Abstract
d-serine is present in the vertebrate retina and serves as a coagonist for the N-methyl-d-aspartate (NMDA) receptors of ganglion cells. Although the enzyme d-amino acid oxidase (DAO) has been implicated as a pathway for d-serine degradation, its role in the retina has not been established. In this study, we investigated the role of DAO in regulating d-serine levels using a mutant mouse line deficient in DAO (ddY/DAO−) and compared these results with their wild-type counterparts (ddY/DAO+). Our results show that DAO is functionally present in the mouse retina and normally serves to reduce the background levels of d-serine. The enzymatic activity of DAO was restricted to the inner plexiform layer as determined by histochemical analysis. Using capillary electrophoresis, we showed that mutant mice had much higher levels of d-serine. Whole cell recordings from identified retinal ganglion cells demonstrated that DAO-deficient animals had light-evoked synaptic activity strongly biased toward a high NMDA-to-AMPA receptor ratio. In contrast, recordings from wild-type ganglion cells showed a more balanced ratio between the two receptor subclasses. Immunostaining for AMPA and NMDA receptors was carried out to compare the two receptor ratios by quantitative immunofluorescence. These studies revealed that the mutant mouse had a significantly higher representation of NMDA receptors compared with the wild-type controls. We conclude that 1) DAO is an important regulatory enzyme and normally functions to reduce d-serine levels in the retina, and 2) d-serine levels play a role in the expression of NMDA receptors and the NMDA-to-AMPA receptor ratio.
Keywords: d-amino acid oxidase, d-serine, glutamate receptors, mouse, retinal ganglion cells
n-methyl-d-aspartate (NMDA) receptors are the only subclass of glutamate receptors that require the presence of a coagonist in addition to glutamate to gate the ion channel properly. Originally, this coagonist was thought to be glycine (Johnson and Ascher 1987), but additional studies have now emphasized the importance of d-serine as a coagonist (Hashimoto et al. 1992a,b), and recent studies in the brain have suggested that both coagonists may serve at synaptic (d-serine) and nonsynaptic (glycine) sites (Papouin et al. 2012). In the vertebrate retina, NMDA receptors are found on retinal ganglion cells (RGCs) and a subset of amacrine cells (Dixon and Copenhagen 1992; Massey and Miller 1990; Mittman et al. 1990; Slaughter and Miller 1983) as well as in horizontal cells of catfish and carp (Shen et al. 2006).
Shortly after the mechanism of d-serine synthesis was established with the discovery of serine racemase (Wolosker et al. 1999), studies in the retina revealed the presence of serine racemase in Müller cells and astrocytes. Evidence also showed that d-serine served as an NMDA receptor coagonist and that since the addition of exogenous d-serine enhanced light-evoked NMDA receptor currents in whole cell recordings of ganglion cells, the coagonist sites were not saturated in the intact retina (Stevens et al. 2003), as had been previously reported in retinal slice preparations (Gottesman and Miller 1992; Taylor et al. 1995). This observation radically altered our understanding of the relationship between the two agonists necessary for gating the NMDA receptor ion channel, glutamate and either d-serine or glycine. When glycine was the presumed coagonist, it was common to think that the abundance of glycine in the extracellular space meant that NMDA receptor activation was based solely on the spatiotemporal profile of glutamate in the extracellular partition, together with the spatial distribution of NMDA receptors. However, the observation that during light stimulation only a fraction of the potentially activated NMDA receptors are supplied with coagonist means that under at least some stimulus parameters the limiting elements are more complicated: the spatiotemporal profile of the coagonist can also determine the maximum activation profile of NMDA receptors, not just the distribution of glutamate. Since there is now indirect evidence for a light-evoked release of d-serine (Sullivan et al. 2011; Sullivan and Miller 2010, 2012), dynamic spatiotemporal modulation of this coagonist could play a direct and more dynamic role in modulating the pool of NMDA receptors activated by light. Thus it is essential to understand further how NMDA receptor coagonists are regulated in the retina and how such regulation might control the functional expression of NMDA receptors.
In addition to the d-serine-synthesizing pathway served by serine racemase, a potential degradation pathway through d-amino acid oxidase (DAO) was described in the brain and amphibian retina (Beard et al. 1988; Neims et al. 1966; St Jules et al. 1992), but the functional role of this enzyme in the regulation of d-serine in the retina has not been established. DAO expression is inversely related to d-serine levels and to activity at NMDA receptors in the brain. Whereas low DAO levels in the rat cortex corresponded with high d-serine levels, d-serine levels in cerebellar Bergmann glia are high early in development and peak around P12–14, at which point DAO expression increases and d-serine levels fall by 97% in the adult (Schell et al. 1997).
A unique strain of mice that lacks DAO activity due to a point mutation (G181R) was identified (Konno and Yasumura 1983). This ddY/DAO− mouse has increased levels of d-serine in serum and brain samples with no obvious compensatory changes in d-serine-associated proteins. Several differences were reported between the mutant and wild-type mice, including enhanced hippocampal LTP and improved spatial learning in the mutant animals (Almond et al. 2006). These results suggest that d-serine levels may be regulated in part by DAO activity and that increased d-serine may affect NMDA receptor function. However, the actions of DAO in regulating d-serine levels in the retina have not been explored.
In this study, we aimed to characterize the effects that the ddY/DAO− mutation has on the functional organization of the retina and thereby examine the importance of DAO in the regulation and availability of d-serine. We used an assay of oxidase activity to compare DAO activity in wild-type and mutant retinal sections. Capillary electrophoresis was used to measure d-serine levels in tissue homogenates and in the extracellular space. We used whole cell recordings from ganglion cells in wild-type and mutant mice to assess the role of d-serine and to compare the occupancy level of NMDA receptor coagonist sites in ganglion cells. Our observations of these two mouse strains compelled us to compare the NMDA-to-AMPA receptor ratios of the light-evoked responses as well as the expression of NMDA and AMPA receptor subunits through Western blots and quantitative immunofluorescent labeling of retinal sections. These results reveal a powerful regulatory influence of background d-serine levels on the ratio of light-evoked NMDA to AMPA receptors with far more dominance of NMDA receptors in the mutant DAO animals compared with the more balanced ratio observed in wild-type retinas.
METHODS
Experiments were done in accordance with Association for Research in Vision and Ophthalmology (ARVO) guidelines and animal protocols approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Mice were obtained from the Konno laboratory and bred in-house at the University of Minnesota. Mice were killed using an overdose of Nembutal (100 mg/kg).
