig. 2 A; Duffield et al., 2008; Gonzalez and Rodriguez-Boulan, 2009). Notably, sequences conforming to these motifs also mediate rapid endocytosis from the cell surface as well as sorting to lysosomes and lysosome-related organelles (Traub and Bonifacino, 2013). Some basolateral signals, however, do not fit any known motif and are functionally distinct from endocytic/lysosomal-sorting signals. For example, the basolateral signal of the transferrin receptor (TfR) is not the endocytic signal YTRF (a YXXØ motif) but the noncanonical sequence GDNS (Fig. 2 A; Odorizzi and Trowbridge, 1997). Likewise, the basolateral sorting signal of the low density lipoprotein receptor (LDLR) is not the endocytic signal FDNVPY (a [YF]XNPX[YF] motif) but a bipartite signal comprising a proximal determinant and a distal determinant, both having critical tyrosine and acidic amino acid residues (Fig. 2 A; Matter et al., 1992). Finally, the polymeric immunoglobulin receptor (pIgR) has a noncanonical basolateral signal consisting of a 17–amino acid sequence with a critical HRRNV core that is not involved in endocytosis (Fig. 2 A; Casanova et al., 1991; Aroeti et al., 1993). Mutations in these signals abrogate basolateral sorting, resulting in nonpolarized or apical distribution of the corresponding proteins. An example that is relevant to human disease is the mutation of glycine-823 to aspartic acid in the distal determinant of the LDLR in patients with familial hypercholesterolemia–Turku variant, which causes missorting of the LDLR to the apical surface of epithelial cells and the bile canalicular surface of hepatocytes (Koivisto et al., 2001). Decreased basolateral endocytosis of LDL leads to reduced cholesterol clearance from the bloodstream and ensuing hypercholesterolemia.
Figure 2.
Signals and adaptor proteins involved in polarized sorting. (A) Sequences of the cytosolic tails of the indicated proteins, with critical residues of polarized sorting signals highlighted in red. Signals are categorized as YXXØ, [DE]XXXL[LI], or noncanonical. The transmembrane domain (TM) and the number of additional residues in each tail are indicated. For the TfR, the basolateral sorting signal is GDNS, but the somatodendritic sorting signal is YTRF. The LDLR has a proximal signal with one tyrosine residue and an acidic cluster (EDE) and a distal signal with two tyrosine residues and another acidic cluster (EED). (B) Adaptor proteins that have been implicated in polarized sorting. AP-1 and AP-4 are composed of four homologous subunits, as reflected by the color scheme. The core, hinge, and ear domains of the AP complexes and the PTB domain of ARH and Numb are indicated. Folded regions are represented by geometric shapes and disordered regions by wavy lines. The AP-2, AP-3, AP-5, and COPI-F complexes are homologous to AP-1 and AP-4, but to date they have not been directly implicated in polarized sorting. Three of the AP-1 subunits occur as isoforms encoded by different genes: γ1 and γ2; μ1A and μ1B; and σ1A, σ1B, and σ1C. PM, plasma membrane. (C) Schematic representation of a clathrin-coated bud on the TGN/RE containing AP-1, Numb, and ARH as adaptor proteins. Arf proteins recruit AP-1 to membranes and promote its conformational activation. Clathrin triskelia polymerize onto the adaptor proteins to form a polyhedral coat. Cargos are gathered into the coated buds by interaction of sorting signals with the adaptor proteins.
Role of AP-1 in basolateral sorting
What is the nature of the sorting devices that decode basolateral signals? Before the identification of the basolateral-sorting adaptors, it was known that endocytic/lysosomal-sorting signals fitting the YXXØ and [DE]XXXL[LI] motifs were recognized by some, although not all, members of the family of heterotetrameric AP complexes, which includes AP-1, AP-2, AP-3, AP-4, AP-5, and COPI-F (Fig. 2 B; Robinson, 2004; Hirst et al., 2013). AP family complexes are components of protein coats that mediate cargo selection and coated vesicle formation at different stages of the endomembrane system (Fig. 2, B and C). It was then logical to assume that basolateral sorting would be mediated by one or more of these complexes—but which one(s)? The first hint to the identity of a basolateral-sorting adaptor was provided by the identification of μ1B, an isoform of the μ1 subunit of AP-1 (Fig. 2 B) that is specifically expressed in a subset of polarized epithelial cells in mammals (Ohno et al., 1999). This was in contrast to the μ1A isoform and the other subunits of AP-1 (γ, β1, and σ1, and their various isoforms; Fig. 2 B), which were expressed in all cell types. Initial analysis of the role of μ1B in basolateral sorting was enabled by the use of the μ1B-positive MDCK and the μ1B-negative LLC-PK1 epithelial cell lines, derived from kidney distal and proximal tubule cells, respectively. Both cell lines can be cultured as monolayers of polarized columnar cells, but they sort some cargos differently (Roush et al., 1998; Fölsch et al., 1999). For instance, the TfR and LDLR are basolateral in MDCK cells but apical in LLC-PK1 cells (Fölsch et al., 1999). Strikingly, expression of μ1B in LLC-PK1 redirected the TfR and LDLR to the basolateral surface, thus demonstrating the critical role of this μ1 isoform in basolateral sorting (Fölsch et al., 1999). Later studies showed that μ1B knockdown also impaired basolateral sorting of several cargos, including the TfR and LDLR, in MDCK cells, and that this defect was exacerbated by simultaneous μ1A knockdown (Gravotta et al., 2012; Carvajal-Gonzalez et al., 2012). These findings thus demonstrated that μ1A and μ1B play partly complementary roles in basolateral sorting as alternative subunits of two AP-1 complex variants named AP-1A and AP-1B, respectively. Consistent with the role of AP-1 as a clathrin adaptor (Fig. 2 C), the scaffolding protein clathrin was also found to be required for basolateral sorting (Deborde et al., 2008).
The μ1B isoform is expressed in some mammalian epithelial cells (e.g., kidney distal and intestinal epithelial cells) but not in other epithelial cells (e.g., kidney proximal and retinal pigmented epithelial cells) or other polarized cell types (e.g., neurons, hepatocytes). This expression pattern is different from that of μ1A, which is expressed in all cells (Ohno et al., 1999; Diaz et al., 2009; Schreiner et al., 2010). Why did some epithelial cells evolve to express μ1B? Several studies showed predominant localization of μ1A and μ1B to the TGN and RE, respectively (Fölsch et al., 2001, 2003; Gravotta et al., 2012), leading to the notion that AP-1A may be mainly involved in biosynthetic sorting at the TGN and AP-1B in recycling to the basolateral surface from RE. Additionally, AP-1B was shown to be specifically regulated by the small GTP-binding protein Arf6 (Shteyn et al., 2011) and the phosphoinositide phosphatidylinositol 3,4,5-trisphosphate (PIP3; Fields et al., 2010), and to recognize the proximal basolateral sorting signal of the LDLR indirectly thorough interaction with ARH (Fig. 2 B; Kang and Fölsch, 2011). However, AP-1B was also shown to promote export of the LDLR from the TGN in the absence of AP-1A (Gravotta et al., 2012), and AP-1A was found to participate in endosomal sorting events in other cell types (Eskelinen et al., 2002; Delevoye et al., 2009; Hirst et al., 2012). Consistent with these findings, a recent study using high-resolution microscopic and biochemical methods presented an alternative explanation to the μ1A–μ1B conundrum: both AP-1A and AP-1B localize indistinctly to the TGN and RE and exhibit similar regulation by Arf family members, but display preferential interactions with some cargos (X. Guo et al., 2013). In particular, AP-1B directly and preferentially binds to basolateral signals from cargos that are sorted in a μ1B-dependent manner in some cell types. For example, AP-1B binds the noncanonical signals from the LDLR approximately fivefold more strongly than AP-1A (X. Guo et al., 2013). According to these findings, then, expression of μ1B enables recognition of cargos that are not efficiently recognized by μ1A. Differential expression of μ1B may allow alternative sorting of some receptors in different epithelial cell types, as is the case for the LDLR, which is nonpolarized (i.e., both basolateral and apical) in μ1B-negative proximal kidney tubule cells but exclusively basolateral in μ1B-positive intestinal epithelial cells (Pathak et al., 1990; Hase et al., 2013). Because cargo binding stabilizes the conformationally active, membrane-associated form of AP-1 (Lee et al., 2008; Ren et al., 2013), AP-1A and AP-1B could function at either the TGN or RE, depending on where they meet their preferred cargos.
