Abstract
Background
A chronic or acute insult may affect the regulatory processes that guide motor and behavioral performance, leading to increased intra-individual variability (IIV). Increased variability is often interpreted as an indication of regulatory dysfunction. Iron plays an important role in the regulatory processes of the nervous system and affects motor activity. To our knowledge, no study has examined the long-lasting patterns and IIV of motor activity following iron-deficiency anemia in human infants.
Aims
This study compared 48-hour motor activity and variability in preschool-aged children with or without iron-deficiency anemia (IDA) in infancy.
Methods
Motor activity was recorded through actigraphs during two week-days in 47 4-year-old Chilean children (23 former IDA and 24 non-anemic in infancy). All were given oral iron as infants. Sleep-wake states were identified by means of automated software. The frequency of movement units per minute was determined for each waking/sleep state during the individual day and night periods; data were examined in blocks of 15 minutes. Analyses of mean frequency and duration and intra-individual variability were conducted using multivariate mixed models.
Results
For daytime sleep, former IDA children were more active without a difference in the total duration. They also spent less time awake throughout the individual day period. Motor activity intra-individual variability was higher in former IDA children.
Conclusions
The findings suggest that IDA in infancy sets the stage for long lasting dysfunction in the neural processes regulating sleep-wake states and spontaneous motor activity patterns.
Keywords: Iron-deficiency, activity patterns, actimeters
Introduction
Being physically active is a critical component of proper development. Motor activity, especially in the first years of life, has a positive impact on child health and motor, cognitive, and socio-emotional well-being [1-6]. Iron deficiency anemia (IDA) in infancy, a common nutrient disorder worldwide, is associated with altered motor activity in animal models and humans [7-9]. However, long-lasting effects of early IDA on motor activity patterns have not been examined in the human. The effect of this nutritional insult on intra-individual variability in motor activity, a marker of the stability of regulatory processes, has also not been investigated.
Iron deficiency and motor activity
Changes in motor activity associated with early IDA have been reported in animal models. Studies in rodent models have reported a wide range of modifications ranging from reduced locomotor activity during the period of IDA [10] to altered circadian patterns of motor activity [11,12]. Two studies that systematically varied the timing of IDA during early development found that IDA rat pups showed decreased activity [13] and moved less in a home-orienting task [12]. In the primate, prenatally iron-deprived infant monkeys showed reduced motor activity and postnatally iron-deprived monkeys slept more at night over a 48-hour period, compared to controls [9]. In addition, a small pilot project involving juvenile monkeys reported dramatic decreases in running and playing, even in those animals with mild iron deficiency anemia [14].
In the adult human, maximal physical performance, submaximal endurance, and work productivity have been shown to be reduced in individuals with IDA and, in some cases, iron deficiency (ID) without anemia [15-19]. Treatment with iron improved physical performance and endurance [20]. Only a few studies have reported motor activity in human infants and children while IDA or after treatment. In a study of play behavior and mother-infant interaction, Lozoff et al. (1998) found lower activity in IDA infants using crude measures (crossing gridlines in a play room, moving beyond arm's length from the mother, etc.) [21]. In a study of 12- to 18-month-old undernourished infants with IDA, Harahap et al. (2000) found lower motor test scores and motor activity scores compared to controls before iron therapy. Following iron treatment, the activity of IDA infants increased to a larger extent than controls [22]. Similar results of lower activity in IDA children were found in Zanzibari toddlers as they initiated independent walking [23]. In a study performed in Chile using actigraph recordings in the home, we found overall increased activity during the day and night, in both waking and sleep states, in 6-month-old infants while IDA [7, 24]. In contrast, they showed decreased motor activity in a laboratory (i.e., unfamiliar) setting after iron treatment (at 12 and 18 months). The few published studies of concurrent iron deficiency and motor activity in school-aged children report lower activity [25].
Motor activity and intra-individual variability
Activity in young children is typically intermittent and includes frequent transitions across different magnitudes of activity [26]. This pattern generates intra-individual variability (IIV). A chronic or acute insult may affect the regulatory processes that guide motor and behavioral performance, leading to altered IIV patterns and, in particular, increased IIV. Several studies show increased IIV in the motor behaviors of aging populations [27] and populations with diseases or disorders, such as stroke [28], post-traumatic brain injury [29], or ADHD in children [30]. For instance, children with ADHD show more IIV in motor timing tasks compared to typically developing peers. In fact, IIV was a better indicator of ADHD than performance alone [30].
In terms of development, IIV shows a U-shaped pattern from birth to adulthood [31-32]. In contrast, magnitude of motor activity shows the opposite, that is, an inverted U-shaped pattern [2]. Such an inverse relationship is unlikely to be explained by a ceiling effect, since physical activity levels can be greatly increased with purposeful exercise practice even at young ages [33]. Typically, children's motor activity increases and IIV decreases with advancing age up to approximately 7-9 years of age [34]. Given the role of iron in myelination, neurometabolism, and neurotransmitter function [35], iron deficiency in infancy might contribute to increased IIV in motor activity. To our knowledge, no study to date has considered this question.
