Abstract
Recent studies across several mammalian species have revealed a distributed network of cortical and subcortical brain regions responsible for sensorimotor decision making. Many of these regions have been shown to be interconnected with the pedunculopontine tegmental nucleus (PPTg), a brain stem structure characterized by neuronal heterogeneity and thought to be involved in several cognitive and behavioral functions. However, whether this structure plays a general functional role in sensorimotor decision making is unclear. We hypothesized that, in the context of a sensorimotor task, activity in the PPTg would reflect task-related variables in a similar manner as do the cortical and subcortical regions with which it is anatomically associated. To examine this hypothesis, we recorded PPTg activity in mice performing an odor-cued spatial choice task requiring a stereotyped leftward or rightward orienting movement to obtain a reward. We studied single-neuron activity during epochs of the task related to movement preparation, execution, and outcome (i.e., whether or not the movement was rewarded). We found that a substantial proportion of neurons in the PPTg exhibited direction-selective activity during one or more of these epochs. In addition, an overlapping population of neurons reflected movement direction and reward outcome. These results suggest that the PPTg should be considered within the network of brain areas responsible for sensorimotor decision making and lay the foundation for future experiments to examine how the PPTg interacts with other regions to control sensory-guided motor output.
Keywords: pedunculopontine tegmental nucleus, decision making, basal ganglia, sensorimotor
an organism's capacity to select, prepare, and execute a response to an environmental stimulus is an important property of the vertebrate nervous system. Studies across several species have elucidated the contributions of a network of brain regions within the cortex, basal ganglia, and midbrain to reflexive and complex voluntary behaviors (Cisek and Kalaska 2010; Gold and Shadlen 2007). However, fewer studies have examined the activity of brain stem nuclei in the context of sensorimotor decisions, despite their well-defined roles in representing sensory input and motor output. Although it is conventional to conceptualize decision making as a hierarchical process in which decisions are made by higher-level regions (e.g., cortex) and carried out at lower levels (e.g., brain stem) (Fodor 1983; Miller et al. 1960), much neurophysiological evidence argues against such an organization (Cisek and Kalaska 2010). In this report, we examine whether and how a brain stem structure itself, the pedunculopontine tegmental nucleus (PPTg), which is interconnected with several regions known to be involved in decision making, may contribute to this process.
The PPTg is composed of a heterogeneous population of cholinergic, GABAergic and glutamatergic cells that give rise to efferent projections to basal ganglia nuclei (substantia nigra pars reticulata and subthalamic nucleus), midbrain regions (superior colliculus and ventral tegmental area), thalamus, deep cerebellar nuclei, medullary targets, and cortical regions (Beninato and Spencer 1987; Edley and Graybiel 1983; Garcia-Rill 1991; Martinez-Gonzalez et al. 2011; Rye et al. 1987; Woolf and Butcher 1986). The reciprocal connectivity with the basal ganglia, in particular, suggests that the PPTg plays a role in motor control and reward processing (Garcia-Rill et al. 1987; Garcia-Rill and Skinner 1987; Garcia-Rill 1991; Inglis and Winn 1995; Mena-Segovia et al. 2004). Indeed, electrophysiological studies in several mammalian species performing operant motor tasks have found that single PPTg neurons are phasically active at the time of eye (Kobayashi et al. 2002; Okada et al. 2009), limb (Dormont et al. 1998; Matsumura et al. 1997), and body movements (Norton et al. 2011) and in response to reward delivery (Kobayashi et al. 2002; Kobayashi and Okada 2007; Norton et al. 2011; Pan and Hyland 2005). However, the widespread connectivity of the PPTg, as well as its modulation by dopaminergic, serotonergic, and noradrenergic input (Blaha and Winn 1993; Grace et al. 2012; Inglis et al. 1993; Matulewicz et al. 2010; Steiniger and Kretschmer 2004), suggests that the PPTg may contribute more broadly to sensorimotor decision making rather than simply represent motor and reward information (Steckler et al. 1994). In this study, we employed a sensory-cued spatial-choice task (Uchida and Mainen 2003) to examine how and when PPTg activity reflects “task-related variables,” defined as quantities underlying goal-directed movements in response to sensory stimuli (e.g., movement direction and outcome).
We found that PPTg activity reflects the direction of movement before, during, and after the choice, as well as the presence or absence of reward. Moreover, the activity of individual neurons often reflected multiple task-related variables, either simultaneously or across time. We discuss these results within the context of the interconnected network of brain regions underlying sensorimotor decisions.
MATERIALS AND METHODS
Animals
All experiments were performed according to protocols approved by the University of Colorado School of Medicine Institutional Animal Care and Use Committee. Mice were bred in the animal facilities of the University of Colorado Anschutz Medical Campus or purchased from a commercial breeder (Jackson Laboratory). We used male adult C57BL/6J mice (n = 5; age at the start of experimental use = 124–186 days). The animals were housed in a vivarium with a 12:12-h light-dark cycle with lights on at 5:00 AM. Food (Teklad Global Rodent Diet no. 2918; Harlan) was available ad libitum. Access to water was restricted to the behavioral session; however, if mice did not obtain ∼1 ml of water during the behavioral session, additional water was provided for ∼2–5 min following the behavioral session (Smear et al. 2011). All mice were weighed daily and received sufficient water during behavioral sessions to maintain >85% of pre-water restriction weight (predeprivation, 26.43 ± 2.05 g; postdeprivation, 26.41 ± 1.73 g; means ± SD).
Behavior (Odor-Guided Spatial Choice Task)
All mice were trained using operant conditioning to discriminate the dominant odor from the mixture of two odors and associate an odor stimulus with reward port location. Behavioral training and testing used water restriction for motivation. Performance parameters, odor valve operation, and water delivery were controlled using custom software written in MATLAB (The MathWorks, Natick, MA).
Each mouse was trained to interact with three ports raised 2 cm from the platform floor along one wall of a behavioral chamber (24.5 × 20 × 19 cm). A light turning on in the center port indicated that the mouse could initiate a trial by entering the center port, which triggered the delivery of an odor [empirically measured to arrive to the mouse at 75–100 ms following port entry using a photo-ionization-detector; similar to previous reports (Uchida and Mainen 2003)]. Between 200 and 600 ms (range; mean ± SD, 434 ± 68 ms) after the odor valve was opened, a go signal (center port light off) indicated that the mouse was permitted to exit the odor port and move toward a reward port. Premature exits from the odor port resulted in the unavailability of reward on that trial. On the basis of attempts to train mice on a reaction-time version of the task (i.e., without a go signal), we believe that the go signal is useful for training some mice to attend to the odor, even if they were not always successful at waiting for the cue. Odors comprised binary mixtures of (+)-carvone and (−)-carvone, commonly perceived as caraway and spearmint, respectively; an enantiomeric odor pair was selected to control for differences in molecular structure of odorant stimuli. Mice were trained to associate odor combinations wherein (+)-carvone > (−)-carvone indicated right port reward availability and (−)-carvone > (+)-carvone indicated left port reward availability. On trials in which (+)-carvone = (−)-carvone, the probability of reward at the left and right ports, independently, was 0.5. Reward, consisting of 5 μl of water, was delivered by transient opening of a calibrated water valve 1–40 ms after water port entry.
In this experiment, we used seven odor mixtures comprising varying concentrations of the two carvones [(+/−)-carvone: 95/5, 80/20, 60/40, 50/50, 40/60, 20/80, 5/95]. Twenty microliters of each odor mixture were diluted 10-fold in light mineral oil (Fisher Scientific) and pipetted onto a syringe filter that was connected to a manifold used by the olfactometer (Island Motion, Tappan, NY). The olfactometer delivered air at 100 ml/min through individual polyethylene tubes connected to each odorant syringe filter. This odorized air was further diluted eightfold in carrier air provided by a standard air cylinder for a final odorant delivery of 800 ml/min through the odor valve. Across a single session all odorants were delivered with equal likelihood; on individual trials the odorant presented was selected pseudorandomly.
Details of the behavioral training protocol are described by Stubblefield et al. (2013). Briefly, after acclimation to 5 days of water restriction (1 ml of water per day), all mice were trained to perform the odor-cued spatial choice task with the following initial stages. 1) Train mice to obtain water reward at side reward ports: the center odor port was obstructed and mice were rewarded for entering into either the left or right port. This stage continued until mice obtained ∼1 ml of water within one session (2–3 days). 2) Train mice to sample odor in the center port and subsequently obtain water in one of the side reward ports: the center odor port was unobstructed and mice were exposed to alternating blocks of 50 trials, where blocks of trials would alternate between delivery of a left odor [(+/−)-carvone: 5/95], indicating that water was available at the left reward port, and a right odor [(+/−)-carvone: 95/5], indicating that water was available at the right reward port. During a block of left-rewarded trials the right reward port would be blocked, and vice versa. 3) Train mice to increase odor sampling time: a required odor sampling time (measured from odor valve open to go signal) indicated when the mouse could exit the center port; premature exits resulted in the absence of reward delivery. The initial required odor sampling time was set to 0.01 s and gradually increased in 0.015-s increments based on performance until a required odor sampling time of 0.3–0.5 s was achieved. Blocks of left and right trials were continued in this stage, but the nonrewarded side was now accessible (i.e., not obstructed). 4) Train mice to perform task with randomized presentation of odor mixtures: we first used blocks of 50 trials with the same odor mixture [(+/−)-carvone: 5/95 or 95/5, left or right reward, respectively] and gradually reduced the trials per block to 5. Once mice performed 70% correct trials across 2 sessions, block trials were replaced with pseudorandom odor mixture presentation (equivalent to a block size of 1). Once mice performed 70% correct trials for 2 sessions, the second pair of odor mixtures [(+/−)-carvone: 20/80 and 80/20] were pseudorandomly interleaved (among the 95/5 and 5/95 mixtures) during subsequent sessions. This step was repeated for the final pair of odor mixtures (40/60 and 60/40) and, finally, the 50/50 odor mixture. Mice completed 258.21 ± 57.23 (mean ± SD) successful trials per session depending on motivation and completed training in 6–8 wk.
