Introduction and definitions
It is almost becoming a truism to state that the unique opportunities made possible by continuing progresses in biotechnologies, the “omics”, biomedical imaging, computer technologies and nanotechnologies, open avenues to a new management of many diseases, switching from a “population treatment” approach to the concept of “personalized medicine”. Indeed, theranostics is at the heart of Quantitative Imaging in Medicine and Surgery’s scope. The concept of “theranostics” was coined by the US consultant John Funkhouser, in a press release from the company Cardiovascular Diagnostics in August 1998, to describe a material that allows the combined diagnosis, treatment and follow up of a disease (1). This approach allows the selection of the sub-population of patients most likely to benefit from a targeted therapy in accordance with their “molecular profile” at a given time-point, or, conversely, those patients for whom the risk of adverse effects is higher. Personalized medicine has obvious critical consequences for patients’ management and for health economics as well. Personalised medicine emphasises the systematic use of molecular information about one individual patient to select or optimise that patient’s medical care.
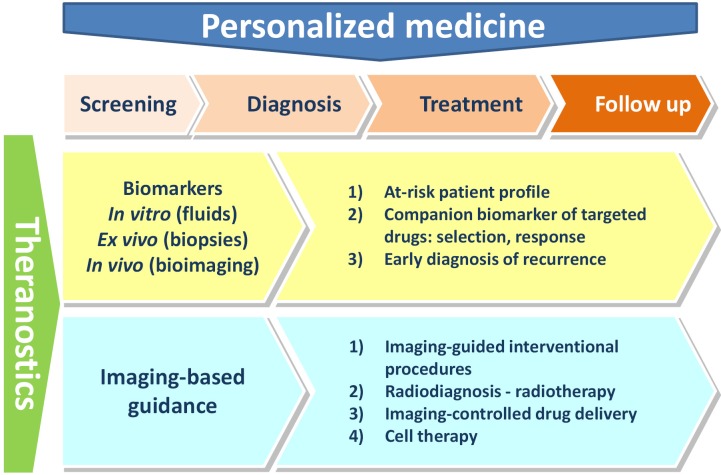
The concept of theranostics evolved over the time, and, nowadays, it integrates two distinct approaches that both encompass all steps of patients’ management (Figure 1).
Figure 1.
Current concept of theranostics.
The biomarker approach, to diagnose the disease, determine the best course of treatment, follow up patient’s response, detect potential recurrence of the disease and to anticipate potential adverse effects. This approach includes in vitro measurements performed on biological fluids, ex vivo studies (e.g., histopathological studies) and, last but not least, medical imaging procedures performed with or without contrast agents.
Basically, companion diagnostics have four distinct indications:
To select patients for a specific/targeted treatment;
As staging markers to adapt the therapeutic strategy;
As predictive markers for drug response, resistance and safety;
-
As follow-up markers to check the therapeutic response and to adjust therapy (alternative, dosing, duration) if required.
The planning, per-operative guidance and follow-up of the therapeutic action (e.g., local delivery of a drug, a medical device or cell therapy). Of course, medical imaging is the prerequisite for such approach, which can be summarized by the classical motto “plan, guide, treat and predict”.
Contrast agents in theranostics: some examples and perspectives
Biomarker approach
According to the classical definition of the US National Institutes of Health, a biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” (2). Companion diagnostics can be defined as a biomarker which provides biological and/or clinical information enabling appropriate and timely decision making about the early drug development (for drug companies) and use of individual drug therapy in routine clinical care (for clinicians) (3,4). “Personalized medicine” is defined as:
A medicine that has a label from regulatory authorities stating that its selection as a treatment must be governed by results from a companion diagnostic;
A medicine recommended for use by at least one professional organization after a companion diagnostic test has been performed (5).
For the period 1998-2009, a total of 27 personalized medicine treatments have been identified worldwide, including 22 treatments for oncology indications. Interestingly, the pace of emergence of personalized medicine treatments seems to be slower in the US than in Europe (the so-called “EU5 market”, i.e., France, Germany, Italy, Spain and the United Kingdom) and Japan (5).
Specific contrast agents can bind to the same (cellular or molecular) target as drug therapies. The most salient example of this approach is the vintafolide/etarfolatide couple. Vintafolide (EC145) is a conjugate of folic acid and the vinca alkaloid desacetylvinblastine hydrazide. It targets the folate receptor-α (FR-α), a major membrane marker for ovarian cancer (and other solid cancer types of epithelial origin). The radiolabelled contrast agent etarfolatide (99mTc-EC20, FolateScan®) is its companion imaging marker (6). Vintafolide and etarfolatide are currently under review with the European Medicine Agency. These two molecules have been granted orphan drug status by the European Commission. Another example is octreotide (Octreoscan®), an 111In-labelled analogue of somatostatin. Octreotide is the gold standard for imaging of several neuroendocrine tumours (gastrinoma, VIPoma, and glucagonoma) (7). This agent paved the way for research on peptide receptor radionuclide therapy. The high sensitivity associated with contrast agents for nuclear medicine has been a major asset for their approval by health authorities, in comparison with targeted contrast agents for magnetic resonance imaging (MRI).