Altered epithelial cell polarity in AP-1 mutant animals
The involvement of AP-1 in polarized sorting in epithelial cells has received strong confirmation from recent studies on whole animals. Knock-out (KO) of the genes encoding the γ1 (AP1G1) or μ1A (AP1M1) subunits of AP-1 in mouse caused early embryonic lethality (Zizioli et al., 1999; Meyer et al., 2000). In contrast, μ1B (AP1M2) KO mice were born at term, although half died before eight weeks of age and the surviving half showed stunted growth (Hase et al., 2013). This reduced viability and growth retardation were due to severe defects in the intestinal epithelial cell layer, including hyperplasia and morphological abnormalities with ensuing nutrient malabsorption. In line with the cell culture studies discussed above, basolateral sorting of some cargos such as the LDLR, the ephrin receptor Eph2B, the adhesion protein E-cadherin, and the interleukin 6 signal transducer IL-6st was impaired in intestinal epithelial cells from the μ1B KO mice (Takahashi et al., 2011; Hase et al., 2013). Missorting of E-cadherin disrupted its complex with β-catenin at adherens junctions, causing nuclear translocation of β-catenin and increased transcription of β-catenin–regulated genes (Hase et al., 2013). Activation of this signaling pathway is likely responsible for the hyperproliferative phenotype of the intestinal epithelial cells in the μ1B KO mice. The presence of duodenal polyps in μ1B KO mice and the reduced expression of μ1B in some human colorectal tumors suggest that hyperproliferation caused by loss of μ1B may contribute to the pathogenesis of intestinal cancer (Mimura et al., 2012; Hase et al., 2013). The μ1B KO mice exhibited an additional pathology: chronic colitis due to missorting of cytokine receptors such as IL-6st, reduced expression of antimicrobial peptides, and impaired secretion of immunoglobulin A (IgA) in the intestine (Takahashi et al., 2011). These perturbations allowed increased penetration of commensal bacteria through the intestinal mucosa, triggering a strong inflammatory response (Takahashi et al., 2011). The inflammatory colitis in μ1B KO mice is reminiscent of that in humans with Crohn’s disease. Indeed, analysis of colonic mucosa from Crohn’s patients showed reduced levels of μ1B mRNA, possibly implicating changes in μ1B expression in this pathology (Takahashi et al., 2011).
Studies using Caenorhabditis elegans (Shafaq-Zadah et al., 2012; Zhang et al., 2012) and zebrafish (Clemens Grisham et al., 2013) have provided additional support for the role of AP-1 and clathrin in the regulation of epithelial cell polarity. RNAi-mediated depletion of C. elegans AP-1 subunits, including the APM-1 μ1 isoform, decreased the polarization of several basolateral transmembrane proteins (Shafaq-Zadah et al., 2012; Zhang et al., 2012). Mutations in the gene encoding the β1 subunit of AP-1 in zebrafish also mislocalized the basolateral Na+/K+ ATPase to the apical surface in auditory and lateral-line hair cells, which are specialized epithelial cells (Clemens Grisham et al., 2013). Mislocalization of the Na+/K+ ATPase and other basolateral proteins likely underlie other phenotypes such as abnormal ion homeostasis and mechanotransduction as well as degeneration of the mutant hair cells (Clemens Grisham et al., 2013).
Unexpectedly, μ1B KO mice (Hase et al., 2013) and AP-1–deficient C. elegans (Shafaq-Zadah et al., 2012; Zhang et al., 2012) also displayed missorting of apical proteins to the basolateral surface or to intracellular vesicles in intestinal epithelial cells. These included transmembrane proteins (e.g., sucrase, NHX-2, PEPT-1, AQP-4) as well as cytoskeletal proteins (e.g., villin, actin) and the polarity-determinant protein PAR-6. Moreover, loss of AP-1 subunits in both organisms resulted in the appearance of ectopic apical-like membranes along the lateral surface or as invaginations from the lateral membrane (Shafaq-Zadah et al., 2012; Zhang et al., 2012; Hase et al., 2013). Similar phenotypes were observed upon clathrin depletion in C. elegans (Zhang et al., 2012). These apical abnormalities resembled those resulting from interference with glycosphingolipid biosynthesis, which affects the lipid raft mechanism of apical sorting (Cao et al., 2012; Zhang et al., 2012). Thus, AP-1 is required for the sorting of both basolateral and apical proteins in intestinal epithelial cells of whole animals. The role of AP-1 in basolateral sorting is most likely direct because of its ability to interact with basolateral sorting signals. How AP-1 mediates apical sorting is less clear. Direct recognition of apical sorting determinants seems unlikely because these determinants are most often found in the luminal or transmembrane domains. Instead, AP-1–dependent sorting of one or more key proteins to the basolateral membrane might be required for exclusion of apical proteins from this membrane domain. Alternatively, AP-1 defects could alter the global properties of TGN or RE membranes such that transport to the basolateral and apical membranes becomes randomized.
