Abstract
Several studies have linked stress with Alzheimer’s disease (AD) vulnerability; however, the mechanism remains to be fully elucidated. In the current paper, we investigated the role of glucocortitcoids on the AD-like phenotype. We administered the glucocorticoid dexamethasone to Tg2576 mice for 4 weeks and then investigated its effect on memory, amyloid-β and tau levels, and metabolism. At the end of the treatment period, we observed that mice receiving dexamethasone had a significant impairment in the fear conditioning paradigm compared with controls. Dexamethasone-treated animals showed a significant increase in the amount of brain soluble Aβ40 levels, but no alteration in the steady state levels of its precursor protein, AβPP, or in the major protease enzymes involved in its metabolism (i.e., ADAM-10, BACE-1, or γ-secretase complex). While total tau protein levels were unaltered between the two groups, we found that dexamethasone significantly reduced tau phosphorylation at specific sites that were mediated by decreases in glycogen synthase kinase-3β protein level and activity. Finally, we observed a direct correlation between memory impairments and tau phosphorylation levels. Our study highlights the significant role that glucocorticoids play in exacerbating AD-like cognitive impairments via alteration of tau protein phosphorylation state.
Keywords: Alzheimer’s disease, transgenic animal model, stress, glucocorticoid
INTRODUCTION
In its sporadic form, Alzheimer’s disease (AD) is considered a chronic neurodegenerative disorder with dementia resulting from the combination of both genetic and environmental risk factors. Psychosocial stress has been suggested to be one important environmental factor that can influence AD age of onset and/or development [1]. Several clinical studies have linked dysregulation of stress hormone levels, such as glucocorticoids, with AD vulnerability. Plasma cortisol levels are increased in subjects with mild cognitive impairment and in AD patients, and conditions in which there is dysfunction of the hypothalamus-pituitary-adrenal axis are associated with a higher risk of AD [2–4]. Glucocorticoids are known to alter neuronal plasticity, impair learning and memory, and produce atrophy in several areas of the brain, but the precise mechanism by which they contribute to AD remains to be fully elucidated [5–7]. Some studies have demonstrated that glucocorticoids promote amyloid-β (Aβ) deposition and impairments in learning and memory in rodent models of AD, but few have directly investigated how glucocorticoids modulate endogenous tau protein in the presence of pathologic Aβ peptides [8–11]. AD rodent models that contain the tauP301L mutation form hyperphosphorylated tau species and develop neurofibrillary tangles (NFTs) upon glucocorticoid administration, but limit understanding of the role of endogenous tau in this context as mutations in tau have not been described in AD patients. To better understand the role of endogenous tau in the context of AD, we treated Tg2576 mice, which express the AβPPK670L/M671L mutation and endogenous mouse tau, with a chronic course of dexamethasone. We observed that dexamethasone treatment resulted in memory impairment as well as an elevation in Aβ peptides in the brains of Tg2576 animals compared to controls. Surprisingly, we also found hypophosphorylation of certain tau species mediated by changes in glycogen synthase kinase 3β (GSK3β) activity.
MATERIALS AND METHOD
Animals
Studies were approved by the Institutional Animal Care and Usage Committee of the Temple University School of Medicine, in accordance with NIH guidelines. Female Tg2576 mice (mice expressing the human AβPPK670L/M671L Swedish mutation transgene) aged 10–12 months were intraperitoneally either given phosphate-buffered saline as control, or dexamethasone (Dex; Sigma-Aldrich) at a dose of 5 mg/kg for 28 d (n = 5 control, n = 6 Dex). On days 29 and 30, learning and memory was assessed using the fear conditioning paradigm as previously described [12]. On day 31, mice were sacrificed and their brains were removed. Cortices were dissected from one brain hemisection and stored at −80° C while the other hemisection was paraformaldehyde-fixed overnight, processed and paraffin-embedded, and used for immunohistochemistry or immunofluorescence assays.
Biochemical analyses
Mouse brain cortical homogenates were sequentially extracted first in radio-immunoprecipitation assay buffer (RIPA) containing EDTA-free protease inhibitor (Roche) and phosphatase inhibitor (Thermo Fisher) for the Aβ soluble fractions and then in formic acid (FA) for the Aβ insoluble fractions as previously described [12, 13]. Aβ1–40 and Aβ1–42 levels were assayed by a sensitive sandwich enzyme-linked immunosorbent assay (ELISA) kit (Wako Chemicals, USA) in accordance to the manufacturer’s protocols.