Drugs that target mutations associated with cancer provide substantial clinical benefit. The standard of care requires the assessment of epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocations in tumors of patients with advanced non-small cell lung cancer (NSCLC) (8). Undoubtedly, the current research on targeted therapies for NSCLC would benefit from a simultaneous (or earlier) development of companion imaging markers.
Iron oxide nanoparticles like ferumoxtran-10 (an ultra small superparamagnetic iron oxide, USPIO), allow “pleiotropic” clinical applications as MRI contrast agents because of their composition and size, which influence their biodistribution (9). USPIO allows imaging of liver tumour, metastatic lymph nodes and inflammatory atherosclerotic plaques, functional MRI, MR angiography, measurement of tumour microvascular permeability and blood volume, and cell tracking (stem cell migration and immune cell trafficking), etc. Clinical benefit from these particles has moved far beyond the single proof of concept stage. In patients with carotid stenosis >40%, the USPIO ferumoxtran-10-enhanced MR signal loss in carotid plaques was found to be reduced by 12 weeks of treatment with high-dose atorvastatin while a low-dose had no effect (10).
Imaging-guided therapy
The concept of theranostics perfectly applies to Lipiodol-based transcatheter arterial chemoembolization (TACE) of hepatocellular carcinoma (HCC) (11,12). Actually, it was probably the first example of the clinical validity of the theranostics concept, when a Japanese team dissolved a lipophilic polymer derived from the cytotoxic agent neocarzinostatin in Lipiodol and demonstrated outstanding results on the VX-2 tumour model in rabbits, following intra-arterial infusion (13). Subsequently, a huge number of nonclinical and clinical studies confirmed the value of TACE associating Lipiodol with several cytotoxic drugs (12). TACE is currently considered to be the gold standard treatment of asymptomatic patients with intermediate-stage HCC (14). Lipiodol has unique profile as it combines three major characteristics: (I) transient and plastic embolization of tumour vessels; (II) radioopacity and (III) drug delivery (11,12). In addition to the ability of this oily matrix to gradually deliver drugs at the tumour site, it also allows determining exact tumour location, size, and intrahepatic spread and has the potential to estimate the amount of drug delivered locally (15). Furthermore, the degree of Lipiodol labelling of the tumour was found to be an independent prognostic parameter for survival in HCC patients (16,17).
Given the radio-opacity and drug delivery capacity of Lipiodol, several perspectives can be considered. One is thermoembolization (TE). This procedure is based on targeted deposition of magnetic nanoparticles into the lesion, followed by application of an alternating magnetic field inducing focused temperature rising by Neel or Brown relaxation mechanisms. If the temperature is sufficiency high (above 42 °C) this modality may lead to cell necrosis (18). Moreover, the formation of an embolus potentiates the hyperthermia efficiency since the blood flow induces tissue cooling. The presence of both iodine and iron in the tumour-deposited emulsion may also allow dual (computed tomography and MRI) imaging. Lastly, given that heating enhances the tumor cells sensitivity to chemo-therapy, TE may also potentiate the effects of a cytotoxic drug (19). Immunoembolization is another perspective. In one phase I study, the administration of Lipiodol-emulsified granulocyte-macrophage colony-stimulating factor (GM-CSF) and associated with embolic particles into the hepatic artery, led to promising results in patients with liver metastases from primary uveal melanoma (20,21). Gene therapy may also benefit from a radio-opaque and oily drug delivery matrix. At the preclinical stage, the therapies tested so far include p53 (22-24), interleukin-12 (25), Rb (23) and double suicide gene therapy with both TK and CD fusion gene (24).
Another example is ultrasound contrast agents which can be functionalized and designed to carry drugs or genes, thus allowing the combination of diagnosis and treatment. The sonoporation phenomenon associated with the application of ultrasound on the lesion targeted with the functionalized microbubble may lead to local release of high concentration of drugs or gene vectors (26).
Fluorescence-guided cystoscopy, using a porphyrin, hexylaminolevulinate (Hexvix®) has been found to improve the detection of bladder carcinoma in situ, compared with standard white-light cystoscopy, as well as the quality of transurethral resection of the tumour (27). Since δ-aminolaevulinic acid derivatives both fluoresce and can photosensitize the tumour lesions, porphyrin-based theranostics is an active and promising field of research (28).