Role of AP-1 in cell fate specification during asymmetric cell division
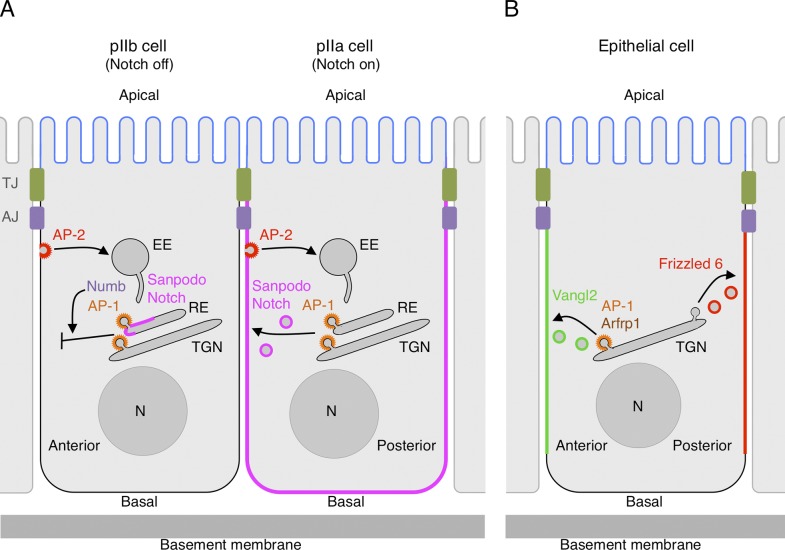
The ability of AP-1 to regulate polarized sorting to different plasma membrane domains has been recently linked to Notch-dependent cell fate specification after asymmetric division of Drosophila sensory organ precursor (SOP) cells (Fig. 3 A; Benhra et al., 2011; Cotton et al., 2013). SOP cells arise in the dorsal neuroepithelium (i.e., notum) of Drosophila pupae and undergo a series of asymmetric divisions to generate the four specialized cells that make up the mature sensory organ. The first division generates two daughter cells named pIIa (posterior) and pIIb (anterior). Notch, a transmembrane signaling receptor for Delta family ligands, is activated in pIIa cells and inhibited in pIIb cells, leading each cell along a different specification pathway. Activation of Notch requires another transmembrane protein named Sanpodo. How is signaling by Notch/Sanpodo differentially regulated in pIIa and pIIb cells? During asymmetric division of the SOP cell, the monomeric PTB-containing adaptor Numb (Fig. 2 B) is unequally partitioned into the pIIb cell, where it prevents Notch and Sanpodo localization to the cell surface, thus suppressing Notch signaling (Fig. 3 A). Notch/Sanpodo are internalized in an AP-2–dependent manner, but expression of Numb has no effect on the rate of endocytosis (Berdnik et al., 2002; Cotton et al., 2013; Couturier et al., 2013). Instead, Numb regulates Notch/Sanpodo traffic through effects on AP-1 (Benhra et al., 2011; Cotton et al., 2013). In the SOP and pIIb cells Sanpodo is mainly localized to endosomes, whereas in the pIIa cell it localizes to the basolateral plasma membrane. Silencing or mutation of AP-1 subunit genes causes Sanpodo redistribution to the apical surface of all of these cells, indicating that AP-1 mediates basolateral sorting as in other polarized epithelia (Benhra et al., 2011). In the pIIb cell, Numb inhibits the ability of AP-1 to return Sanpodo to the basolateral surface, shifting its steady-state localization to endosomes. This function is dependent on the YTNPAF sequence (fitting a [FY]XNPX[YF] motif) in the Sanpodo N-terminal tail, which binds to the Numb PTB domain (Tong et al., 2010; Benhra et al., 2011). Redistribution of Sanpodo to the plasma membrane by mutation of genes encoding Numb or AP-1 subunits causes Notch activation, resulting in pIIb-to-pIIa cell fate transformation (Benhra et al., 2011; Cotton et al., 2013). Regulation of Notch activation by this process starts during cytokinesis of the SOP cell (Couturier et al., 2013), suggesting that AP-1 exerts its role even before separation of the daughter cells. Thus, Numb functions in endosomes to prevent AP-1–mediated recycling of Notch/Sanpodo to the pIIb cell surface, in line with previous studies implicating Numb in the regulation of endosomal traffic in mammals (McGill et al., 2009) and C. elegans (Nilsson et al., 2008). These findings demonstrate that some adaptors (e.g., Numb) can counteract the ability of other adaptors (e.g., AP-1) to promote transport. The molecular mechanisms involved in such inhibitory effects, however, remain to be elucidated.
Figure 3.
Role of AP-1 in cell fate specification and planar cell polarity. (A) Asymmetric inheritance of Numb in Drosophila sensory organ precursor (SOP) daughter cells determines AP-1–dependent inhibition of Sanpodo and Notch recycling in the pIIb cell (Numb positive) and allows AP-1–dependent recycling of Sanpodo and Notch in the pIIa cell (Numb negative; Benhra et al., 2011; Cotton et al., 2013). Notch signaling is thus inhibited in the pIIb cell and activated in the pIIa cell. The related AP-2 complex mediates Notch and Sanpodo internalization, but Numb does not appear to regulate this process (Cotton et al., 2013; Couturier et al., 2013). The anterior or posterior orientation of the SOP daughter cells in the plane of the notum is indicated. EE, early endosome. (B) AP-1 in association with the Arf-like protein Arfrp1 mediates export of the planar cell polarity (PCP) regulator Vangl2 from the TGN to the anterior (or proximal) surface of the lateral membrane domain in epithelial cells (Y. Guo et al., 2013). Another PCP regulator, Frizzled 6, is targeted to the posterior (or distal) surface by an unknown mechanism. AJ, adherens junction; EE, early endosome; N, nucleus; TJ, tight junction.
Function of AP-1 in sorting to the neuronal somatodendritic domain
The mechanisms of protein sorting to the axonal and somatodendritic domains of neurons are thought to be similar, although not identical, to those of sorting to the apical and basolateral domains of epithelial cells, respectively (Fig. 1 B; Dotti and Simons, 1990; Jareb and Banker, 1998). The similarities are particularly apparent for somatodendritic sorting signals, which also map to the cytosolic domains of the proteins and in some cases are identical to basolateral sorting signals (Jareb and Banker, 1998). For example, the sequence YNQV (a YXXØ motif) in the cytosolic tail of the Coxsackie and adenovirus receptor (CAR; Fig. 2 A) mediates both basolateral (Cohen et al., 2001; Carvajal-Gonzalez et al., 2012) and somatodendritic sorting (Farías et al., 2012). In other cases, somatodendritic and basolateral sorting signals differ. For instance, the somatodendritic sorting signal of the TfR is the sequence YTRF (a YXXØ motif) and not the basolateral signal GDNS (Fig. 2 A; Farías et al., 2012). Both the CAR and TfR somatodendritic signals are directly recognized by μ1A (Carvajal-Gonzalez et al., 2012; Farías et al., 2012), the only μ1 subunit isoform expressed in the brain (Ohno et al., 1999). Expression of a μ1A dominant-negative mutant or RNA-mediated knockdown of the γ1 subunit of AP-1 abrogates the somatodendritic polarity of TfR and CAR, as well as that of metabotropic and NMDA-type glutamate receptor proteins, causing them to become evenly distributed between the somatodendritic and axonal domains (Farías et al., 2012). Analysis of TfR traffic showed that this loss of polarity upon AP-1 interference is due to misincorporation of TfR into axonal transport carriers, from which the receptor is normally excluded. Similar effects were elicited by expression of a clathrin dominant-negative mutant, indicating that, as in basolateral sorting, AP-1 and clathrin function together in signal-mediated cargo sorting to the somatodendritic domain (Farías et al., 2012).
AP-1 and clathrin have also been implicated in sorting of various receptors and transporters to dendrites in C. elegans neurons (Dwyer et al., 2001; Bae et al., 2006; Margeta et al., 2009). The μ1 isoform involved in dendritic sorting is UNC-101 and not the basolateral-sorting APM-1 (Shafaq-Zadah et al., 2012; Zhang et al., 2012), providing another example of the tissue-specific roles of different μ1 isoforms. Also in this organism, dendritic sorting is mediated by the cytosolic tails of proteins. Mutation of either UNC-101 or the cytosolic tails of the proteins resulted in even distribution between dendrites and axons. In some neurons (e.g., AWA) UNC-101 mutation blocked formation of dendritic carriers (Dwyer et al., 2001), whereas in others (e.g., RIA) it prevented retrieval of the proteins from axonal presynaptic sites (Margeta et al., 2009), suggesting that the mechanisms of AP-1 action may vary in different types of neurons. Another monomeric clathrin adaptor, UNC-11 (homologous to mammalian AP180), was implicated in axonal sorting of the synaptic vesicle protein synaptobrevin (Dwyer et al., 2001) but not dendritic sorting of several receptors (Dwyer et al., 2001; Margeta et al., 2009) in C. elegans.