The purpose of the present study was to assess the magnitude of spontaneous motor activity and IIV for 48 continuous hours in the home among preschool-aged children who did or did not experience IDA in infancy. On the basis of the revised literature, we hypothesized that former IDA children would demonstrate lower levels of motor activity but higher levels of IIV compared to control children.
Methods
Participants
This study of motor activity was conducted in conjunction with the neurophysiological components [21] of a larger study in Chile on the long-term behavioral and neuromaturational effects of IDA in infancy. Activity could be measured in only a subset of the children at the preschool follow-up, as funding limitations precluded activity monitoring in all. Nonetheless, we considered that studying spontaneous motor activity for 48 continuous hours with more advanced techniques promised to provide new information about the long-lasting effects of IDA in infancy. Detailed descriptions of the population and other findings during infancy and the preschool period have been published elsewhere [7, 24, 36-38].
Participants in this study had been born healthy, at term, weighing ≥ 3.0 kg and were free of acute or chronic health problems as infants (for further details of inclusion criteria, see [35-36]). Based on a venous blood specimen at 6, 12, or 18 months, IDA and control children were identified. Anemia was defined as a low hemoglobin for age (a venous hemoglobin ≤ 100 g/L at 6 months or < 110 g/L at 12 and 18 months [39]). Iron deficiency was defined as two or more iron measures in the deficient range (mean cell volume < 70 fl [40], erythrocyte protoporphyrin ≥ 1.77 μmol/L [100 μg/dL] red blood cells [41], serum ferritin < 12 μg/L [41]), or an increase in hemoglobin ≥ 10 g/L after 6 months of iron therapy [40]). IDA was defined as anemia plus iron deficiency. Seven infants were identified as IDA at 6 months, 11 at 12 months, and 5 at 18 months. The control group consisted of randomly chosen children who were clearly nonanemic (venous Hb ≥ 115 g/L). Six-month-old infants were treated orally for one year with 15 mg/day of elemental iron as oral ferrous sulphate (Fer-in-Sol®). A venipuncture was repeated at 12 months to determine response to therapy, using hemoglobin level and iron status measures. A finger-stick hemoglobin level was obtained at 18 months to monitor maintenance of response [see refs. 36-37 for full details]. Infants identified at 12 or 18 months were treated with oral iron (30 mg/day) for a minimum of 6 months. Finger-stick hemoglobin levels were also obtained after treatment to monitor maintenance of response. Given that IDA in infancy was very common in the population at the time, infants from the control group underwent the same iron treatment in order to assure they did not become anemic with advancing age. Neither parents nor project personnel were informed of an infant's hematologic status.
All aspects of the study were explained to parents of qualifying children, and signed informed consent was obtained. The research protocol was approved by the Institutional Review Boards of the University of Michigan Medical Center, Ann Arbor, of INTA, University of Chile, Santiago, and of the Office of Protection from Research Risks, NIH.
Of the available preschool-aged children who had been part of the infant studies (former IDA and control groups), we conducted activity monitoring in those who were under 5 years of age, depending on availability of a device. Data were collected for 23 children who had IDA at 6, 12, or 18 months of age and 24 children who had been non-anemic throughout infancy. No child had IDA when blood was collected at 5 ½ years (blood was not obtained at earlier ages in the preschool follow-up). The children were well-nourished with growth at the U.S. 60-70th percentile, on average, upon entry into the study and at the childhood testing time. Their overall development was comparable to that of U.S. infants and children as assessed by the Bayley Scale of Infant Development II [42].
Procedures
As part of the preschool-age follow-up, children were scheduled for an overnight polysomnographic sleep recording [38]. Upon waking in the morning, an actigraph (Ambulatory Monitoring, Inc.) was attached to the child's right ankle with a Velcro band for 2 consecutive days. These actigraphs are computerized activity monitors with a piezo-electric sensor sensitive to accelerations above .01 g per radians/second and an internal memory. The actigraph counted each such acceleration, digitizing and storing in memory the total number of accelerations-decelerations (movement units) per 2-second interval. The weight and dimensions of this device are minimal (56.7 gm, 4.45×3.3×.97 cm) and do not interfere with children' actions.
The subset of children under 5 years of age who received activity monitoring was determined solely by the availability of our limited number of actigraphs. Further, a few actigraphic recordings were technically inadequate. Satisfactory recordings at preschool age were obtained for 23 IDA and 24 controls. There were no differences between children who did or did not have activity data with respect to factors including gender, birth weight, growth, and family background. The exception was the home environment (HOME): children with activity data had more supportive home environments in infancy than those who participated in the preschool follow-up but had no activity data.