Immunoblotting
Mouse cortical homogenate RIPA samples were electrophoretically separated using 10% Bis-Tris gels or 3% to 8% Tris-acetate gel (Bio-Rad), according to the molecular weight of the target molecule, and then transferred onto nitrocellulose membranes (Bio-Rad). Membranes were blocked with Odyssey blocking buffer and incubated with primary antibodies overnight at 4°C. After 3 washing cycles, membranes were incubated with IRDye secondary antibodies (LI-COR) at 22°C for 1 h. Signals were developed with Odyssey Infrared Imaging Systems (LI-COR). Actin was always used as an internal loading control. Antibodies and dilutions were as follows: anti-AβPP N-terminal raised against amino acids 66–81 for total AβPP (22C11; 1 : 1500; Chemicon International), BACE-1 (1 : 200; IBL), ADAM-10 (1 : 500 dilution; Chemicon International), PS1 (1 : 200; Cell Signaling), nicastrin (1 : 200; Cell Signaling), Pen2 (1 : 200; Invitrogen), APH-1 (1 : 200; Millipore); CREB (1 : 200; Cell Signaling), p-CREB (1 : 200; Cell Signaling), actin (1 : 1,000; Santa Cruz), Tau 1 (1 : 200, Millipore), AT8 (1 : 200; Pierce), AT180 (1 : 200; Pierce), AT270 (1 : 200; Pierce), ser396 (1 : 200; Pierce), GSK3 (1 : 200; Santa Cruz), pGSK3 (1 : 200; Santa Cruz), cdk5 (1 : 200; Santa Cruz), p35/p25 (1 : 200; Santa Cruz).
Immunohistochemistry
Immunohistochemistry analysis was performed as previously described [13]. Briefly, brain sections were deparaffinized, hydrated, and after blocking with 2% serum, incubated with primary antibody against 4G8 (1 : 200; Cell Sciences), tau 1 (1 : 100; Pierce), AT8 (1 : 100; Pierce), or ser396 (1 : 100; Pierce) overnight at 4°C. Formic acid was used to retrieve antigen for slides using 4G8, and citric acid was used to retrieve antigen for tau 1, AT8 and ser396. After 3 washings, sections were incubated with appropriate secondary antibody and finally developed using the avidin-biotin complex method (Vector Laboratories) with 3,3-diaminobenzidine as chromogen.
Immunofluorescence
Immunofluorescence was performed on 6-μm paraffin sections of brain tissue as previously described [14]. Briefly, sections were first blocked in buffer containing 2% BSA, and 0.1% Triton X-100 in TBS for 1 h at room temperature. Using the same buffer, sections were incubated overnight at 4°C with the primary antibody anti-synaptophysin (SYP-38, 1 : 250; Sigma-Aldrich). Sections were then rinsed and incubated for 1 h with secondary Alexa Fluor–conjugated antibodies (1 : 1000; Invitrogen) at room temperature. Finally, sections were mounted onto slides using a 9 : 1 glycerine/PBS mounting medium and examined under Olympus BX60 fluorescent microscope. Fluorescence emission was collected at 425–475 nm for 4′,6-diamidino-2-phenylindole and 555–655 nm for Alexa546. Immunofluorescence was assessed at similar brain coronal levels.
GSK3β kinase activity
GSK3β kinase activity was carried out as previously described [15, 16]. Briefly, cortical brain lysate was extracted as described above using RIPA with pro-tease and phosphatase inhibitor. The samples were centrifuged at 12,000 g at 4°C for 20 min and the supernatant (400 μg protein) were incubated with 8 μg anti-GSK3β antibody (Santa Cruz) at 4°C for 2 h. Protein A agarose beads (Invitrogen) were added and then incubated for another hour. The immunoprecipitates were then washed three times with lysate buffer (Tris-HCl pH 7.4, 50 MgCl2, 5 mM EDTA, protease inhibitor and phosphatase inhibitor) and once with HBS buffer (10 mM HEPES pH 7.4, 150 mM NaCl). The kinase activity of the immunoprecipitates GSK3β was determined by using GSK3β substrate phosphor-glycogen synthase peptide 2 (Upstate). Beads were incubated with 5 μg phosphor-glycogen synthase peptide 2 (Upstate) in HBS (20 μl) containing 15 mM MgCl2, 5 μCi [γ32P] ATP (0.5 mM), and 1 mM dithiothretol. After 30 min incubation at 30°C, the reaction was stopped and its products were determined by liquid scintillation counter.
Data analysis
Data are expressed as mean ± standard error of the mean and were analyzed and reported using GraphPad Prism. Student’s t-tests were used to test for significant differences in means between control and dexamethasone-treated animals in experiments shown in Figs. 1–4. Correlation was assessed using the Pearson correlation coefficient for the experiment reported in Fig. 5. For all statistical tests, p < 0.05 was used as the threshold for significance.