Defects in somatodendritic sorting may underlie two human neurodevelopmental disorders caused by mutations in isoforms of the σ1 subunit of AP-1 (Fig. 2 B): the MEDNIK syndrome (σ1A mutation; Montpetit et al., 2008) and Fried-type X-linked mental retardation (σ1B mutation; Tarpey et al., 2006). Both diseases present with intellectual disability and other neurological abnormalities. In addition, MEDNIK patients exhibit enteropathy and skin conditions. The organs affected (i.e., brain, spinal cord, intestines, skin) are all consistent with the role of AP-1 in polarized sorting in neurons and epithelial cells. Because isoforms of σ1 exhibit distinct preferences for subsets of [DE]XXXL[LI] signals (Mattera et al., 2011), it is likely that the pathogenesis of these diseases involves defective polarized sorting of select transmembrane cargos bearing [DE]XXXL[LI] or related signals.
Models for AP-1 function in epithelial and neuronal polarized sorting
AP-1 has been localized to both the TGN and RE (Klumperman et al., 1993; Futter et al., 1998), where it likely functions to select basolateral or somatodendritic cargos for packaging into a population of clathrin-coated vesicles (CCVs) or pleiomorphic transport carriers having clathrin-coated buds (Fig. 2 C and Fig. 4 A; Polishchuk et al., 2006). These intermediates could themselves deliver cargos to the corresponding plasma membrane domain. Alternatively, they could uncoat soon after budding and undergo homotypic or heterotypic fusion to generate the actual transport carriers. Importantly, in many cases AP-1 and clathrin are not strictly required for cargo transport to the basolateral or somatodendritic domain; rather, they are required for exclusion of these cargos from the apical and axonal domains (Deborde et al., 2008; Carvajal-Gonzalez et al., 2012; Farías et al., 2012; Gravotta et al., 2012). Therefore, the main role of AP-1 might be to capture cargos into CCVs so that they are excluded from apical/axonal carriers. AP-1 could also function to retrieve basolateral/somatodendritic cargos from apical/axonal carriers budding from the TGN/RE (Fig. 4 B) in a manner analogous to the retrieval of mannose 6-phosphate receptors from immature secretory vesicles (Klumperman et al., 1998). Finally, AP-1 could function not just to form CCVs but to differentiate a domain of the TGN/RE into which basolateral/somatodendritic cargos are segregated away from sites of apical/axonal-carrier formation (Fig. 4 C). In this latter model, interference with AP-1 or clathrin could disrupt not only the sorting of basolateral/somatodendritic cargos but also the membrane domain organization of the TGN/RE, leading to concomitant missorting of apical/axonal cargos, as observed in AP-1–deficient mice (Hase et al., 2013) and C. elegans (Shafaq-Zadah et al., 2012; Zhang et al., 2012).
Figure 4.
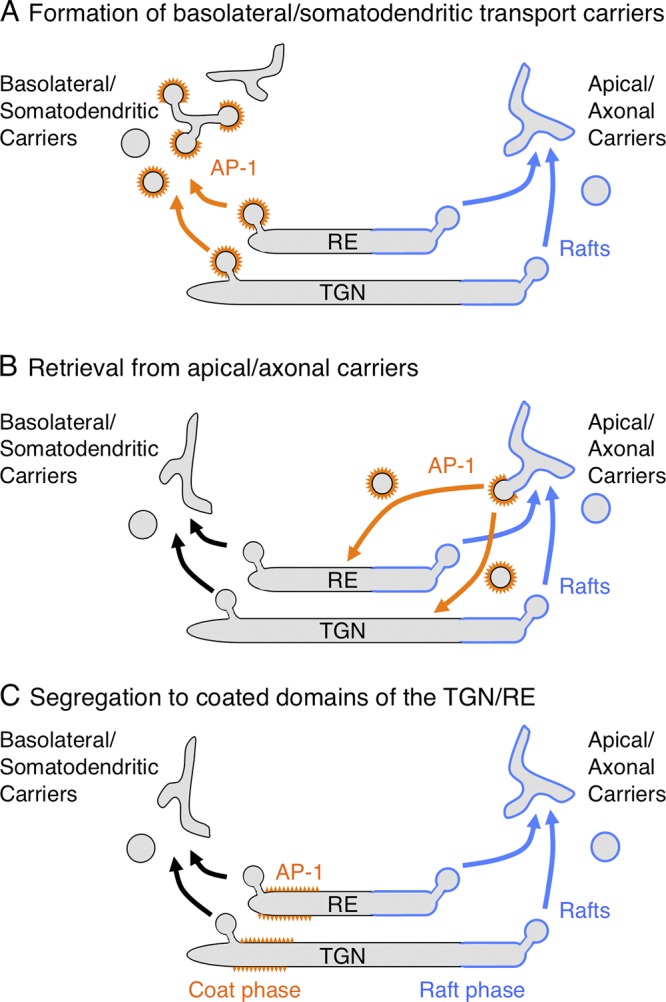
Models of AP-1 function in polarized sorting at the TGN and RE. The schemes depict three models for the function of AP-1 in sorting to the basolateral domain of epithelial cells and the somatodendritic domain of neurons. In model A, AP-1 sorts cargos into clathrin-coated vesicles or pleiomorphic transport carriers at the TGN/RE. These intermediates lose their coats and eventually deliver their cargos to the basolateral or somatodendritic plasma membrane domains. In model B, AP-1 removes basolateral or somatodendritic cargos from apical or axonal transport carriers to return them to the TGN/RE. In model C, AP-1 segregates basolateral or somatodendritic cargos into a “coat phase” of the TGN/RE, away from the “raft phase” that gives rise to apical or axonal carriers.
Role of AP-4 in polarized sorting
AP-4 is another member of the heterotetrameric AP family that is associated with the TGN (Fig. 2 B; Dell’Angelica et al., 1999; Hirst et al., 1999). Unlike AP-1, however, AP-4 does not associate with clathrin and might therefore be part of a nonclathrin coat. The μ4 subunit of AP-4 was shown to interact with the cytosolic tails of several basolateral cargos, including the LDLR, and antisense-RNA–mediated knockdown of μ4 caused mislocalization of those cargos to the apical surface (Simmen et al., 2002). However, ablation of the gene encoding the β4 subunit of AP-4 in mouse did not cause any obvious defects in epithelia (Matsuda et al., 2008; Hase et al., 2013). In fact, these mice were perfectly viable and only exhibited mild motor impairment (Matsuda et al., 2008). Histological analysis showed mislocalization of the normally dendritic AMPA-type glutamate receptors and their regulatory proteins, TARPs, to axonal autophagosomes in cerebellar Purkinje neurons (Matsuda et al., 2008). The dendritic Delta2 glutamate receptor and LDLR were also mislocalized to axons, whereas metabotropic and NMDA-type glutamate receptor proteins were not affected (Matsuda and Yuzaki, 2008). Mutations in each of the four subunits of AP-4 have recently been identified as causes of a neurodevelopmental disorder with characteristics of hereditary spastic paraplegia in humans (Verkerk et al., 2009; Abou Jamra et al., 2011; Moreno-De-Luca et al., 2011). In contrast to the mild neurological impairment of β4 KO mice, the symptoms of this disease in humans are very severe, including microcephaly, cerebral palsy, and profound intellectual disability. Post-mortem immunohistochemical analysis also showed increased accumulation of Delta2 glutamate receptors in the soma of Purkinje neurons from an affected patient (Verkerk et al., 2009), consistent with decreased transport to the dendrites. The μ4 subunit interacts with the cytosolic tails of the TARPs and the Delta2 glutamate receptor via noncanonical sequences containing phenylalanine and tyrosine residues (Yap et al., 2003; Matsuda et al., 2008), indicating that AP-4 may function in a manner similar to AP-1 to sort a subset of neuronal cargos into dendritic transport carriers (Figs. 1 A and 4).