Surgery
Mice were placed in a ventilated chamber and briefly exposed to a volatile anesthesia (isoflurane, 2%). Immediately following the onset of deep anesthesia (verified by toe pinch), the animal was placed in the mouth bar of a stereotaxic device with a nose cone that continuously delivered 1–1.5% isoflurane to maintain an anesthetized state. When the animal was fully unresponsive to foot pinch and appeared to maintain a consistent breathing rate, the fur on the surface of the scalp from the midline of the orbits to the midpoint between the ears was removed, and topical antiseptic (Betadine) was applied along with ophthalmic ointment on the eyes. Before the skull was exposed, a bolus of topical anesthetic (2% lidocaine; 250 μl) was injected under the surface of the scalp. With the skull exposed by central incision and scalp retraction, we aligned bregma and lambda along the horizontal plane (z coordinate) and drilled out a 1.5 × 1.5-mm cranial window with center point targeted to coordinates for the PPTg (4.5 mm posterior from bregma, 1.1 mm lateral from the midline; Paxinos and Franklin 2004) in the left hemisphere.
Once a cranial aperture was completed, the dural membrane was excised and a Versa Drive 4 (Neuralynx, Bozeman, MT) micro drive (which contains 4 independently adjustable tetrodes) was affixed to the skull using two small skull screws, a layer of luting (3M), and a final layer of dental acrylic (A-M Systems). One skull screw was used to attach a ground wire to the micro drive with solder. Before implantation, tetrodes protruding from the base of the micro drive were positioned to within 100–200 μm from the dorsal surface of the PPTg (2.4–2.6 mm from brain surface). After the dental acrylic hardened, a topical antibiotic was applied to the scalp around the drive implant, the isoflurane was turned off, and oxygen alone was delivered to the animal to gradually alleviate anesthetic state. Immediately following surgery, animals were administered sterile isotonic saline (0.9%) for rehydration and an analgesic (Ketofen; 5 mg/kg) for pain management. Postoperative care, including analgesic administration, and topical antibiotic application to the scalp were continued for up to 5 days after surgery, and animals were closely monitored for signs of distress.
Electrophysiology
Electrophysiological recordings were collected using four tetrodes, wherein each tetrode consisted of four polyimide-coated nichrome wires (Sandvik, Palm Coast, FL; single-wire diameter 12.5 μm) gold plated to 0.2–0.4 MΩ impedance. Electrical signals were amplified and recorded using the Digital Lynx S multichannel acquisition system (Neuralynx) in conjunction with Cheetah data acquisition software (Neuralynx).
Tetrode depths were adjusted ∼12 h before each recording session to sample an independent population of neurons across sessions. To estimate tetrode depths during each session, we calculated distance traveled with respect to rotation fraction of the screw that was affixed to the shuttle holding the tetrode. One full rotation moved the tetrode ∼250 μm, and tetrodes were moved on average 62.5 μm between sessions. The final tetrode location was confirmed through histological assessment using electrolytic lesions and tetrode tracks.
Offline spike sorting and cluster quality analysis was performed using MClust software (A. D. Redish, University of Minnesota) in MATLAB. Briefly, for each tetrode, single units were isolated by manual cluster identification based on spike features derived from sampled waveforms. Identification of single units through examination of spikes in high-dimensional feature space allowed us to refine the delimitation of identified clusters by examining all possible two-dimensional combinations of selected spike features. We used standard spike features for single unit extraction: peak amplitude, energy (square root of the sum of squares of each point in the waveform, divided by the number of samples in the waveform), and the first principal component normalized by energy. Spike features were derived separately for individual leads. To assess the quality of identified clusters, we calculated two standard quantitative metrics: L-ratio and isolation distance (Schmitzer-Torbert et al. 2005). Clusters with an L-ratio <0.75 and isolation distance >12 were deemed single units, which resulted in the exclusion of 30% of the identified clusters. Although units were clustered without knowledge of interspike interval (ISI), only clusters with ISI >1 ms were considered for further examination. Furthermore, we excluded the possibility of including data from the same neuron twice by ensuring that both the waveforms and response properties sufficiently changed across sessions. If they did not, we conservatively assumed that we were recording from the same neuron and only included data from one session.
Lesion and Histology
To verify the final location of implanted tetrodes, we performed electrolytic lesions (100 μA, ∼1.5 min per lead) after the last recording session. One day following lesion, mice were overdosed with an intraperitoneal injection of pentobarbital sodium (100 mg/kg) and transcardially perfused with saline followed by ice-cold 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). After perfusion, brains were submerged in 4% PFA in 0.1 M PB for 3.5 h for postfixation and then cryoprotected overnight by immersion in 20% sucrose in 0.1 M PB. The brain was embedded in optimal cutting temperature compound (OCT; ThermoFisher Scientific, Waltham, MS) and frozen rapidly on dry ice. Serial coronal sections (50 μm) were cut on a cryostat for reconstruction of the lesion site and tetrode tracks.
For all animals, fluorescent Nissl (NeuroTrace; catalog no. N-21480, Invitrogen) was used to identify cytoarchitectural features of the PPTg and verify tetrode tracks and lesion damage within or below the PPTg. Briefly, after three 10-min washes in 0.1 M PBS, sections were incubated in Alexa 555 fluorescent Nissl (1:500) in 0.1 M PBS (phosphate buffer with 0.9% saline) for 25 min. After incubation, sections were washed 3 times for 10 min in 0.1 M PBS and finally for 10 min in 0.1 M PB. After the final PB wash, sections were mounted serially onto slides and coverslipped with Fluormount-G (Southern Biotechnology, Birmingham, AL).
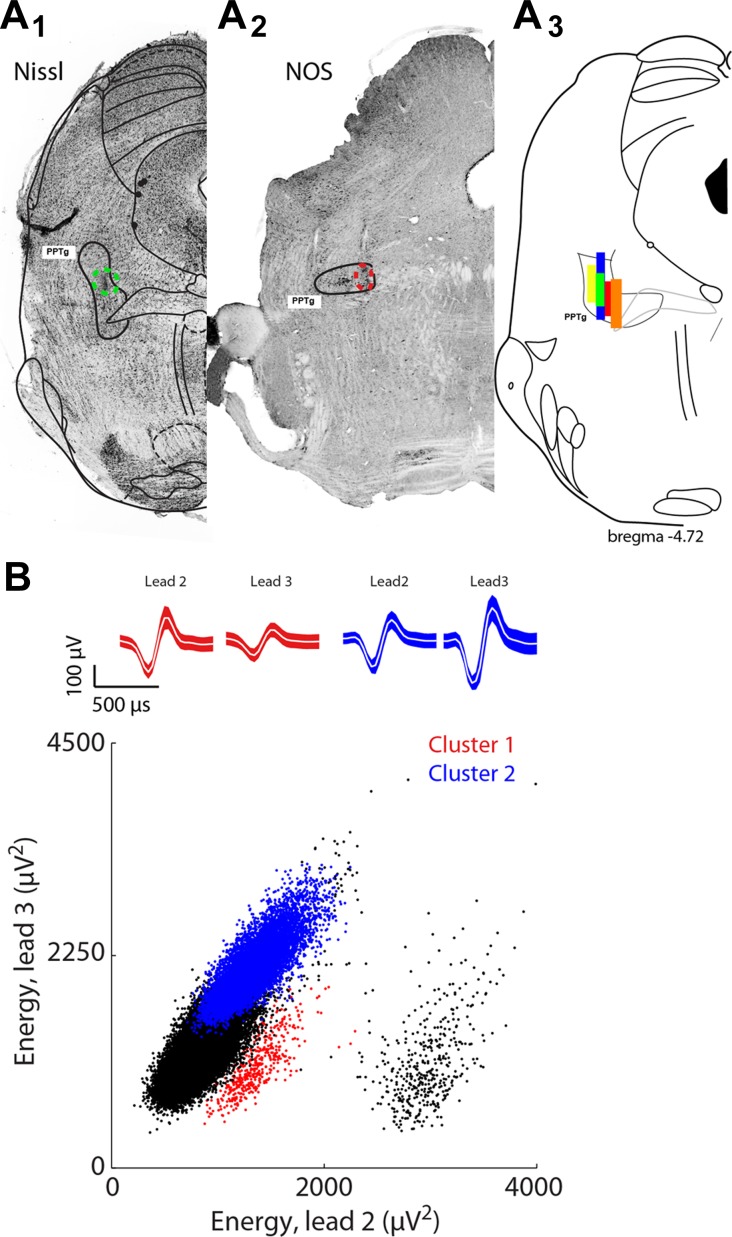
In two of five animals, immunocytochemistry was used to verify the anatomical location of the PPTg. To confirm the anatomic location of PPTg cell bodies, we used anti- nitric oxide synthase (NOS; see Fig. 2A2) (Dawson et al. 1991; Hope et al. 1991; Sugaya and McKinney 1994; Vincent et al. 1983). Briefly, to assess a rabbit anti-NOS I polyclonal antibody (working dilution 1:1,000; catalog no. AB1552, lot no. NG1827382, Millipore) in the PPTg, after three 10-min washes in 0.1 M PBS, coronal sections of tissue containing PPTg were incubated in blocking solution containing 1% BSA, 2% normal goat serum, 2% normal donkey serum, and 0.3% Triton X-100 in 0.1 PBS for 1 h and then incubated in a rabbit anti-NOS for 48 h at −4°. After incubation in antisera, sections were washed three times for 10 min each in 0.1 M PBS. Then sections were incubated in a secondary antibody mixture (goat anti-rabbit Alexa 488, 1:500 and 555 fluorescent Nissl stain solution, 1:500) for 2 h. After secondary antibody incubation, sections were washed three times in 0.1 PBS and once in 0.1 M PB, mounted in serial order onto slides, and coverslipped with Fluormount-G. Images of PPTg used for assessment of tetrode location were captured with a ×10 objective lens, using Surveyor software (Objective Imaging, Cambridge, UK), which controlled a Leica DFC365 FX camera attached to a Leica DM6000B microscope.