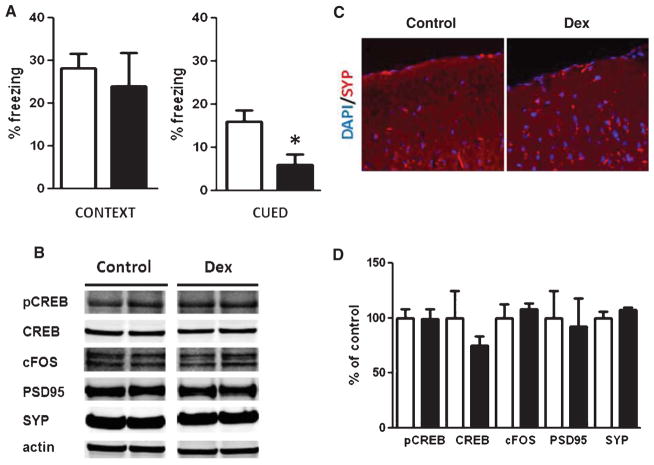
Fig. 1.
Dexamethasone effects on behavior and memory related proteins. A) Context and cued recall freezing responses in the fear conditioning behavioral paradigm of Tg2576 mice treated with either saline (n = 5 open bars) or 5 mg/kg dexamethasone (n = 6, closed bars) for 28 d (*p < 0.02). B) Representative western blots for pCREB, CREB, cFOS, PSD95, or synaptophysin (SYP) in brain homogenates from Tg2576 mice receiving dexamethasone (Dex) or PBS (control). C) Representative immunofluorescence staining of synaptophysin (SYP) in brain sections from Tg2576 mice receiving dexamethasone (Dex) or PBS (control). D) Densitometric analyses of the immunoreactivities to the antibodies shown in panel B (control: open bars; dexamethasone: closed bars).
Fig. 4.
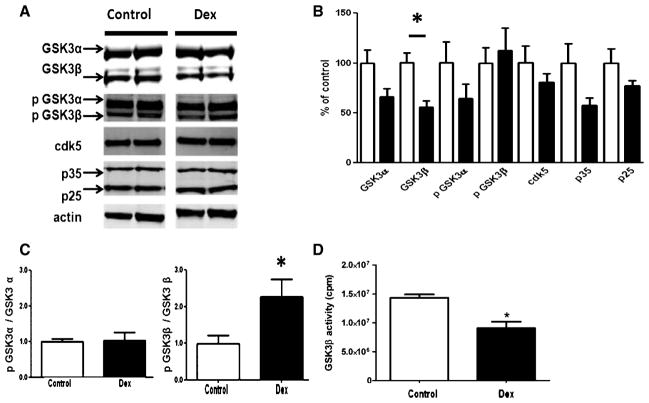
Dexamethasone treatment alters GSK3β level and activity. A) Representative western blots for GSK3α, GSK3β, p-GSK3α, p-GSK3β, cdk5, p35, and p25 in brain homogenates from Tg2576 mice treated with PBS (control) (n = 5 open bars) or Dexamethasone (Dex) (n = 6 closed bars). B) Densitometric analyses of the immunoreactivities to the antibodies shown in A (control: open bars; dexamethasone: closed bars) (p < 0.004). C) Ratios between total and phosphorylated forms of GSK-3 proteins (control: open bars; dexamethasone: closed bars) (p < 0.004). D) GSK3β activity in brain homogenates from Tg2576 mice treated with PBS (control) (n = 5 open bars) or Dexamethasone (Dex) (n = 6 closed bars) (*p < 0.005).
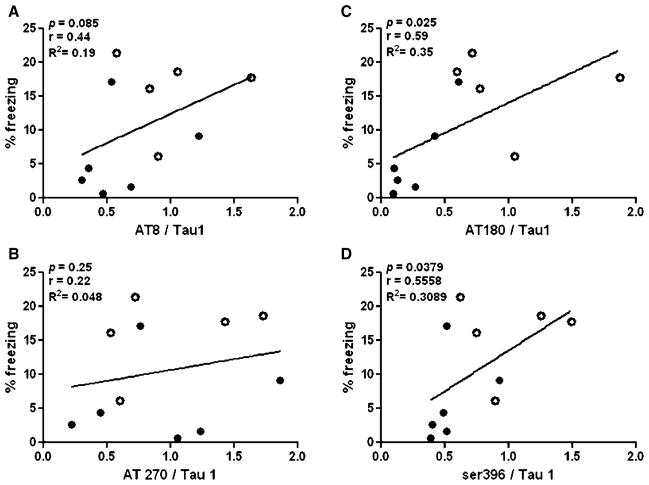
Fig. 5.
