Abstract
Background
Pulmonary emphysema is divided into three major subtypes at autopsy: centrilobular, paraseptal and panlobular emphysema. These subtypes can be defined by visual assessment on computed tomography (CT); however, clinical characteristics of emphysema subtypes on CT are not well-defined. We developed a reliable approach to visual assessment of emphysema subtypes on CT and examined if emphysema subtypes have distinct characteristics.
Methods
The Multi-Ethnic Study of Atherosclerosis COPD Study recruited smokers with COPD and controls age 50–79 years with ≥10 pack-years. Participants underwent CT following a standardized protocol. Definitions of centrilobular, paraseptal and panlobular emphysema were obtained by literature review. Six-minute walk distance and pulmonary function were performed following guidelines.
Results
Twenty-seven percent of 318 smokers had emphysema on CT. Inter-rater reliability of emphysema subtype was substantial (K:0.70). Compared to participants without emphysema, individuals with centrilobular or panlobular emphysema had greater dyspnea, reduced walk distance, greater hyperinflation, and lower diffusing capacity. In contrast, individuals with PSE were similar to controls, except for male predominance. Centrilobular but not panlobular or paraseptal emphysema was associated with greater smoking history (+21 pack-years P<0.001). Panlobular but not other types of emphysema was associated with reduced body mass index (−5 kg/m2;P=0.01). Other than for dyspnea, these findings were independent of the forced expiratory volume in one second. Seventeen percent of smokers without COPD on spirometry had emphysema, which was independently associated with reduced walk distance.
Conclusions
Emphysema subtypes on CT are common in smokers with and without COPD. Centrilobular and panlobular emphysema but not paraseptal emphysema have considerable symptomatic and physiological consequences.
KEWWORDS: Emphysema, computed tomography, centrilobular, paraseptal, panlobular
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) and emphysema are, together, the third leading cause of death in the United States.1 COPD is defined by airflow limitation that is not fully reversible on spirometry and overlaps partially with pulmonary emphysema, which is defined anatomically as permanent enlargement of air spaces distal to the terminal bronchiole, accompanied by destruction of their walls.2,3 Although emphysema is classically assessed on gross and microscopic pathology, computed tomography (CT) allows in vivo assessment of pulmonary emphysema at the macroscopic level.4,5
Subtypes of emphysema have been described, but there is no universally accepted classification system: centrilobular emphysema is commonly described as an abnormal enlargement of airspaces centered on the respiratory bronchiole with coalescence of destroyed lobules in severe cases; panlobular emphysema is often characterized as abnormal dilation distributed throughout the pulmonary lobule; and paraseptal emphysema refers to emphysematous change adjacent to a pleural surface.3,6–10
The classic, autopsy-based literature suggests that centrilobular emphysema is predominantly smoking-related, whereas panlobular emphysema is not related to smoking in the general population,11,12 being caused, in a minority of cases, by variants in the SERPINA1 gene (alpha1-antitrypsin deficiency).13 The clinical significance of paraseptal emphysema is uncertain, though spontaneous pneumothorax is thought to result from rupture of a paraseptal bleb/bulla.14 Autopsy studies, however, have obvious limitations and it is unclear whether emphysema subtypes defined by radiologist interpretation on CT have similarly distinct clinical characteristics.
Emphysema detected by radiologist interpretation on CT has previously been correlated with gross pathology,5,15–20 and is associated with important clinical outcomes, including mortality,21 lung cancer,22 and airflow obstruction.5,15,23–26 However, there is often only modest inter-rater agreement among radiologists for emphysema subtypes.5,17–19,27,28
We therefore developed a reliable approach to visual assessment of emphysema subtypes on CT in order to examine clinical characteristics of emphysema subtypes in a multicenter study of smokers drawn predominantly from the general population.
MATERIALS AND METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study recruited cases of COPD and controls predominantly from MESA, a population-based prospective cohort study of subclinical atherosclerosis,29 and the Emphysema and Cancer Action Project (EMCAP), a separate, non-overlapping lung cancer screening study,30 and also from the outpatient community at Columbia University Medical Center. Included participants were 50–79 years of age with ≥10 pack-year smoking history. Exclusion criteria were clinical cardiovascular disease, stage IIIb-V chronic kidney disease, asthma prior to age 45 years, prior lung resection, contraindication to magnetic resonance imaging and pregnancy.
Protocols for this study were approved by the institutional review board of participating institutions and by the National Heart, Lung, and Blood institute. Written informed consent was obtained from all participants.
Visual Assessment of Emphysema Subtypes (See Supplementary Appendix for Additional Details)
Reliability of emphysema subtype assessment was assessed first in a training set of 40 CT scans from participants selected randomly in EMCAP30 who were not in the MESA COPD Study and verified in an independent validation set of all 127 participants who were recruited into the MESA COPD Study from EMCAP and the community. Scans for the remaining 192 MESA COPD Study participants were read by a single rater (J.H.M.A.).
CT Acquisition
All thoracic CT scans were acquired at suspended inspiration without intravenous contrast and reconstructed using a high spatial contrast algorithm with 0.75 mm slice thickness. The training set scans were acquired on a Siemens 16 multi-detector scanner and all MESA COPD Study scans were acquired on Siemens and GE 64-slice scanners. All scans were acquired at 120 kVp, 0.5 seconds, with milliamperes (mA) set by body mass index for MESA participants (145 for <20 kg/m2, 180 for 20–30 kg/m2 and 270 for >30 kg/m2) following the MESA Lung/SPIROMICS protocol,31 and 200 mA for EMCAP participants.
Raters
Four chest radiologists from two academic medical centers independently assessed emphysema subtypes at CT without clinical information. Measurement of image density was not permitted.
Visual Emphysema Subtype Assessment
Radiologists used an electronic score sheet to record the extent of each emphysema subtype assessed visually on CT (Supplementary Table S1). Definitions of emphysema subtypes were based on review of the literature: 3,6–10
Centrilobular emphysema
Focal regions of low attenuation, surrounded by normal lung attenuation, located within the central portion of secondary pulmonary lobules. As severity increases, vessels appear “pruned” and low attenuation regions enlarge.
Panlobular emphysema
Diffuse regions of low attenuation involving entire secondary pulmonary lobules. As severity increases, paucity of peripheral vessels increases.
Paraseptal emphysema
Regions of low attenuation adjacent to visceral pleura (including fissures).
Raters assigned separate scores for the upper, mid and lower zones of the right and left lung. The extent of emphysema was defined as the percentage (0 to 100%) of the lung zone affected by each subtype.
Upon completion of training set assessment by all raters, reference images were selected based on all four raters independently agreeing on the isolated presence of each emphysema subtype (Figure 1).