Fig. 2.
Confirmation of recording sites and spike clustering. A1: representative electrolytic lesion administered after final recording session (green dotted polygon), within pedunculopontine tegmental nucleus (PPTg). Coronal section, 4.6–4.9 mm caudal from bregma. A2: representative anti-nitric oxide synthase (NOS) reaction used to enhance the distinction of the PPTg boundaries (red dotted polygon shows electrolytic lesion administered after final recording session). Coronal section, 4.6–4.9 mm caudal from bregma. A3: colored boxes show estimated extent of tetrode recording for each experimental animal. B: energy of waveforms from lead 2 plotted against energy of waveforms from lead 3 of one tetrode for a representative recording session. Blue and red points show waveform energies recorded from neurons considered to be distinct; corresponding waveforms (mean ± SD) shown above. Clustering quality was enforced by requiring clusters to meet thresholds for L-ratio and isolation distance (see materials and methods).
Data Analysis and Statistics
Behavioral analysis.
To assess the performance of each animal across sessions, psychometric functions were fit for each session using p = 1/(1 + e−a − bx), where x is the proportion of the right odor [(+)-carvone] in the mixture, p is the fraction of right choices, and a and b are the best-fit free parameters. For each session we also calculated the fraction correct choices [no. of correct choices/(no. of correct choices + no. of incorrect choices)] and the fraction complete choices [(no. of correct choices + no. of incorrect choices)/total attempts; total attempts = no. of correct choices + no. of incorrect choices + no. of instances in which the mouse exited the center port before the go signal].
Epoch selection.
After isolating spike times for single neurons, the firing rate of each neuron was examined during different task epochs. We segmented each trial into epochs based on events triggered by the animal. The delay epoch was defined as the window between 100 ms after the odor valve was opened (to account for the time required for the odor to reach the port) to the time when the mouse exited the odor port (Fig. 1A). The movement epoch was defined as the window between when the mouse exited the odor port and entered one of the two reward ports (Fig. 1A). The reward epoch was defined as the 750 ms following reward port entry (Fig. 1A; this window allowed us to measure neural activity during error trials in which reward port departure could be short in the absence of a reward).
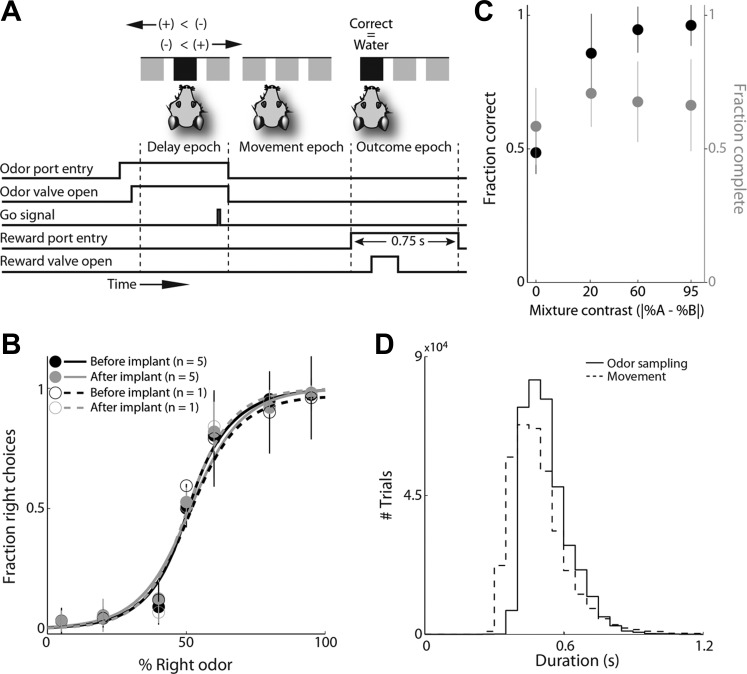
Fig. 1.
Behavioral performance on the odor-cued spatial choice task. A: schematic of trial events and task epochs. In each trial, after entering the odor port, the mouse receives an odor, waits for the go signal, exits the odor port, moves to one of the reward ports, and receives a water reward following correctly performed trials. When (+)-carvone > (−)-carvone in the mixture, reward is available at the right; when (+)-carvone < (−)-carvone, reward is available at the left; and when (+)-carvone = (−)-carvone, the probability of reward at the left and right ports, independently, is 0.5. The delay epoch begins 100 ms after odor valve open. B: choice behavior is unaffected by drive implant. Behavioral performance for each session best fit to p = 1/(1 + e−a − bx), where x is the proportion of (+)-carvone in the mixture ratio, p is the fraction of right choices, and a and b are free parameters. Each line is the average fit across all sessions for either 1 example mouse or all 5 mice. Error bars represent SD. C: gray circles indicate the fraction of completed trials (in which the mouse successfully waited for the go signal before exiting the odor port) as a function of discrimination difficulty, across sessions. Black circles indicate the fraction of completed trials in which the correct reward port was chosen, across sessions. At a mixture contrast of 0, the fraction of rewarded trials is shown (reward was equally likely at left and right on 50/50 trials). Error bars denote SD across sessions. D: solid line shows the distribution of odor sampling durations (from odor valve open until odor port exit) across trials, for all recording sessions (5 mice). In each trial, the delay epoch is delimited by the odor sampling duration. Dotted line shows the distribution of movement durations (from odor port exit to water port entry) across trials, for all recording sessions (5 mice).
Preference analyses.
To quantify the selectivity of single neurons for task variables (direction of movement, outcome), we used an algorithm based on receiver operating characteristic (ROC) analysis (Feierstein et al. 2006; Felsen and Mainen 2008; Green and Swets 1966). This analysis calculates the ability of an ideal observer to classify whether a given spike rate was recorded in one of two conditions (e.g., during leftward or rightward movement). We defined “preference” as 2(ROCarea − 0.5), a measure ranging from −1 to 1, where −1 signifies the strongest possible preference for one alternative, 1 signifies the strongest possible preference for the other alternative, and 0 signifies no preference. This analysis was used to test left vs. right direction preference (left = −1, right = 1) and outcome preference (no reward = −1, reward = 1). Statistical significance was determined with a permutation test: we recalculated the preference after randomly reassigning all firing rates to either of the two groups arbitrarily, repeated this procedure 1,000 times to obtain a distribution of values, and calculated the fraction of random values exceeding the actual value. For all analyses, we tested for significance at alpha = 0.05. Only neurons with a minimum number of four trials for each analyzed condition and a firing rate above 2 spikes/s for either of the analyzed conditions were included in the analysis. For analyses based on movement from the odor port to reward port, trials in which the movement time was >1.5 s were excluded.
Speed/firing rate correlations.
To determine the relationship between speed of movement toward a reward port and firing rate either preceding or during movement, we calculated speed as distance divided by movement time (distance = 51 mm from odor to reward port; movement time was calculated as time from odor port exit to reward port entry; Fig. 1D). For each direction-selective neuron within either the delay epoch or movement epoch, we extracted firing rates in trials corresponding to the preferred direction of the neuron and correlated firing rate with speed in those trials. The same analysis was performed separately for trials in the anti-preferred direction of the neuron. Therefore, two correlation coefficient values were calculated for each neuron within each epoch. We derived and report P values for the correlation coefficients using the MATLAB function corrcoef (Feierstein et al. 2006).
RESULTS
Mice were trained on a delayed-response version of an odor-guided spatial choice task (Fig. 1A; Rinberg et al. 2006; Uchida and Mainen 2003). In each trial of a session the mouse entered the central port, breaking a photobeam and triggering the delivery of an odor. After a short delay (300–500 ms) during which odor was continuously delivered, a go signal (light off) indicated that the mouse could leave the odor port and move to either the left or right reward port where water would be delivered for a correct response (Fig. 1A). Choice accuracy varied with odor discrimination difficulty but was not impaired by microdrive implantation (Fig. 1B). If the mouse prematurely exited the odor port, the trial was aborted and water was not delivered to either reward port. Mice completed 61.7 ± 8.7% (mean ± SD, across all sessions) of the trials during each session. Whether the mice attended to the go signal or simply learned to delay movement initiation for long enough is not relevant to our subsequent analyses; more importantly, the number of trials completed for each odor mixture was sufficient (Fig. 1C). Aborted trials were excluded before neurophysiological analysis. During recording, mice responded correctly in 87.9 ± 6.8% (mean ± SD, across all sessions) of the completed trials, with a higher fraction of correct responses for easier trials (Fig. 1C).
We recorded from 490 well-isolated neurons in the left PPTg during 210 sessions performed by 5 mice (materials and methods; Fig. 2B). In the following sections, we describe our analyses of the neural activity recorded during epochs preceding, during, and following the odor-cued movement. Briefly, we found that the activity of PPTg neurons reflected task-related variables during each of these epochs (Fig. 3), extending our understanding of the role of the PPTg in goal-directed movements.