Data reduction
The 48-hour actigraph data were downloaded to a PC computer using a special interface unit (Ambulatory Monitoring, AMI, USA). Software from the same company was used to classify each data point as waking, quiet sleep and active sleep on a minute-by-minute basis [43-44]. Following the standard procedures of this software, the actigraph filter was set to a level of 18 to be compatible with this automated algorithm. The software created output summaries for each 15-minute block. Each data file was separated into day 1 and day 2. After each minute was classified as a particular state (wake, active sleep or quiet sleep), we further divided each 24-hour period into day and night on an individual basis (iDay and iNight). iDay was defined as the longest period of waking and iNight as the longest period of sleep for a particular child. To determine the “iDay/iNight” breaking point for each child, we defined an hour as iDay if it had more than 40 minutes of waking or 20-40 minutes of waking followed by an awake hour. Similarly, an hour was defined as iNight if it had 20 or fewer minutes of waking or 20-40 waking minutes preceded by an hour of sleep. Within the hour that contained the “iDay-to-iNight” change, we examined the total number of wake and sleep minutes within each of the four 15-minute blocks. If there were seven wake minutes or less, then the iDay-to-iNight transition was defined at this particular time. Similarly, for the hour that contained the “iNight-to-iDay” change, the transition time was considered to be the 15-minute block in which the total number of wake minutes was more than seven minutes. In 15-minute blocks for each day, we then calculated the total amount of time that a given child was in the following states: awake, active sleep, and quiet sleep during both the iDay and the iNight. We also computed the total number of movement units per 15 minute for each state. The intra-individual variability of each variable was based on the child's standard deviation within the 15-minute blocks of that state.
Statistical analysis
We tested for group differences in child and family background characteristics using the chi-square test for dichotomous and categorical variables and the student t-test for continuous variables. The pooled t-test was selected for equal variances and the Satterthwaite t-test for unequal variances, based on the F-test.
There were no significant differences in motor activity duration between the 1st and 2nd 24-hour periods. We therefore utilized the mean of days 1and 2 in all further analyses. Multivariate analyses of covariance, with gender and age of testing as covariates, were used to determine differences between the former IDA and non-anemic control groups. Data were appropriate for parametric analysis since variables were normally distributed and variances were homogeneous. All analyses were carried out with SAS 9. An alpha level of 0.05 was defined for tests of statistical significance.
Results
Children and family characteristics
There were no differences between IDA and control groups in gender, birth weight and height, gestational age, or growth measures in infancy. There were no differences in mothers' or fathers' education or the home environment (HOME) [45] in infancy, but there was suggestive trend for the IDA group to have somewhat lower socioeconomic status than controls. At the preschool-age follow-up, there were no significant differences in age (approximately 3.7 years in both groups), or gender distribution (see Table 1). Socioeconomic status did not correlate with our dependent variables, but gender and age of testing did, as has been reported by others [2, 33, 46-48]. We therefore used gender and age of testing as covariates in further analyses.
Table 1. Background characteristics of children and family1.
| Group | Former IDA | Control | p-value |
|---|---|---|---|
| Total n | 23 | 24 | |
| Child | |||
| Gender (% male) (n) | 78.3%(18) | 58.3%(14) | .212 |
| Age at preschool follow-up (years) | 3.74±.45 | 3.67±.48 | .529 |
| Characteristics in infancy | |||
| Birth weight (kg) | 3.43±.39 | 3.5±.35 | .513 |
| Birth length (cm) | 50.6±2.42 | 50.9±1.51 | .545 |
| Gestational age (weeks) | 39.3±.96 | 39.5±1.1 | .513 |
| Growth at 6 months | |||
| Weight (kg) | 8.2±.85 | 8.3±.83 | .740 |
| Length (cm) | 67.3±2.09 | 67.2±2.15 | .900 |
| Weight gain birth to 6 months (kg) | 4.8±.73 | 4.8±.74 | .963 |
| Growth in infancy (12 months) | |||
| Weight-for-Age (Z-score) | 0.49±2.34 | 0.27±0.84 | .668 |
| Height-for-Age (Z-score) | 0.41±2.32 | -0.13±0.93 | .322 |
| Iron status at study entry in infancy | |||
| Hemoglobin (g/L) | 101.8±6.4 | 121.3±7.1 | <.001 |
| Mean corpuscular volume (fL) | 68.0±4.1 | 75.3±3.2 | <.001 |
| Erythrocyte protoporphyrin (μg/dL rbcs) | 164.1±97.5 | 86.6±22.1 | .001 |
| Ferritin (μg/L) | 7.7±8.2 | 14.4±11 | .025 |
| Family | |||
| Socioeconomic status index | 29.7±5.69 | 26.0±5.76 | .043 |
| HOME score in infancy | 30.3±4.89 | 32.0±1.00 | .447 |
| Father's education (years) | 9.6±3.17 | 8.68±3.80 | .386 |
| Mother's education (years) | 10.4±2.27 | 9.58±3.02 | .333 |
| Mother's IQ | 85.5±12.74 | 83.9±11.79 | .687 |
Values are means ± SD for continuous variables and percentages (n) for categorical variables. Statistical significance was determined by the Student t-test or Chi square. By design, IDA and nonanemic control group differed in hematologic status with all iron measures being significant. Ns vary due to missing background data in infancy, especially HOME score. Higher scores on the socioeconomic status index indicate lower status.