Other sorting events, other coats
AP-1 has been implicated in many other sorting processes, some related, some unrelated, to cell polarity. In the related category is the requirement of AP-1 and clathrin for secretion of the signaling protein Wnt3a from the basolateral surface in epithelial cells, probably through a role of AP-1 and clathrin in basolateral sorting of the Wntless receptor (Yamamoto et al., 2013). AP-1 is also required for lysosome exocytosis from the basolateral surface, likely due to its role in basolateral sorting of the target-SNARE syntaxin 4 (Reales et al., 2011; Xu et al., 2012). In addition, AP-1 promotes delivery of another syntaxin, KNOLLE, to the cell plate in dividing plant cells (Teh et al., 2013). Moreover, AP-1 may be involved in some forms of planar cell polarity (PCP; Y. Guo et al., 2013) in which cellular structures are asymmetrically distributed within the plane of the epithelium (Y. Guo et al., 2013). PCP involves further differentiation of the lateral membrane into anterior/proximal and posterior/distal domains (Fig. 3 B). In this regard, AP-1, in conjunction with the Arf-related protein Arfrp1/Arl3, has recently been shown to promote export of the anterior/proximal signaling protein Vangl2 from the TGN to the plasma membrane through recognition of a YYXXF motif (Y. Guo et al., 2013). Another TGN-associated coat complex named “exomer” mediates export of the chitin synthase Chs3p to the bud neck and the cell fusion protein Fus1p to mating projections in yeast (Wang et al., 2006; Barfield et al., 2009). In the absence of exomer, these cargos are retained in the TGN or RE, but mutation of AP-1 subunit genes restores their transport to the plasma membrane (Valdivia et al., 2002). Thus, under these conditions AP-1 mediates intracellular retention of Chs3p, through recognition of a [DE]XXXL[LI] signal in the Chs3p tail (Starr et al., 2012). Taken together, these findings indicate that AP-1 can exert both positive and negative effects on cargo transport from the TGN/RE to different cell surface domains. The list of functions ascribed to AP-1 is much longer and also includes bidirectional transport between the TGN and endosomes (Hirst et al., 2012), sorting to maturing melanosomes (Delevoye et al., 2009), biogenesis of Weibel-Palade bodies (Lui-Roberts et al., 2005), and many others. How could AP-1 mediate so many different processes? A likely explanation is that the activity of AP-1 is modified by interactions with other adaptors or accessory proteins, as exemplified by ARH (Kang and Fölsch, 2011), Numb (Cotton et al., 2013), and Arfrp1 (Y. Guo et al., 2013) in the cases discussed in the previous sections. The outcome of a particular AP-1 signal-recognition event would thus depend on what other interacting proteins are involved. Another possibility is that AP-1 functions more generally as a domain organizer at the TGN/RE, enabling sorting to occur by additional mechanisms.
This discussion has focused mainly on AP-1 and related proteins because their roles in polarized sorting are the best understood. However, other coats are also likely to be involved. One of these is the “retromer”, a structurally distinct protein complex that primarily mediates retrograde transport from endosomes to the TGN (Seaman et al., 1998). Analyses of polarized sorting have shown that retromer is required for maintenance of the TGF-β receptor II at the basolateral surface (Yin et al., 2013), basolateral-to-apical transcytosis of the polymeric immunoglobulin receptor in polarized epithelial cells (Vergés et al., 2004), and trafficking of the polarity determinants Crumbs, Par6, and aPKC to the apical surface of epithelial cells (Pocha et al., 2011). Finally, the sorting nexin SNX17, which is structurally related to two of the retromer subunits, directs recycling of the LDLR-related protein LRP1 from endosomes to the basolateral surface through interaction of an IXNPXY motif (a variant of the [FY]XNPX[YF] motif) in the LRP1 tail with the FERM domain of SNX17 (Donoso et al., 2009; Farfán et al., 2013).
Concluding remarks
The evidence reviewed here makes it abundantly clear that sorting of transmembrane proteins to the basolateral surface of epithelial cells, the somatodendritic surface of neurons, and various specialized plasma membrane domains in other cells is dependent on specific interactions of the transmembrane protein tails with adaptors or other coat proteins associated with the cytosolic aspect of the TGN and RE. The molecular mechanisms involved in this process are thus similar to those responsible for clathrin-mediated endocytosis from the cell surface (Traub and Bonifacino, 2013). The AP-1 clathrin adaptor appears to play a central role in polarized sorting in the same manner that AP-2 functions as a core component of the endocytic machinery. However, many outstanding issues remain to be investigated, including the exact compartment where polarized sorting takes place, the relationship of polarized sorting to other sorting events mediated by AP-1, how AP-1 could mediate both export from and retention at the TGN/RE, the mechanisms by which AP-4 and retromer participate in polarized sorting, and the potential involvement of other coat proteins.
Future work should also address whether adaptor and coat proteins influence events that follow the sorting of cargo into transport carriers, such as the translocation of the carriers through the cytoplasm and their fusion with the corresponding target organelles. In this regard, AP-1 has been shown to interact directly or indirectly with components of the microtubule motors kinesin-1 (Schmidt et al., 2009) and kinesin-3 (Nakagawa et al., 2000), although the role of these interactions in polarized sorting remains to be determined. Adaptors or other coat proteins are also likely to select the SNARE proteins that mediate fusion of transport carriers with their target organelles. For example, syntaxin 4 is sorted to the basolateral surface of epithelial cells by virtue of interaction with AP-1 (Xu et al., 2012). Assembly of the vesicle-SNARE cellubrevin/VAMP3 with syntaxin 4 and other cognate SNAREs then allows for the delivery of AP-1B cargos to the basolateral surface (Fields et al., 2007). The exocyst complex, which promotes tethering and fusion of transport carriers with the basolateral plasma membrane, is also recruited to recycling endosomes in an AP-1B–dependent manner (Fölsch et al., 2003). The molecular details of these interactions and their exact roles in polarized sorting are, however, poorly understood. The elucidation of these mechanisms should help explain how failure of polarized sorting underlies various genetic and acquired diseases. With powerful molecular, structural, genetic, and imaging tools now at hand, we can expect many exciting developments in this area of research in the coming years.
Acknowledgments
I thank all the colleagues in my laboratory and other laboratories who contributed to the research described in this article. I also thank Roland Le Borgne for helpful discussions.