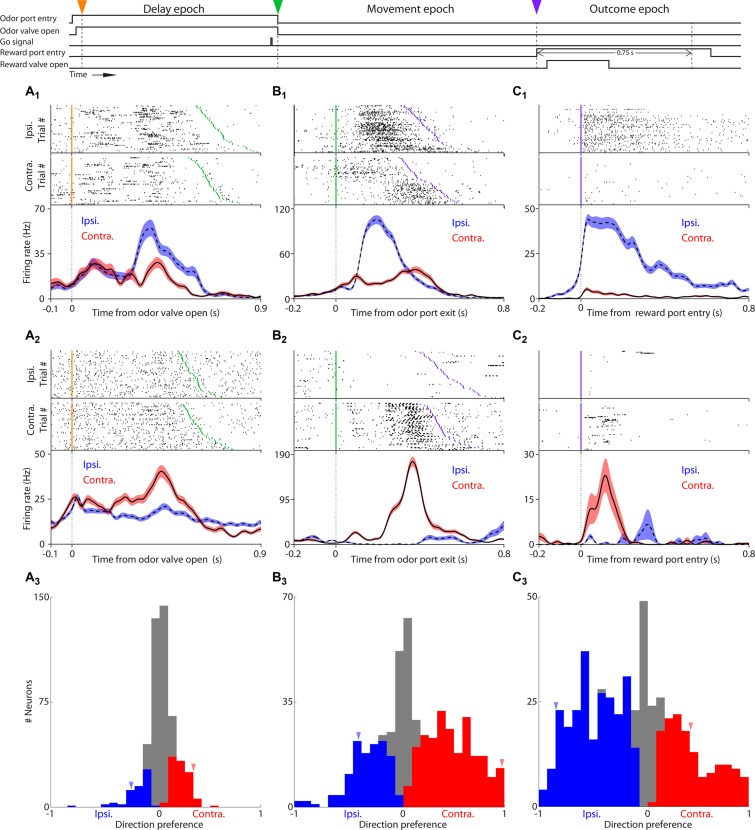
Fig. 3.
PPTg neurons exhibit direction preference during each task epoch. A1, top: rasters grouped by movement choice for an example neuron preferring ipsilateral movement during the delay epoch. Each row shows spikes (black ticks) in 1 trial, aligned to time of odor valve open and sorted by odor sampling duration. Orange ticks, times of odor valve open; green ticks, times of go signal; 50 pseudorandomly selected trials are shown per group. Bottom, perievent histograms show average activity separately for ipsilateral and contralateral trials. Shading indicates ±SE. Histograms are smoothed with a Gaussian filter (σ = 15 ms). A2: as in A1, for an example neuron preferring contralateral movement. A3: histogram showing direction preferences during delay epoch across population of neurons that met criteria for trials and firing rate (materials and methods). Blue and red bars indicate neurons with a significant preference for ipsilateral and contralateral movement, respectively (P < 0.05). Blue and red arrowheads indicate preference for example neurons shown in A1 and A2, respectively. B1–B3: as in A1–3, with respect to the movement epoch. C1–C3; as in A1–A3, with respect to the outcome epoch. Ipsi, ipsilateral; Contra, contralateral.
Neural Activity Preceding Movement
We first examined neural activity recorded during the delay epoch (Fig. 1A), during which the mouse samples the odor stimulus and selects and prepares its movement toward a reward port. During this epoch, we found that the activity of many PPTg neurons was selective for the direction of upcoming movement. Figure 3A1 shows data from an example neuron that exhibited a higher firing rate preceding ipsilateral (i.e., leftward) movement, and Fig. 3A2 shows another neuron that exhibited a higher firing rate preceding contralateral (i.e., rightward) movement. To quantify direction preference across the population, we used an ROC-based measure that ranges from −1 to 1; negative values indicate ipsilateral preference, positive indicate contralateral preference, and the magnitude reflects the strength of the preference (materials and methods; Green and Swets 1966). We found that 167/454 neurons (36.8%) exhibited significant direction preference preceding movement (P < 0.05), with more preferring contralateral than ipsilateral movement (P < 0.05, χ2 test; 104 prefer contralateral, 63 prefer ipsilateral; Fig. 3A3).
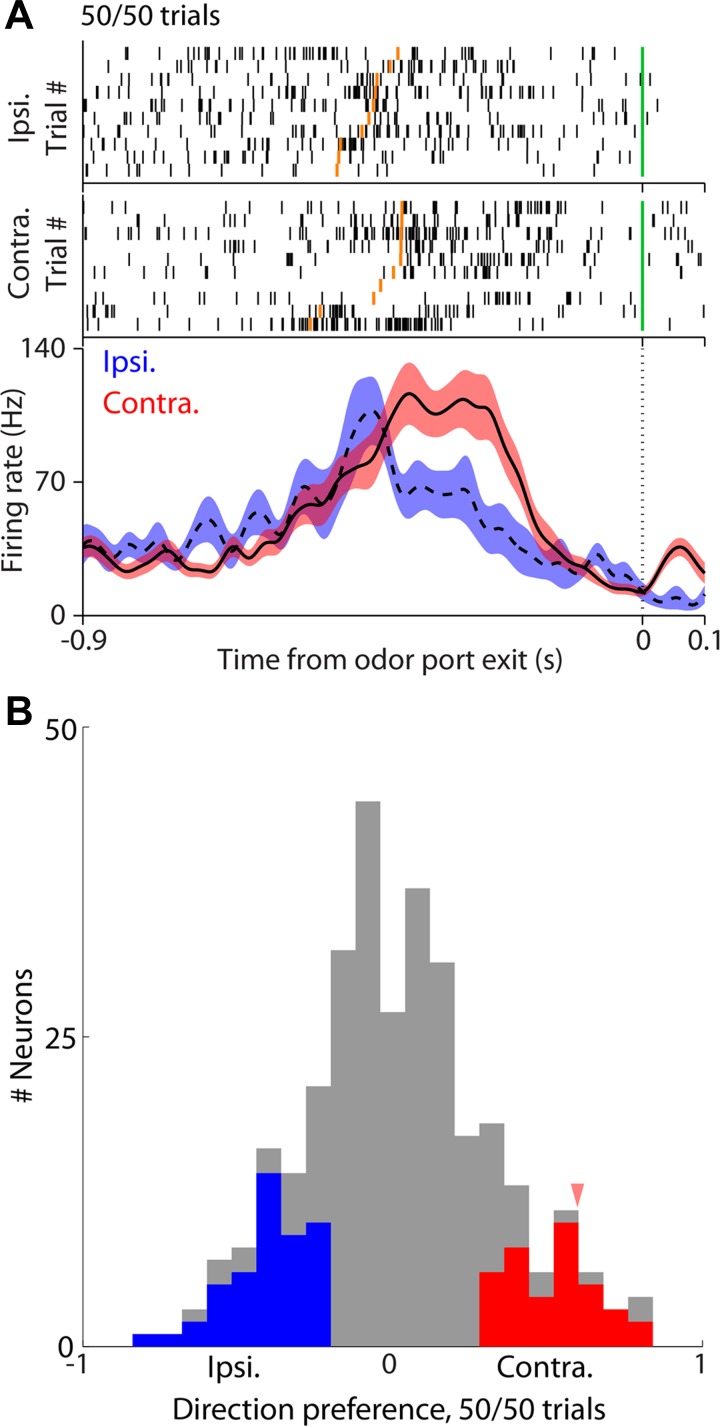
Although PPTg neurons are not known to be odor selective, in theory the activity we observed could reflect the odor and not the direction of movement with which the odor is correlated (Fig. 1B). To disambiguate whether preference indicated odor identity or direction of movement, we examined direction preference during presentation of 50/50 odors. On these trials, the odor was held constant but movement direction varied; thus, if the selectivity we have observed (Fig. 3A) is actually for odor and not direction, there should be no difference in activity between trials in which the left or right reward port was selected. In Fig. 4A, the spiking activity for a representative PPTg neuron recorded during both ipsilateral and contralateral movements following the delivery of a 50/50 odor stimulus shows direction preference despite the absence of an explicit odor/direction association. Indeed, across the population we found that many neurons retained direction preference during 50/50 trials (Fig. 4B). Although we cannot entirely exclude the possibility that some PPTg neurons are odor selective, this analysis of 50/50 trials suggests that odor selectivity is not sufficient to explain the preference demonstrated in Fig. 3A3; these neurons are therefore likely to be direction selective.
Fig. 4.
Activity during delay epoch reflects upcoming direction, and not odor identity. A, top: rasters corresponding to 50/50 trials grouped by movement choice for an example neuron. Bottom, perievent histograms showing average activity on 50/50 trials separately for ipsilateral and contralateral trials. Conventions are the same as in Fig. 3A1. B: direction preference calculated during 50/50 trials, in which the odor does not vary across trials. Arrowhead indicates preference for example neuron shown in A.
Neural Activity During Movement
We next examined neural activity recorded during the movement epoch (Fig. 1A), as the mouse moves from the odor to the reward port. Figure 3, B1 and B2, shows data from two example neurons that exhibit direction selectivity during this epoch. Applying the ROC analysis described above to this epoch, we again found that the firing rate of many recorded neurons depended on whether movement was toward the ipsilateral or contralateral reward port (78.2%; 365/467: 127 ipsilateral and 238 contralateral; Fig. 3B3). Similar to the delay epoch, the proportion of contralateral preferring neurons was significantly larger than ipsilateral preferring neurons (P < 0.05, χ2 test).