Comments
Ideally and conceptually, the theranostics approach should differ from the classical drug/diagnostic test combination (e.g., vitamin K antagonists and international normalized ratio monitoring) by:
The simultaneous and fully integrated development of the drug and its diagnostic counterpart;
The consideration, by health authorities, of the coupled combination of the new drug with its diagnostic test in the marketing authorization process (29).
In practice, it is disappointing to note that the development of the drug and its diagnostic counterpart are rarely in synchrony and that, in most countries, the authorities in charge of market access of drugs, medical devices and in vitro/ex vivo diagnostics tests as well as those in charge of reimbursement authorization are distinct bodies which rarely work in a fully coordinated way. Indeed, progress is also needed, in terms of coordination, among the companies or academic centres which develop drugs and their companion counterparts. The drug/test couple should ideally be conceptualized from the very start of the project, thus allowing a prospective and coordinated development, including the validation of the companion marker, a long, complex and costly process.
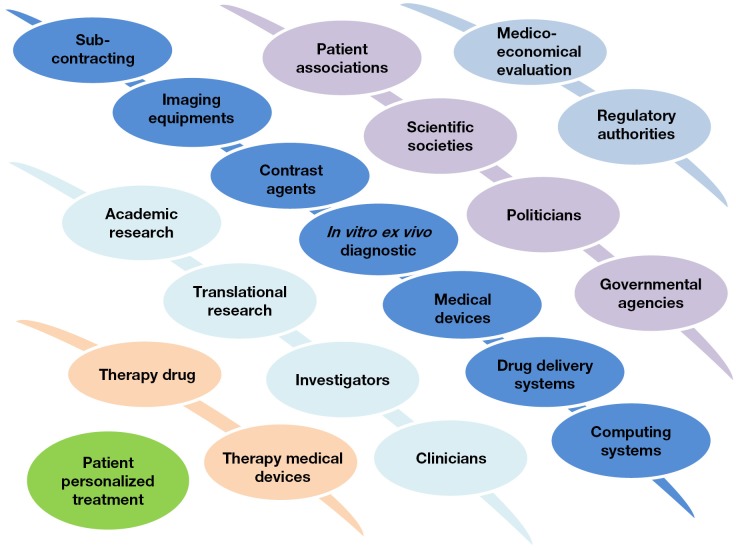
The shift from population to individual patient treatment necessarily implies the involvement of a complex interplay between multiple actors and disciplines (Figure 2) which individually, do not have the capacity to propose a comprehensive offer. This is particularly true in the field of contrast agents which must fit into a complex multitechnological healthcare offer (30).
Figure 2.
The new and complex interplay resulting from the multitechnological paradigm of personalized medicine [From reference (30), with permission].
While, at the patient’s level, the benefits of personalized medicine are undisputable, its implementation at the community level is in its infancy and its impact, especially in terms of health economics, remains to be called into question. In spite of extensive research efforts from industrial and academic teams worldwide, no MR contrast agents that meet the definition of companion diagnostics have been approved so far. Only one USPIO (ferumoxytol, Feraheme®) is currently marketed, for a clinical application (treatment of iron deficiency anaemia) obviously not related to its properties as a MR contrast agent. The clinical development of numerous USPIOs (VSOP, ferumoxtran-10, ferucarbotran S, feruglose) has been stopped for regulatory and/or economic reasons which generally were not related with the intrinsic value of these nanoparticular compounds as contrast agents. With respect to molecular imaging associated with pharmacological or any other category of treatment, there is crucial need for definition and implementation of new, innovative business models compatible with the needs of the numerous partners involved in the new landscape. This need goes far beyond the traditional debates on private-public partnerships in translational medicine (31). Lastly, these issues must definitely not hide the exciting aspects of contrast agent-associated theranostics that certainly will find its place in tomorrow’s personalized medicine.
Acknowledgements
All authors are employees of Guerbet.
Disclosure: The authors declare no conflict of interest.