FIGURE 1. Reference Images for Absence of Emphysema and Emphysema Subtypes.
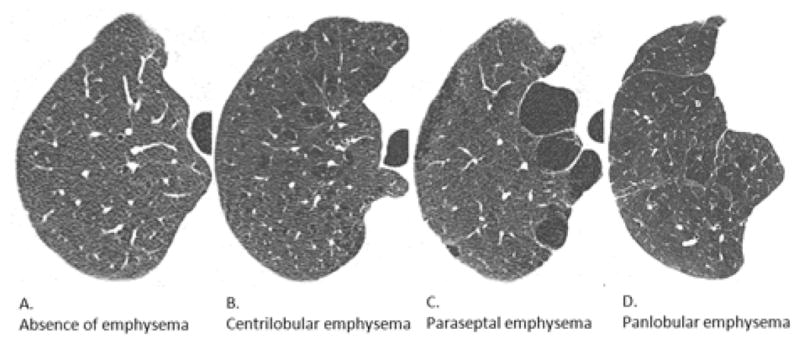
Axial CT images were selected from training set scans in which all four raters independently agreed on the absence or isolated presence of each emphysema subtype. A. Absence of emphysema; B. Centrilobular emphysema; C. Paraseptal emphysema; D. Panlobular emphysema.
Abbreviation: CT denotes computed tomography.
Emphysema Subtypes and Clinical Characteristics
Lung function and six-minute walk test
Body plethysmography, single breath diffusing capacity of carbon monoxide (DLCO), post-bronchodilator spirometry, and six-minute walk distance (6MWD) were assessed following American Thoracic Society (ATS) recommendations.32–35 Predicted lung function and 6MWD values were calculated using reference equations.36–40 COPD was defined as post-bronchodilator ratio of forced expired volume in one second to forced vital capacity (FEV1/FVC) less than 0.7 and spirometric severity as mild (FEV1≥80% predicted), moderate (50%≤FEV1<80% predicted, severe or very severe (<50% predicted).41
Lung Density Assessment
Attenuation was assessed using standard reconstruction CT images with APOLLO software (VIDA Diagnostics, Coralville, IA).42 Percent of emphysema-like lung was defined as the percentage of total voxels within the lung field below −950 Hounsfield units (percent emphysema-950HU).43
Anthropometry, Demographics, and other Co-Variates
Height, weight, and white blood cell (WBC) count were measured, and body mass index (BMI) calculated by standardized protocol. Race-ethnicity was self-reported and dyspnea was assessed using the 5 level (0 to 4) modified Medical Research Council (mMRC) dyspnea scale.44 Resting arterial hemoglobin saturation was estimated by pulse oximetry (SpO2, CMS-50F, Contec Medical Systems, Hebei, China). Low SpO2 was defined as a saturation ≤95% while breathing ambient air or long-term use of supplemental oxygen. Smoking history was confirmed with plasma or urine cotinine levels.45
Statistical Analysis
Dichotomous variables are presented as proportions and continuous variables as means with standard deviation unless otherwise indicated.
Primary analysis assessed the reliability of visual assessment of centrilobular, paraseptal and panlobular emphysema affecting both lungs by summing the percent severity for upper, mid and lower zones of both lungs and dividing by six. For dichotomous analyses the presence of an emphysema subtype was defined as ≥1.0% of the lung volume affected. Unweighted Cohen’s K statistic was computed for presence of an emphysema subtype. The level of agreement was interpreted as follows: >0.2: poor, 0.21–0.4: fair, 0.41–0.6: moderate, 0.61–0.80: substantial, and 0.81–1.0: excellent.46 Reliability of subtype severity assessment was estimated with intra-class correlation coefficient (ICC).
For associations between clinical characteristics and visually detected emphysema subtypes, emphysema subtype scores were averaged for CT scans with multiple raters. The predominant emphysema subtype was defined as the subtype affecting the greatest percentage of lung. In order to obtain unbiased estimates of emphysema subtype prevalence in the source population, analyses were weighted by the ratio of COPD prevalence in the source study to that in the MESA COPD Study, as previously described.47 Clinical characteristics of individuals with centrilobular-, paraseptal-, and panlobular-predominant emphysema were compared to individuals without emphysema using bivariate and multivariate regression, adjusting for age, gender, race-ethnicity and smoking status. Additional adjustment for percent predicted FEV1 and percent emphysema<-950HU was performed in sensitivity analyses. Dunnett’s procedure was used to adjust P-values for multiple pairwise comparisons against participants without emphysema.
All calculations were performed using SAS 9.3 (Cary, NC) with a hypothesis testing alpha level of 0.05.
RESULTS
Reliability of Visual Emphysema Subtype Assessment
Characteristics of participants included in the training and validation sets are summarized in Table S2. The mean age was 68 years in both groups and approximately half were male.
In the training set, intra-reader agreement for the presence of centrilobular, paraseptal, and panlobular emphysema was substantial-to-excellent (Table S3). Inter-reader agreement was moderate-to-substantial for centrilobular and paraseptal emphysema although poor for panlobular emphysema. Nonetheless, reliability of severity of emphysema was moderate-to-excellent for all subtypes for both intra-reader and inter-reader assessment. In the validation set, findings were generally similar (Table S4). The inter-reader agreement for predominant emphysema subtype was substantial (κ:0.70; 95%CI:0.59 to 0.80).
Prevalence of Emphysema Subtypes
Of 321 MESA COPD Study participants, three CT scans had excessive motion artifact preventing assessment of emphysema subtypes. Among the 318 participants included in the analysis, mean age was 68±7 years, 60% were male, and 48% had COPD that was predominantly moderate in severity (39% mild, 47% moderate, and 14% severe).
The estimated population prevalence of emphysema was 27% (95%CI:21–32%), with centrilobular-predominant emphysema being most common (14%; 95%CI:10–18%), followed by paraseptal-predominant (9%; 95%CI:6–12%), and panlobular-predominant emphysema (4%; 95%CI:1–6%). Among participants with any emphysema, multiple subtypes were present in 57%, with co-existent centrilobular and paraseptal emphysema being most frequent (Figure 2).
FIGURE 2. Proportions of MESA COPD Study Participants with One or Multiple Subtypes of Emphysema.
