Abstract
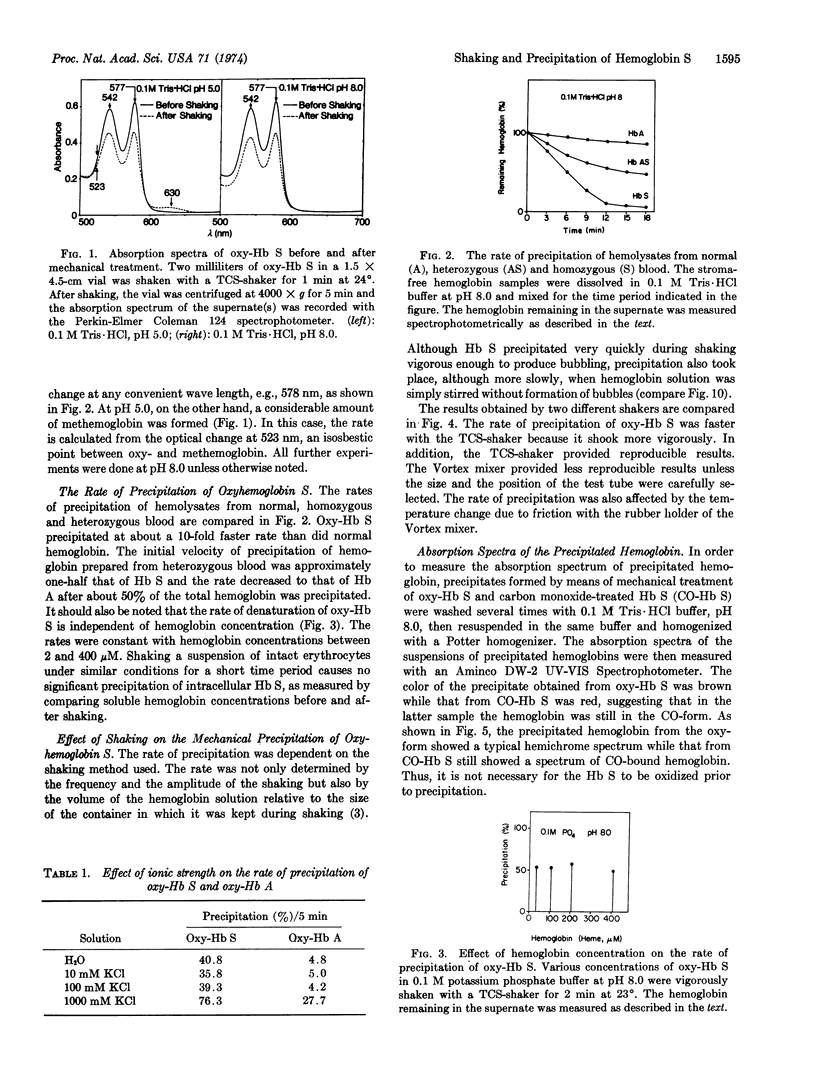
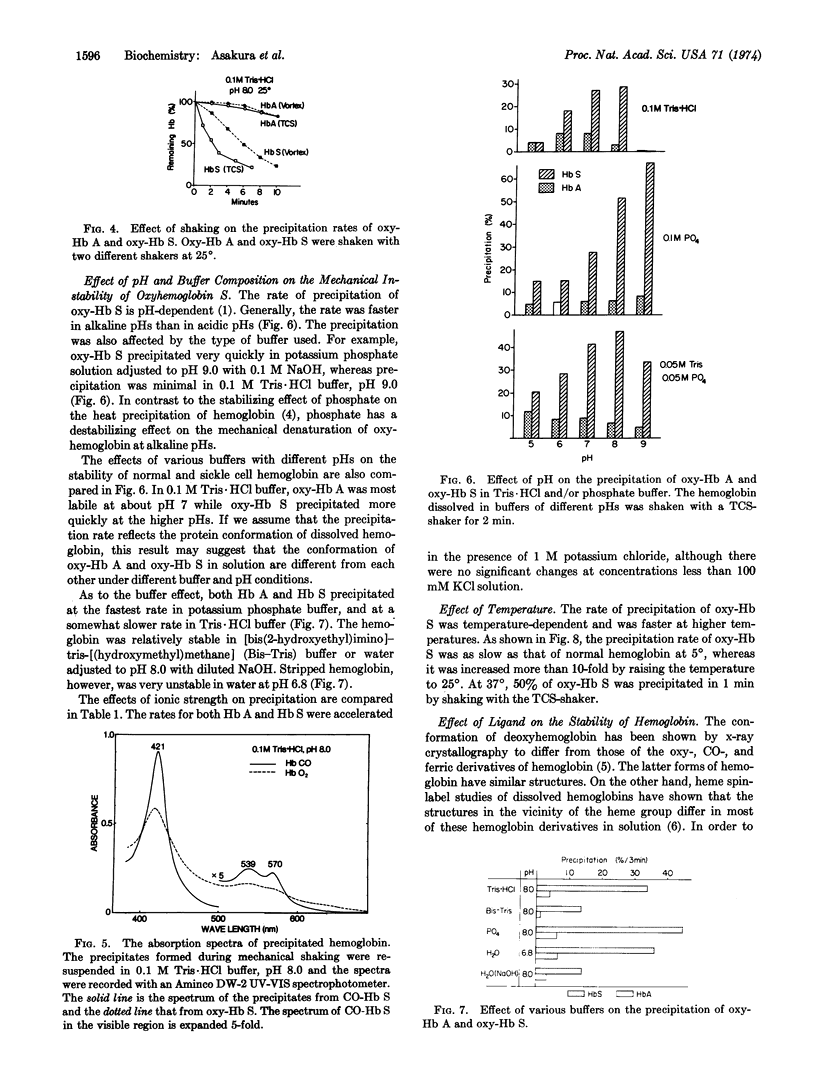
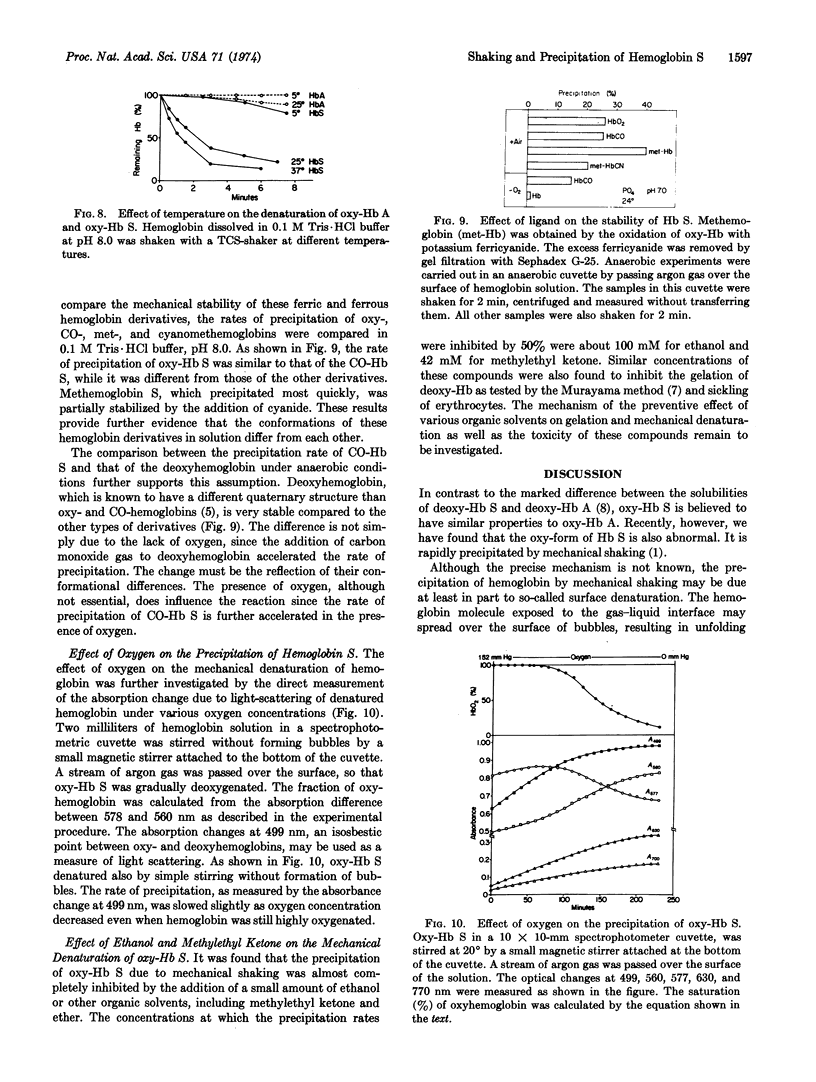
The oxy-form of sickle hemoglobin (Hb S) is abnormally unstable and precipitates at a 10-fold faster rate than does oxyhemoglobin A (oxy-Hb A) during mechanical shaking. The apparent rate of precipitation of heterozygous hemolysate (AS) is approximately half that of oxy-Hb S. The deoxy-form of Hb S, on the other hand, is resistant to the mechanical treatment. This stabilization is attributed to the conformational change of hemoglobin rather than the lack of oxygen, because carbonmonoxide hemoglobin S, which is known to have conformational properties similar to those of oxy-Hb, is unstable even under anaerobic conditions. Methemoglobin S is most unstable, although addition of cyanide stabilizes the protein. The precipitation of oxy-Hb S is inhibited by ethanol and other organic solvents. The relationship of the mechanical instability of sickle oxyhemoglobin to intraerythrocytic denaturation and vaso-occlusive phenomena in sickle cell disease to be determined.