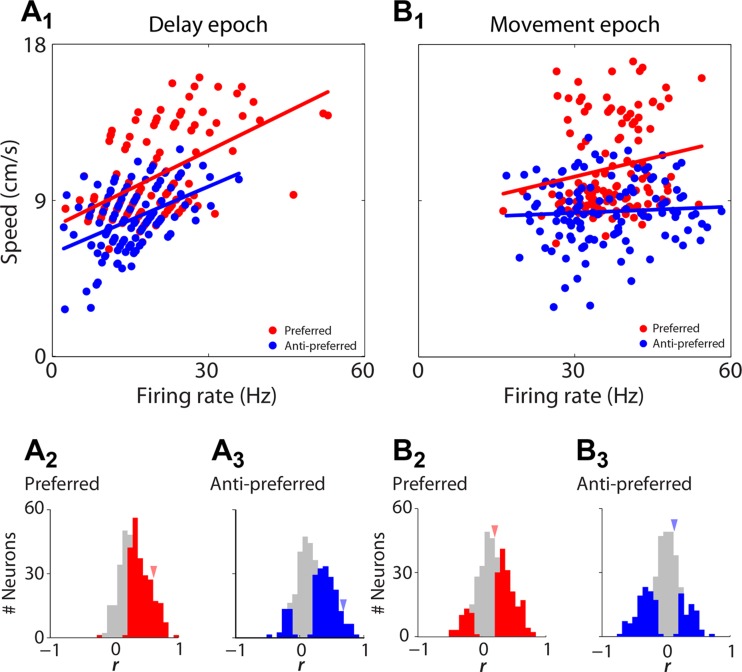
We then examined whether neural activity during the delay and movement epochs was associated with movement speed, which would be consistent with a role for the PPTg in movement preparation and execution. Data from an example neuron exhibiting positive correlation between firing rate during a fixed duration at the end of the delay epoch and upcoming movement speed are depicted in Fig. 5A1. Note that this correlation is positive for trials in which the movement was in either the preferred or anti-preferred directions. Across the population of neurons that were direction selective during the delay epoch (Fig. 3A3, colored bars), we found that, for both preferred- and anti-preferred-direction trials, nearly all neurons in which delay-epoch firing rate and speed of upcoming movement were significantly correlated exhibited a positive correlation (Fig. 5, A2 and A3, P < 0.001, χ2 test; both preferred and anti-preferred-direction trials). We then repeated this analysis for firing rate recorded during the movement epoch. Figure 5B1 shows data from the same example neuron in Fig. 5A1, but for firing rates during the movement epoch. We again found that firing rate was positively correlated with movement speed for movements in the preferred direction of the neuron but was not correlated with movement speed for movements in the anti-preferred direction of the neuron. This pattern of correlations was representative of the population of neurons that showed direction preference during the movement epoch (Fig. 3B2, colored bars); for preferred-direction trials, more neurons exhibited a positive firing rate-speed correlation than a negative correlation (Fig. 5B2, P < 0.001, χ2-test), whereas during anti-preferred trials more neurons exhibited a negative than a positive firing rate-speed correlation (Fig. 5B3, P < 0.01, χ2-test). Together, these data demonstrate that the activity of a population of PPTg neurons reflects the direction and speed of movements as they are being planned and executed.
Fig. 5.
Movement speed correlates with activity during delay and movement epochs. A1: speed of movement during each trial plotted against activity of an example neuron during the final 267.5 ms of the delay epoch (the shortest odor sampling duration in our data set) of the corresponding trial, separated by trials in which movement was in the preferred and anti-preferred direction of the neuron. Each circle corresponds to 1 trial. Best-fit lines are shown for each group of trials. A2: correlation between speed and firing rate for all neurons that were direction selective during the delay epoch (colored bars in Fig. 3A3) for preferred direction trials. Red bars indicate neurons with a significant correlation on preferred (P < 0.05). Arrowheads indicate correlations for example neuron shown in A1. A3: correlation between speed and firing rate for all neurons that were direction selective during the delay epoch (colored bars in Fig. 3A3) for anti-preferred-direction trials. Blue bars indicate neurons with a significant correlation on anti-preferred-direction trials (P < 0.05). Arrowheads indicate correlations for example neuron shown in A1. B1: same example neuron as in A1, showing activity during the movement epoch. B2: as in A2, for activity during the movement epoch. B3: as in A3, for activity during the movement epoch.
Neural Activity Following Movement
Finally, we examined neural activity recorded during the outcome epoch (Fig. 1A), when the mouse either obtains or does not obtain the anticipated reward. Figure 3, C1 and C2, shows data from two example neurons that are selective for side (contralateral vs. ipsilateral) during this epoch. Across the population, we quantified side preference during the 750 ms following reward port entry. We observed a higher proportion of neurons with a significant ipsilateral side preference during the outcome epoch (245/473 preferred ipsilateral and 147/473 preferred contralateral; P < 0.05, χ2 test; Fig. 3C3). The overall percentage of side-selective neurons during this epoch (82.9%) was higher than either the delay or the movement epochs. Interestingly, in contrast to the delay and movement epochs, the proportion of ipsilateral-preferring neurons was significantly larger than contralateral-preferring neurons (P < 0.05, χ2 test).
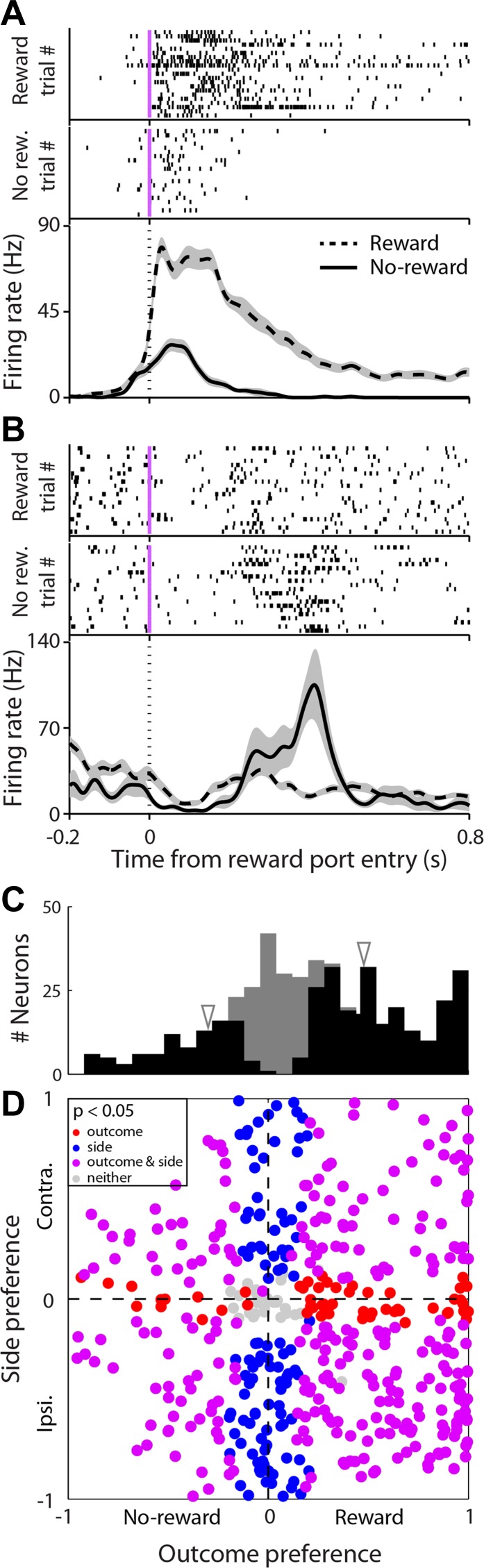
Some electrophysiological studies suggest that PPTg activity reflects reward-related information (i.e., magnitude: Okada et al. 2009, 2011; magnitude and context: Norton et al. 2011), whereas others suggest that the activity reflects sensory and motor task components rather than reward (Matsumura et al. 1997, Pan and Hyland 2005). We therefore examined whether activity depended on trial outcome (i.e., reward vs. no reward; Fig. 6). Figure 6A shows data from an example neuron that exhibited a higher firing rate during trials in which reward was obtained, whereas Fig. 6B shows a neuron that fired more when reward was not obtained. Across the population, we applied the same ROC-based analysis used above and found that 343/478 neurons (72%) appear to be outcome selective (materials and methods; note that some variation in activity may be due to different movements during rewarded and nonrewarded trials). Within this population, we found that 28% (96/343) preferred no reward (Fig. 6B), meaning that the firing rate of these neurons increased when reward was not obtained. However, a majority of the outcome-selective neurons (72%; 247/343) preferred reward (Fig. 6B), indicating that the firing rate of these neurons increased during reward acquisition. The proportion of reward-preferring neurons was significantly larger than that of no-reward-preferring neurons (Fig. 6C; P < 0.05, χ2 test).
Fig. 6.
Outcome-epoch activity reflects the presence or absence of reward. A, top: rasters are grouped by outcome (rewarded or nonrewarded) for an example neuron that prefers reward during the outcome epoch, aligned to time of reward port entry (red line). Bottom, perievent histograms showing average activity separately for rewarded and nonrewarded trials. Conventions are the same as in Fig. 3A1. B: as in A, for an example neuron that prefers no reward. C: histogram showing outcome preferences during outcome epoch across population of neurons that met criteria for trials and firing rate (materials and methods). Black bars indicate neurons with a significant preference for reward or no reward (P < 0.05). Arrowheads indicate preferences for example neurons shown in A and B. D: joint histogram showing outcome and direction preferences during outcome epoch across population of neurons that met criteria for trials and firing rate (materials and methods). Each circle represents 1 neuron. Marginal histograms for outcome and direction preference are shown in C and Fig. 3C3, respectively. A large fraction of neurons (purple) was selective for both outcome and direction.
Given that most PPTg neurons exhibited outcome selectivity (Fig. 6C), and most also exhibited direction selectivity during the same epoch (Fig. 3C3, colored bars), we sought to determine the extent to which individual neurons jointly represent information from both contexts. For this analysis, we excluded neurons that failed to meet our firing rate and trial number criteria in either condition (materials and methods). We found that 72.5% (343/473) of the neurons were outcome selective, 82.9% (392/473) were side selective, and 60.3% (285/473) were selective for both side and outcome (Fig. 6D). These results indicate that the activity of single PPTg neurons may reflect information about both the location and the outcome associated with movement choice.
