Abstract
Melanocyte stimulating hormone coupled to Sepharose effects an increase in tryosinase (EC 1.14.18.1; monophenol monoxygenase) activity of cultivated mouse melanoma cells. Synchronized cells are found to respond to melanocyte stimulating hormone only in the G2 phase of the cell cycle, although their response to cyclic AMP is independent of position in the cell cycle. The binding of 125I-labeled melanocyte stimulating hormone occurs predominantly in G2. These observations are satisfied by a model in which the hormone can activate adenylate cyclase (EC 4.6.1.1) by binding to a melanocyte stimulating hormone receptor only in G2; the events distal to cyclic AMP production can occur throughout the cell cycle.
Keywords: hormone receptor, cyclic AMP, tryosinase
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Butcher R. W., Nicholson W. E., Baird C. E., Liddle R. A., Liddle G. W. Adenosine 3',5'-monophosphate (cyclic AMP) as the mediator of the actions of melanocyte stimulating hormone (MSH) and norepinephrine on the frog skin. Endocrinology. 1969 Feb;84(2):362–368. doi: 10.1210/endo-84-2-362. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H., Summers D. F. Purification and properties of HeLa cell plasma membranes. J Biol Chem. 1971 Aug 25;246(16):5162–5175. [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Burstein S. R. Effects of cyclic adenosine monophosphate and melanocyte-stimulating hormone on frog skin in vitro. Nature. 1965 Dec 25;208(5017):1282–1284. doi: 10.1038/2081282a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Rosenthal M., Lengle N. Cyclic adenosine 3',5'-monophosphate in the control of melanoma cell replication and differentiation. J Natl Cancer Inst. 1973 Feb;50(2):555–558. doi: 10.1093/jnci/50.2.555. [DOI] [PubMed] [Google Scholar]
- Lande S., Lerner A. B. The biochemistry of melanotropic agents. Pharmacol Rev. 1967 Mar;19(1):1–20. [PubMed] [Google Scholar]
- Martin D., Jr, Tomkins G. M., Granner D. Synthesis and induction of tyrosine aminotransferase in synchronized hepatoma cells in culture. Proc Natl Acad Sci U S A. 1969 Jan;62(1):248–255. doi: 10.1073/pnas.62.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
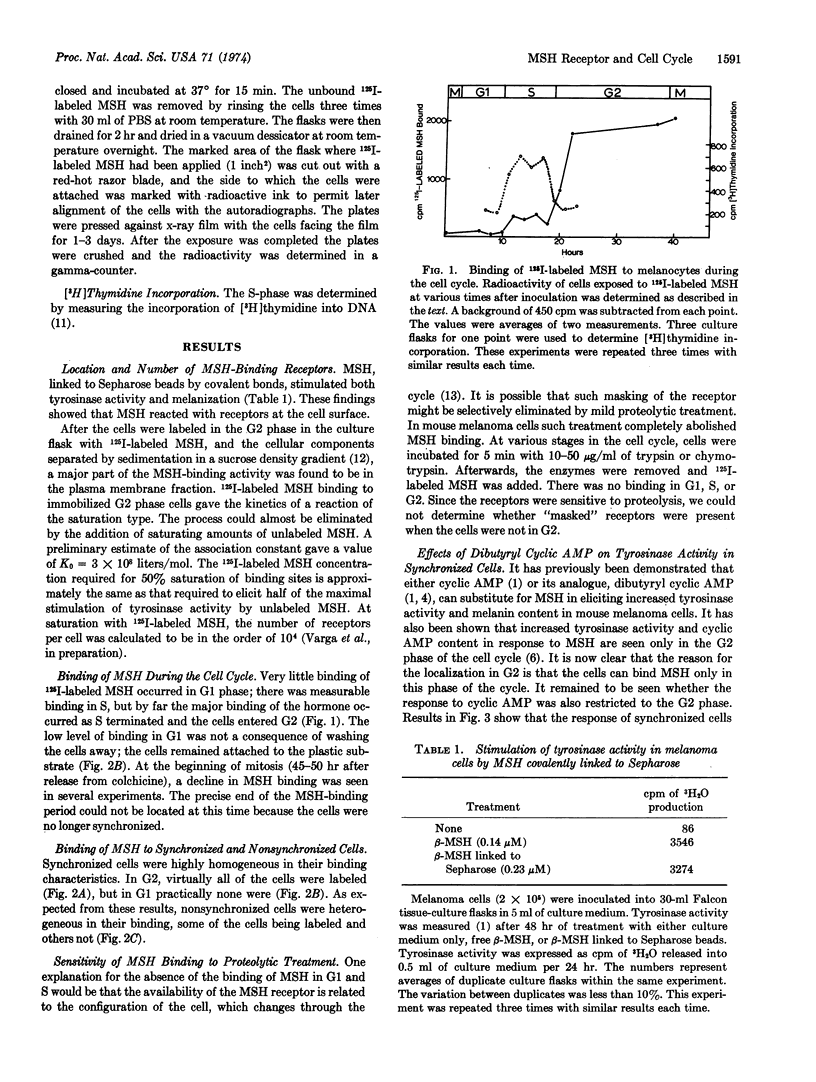
- Pawelek J., Wong G., Sansone M., Morowitz J. Molecular biology of pigment cells. Molecular controls in mammalian pigmentation. Yale J Biol Med. 1973 Dec;46(5):430–443. [PMC free article] [PubMed] [Google Scholar]
- Singer F. R., Habener J. F., Greene E., Godin P., Potts J. T., Jr Inactivation of calcitonin by specific organs. Nat New Biol. 1972 Jun 28;237(78):269–270. doi: 10.1038/newbio237269a0. [DOI] [PubMed] [Google Scholar]
- Wong G., Pawelek J. Control of phenotypic expression of cultured melanoma cells by melanocyte stimulating hormones. Nat New Biol. 1973 Feb 14;241(111):213–215. doi: 10.1038/newbio241213a0. [DOI] [PubMed] [Google Scholar]