Hematologic status
Data on hematologic status at the time of IDA was detected in infancy (6, 12, or 18 months) are provided in Table 1. All of the non-anemic infants were iron-sufficient. As expected, all iron-related parameters were significantly worse in the IDA group.
Length of iDay and iNight
There was a suggestive trend for the length of the iDay to be shorter in former IDA children compared to controls (786.9 min ± 81.4 vs. 818.8 min ± 90.1, F(1,43)=3.39, p=.07). Gender was a significant covariate, with females showing shorter length of the iDay compared to males (F(1,43)=5.81, p=.02). Length of the iNight tended to be longer for former IDA compared to controls (654.6 min ± 81.2 vs. 617.9 min ± 104.7) and for females than males (both approaching significance, F(1,43)=3.09, p=.06 and F(1,43)=3.61, p=.08, respectively).
Time of waking and sleeping during the iDay and iNight
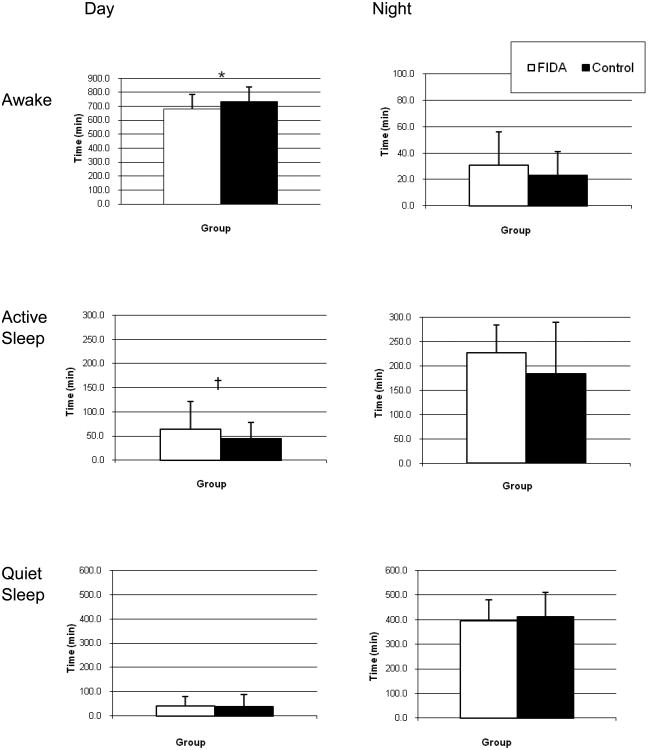
Former IDA children spent less time awake in the iDay than control children (682.1 min ± 102.2 vs. 734.9 min ± 100.9, F(1,43)=4.96, p=.03) (see Figure 1). Gender was a significant covariate with females demonstrating reduced amount of time than males (674.0 min ± 137.9 vs. 725.5 min ± 80.9, F(1,43)=4.47, p=.04). Former IDA children spent 63.9 min ± 57.8 in active sleep during the iDay compared to 44.1 min ± 34.6, in control children. However, this difference was at the limit of a suggestive trend (F(1,43)=2.74, p=.10). Neither gender nor age of testing was significant in the model. There were no other differences between former IDA children and controls in the amount of time spent in each state.
Figure 1.
Total amount of time (mean ± SD) of each waking/sleep state during 24 hours for preschool-aged children with IDA in infancy and controls. * p < 0.05, † suggestive trend (p < .10).
Total motor activity by state during the iDay and iNight
Former IDA children had more leg movements in active sleep during the iDay than controls (3.5 Movement units/15min ± 3.9 vs. 2.0 Movement units/15min ± 1.6, F(1,43)=4.4, p=.04) (see Figure 2). Again, gender was a significant covariate (F(1,43)=4.02, p=.05) with females demonstrating greater motor activity in active sleep during the iDay than males (3.8 Movement units/15min ± 4.8 vs. 2.3 Movement units/15min ± 1.6, respectively). Age of testing was a significant covariate for motor activity in quiet sleep during the iDay, with motor activity in quiet sleep during the iDay decreasing as children's age increased between 38 and 60 months (F(1,43)=7.52, p<.01). There were no other differences between former IDA and controls in the amount of motor activity spent in each state.
Figure 2.
Total motor activity (mean ± SD) in each waking/sleep state during 24 hours for preschool-aged children with IDA in infancy and controls. * p < 0.05.