Dynamics of Direction Preference Throughout the Trial
We have demonstrated that populations of PPTg neurons exhibit direction preference across multiple task epochs (Figs. 3, 4, and 6). In addition, we have shown that individual PPTg neurons may represent more than one variable associated with a given epoch of the decision task (i.e., outcome and side preference during the outcome epoch; Fig. 6). Given our findings that PPTg neurons reflect direction preference across all measured epochs of our task and appear to show laterality (e.g., there is a contralateral bias within the population of direction-selective neurons in the delay epoch), we now address two complementary questions: 1) what are the fine-scale dynamics of direction preference during movement preparation and execution? and 2) considering across-epoch dynamics for individual PPTg neurons, what fraction of PPTg neurons show direction or corresponding side preference that persists across epochs?
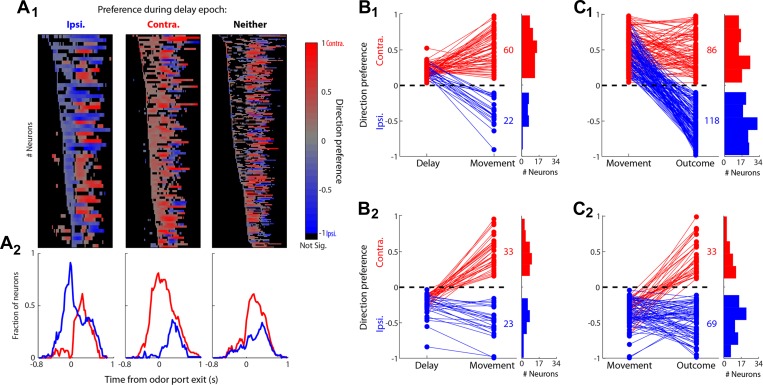
To address the first question, we calculated a “preference curve” for each neuron that exhibited selectivity during the delay epoch and grouped neurons by preferred direction during the delay epoch (e.g., ipsilateral, contralateral and neither; Fig. 7A1). We computed preference in short (200 ms) overlapping time bins between 800 ms preceding and 1,000 ms following odor port exit (Fig. 7A1; Felsen and Mainen 2008; Wallis and Miller 2003), which encompassed both the delay and movement epochs. This analysis describes how direction preference emerges and changes as the movement is planned and executed and reveals whether and when a switch in preferred direction occurs. In each panel of Fig. 7A1, neurons were sorted by the earliest bin with a significant direction preference. For both ipsilateral- and contralateral-preferring neurons, those neurons that exhibited a switch in their direction preference tended to do so ∼300 ms after initiation, as the movement was being executed (Fig. 7A2). Interestingly, some neurons that were not direction selective when the delay epoch was considered in its entirety often exhibited a preference during individual bins within the epoch (Fig. 7A1, right).
Fig. 7.
Dynamics of direction preference throughout trial. A1: preference curves for all neurons exhibiting significant preference for the ipsilateral, contralateral, or neither direction during the entire delay epoch (blue, red, and gray bars in Fig. 3A3, respectively), sorted by earliest emergence of preference. Each row corresponds to 1 neuron. Preference curves were calculated by sliding the 200-ms window by 20-ms increments. Trials are aligned to odor port exit. Color scale shows significant preferences (P < 0.05). Black boxes indicate bins with nonsignificant preferences (P > 0.05) or with fewer than 4 ipsilateral or contralateral trials. Activity that occurred before odor valve open or after reward port entry was excluded. A2: fraction of neurons shown in corresponding panel of A1 preferring either the ipsilateral (blue) or contralateral (red) direction in each time bin. B1: direction preference during the movement epoch for all neurons that preferred upcoming contralateral movement during the delay epoch. Here and in B2, C1, and C2, numbers indicate total number of neurons exhibiting a significant preference (red represents contralateral and blue ipsilateral). B2: direction preference during the movement epoch for all neurons that preferred upcoming ipsilateral movement during the delay epoch. C1: side preference during the outcome epoch for all neurons that preferred contralateral movement during the movement epoch. C2: side preference during the outcome epoch for all neurons that preferred ipsilateral movement during the movement epoch.
This analysis demonstrates that the direction preference of a sizeable fraction of neurons appears to switch between the delay and movement epochs. We next examined the prevalence of this phenomenon across all three epochs by asking whether the direction preference exhibited by a given neuron in one epoch was maintained during the subsequent epoch. In other words, is the preferred direction (or preferred side) of a given neuron independent from one epoch to the next, or can it be predicted from the preferred direction in the preceding epoch? We found that, among the neurons that preferred the contralateral direction during the delay epoch, most maintained their preference during the movement epoch (Fig. 7B1). In contrast, most neurons that preferred the ipsilateral direction during the delay epoch switched their preference during the movement epoch (Fig. 7B2).
Thus there exist subpopulations of PPTg neurons that both reverse and maintain direction preference. However, given the bias toward contralateral preference (i.e., contralaterality) observed in the overall population of direction-selective neurons during the movement epoch (Fig. 3B3), these results do not differ from what would be expected under the assumption that preference during the delay and movement epochs are independent. We compared the distributions of preferred directions during the movement epoch between all neurons selective for direction in this epoch (Fig. 3B3, colored bars) and only those neurons that showed a contralateral direction preference during the delay epoch (Fig. 7B1), and we found that these distributions were not significantly different (P = 0.13, χ2 test). We also found no difference in the distributions of preferred directions during the movement epoch between all neurons selective for direction in this epoch (Fig. 3B3, colored bars) and only those neurons that showed an ipsilateral direction preference during the delay epoch (Fig. 7B2) (P = 0.37, χ2 test).
Furthermore, we found that, among the neurons that preferred the contralateral direction during the movement epoch, most switched their side preference during the outcome epoch (Fig. 7C1), whereas among the neurons that preferred the ipsilateral direction during the movement epoch, most maintained their side preference during the outcome epoch (Fig. 7C2). These results demonstrate the prevalence of exhibiting a preference switch, but given the bias toward ipsilateral preference observed in the overall population of side-selective neurons during the outcome epoch (Fig. 3C3), they are again consistent with our expectation under the assumption that direction preference during the movement epoch and side preference during the outcome epoch are independent. We compared the distributions of preferred sides during the outcome epoch between all neurons selective for side in this epoch (Fig. 3C3, colored bars) and only those neurons that showed a contralateral side preference during the movement, and we found that these distributions were not significantly different (P = 0.17, χ2 test). We also found no difference in the distributions of preferred sides during the outcome epoch between all neurons selective for side in this epoch and only those neurons that showed an ipsilateral side preference during the movement epoch (P = 0.37, χ2 test). These results indicate that the preferred direction of individual neurons varies independently across epochs, suggesting that PPTg neurons may participate in multiple distinct processes, throughout the trial, related to goal-directed motor output.
DISCUSSION
Recordings from the PPTg of mice engaged in a spatial choice task revealed overlapping populations of neurons that exhibited direction preference preceding, during, and subsequent to movement choice (Figs. 3–6), with a diversity of temporal dynamics (Fig. 7), indicating a broad role for the PPTg in planning and executing movements. The multiple representations of overt task variables by individual neurons (Figs. 6D and 7), in particular, suggest that the PPTg may contribute to several sensorimotor processes. These results are consistent with the idea that the PPTg is critical for motor control (Garcia-Rill 1991) and supported by known projections of the PPTg to other motor-related regions, such as the SC, medulla, and striatum (Martinez-Gonzalez et al. 2011; Matsumura et al. 2000; Moriizumi et al. 1988; Rye et al. 1987, 1988; Steininger et al. 1992). In addition, we found that PPTg neurons may be selective for movement outcome (i.e., whether or not the choice was rewarded; Fig. 6, A–C), often while simultaneously reflecting the chosen side (left or right; Fig. 6D). This finding supports and may extend previous studies identifying a role for the PPTg in reward processing (Kobayashi and Okada 2007; Norton et al. 2011; Okada and Kobayashi 2009; Okada et al. 2009, 2011; Pan and Hyland 2005) and is consistent with its well-established interconnectivity with reward-related regions [substantia nigra pars compacta (SNc) and ventral tegmental area (VTA); Alam et al. 2011; Beninato and Spencer 1986, 1987; Blaha and Winn 1993; Inglis and Winn 1995; Parker et al. 1993; Steininger et al. 1992]. These findings support our hypothesis that the PPTg may contribute to the interconnected network of cortical and subcortical regions responsible for sensorimotor decision making (Cisek and Kalaska 2010; Gold and Shadlen 2007; MacLaren et al. 2013).
PPTg Function Across Species
The PPTg as a brain stem structure has been conserved across many taxa (Alam et al. 2011; Stephenson-Jones et al. 2012). Electrophysiological studies across species and in a variety of behavioral paradigms implicate the PPTg in goal-directed motor control (Martinez-Gonzalez et al. 2011; Mena-Segovia et al. 2004; Winn 2006, 2008), consistent with our observations.
In rats, although ablation of bilateral PPTg does not impair spontaneous locomotion (Inglis et al. 1994; Keating and Winn 2002), both pharmacological (Steckler et al. 1994; Steiniger and Kretschmer 2004; Winn 2006) and electrical (Garcia-Rill et al. 1987) manipulation of the PPTg affect goal-directed locomotor behavior. Consistent with these results, a recent electrophysiological study in rats navigating a radial-arm maze found activity correlated with turning behavior (Norton et al. 2011). Similar findings have been observed in other species. In cats performing a conditioned forelimb movement, unilateral PPTg inactivation induced transient arrest of performance (Conde et al. 1998), and recordings revealed PPTg activity preceding movement initiation (Dormont et al. 1998). In primates performing a lever press/release task, PPTg activity was observed preceding movements of both the ipsilateral and contralateral arm (Matsumura et al. 1997). Finally, recordings in the PPTg of primates performing a visually guided saccade task found activity correlated with the time of saccade initiation (Kobayashi et al. 2002; Okada and Kobayashi 2009). Although few studies have systematically examined whether the activity of individual PPTg neurons reflects a specific direction of movement, Okada and Kobayashi (2009) found in primates that more neurons preferred contralateral than ipsilateral saccades, consistent with our results in freely moving mice (Fig. 3, A3 and B3).