Electrophysiology.
Whole cell voltage- and current-clamp recordings were made from RGCs using an inverted retina-eyecup preparation. Following the removal of the front of the eye and lens, the eyecup was treated with an enzyme solution containing collagenase and hyaluronidase (120 and 465 U/ml, respectively; Worthington Biochemical, Lakewood, NJ). After rinsing, the eyes were inverted over a dome in a custom perfusion chamber and continuously perfused with a 95% O2-5% CO2-bubbled Ringer solution containing, in mM, 111 NaCl, 32 NaHCO3, 3 KCl, 2 CaCl2, 1 MgSO4, 0.5 NaH2PO4, and 15 glucose at a rate ∼2.5 ml/min. Retinas were viewed using infrared optics, and because the mice were albinos we were able to visualize retinal cells by projecting the light through the chamber from below. Patch electrodes were pulled on a commercial electrode puller (Sutter P-97) with tip resistances of 4–10 MΩ; electrodes were filled with a solution consisting of, in mM, 128 KCH3SO4, 5 NaCH3SO4, 2 MgCl2, 5 EGTA, 5 HEPES, 1 glutathione, 2 ATP-Mg2+, 0.2 GTP(3Na), and Alexa Fluor 568 or 488 dye to identify the cell type. With electrodes in place, after breaking the gigaseal, the spiking pattern of each cell was recorded in normal Ringer with Mg2+ (1.0 mM) present. After these recordings were obtained, TTX and strychnine were introduced with a Mg2+-free perfusion environment. All cells in this study displayed an ON response (21 transient ON, 3 sustained ON, and 14 ON/OFF). No difference was seen in ON vs. OFF responses, but due to lower numbers of OFF responses only ON responses are included in these results. Current-clamp recordings were made in normal Ringer solution, and voltage-clamp recordings were carried out in a magnesium-free Ringer solution with TTX (1 μM) and strychnine (10 μM). Voltage-clamp studies were carried out after adjusting the standing current to 0 (average membrane potential = −64.7 ± 0.9 mV, n = 38) using an Axoclamp 700A amplifier with a 10-kHz low-pass Bessel filter at a sampling frequency of 4–10 kHz, digitized with a Digidata 1320, and recorded in pClamp 9.0 (all Molecular Devices). Series resistance was uncompensated. Fluorescence microscopy was used to visualize ganglion cell axons, and only axon-bearing cells are included in the results. Reported data are not corrected for a liquid junction potential of −8.3 mV that was calculated with pClamp. All experiments were performed at room temperature (∼22°C).
Light stimulation was provided by a computer-controlled LCD projector system using a tungsten-halogen light source (Burkhardt et al. 1998). The diameter of the light stimulus was 200 μm. The intensity was adjusted to elicit a less than maximal response but was ∼1,900 cd/m2. The duration of light stimulation was 2 or 4 s with an interstimulus interval of 20 s. Responses to the onset of the stimulus were evaluated as peak amplitude and as total charge, which was determined by integrating the light-evoked currents.
DAO activity assay.
Freshly dissected eyecups were immersion-fixed in 2% paraformaldehyde for 30 min, washed in PBS, and cryoprotected in a sucrose series. Fixed eyecups were embedded in 5% agarose, and transverse retinal sections (100 μm) were cut on a vibratome. Sections were incubated 20 h in a reaction mixture that included the following with a final volume of 500 μl: 0.05 M Tris·HCl, pH 7.6, containing 0.1% horseradish peroxidase (Type VI; HRP), 0.005% 3–3′ diaminobenzidine (DAB), 0.065% sodium azide, 0.6% nickel ammonium sulfate, and 22 mM d-proline. Reactions were stopped with two rinses of 0.1 M PBS. Sections were mounted on polylysine-coated slides, dehydrated through an ethanol-xylene series, and coverslipped. Bright- and dark-field images were acquired on an Olympus Vanox microscope equipped with a SPOT camera (SPOT Imaging Solutions, Sterling Heights, MI). Images were taken with identical settings and edited simultaneously in Adobe Photoshop.
Immunofluorescence.
Eyes were dissected by cutting away the front half of the eye and removing the lens to yield an eyecup. Eyecups prepared from age-matched mutant and wild-type mice (7 of each genotype) were fixed (15 min in 4% wt/vol paraformaldehyde) and cryoprotected in parallel and then embedded and cryosectioned together in the same block. Slides were air dried and stored at −80°C until use. After thawing, sections were immunolabeled similar to previously described methods (Morgans et al. 2006). Primary antibodies were mixed together at the dilutions 1:200 anti-GluN2A/B (host: rabbit; Chemicon) and 1:150 anti-GluA2 (host: mouse; Millipore) and applied similarly to all retina sections for 1–2 h at room temperature. Secondary antibodies, anti-rabbit IgG coupled to CY3 (1:1,000; Jackson ImmunoResearch) and anti-mouse IgG coupled to Alexa Fluor 488 (1:1,000; Invitrogen), were applied for 1 h at room temperature. Images were collected with an Olympus FluoView 1000 confocal microscope using a ×40 oil-immersion lens. Identical settings and conditions were used for collecting images from wild-type and mutant sections. Image optimization (contrast and brightness) was performed identically for all wild-type and mutant images. The Color Histogram tool in ImageJ was used to determine the ratio of red to green pixels in the inner plexiform layer (IPL) of images. Ratios were determined from multiple images collected from at least three animals for each genotype; averages and standard errors were calculated from DAO wild-type (n = 7) and DAO mutant (n = 7) mice.
Western blot.
Mouse retinal proteins were separated by SDS-PAGE on NuPAGE Bis-Tris 4–12% gradient gels (Invitrogen Life Technologies), transferred to polyvinylidene difluoride membrane, and probed with antibodies against GluA2 (1:200) and GluN2A/B (1:1,000). Secondary antibodies were coupled to infrared fluorescent dyes, and immunoreactivity was detected with an Odyssey imaging system (LI-COR Biosciences).