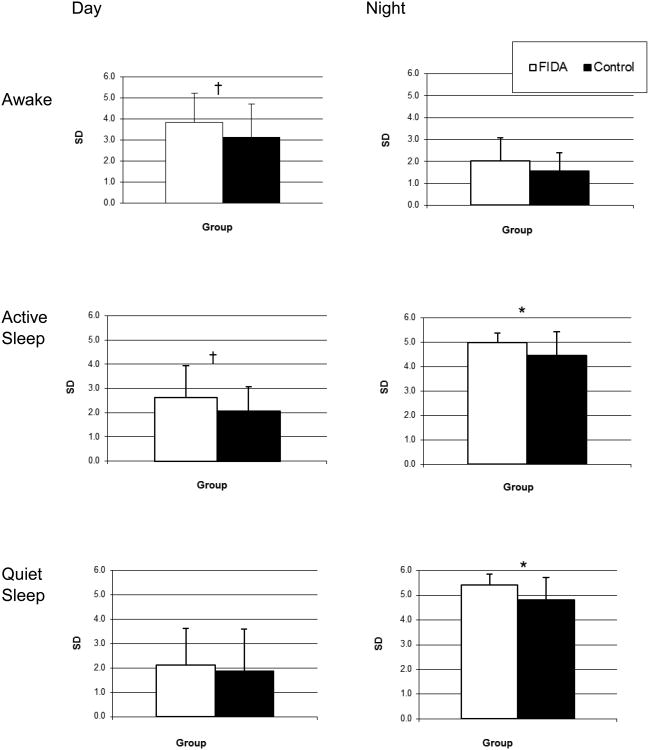
Intra-individual variability (IIV)
Overall, former IDA children showed more IIV in time variables than the control group (see Figure 3). IIV in the amount of time of both active and quiet sleep at iNight was significantly larger in former IDA children than controls (5.0 ± 0.4 vs. 4.4 ± 1.0, 5.4 ± 0.4 vs. 4.8 ± 0.9; F(1,43)=4.4 p=.05, F(1,43)=4.4 p=.02, respectively). During the iDay, awake and active sleep times showed suggestive trends toward larger IIV in former IDA compared to control children (3.8 ± 1.4 vs. 3.1 ± 1.6, F(1,43)=2.7, p=.10; and 2.6 ± 1.3 vs. 2.0 ± 1.0, F(1,43)=3.3, p=.07, respectively). For IIV in the motor activity variables, former IDA children showed larger IIV in active sleep during the iDay compared to controls (114.3 ± 69.3 vs. 83.4 ± 46.2, F(1,43)=4.4, p=.04).
Figure 3.
Intra-individual variability (mean ± SD) for the time variables per waking/sleep state during 24 hours for preschool-aged children with IDA in infancy and controls. * p < 0.05, † suggestive trend (p < .10).
Discussion
This study used objective quantitative techniques to assess long-term effects of IDA in infancy on motor activity and IIV in the home setting. We found that former IDA children spent less time awake during the day and had a higher frequency of leg movements while sleeping during the day than control children. These results seem to suggest that IDA at 6-18 months of age does not produce an overall reduction in motor activity at a later preschool age. However, we consider that lasting effects of IDA depend on the timing and severity of IDA.
In monkeys, for example, prenatally iron-deprived infants showed a reduction in motor activity, but those postnatally deprived demonstrated a tendency to increase motor activity and sleep more during the night [9]. Our results appear in agreement with this postnatal model of ID: former IDA children showed increased activity while asleep during the day, less time awake in the day, and a tendency for more time sleeping at night. These results are also in agreement with our observations in infancy [7] in that we found longer durations of waking and sleep during the night and higher motor activity in the day in IDA infants at 6 months, during the period of IDA. After 6 or 12 months of iron treatment (at 12 or 18 months) only the durations of waking and sleep at night remained increased in former IDA infants. It thus appears that early postnatal IDA derails the normal progression of sleep-wake patterning, as it was also the case for the nighttime temporal organization of sleep states in formerly IDA children at 4 years of age [38]. A tendency towards longer sleep durations at night was found, similar to the monkeys in the postnatal model [9].
Our results also showed that IIV of night-time durations of both active and quiet sleep, were higher in former IDA children compared to controls. This same pattern of increased IIV was also observed while children were awake or in active sleep during the day. Because these states combined account for practically the entire 24-hour period, we can say that the overall effect observed was increased IIV across the 24-hour period. Increased IIV could be interpreted as a neurodevelopmental delay in former IDA children, since IIV should be following a decreasing tendency by this age [31]. The functional relevance of decreasing IIV in childhood has been demonstrated; there is a strong association between improved motor and cognitive performance and reduced IIV [31]. The reduction of IIV with age has been linked to two potential neuronal processes. First, completion of myelination of the CNS could reduce the amount of IIV in the sensorimotor system [49]. Second, elimination of transient connections in the CNS (i.e., pruning) may also decrease IIV, since excess connections create noise and interfere with signal transmission [50]. There is an increase in white matter density in the brain of children with advancing age, suggesting progressive myelination within the brain during childhood [51-53]. Since iron is required for normal myelination, more IIV in former IDA children may indicate a delay in the maturation of the CNS with respect to myelination.
There is only one other study that examined IIV in the context of iron deficiency [54]. In that study, infants' IIV in temperament responses during the first year of life was assessed and related to maternal or newborn iron status. At 3 months, but not at 6 and 12 months, higher IIV correlated with higher maternal hemoglobin during pregnancy and higher neonatal ferritin. Because IIV should be higher in the very early phase of development, it is not surprising such a positive correlation was observed only at the 3-month assessment. In our study, IIV may not have reduced to the expected level for age in IDA children, but longitudinal studies would be necessary to confirm this possibility. Furthermore, neural processes involved in the developing progression and establishment of temperament and IIV in either the patterning of sleep-wake episodes length or motor activity modulation are likely to be different.