Work in my laboratory is funded by the Intramural Program of the National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Abbreviations used in this paper:
- AP
- adaptor protein
- KO
- knock-out
- LDLR
- low density lipoprotein receptor
- RE
- recycling endosomes
- SOP
- sensory organ precursor
- TfR
- transferrin receptor
References
- Abou Jamra R., Philippe O., Raas-Rothschild A., Eck S.H., Graf E., Buchert R., Borck G., Ekici A., Brockschmidt F.F., Nöthen M.M., et al. 2011. Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am. J. Hum. Genet. 88:788–795 10.1016/j.ajhg.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E., Maday S., Sfakianos J., Hull M., Winckler B., Sheff D., Fölsch H., Mellman I. 2005. Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways. J. Cell Biol. 170:595–605 10.1083/jcb.200506051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang A.L., Taguchi T., Francis S., Fölsch H., Murrells L.J., Pypaert M., Warren G., Mellman I. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 167:531–543 10.1083/jcb.200408165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G., Gallo L.I., Bryant D.M. 2012. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat. Cell Biol. 14:1235–1243 10.1038/ncb2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N., Kaibuchi K. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8:194–205 10.1038/nrn2056 [DOI] [PubMed] [Google Scholar]
- Aroeti B., Kosen P.A., Kuntz I.D., Cohen F.E., Mostov K.E. 1993. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J. Cell Biol. 123:1149–1160 10.1083/jcb.123.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y.K., Qin H., Knobel K.M., Hu J., Rosenbaum J.L., Barr M.M. 2006. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 133:3859–3870 10.1242/dev.02555 [DOI] [PubMed] [Google Scholar]
- Barfield R.M., Fromme J.C., Schekman R. 2009. The exomer coat complex transports Fus1p to the plasma membrane via a novel plasma membrane sorting signal in yeast. Mol. Biol. Cell. 20:4985–4996 10.1091/mbc.E09-04-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhra N., Lallet S., Cotton M., Le Bras S., Dussert A., Le Borgne R. 2011. AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors. Curr. Biol. 21:87–95 10.1016/j.cub.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Berdnik D., Török T., González-Gaitán M., Knoblich J.A. 2002. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell. 3:221–231 10.1016/S1534-5807(02)00215-0 [DOI] [PubMed] [Google Scholar]
- Bryant D.M., Mostov K.E. 2008. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9:887–901 10.1038/nrm2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Surma M.A., Simons K. 2012. Polarized sorting and trafficking in epithelial cells. Cell Res. 22:793–805 10.1038/cr.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Gonzalez J.M., Gravotta D., Mattera R., Diaz F., Perez Bay A., Roman A.C., Schreiner R.P., Thuenauer R., Bonifacino J.S., Rodriguez-Boulan E. 2012. Basolateral sorting of the Coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc. Natl. Acad. Sci. USA. 109:3820–3825 10.1073/pnas.1117949109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.E., Apodaca G., Mostov K.E. 1991. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 66:65–75 10.1016/0092-8674(91)90139-P [DOI] [PubMed] [Google Scholar]
- Clemens Grisham R., Kindt K., Finger-Baier K., Schmid B., Nicolson T. 2013. Mutations in ap1b1 cause mistargeting of the Na(+)/K(+)-ATPase pump in sensory hair cells. PLoS ONE. 8:e60866 10.1371/journal.pone.0060866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C.J., Gaetz J., Ohman T., Bergelson J.M. 2001. Multiple regions within the Coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J. Biol. Chem. 276:25392–25398 10.1074/jbc.M009531200 [DOI] [PubMed] [Google Scholar]
- Cotton M., Benhra N., Le Borgne R. 2013. Numb inhibits the recycling of Sanpodo in Drosophila sensory organ precursor. Curr. Biol. 23:581–587 10.1016/j.cub.2013.02.020 [DOI] [PubMed] [Google Scholar]
- Couturier L., Mazouni K., Schweisguth F. 2013. Numb localizes at endosomes and controls the endosomal sorting of notch after asymmetric division in Drosophila. Curr. Biol. 23:588–593 10.1016/j.cub.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez-Boulan E. 2008. Clathrin is a key regulator of basolateral polarity. Nature. 452:719–723 10.1038/nature06828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C., Hurbain I., Tenza D., Sibarita J.B., Uzan-Gafsou S., Ohno H., Geerts W.J., Verkleij A.J., Salamero J., Marks M.S., Raposo G. 2009. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J. Cell Biol. 187:247–264 10.1083/jcb.200907122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Mullins C., Bonifacino J.S. 1999. AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem. 274:7278–7285 10.1074/jbc.274.11.7278 [DOI] [PubMed] [Google Scholar]
- Diaz F., Gravotta D., Deora A., Schreiner R., Schoggins J., Falck-Pedersen E., Rodriguez-Boulan E. 2009. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc. Natl. Acad. Sci. USA. 106:11143–11148 10.1073/pnas.0811227106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso M., Cancino J., Lee J., van Kerkhof P., Retamal C., Bu G., Gonzalez A., Cáceres A., Marzolo M.P. 2009. Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol. Biol. Cell. 20:481–497 10.1091/mbc.E08-08-0805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C.G., Simons K. 1990. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 62:63–72 10.1016/0092-8674(90)90240-F [DOI] [PubMed] [Google Scholar]
- Duffield A., Caplan M.J., Muth T.R. 2008. Protein trafficking in polarized cells. Int Rev Cell Mol Biol. 270:145–179 10.1016/S1937-6448(08)01404-4 [DOI] [PubMed] [Google Scholar]
- Dwyer N.D., Adler C.E., Crump J.G., L’Etoile N.D., Bargmann C.I. 2001. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 31:277–287 10.1016/S0896-6273(01)00361-0 [DOI] [PubMed] [Google Scholar]
- Eskelinen E.L., Meyer C., Ohno H., von Figura K., Schu P. 2002. The polarized epithelia-specific mu 1B-adaptin complements mu 1A-deficiency in fibroblasts. EMBO Rep. 3:471–477 10.1093/embo-reports/kvf092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfán P., Lee J., Larios J., Sotelo P., Bu G., Marzolo M.P. 2013. A sorting nexin 17-binding domain within the LRP1 cytoplasmic tail mediates receptor recycling through the basolateral sorting endosome. Traffic. 14:823–838 10.1111/tra.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías G.G., Cuitino L., Guo X., Ren X., Jarnik M., Mattera R., Bonifacino J.S. 2012. Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons. Neuron. 75:810–823 10.1016/j.neuron.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields I.C., Shteyn E., Pypaert M., Proux-Gillardeaux V., Kang R.S., Galli T., Fölsch H. 2007. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J. Cell Biol. 177:477–488 10.1083/jcb.200610047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields I.C., King S.M., Shteyn E., Kang R.S., Fölsch H. 2010. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol. Biol. Cell. 21:95–105 10.1091/mbc.E09-01-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H., Ohno H., Bonifacino J.S., Mellman I. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 99:189–198 10.1016/S0092-8674(00)81650-5 [DOI] [PubMed] [Google Scholar]