Capillary electrophoresis (CE) was used to measure d-serine in the retina. Previously described protocols were used to determine d-serine levels in homogenized tissue (Sullivan et al. 2011) or accumulation of extracellular d-serine (Sullivan and Miller 2010). For tissue measurements, retinas were homogenized in 0.6 M perchloric acid (PCA) using a sonicator probe. KOH was added to neutralize the PCA. The mixture was spun down in a tabletop centrifuge, and the supernatant was removed. The remaining pellet was resuspended in 2 M NaOH for protein determination using a Pierce Biotechnology (Rockford, IL) bicinchoninic acid assay. For extracellular preparations, two retinas were incubated in 100 μl of Ringer solution in an oxygenated, humidified chamber for 50 min. The media were then collected, and the retinas were processed for protein determination. Amino acids in the supernatant or incubation media were fluorescently derivatized at 60°C for 15 min with 4-fluoro-7-nitrobenzofurazan (NBD-F; Molecular Probes, Eugene, OR). CE separations were performed on a commercial CE instrument (MDQ; Beckman Coulter, Fullerton, CA) with laser-induced fluorescence detection in a (2-hydroxypropyl)-β-cyclodextrin buffer at 15 kV (70 μA). Fluorescent signals were detected by a photomultiplier tube and digitally plotted as the magnitude of the fluorescence signal vs. time (electropherogram). For all determinations, aminoadipic acid (aaa) was added as an internal standard used to compare different electropherograms. To determine the d-serine concentration in each sample, the d-serine peaks were compared with the peaks formed by a set of d-serine standards. Each retina was processed for protein determination, and the result was expressed as nanomoles per gram protein. Data were displayed and peak integration was performed using 32 Karat software (Beckman Coulter).
d-serine deaminase (DsdA) was provided by Dr. Herman Wolosker (Technion-Israel Institute of Technology, Haifa, Israel). All other chemicals and agents used in these experiments were purchased from Sigma Chemical (St. Louis, MO) except KCH3SO4 (Pfaltz & Bauer) and TTX, d,l-2-amino-phosphonoheptanoate (AP7), and 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) from Tocris Bioscience (Ellisville, MO).
All results are reported as means ± SE. One-directional t-tests were used for comparisons. A P value <0.05 was considered significant.
RESULTS
Retinas from ddY/DAO− mutant mice have increased levels of d-serine.
Retinal slices from fixed eyecups were used to measure DAO activity in wild-type and mutant retinas (Horiike et al. 1985). Figure 1A shows contrast-reversed, dark-field images of sections of mouse retinas that had been assayed for oxidase activity. Following exposure to d-proline, an HRP-DAB reaction product was visible in the IPL of the wild-type retina but not in the retinal slice from the ddY/DAO− mutant animal. The lack of product in the mutant retinas indicated an absence of endogenous DAO activity that, in the wild-type retinas, created hydrogen peroxide as a product of the oxidation of d-proline. The IPL is a fitting location for DAO activity since the dendrites of the RGCs that contain NMDA receptors receive input from the bipolar cells throughout this region. The middle of Fig. 1A illustrates the basic structure of the retina, with the main, three-cell neuronal circuit colored in black. At the top, we see the photoreceptor processes (PR) meeting the bipolar cell (BC) dendrites in the outer plexiform layer (OPL). The bipolar cells then synapse with ganglion cells (RGC) throughout the IPL, with stratifications based on ON vs. OFF properties of the cells. In this case, we have illustrated the ON pathway with its characteristic dendritic arborization in the region close to the ganglion cell layer. Main interneurons of the retina can be seen in gray, horizontal cell processes at the OPL and amacrine cells in the IPL.
Fig. 1.
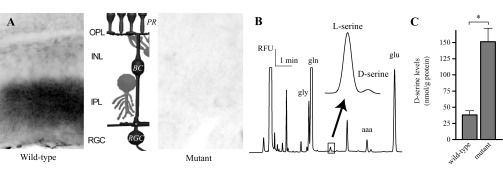
A lack of d-amino acid oxidase (DAO) activity results in an increase in d-serine. A: a horseradish peroxidase (HRP)-3–3′ diaminobenzidine (DAB) reaction product, the end product in a DAO activity assay, was visible in the inner plexiform layer (IPL) of wild-type retina but not the ddY/DAO− mutant retina. The middle shows a schematic of the basic retinal circuitry. The main 3-neuron circuit is presented in black: with light response flowing from photoreceptor, PR; to bipolar cell, BC; to retinal ganglion cell, RGC. OPL, outer plexiform layer; INL, inner nuclear layer. B: CE, capillary electrophoresis. An electropherogram, time vs. relative fluorescent units (RFU) showing the separation of l- and d-serine from homogenized mouse retina. Also noted: gly, glycine; gln, glutamine; aaa, aminoadipic acid; glu, glutamate. C: cumulative results of d-serine levels measured from homogenized wild-type and mutant retinas normalized to protein levels. *P < 0.05 between genotypes.
To determine directly the effect of the DAO mutation on retinal d-serine, we used CE to measure the d-serine levels of retinal tissue homogenates from the mutant and wild-type retinas. An example trace from one CE experiment is presented in Fig. 1B to show how the separation of the d- and l-enantiomers is obtained, with a closeup of the region of interest showing the separation of d- and l-serine. An aaa standard was added to each sample to enable normalization of d-serine peaks across trials. By calibrating the integrated peak values against those of a series of d-serine standards, we were able to determine the concentration of d-serine and demonstrate that it was increased in the mutant retinas. Cumulative results from six sets of experiments showed that d-serine levels were roughly four times higher in the mutant retinas compared with those in the wild-type (Fig. 1C; mutant = 151.3 ± 20.9 nmol/g protein, wild-type 38.1 ± 6.5 nmol/g protein, n = 6; P = 0.0002).
NMDA receptor coagonist sites are saturated in RGCs of ddY/DAO− mutant mice.
We examined what impact the lack of DAO activity and the subsequent increase in d-serine levels had on light-evoked RGC activity. Figure 2A shows whole cell, voltage-clamp responses of RGCs from a ddY/DAO+ wild-type and a ddY/DAO− mutant mouse. The light stimulus timing is displayed as a line above the traces. The addition of a saturating dose of exogenous d-serine (100 μM, gray trace) to the bathing solution enhanced the light response of the wild-type ganglion cell compared with the response in control Ringer (black trace). In contrast, the addition of d-serine failed to augment the light response in the mutant. Cumulative results from these experiments (Fig. 2C) show that in ganglion cells from wild-type retinas, d-serine significantly enhanced the light responses with the total charge increased by 24.5 ± 3.8% (P = 0.0001) and the peak of the response by 49.2 ± 11.3% (P = 0.001; n = 9) compared with control. In the mutant mouse, d-serine failed to potentiate the responses of ganglion cells, which showed insignificant decreases of 2.4 ± 3.7% of the total charge (P = 0.7) and 3.6 ± 2.3% of the peak amplitude (P = 0.9; n = 7) in the presence of exogenous d-serine.