Anatomic and physiological evidence has shown that the PPTg provides a key input to reward-related midbrain dopaminergic neurons (Lokwan et al. 1999; Maskos 2008; Mena-Segovia et al. 2008; Pan and Hyland 2005). Recently, recording studies in behaving rats and primates have examined the functional relevance of this projection. These studies have demonstrated that PPTg activity is modulated by reward magnitude (Norton et al. 2011), as well as by either predicted or actual reward (Okada et al. 2009), consistent with our observation of a large population of neurons selective for task outcome (Fig. 6).
Our results demonstrate that the PPTg in the mouse may play a broadly similar role in full-body orienting movements as it does in other species for orienting eye and limb movements (Conde et al. 1998; Dormont et al. 1998; Kobayashi et al. 2002; Matsumura et al. 1997; Okada and Kobayashi 2009). However, in contrast to previous studies in the PPTg of other species, our paradigm revealed widespread preference for movement direction during multiple task epochs. Although this finding may indicate a difference in function across species, it is also possible that equally prevalent direction preference would be observed in the PPTg of other species performing more natural gaze-displacing movements, as opposed to movements restricted to one component of the orienting system (e.g., the eyes; Sparks 1999). Although ours is the first to study the PPTg in behaving mice, given the compatibility of our results with those of previous studies, as well as the advantages afforded by the behaving mouse model (Busse et al. 2011; Madisen 2012; O'Connor et al. 2009;), mice may provide a complementary model system for further elucidating PPTg function. Ultimately, understanding the PPTg in a genetically accessible model may be useful for informing PPTg deep brain stimulation therapies for movement disorders (Benarroch 2013; Fasano et al. 2012; Gradinaru et al. 2009; Insola et al. 2012; Khan et al. 2012; Lourens et al. 2011; Nandi et al. 2008; Peppe et al. 2010; Thevathasan et al. 2010, 2011a, 2011b, 2012a, 2012b; Wilcox et al. 2011).
Relationship With Activity in Other Sensorimotor Decision Regions
Our results show that the PPTg appears to represent multiple task-related variables associated with sensorimotor decision making, simultaneously (Fig. 6D) and across time (Fig. 7), suggesting that the PPTg may lie within the network of decision-making regions involved in initiating movement and evaluating their outcome. Given this interconnectivity of the PPTg with other sensorimotor regions, how does the activity that we have described in the PPTg compare to activity in well-studied areas associated with sensorimotor processing during comparable tasks? We focus primarily on data from rodents, because these are most comparable to the present study.
Previous work suggests that the brainstem cholinergic system modulates motor output via the projection from cholinergic PPTg neurons to the intermediate layers of the superior colliculus (SC; Beninato and Spencer 1986; Hall et al. 1989; Ma et al. 1991; Sooksawate and Isa 2006). Furthermore, motor-preparatory activity has been observed in the SC of several species (Dorris et al. 1997; Glimcher and Sparks 1992; Harris 1980; Horwitz and Newsome 2001; Munoz et al. 1991), including rodents performing a similar task to that used here (Felsen and Mainen 2008, 2012; Hirokawa et al. 2011), with a comparable preponderance of preference for upcoming contralateral movements. PPTg activity observed during the delay epoch (Figs. 3A, 4, and 5A) may reflect a modulatory influence on motor preparation in the SC, in support of the hypothesis. The diversity of the timing of this activity (Fig. 7A) may reflect the fact that the PPTg also consists of glutamatergic and GABAergic neurons and projects to other motor-related regions, such as the thalamus, subthalamic nucleus, and reticular formation (Edley and Graybiel 1983; Hallanger et al. 1987; Jones 1990; Mesulam et al. 1983; Moriizumi et al. 1988; Rye et al. 1987, 1988; Steininger et al. 1992), and receives input from cortical regions involved in orienting movements (Matsumura et al. 2000) that also exhibit motor-preparatory activity in rodents performing a similar task (Erlich et al. 2011). Future experiments focusing on neurotransmitter- or projection-defined subpopulations of PPTg neurons may disambiguate the potentially distinct roles of these PPTg afferents and efferents (Cardin et al. 2010; Cohen et al. 2012; Lima et al. 2009).
It has also been proposed that, via its inputs to the VTA and SNc (Beninato and Spencer 1987; Futami et al. 1995; Sesack et al. 2003; Woolf 1991), the PPTg contributes to representing the difference between actual and expected reward (Brown et al. 1999; Kobayashi and Isa 2002; Kobayashi and Okada 2007; Norton et al. 2011; Okada et al. 2009; Schultz et al. 1997). The outcome-selective activity that we observed (Fig. 6) is consistent with this idea. Furthermore, our finding that the activity of many neurons jointly reflects the choice and its outcome (Fig. 6D) suggests that the PPTg, along with other regions implicated in performance monitoring, may be involved in associating actions with their values (Feierstein et al. 2006; Horst and Laubach 2012; Ito et al. 2003; Schoenbaum and Setlow 2001; Sul et al. 2010). These data are consistent with the idea that some PPTg neurons contribute to the computation of reward prediction error.
The fact that several cortical, midbrain, and brain stem regions exhibit similar neural representations of task-related variables is consistent with the idea that action selection is a distributed process incorporating areas traditionally associated with both higher-order cognition and motor control (Cisek 2012; Cisek and Kalaska 2010). Future studies can build on ours by leveraging the advantages of the mouse model to understand the roles of distinct cell types within the PPTg and, ultimately, to address how the PPTg interacts with these other regions to mediate sensorimotor decisions.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS079518 and the Boettcher Foundation's Webb-Waring Biomedical Research Award (to G. Felsen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.T. and G.F. conception and design of research; J.A.T. performed experiments; J.A.T. and G.F. analyzed data; J.A.T. and G.F. interpreted results of experiments; J.A.T. and G.F. prepared figures; J.A.T. and G.F. drafted manuscript; J.A.T. and G.F. edited and revised manuscript; J.A.T. and G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jamie Costabile, Beth Stubblefield, Mario Lintz, and Steven Ojemann for helpful discussions and comments on the manuscript.
REFERENCES
- Alam M, Schwabe K, Krauss JK. The pedunculopontine nucleus area: critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 134: 11–23, 2011 [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology 80: 1148–1155, 2013 [DOI] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. A cholinergic projection to the rat substantia nigra from the pedunculopontine tegmental nucleus. Brain Res 412: 169–174, 1987 [DOI] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol 253: 525–538, 1986 [DOI] [PubMed] [Google Scholar]
- Blaha CD, Winn P. Modulation of dopamine efflux in the striatum following cholinergic stimulation of the substantia nigra in intact and pedunculopontine tegmental nucleus-lesioned rats. J Neurosci 13: 1035–1044, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bullock D, Grossberg S. How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. J Neurosci 19: 10502–10511, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse L, Ayaz A, Dhruv NT, Katzner S, Saleem AB, Scholvinck ML, Zaharia AD, Carandini M. The detection of visual contrast in the behaving mouse. J Neurosci 31: 11351–11361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc 5: 247–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298, 2010 [DOI] [PubMed] [Google Scholar]
- Cisek P. Making decisions through a distributed consensus. Curr Opin Neurobiol 22: 927–936, 2012 [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482: 85–88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde H, Dormont JF, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. II. Effects of reversible inactivation by intracerebral microinjections. Exp Brain Res 121: 411–418, 1998 [DOI] [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA 88: 7797–7801, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito JL, Anderson ME, Walsh KE. A horseradish peroxidase study of afferent connections of the globus pallidus in Macaca mulatta. Exp Brain Res 38: 65–73, 1980 [DOI] [PubMed] [Google Scholar]
- Dormont JF, Conde H, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res 121: 401–410, 1998 [DOI] [PubMed] [Google Scholar]
- Dorris MC, Pare M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edley SM, Graybiel AM. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol 217: 187–215, 1983 [DOI] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron 72: 330–343, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson's disease with deep brain stimulation. Lancet Neurol 11: 429–442, 2012 [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron 51: 495–507, 2006 [DOI] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Midbrain contributions to sensorimotor decision making. J Neurophysiol 108: 135–147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron 60: 137–148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor JA. Modularity of Mind. Cambridge, MA: MIT Press, 1983 [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res 21: 331–342, 1995 [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Houser CR, Skinner RD, Smith W, Woodward DJ. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull 18: 731–738, 1987 [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res 411: 1–12, 1987 [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. The pedunculopontine nucleus. Prog Brain Res 36: 363–389, 1991 [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature 355: 542–545, 1992 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007 [DOI] [PubMed] [Google Scholar]
- Grace KP, Liu H, Horner RL. 5-HT1A receptor-responsive pedunculopontine tegmental neurons suppress REM sleep and respiratory motor activity. J Neurosci 32: 1622–1633, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of Parkinsonian neural circuitry. Science 324: 354–359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol 287: 495–514, 1989 [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol 262: 105–124, 1987 [DOI] [PubMed] [Google Scholar]
- Harris LR, Blakemore C, Donaghy M. Integration of visual and auditory space in the mammalian superior colliculus. Nature 288: 56–59, 1980 [DOI] [PubMed] [Google Scholar]