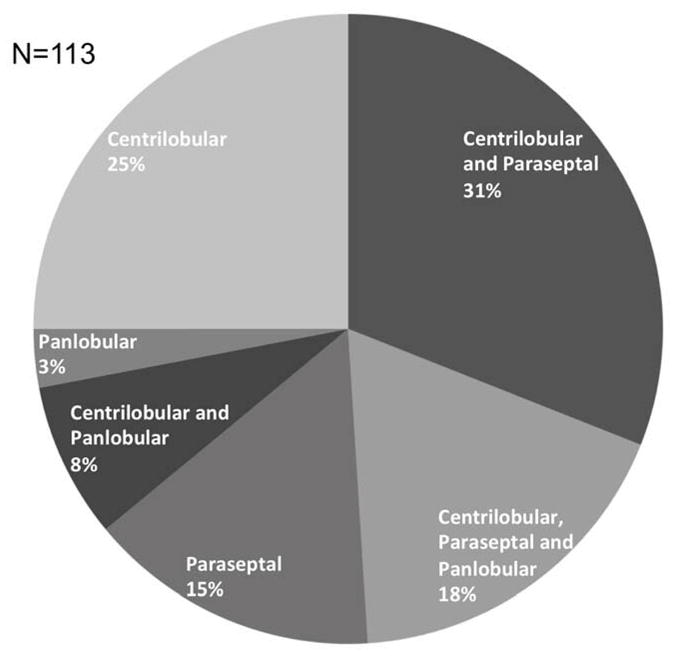
Presence of each emphysema subtype was defined as ≥1% of the lung volume affected. Proportions are weighted to reflect distribution in the source population (see methods for details).
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, and COPD chronic obstructive pulmonary disease.
Clinical Characteristics of Emphysema Subtypes
Age and race/ethnicity were similar across predominant emphysema subtypes when compared to participants without emphysema, whereas paraseptal emphysema occurred more frequently in men (Table 1). Current smoking was more common among participants with all emphysema subtypes compared to participants without emphysema.
Table 1.
Clinical Characteristics of MESA COPD Study Participants by Predominant Emphysema Subtype.
| Mean or proportion of clinical characteristic† P-value adjusted for multiple subtype comparisons* |
Global P-value** | ||||
|---|---|---|---|---|---|
| Clinical characteristic | Absence of emphysema N=205 (73%) |
Centrilobular predominant emphysema N=65 (14%) |
Paraseptal predominant emphysema N=33 (9%) |
Panlobular predominant emphysema N=15 (4%) |
|
| Age – years | 68±7 Reference |
68±5 P=1.00 |
68±7 P=1.00 |
69±5 P=0.97 |
0.56 |
| Proportion male – % | 120 (54) Reference |
35 (53) P=1.00 |
28 (78) P=0.02 |
8 (58) P=0.99 |
0.02 |
| Race/ethnicity – no. (%) | |||||
| Caucasian | 101 (51) Reference |
43 (56) P=0.92 |
13 (44) P=0.82 |
11 (57) P=0.97 |
0.74 |
| African American | 50 (22) Reference |
18 (33) P=0.30 |
14 (35) P=0.32 |
3 (23) P=1.00 |
0.25 |
| Other | 54 (27) Reference |
4 (11) P=0.08 |
6 (21) P=0.90 |
1 (20) P=0.95 |
0.10 |
| Proportion of current smokers – % | 45 (17) Reference |
25 (40) P=0.003 |
15 (45) P=0.004 |
8 (53) P=0.02 |
<0.001 |
Means and proportions are weighted to reflect distribution in the source population (see methods for details).
P-values comparing emphysema subtypes to a control group (absence of emphysema) are adjusted for multiple comparisons using Dunnett’s procedure.
Global p-values are from Kruskal- Wallis, Chi-square, or Fisher exact tests where appropriate.
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, and COPD chronic obstructive pulmonary disease.
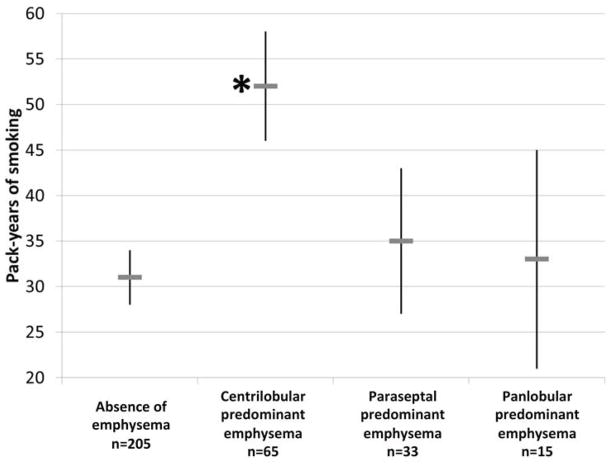
Individuals with centrilobular-predominant emphysema had significantly higher number of pack-years compared to participants without emphysema (Figure 3A). Individuals with centrilobular-predominant emphysema were also more likely to report grade 2 or higher mMRC dyspnea and have a shorter 6MWD compared to controls, in addition to greater hyperinflation, lower diffusing capacity, higher percent emphysema<-950HU, and higher WBC count after adjustment for age, gender, race-ethnicity and current smoking status (Table 2). Associations of centrilobular-predominant emphysema with pack-years, 6MWD, hyperinflation, diffusing capacity, lung density, and WBC count remained significant after additional adjustment for percent predicted FEV1 (Table 3). The proportion of individuals with low SpO2 was also greater with centrilobular--predominant emphysema compared to those without emphysema, but this comparison did not achieve statistical significance (Table 2).
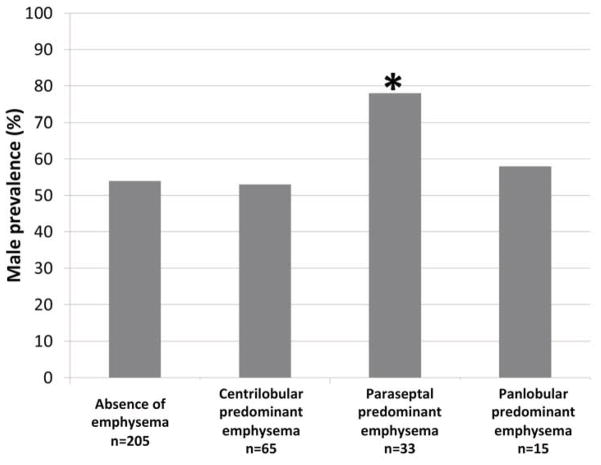
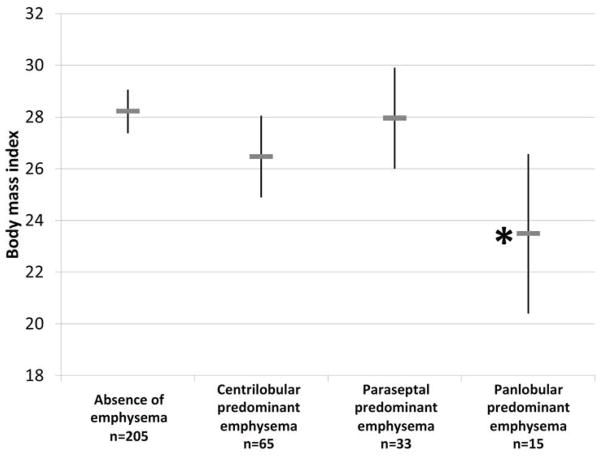
FIGURE 3. Male Gender, Body Mass Index and Pack-Years of Smoking among MESA COPD Study Participants by Emphysema Subtype.