Fig. 2.
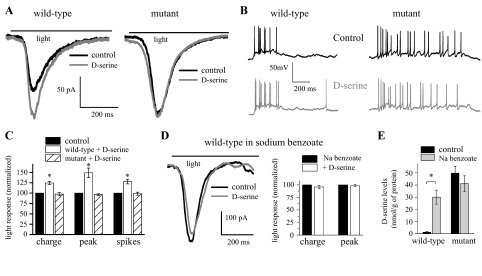
DAO activity regulates N-methyl-d-aspartate (NMDA) receptor coagonist site occupancy. A: bath application of d-serine (100 μM) potentiated the light response of a voltage-clamped RGC from a wild-type retina but did not affect the response in a mutant retina. B: current-clamp recordings of light responses from RGCs of a wild-type (left) and mutant (right) retinas. Normal, control Ringer is shown in black, and the addition of 100 μM d-serine in gray. C: cumulative results recorded following the application of d-serine shown as total charge, peak amplitude, and spikes. D: bath application of d-serine did not alter the light response of a voltage-clamped RGC from a wild-type retina incubated with sodium (Na) benzoate. Normalized results of RGCs from wild-type retinas incubated in sodium benzoate (200 μM) with and without d-serine (100 μM). E: cumulative CE results of d-serine levels following 50-min well accumulation from wild-type and mutant retinas in control Ringer and in Ringer with sodium benzoate. *P < 0.05 compared with control.
In our whole cell voltage-clamp experiments, we used a magnesium-free Ringer solution to maximize the NMDA receptor currents as well as TTX and strychnine. To investigate whether the unsaturated state of the NMDA receptors we found in our voltage-clamp experiments was also present under more physiological conditions, we studied light-evoked ganglion cell impulse activity in current-clamp configuration using normal magnesium Ringer (1 mM) without TTX and strychnine. Figure 2B shows example traces from ganglion cells obtained in normal Ringer. In response to the light stimulus, the wild-type cell fired 8 action potentials. Following the addition of d-serine (100 μM), the number of spikes resulting from an identical stimulus increased to 12. In a cell from a mutant mouse, 18 spikes were recorded at the onset of the 2-s light stimulus in normal Ringer, and 17 in the presence of d-serine. On average, the number of action potentials recorded from wild-type ganglion cells in the presence of d-serine was 28.0 ± 5.8% greater compared with responses in normal Ringer (Fig. 2C; P = 0.004; n = 6), whereas d-serine had no effect on spikes in the mutant mice (98.6 ± 3.6% of control, n = 6). Thus the enhanced excitability of wild-type RGCs in the presence of d-serine was not created by the absence of Mg2+ in the bathing solution.
In an attempt to mimic the effects of the DAO mutation, we investigated the impact of the DAO inhibitor sodium benzoate (Moses et al. 1996). Previous unpublished results from our laboratory demonstrated that the bath application of sodium benzoate for up to 30 min had no effect on extracellular recordings of light responses. We therefore increased the exposure time of the retinas by incubating them in sodium benzoate (200 μM) for 2 h before beginning our experiments. Figure 2D illustrates how sodium benzoate incubation eliminated the ability of d-serine to enhance the light-evoked responses in a wild-type retina, resulting in traces similar to those seen in the mutant (Fig. 2A). Unlike the results seen in wild-types above (Fig. 2C), the addition of d-serine did not augment the responses of wild-type retinas incubated with sodium benzoate (decreases of 4.2 ± 3.3%, P = 0.9 for the total charge and of 1.3 ± 1.9%, P = 0.7 for the peak amplitude, n = 5; Fig. 2D). The responses of RGCs from mutant mice treated with sodium benzoate remained saturated (data not shown). Using CE, we measured the effect that sodium benzoate had on the extracellular accumulation of d-serine produced by incubating retinas in small wells. Following 2 h in sodium benzoate, the amount of d-serine measured in the well solution after a 50-min additional incubation was greatly enhanced (∼10 times) in samples from the wild-type retinas but not affected in those from mutants (Fig. 2E). This suggests that DAO is actively degrading d-serine in the wild-type mice and maintains d-serine levels below that needed to saturate the NMDA receptor coagonist sites.
d-serine is an essential ligand for NMDA receptors in the mouse retina.
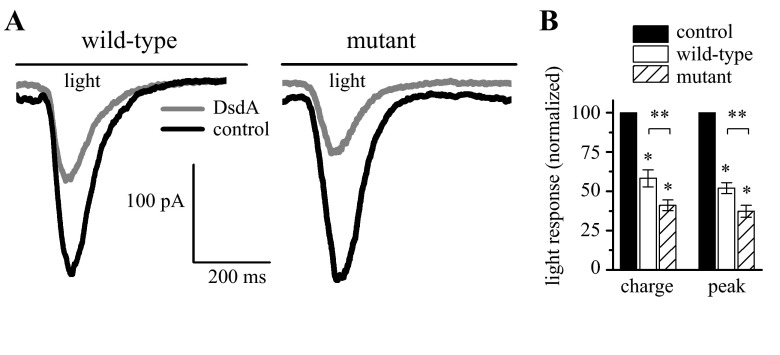
A previous study of salamander RGCs used the specific d-serine-degrading enzyme DsdA to demonstrate that d-serine was essential for normal light-evoked activity (Gustafson et al. 2007). Similarly, we used DsdA to determine the actions of endogenous d-serine on light-evoked, whole cell responses in the wild-type and mutant mice used in this study. Figure 3A shows the results from experiments in which whole cell, voltage-clamp recordings were made from RGCs. The application of DsdA (gray trace) resulted in a large decrease in the current response compared with the control (black) in both the wild-type and mutant retinas. The cumulative results of RGC responses from six wild-type and seven mutant retinas are presented in Fig. 3B. Wild-type responses decreased significantly following DsdA application with the total charge reduced by 41.7 ± 5.4% and the peak by 49.3 ± 6.7% (P = 0.01; n = 6). A similar but larger effect was seen in the mutant retinas where the total charge was reduced by 58.8 ± 3.4% and the peak responses were reduced by 62.6 ± 3.8% in DsdA (P = 0.005 charge, 0.0008 peak; n = 7). The decreases in the mutant retina responses were significantly larger than what we observed in the wild-type (P = 0.009 both charge and peak). DsdA had no effect on the light responses when NMDA receptor currents were blocked with AP7 (data not shown). These attenuated responses of RGCs following DsdA application illustrate that d-serine is essential for normal light-evoked responses in these mice.