The tendency towards shorter day length and longer night length in the IDA group compared to controls may indicate alterations in the organization or regulation of the circadian pattern. IDA has been reported by some investigators to alter or even reverse the diurnal pattern of motor activity in animal models [11,12]. Taken together, the long-lasting effects of early IDA imply temporal dysregulation at the level of day/night (diurnal patterns) and the level of individual variability in state durations.
Iron is involved in many central nervous system processes that could affect infant behavior and development [35]. Since IDA alters the functioning of several central neurotransmission systems, neuronal metabolism, and both the quantity and quality of myelin, it may disrupt the modulation of motor activity and IIV within waking and sleep. There is ample reason to suspect that iron deficiency alters spontaneous motor activity [7-10], as well as sensory systems [36] and sleep patterns [39,55], all of which are critical contributors to well-regulated behavior throughout a 24-hour period. Furthermore, the lasting effects of early IDA apparently depend on timing, with different effects for IDA or iron deprivation pre- or post-natally. These interpretations would benefit from study replication. Such possibilities should be considered in future longitudinal studies or randomized controlled trials of iron supplementation.
Several limitations should be recognized in this study. The sample is small, and therefore caution should be taken in generalizing the findings. IIV was evaluated as the standard deviation value; other methodologies in the frequency domain may have been more informative about the structure of the variability, but the nature of our data did not allow such analyses. Furthermore, actigraphy is an adequate method to evaluate sleep/wake patterns, but when other more specific sleep parameters are needed it produces inconsistent results. Namely the relative inability to identify sleep and wake during short periods of high motility during sleep, as well as during periods of wakefulness without motion, and a tendency to overestimate activity during sleep. However, the same methodology was applied to both groups.
Conclusions
Altered motor activity patterns, day/night wake/sleep durations, and higher IIV in the former IDA group point to previously unrecognized but long-lasting effects of early IDA. Given the functional relevance of IIV for motor and cognitive performance (higher IIV associated with poorer performance at this age) [32], the results of this study add to the growing evidence of enduring consequences of early IDA.
As a final note, many studies of physical activity levels in preschool children ([44-45] for recent studies and review) examine daytime activity without considering sleep patterns. Conversely, “the mysteries of sleep may be lost to the sleep researcher who does not also study wakefulness” [56]. An approach that ignores either wakefulness or sleeping may not be as informative or relevant for overall performance in childhood and perhaps even throughout the life span.
Acknowledgments
This research was funded by grants from the U.S. National Institutes of Health (R01 HD33487 and T37 TW00035) and the Chilean Agency for Funding in Science and Technology (FONDECYT,# 1110513). We are grateful to the children and their parents for their cooperation and to Agustin Calatroni for valuable statistical assistance.
Funded by the U.S. National Institutes of Health (R01 HD33487 and T37 TW00035) and the Chilean Agency for Funding in Science and Technology (FONDECYT 1110513) grants.
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflict of interest
Authors' Contributions: RAB carried out the data reduction, participated in the data analysis and drafted the manuscript. PP and CA participated in the design of the study and carried out data collection. NK performed the statistical analysis. BL conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
R.M. Angulo-Barroso, Email: rangulo@gencat.cat, rangulo@umich.edu.