*Indicates p<0.05 for bivariate comparison of emphysema subtype versus control group (no emphysema) adjusted for multiple subtype comparisons using Dunnett’s procedure. Pack-years, gender prevalence, and body mass are weighted to reflect distribution in the source population (see methods for details). A. Pack-years of smoking; B. Gender; and C. Body mass index.
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, COPD chronic obstructive pulmonary disease.
Table 2.
Clinical Characteristics of MESA COPD Study Participants by Predominant Emphysema Subtype.
| Clinical characteristic | Predicted mean or proportion of clinical characteristic adjusted for age, gender, race-ethnicity and smoking status† (95% CI) P-value adjusted for multiple subtype comparisons* |
Global P-value** | |||
|---|---|---|---|---|---|
| Absence of emphysema N=205 (73%) |
Centrilobular predominant emphysema N=65 (14%) |
Paraseptal predominant emphysema N=33 (9%) |
Panlobular predominant emphysema N=15 (4%) |
||
| Height – cm | 166 (165 to 167) Reference |
168 (166 to 169) P=0.41 |
165 (163 to 167) P=0.82 |
169 (166 to 173) P=0.27 |
0.13 |
| Weight – kg | 78 (75 to 80) Reference |
75 (70 to 79) P=0.51 |
77 (71 to 82) P=0.96 |
67 (58 to 76) P=0.07 |
0.11 |
| Body mass index | 28 (27 to 29) Reference |
26 (25 to 28) P=0.13 |
28 (26 to 30) P=0.99 |
23 (20 to 27) P=0.01 |
0.009 |
| Pack-years | 31 (28 to 34) Reference |
52 (46 to 58) P<0.001 |
35 (27 to 43) P=0.70 |
33 (21 to 45) P=0.99 |
<0.001 |
| Proportion with mMRC dyspnea scale ≥2 – %a | 7.9 (2.2 to 25) Reference |
26 (14 to 44) P<0.001 |
7.5 (2.0 to 24) P=1.00 |
29 (9.4 to 62) P=0.03 |
0.001 |
| Proportion with COPD – % | 15 (4 to 47) Reference |
54 (32 to 74) P<0.001 |
29 (12 to 55) P=0.08 |
48 (16 to 82) P=0.04 |
<0.001 |
| Percent predicted FEV1 | 97 (93 to 100) Reference |
87 (81 to 93) P=0.009 |
91 (93 to 98) P=0.37 |
86 (74 to 98) P=0.23 |
0.008 |
| Percent predicted FVC | 96 (94 to 99) Reference |
100 (95 to 105) P=0.33 |
95 (89 to 101) P=0.99 |
104 (94 to 114) P=0.33 |
0.20 |
| FEV1/FVC | 0.76 (0.75 to 0.77) Reference |
0.64 (0.61 to 0.67) P<0.001 |
0.73 (0.69 to 0.76) P=0.20 |
0.62 (0.56 to 0.68) P<0.001 |
<0.001 |
| Percent predicted RV‡ | 82 (74 to 90) Reference |
101 (90 to 113) P=0.01 |
96 (78 to 114) P=0.36 |
110 (90 to 130) P=0.02 |
<0.001 |
| Percent predicted FRC‡ | 91 (86 to 96) Reference |
108 (101 to 116) P<0.001 |
95 (82 to 107) P=0.89 |
125 (111 to 138) P<0.001 |
<0.001 |
| Percent predicted TLC‡ | 92 (88 to 95) Reference |
102 (97 to 107) P=0.002 |
98 (89 to 106) P=0.41 |
104 (95 to 113) P=0.03 |
<0.001 |
| Percent predicted DLCO/VA‡ | 79 (74 to 83) Reference |
64 (59 to 70) P<0.001 |
81 (72 to 90) P=0.96 |
65 (56 to 75) P=0.03 |
<0.001 |
| Percent predicted 6MWDa | 92 (88 to 95) Reference |
80 (74 to 87) P=0.005 |
89 (81 to 98) P=0.91 |
73 (59 to 87) P=0.03 |
<0.001 |
| Percent emphysema<-950HU | 1.0 (0.8 to 1.2) Reference |
2.7 (1.9 to 3.8) P<0.001 |
1.1 (0.7 to 1.7) P=0.96 |
2.6 (1.4 to 5.1) P=0.02 |
<0.001 |
| Proportion with SpO2 ≤95% – %a | 17 (5.2 to 42) Reference |
30 (15 to 50) P=0.11 |
18 (5.5 to 44) P=1.00 |
32 (9.1 to 70) P=0.44 |
0.07 |
| White blood cell count – · 109/L | 6.3 (6.0 to 6.6) Reference |
7.1 (6.6 to 7.7) P=0.02 |
7.0 (6.3 to 7.7) P=0.15 |
6.7 (5.6 to 7.7) P=0.89 |
0.02 |
Predicted means and proportions are weighted to reflect distribution in the source population (see methods for details).
P-values comparing emphysema subtypes to a control group (absence of emphysema) are adjusted for multiple comparisons using Dunnett’s procedure.
Global p-values are from likelihood ratio tests comparing models with and without emphysema subtype term.
Plethysmography and diffusing capacity were measured on validation participants only.
6MWD was measured on 263 participants, and mMRC and resting SpO2 measured on 297 participants.
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, COPD chronic obstructive pulmonary disease, CI confidence interval, mMRC modified Medical Research Council, FEV1 forced expired volume in the first second, FVC forced vital capacity, RV residual volume, FRC functional residual capacity, TLC total lung capacity, DLCO/VA diffusing capacity of the lung for carbon monoxide divided by alveolar volume, 6MWD six minute walk distance, HU Hounsfield units, and SpO2 pulseoximeter estimated arterial oxygen-hemoglobin saturation.
Table 3.