Fig. 3.
d-serine degradation reduces RGC light responses. A: degradation of d-serine by d-serine deaminase (DsdA) reduced the ganglion cell light responses from a wild-type retina and from a mutant retina. B: cumulative results of total charge and peak amplitude show significant differences between control and DsdA treated retinas (*) as well as between similarly treated wild-type and mutant animals (**).
NMDA receptor component of the light response is greater in ddY/DAO− mutant mice.
As noted above, DsdA had no effect on light responses when the NMDA receptor component was blocked by bath application of AP7. What we did notice when performing these experiments was that the effect of AP7 was noticeably larger in the mutant vs. the wild-type retinas. We therefore analyzed the NMDA receptor contribution to the light response by measuring the response of ganglion cells in the presence of the NMDA receptor antagonist AP7 (100 μM). In Fig. 4A, the light responses of cells from a ddY/DAO+ wild-type and a ddY/DAO− mutant retina are presented. On the left, the addition of AP7 to the bathing media (gray) resulted in a measurable decrease in the light-evoked ganglion cell current recorded from a wild-type retina compared with the control response (black). The light response returned to control levels after a short wash in control Ringer (data not shown). A larger decrease was seen in a recording of a cell from a mutant retina. We again used current-clamp recordings of ganglion cells to verify that NMDA receptor blockade had a similar effect in normal Ringer. Spike traces in Fig. 4B show a cell from a wild-type retina that fired 8 spikes in control Ringer and only 6 when AP7 was added, whereas the response of a cell in a mutant retina was decreased from 10 to 4 spikes. Blocking NMDA receptor currents with AP7 had a significant effect on the responses in all RGCs recorded (Fig. 4C). Light responses decreased by about one-third in wild-type cells following NMDA receptor block (33.4 ± 6.1% charge, 37.0 ± 6.2% peak; P < 0.001; n = 8). In mutant cells, the decreased response in AP7 was far greater (decrease of 78.8 ± 3.3% charge and 79.7 ± 3.8% peak; P < 0.001; n = 7). This decrease measured between the mutant and wild-type animals in whole cell responses was significant (wild-type vs. mutant: charge P = 0.005 and peak P = 0.007). On average, AP7 application decreased RGC spike count by 39.4 ± 5.8% in wild-type and 50.4 ± 4.6% in mutant retinas (wild-type P = 0.002, n = 6, mutant P < 0.001, n = 5; the difference between wild-type and mutant spiking activity was not significant, P = 0.18).
Fig. 4.
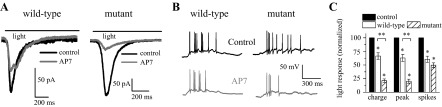
NMDA receptors have a significant role in RGC light responses. A: the application of 100 μM d,l-2-amino-phosphonoheptanoate (AP7) reduced the light responses recorded in voltage-clamp recordings from RGCs in wild-type and mutant retinas. B: current-clamp recordings of light responses from RGCs of wild-type and mutant retinas in normal Ringer and with the addition of AP7. C: light responses, normalized to control, recorded following the application of AP7 shown as total charge, peak amplitude, and spikes. Significance: compared with control (*) and between wild-type and mutant (**).
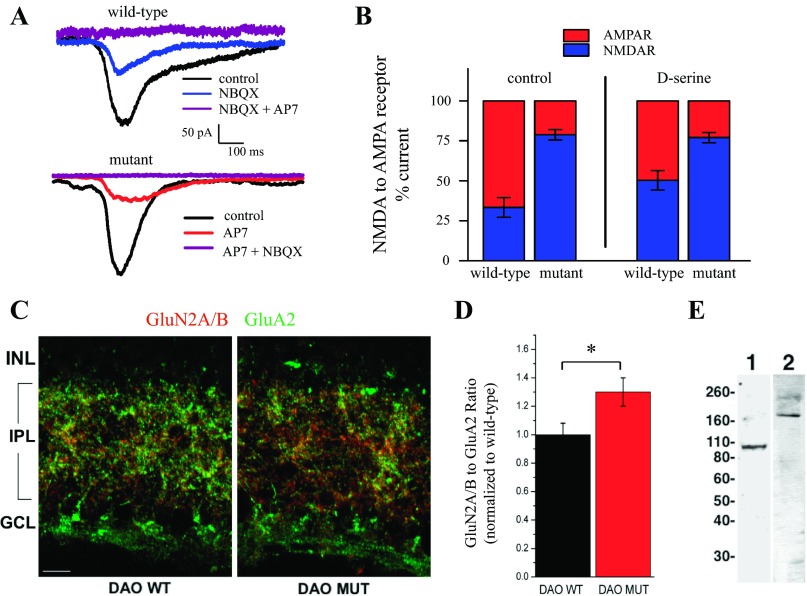
The NMDA-to-AMPA receptor current ratio is altered in ddY/DAO− mutant mice.
The difference observed in the presence of AP7 in the mutant vs. wild-type retinas suggested an alteration in the NMDA receptor activity in these two mice. One possible explanation for the difference in the NMDA receptor component of the light response may simply have been the availability of coagonist. The responses measured in the mutant retinas were under a saturating dose of d-serine (since exogenous d-serine application did not augment the responses), whereas those in the wild-type retinas were not. Given that the light-evoked currents in both ddY/DAO+ and ddY/DAO− mice were entirely blocked when AP7 and the AMPA receptor antagonist NBQX (10 μM) were added in combination (Fig. 5A), we used the resulting NMDA-to-AMPA receptor current ratios to examine to what degree the difference in NMDA receptor activity between the wild-type and mutant mice was the result of coagonist availability. On the left of Fig. 5B, the ratios in control conditions show the same twofold increase in NMDA receptor currents measured in wild-type and mutant mice presented above in Fig. 4 (percentage of current mediated by AMPA receptors in red, by NMDA receptors in blue). Since the coagonist sites of the NMDA receptors in the wild-type retinas were not saturated in the wild-type animals (Fig. 2A), we also examined the ratio of the responses in a saturating dose of d-serine (100 μM; right 2 bars of Fig. 5B) to determine whether the difference in NMDA receptor contribution was simply the result of coagonist availability. Saturation of the coagonist sites with exogenous d-serine slightly changed the fraction of the light response contributed by NMDA receptors in wild-type retinas to 50.3 ± 5.2%, but this change did not bring the wild-type responses to the same level we observed in the mutant ganglion cells. These results suggest that there had been a permanent change in the NMDA-to-AMPA receptor ratios in ganglion cells of the mutant retinas that cannot be explained by whether the coagonist sites of NMDA receptors are saturated.