- Fölsch H., Pypaert M., Schu P., Mellman I. 2001. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 152:595–606 10.1083/jcb.152.3.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H., Pypaert M., Maday S., Pelletier L., Mellman I. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 163:351–362 10.1083/jcb.200309020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C.E., Gibson A., Allchin E.H., Maxwell S., Ruddock L.J., Odorizzi G., Domingo D., Trowbridge I.S., Hopkins C.R. 1998. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 141:611–623 10.1083/jcb.141.3.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., McGraw T.E., Rodriguez-Boulan E. 2002. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 4:605–609 [DOI] [PubMed] [Google Scholar]
- Garrido J.J., Fernandes F., Giraud P., Mouret I., Pasqualini E., Fache M.P., Jullien F., Dargent B. 2001. Identification of an axonal determinant in the C-terminus of the sodium channel Na(v)1.2. EMBO J. 20:5950–5961 10.1093/emboj/20.21.5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Rodriguez-Boulan E. 2009. Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 583:3784–3795 10.1016/j.febslet.2009.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D., Carvajal-Gonzalez J.M., Mattera R., Deborde S., Banfelder J.R., Bonifacino J.S., Rodriguez-Boulan E. 2012. The clathrin adaptor AP-1A mediates basolateral polarity. Dev. Cell. 22:811–823 10.1016/j.devcel.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Mattera R., Ren X., Chen Y., Retamal C., González A., Bonifacino J.S. 2013. The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev. Cell. 27:353–366 10.1016/j.devcel.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Zanetti G., Schekman R. 2013. A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. Elife. 2:e00160 10.7554/eLife.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase K., Nakatsu F., Ohmae M., Sugihara K., Shioda N., Takahashi D., Obata Y., Furusawa Y., Fujimura Y., Yamashita T., et al. 2013. AP-1B-mediated protein sorting regulates polarity and proliferation of intestinal epithelial cells in mice. Gastroenterology. 145:625–635 10.1053/j.gastro.2013.05.013 [DOI] [PubMed] [Google Scholar]
- Hirst J., Bright N.A., Rous B., Robinson M.S. 1999. Characterization of a fourth adaptor-related protein complex. Mol. Biol. Cell. 10:2787–2802 10.1091/mbc.10.8.2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Borner G.H., Antrobus R., Peden A.A., Hodson N.A., Sahlender D.A., Robinson M.S. 2012. Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr. Biol. 22:1711–1716 10.1016/j.cub.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Irving C., Borner G.H. 2013. Adaptor protein complexes AP-4 and AP-5: new players in endosomal trafficking and progressive spastic paraplegia. Traffic. 14:153–164 10.1111/tra.12028 [DOI] [PubMed] [Google Scholar]
- Jareb M., Banker G. 1998. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron. 20:855–867 10.1016/S0896-6273(00)80468-7 [DOI] [PubMed] [Google Scholar]
- Kang R.S., Fölsch H. 2011. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J. Cell Biol. 193:51–60 10.1083/jcb.201012121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Hille A., Veenendaal T., Oorschot V., Stoorvogel W., von Figura K., Geuze H.J. 1993. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J. Cell Biol. 121:997–1010 10.1083/jcb.121.5.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Kuliawat R., Griffith J.M., Geuze H.J., Arvan P. 1998. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J. Cell Biol. 141:359–371 10.1083/jcb.141.2.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto U.M., Hubbard A.L., Mellman I. 2001. A novel cellular phenotype for familial hypercholesterolemia due to a defect in polarized targeting of LDL receptor. Cell. 105:575–585 10.1016/S0092-8674(01)00371-3 [DOI] [PubMed] [Google Scholar]
- Lasiecka Z.M., Yap C.C., Vakulenko M., Winckler B. 2009. Compartmentalizing the neuronal plasma membrane from axon initial segments to synapses. Int Rev Cell Mol Biol. 272:303–389 10.1016/S1937-6448(08)01607-9 [DOI] [PubMed] [Google Scholar]
- Lee I., Doray B., Govero J., Kornfeld S. 2008. Binding of cargo sorting signals to AP-1 enhances its association with ADP ribosylation factor 1-GTP. J. Cell Biol. 180:467–472 10.1083/jcb.200709037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui-Roberts W.W., Collinson L.M., Hewlett L.J., Michaux G., Cutler D.F. 2005. An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells. J. Cell Biol. 170:627–636 10.1083/jcb.200503054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta M.A., Wang G.J., Shen K. 2009. Clathrin adaptor AP-1 complex excludes multiple postsynaptic receptors from axons in C. elegans. Proc. Natl. Acad. Sci. USA. 106:1632–1637 10.1073/pnas.0812078106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Yuzaki M. 2008. AP-4: autophagy-four mislocalized proteins in axons. Autophagy. 4:815–816 [DOI] [PubMed] [Google Scholar]
- Matsuda S., Miura E., Matsuda K., Kakegawa W., Kohda K., Watanabe M., Yuzaki M. 2008. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron. 57:730–745 10.1016/j.neuron.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. 1992. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 71:741–753 10.1016/0092-8674(92)90551-M [DOI] [PubMed] [Google Scholar]
- Mattera R., Boehm M., Chaudhuri R., Prabhu Y., Bonifacino J.S. 2011. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J. Biol. Chem. 286:2022–2030 10.1074/jbc.M110.197178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M.A., Dho S.E., Weinmaster G., McGlade C.J. 2009. Numb regulates post-endocytic trafficking and degradation of Notch1. J. Biol. Chem. 284:26427–26438 10.1074/jbc.M109.014845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Nelson W.J. 2008. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 9:833–845 10.1038/nrm2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C., Zizioli D., Lausmann S., Eskelinen E.L., Hamann J., Saftig P., von Figura K., Schu P. 2000. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19:2193–2203 10.1093/emboj/19.10.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura M., Masuda A., Nishiumi S., Kawakami K., Fujishima Y., Yoshie T., Mizuno S., Miki I., Ohno H., Hase K., et al. 2012. AP1B plays an important role in intestinal tumorigenesis with the truncating mutation of an APC gene. Int. J. Cancer. 130:1011–1020 10.1002/ijc.26131 [DOI] [PubMed] [Google Scholar]
- Montpetit A., Côté S., Brustein E., Drouin C.A., Lapointe L., Boudreau M., Meloche C., Drouin R., Hudson T.J., Drapeau P., Cossette P. 2008. Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet. 4:e1000296 10.1371/journal.pgen.1000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-De-Luca A., Helmers S.L., Mao H., Burns T.G., Melton A.M., Schmidt K.R., Fernhoff P.M., Ledbetter D.H., Martin C.L. 2011. Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J. Med. Genet. 48:141–144 10.1136/jmg.2010.082263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Setou M., Seog D., Ogasawara K., Dohmae N., Takio K., Hirokawa N. 2000. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell. 103:569–581 10.1016/S0092-8674(00)00161-6 [DOI] [PubMed] [Google Scholar]
- Nilsson L., Conradt B., Ruaud A.F., Chen C.C., Hatzold J., Bessereau J.L., Grant B.D., Tuck S. 2008. Caenorhabditis elegans num-1 negatively regulates endocytic recycling. Genetics. 179:375–387 10.1534/genetics.108.087247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G., Trowbridge I.S. 1997. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J. Cell Biol. 137:1255–1264 10.1083/jcb.137.6.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Tomemori T., Nakatsu F., Okazaki Y., Aguilar R.C., Foelsch H., Mellman I., Saito T., Shirasawa T., Bonifacino J.S. 1999. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 449:215–220 10.1016/S0014-5793(99)00432-9 [DOI] [PubMed] [Google Scholar]