Clinical Characteristics of MESA COPD Study Participants by Predominant Emphysema Subtype with Additional Adjustment for Percent Predicted FEV1.
| Clinical characteristic | Predicted mean or proportion of clinical characteristic adjusted for age, gender, race-ethnicity, smoking status and percent predicted FEV1† (95% CI) Adjusted P-value for multiple comparisons of subtypes to control group* |
Global P-value** | |||
|---|---|---|---|---|---|
| Absence of emphysema N=205 (73%) |
Centrilobular predominant emphysema N=65 (14%) |
Paraseptal predominant emphysema N=33 (9%) |
Panlobular predominant emphysema N=15 (4%) |
||
| Body mass index | 28 (27 to 29) Reference |
26 (25 to 28) P=0.08 |
28 (26 to 30) P=0.97 |
23 (20 to 26) P=0.008 |
0.006 |
| Pack-years | 31 (28 to 35) Reference |
51 (45 to 57) P<0.001 |
35 (27 to 42) P=0.81 |
32 (20 to 44) P=1.00 |
<0.001 |
| Proportion with mMRC dyspnea scale ≥2 – %a | 5.7 (1.2 to 23) Reference |
9.5 (4.1 to 21) P=0.23 |
3.8 (0.9 to 15) P=0.92 |
7.5 (1.7 to 28) P=0.72 |
0.53 |
| Percent predicted RV‡ | 83 (75 to 90) Reference |
99 (88 to 110) P=0.02 |
93 (76 to 111) P=0.56 |
107 (88 to 126) P=0.05 |
0.01 |
| Percent predicted FRC‡ | 91 (86 to 96) Reference |
109 (101 to 116) P<0.001 |
96 (84 to 108) P=0.80 |
126 (112 to 139) P<0.001 |
<0.001 |
| Percent predicted TLC‡ | 91 (88 to 95) Reference |
103 (98 to 108) P<0.001 |
99 (91 to 107) P=0.14 |
106 (97 to 114) P=0.004 |
<0.001 |
| Percent predicted DLCO/VA‡ | 81 (77 to 85) Reference |
64 (58 to 70) P<0.001 |
81 (72 to 91) P=1.00 |
65 (55 to 75) P<0.001 |
<0.001 |
| Percent predicted 6MWDa | 92 (88 to 95) Reference |
81 (74 to 87) P=0.009 |
89 (81 to 97) P=0.93 |
74 (60 to 88) P=0.04 |
0.002 |
| Percent emphysema-950 HU | 1.0 (0.8 to 1.2) Reference |
2.5 (1.8 to 3.6) P<0.001 |
1.1 (0.7 to 1.6) P=0.99 |
2.5 (1.3 to 4.8) P=0.03 |
<0.001 |
| White blood cell count – ·109/L | 6.3 (6.1 to 6.6) Reference |
7.1 (6.5 to 7.6) P=0.05 |
7.0 (6.3 to 7.7) P=0.21 |
6.6 (5.5 to 7.6) P=0.97 |
0.02 |
Predicted means and proportions are weighted to reflect distribution in the source population (see methods for details).
P-values comparing emphysema subtypes to a control group (absence of emphysema) are adjusted for multiple comparisons using Dunnett’s procedure.
Global p-values are from likelihood ratio tests comparing models with and without emphysema subtype term.
Plethysmography and diffusing capacity were measured on validation participants only.
6MWD was measured on 263 participants, and mMRC and resting SpO2 measured on 297 participants.
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, COPD chronic obstructive pulmonary disease, CI confidence interval, mMRC modified Medical Research Council, FEV1 forced expired volume in the first second, RV residual volume, FRC functional residual capacity, TLC total lung capacity, DLCO diffusing capacity of the lung for carbon monoxide, 6MWD six minute walk distance, HU Hounsfield units, and SpO2 pulse-oximeter estimated arterial oxygen-hemoglobin saturation.
Paraseptal-predominant emphysema was significantly more common among men compared to women, unlike other forms of emphysema (Figure 3B). Participants with paraseptal-predominant emphysema were otherwise similar to those without emphysema, having no increased symptoms or physiologic abnormalities (Table 2 and 3).
Individuals with panlobular-predominant emphysema had a similar history of smoking to participants without emphysema (Figure 3A) but a significantly lower BMI (Figure 3C). Similar to individuals with centrilobular-predominant emphysema, they were more likely to have dyspnea, shorter 6MWD, hyperinflation, lower diffusing capacity, and higher percent emphysema<-950HU compared to those without emphysema (Table 2). Associations of panlobular-predominant emphysema with BMI, percent predicted 6MWD, hyperinflation, lung density, and diffusing capacity remained significant after additional adjustment for percent predicted FEV1 (Table 3).
Adjustment for percent emphysema<-950HU was performed to determine if functional and systemic characteristics significantly associated with predominant subtypes were due to differences in emphysema severity. Subtype-specific associations with BMI, pack-years of smoking, 6MWD, and WBC count remained statistically significant (Table S5).
Emphysema Subtypes and COPD
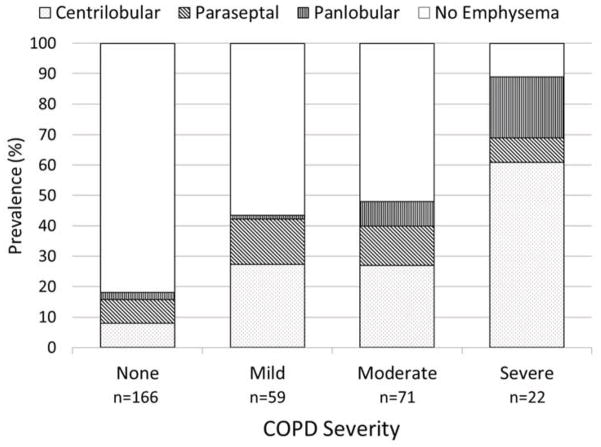
Emphysema prevalence was higher among those with COPD on spirometry (51%), and increased with COPD severity (Figure 4). This increase was due to differences across categories of COPD severity in prevalence of centrilobular (P<0.001) and panlobular emphysema (P=0.007), whereas paraseptal emphysema was similar across stages of COPD (P=0.50).
FIGURE 4. Prevalence of Predominant Emphysema Subtypes among MESA COPD Study Participants by COPD Severity.
Predominant emphysema subtype was defined as the subtype affecting the greatest percentage of lung in each participant and severity of COPD was defined by GOLD spirometric criteria. Proportions are weighted to reflect distribution in the source population (see methods for details).
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, COPD chronic obstructive pulmonary disease, GOLD global initiative for chronic obstructive lung disease.
Emphysema was detected in 17% of participants without COPD on spirometry, in whom paraseptal- and centrilobular-predominant emphysema subtypes were most common (46% and 43%, respectively). Among participants without COPD, those with emphysema were more likely to be current and heavier smokers, have shorter 6MWD and higher WBC count compared to those without emphysema, and DLCO did not differ (Table S6). Similar associations were observed adjusting for age, gender, race/ethnicity and smoking status, however, the higher WBC was no longer statistically significant (Table 4).