Fig. 5.
NMDA receptor component of the light response is greater in ddY/DAO− mice. A: the combination of AP7 and 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) completely blocks current responses of RGCs in wild-type and mutant mice. B: the percentage of the current mediated by NMDA (blue) and AMPA (red) receptors (R) under different conditions. The left side represents the percentage of currents recorded in control Ringer for both wild-type and mutant mice. The right side compares the percentages in relation to a Ringer solution containing 100 μM d-serine to maximize the NMDA receptor contribution. C: retina cryosections from DAO wild-type (DAO WT) and DAO mutant (DAO MUT) mice were double-labeled by immunofluorescence for GluN2A/B (red) and GluA2 (green) receptor subunits. GCL, ganglion cell layer. The scale bar in C represents 10 μm. D: changes in the ratio of GluN2A/B to GluA2 immunofluorescence of mutant animals compared with their wild-type counterparts (DAO WT, n = 7; DAO MUT, n = 7). *P < 0.05 between genotypes. E: Western blots of mouse retinal proteins probed with antibodies against GluA2 (lane 1) and GluN2A/B (lane 2). The molecular weights (in kilodaltons) and position in the gel of protein standards are indicated to the left.
The possibility that a permanent change in NMDA receptor availability had taken place in the mutant mouse prompted us to use immunostaining to examine the expression of NMDA and AMPA receptors in these mice and to quantify any differences we could detect in the labeling pattern within the IPL. We compared the labeling of two glutamate receptor antibodies in fixed retinal cryosections to examine whether receptor expression could be partly responsible for this difference in NMDA and AMPA receptor currents. Retina sections from ddY/DAO+ and ddY/DAO− mice were double-labeled with one antibody recognizing both the GluN2A and GluN2B subunits of the NMDA receptor (red, Fig. 5D) and the other labeling the GluA2 subunit of AMPA receptors (green, Fig. 5D). Although it might seem relevant to do quantitative work using Western blots, the GluA2 subunits are highly expressed in the outer retina (Haverkamp et al. 2000), where NMDA receptors are negligible (Fletcher et al. 2000), thus making such measurements difficult to interpret. So, to isolate the expression of AMPA and NMDA receptors that contribute directly to RGC activation, it was essential to limit the quantitative comparisons of subunits to the IPL. In doing this, the ratio of intensities corresponding to GluN2A/B vs. GluA2 subunits was found to be ∼30% greater in the mutant retinas compared with those of the wild-type (Fig. 5D). The Western blots of retinas shown in Fig. 5E used the same antibodies for protein detection and show the appropriate bands for GluA2 in lane 1 and for GluN2A/B in lane 2.
DISCUSSION
The present study has demonstrated that the enzymatic activity of DAO is found in the IPL of the mouse retina, a region rich in NMDA receptors, whereas the oxidase activity was undetectable in the ddY/DAO− mutant retina. Since d-serine levels are about four times higher in the mutant mice, our observations provide the first evidence for a functional role of DAO in regulating d-serine in the retina. Furthermore, it appears that the regulatory role of DAO is carried out in such a way that the coagonist sites of ganglion cell NMDA receptors are not fully occupied, since adding exogenous d-serine enhanced the NMDA currents in wild-type retinas. In contrast, the NMDA receptor currents in the mutant mice were not affected when tested with exogenous d-serine. We know that the synthetic pathway of d-serine through serine racemase plays an important role in regulating d-serine availability. Indeed, in the retina, mice in which serine racemase has been knocked out show reduced levels of d-serine and a corresponding shift toward AMPA receptors as the dominant glutamate receptor subtype that mediates light-evoked synaptic activity in ganglion cells (Sullivan et al. 2011). Furthermore, many elements can regulate d-serine levels through interactions with the serine racemase pathway, including metabotropic glutamate receptor activation influence on PIP2, nitric oxide activity, and the interaction of ephrinBs and Eph receptors (Mustafa et al. 2007, 2009; Zhuang et al. 2010). Our results here suggest that the degradation pathway involving DAO also plays an integral role in d-serine availability and retinal function. Previous studies of DAO in the retina were limited to describing its localization in Müller cells and cone photoreceptors of amphibians (Beard et al. 1988; St Jules et al. 1992). These studies neither investigated nor suggested a role for the enzyme in retinal function, understandably, since they appeared before we knew about the special role that d-serine plays in the retina.
Studies of intact retinas have reported increases in ganglion cell responses when exogenous d-serine was added to the bathing medium (Reed et al. 2009; Stevens et al. 2003). Many of these studies were carried out in reduced external Mg2+ to enhance the NMDA receptor currents. In this study, whole cell recordings of RGCs from the wild-type mice were initially recorded in a normal Ringer environment in the presence of Mg2+; based on the light-evoked impulse pattern, ganglion cell spiking behavior was increased when d-serine was added to the environment. Thus the d-serine enhancement of activity in normal Mg2+ indicates that it does not depend on a Mg2+-free environment to exert its enhancing actions. The increased responses we observed in wild-type mice when exogenous d-serine was added mean that there was insufficient d-serine to match the amount of glutamate being released, indicating that the coagonist site is available for neuromodulation. In the mutant mice, on the other hand, application of exogenous d-serine had no effect on the light-evoked responses, suggesting that coagonist levels are sufficient to saturate these sites. Thus DAO plays an important role in regulating the occupancy of the NMDA receptor coagonist sites of RGCs.