Deficits in cued recall correlate with tau hypophosphorylation. Percentage freezing time in the cued recall phase of fear conditioning as show in Fig. 1 did not correlated with levels of A) AT8 or B) AT270 but did correlate with levels of AT180 and Ser396. Open circles represent control animals (n = 5) and closed black circles represent dexamethasone-treated animals (n = 6).
RESULTS
Dexamethasone impairs cued recall in the fear conditioning paradigm
We administered dexamethasone intraperitoneally (5 mg/kg) to Tg2576 mice aged 10–12 months daily for 28 days. At the end of this period, we observed that compared with controls the active treatment caused a significant deficit in cued recall but did not significantly alter contextual recall in the fear conditioning paradigm (Fig. 1A). However, no difference was noted in the conditioning phase of this test (data not shown).
Because glucocorticoids are known to modulate a variety of substrates implicated in learning and memory, we assayed for the cellular response element binding protein (CREB), its phosphorylated form (pCREB), the post synaptic density protein 95 (PSD95), and synaptophysin (SYP). As shown in Fig. 1B–D, we found that dexamethasone treatment did not alter the steady-state levels of any of these proteins. These results suggest that the impairment in the cued recall of dexamethasone-treated Tg2576 mice is not due to dysregulation of these molecular targets which are involved networks of cognition.
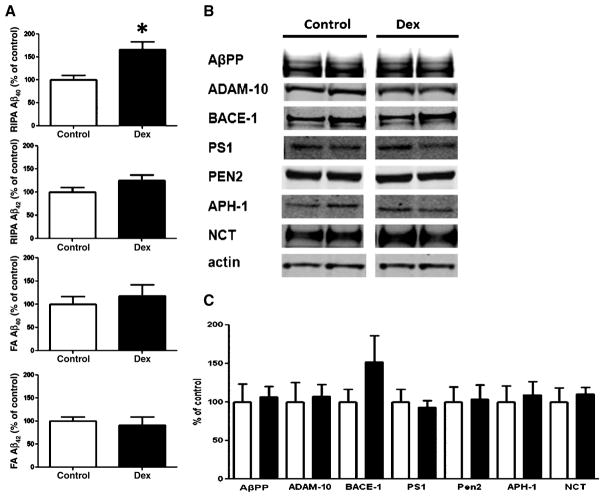
Dexamethasone elevates soluble Aβ40 peptides
Previously studies have shown that behavioral stress or dexamethasone treatment elevates Aβ species in the brains of AD transgenic animals [8–11]. To investigate how Aβ species are modulated in this condition, we assayed homogenized tissue RIPA fractions, which contain soluble amyloid species, and FA fractions, which contain detergent-insoluble amyloid species. In confirmation of earlier work, we found that dexamethasone-treated Tg2576 mice displayed higher levels of brain RIPA soluble Aβ40 than control, however no differences were found in levels of FA Aβ40, RIPA Aβ42, or FA Aβ42 between groups (Fig. 2A). Brain immunostaining for Aβ using the 4G8 antibody revealed similar levels of Aβ for both control as well as dexamethasone-treated animals (supplementary Figure 1A; available online: http://www.j-alz.com/issues/31/vol31-1.html#supplementarydata03). To explore the potential mechanism responsible for the change in Aβ, we assessed the processing of AβPP. We found that, under our experimental condition, dexamethasone did not induce any significant changes in the steady-state levels of AβPP, α-secretase (ADAM-10), β-secretase (BACE-1), or the four components of the γ-secretase complex (presenilin 1, PS1; presenilin enhancer 2, PEN2; anterior pharynx defective 1, APH-1; nicastrin, NCT) (Fig. 2). We also assayed for levels of several proteins implicated in the clearance of Aβ including apolipoprotein E, as well as insulin degrading enzyme and neprilysin, putative Aβ-degrading enzymes, and found that their levels did not significantly differ between dexamethasone-treated animals and controls (supplementary Figure 1B, C).
Fig. 2.
Dexamethasone elevates RIPA-soluble Aβ40 peptides. A) Dexamethasone treatment (n = 6, closed bars) elevates RIPA soluble Aβ40 without altering RIPA soluble Aβ42, FA soluble Aβ40 or FA insoluble Aβ42 in the brains of Tg2576 mice compared to controls (n = 5, open bars) (*p < 0.004). B) Representative western blots for AβPP, ADAM-10, BACE-1, PS1, PEN-2, APH-1, and NCT in brain homogenates from Tg2576 mice treated with either PBS (control) (n = 5 open bars) or 5 mg/kg dexamethasone (Dex) (n = 6, closed bars). C) Densitometric analyses of the immunoreactivities to the antibodies shown in panel B (control: open bars; dexamethasone: closed bars).