P. Peirano, Email: ppeirano@inta.uchile.cl.
C. Algarin, Email: calgarin@inta.uchile.cl.
N. Kaciroti, Email: nicola@umich.edu.
B. Lozoff, Email: blozoff@umich.edu.
References
- 1.Berthenthal BI, Campos JJ, Barrett KC. Self-produced locomotion: An organizer of emotional cognitive, and social development in infancy. In: Emde RN, Harmon RJ, editors. Continuity and discontinuities in development. New York: Plenum; 1984. pp. 175–209. [Google Scholar]
- 2.Campbell DW, Eaton WO, McKeen NA. Motor Activity level and behavioral control in young children. Int J Behav Dev. 2002;26(4):289–296. [Google Scholar]
- 3.Hume C, Okely A, Bagley S, Telford A. Does weight status influence associations between children fundamental movement skills and physical activity? Res Q Exerc Sport. 2008;79(2):158–165. doi: 10.1080/02701367.2008.10599479. [DOI] [PubMed] [Google Scholar]
- 4.Kermoian R, Campos JJ. Locomotor experience: A facilitator of spatial cognitive development. Child Dev. 1988;59:908–917. [PubMed] [Google Scholar]
- 5.Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: A meta-analysis. Ped Research Science. 2003;15(3):243–256. [Google Scholar]
- 6.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev. 2002;70:85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 8.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 9.Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt JR, Zito CA, Erjavec J, Johson LK. Severe or marginal iron deficiency affects spontaneous physical activity in rats. Am J Clin Nutr. 1994;59:413–418. doi: 10.1093/ajcn/59.2.413. [DOI] [PubMed] [Google Scholar]
- 11.Glover J, Jacobs A. Activity pattern of iron-deficient rats. BMJ. 1972;2:627–628. doi: 10.1136/bmj.2.5814.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youdim MBH, Yehuda S, Ben-Uriah Y. Iron deficiency-induced circadian rhythm reversal of dopaminergic mediated behaviours and thermoregulation in rats. Eur J Pharmacol. 1981;74:295–301. doi: 10.1016/0014-2999(81)90048-0. [DOI] [PubMed] [Google Scholar]
- 13.Piñero D, Jones B, Beard J. Variations in dietary iron alter behavior in developing rats. J Nutr. 2001;131(2):311–318. doi: 10.1093/jn/131.2.311. [DOI] [PubMed] [Google Scholar]
- 14.Munro N. A three year study of iron deficiency and behavior in rhesus monkeys. Int J Biosoc Res. 1987;9:35–62. [Google Scholar]
- 15.Lozoff B, Brittenham GM. Behavioral aspects of iron deficiency. Prog Hematol. 1986;14:23–53. [PubMed] [Google Scholar]
- 16.Dallman PR. Iron deficiency: distinguishing the effects of anemia from muscle iron deficiency on work performance. In: Saltman P, Hegenauer J, editors. The biochemistry and physiology of iron. Amsterdam: Elsevier North Holland; 1982. pp. 509–523. [Google Scholar]
- 17.Edgerton VR, Gardner GW, Ohira Y, Gunawardena KA, Senewiratne B. Iron deficiency anemia and its effect on worker productivity and activity patterns. BMJ. 1979;2:1546–1549. doi: 10.1136/bmj.2.6204.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu YI, Haas JD. Altered metabolic response of iron-depleted nonanemic women during a 15-Km time trial. J Appl Psychol. 1998;256:E401–405. doi: 10.1152/jappl.1998.84.5.1768. [DOI] [PubMed] [Google Scholar]
- 19.Haas JD, Brownlie T. J Nutre. 2S-II. Vol. 131. Geneva: WHO; 2001. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Belmont Conference: critical review of evidence that iron deficiency anemia causes reduced work capacity; pp. 676S–690S. [Google Scholar]
- 20.Li R, Chen X, Yan H, Deurenberg P, Garby L, Hautvast JG. Functional consequences of iron supplementation in iron-deficient female cotton mill workers in Beijing, China. Am J Clin Nutr. 1994;59(4):908–913. doi: 10.1093/ajcn/59.4.908. [DOI] [PubMed] [Google Scholar]
- 21.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 22.Harahap H, Jahari AB, Husaini M, Saco-Pollitt C, Pollitt E. Effects of an energy and micronutrient supplement on iron deficiency anemia, physical activity, and motor and mental development in undernourished children in Indonesia. Eur J Clin Nutr. 2000;54:S114–119. doi: 10.1038/sj.ejcn.1601011. [DOI] [PubMed] [Google Scholar]
- 23.Olney DK, Pollit E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Mast D, Allen LH, Stoltzfus RJ. Young Zanzibari children with iron deficiency, iron deficiency anemia, stunting, or malaria have lower motor activity scores and spend less time in locomotion. J Nutr. 2007;137(12):2756–2762. doi: 10.1093/jn/137.12.2756. [DOI] [PubMed] [Google Scholar]
- 24.Angulo-Kinzler RM, Peirano P, Lin E, Garrido M, Lozoff B. Motor activity in human infants with iron-deficiency anemia. Early Hum Dev. 2002;66(2):67–79. doi: 10.1016/s0378-3782(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia D, Seshadri S. Anemia, undernutrition and physical work capacity of young boys. Indian Pediatr. 1987;(24):133–139. [PubMed] [Google Scholar]
- 26.Bailey R, Olson J, Pepper S, Porszasz J, Barstow T, Cooper D. The level and tempo of children's physical activities: An observational study. Med Sci Sports Exerc. 1995;27:1033–1041. doi: 10.1249/00005768-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Newell KM, Mayer-Kress G, Liu YT. Aging, time scales and sensorimotor variability. Psychol Aging. 2009;24(4):809–818. doi: 10.1037/a0017911. [DOI] [PubMed] [Google Scholar]