Table 4.
Clinical Characteristics of Participants without COPD by Spirometric Criteria by Presence of Emphysema on CT.
| Clinical characteristic | Mean or proportion of clinical characteristic among MESA COPD Study participants without COPD adjusted for age, gender, race-ethnicity, and smoking status (95% CI) | P-value* | |
|---|---|---|---|
| Emphysema absent N=138 |
Emphysema present N=28 |
||
| Body mass index | 28 (26 to 30) | 27 (26 to 29) | 0.41 |
| Pack-years | 31 (27 to 35) | 40 (33 to 47) | 0.03 |
| Proportion with mMRC dyspnea scale ≥2 – %a | 7.0 (2.9 to 11) | 0.0 (0.0 to 9) | 0.36 |
| Percent predicted FEV1 | 99 (95 to 102) | 101 (95 to 107) | 0.51 |
| Percent predicted FVC | 96 (93 to 99) | 100 (94 to 106) | 0.20 |
| FEV1/FVC | 0.78 (0.77 to 0.79) | 0.77 (0.75 to 0.79) | 0.16 |
| Percent predicted RV‡ | 74 (61 to 87) | 86 (69 to 103) | 0.17 |
| Percent predicted FRC‡ | 90 (80 to 101) | 104 (90 to 118) | 0.11 |
| Percent predicted TLC‡ | 89 (82 to 96) | 98 (88 to 107) | 0.07 |
| Percent predicted DLCO/VA‡ | 79 (72 to 86) | 73 (64 to 82) | 0.20 |
| Percent predicted 6MWDa | 94 (90 to 99) | 83 (75 to 91) | 0.01 |
| Percent emphysema<-950HU | 1.0 (0.8 to 1.3) | 0.9 (0.6 to 1.4) | 0.63 |
| Proportion with SpO2 ≤ 95% - % | 3.3 (0.1 to 15) | 12 (2.5 to 40) | 0.10 |
| White blood cell count – ·109/L | 6.2 (5.8 to 6.5) | 6.9 (6.2 to 7.5) | 0.06 |
P-values represent bi-variate comparison of mean or proportion using Chi-square, Fisher’s exact or Student t-test where appropriate.
Plethysmography and diffusing capacity were measured on validation participants only.
6MWD and mMRC dyspnea scale were measured in a subset of participants (n=145 and n=156 participants, respectively).
Abbreviations: MESA denotes Multi-Ethnic Study of Atherosclerosis, COPD chronic obstructive pulmonary disease, CI confidence interval, mMRC modified Medical Research Council, FEV1 forced expired volume in the first second, FVC forced vital capacity, RV residual volume, FRC functional residual capacity, TLC total lung capacity, DLCO/VA diffusing capacity of the lung for carbon monoxide divided by alveolar volume, 6MWD six minute walk distance, HU Hounsfield units, and SpO2 pulse-oximeter estimated arterial oxygen-hemoglobin saturation.
Regional Distribution of Emphysema Subtypes
Centrilobular and paraseptal emphysema severity were greater in the right lung compared to left lung (p<0.001 for both), whereas panlobular emphysema severity did not differ by side (p=0.10). Centrilobular and paraseptal emphysema severity were also greater in higher lung zones than lower lung zones (p<0.001 for both), whereas severity of panlobular emphysema did not vary by lung zone (p=0.84).
DISCUSSION
Characteristics of emphysema subtypes on CT were clinically important and varied substantially by predominant emphysema subtype. Centrilobular and panlobular emphysema were associated with increased dyspnea and lower functional capacity on the 6MWD; however, only centrilobular emphysema was associated with smoking history, and only panlobular emphysema was associated with markedly reduced BMI. In contrast, paraseptal emphysema was associated with no increased symptoms or reduced function, and only differed from controls with respect to a male predominance. In addition, emphysema was observed in a substantial minority of smokers without spirometrically defined COPD and these individuals had a significantly lower 6MWD.
To our knowledge, this is the largest study to date of the reliability and clinical characteristics of visually assessed emphysema subtypes, and has additional strengths of a multicenter and largely population-based design. The metrics of inter-rater reliability observed for total emphysema in the present study are as good, if not better, than prior studies.5,17–19,27,28,48 While these measures of reliability fall below those of automated densitometry,19,28,49 visual assessment at CT is the clinical gold-standard and has been demonstrated to be valid compared to pathologic specimens with centrilobular and panlobular emphysema.15,16,20
The present study observed subtype-specific associations with clinical characteristics that support the notion of distinct pulmonary and systemic phenotypes according to emphysema subtype. For example, cigarette smoking is the cardinal risk factor for centrilobular emphysema but not panlobular emphysema,11,12 and we observed a significantly greater number of pack-years among participants with centrilobular-predominant but not panlobular-predominant emphysema. The panlobular-predominant emphysema subtype was uniquely and strongly associated with lower BMI compared to individuals without emphysema. This finding is consistent with early reports of low body weight adjusted for height among subjects with α-1- antitrypsin deficiency.50,51 Further, in our study, panlobular emphysema was not associated with zonal predominance, whereas centrilobular emphysema was more common in right and upper zones, all of which are consistent with prior pathologic studies.52,53 The paraseptal-predominant emphysema subtype was uniquely associated with significantly greater proportion of men, but no increased dyspnea and no functional impairment. While this study is the first to report gender by emphysema subtype, spontaneous pneumothorax, a condition thought to arise from paraseptal blebs/bullae,14 also occurs more commonly in men.14,54 Finally, individuals with centrilobular-predominant emphysema had the highest WBC count. While COPD was also more common in this group, our finding is consistent with observations by Saetta and colleagues, where resected lung specimens with centrilobular emphysema had significantly higher airway inflammatory cell content compared to specimens with panlobular emphysema.55 Together, these observations suggest that emphysema subtypes on CT have distinct pathophysiology, as well as clinical manifestations.
Emphysema in the absence of COPD is increasingly recognized as a prevalent and clinically important entity.56–59 Studies have demonstrated emphysema in the absence of COPD to be associated with significant physiologic impairment, including left ventricular under filling compatible with low preload state.56,57 De Torres and colleagues observed a specific plasma cytokine profile including lower endothelial growth factor, IL-15, and IL-8, among subjects with emphysema and normal spirometry.58 In the current study 17% of participants without COPD had emphysema. This finding is remarkably similar to a Japanese health screening study that reported an 18.6% prevalence of emphysema on CT among smokers without COPD.59 Furthermore, we observed greater proportion of current smokers, higher number of pack-years, shorter 6MWD, and higher WBC count among non-COPD participants with emphysema. While inflammatory responses to cigarette smoke may contribute to the pathogenesis of this under-recognized clinical entity, further mechanistic studies are needed.