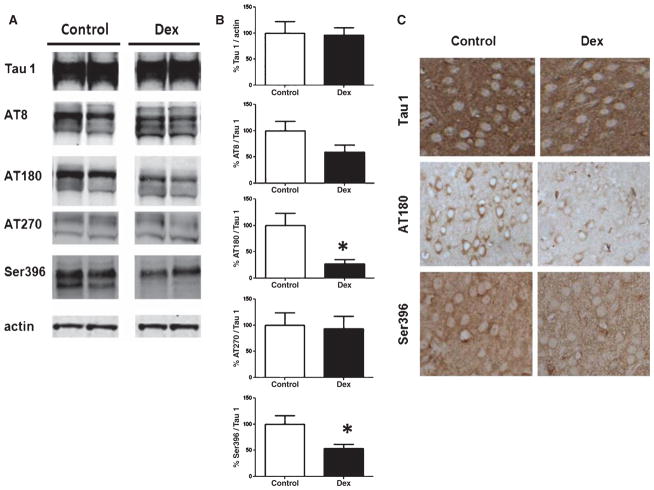
Dexamethasone decreases tau phosphorylation
While a large body of literature indicates that Aβ peptides may be initiators in the learning and memory deficits seen in AD, significant work and clinical data have suggested that tau pathology also figures prominently in cognitive deficits. To that end, we investigated total tau as well as several AD-relevant phosphorylated tau epitopes in the two groups of mice. Dexamethasone did not alter total tau, recognized by Tau 1, or the tau phosphorylated at ser202/thr205, recognized by the AT8 antibody, or at thr181, recognized by the AT270 antibody, in the brains of Tg2576 mice significantly from control. By contrast, we found significantly decreased levels of tau phosphorylated at the thr231/ser235, recognized by the AT180, and at ser396 in dexamethasone-treated animals compared to controls (Fig. 3A, B). Supporting this observation, compared with control mice, immunohistochemistry analysis also revealed decreased staining in the soma of cortical neurons for AT180 and ser396 in dexamethasone-treated animals, while no differences were observed for Tau 1 staining (Fig. 3C).
Fig. 3.
Dexamethasone decreases tau phosphorylation. A) Representative western blots for total tau (Tau 1), phosphorylated tau at ser202/thr205 (AT8), at thr181 (AT270), at thr231/ser235 (AT180), and at ser 396 in brain homogenates from Tg2576 mice treated with either PBS (control) (n = 5 open bars) or 5 mg/kg dexamethasone (Dex) (n = 6, closed bars). B) Ratios for the densitometric analyses of the immunoreactivities to the antibodies shown in B (control: open bars; dexamethasone: closed bars) (*p < 0.001 for AT180, and p < 0.02 for ser396). C) Immunohistochemistry of brain sections from Tg2576 mice treated with either PBS (control) or dexamethasone (Dex) reveals similar tau 1 but diminished AT180 and ser396 immunostaining in Dex-treated mice.
Dexamethasone treatment alters GSK3β level and activity
To elucidate the molecular basis underlying the dexamethasone-mediated tau hypophosphorylation, we assayed levels and activities of two major tau kinases which have been shown to be involved in tau phosphorylation (and dysregulated in the brains of AD patients), glycogen synthase kinase 3 (GSK3) and cyclin-dependent kinase 5 (cdk5). Compared with control mice, dexamethasone did not alter steady state levels of cdk5, its normal coactivator p35, or the truncated coactivator p25 (Figs. 4A, 3B).
By contrast, while we observed no significant change in levels of GSK3α, a significant decrease in levels of GSK3β was detected in the dexamethasone-treated animals compared with controls. GSK3 activity is modulated by its phosphorylation, with phosphorylation on the serine 21 residue of GSK3α (p GSK3α) or the serine 9 residue of GSK3β (p GSK3β) inhibiting their kinase activity, respectively. In our study we found that steady state levels of both phosphorylated forms were not significantly different between the two groups (Fig. 4B). However, analysis of the ratios of phosphorylated GSK3α to total GSK3α and phosphorylated GSK3β to total GSK3β revealed that dexamethasone treatment significantly increased the phosphorylated fraction of GSK3β without influencing the phosphorylated fraction of GSK3β (Fig. 4C). This result would suggest that the activity of this kinase was reduced by the dexamethasone treatment. To confirm this hypothesis, we directly measured GSK3β kinase activity from brain homogenates of dexamethasone-treated and control mice. As shown in Fig. 4D, we found that GSK3β activity was significantly diminished in the dexamethasone-treated animals.