Studies using sodium benzoate to suppress DAO served as an important set of experiments to emphasize further the role of DAO as a regulatory enzyme for controlling the level of d-serine. Sodium benzoate suppresses the activity of DAO (Moses et al. 1996), and when we applied it to the retina we observed a substantial increase in d-serine levels. In fact, sodium benzoate increased the background levels of d-serine enough so that the NMDA receptor coagonist sites were saturated in a manner similar to what we observed in the mutant mouse. It is inescapable that DAO provides regulatory control of d-serine levels by decreasing d-serine below the level required to occupy fully the coagonist sites of ganglion cell NMDA receptors. It would seem, however, that fine tuning of d-serine levels must involve feedback with its synthesis, and the mechanism(s) by which this is accomplished have not been established.
Our studies demonstrate that in the mouse retina, d-serine is a major coagonist subserving ganglion cell NMDA receptors. We used DsdA to reduce endogenous d-serine and revealed that it is an essential player as a coagonist for light-activated NMDA receptors of RGCs. This finding confirms an earlier report that established the same relationship between d-serine and NMDA receptors of ganglion cells in the salamander retina (Gustafson et al. 2007). The application of DsdA in the mouse was comparable with blocking the NMDA receptors with a competitive antagonist such as AP7. This suggests that under the stimulus parameters we used in this study, d-serine plays the dominant role, since blocking NMDA receptors and reducing d-serine through enzymatic degradation gave similar reductions in the light response and applying the NMDA receptor antagonist in the presence of DsdA did not reduce the light response any further. Thus, for the NMDA receptors activated under our stimulus conditions, any role for glycine as a coagonist must be very small, but we cannot eliminate a role for glycine entirely. Our results point out a difference from those shown in a study of slices from rat retinas in which enzymatic degradation of d-serine with bath application of dao reduced the holding current of ON ganglion cells but did not affect evoked responses (Kalbaugh et al. 2009). Although it is difficult to make direct comparisons between the two studies, given the differences in species, enzyme, preparation (slices vs. intact eyecup), and stimulus methods used, the conclusion that glycine plays an important role under certain conditions cannot be overlooked. In fact, our recent report (Sullivan et al. 2011) raised the possibility that glycine might play a role as a coagonist for nonsynaptic NMDA receptors. This interesting possibility deserves further study, especially considering that studies in other regions of the central nervous system have implicated d-serine as a coagonist for synaptic NMDA receptors and glycine as a coagonist for nonsynaptic receptors (Papouin et al. 2012). It is worth emphasizing that all one has to do to have glycine serve as the coagonist for NMDA receptors is to limit the role of the high-affinity GlyT1 uptake system for glycine (Reed et al. 2009; Stevens et al. 2010). This system undoubtedly plays an important role in these mice since earlier reports showed that expression of the GlyT1 transporter protein was not altered in the brain of these ddY/DAO− mice (Almond et al. 2006) and that the well accumulation of glycine, although vastly higher than that of d-serine, was similar in both the wild-type and mutant animals (Sullivan and Miller 2010).
The exposure to chronically high d-serine in the mutant mice had an effect on the expression of NMDA receptors. As expected, the NMDA receptor antagonist AP7 decreased light-evoked responses of ganglion cells. However, the decrease observed in mutant ddY/DAO− mice was far greater than that in the ddY/DAO+ wild-type. In the wild-type cells, only one-third of the light-evoked current was carried by NMDA receptors, whereas more than three-fourths of the current was NMDA receptor-mediated in the mutant mice. Since the NMDA receptors of the mutant mice were presented with a saturating dose of coagonist, it was possible that the difference in NMDA receptor currents in these two mice strains was simply the result of insufficient occupancy of the NMDA coagonist sites in the wild-type retinas. However, when we added a saturating dose of d-serine to saturate the coagonist sites, although it enhanced the NMDA receptor response, the difference between the mutant and wild-type was still substantial (∼50% NMDA receptor current in the wild-type but >75% in the mutant). These results argue that the synaptic NMDA receptors have become more dominant in the mutant compared with the wild-type retinas. We found strong support for this conclusion when we evaluated the immunostaining pattern for GluA2 AMPA receptor subunits compared with the labeling pattern of the GluN2A/B NMDA subunit in the IPL. This phase of our study showed an increase by ∼30% favoring more NMDA receptor labeling within the IPL. It is likely, therefore, that elevated levels of d-serine, caused by a mutation in the gene for DAO, resulted in chronic elevation of d-serine, which altered the expression of NMDA and/or AMPA receptors. Our results do not allow us to state definitively what the changes are that have created this increase in NMDA receptor currents and immunostaining ratio. One explanation may be that mutant mice simply have a higher number of NMDA vs. AMPA receptors. Another possibility is that the subunit expression of NMDA receptors has been changed in these mutant mice. In the Kalbaugh et al. (2009) study mentioned above, a predominance of GluN2B-containing NMDA receptors was reported in the ON pathway of the retina. Receptors containing these subunits have conductance properties that differ from those with GluN2A subunits (Meguro et al. 1992), and, although d-serine is more efficacious than glycine across all NMDA receptor subtypes (Matsui et al. 1995), the glycine affinity for GluN2B subunits is higher than that of GluN2As (Priestley et al. 1995). Such a subunit change could account for the differences in the ganglion cell recordings we found here as well as the alterations in the immunostaining since the affinities of the pan-GluN2A/B antibody to the distinct subunits cannot be assumed to be equal. Although other alternative interpretations are possible, such as a more direct effect of DAO on receptor expression, the parsimonious explanation is that d-serine itself plays a regulatory role in NMDA receptor expression. This should not be surprising since d-serine has been suggested to play a role in synaptogenesis in the retina (Diaz et al. 2007) due to its presence during this early developmental period.
It is worth mentioning that mice in which serine racemase has been knocked out show a reduction in d-serine compared with wild-type controls, and, under those conditions, the light-evoked response is generated predominantly by AMPA receptors (Sullivan et al. 2011). This, too, points to a role for d-serine in regulating the ratio of NMDA to AMPA receptors for generating light responses in RGCs. Knowledge of the developmental history of d-serine availability would be useful in future considerations of this important issue.