- 28.Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby G. Allowing intralimb kinematic variability during locomotion training poststroke improves kinematic consistency: A subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89(8):829–839. doi: 10.2522/ptj.20080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz-Leure M, Rotem H, Keren O, Meyer S. The relationship between step variability, muscle strength and functional walking performance in children with post-traumatic brain injury. Gait Posture. 2009;29:154–157. doi: 10.1016/j.gaitpost.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Rommelse NNJ, Altink ME, Oosterlaan J, Beem L, Buschgens CJM, Buitelaar J, Sergeant JA. Speed, variability, and timing of motor output in ADHD: Which measures are useful for endophenotypic research? Behav Genet. 2008;38:121–132. doi: 10.1007/s10519-007-9186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deutsch KM, Newell KM. Noise, variability, and the development of children's perceptual-motor skills. Dev Reviews. 2005;25:155–180. [Google Scholar]
- 32.Nesselroade JR, Salthouse TA. Methodological and theoretical implications of intraindividual variability in perceptual-motor performance. Journal of Gerontology: Physiological sciences. 2004;59B(2):49–55. doi: 10.1093/geronb/59.2.p49. [DOI] [PubMed] [Google Scholar]
- 33.Davies PSW, Feng JY, Crisp JA, Day JME, Laidlaw A, Chen JD, Liu XP. Total energy expenditure and physical activity in young Chinese gymnasts. Pediatric exercise science. 1997;9(3):243–252. [Google Scholar]
- 34.Eaton WO, McKeen NA, Campbell DW. The waxing and waning of movement: Implications for psychological development. Dev Review. 2001;21:205–223. [Google Scholar]
- 35.Beard JL, Connor JR. Iron status and neural functioning. Ann Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 36.Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron Deficiency Anemia in Infancy: Long-Lasting Effects on Auditory and Visual System Functioning. Pediatr Res. 2003;53(2):217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- 37.Walter T, Pino P, Pizarro F, Lozoff B. Prevention of iron-deficiency anemia: comparison of high- and low iron formula in full-term healthy infants. J Pediatr. 1998;132:635–640. doi: 10.1016/s0022-3476(98)70352-x. [DOI] [PubMed] [Google Scholar]
- 38.Peirano PD, Algarin CR, Garrido MI, Lozoff B. Iron deficiency anemia in infancy is associated with altered temporal organization of sleep states in childhood. Pediatr Res. 2007;62(6):715–719. doi: 10.1203/PDR.0b013e3181586aef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan DG, Orkin SH. Hematology of infancy and childhood. Philadelphia: Saunders; 1998. [Google Scholar]
- 40.Dallman PR, Reeves JD, Driggers DA, Lo YET. Diagnosis of iron deficiency: the limitations of laboratory tests in predicting response to iron treatment in 1-year-old infants. J Pediatr. 1981;98:376–381. doi: 10.1016/s0022-3476(81)80321-6. [DOI] [PubMed] [Google Scholar]
- 41.Deinard AS, Schwartz S, Yip R. Developmental changes in serum ferritin and erythrocyte protoporphyrin in normal (nonanemic) children. Am J Clin Nutr. 1983;38:71–75. doi: 10.1093/ajcn/38.1.71. [DOI] [PubMed] [Google Scholar]
- 42.Bayley N. Bayley Scales of Infant Development. 2nd. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- 43.Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep –wake patterns. Pediatrics. 1991;87:494–499. [PubMed] [Google Scholar]
- 44.Sadeh A, Acebo C, Seifer R, Aytur S, Carskadon MA. Activity based assessment of sleep–wake patterns during 1st year of life. Infant Beh Dev. 1995;18:329–337. [Google Scholar]
- 45.Caldwell BM, Bradley RH, et al. Home observation for measurement of the environment. Little Rock, AK: University of Arkansas; 1984. [Google Scholar]
- 46.Cardon GM, De Bourdeaudhuij IMM. Are preschool children active enough?Objectively measured physical activity levels. Res Q Exerc Sport. 2008;79:326–332. doi: 10.1080/02701367.2008.10599496. [DOI] [PubMed] [Google Scholar]
- 47.Tucker P. The physical activity levels of preschool-aged children: A systematic Review. Early Chil Res Q. 2008;23:547–558. [Google Scholar]
- 48.Trost SG, Sirard JR, Dowda M, Pfeiffer KA, Pate RR. Physical activity in overweight and nonoverweight preschool children. Int J Obes Relat Metab Disord. 2003;27(7):834–839. doi: 10.1038/sj.ijo.0802311. [DOI] [PubMed] [Google Scholar]
- 49.Plude DJ, Enns JT, Brodeur D. The development of selective attention: A life-span overview. Acta Psychol. 1994;(86):227–272. doi: 10.1016/0001-6918(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 50.Kail R. The neural noise hypothesis: Evidence from processing speed in adults with multiple sclerosis. Aging Neuropsychol Cogn. 1997;4:157–165. [Google Scholar]
- 51.Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 52.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 53.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific Publications; 1967. pp. 3–70. [Google Scholar]
- 54.Wachs TD, Creed Kanashiro H, Gurkas P. Intra-individual variability in infancy: Structure, stability and nutritional correlates. Dev Psychobiol. 2008;50(3):217–231. doi: 10.1002/dev.20284. [DOI] [PubMed] [Google Scholar]
- 55.Peirano PD, Algarin CR, Chamorro RA, Reyes SC, Durán SA, Garrido MI, Lozoff B. Sleep alterations and iron deficiency anemia in infancy. Sleep Med. 2010;11:637–642. doi: 10.1016/j.sleep.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleitman N. Study wakefulness Study the rest-activity cycle. Don't just study sleep. Int Psychiatry Clin. 1970;7:381–384. [PubMed] [Google Scholar]