Deficits in cued recall are associated with tau hypophosphorylation
Tau stabilizes axonal microtubules, with its phosphorylation status modulating microtubule affinity. Because proper microtubule functioning is critical for cellular cargo trafficking from neuronal cell body to synapse, we investigated whether the phosphorylation status of tau correlated with memory impairments in the cued recall of the fear conditioning paradigm. We found that there was a significant correlation between levels of AT180 and ser396 phosphoepitopes and freezing times in the cued recall phase of fear conditioning (Fig. 5C, D), with lower levels of those phosphoepitopes correlating with decreased freezing time. By contrast, we found no correlation between the AT8 or AT270 phosphotau epitopes and freezing times in cued recall (Fig. 5A, B). Additionally, we did not find a significant relationship between soluble Aβ40 levels and cued recall (p > 0.49, r = 0.0053, R2 = 2.87 × 10−5).
DISCUSSION
In the current study, we show that chronic administration of dexamethasone to Tg2576 mice results in cued recall deficits in the fear conditioning task, an elevation of soluble Aβ40, but a significant decrease in the levels of the thr231/ser235 and ser396 phosphorylated tau epitopes, secondary to a reduction in GSK3β levels and activity. Additionally, we show that performance in the fear conditioning behavioral paradigm directly correlates with the degree of phosphorylation of these specific tau epitopes. Taken together, our results represent the first description of an association between stress, lower phosphorylated tau species, and memory impairment in a transgenic mouse model of AD.
An emerging body of work has described the interaction between stress and AD. Stress, pharmacologically through corticosteroid administration, or, behaviorally through various stress paradigms, increases Aβ pathology in AβPP transgenic models of AD [8–11]. In transgenic AD models that express the human mutant tauP301L, stress also results in the accumulation of NFTs [8, 17]. While our work describes an elevation in Aβ in response to elevated glucocorticoids that is consistent with previous studies, our findings that dexamethasone decreases certain tau phosphorylated AD-relevant sites runs counter to expected findings. This discrepancy may be due to the wide variety of different stressors, age of treatment, treatment paradigms and lengths of treatment in those reports, and the complexity and time course of glucocorticoid action. For example, Lee and colleagues have reported that restraint stress for 2 h/day for 16 days results in higher levels of ser199, thr231, and ser296 phosphorylated tau but not the ser202 tau phosphoepitope in the Tg2576 mice at ~15 months of age [11]. On the other hand, Jeong and colleagues have reported that transgenic mice expressing the AβPPV717I London mutation displayed memory impairment and increased tau phosphorylation at the ser202/thr205 site after 8 months of immobilization and isolation stress, starting at 3 months, for 6 h/day for 4 d/week [9]. Furthermore, in the PS19 mouse model of tauopathy that expresses only tauP301L, only 30 days of chronic restrain/isolation stress, but not subcutaneous pellets of slow-release corticosterone or dexamethasone (5 mg/kg), increases phosphorylation of tau species and pathologic tau inclusions [17]. These inconsistencies in tau/phosphorylated tau indicate a complex interplay between tau metabolism and stress, and hint that different behavioral stress paradigms may modulate tau phosphorylation differently.
In association with a decrease in tau phosphorylation, we found that dexamethasone decreased GSK3β level and activity. GSK3β is a major kinase that phosphorylates tau and has been shown to be dysregulated in the brains of AD patients, but no work to date has directly examined the role of GSK3β in vivo in the glucocorticoid stress paradigm in Tg2576 mice [18]. Published literature has described conflicting reports of the relationship between glucocorticoids and GSK3β function. In some contexts, glucocorticoids activate GSK3β, releasing it from serine 9 phosphorylation; in others, glucocorticoids induce serine 9-mediated degradation of GSK3β [19–23]. Our work supports the latter body of work in this particular AD-like model and disease context. Of note is our observation that while GSK3β was significantly altered in dexamethasone-treated groups, there was no significant difference in cdk5 or its p35 and p25 co-activators, implying that dexamethasone selectively alters the GSK3β pathway. This finding may also explain why only certain phosphorylated tau epitopes were decreased upon dexamethasone treatment. Future work investigating the exact mechanism of the glucocorticoid-mediated down-regulation of GSK3β level/function would be particularly instructive in this regard.
Though still far from settled, the totality of the work on Aβ and tau in AD seems to currently suggest that tau pathology occurs downstream of the changes caused by Aβ peptides [24, 25]. To that end, Aβ is thought to be the principle molecular player in the impairments of learning and memory. However, since corticosteroids are known to alter neuronal plasticity, impair learning and memory, we first explored some of the molecular mechanism by which dexamethasone could have modulated the memory impairments in our experimental setting. To this end, no changes in the levels of transcription factors involved in memory formation such as c-Fos, CREB, p-CREB, PSD-95, and synaptophysin were observed, ruling out a role for these factors.