Inter-rater reliability of panlobular emphysema detection was not as good as centrilobular or paraseptal emphysema, which may reflect the uniform destruction of alveolar walls within the pulmonary lobule in panlobular emphysema.3 The lack of visual contrast between normal lung density and emphysematous low attenuation within pulmonary lobules, as is seen in centrilobular emphysema, may make visual assessment of panlobular emphysema difficult unless it is advanced.20 CT densitometry may be better capable of detecting the diffuse low attenuation of panlobular emphysema, particularly at early stages of disease.60 In support of this hypothesis, greater severity of panlobular emphysema in the present study was independently associated with percent emphysema<-950HU despite poor reliability of visual detection.
A limitation of the present study is that we did not assess the validity of our emphysema subtype detection method against pathologic specimens. Gold-standard quantification of multiple emphysema subtypes would require microscopic examination of whole lung specimens and was not performed in this population-based sample. We did, however, observe subtype specific associations with smoking, zonal distribution that were consistent with autopsy descriptions.11,12,52,53
Differences in disease severity assessed by lung function and percent emphysema, in addition to differences in current smoking, across predominant subtypes may have contributed in part to the observed clinical associations; however, associations generally remained significant with additional adjustment for the FEV1, percent emphysema<-950HU and current smoking, the latter being confirmed with cotinine. These findings support a distinct pathobiology and clinical significance of emphysema subtypes as well as emphysema in the absence of COPD.
Measures of reliability can be influenced by the spectrum of disease severity. We reported multiple metrics of reliability. In contrast to prior studies of reliability that included patients undergoing lung resection or autopsy,5,17–19 our study enrolled a population-based sample of current and former smokers, which increases the applicability of our findings and the importance of studying emphysema subtypes early in the course of disease.
In summary, a reliable method for detecting emphysema subtypes at CT identified both novel and classically described clinical characteristics that suggest distinct pathophysiology and clinical significance. Centrilobular and panlobular emphysema were associated with increased symptoms, as well as reduced exercise capacity independent of airflow obstruction; whereas paraseptal emphysema, although common, was of little physiologic significance. Emphysema was also observed in a substantial minority of individuals without COPD and was associated with functional impairment.
Supplementary Material
Clinical Significance.
Centrilobular and panlobular emphysema detected visually on CT were associated with increased symptoms and reduced exercise capacity.
Paraseptal emphysema, although common, was of little physiologic significance.
Emphysema on CT was also observed among 17% of participants without spirometry-defined COPD and was associated with functional impairment.
Acknowledgments
FUNDING SOURCES NIH/NHLBI R01-HL093081, R01-HL077612, R01-HL075476, and N01-HC95159-HC95169. Fonds de la recherche en santé Québec.
Footnotes
DATA ACCESS All authors had access to the data and a role in writing the manuscript
POTENTIAL CONFLICTS OF INTEREST RGB holds grants from the National Institutes of Health (NIH) US Environmental Protection Agency and Alpha-1 Foundation. EAH is a founder and shareholder of VIDA diagnostics, whose software was used in secondary analyses of this paper, and holds grants from NIH, Alpha-1 Foundation and the American Lung Association. JDN holds grants from NIH and Seimens Healthcare, and is a paid consultant with patent pending for VIDA diagnostics. BMS receives funding from the Fonds de la recherche en santé Québec. For the remaining authors, no potential conflicts were declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoyert DL, Xu J. Deaths: Preliminary Data for 2011. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2012;61:1–52. [PubMed] [Google Scholar]
- 2.Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182–5. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- 4.Hayhurst MD, MacNee W, Flenley DC, et al. Diagnosis of pulmonary emphysema by computerised tomography. Lancet. 1984;2:320–2. doi: 10.1016/s0140-6736(84)92689-8. [DOI] [PubMed] [Google Scholar]
- 5.Bergin C, Müller N, Nichols DM, et al. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986;133:541–6. doi: 10.1164/arrd.1986.133.4.541. [DOI] [PubMed] [Google Scholar]
- 6.Leopold JG, Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957;12:219–35. doi: 10.1136/thx.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher CM, Gilson JGPH-J, Scadding JG. Terminology, definitions, and classification of chronic pulmonary emphysema and related conditions: a report of the conclusions of a CIBA guest symposium. Thorax. 1959;14:286–9. [Google Scholar]
- 8.Pratt PC, Kilburn KH. A modern concept of the emphysemas based on correlations of structure and function. Hum Pathol. 1970;1:443–63. doi: 10.1016/s0046-8177(70)80077-6. [DOI] [PubMed] [Google Scholar]
- 9.Heard BE, Khatchatourov V, Otto H, Putov NV, Sobin L. The morphology of emphysema, chronic bronchitis, and bronchiectasis: definition, nomenclature, and classification. J Clin Pathol. 1979;32:882–92. doi: 10.1136/jcp.32.9.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of terms tor thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AE, Jr, Foraker AG. Centrilobular emphysema and panlobular emphysema: two different diseases. Thorax. 1973;28:547–50. doi: 10.1136/thx.28.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AE, Jr, Hernandez JA, Eckert P, Foraker AG. Emphysema in Lung Macrosections Correlated with Smoking Habits. Science. 1964;144:1025–6. doi: 10.1126/science.144.3621.1025. [DOI] [PubMed] [Google Scholar]
- 13.Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–59. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 14.Light RW. Management of spontaneous pneumothorax. Am Rev Respir Dis. 1993;148:245–8. doi: 10.1164/ajrccm/148.1.245. [DOI] [PubMed] [Google Scholar]
- 15.Foster WL, Jr, Pratt PC, Roggli VL, Godwin JD, Halvorsen RA, Jr, Putman CE. Centrilobular emphysema: CT-pathologic correlation. Radiology. 1986;159:27–32. doi: 10.1148/radiology.159.1.3952318. [DOI] [PubMed] [Google Scholar]
- 16.Hruban RH, Meziane MA, Zerhouni EA, et al. High resolution computed tomography of inflation-fixed lungs. Pathologic-radiologic correlation of centrilobular emphysema. Am Rev Respir Dis. 1987;136:935–40. doi: 10.1164/ajrccm/136.4.935. [DOI] [PubMed] [Google Scholar]
- 17.Miller RR, Müller NL, Vedal S, Morrison NJ, Staples CA. Limitations of computed tomography in the assessment of emphysema. Am Rev Respir Dis. 1989;139:980–3. doi: 10.1164/ajrccm/139.4.980. [DOI] [PubMed] [Google Scholar]