- Pathak R.K., Yokode M., Hammer R.E., Hofmann S.L., Brown M.S., Goldstein J.L., Anderson R.G. 1990. Tissue-specific sorting of the human LDL receptor in polarized epithelia of transgenic mice. J. Cell Biol. 111:347–359 10.1083/jcb.111.2.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha S.M., Wassmer T., Niehage C., Hoflack B., Knust E. 2011. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr. Biol. 21:1111–1117 10.1016/j.cub.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Polishchuk R.S., San Pietro E., Di Pentima A., Teté S., Bonifacino J.S. 2006. Ultrastructure of long-range transport carriers moving from the trans Golgi network to peripheral endosomes. Traffic. 7:1092–1103 10.1111/j.1600-0854.2006.00453.x [DOI] [PubMed] [Google Scholar]
- Reales E., Sharma N., Low S.H., Fölsch H., Weimbs T. 2011. Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity. PLoS ONE. 6:e21181 10.1371/journal.pone.0021181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Farías G.G., Canagarajah B.J., Bonifacino J.S., Hurley J.H. 2013. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell. 152:755–767 10.1016/j.cell.2012.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S. 2004. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14:167–174 10.1016/j.tcb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Roush D.L., Gottardi C.J., Naim H.Y., Roth M.G., Caplan M.J. 1998. Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells. J. Biol. Chem. 273:26862–26869 10.1074/jbc.273.41.26862 [DOI] [PubMed] [Google Scholar]
- Sampo B., Kaech S., Kunz S., Banker G. 2003. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 37:611–624 10.1016/S0896-6273(03)00058-8 [DOI] [PubMed] [Google Scholar]
- Schmidt M.R., Maritzen T., Kukhtina V., Higman V.A., Doglio L., Barak N.N., Strauss H., Oschkinat H., Dotti C.G., Haucke V. 2009. Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc. Natl. Acad. Sci. USA. 106:15344–15349 10.1073/pnas.0904268106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner R., Frindt G., Diaz F., Carvajal-Gonzalez J.M., Perez Bay A.E., Palmer L.G., Marshansky V., Brown D., Philp N.J., Rodriguez-Boulan E. 2010. The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int. 78:382–388 10.1038/ki.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N., McCaffery J.M., Emr S.D. 1998. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142:665–681 10.1083/jcb.142.3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaq-Zadah M., Brocard L., Solari F., Michaux G. 2012. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development. 139:2061–2070 10.1242/dev.076711 [DOI] [PubMed] [Google Scholar]
- Shteyn E., Pigati L., Fölsch H. 2011. Arf6 regulates AP-1B-dependent sorting in polarized epithelial cells. J. Cell Biol. 194:873–887 10.1083/jcb.201106010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T., Höning S., Icking A., Tikkanen R., Hunziker W. 2002. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 4:154–159 10.1038/ncb745 [DOI] [PubMed] [Google Scholar]
- Starr T.L., Pagant S., Wang C.W., Schekman R. 2012. Sorting signals that mediate traffic of chitin synthase III between the TGN/endosomes and to the plasma membrane in yeast. PLoS ONE. 7:e46386 10.1371/journal.pone.0046386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D., Hase K., Kimura S., Nakatsu F., Ohmae M., Mandai Y., Sato T., Date Y., Ebisawa M., Kato T., et al. 2011. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology. 141:621–632 10.1053/j.gastro.2011.04.056 [DOI] [PubMed] [Google Scholar]
- Tarpey P.S., Stevens C., Teague J., Edkins S., O’Meara S., Avis T., Barthorpe S., Buck G., Butler A., Cole J., et al. 2006. Mutations in the gene encoding the Sigma 2 subunit of the adaptor protein 1 complex, AP1S2, cause X-linked mental retardation. Am. J. Hum. Genet. 79:1119–1124 10.1086/510137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh O.K., Shimono Y., Shirakawa M., Fukao Y., Tamura K., Shimada T., Hara-Nishimura I. 2013. The AP-1 μ adaptin is required for KNOLLE localization at the cell plate to mediate cytokinesis in Arabidopsis. Plant Cell Physiol. 54:838–847 10.1093/pcp/pct048 [DOI] [PubMed] [Google Scholar]
- Tong X., Zitserman D., Serebriiskii I., Andrake M., Dunbrack R., Roegiers F. 2010. Numb independently antagonizes Sanpodo membrane targeting and Notch signaling in Drosophila sensory organ precursor cells. Mol. Biol. Cell. 21:802–810 10.1091/mbc.E09-09-0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L.M., Bonifacino J.S. 2013. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 5:a016790 10.1101/cshperspect.a016790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia R.H., Baggott D., Chuang J.S., Schekman R.W. 2002. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell. 2:283–294 10.1016/S1534-5807(02)00127-2 [DOI] [PubMed] [Google Scholar]
- Vergés M., Luton F., Gruber C., Tiemann F., Reinders L.G., Huang L., Burlingame A.L., Haft C.R., Mostov K.E. 2004. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat. Cell Biol. 6:763–769 10.1038/ncb1153 [DOI] [PubMed] [Google Scholar]
- Verkerk A.J., Schot R., Dumee B., Schellekens K., Swagemakers S., Bertoli-Avella A.M., Lequin M.H., Dudink J., Govaert P., van Zwol A.L., et al. 2009. Mutation in the AP4M1 gene provides a model for neuroaxonal injury in cerebral palsy. Am. J. Hum. Genet. 85:40–52 10.1016/j.ajhg.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.W., Hamamoto S., Orci L., Schekman R. 2006. Exomer: A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J. Cell Biol. 174:973–983 10.1083/jcb.200605106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz O.A., Rodriguez-Boulan E. 2009. Apical trafficking in epithelial cells: signals, clusters and motors. J. Cell Sci. 122:4253–4266 10.1242/jcs.032615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Toops K.A., Diaz F., Carvajal-Gonzalez J.M., Gravotta D., Mazzoni F., Schreiner R., Rodriguez-Boulan E., Lakkaraju A. 2012. Mechanism of polarized lysosome exocytosis in epithelial cells. J. Cell Sci. 125:5937–5943 10.1242/jcs.109421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Awada C., Hanaki H., Sakane H., Tsujimoto I., Takahashi Y., Takao T., Kikuchi A. 2013. The apical and basolateral secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by different mechanisms. J. Cell Sci. 126:2931–2943 10.1242/jcs.126052 [DOI] [PubMed] [Google Scholar]
- Yap C.C., Murate M., Kishigami S., Muto Y., Kishida H., Hashikawa T., Yano R. 2003. Adaptor protein complex-4 (AP-4) is expressed in the central nervous system neurons and interacts with glutamate receptor delta2. Mol. Cell. Neurosci. 24:283–295 10.1016/S1044-7431(03)00164-7 [DOI] [PubMed] [Google Scholar]
- Yap C.C., Wisco D., Kujala P., Lasiecka Z.M., Cannon J.T., Chang M.C., Hirling H., Klumperman J., Winckler B. 2008. The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. J. Cell Biol. 180:827–842 10.1083/jcb.200707143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Murphy S.J., Wilkes M.C., Ji Y., Leof E.B. 2013. Retromer maintains basolateral distribution of the type II TGF-β receptor via the recycling endosome. Mol. Biol. Cell. 24:2285–2298 10.1091/mbc.E13-02-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kim A., Abraham N., Khan L.A., Hall D.H., Fleming J.T., Gobel V. 2012. Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development. 139:2071–2083 10.1242/dev.077347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizioli D., Meyer C., Guhde G., Saftig P., von Figura K., Schu P. 1999. Early embryonic death of mice deficient in gamma-adaptin. J. Biol. Chem. 274:5385–5390 10.1074/jbc.274.9.5385 [DOI] [PubMed] [Google Scholar]