Interestingly, our work implies that the degree of phosphorylation of two tau epitopes correlates with impairments in learning and memory. Thus we found that the levels of those tau species correlated with not only the learning and memory impairments in dexamethasone-treated animals but also recall of control animals, albeit with modest correlation coefficients. Current evidence suggests that there is a hierarchy of tau phosphorylation as it relates to pathologic NFT progression, with certain phosphorylated tau epitopes corresponding with early pre-NFT pathology, intermediate intraneuronal NFT pathology, or late-stage extraneuronal NFT pathology. Ser202/205 and thr 231 epitopes, which were not correlated with memory impairments in our study, have been associated with late and intermediate stage NFTs, respectively, while thr231/ser235 and ser396 epitopes, which did correlate with memory impairments, have been correspondingly observed with early and late NFTs [31]. Taking this into account, our findings are not easily explained by the present understanding of how phosphorylated tau epitopes are thought to contribute to tau pathology in AD.
Recent work has shown that tau, aside from being a protein important in cytoskeletal stability, may have other functions, which include intracellular signaling and protein interaction [26, 27]. The possibility that phosphorylation of tau imparts a cognition-relevant functional modification aside from alteration of micro-tubule affinity in the AD context is an interesting supposition.
Human brain biopsies suggest that some degree of tau phosphorylation is homeostatic, and phosphorylation of some residues on tau has been implicated in protecting it from aggregating into neurofibrillary tangles [28, 29]. Phosphorylation of tau at the thr231 and ser396 sites has also been observed during brain development, where those phosphoepitopes are associated with immature neurons in loci of neurogenesis [30]. Because glucocorticoids are well known to alter axonal functioning and dendritic spine morphology, it is possible that they may interfere with the balance between the pool of total tau and phosphorylated tau such that microtubule formation, a highly dynamic process that is critical in proper neuronal development and functional network integration, is altered [32].
Another interesting finding is that dexamethasone in our paradigm modulated cued recall, which is thought to involve the amygdala, while sparing contextual recall, which is more hippocampal-based. While degeneration of both limbic structures are known to occur in AD, little is known whether and how stress may selectively confer cognitive insults in AD and other related dementias, and further studies must carried out to investigate this relationship [33].
One limitation of our study is the use of dexamethasone to simulate a stress phenotype. The dominant stress hormones in humans and rodents are cortisol and corticosterone, respectively, which physiologically act on mineralocorticoid receptors in addition to glucocorticoid receptors. While dexamethasone is much more selective for glucocorticoid receptors and produces responses in the hypothalamus-pituitary-adrenal axis, it is the substrate for p-glycoprotein in the epithelium of the blood-brain barrier, which diminishes dexamethasone’s brain penetration [34, 35]. Therefore, it is unclear how dexamethasone administration regulates the endogenous steroids in the AD brain, which may be involved in modulating AD pathology.
In summary, our work reveals a novel consequence of chronic glucocorticoid administration in an AD mouse model involving the modulation of GSK3&BETA activity and phosphorylation status of tau, which is associated with impairments in memory. Future work exploring a role for tau distinct from its accepted cytoskeletal function is critical to better understand whether and how tau contributes to cognition in the AD context as well as in other neurologic conditions.
Supplementary Material
Acknowledgments
This work was funded in part by a grant from the National Institute of Health (AG033568). No role was played by the funding body in the design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Supplementary data available online: http://www.j-alz.com/issues/31/vol31-1.html#supplementarydata03
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1248).