GRANTS
This work was supported by the National Eye Institute (Grants R01-EY-003014 to R. F. Miller and T32-EY-07133 to E. C. Gustafson and S. J. Sullivan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.C.G., C.W.M., S.J.S., M.E., R.K., and R.F.M. conception and design of research; E.C.G., C.W.M., M.T., S.J.S., and M.E. performed experiments; E.C.G., C.W.M., S.J.S., M.E., and R.F.M. analyzed data; E.C.G., C.W.M., M.E., and R.F.M. interpreted results of experiments; E.C.G., C.W.M., M.E., and R.F.M. prepared figures; E.C.G. and R.F.M. drafted manuscript; E.C.G. and R.F.M. edited and revised manuscript; E.C.G., C.W.M., M.T., S.J.S., M.E., R.K., and R.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Derek Miller for editorial support.
REFERENCES
- Almond SL, Fradley RL, Armstrong EJ, Heavens RB, Rutter AR, Newman RJ, Chiu CS, Konno R, Hutson PH, Brandon NJ. Behavioral and biochemical characterization of a mutant mouse strain lacking D-amino acid oxidase activity and its implications for schizophrenia. Mol Cell Neurosci 32: 324–334, 2006 [DOI] [PubMed] [Google Scholar]
- Beard ME, Davies T, Holloway M, Holtzman E. Peroxisomes in pigment epithelium and Müller cells of amphibian retina possess D-amino acid oxidase as well as catalase. Exp Eye Res 47: 795–806, 1988 [DOI] [PubMed] [Google Scholar]
- Burkhardt DA, Fahey PK, Sikora M. Responses of ganglion cells to contrast steps in the light-adapted retina of the tiger salamander. Vis Neurosci 15: 219–229, 1998 [DOI] [PubMed] [Google Scholar]
- Diaz CM, Macnab LT, Williams SM, Sullivan RK, Pow DV. EAAT1 and D-serine expression are early features of human retinal development. Exp Eye Res 84: 876–885, 2007 [DOI] [PubMed] [Google Scholar]
- Dixon DB, Copenhagen DR. Two types of glutamate receptors differentially excite amacrine cells in the tiger salamander retina. J Physiol 449: 589–606, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol 420: 98–112, 2000 [PubMed] [Google Scholar]
- Gottesman J, Miller RF. Pharmacological properties of N-methyl-d-aspartate receptors on ganglion cells of an amphibian retina. J Neurophysiol 68: 596–604, 1992 [DOI] [PubMed] [Google Scholar]
- Gustafson EC, Stevens ER, Wolosker H, Miller RF. Endogenous d-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol 98: 122–130, 2007 [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. The presence of free D-serine in rat brain. FEBS Lett 296: 33–36, 1992a [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert.-butyloxycarbonyl-l-cysteine and o-phthaldialdehyde. J Chromatogr 582: 41–48, 1992b [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wassle H. The cone pedicle, a complex synapse in the retina. Neuron 27: 85–95, 2000 [DOI] [PubMed] [Google Scholar]
- Horiike K, Arai R, Tojo H, Yamano T, Nozaki M, Maeda T. Histochemical staining of cells containing flavoenzyme d-amino acid oxidase based on its enzymatic activity: application of a coupled peroxidation method. Acta Histochem Cytochem 18: 539–550, 1985 [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529–531, 1987 [DOI] [PubMed] [Google Scholar]
- Kalbaugh TL, Zhang J, Diamond JS. Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells. J Neurosci 29: 1469–1479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno R, Yasumura Y. Mouse mutant deficient in D-amino acid oxidase activity. Genetics 103: 277–285, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC, Miller RF. N-methyl-d-aspartate receptors of ganglion cells in the rabbit retina. J Neurophysiol 63: 16–30, 1990 [DOI] [PubMed] [Google Scholar]
- Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem 65: 454–458, 1995 [DOI] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature 357: 70–74, 1992 [DOI] [PubMed] [Google Scholar]
- Mittman S, Taylor WR, Copenhagen DR. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol 428: 175–197, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Ren G, Akileswaran L. Localization of nyctalopin in the mammalian retina. Eur J Neurosci 23: 1163–1171, 2006 [DOI] [PubMed] [Google Scholar]
- Moses J, Siddiqui A, Silverman PB. Sodium benzoate differentially blocks circling induced by d- and l-dopa in the hemi-parkinsonian rat. Neurosci Lett 218: 145–148, 1996 [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci USA 104: 2950–2955, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, Chakraborty A, Barrow RK, Amzel LM, Snyder SH. Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci USA 106: 2921–2926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neims AH, Zieverink WD, Smilack JD. Distribution of D-amino acid oxidase in bovine and human nervous tissues. J Neurochem 13: 163–168, 1966 [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150: 633–646, 2012 [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdellés B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-d-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol 48: 841–848, 1995 [PubMed] [Google Scholar]
- Reed BT, Sullivan SJ, Tsai G, Coyle JT, Esguerra M, Miller RF. The glycine transporter GlyT1 controls N-methyl-d-aspartic acid receptor coagonist occupancy in the mouse retina. Eur J Neurosci 30: 2308–2317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Brady RO, Jr, Molliver ME, Snyder SH. D-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci 17: 1604–1615, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Zhang M, Jin Y, Yang XL. Functional N-methyl-d-aspartate receptors are expressed in cone-driven horizontal cells in carp retina. Neurosignals 15: 174–179, 2006 [DOI] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci 3: 1701–1711, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jules R, Kennard J, Setlik W, Holtzman E. Frog cones as well as Müller cells have peroxisomes. Exp Eye Res 54: 1–8, 1992 [DOI] [PubMed] [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA 100: 6789–6794, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ER, Gustafson EC, Miller RF. Glycine transport accounts for the differential role of glycine vs. D-serine at NMDA receptor coagonist sites in the salamander retina. Eur J Neurosci 31: 808–816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ, Esguerra M, Wickham RJ, Romero GE, Coyle JT, Miller RF. Serine racemase deletion abolishes light-evoked NMDA receptor currents in retinal ganglion cells. J Physiol 589: 5997–6006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ, Miller RF. AMPA receptor mediated D-serine release from retinal glial cells. J Neurochem 115: 1681–1689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SJ, Miller RF. AMPA receptor-dependent, light-evoked d-serine release acts on retinal ganglion cell NMDA receptors. J Neurophysiol 108: 1044–1051, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Chen E, Copenhagen DR. Characterization of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. J Physiol 486: 207–221, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci USA 96: 721–725, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang B, Theus MH, Sick JT, Bethea JR, Sick TJ, Liebl DJ. EphrinBs regulate D-serine synthesis and release in astrocytes. J Neurosci 30: 16015–16024, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]