References
- 1.Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem 2011;22:1879-903 [DOI] [PubMed] [Google Scholar]
- 2.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95 [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A.Do companion diagnostics make economic sense for drug developers? N Biotechnol 2012;29:695-708 [DOI] [PubMed] [Google Scholar]
- 4.Zieba A, Grannas K, Söderberg O, et al. Molecular tools for companion diagnostics. N Biotechnol 2012;29:634-40 [DOI] [PubMed] [Google Scholar]
- 5.Hu SX, Aitken ML, Epstein AM, et al. Market watch: Defining and quantifying the use of personalized medicines. Nat Rev Drug Discov 2013;12:896-7 [DOI] [PubMed] [Google Scholar]
- 6.Teng L, Xie J, Teng L, et al. Clinical translation of folate receptor-targeted therapeutics. Expert Opin Drug Deliv 2012;9:901-8 [DOI] [PubMed] [Google Scholar]
- 7.Laverman P, Sosabowski JK, Boerman OC, et al. Radiolabelled peptides for oncological diagnosis. Eur J Nucl Med Mol Imaging 2012;39Suppl 1:S78-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadgeel SM. New targets in non-small cell lung cancer. Curr Oncol Rep 2013;15:411-23 [DOI] [PubMed] [Google Scholar]
- 9.Corot C, Robert P, Idée JM, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 2006;58:1471-504 [DOI] [PubMed] [Google Scholar]
- 10.Tang TY, Howarth SP, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol 2009;53:2039-50 [DOI] [PubMed] [Google Scholar]
- 11.Wang YXJ. Transcatheter embolization therapy in liver Cancer. Rec Pat Med Imaging 2012;2:150-62 [Google Scholar]
- 12.Idée JM, Guiu B. Use of lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol 2013;88:530-49 [DOI] [PubMed] [Google Scholar]
- 13.Iwai K, Maeda H, Konno T.Use of oily contrast medium for selective drug targeting to tumor: enhanced therapeutic effect and X-ray image. Cancer Res 1984;44:2115-21 [PubMed] [Google Scholar]
- 14.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43 [DOI] [PubMed] [Google Scholar]
- 15.Maeda H.Vascular permeability in Cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc Jpn Acad Ser B Phys Biol Sci 2012;88:53-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondazzi L, Bottelli R, Brambilla G, et al. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology 1994;19:1115-23 [PubMed] [Google Scholar]
- 17.Dumortier J, Chapuis F, Borson O, et al. Unresectable hepatocellular carcinoma: survival and prognostic factors after lipiodol chemoembolisation in 89 patients. Dig Liver Dis 2006;38:125-33 [DOI] [PubMed] [Google Scholar]
- 18.Moroz P, Jones SK, Winter J, et al. Targeting liver tumors with hyperthermia: ferromagnetic embolization in a rabbit liver tumor model. J Surg Oncol 2001;78:22-9; discussion 30-1 [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt R.Hyperthermia and drugs. Recent Results Cancer Res 1987;104:136-203 [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Eschelman DJ, Gonsalves CF, et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol 2008;26:5436-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto A, Chervoneva I, Sullivan KL, et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology 2009;252:290-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu T, Li CX, Feng Y, et al. Trans-arterial gene therapy for hepatocellular carcinoma in a rabbit model. World J Gastroenterol 2007;13:2113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong S, Tang Q, Long M, et al. The cooperative effect of p53 and Rb in local nanotherapy in a rabbit VX2 model of hepatocellular carcinoma. Int J Nanomedicine 2013;8:3757-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu HX, Du T, Xu ZF, et al. Role of wild type p53 and double suicide genes in interventional therapy of liver Cancer in rabbits. Acta Cir Bras 2012;27:522-8 [DOI] [PubMed] [Google Scholar]
- 25.Xia X, Li X, Feng G, et al. Intra-arterial interleukin-12 gene delivery combined with chemoembolization: anti-tumor effect in a rabbit hepatocellular carcinoma (HCC) model. Acta radiol 2013;54:684-9 [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Chen ZY, Yang F. Ultrasound-based multimodal molecular imaging and functional ultrasound contrast agents. Curr Pharm Des 2013;19:3342-51 [DOI] [PubMed] [Google Scholar]
- 27.Witjes JA, Douglass J. The role of hexaminolevulinate fluorescence cystoscopy in bladder Cancer. Nat Clin Pract Urol 2007;4:542-9 [DOI] [PubMed] [Google Scholar]
- 28.Josefsen LB, Boyle RW. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012;2:916-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landais P, Méresse V, Ghislain JC. Evaluation and validation of diagnostic tests for guiding therapeutic decisions. Therapie 2009;64:187-201 [DOI] [PubMed] [Google Scholar]
- 30.Corot C, Warlin D.Superparamagnetic Iron oxide nanoparticles for MRI: contrast media pharmaceutical company R&D perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2013;5:411-22 [DOI] [PubMed] [Google Scholar]
- 31.Luijten PR, van Dongen GA, Moonen CT, et al. Public-private partnerships in translational medicine: concepts and practical examples. J Control Release 2012;161:416-21 [DOI] [PubMed] [Google Scholar]