References
- 1.Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennet DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 2.Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Aznar S, Knudsen GM. Depression and Alzheimer’s disease: Is stress the initiating factor in a common neuropathological cascade? J Alzheimers Dis. 2011;23:177–193. doi: 10.3233/JAD-2010-100390. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothman SM, Mattson MP. Adverse stress, hippocampal networks and Alzheimer’s disease. Neuromolecular Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, Lee SH, Emson PC, Suh YH. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J. 2006;20:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- 10.Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KW, Kim JB, Seo JS, Kim TK, Im JY, Baek IS, Kim KS, Lee JK, Han PL. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J Neurochem. 2009;108:165–175. doi: 10.1111/j.1471-4159.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Zhou JM, Chu J, Chinnici C, Pratico D. Amelioration of the Alzheimer’s disease phenotype by absence of 12/15-lipoygenase. Biol Psychiatry. 2010;68:922–929. doi: 10.1016/j.biopsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Chu J. Pratico D. 5-lipoygenase as an endogenous modulator of amyloid beta formation in vivo. Ann Neurol. 2011;69:34–46. doi: 10.1002/ana.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros R, Kitazawa M, Caccamo A, Baglietto-Vargas D, Estrada-Hernandez T, Cribbs DH, Fisher A, LaFerla FM. Loss of muscarinic M1 receptor exacerbates Alzheimer’s disease-like pathology and cognitive decline. Am J Pathol. 2011;179:980–981. doi: 10.1016/j.ajpath.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Wang H, Feng Y, Chen J. Increased expression of cdk5/p25 in N2a cells leads to hyperphosphorylation and impaired axonal transport of neurofilament proteins. Life Sci. 2010;86:532–537. doi: 10.1016/j.lfs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Louis JV, Martens E, Borghgraef P, Lambrecht C, Sents W, Longin S, Zwaenepoel K, Pijnenborg R, Landrieu I, Lippens G, Ledermann B, Götz J, Van Leuven F, Goris J, Janssens V. Mice lacking phosphatase PP2A subunit PR61/B’delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta. Proc Natl Acad Sci U S A. 2011;108:6957–6962. doi: 10.1073/pnas.1018777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, Trojanowski JQ. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model or tauopathy. J Neurosci. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Yun SI, Yoon HY, Jeong SY, Chung YS. Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3beta. J Bone Miner Metab. 2009;27:140–148. doi: 10.1007/s00774-008-0019-5. [DOI] [PubMed] [Google Scholar]
- 20.Glliher-Beckley AJ, Williams JG, Collins JB, Cidlowki JA. Glycogen synthase kinase 3beta-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Mol Cell Biol. 2008;28:7609–7322. doi: 10.1128/MCB.00808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith E, Coetzee GA, Frenkel B. Glucocorticoids inhibit cell cycle progression in differentiating osteoblasts via glycogen synthase kinase-3beta. J Biol Chem. 2002;277:18191–18197. doi: 10.1074/jbc.M109708200. [DOI] [PubMed] [Google Scholar]
- 22.Spokoini R, Kfir-Erenfeld S, Yefenof E, Sionov RV. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol Endocrinol. 2010;24:1136–1150. doi: 10.1210/me.2009-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Failor KL, Desyantnikov Y, Finger LA, Firestone GL. Glucocorticoid-induced degradation of glycogen synthase kinase-3 protein is triggered by serum- and glucocorticoid-induced protein kinase and akt signaling and contros beta-catenin dynamics and tight junction formation in mammary epithelial tumor cells. Mol Endocrinol. 2007;21:2403–2415. doi: 10.1210/me.2007-0143. [DOI] [PubMed] [Google Scholar]
- 24.Busciglio J, Lorenzo A, Yeh J, Yanker BA. beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14:878–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 25.De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, Bigio EH, Jerecic J, Acton PJ, Shughrue PJ, Chen-Dodson E, Kinney GG, Klein WL. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leugers CJ, Lee G. Tau potentiates nerve growth factor-induced mitogen-activated protein kinase signaling and neurite initiation without a requirement for microtubule binding. J Biol Chem. 2010;285:19125–19134. doi: 10.1074/jbc.M110.105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J, Combs CK, Pilcher WH, Song LY, Utal AK, Coleman PD. Low initial tau phosphorylation in human brain biopsy samples. Neurobiol Aging. 1997;18:475–481. doi: 10.1016/s0197-4580(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 29.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (ser262, ser214) also protects it against aggregation into Alzheimer paired helical fragments. Biochemistry. 2008;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 30.Hong XP, Peng CX, Wei W, Tian Q, Liu YH, Yao XQ, Zhang Y, Cao FY, Wang Q, Wang JZ. Essential role of tau phosphorylation in adult hippocampal neurogenesis. Hippocampus. 2010;20:1339–1349. doi: 10.1002/hipo.20712. [DOI] [PubMed] [Google Scholar]
- 31.Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 32.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108:16074–16074. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulin P, Zakzanis KK. In vivo neuroanatomy of Alzheimer’s disease: Evidence from structural and functional brain imaging. Brain Cogn. 2002;49:220–225. [PubMed] [Google Scholar]
- 34.De Kloet ER. Why dexamethasone poorly penetrates in brain. Stress. 1997;2:13–20. doi: 10.3109/10253899709014734. [DOI] [PubMed] [Google Scholar]
- 35.Karssen AM, Meijer OC, van der Sandt IC, Lucassen PJ, de Lange EC, de Boer AG, de Kloet ER. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology. 2001;142:2686–2694. doi: 10.1210/endo.142.6.8213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.