Keywords: denaturation, vaso-occlusion, sickle cell disease, ethanol, methylethyl ketone
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura T., Agarwal P. L., Relman D. A., McCray J. A., Chance B., Schwartz E., Friedman S., Lubin B. Mechanical instability of the oxy-form of sickle haemoglobin. Nature. 1973 Aug 17;244(5416):437–438. doi: 10.1038/244437a0. [DOI] [PubMed] [Google Scholar]
- BASU A. K., WOODRUFF A. W. EFFECT OF PYREXIA ON SICKLAEMIC STATES. Lancet. 1963 Nov 23;2(7317):1088–1090. doi: 10.1016/s0140-6736(63)92858-7. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Tam J. W. Acetylation of sickle cell hemoglobin by aspirin. Proc Natl Acad Sci U S A. 1973 May;70(5):1313–1315. doi: 10.1073/pnas.70.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAYAMA M. A MOLECULAR MECHANISM OF SICKLED ERYTHROCYTE FORMATION. Nature. 1964 Apr 18;202:258–260. doi: 10.1038/202258a0. [DOI] [PubMed] [Google Scholar]
- Macleod R. M., Hill R. J. Demonstration of the hybrid hemoglobin 2 A A S . J Biol Chem. 1973 Jan 10;248(1):100–103. [PubMed] [Google Scholar]
- Nalbandian R. M., Henry R. L., Nichols B. M., Camp F. R., Jr, Wolf P. L. Molecular basis for a simple, specific test for S hemoglobin: the Murayama test. Clin Chem. 1970 Nov;16(11):945–950. [PubMed] [Google Scholar]
- PERUTZ M. F., MITCHISON J. M. State of haemoglobin in sickle-cell anaemia. Nature. 1950 Oct 21;166(4225):677–679. doi: 10.1038/166677a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. The Croonian Lecture, 1968. The haemoglobin molecule. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):113–140. doi: 10.1098/rspb.1969.0043. [DOI] [PubMed] [Google Scholar]
- Schneider R. G., Takeda I., Gustavson L. P., Alperin J. B. Intraerythrocytic precipitations of haemoglobins S and C. Nat New Biol. 1972 Jan 19;235(55):88–90. doi: 10.1038/newbio235088a0. [DOI] [PubMed] [Google Scholar]
- Schneiderman L. J., Junga I. G., Fawley D. E. Effect of phosphate and non-phosphate buffers on thermolability of unstable haemoglobins. Nature. 1970 Mar 14;225(5237):1041–1042. doi: 10.1038/2251041a0. [DOI] [PubMed] [Google Scholar]