- Hirokawa J, Sadakane O, Sakata S, Bosch M, Sakurai Y, Yamamori T. Multisensory information facilitates reaction speed by enlarging activity difference between superior colliculus hemispheres in rats. PLoS One 6: e25283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA 88: 2811–2814, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Laubach M. Working with memory: evidence for a role for the medial prefrontal cortex in performance monitoring during spatial delayed alternation. J Neurophysiol 108: 3276–3288, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: direction-selective visual responses in the superior colliculus. J Neurophysiol 86: 2527–2542, 2001 [DOI] [PubMed] [Google Scholar]
- Inglis WL, Dunbar JS, Winn P. Barbiturate anaesthesia reduces the neurotoxic effects of quinolinate but not ibotenate in the rat pedunculopontine tegmental nucleus. Neurosci Lett 156: 78–82, 1993 [DOI] [PubMed] [Google Scholar]
- Inglis WL, Dunbar JS, Winn P. Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by d-amphetamine. Neuroscience 62: 51–64, 1994 [DOI] [PubMed] [Google Scholar]
- Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 47: 1–29, 1995 [DOI] [PubMed] [Google Scholar]
- Insola A, Valeriani M, Mazzone P. Targeting the pedunculopontine nucleus: a new neurophysiological method based on somatosensory evoked potentials to calculate the distance of the deep brain stimulation lead from the Obex. Neurosurgery 71: 96–103, 2012 [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science 302: 120–122, 2003 [DOI] [PubMed] [Google Scholar]
- Jones BE. Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol 295: 485–514, 1990 [DOI] [PubMed] [Google Scholar]
- Keating GL, Winn P. Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience 112: 687–696, 2002 [DOI] [PubMed] [Google Scholar]
- Khan S, Gill SS, Mooney L, White P, Whone A, Brooks DJ, Pavese N. Combined pedunculopontine-subthalamic stimulation in Parkinson disease. Neurology 78: 1090–1095, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28: 2991–3007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Inoue Y, Yamamoto M, Isa T, Aizawa H. Contribution of pedunculopontine tegmental nucleus neurons to performance of visually guided saccade tasks in monkeys. J Neurophysiol 88: 715–731, 2002 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Isa T. Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Netw 15: 731–741, 2002 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Okada K. Reward prediction error computation in the pedunculopontine tegmental nucleus neurons. Ann NY Acad Sci 1104: 310–323, 2007 [DOI] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One 4: e6099, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokwan SJ, Overton PG, Berry MS, Clark D. Stimulation of the pedunculopontine tegmental nucleus in the rat produces burst firing in A9 dopaminergic neurons. Neuroscience 92: 245–254, 1999 [DOI] [PubMed] [Google Scholar]
- Lourens MA, Meijer HG, Heida T, Marani E, van Gils SA. The pedunculopontine nucleus as an additional target for deep brain stimulation. Neural Netw 24: 617–630, 2011 [DOI] [PubMed] [Google Scholar]
- Ma TP, Graybiel AM, Wurtz RH. Location of saccade-related neurons in the macaque superior colliculus. Exp Brain Res 85: 21–35, 1991 [DOI] [PubMed] [Google Scholar]
- MacLaren DA, Wilson DI, Winn P. Updating of action-outcome associations is prevented by inactivation of the posterior pedunculopontine tegmental nucleus. Neurobiol Learn Mem 102C: 28–33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15: 793–802, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat 5: 22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol 153, Suppl 1: S438–S445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Nambu A, Yamaji Y, Watanabe K, Imai H, Inase M, Tokuno H, Takada M. Organization of somatic motor inputs from the frontal lobe to the pedunculopontine tegmental nucleus in the macaque monkey. Neuroscience 98: 97–110, 2000 [DOI] [PubMed] [Google Scholar]
- Matsumura M, Watanabe K, Ohye C. Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res 28: 155–165, 1997 [DOI] [PubMed] [Google Scholar]
- Matulewicz P, Kasicki S, Hunt MJ. The effect of dopamine receptor blockade in the rodent nucleus accumbens on local field potential oscillations and motor activity in response to ketamine. Brain Res 1366: 226–232, 2010 [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci 27: 585–588, 2004 [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Winn P, Bolam JP. Cholinergic modulation of midbrain dopaminergic systems. Brain Res Rev 58: 265–271, 2008 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214: 170–197, 1983 [DOI] [PubMed] [Google Scholar]
- Miller GA, Galanter E, Pribram KH. Plans and Structure of Behavior. New York: Holt, Rinehart and Winston, 1960 [Google Scholar]
- Moriizumi T, Nakamura Y, Tokuno H, Kitao Y, Kudo M. Topographic projections from the basal ganglia to the nucleus tegmenti pedunculopontinus pars compacta of the cat with special reference to pallidal projection. Exp Brain Res 71: 298–306, 1988 [DOI] [PubMed] [Google Scholar]
- Munoz DP, Pelisson D, Guitton D. Movement of neural activity on the superior colliculus motor map during gaze shifts. Science 251: 1358–1360, 1991 [DOI] [PubMed] [Google Scholar]
- Nandi D, Jenkinson N, Stein J, Aziz T. The pedunculopontine nucleus in Parkinson's disease: primate studies. Br J Neurosurg 22, Suppl 1: S4–S8, 2008 [DOI] [PubMed] [Google Scholar]
- Norton AB, Jo YS, Clark EW, Taylor CA, Mizumori SJ. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci 33: 1885–1896, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Huber D, Svoboda K. Reverse engineering the mouse brain. Nature 461: 923–929, 2009 [DOI] [PubMed] [Google Scholar]
- Okada K, Kobayashi Y. Characterization of oculomotor and visual activities in the primate pedunculopontine tegmental nucleus during visually guided saccade tasks. Eur J Neurosci 30: 2211–2223, 2009 [DOI] [PubMed] [Google Scholar]
- Okada K, Nakamura K, Kobayashi Y. A neural correlate of predicted and actual reward-value information in monkey pedunculopontine tegmental and dorsal raphe nucleus during saccade tasks. Neural Plast 2011: 579840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y. Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci 29: 4858–4870, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci 25: 4725–4732, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GC, Inglis WL, Winn P. A comparison of behaviour following stimulation of the anterior substantia nigra by direct cholinergic agonists and anticholinesterases. Psychopharmacology 112: 242–248, 1993 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates (compact 2nd ed.). Amsterdam: Elsevier Academic, 2004 [Google Scholar]
- Peppe A, Pierantozzi M, Chiavalon C, Marchetti F, Caltagirone C, Musicco M, Stanzione P, Stefani A. Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: effects on gait in Parkinson's disease. Gait Posture 32: 512–518, 2010 [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron 51: 351–358, 2006 [DOI] [PubMed] [Google Scholar]
- Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 269: 315–341, 1988 [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol 259: 483–528, 1987 [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Integrating orbitofrontal cortex into prefrontal theory: common processing themes across species and subdivisions. Learn Mem 8: 134–147, 2001 [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci 1003: 36–52, 2003 [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O'Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature 479: 397–400, 2011 [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Isa T. Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus. Eur J Neurosci 24: 3096–3108, 2006 [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol 9: 698–707, 1999 [DOI] [PubMed] [Google Scholar]
- Steckler T, Inglis W, Winn P, Sahgal A. The pedunculopontine tegmental nucleus: a role in cognitive processes? Brain Res Brain Res Rev 19: 298–318, 1994 [DOI] [PubMed] [Google Scholar]
- Steiniger B, Kretschmer BD. Effects of ibotenate pedunculopontine tegmental nucleus lesions on exploratory behaviour in the open field. Behav Brain Res 151: 17–23, 2004 [DOI] [PubMed] [Google Scholar]
- Steiniger B, Kretschmer BD. Glutamate and GABA modulate dopamine in the pedunculopontine tegmental nucleus. Exp Brain Res 149: 422–430, 2003 [DOI] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol 321: 515–543, 1992 [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: dual-output pathways conserved throughout vertebrate phylogeny. J Comp Neurol 520: 2957–2973, 2012 [DOI] [PubMed] [Google Scholar]
- Stubblefield EA, Costabile JD, Felsen G. Optogenetic investigation of the role of the superior colliculus in orienting movements. Behav Brain Res 255C: 55–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, McKinney M. Nitric oxide synthase gene expression in cholinergic neurons in the rat brain examined by combined immunocytochemistry and in situ hybridization histochemistry. Brain Res Mol Brain Res 23: 111–125, 1994 [DOI] [PubMed] [Google Scholar]
- Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron 66: 449–460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, Brittain JS, Coyne TJ, Silburn PA, Aziz TZ, Kerr G, Brown P. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain 135: 1446–1454, 2012a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, Silburn PA. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 69: 1248–1253, 2011a [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, Silburn PA, Aziz TZ, Brown P. A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain 134: 2085–2095, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Foltynie T, Limousin P, Bogdanovic M, Zrinzo L, Green AL, Aziz TZ, Brown P. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 135: 148–160, 2012b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Silburn PA, Brooker H, Coyne TJ, Khan S, Gill SS, Aziz TZ, Brown P. The impact of low-frequency stimulation of the pedunculopontine nucleus region on reaction time in parkinsonism. J Neurol Neurosurg Psychiatry 81: 1099–1104, 2010 [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229, 2003 [DOI] [PubMed] [Google Scholar]
- Vincent SR, Satoh K, Armstrong DM, Fibiger HC. NADPH-diaphorase: a selective histochemical marker for the cholinergic neurons of the pontine reticular formation. Neurosci Lett 43: 31–36, 1983 [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci 18: 2069–2081, 2003 [DOI] [PubMed] [Google Scholar]
- Wilcox RA, Cole MH, Wong D, Coyne T, Silburn P, Kerr G. Pedunculopontine nucleus deep brain stimulation produces sustained improvement in primary progressive freezing of gait. J Neurol Neurosurg Psychiatry 82: 1256–1259, 2011 [DOI] [PubMed] [Google Scholar]
- Winn P. Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat Disord 14, Suppl 2: S194–S198, 2008 [DOI] [PubMed] [Google Scholar]
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci 248: 234–250, 2006 [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37: 475–524, 1991 [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull 16: 603–637, 1986 [DOI] [PubMed] [Google Scholar]