- 18.Kuwano K, Matsuba K, Ikeda T, et al. The diagnosis of mild emphysema. Correlation of computed tomography and pathology scores. Am Rev Respir Dis. 1990;141:169–78. doi: 10.1164/ajrccm/141.1.169. [DOI] [PubMed] [Google Scholar]
- 19.Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology. 1999;211:851–8. doi: 10.1148/radiology.211.3.r99jn05851. [DOI] [PubMed] [Google Scholar]
- 20.Spouge D, Mayo JR, Cardoso W, Müller NL. Panacinar emphysema: CT and pathologic findings. J Comput Assist Tomogr. 1993;17:710–3. [PubMed] [Google Scholar]
- 21.Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141:1216–23. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77:58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Haraguchi M, Shimura S, Hida W, Shirato K. Pulmonary function and regional distribution of emphysema as determined by high-resolution computed tomography. Respiration. 1998;65:125–9. doi: 10.1159/000029243. [DOI] [PubMed] [Google Scholar]
- 24.Aziz ZA, Wells AU, Desai SR, et al. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am J Roentgenol. 2005;185:1509–15. doi: 10.2214/AJR.04.1578. [DOI] [PubMed] [Google Scholar]
- 25.Omori H, Nakashima R, Otsuka N, et al. Emphysema detected by lung cancer screening with low-dose spiral CT: prevalence, and correlation with smoking habits and pulmonary function in Japanese male subjects. Respirology. 2006;11:205–10. doi: 10.1111/j.1440-1843.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66:782–7. doi: 10.1136/thx.2010.145995. [DOI] [PubMed] [Google Scholar]
- 27.Barr RG, Berkowitz EA, Bigazzi F, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9:151–9. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavigli E, Camiciottoli G, Diciotti S, et al. Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol. 2009;19:1686–92. doi: 10.1007/s00330-009-1320-y. [DOI] [PubMed] [Google Scholar]
- 29.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 30.Mesia-Vela S, Yeh CC, Austin JH, et al. Plasma carbonyls do not correlate with lung function or computed tomography measures of lung density in older smokers. Biomarkers. 2008;13:422–34. doi: 10.1080/13547500802002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieren JP, Hoffman EA, Baumhauer H, Barr RG, Goldin JG, Rennard S. CT imaging protocol standardization for use in a multicenter study: Spiromics. Radiological Society of North America; Chicago IL: 2011. [Google Scholar]
- 32.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 33.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 34.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 35.Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 36.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multi-ethnic sample of adults: the multiethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137:138–45. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–25. [PubMed] [Google Scholar]
- 38.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–9. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 39.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Rio F, Dorgham A, Pino JM, Villasante C, Garcia-Quero C, Alvarez-Sala R. Lung volume reference values for women and men 65 to 85 years of age. Am J Respir Crit Care Med. 2009;180:1083–91. doi: 10.1164/rccm.200901-0127OC. [DOI] [PubMed] [Google Scholar]
- 41.Vestbo J, Hurd SS, Agusti AG, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease GOLD Executive Summary. Am J Resp Crit Care. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–32. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–7. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2:257–66. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomashow MA, Shimbo D, Parikh MA, et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am J Respir Crit Care Med. 2013;188:60–8. doi: 10.1164/rccm.201209-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 47.Smith BM, Kawut SM, Bluemke DA, et al. Pulmonary Hyperinflation and Left Ventricular Mass: The Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127:1503–11. doi: 10.1161/CIRCULATIONAHA.113.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SS, Seo JB, Lee HY, et al. Chronic Obstructive Pulmonary Disease: Lobe-based Visual Assessment of Volumetric CT by Using Standard Images--Comparison with Quantitative CT and Pulmonary Function Test in the COPDGene Study. Radiology. 2013;266:626–35. doi: 10.1148/radiol.12120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–99. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welch MH, Reinecke ME, Hammarsten JF, Guenter CA. Antitrypsin deficiency in pulmonary disease: the significance of intermediate levels. Ann Intern Med. 1969;71:533–42. doi: 10.7326/0003-4819-71-3-533. [DOI] [PubMed] [Google Scholar]
- 51.Hutchison DC, Cook PJ, Barter CE, Harris H, Hugh-Jones P. Pulmonary emphysema and alpha 1-antitrypsin deficiency. Br Med J. 1971;1:689–94. doi: 10.1136/bmj.1.5751.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snider GL. Pulmonary tuberculosis and centrilobular emphysema. The “upright theory” of apical localization. Arch Intern Med. 1963;111:762–71. doi: 10.1001/archinte.1963.03620300082014. [DOI] [PubMed] [Google Scholar]
- 53.Anderson AE, Jr, Hernandez JA, Holmes WL, Foraker AG. Pulmonary emphysema. Prevalence, severity, and anatomical patterns in macrosections, with respect to smoking habits. Archives of Environmental Health. 1966;12:569–77. doi: 10.1080/00039896.1966.10664435. [DOI] [PubMed] [Google Scholar]
- 54.Melton LJ, 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379–82. doi: 10.1164/arrd.1979.120.6.1379. [DOI] [PubMed] [Google Scholar]
- 55.Saetta M, Kim WD, Izquierdo JL, Ghezzo H, Cosio MG. Extent of centrilobular and panacinar emphysema in smokers’ lungs: pathological and mechanical implications. Eur Respir J. 1994;7:664–71. doi: 10.1183/09031936.94.07040664. [DOI] [PubMed] [Google Scholar]
- 56.Smith BM, Prince MR, Hoffman EA, et al. Impaired Left Ventricular Filling in COPD and Emphysema: Is it the heart or the lungs?: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. Chest. 2013 doi: 10.01378/chest.13-0183. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castaldi PJ, San Jose Estepar R, Mendoza CS, et al. Distinct Quantitative CT Emphysema Patterns are Associated with Physiology and Function in Smokers. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201305-0873OC. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Torres JP, Blanco D, Alcaide AB, et al. Smokers with CT detected emphysema and no airway obstruction have decreased plasma levels of EGF, IL-15, IL-8 and IL-1ra. PLoS One. 2013:8. doi: 10.1371/journal.pone.0060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsushima K, Sone S, Fujimoto K, et al. Identification of occult parechymal disease such as emphysema or airway disease using screening computed tomography. COPD. 2010;7:117–25. doi: 10.3109/15412551003631717. [DOI] [PubMed] [Google Scholar]
- 60.Holme J, Stockley RA. CT scan appearance, densitometry, and health status in protease inhibitor SZ alpha1-antitrypsin deficiency. Chest. 2009;136:1284–90. doi: 10.1378/chest.09-0057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.